Abstract
We conducted a longitudinal examination to assess the relationship between lifestyle habits, including exercise habits, and the incidence of undernutrition after the Great East Japan Earthquake in March 2011. Of the 31,411 participants aged ≥60 years who lived in the municipalities’ evacuation areas before the disaster and had undergone health examinations, 17,622 persons with a body mass index of 20–25 kg/m2 were followed up through the FY 2017 (a mean follow-up of 6.9 years). The analysis involved 13,378 individuals who could be followed. The associations between undernutrition after the disaster and lifestyle factors were estimated via multivariable-adjusted analysis using the Cox proportional hazard regression model. The dependent variable was the proportion of undernutrition after the disaster, whereas independent variables included evacuation, exercise habits/physical activity, alcohol consumption, smoking, meals before bedtime, gastrointestinal surgery history, history of lifestyle-related diseases, and two or more subjective symptoms. In total, 1712 of the 13,378 participants were newly undernourished after the disaster. The statistically significant variables influencing the occurrence of undernutrition were non-evacuation (hazard ratio (HR), 1.31; 95% confidence index (CI) 1.17–1.47), poor exercise habits (HR, 1.14; 95% CI 1.03–1.50), and poor physical activity (HR, 1.12; 95% CI 1.01–1.25). Other significant related variables were drinking habits, surgical history, lifestyle-related diseases, and two or more subjective symptoms. These results suggest that regular exercise and/or physical activity might be important in preventing undernutrition following a disaster, regardless of sex, other lifestyle habits, or past medical history.
Keywords: older people, undernutrition, the Great East Japan earthquake, lifestyle factors, regular exercise, physical activity
1. Introduction
With the increasing age of the Japanese population, extending the healthy life expectancy is a problem. Reportedly, 70% of men and 90% of women in older adults aged 60 years old or more are less independent due to a dysfunction caused by the geriatric syndrome. To maintain the functional independence of the elderly, not only prevention of disease onset but also prevention of geriatric syndrome caused by aging change, are essential issues [1]. Older individuals tend to have a low physical activity, reduced appetite, and low nutrient intake. This results in undernutrition and reduced muscle mass, which in turn lowers the baseline metabolism and limb muscle strength, thus causing reduced walking speed, greater difficulties in getting around, and lower activity. This situation is known as “the frailty cycle” [2,3]. A survey of 5104 people aged 65 years and older (mean age at onset, 71 years old) was conducted in 2011–2012 in Japan. The result showed that 11.3% fell into the category of frailty, according to the Cardiovascular Health Study criteria [4]. The analysis results that integrated four representative large-scale cohort studies using these criteria in Japan indicated that the frailty prevalence in the community-dwelling older population was 7.4%. The frailty prevalence increases with age and markedly increases after 75 years [5]. In contrast, according to the National Health and Nutrition Survey conducted annually by the government, the percentage of undernourishment (BMI ≤ 20 kg/m2) of those aged 65 years or older was 16.4% in 2017. In addition, about 20% of both men and women aged 80 years or older tend to be undernourished [6], which is a major risk factor for health deterioration. Early detection and intervention for undernutrition and decreased activity are important for interrupting this vicious circle. However, there are no studies that showed the actual weight loss and undernutrition status in the evacuation areas after the disaster and the relationship between lifestyle and undernutrition due to evacuation after the disaster.
After the Great East Japan Earthquake (GEJE), on 11 March 2011, and the subsequent accident at the Fukushima Daiichi Nuclear Power Plant, evacuees from the government-designated evacuation areas were forced to move and change their lifestyle behaviors, including diet, exercise, and other personal habits. Many evacuees had to change jobs, and some received inadequate health check-ups and experienced varying degrees of anxiety about their health. Following the disaster, these changes in the living environment have resulted in increased psychological stress and decreased physical activity [7,8,9,10,11]. According to the Fukushima Health Management Survey (FHMS) conducted in the Fukushima Prefecture after the disaster, lifestyle-related diseases were confirmed to worsen with increasing body weight [12,13,14,15,16,17]. Some residents, however, lost weight and reduced their nutrition intake due to the disaster. The FHMS reported that people in the evacuation centers, temporary housing, apartments, etc., consumed less fruit and vegetables, meat, and soybean and dairy products than those who lived in their own or relative’s home [18]. Therefore, the frailty risk is expected to increase in the older evacuees due to aging, reduced physical activity, decreased nutrient intake, and changes in the living environment [19].
Frailty requires long-term care and is affected by the damage caused by a disaster [19]. Thus, weight loss and undernourishment thinness (BMI ≤ 20.0 kg/m2) in the elderly are essential factors of frailty, and it has been reported that lifestyle is related to weight loss and undernourishment [3].
Therefore, in this study, to investigate the factors associated with the undernutrition onset among older people in the evacuation area after the March 2011 disaster, we used the health examinations results before and after the disaster. Subsequently, we followed them until FY 2017, the year after the disaster, to longitudinally examine the relationship between the undernutrition occurrence and lifestyle factors, such as exercise and dietary behavior. It was hypothesized that individuals who had physical activity and exercise habits before the disaster would have a lower undernutrition risk after the disaster.
2. Materials and Methods
2.1. Study Population
The participants were residents aged 60 years or more living in evacuation-designated areas near the Fukushima Daiichi Nuclear Power Plant in the Fukushima Prefecture before March 2011, when the earthquake occurred. The evacuation area included 13 municipalities: Hirono-machi, Naraha-machi, Tomioka-machi, Kawauchi-mura, Okuma-machi, Futaba-machi, Namie-machi, Katsurao-mura, Iitate-mura, Kawamata-machi, Tamura City, Minami-Soma City, and Date City. Within the evacuation area, those aged 40–74 years had enrolled in the national health insurance, and those aged 75 years or more had enrolled in the medical system for the elderly and underwent annual health check-ups.
Between FY 2008, before the disaster, and FY 2017, after the disaster, a total of 254,161 individuals aged 60 years or more underwent medical examinations. Of these, 68,010 examinees aged 60 years or more underwent at least one health check-up before the disaster during FY 2008–2010 (baseline period). We excluded the second and subsequent results if there was more than one visit during this period. We, therefore, included 31,411 participants (14,350 men and 17,061 women; mean age, 69.8 years) who underwent a health examination during this period.
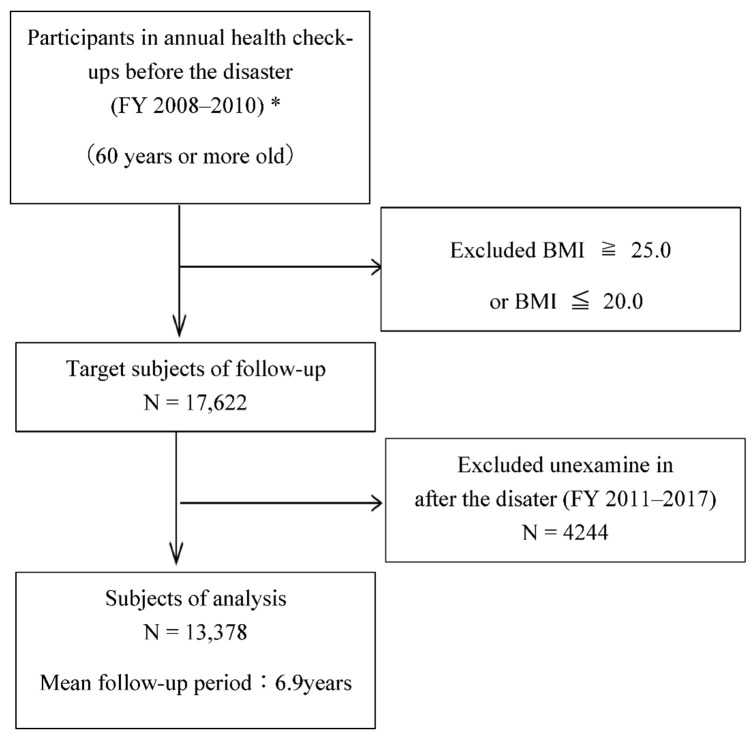
We excluded 13,789 individuals who were overweight (BMI ≥ 25.0 kg/m2) or undernourished (BMI ≤ 20.0 kg/m2); 17,622 were eligible for follow-up examinations that were conducted from FY 2011–FY 2017 after the disaster. Given that 4244 participants did not undergo follow-up examinations, 13,378 (6351 men and 7027 women, 76%) were ultimately eligible for our analysis (Figure 1).
Figure 1.
The selection process for the study patients. * In case of duplication, exclude the data of the second and third exams.
2.2. Measurements/Definitions and Data Collection
The pre-disaster data were provided by the medical examinations conducted by municipalities and included specific health examinations and late-elderly health examinations. For the post-disaster follow-up data, the FHMS and the above data obtained from the municipalities were used. The baseline and follow-up examinations included a medical history review, physical examination, anthropometric measurements, and questionnaire regarding lifestyle behaviors. This study was approved by the Ethics Committee of Fukushima Medical University (#1319, #1916).
2.2.1. Undernutrition
The participants’ body weight and height were measured (with their shoes and excess clothing removed) on the same calibrated scale at baseline and follow-up. BMI was calculated as the body weight (kg) divided by the square of the height (m2). According to the National Health and Nutrition Survey, undernutrition was defined as a BMI ≤ 20.0 kg/m2. Some researchers recommend using BMI as an objective indicator in older individuals [20,21,22]. BMI is not a sensitive indicator in a clinical setting for showing rigorous changes in clinical situations because individuals with a normal or high BMI can have a clinically significant weight loss. Older individuals often have spinal deformity and difficulty standing; therefore, the height measurement required to calculate BMI is unreliable [23]. However, in this study, the target participant was a community-living older individual who could undergo a health check-up. Some studies have used the BMI measured during the health check-up as an indicator of undernutrition [24]. Therefore, this definition was used.
2.2.2. Weight Loss Amount
In previous studies, the weight loss criterion for determining frailty was “2–3 kg in 6 months.” [25,26]. As an “unintended sudden weight loss” for determining geriatric syndrome risk, there was the index “5% weight loss in 6–12 months” [27]. However, identifying the weight loss within such a short period is challenging using the medical examination data in this study. Therefore, the change and rate of change were calculated throughout the observation period as follows: weight loss (≥5 kg or ≥5%) from baseline; amount of weight loss, kg; weight loss per year, kg/year; weight loss rate (loss/weight at baseline); and weight loss rate per year, %/year.
2.2.3. Lifestyle Status
Information on lifestyle factors, including smoking status, exercise habits, physical activities, insufficient sleep, and dietary behaviors, were obtained from a self-administered questionnaire from the standard interview items during the medical checkups [28] and were classified into two categories. Smoking status was shown as regular smoker or nonsmoker. Insufficient sleep was defined by whether the sleep provided rest (yes/no). Dietary behaviors were defined as follows: skipping breakfast (skipping breakfast more than thrice a week), meals before going to bed (having dinner within two hours before going to bed at least thrice a week) and snacking after dinner (more than three snacks after dinner a week). Drinking status was shown by the frequency of drinking and the amount of alcohol consumed per day and was categorized into three groups for the weekly alcohol intake: Never drinks (including quitting); Drinks < 44 g/day; and Drinks > 44 g/day. Other items in the standard interview were as follows: weight gain >10 kg from 20 years of age (yes/no), change in body weight >3 kg in one year (yes/no), and walking speed (faster walking speed than the same age and sex, yes/no). The “Physical Activity reference for Health Promotion 2013” distinguishes between “exercise habits” for sports and physical fitness and those related to living, such as employment, housework, and mobility [29]. The questionnaire items for the medical examination were set according to these criteria: exercise habit was defined by whether the individual lightly sweated and exercised for at least 30 min at least twice a week for more than one year. Physical activity was defined as walking for one or more hours per day or equivalent activity, such as employment, housework, and mobility.
2.2.4. Medical History
Information regarding medical history, surgery, and subjective symptoms was collected through interviews with local public health nurses. A history of gastrointestinal surgery was defined as at least one esophageal occurrence, stomach, duodenal, or colon surgery. A history of lifestyle-related disease was defined as a history of any of the following conditions: hypertension, dyslipidemia, diabetes, hepatic dysfunction, or renal dysfunction. Subjective symptoms over the past year were classified into three categories: none, one, two or more.
2.2.5. Evacuees
There was no information regarding the individuals’ evacuation status based on their residential area in the target municipalities. All residents in areas designated as evacuation areas were defined as evacuees, and those undesignated as evacuation areas were non-evacuees.
2.3. Statistical Analysis
The participants were divided into two groups: undernourished (n = 1712) and not undernourished (n = 13,019). Participants with and without undernutrition were compared using the chi-square test for categorical variables and the t-test for continuous variables. We tested the associations between undernutrition after the disaster and other primary lifestyle factors using a simple, sex/age-adjusted, and multivariate-adjusted analysis using the Cox proportional hazard regression model. Although there were missing values for lifestyle factors, there was no difference in the mean age, BMI, sex distribution, malnutrition, evacuation, exercise habits, and physical activity, even when the missing values were excluded. Therefore, these values were treated as missing, ensuring representativeness. A univariate analysis was performed after deleting the missing data; in the multivariate analysis, the adjustments were made by inserting dummy variables into the missing data. In the multivariate-adjustment model, items associated in a statistically significant manner with undernutrition in the sex-age-adjustment model were employed as adjustment variables. To avoid multicollinearity of exercise habits and physical activity, one of the habits was employed as an adjustment variable if both were statistically significant in a sex-age-adjusted analysis. The condition for censoring was that the follow-up period for undernutrition was defined as the period until the first case of undernutrition was detected. For cases without undernutrition, the condition for censoring was defined as the medical check-up date for the FY 2017 or the final medical check-up date earlier that year. The person years were calculated as the sum of the individual follow-up times until undernutrition incidence or the last examination date.
SAS version 9.4 (SAS Institute, Cary, NC, USA) was employed for all statistical analyses, with two-tailed probability values for the statistical tests. A p-value ≤0.05 was considered statistically significant.
3. Results
3.1. Frequency and Characteristics of Undernutrition after the Disaster
Table 1 indicated the baseline characteristics of the participants with and without undernutrition. Their mean age was 68.4 ± 6.2 years, and 46.6% were men. During the 6.9-year follow-up, 1712 (12.8%) of the participants were undernourished and were significantly older, with a higher percentage of women and a lower percentage of evacuees.
Table 1.
Characteristics at baseline of 13,378 participants with and without incident of undernutrition.
| Total | Undernutrition | Non-Undernutrition | p Values * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants | n | 13,378 | 1712 | (12.8) | 11,666 | (87.2) | |||
| Follow-up period, (years) | 13,378 | 6.9 | (2.2) | 4.9 | (2.0) | 7.2 | (2.1) | <0.001 | |
| Evacuee, (%) | 13,378 | 4586 | (34.3) | 489 | (28.6) | 4097 | (35.1) | <0.001 | |
| Sex(men), n(%) | 13,378 | 6351 | (47.5) | 631 | (36.9) | 5720 | (49.0) | <0.001 | |
| Age, (years) | 13,378 | 68.4 | (6.2) | 68.8 | (6.3) | 68.3 | (6.2) | 0.003 | |
| 75 years old or older, n (%) | 13,378 | 2796 | (20.9) | 389 | (22.7) | 2407 | (20.6) | 0.047 | |
| 80 years old or older, n (%) | 13,378 | 659 | (4.9) | 99 | (5.8) | 560 | (4.8) | 0.08 | |
| Body weight, (kg) | 13,378 | 54.7 | (6.8) | 50.7 | (6.0) | 55.2 | (6.7) | <0.001 | |
| Body mass index, (kg/m2) | 13,378 | 22.7 | (1.3) | 21.3 | (1.0) | 22.9 | (1.3) | <0.001 | |
| Amount of weight loss, (kg) | 13,378 | 0.53 | (3.8) | 4.65 | (3.0) | −0.07 | (3.5) | <0.001 | |
| Rate of weight loss, (%) | 13,378 | 1.01 | (7.0) | 9.02 | (5.4) | −0.16 | (6.4) | <0.001 | |
| Rate of weight loss, (kg/year) | 13,378 | 0.12 | (0.7) | 1.05 | (0.8) | −0.02 | (0.6) | <0.001 | |
| Rate of weight loss, (%/year) | 13,378 | 0.22 | (1.4) | 2.06 | (1.6) | −0.05 | (1.1) | <0.001 | |
| Weight loss ≥ 5 kg, n (%) | 13,378 | 1422 | (10.6) | 645 | (37.7) | 777 | (6.7) | <0.001 | |
| Weight loss ≥ 5 %, n (%) | 13,378 | 3560 | (26.6) | 1268 | (74.1) | 2292 | (19.7) | <0.001 | |
| Exercise habits, n (%) | <30 min/2times/week | 12,488 | 8061 | (64.6) | 1065 | (67.0) | 6996 | (64.2) | 0.03 |
| Physical activities, n (%) | <1 h/day | 12,495 | 7556 | (60.5) | 1010 | (63.5) | 6546 | (60.0) | 0.01 |
| Walking speed, n (%) | fast | 12,490 | 5543 | (44.4) | 693 | (43.6) | 4850 | (44.5) | 0.51 |
| Insufficient sleep, n (%) | yes | 12,532 | 9768 | (77.9) | 1212 | (76.1) | 8556 | (78.2) | 0.06 |
| Weight change from age 20, n (%) | ≥10 kg | 12,493 | 2760 | (22.1) | 161 | (10.1) | 2599 | (23.8) | <0.001 |
| Weight change in 1 year, n (%) | ≥±3 kg | 12,492 | 2055 | (16.5) | 201 | (12.7) | 1854 | (17.0) | <0.001 |
| Meals before going to bed, n (%) | ≥3 times/week | 12,498 | 2835 | (22.7) | 294 | (18.5) | 2541 | (23.3) | <0.001 |
| Snack after dinner, n (%) | ≥3 times/week | 12,514 | 913 | (7.3) | 116 | (7.3) | 797 | (7.3) | 0.98 |
| Lack of breakfast, n (%) | ≥3 times/week | 12,502 | 445 | (3.6) | 60 | (3.8) | 385 | (3.5) | 0.61 |
| Smoking status, n (%) | Current smoker | 13,378 | 1762 | (13.2) | 194 | (11.3) | 1568 | (13.4) | 0.02 |
| Drinking status, n (%) | Non-drinker | 13,378 | 7553 | (56.5) | 1098 | (64.1) | 6455 | (55.3) | <0.001 |
| Current drinker, <44 g/day | 5164 | (38.6) | 547 | (32.0) | 4617 | (39.6) | |||
| Current drinker, <44 g/day | 661 | (4.9) | 67 | (3.9) | 594 | (5.1) | |||
| Digestive surgery, n (%) | yes | 13,378 | 821 | (6.1) | 120 | (7.0) | 701 | (6.0) | 0.11 |
| Lifestyle-related diseases, n (%) | yes | 13,378 | 5586 | (41.8) | 797 | (46.6) | 4789 | (41.1) | <0.001 |
| Subjective symptoms, n (%) | nothing | 13,378 | 10,543 | (78.8) | 1339 | (78.2) | 9204 | (78.9) | 0.04 |
| 1 symptom | 2077 | (15.5) | 254 | (14.8) | 1823 | (15.6) | |||
| 2 or more | 758 | (5.7) | 119 | (7.0) | 639 | (5.5) | |||
Note. Undernutrition; BMI ≤ 20.0 kg/m2, non-undernutrition; BMI > 20.0 kg/m2. The value expressed as mean (standard deviation) or number of people (proportion). * For categorical variables, we used the χ2 test, and Fisher’s exact test was used, and t-test was used for continuous variables.
Compared with the participants without undernutrition, the frequency of meals before going to bed, exercise habits, physical activity, smoking habits, and alcohol consumption were lower among those with undernutrition. The proportion of lifestyle-related diseases was significantly higher among those with undernutrition than among those without undernutrition.
3.2. Lifestyle Factors Associated with Undernutrition
Table 2 shows the sex/age-adjusted and multivariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for each lifestyle factor of undernutrition. The multivariate-adjusted model included variables significantly associated with the sex-age-adjustment model. Since exercise habits and physical activity were categorical variables, we calculated it using Cramer’s V and found it to be 0.753, which we judged to be a strong association. Therefore, if both of these factors were statistically significant in the sex-age-adjustment analysis, only one of them was used as an adjustment variable to avoid multicollinearity.
Table 2.
Hazard ratios (95% confidence intervals) for the incidence of post-disaster undernutrition for lifestyle and sociodemographic factors among 13,378 participants.
| Sex-Age-Adjustment | Multivariable Adjustment (Model 1) * |
Multivariable Adjustment (Model 2) *2 |
Multivariable Adjustment (Model 3) *3 |
Multivariable Adjustment (Model 4) *4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Reference | HR (95% CI) | P Values | HR (95% CI) | p Values | HR (95% CI) | P Values | HR (95% CI) | p Values | HR (95% CI) | p Values |
| Sex (Women) | Men | 1.63 (1.48–1.80) | <0.001 | 1.64 (1.45–1.85) | <0.001 | 1.63 (1.44–1.84) | <0.001 | 1.42 (1.26–1.61) | <0.001 | 1.42 (1.25–1.61) | <0.001 |
| Age | 1 SD (6.2 years) | 1.24 (1.18–1.31) | <0.001 | 1.25 (1.18–1.32) | <0.001 | 1.24 (1.18–1.31) | <0.001 | 1.23 (1.17–1.30) | <0.001 | 1.23 (1.17–1.25) | <0.001 |
| Age (≥75 years old) | <75 years old | 1.60 (1.43–1.79) | <0.001 | ||||||||
| Age (≥80 years old) | <80 years old | 1.96 (1.60–2.41) | <0.001 | ||||||||
| BMI at baseline | −1 SD (1.35 kg/m2) | 0.24 (0.23–0.26) | <0.001 | 0.24 (0.23–0.26) | <0.001 | 0.24 (0.23–0.26) | <0.001 | ||||
| Evacuation (no) | Yes | 1.44 (1.29–1.60) | <0.001 | 1.40 (1.26–1.56) | <0.001 | 1.40 (1.26–1.55) | <0.001 | 1.38 (0.12–1.54) | <0.001 | 1.39 (1.25–1.54) | <0.001 |
| Walking speed (fast) | Slow | 0.94 (0.85–1.03) | 0.18 | ||||||||
| Insufficient sleep (yes) | No | 1.11 (0.99–1.25) | 0.08 | ||||||||
| Exercise habits (<30 min/2 times/week) |
≥30 min/2times/week | 1.16 (1.04–1.29) | 0.006 | 1.14 (1.03–1.27) | 0.02 | 1.11 (1.00–1.24) | |||||
| Physical activities (<1 h/day) | ≥1 h/day | 1.15 (1.04–1.27) | 0.009 | 1.12 (1.01–1.25) | 0.03 | 1.08 (0.98–1.20) | 0.14 | ||||
| Smoking status (yes) | No | 1.20 (1.02–1.41) | 0.03 | 1.16 (0.99–1.37) | 0.07 | 1.16 (0.99–1.37) | 0.07 | 1.01 (0.86–1.19) | 0.93 | 1.01 (0.85–1.18) | 0.95 |
| Drinking status (<44 g/day) | Non-drinker | 0.87 (0.77–0.97) | 0.02 | 0.89 (0.79–1.00) | 0.05 | 0.89 (0.79–1.00) | 0.04 | 0.90 (0.79–1.01) | 0.07 | 0.89 (0.79–1.00) | 0.06 |
| (≥44 g/day) | Non-drinker | 1.03 (0.79–1.34) | 0.86 | 1.07 (0.82–1.39) | 0.64 | 1.06 (0.81–1.39) | 0.66 | 0.96 (0.73–1.25) | 0.75 | 0.95 (0.73–1.25) | 0.71 |
| Meals before going to bed (<3 times/week) |
≥3 times/week | 1.27 (1.12–1.44) | <0.001 | 1.26 (1.11–1.43) | <0.001 | 1.25 (1.10–1.42) | <0.001 | 1.19 (1.05–1.35) | <0.01 | 1.18 (1.04–1.35) | <0.01 |
| Snack after dinner (≥3 time/week) | <3 times/week | 1.01 (0.84–1.22) | 0.90 | ||||||||
| Digestive surgery (yes) | No | 1.27 (1.05–1.53) | 0.01 | 1.24 (1.03–1.50) | 0.02 | 1.24 (1.02–1.49) | 0.03 | 1.02 (0.84–1.23) | 0.85 | 1.02 (0.85–1.23) | 0.83 |
| Lifestyle-related diseases (yes) | No | 1.29 (1.17–1.42) | <0.001 | 1.27 (1.16–1.40) | <0.001 | 1.28 (1.16–1.41) | <0.001 | 1.02 (0.92–1.12) | 0.76 | 1.02 (0.93–1.12) | 0.69 |
| Subjective symptoms (1 symptom) | No symptoms | 0.96 (0.84–1.10) | 0.54 | 0.98 (0.86–1.13) | 0.85 | 0.98 (0.86–1.13) | 0.86 | 0.996 (0.87–1.14) | 0.95 | 0.997(0.87–1.14) | 0.96 |
| (2 or more symptoms) | No symptoms | 1.25 (1.04–1.51) | 0.02 | 1.26 (1.04–1.53) | 0.02 | 1.26 (1.04–1.52) | 0.02 | 1.36 (1.12–1.64) | <0.01 | 1.36 (1.12–1.64) | <0.01 |
Note. HR: hazard ratio; CI: confidence interval; SD: standard deviation. Dependent variable: undernutrition. Independent variable of interest: exercise habits or physical activity. * Model 1: Adjustment variables included in the model: age (continuous variable), sex, evacuation, exercise habits, smoking status, drinking status, meals before going to bed, digestive surgery, lifestyle-related diseases, and subjective symptoms. *2 Model 2: Adjustment variables included in the model: age (continuous variable), sex, evacuation, physical activity, smoking status, drinking status, meals before going to bed, digestive surgery, lifestyle-related diseases, and subjective symptoms. *3 Model 3: Adjustment variables included in the model: age (continuous variable), sex, BMI (at baseline), evacuation, exercise habits, smoking status, drinking status, meals before going to bed, digestive surgery, lifestyle-related diseases, and subjective symptoms. *4 Model 4: Adjustment variables included in the model: age (continuous variable), sex, BMI (at baseline), evacuation, physical activity, smoking status, drinking status, meals before going to bed, digestive surgery, lifestyle-related diseases, and subjective symptoms.
The statistically significant variables influencing the onset of undernutrition in Model 1 were as follows: non-evacuation (HR, 1.31; 95% CI 1.17–1.47), poor (< 30 min/2 times/week) exercise habits (HR, 1.14; 95% CI 1.03–1.27), no/infrequent meals before going to bed (HR, 1.26; 95% CI 1.11–1.43), history of gastrointestinal surgery history (HR, 1.24; 95% CI 1.03–1.50), history of lifestyle-related disease (HR, 1.27; 95% CI 1.16–1.40), and two or more subjective symptoms (HR, 1.26; 95% CI 1.04–1.53). When baseline BMI was added as an adjustment variable in Model 3, the adjusted HR (and 95% CI) of exercise habits remained almost unchanged 1.11 (1.00–1.24; p = 0.005). Furthermore, a multiple regression analysis using the change in BMI as the dependent variable and the same adjustment variables as in Model 1 indicated that gender, age, evacuation, smoking, and late dinners were associated with weight loss. Nevertheless, exercise habit was insignificant (Table S1).
The variables that significantly influenced the onset of undernutrition in Model 2 were as follows: non-evacuation (HR, 1.31; 95% CI 1.17–1.47), poor physical activity (HR, 1.14; 95% CI 1.01–1.25), no/infrequent meals before going to bed (HR, 1.25; 95% CI 1.01–1.42), history of gastrointestinal surgery (HR, 1.24; 95% CI 1.02–1.49), history of lifestyle-related disease (HR, 1.28; 95% CI 1.16–1.41), and two or more subjective symptoms (HR, 1.26; 95% CI 1.04–1.52). In contrast, in Model 4, with the baseline BMI added as an adjustment variable, the adjusted HR (and 95% CI) of physical activity was 1.08 (0.98–1.20; p = 0.142). Furthermore, in a multiple regression analysis, with the change in BMI as the dependent variable (adjusted variables were the same as in Model 2), gender, age, evacuation, and late supper were shown to be associated with weight loss, while exercise habits were not significant (Table S1).
Table 3 indicated the HRs of exercise habits and physical activity with the incidence of undernutrition after the disaster in the multivariate-adjustment model, stratified by each lifestyle factor. Regarding the results of the stratified analysis, the groups that exhibited a significant association between exercise habits/physical activity and undernutrition in each analysis are as follows. In the exercise habit analysis, a significant association (p < 0.05) between exercise habits and undernutrition was found in men (HR, 1.21; 95% CI 1.02–1.44), aged under 75 years (HR, 1.18; 95% CI 1.05–1.33), BMI at baseline <27.0 kg/m2 (HR, 1.15; 95% CI 1.03–1.29), non-evacuation group (HR, 1.19; 95% CI 1.05–1.35), smoking group (HR, 1.54; 95% CI 1.10–2.14), moderate drinking group (HR, 1.22; 95% CI 1.02–1.46), and frequent late dinner group (HR, 1.14; 95% CI 1.02–1.28). The interaction with exercise habit was significant only at baseline BMI. In contrast, for physical activity, there was a significant association (p < 0.05) between physical activity and undernutrition in women (HR, 1.15; 95% CI 1.01–1.31), those under 75 years old (HR, 1.15; 95% CI 1.02–1.29), non-smoking group (HR, 1.14; 95% CI 1.02–1.27), and non-drinking group (HR, 1.19; 95% CI 1.05–1.36). Additionally, no statistically significant interaction with physical activity was confirmed for each lifestyle factor.
Table 3.
Hazard ratios (95% CIs) of exercise habits for undernutrition incidence after disaster among 13,378 participants are stratified by each lifestyle and sociodemographic factor.
| Exercise Habit | Physical Activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥30 min/2times/Week | <30 min/2times/Week | ≥1 h/Day | <1 h/Day | ||||||
| Number of participants | 4427 | 8061 | 4939 | 7556 | |||||
| Number of undernutrition | 525 | 1065 | 580 | 1010 | |||||
| follow-up years | 7.09 | 6.98 | 7.02 | 7.01 | |||||
| Total person years | 31,381 | 56,249 | 34,678 | 53,004 | |||||
| Incidence rate of undernutrition (1000 person years) |
16.7 | 18.9 | 16.7 | 19.1 | |||||
| HR (95% CI) | p values *1 |
p for interaction |
HR (95% CI) | p values *2 |
p for interaction |
||||
| Sex | Men | Reference | 1.21 (1.02–1.44) | 0.03 | 0.48 | Reference | 1.08 (0.92–1.28) | 0.34 | 0.42 |
| Women | Reference | 1.10 (0.96–1.25) | 0.18 | Reference | 1.15 (1.01–1.31) | 0.04 | |||
| Age group | ≥68 years old | Reference | 1.05 (0.90–1.22) | 0.54 | 0.98 | Reference | 1.07 (0.92–1.24) | 0.40 | 0.87 |
| <68 years old | Reference | 1.22 (1.05–1.42) | <0.001 | Reference | 1.16 (1.01–1.34) | 0.04 | |||
| BMI | ≥22.7 | Reference | 0.98 (0.73–1.32) | 0.91 | <0.001 | Reference | 1.04 (0.77–1.39) | 0.81 | 0.87 |
| <22.7 | Reference | 1.15 (1.03–1.29) | 0.02 | Reference | 1.11 (0.99–1.24) | 0.07 | |||
| Evacuation | No | Reference | 1.19 (1.05–1.35) | 0.01 | 0.29 | Reference | 1.10 (0.97–1.24) | 0.13 | 0.37 |
| Yes | Reference | 1.02 (0.85–1.24) | 0.81 | Reference | 1.18 (0.98–1.43) | 0.09 | |||
| Smoking status | No | Reference | 1.10 (0.98–1.23) | 0.09 | 0.08 | Reference | 1.14 (1.02–1.27) | 0.02 | 0.33 |
| Yes | Reference | 1.54 (1.10–2.14) | 0.01 | Reference | 0.98 (0.73–1.32) | 0.90 | |||
| Drinking status | Non-drinker | Reference | 1.09 (0.95–1.24) | 0.22 | Reference | 1.19 (1.05–1.36) | 0.01 | ||
| <44 g/day | Reference | 1.22 (1.02–1.46) | 0.03 | 0.38 | Reference | 1.04 (0.87–1.25) | 0.65 | 0.34 | |
| ≥44 g/day | Reference | 1.46 (0.84–2.54) | 0.18 | 0.35 | Reference | 0.98 (0.59–1.62) | 0.93 | 0.56 | |
| Meals before going to bed | <3 times/week | Reference | 1.16 (0.90–1.47) | 0.25 | 0.67 | Reference | 1.19 (0.94–1.51) | 0.15 | 0.49 |
| ≥3 times/week | Reference | 1.14 (1.02–1.28) | 0.03 | Reference | 1.11 (0.99–1.25) | 0.07 | |||
Note. *1 Adjustment variables included in the model: age (continuous variable), sex, evacuation, exercise habits, smoking status, drinking status, meals before going to bed, digestive surgery, lifestyle-related diseases, and subjective symptoms. *2 Adjustment variables included in the model: age (continuous variable), sex, evacuation, physical activity, smoking status, drinking status, meals before going to bed, digestive surgery, lifestyle-related diseases, and subjective symptoms.
4. Discussion
4.1. Exercise Habits and Physical Activities
In this study, inadequate exercise habits and physical activities affected undernutrition onset after the disaster in older adults. Alternatively, the physical activity was not significantly different when adjusted for baseline BMI. This shows that exercise habits are an important preventive factor for undernutrition among older adults. The interaction with exercise habits was significant only at baseline BMI. Thus, to prevent undernutrition among the elderly after a disaster, it may be necessary to consider the possibility that some elderly people, without an exercise habit to begin with, could be at high risk of undernutrition, regardless of their sex, age, drinking and smoking status, and medical history.
After adjusting for BMI, the association between undernutrition and physical activity disappeared, and only exercise habits were associated with the occurrence of undernutrition. This result proposes that it is important to maintain muscle mass through more intense exercise, rather than physical activity as an extension of daily activities, to prevent undernutrition.
On the other hand, although physical activity was not associated with the occurrence of undernutrition after adjusting for BMI, there is still a possibility that physical activity before the disaster may work to prevent undernutrition. A systematic review found that physical activity promotes appetite control and balanced energy intake [30]. It also reports that physical activity is the least expensive day drug therapy because enhanced physical activity lowers the depression risk and shows a preventive effect [31]. In addition, although undernutrition is one of the risks for decline in life functions in the elderly, it has been reported that increasing the amount of daily physical activity can decrease the risk of age-related decline in life functions, including reduced motor function and dementia, and that people who practice a physically active lifestyle can live longer independently by following a physically active lifestyle [29,32,33,34]. Additionally, exercise habits have been associated with enhanced quality of life and decreased risk of upper respiratory tract infections in older adults. Interventions such as improving walking speed and increasing physical activity improve frailty [35,36,37]. Thus, we believe that having exercise habits and physical activity in daily life is essential for elderly people who are vulnerable to disasters, to reduce the risk of undernutrition caused by the decline in physical function and to improve their QOL, although the mechanisms and the range of effects are different.
4.2. Relationship between Exercise Habits and BMI
In this study, the analysis results, with the occurrence of undernutrition as the outcome, differed from those with the change in BMI as the dependent variable. This result indicates that the effect of exercise habits and physical activity varies depending on the BMI level of the baseline. In fact, as shown in Table 3, when the BMI was analyzed in two groups, exercise habit and physical activity were not associated with undernutrition development in the group with higher-than-average BMI, whereas, they were associated with the development of undernutrition in the group with lower-than-average BMI. In other words, exercise habits and physical activity may not be involved in weight maintenance in all people but may also contribute to weight loss suppression in people who initially had a low BMI and were at high risk of emaciation.
4.3. Association with Evacuation
The prevalence of undernutrition was low among the evacuees after the disaster. According to the FHMS for the same evacuated area residents, a large proportion of the people from the evacuation areas gained weight after the disaster [12,13,14,15,16,17]. The study results show that, in the evacuation areas, even older adults gained weight. However, a certain number of people in the evacuation areas were undernourished. After the disaster, life in the shelters was prolonged, with meals high in carbohydrates [14]. The FHMS reported that living in non-house conditions after the disaster was associated with a poor dietary intake of fruits and vegetables, meat, and soybean, as well as dairy products [18]. A study conducted in Miyagi Prefecture after the GEJE also proposed that while the provision of boxed lunches may supplement the calories and protein that are often lacking in evacuation centers, it may also be insufficient in providing vitamins and vegetables, proposing that there are limits to the nutrients that can be provided using boxed lunches alone [11,38]. Thus, in an environment where the content and form of meals cannot be selected, the evacuees had to eat the available food, regardless of their nutritional balance. The evacuees were therefore likely to gain weight. However, the nutritional intake might decrease in older adults with poor gastrointestinal or swallowing/chewing function. One of the problems was that the disaster management teams tended to focus on gaining weight and may have delayed their response to undernutrition. In situations such as disasters, it is essential not to miss such cases of undernutrition.
4.4. Late Meals
The tendency to eat meals before going to bed fewer than three times a week was a risk factor for undernutrition. Eating within two hours of bedtime is, however, regarded as a dietary habit that promotes obesity due to fat accumulation that results from insulin secretion and action of the appetite hormone leptin. In fact, eating late is known to be an unhealthy eating habit that contributes to obesity and early eating and snacking, and it has been reported to be associated with visceral fat accumulation and obesity [39]. On the other hand, older people have a higher undernutrition risk and/or weight loss than the young counterparts. Therefore, delaying dinner might help prevent undernutrition and weight loss. Nevertheless, going to bed immediately after a meal is not recommended for the long term because it degrades sleep quality and strains the gastrointestinal tract. Therefore, reviewing the dietary balance and lifestyle of older adults is an important step.
4.5. Comparison between the Affected Areas and the Rest of Japan
Since this study was conducted on residents in the evacuation areas and does not directly compare the results with national data, the results obtained in this study on the relationship between the occurrence of undernutrition and exercise and other lifestyle habits cannot be applied to the entire country. However, it was the case that the number of obese people in the evacuation areas, who are the subject of this study, increased more rapidly after the evacuation than in the rest of Japan [12]. In other words, it is essential to note that even in the evacuation area, where the percentage of obese people is much higher than in the rest of Japan, a certain percentage of undernourished people are found, and the results of this study indicate that lifestyle habits, such as exercise, are preventively involved. Since there are more people with undernutrition than the subjects of this study in Japan, it is necessary to verify whether there is a similar association outside the evacuation area. Additionally, it is believed that the results of this study are findings that can be applied to health management of the elderly in Japan, where disasters are common.
4.6. Study Limitations
Firstly, since this study was conducted on residents in the evacuation area and did not directly compare the results with national data, the results obtained in this study on the relationship between undernutrition occurrence and exercise, as well as other lifestyle habits, cannot be applied to the whole of Japan. Although obesity tends to increase after a disaster, and conscious measures are being taken, it is necessary to pay attention to the problem of thinness in the elderly. The results of this study may be applied to health management of the elderly in Japan, where disasters are common. Secondly, in this study, we only dealt with health check-up data, not death data, so the mortality rates are unknown. However, it is expected that there may have been a large number of people who died at an old age who were undernourished. In this case, there is a possibility of estimating a lower frequency of undernutrition. Thirdly, high-risk undernourished adults are more likely not to have undergone a physical check-up, which may be a selection bias in the target population. It has been reported that the most common reason for not undergoing a health check-up is “because I am visiting a medical institution”, and the proportion tends to increase with age, showing a low rate of health check-ups [40,41]. When the characteristics of the 13,378 people who could be followed in this study were compared with those of the 4244 people who could not be followed, the average age was 68.4 vs. 73.3 years, and the subjects who could not be followed were older, so the above hypothesis is possible. The possibility that there is a large percentage of healthy people among those who receive medical check-ups cannot be denied. Consequently, the overall results may be biased toward health, and the percentage of people with a tendency toward undernutrition may be underestimated. It is believed that the effects of exercise habits and physical activity would be more important even if the subjects of this study were a healthier population among the elderly. There was no difference in the distribution of sex, exercise habits, or physical activity in this comparison. Fourth, the undernutrition in certain cases was attributable to other diseases (other than lifestyle diseases); however, those patients from our analysis who might have affected the results could not be ruled out. Fifth, this study employed items from health check-ups. Therefore, there was a lack of detailed information on nutrient intake, which is strongly associated with weight loss and undernutrition. However, these present results were obtained, although only information on eating behavior was used, which suggests that the influence of diet may be underestimated but not overestimated. Sixth, the estimated weight loss (a risk factor for older adults) was 3–5 kg or 5% reduction in body weight over the previous six months to one year [25]. However, the follow-up period differed among the participants, making it challenging to determine how much weight loss occurred and over what period. Lastly, the effects of exercise habits were examined before the disaster (baseline), but the alternations changes in exercise habits after the disaster were not assessed. Therefore, whether the continuation of exercise habits affected the onset of undernutrition could not be determined. It will be necessary to examine, in the future, whether the continuation of exercise habits and physical activity is associated with preventing undernutrition occurrence.
5. Conclusions
The associations between the prevalence of undernutrition and related lifestyle factors after the disaster were examined using health check-up data for residents aged 60 years and above living in the evacuation areas before the disaster. The results suggested that the evacuation itself is associated with the post-disaster undernutrition occurrence, and those that have exercise habits and physical activity before the disaster may prevent post-disaster undernutrition, regardless of other lifestyle habits and gender.
Acknowledgments
We express our deep gratitude to the expert committee members, advisors, staff of the Fukushima Health Survey Group, and municipalities in the evacuation area for conducting this survey and for their support. The findings and conclusions of this article are solely the authors’ responsibility and do not represent the official views of the Fukushima Prefectural Government.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijerph19063399/s1. Table S1: Multiple regression analysis with the change in BMI from baseline as the dependent variable; Table S2: Comparison of the average change in BMI for each factor such as lifestyle factors.
Author Contributions
Conception and methodology, K.O., T.O.; validation, H.N., F.H. and M.N.; formal analysis, K.O.; investigation, A.S., M.S., J.J.K., A.T.; resources, A.S., M.S., J.J.K., A.T.; data curation, A.S., M.S., J.J.K., A.T.; writing—original draft preparation and visualization, K.O.; writing—review and editing, T.O.; supervision, T.O., A.S., M.S., J.J.K., A.T., H.N., F.H., M.N., S.Y., H.O. and K.K.; Project administration and funding acquisition, S.Y., H.O. and K.K.; S.Y., H.O. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National “Health Fund for Children and Adults Affected by the Nuclear Incident,” and a Grant-in-Aid for Scientific Research (M019K10626) from the Japan Society for the Promotion of Science.
Institutional Review Board Statement
This study protocol was approved by the Fukushima Medical University Ethics Committee (approval no. 1319, 1916).
Informed Consent Statement
Informed consent was obtained from the community representatives to conduct an epidemiologic study based on the guidelines of the Council for International Organizations of Medical Science.
Data Availability Statement
The datasets analyzed during the present study are not publicly available because the data from the Fukushima Health Management Survey belongs to the government of Fukushima Prefecture and can only be used within the organization.
Conflicts of Interest
The authors declare that there are no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akiyama H. Concept of science and society in the age of longevity. Kagaku. 2010;80:59–64. [Google Scholar]
- 2.Xue Q.L., Bandeen-Roche K., Varadhan R., Zhou J., Fried L.P. Initial manifestations of frailty criteria and the development of frailty phenotype in the women’s health and aging study II. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 3.Fried L.P., Walston J. Frailty and failure to thrive. In: Hazzard W.R., Blass J.P., Ettinger W.H. Jr., Halter J.B., Ouslander J., editors. Principles of Geriatric Medicine and Gerontology. McGraw Hill; New York, NY, USA: 1998. pp. 1387–1402. [Google Scholar]
- 4.Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Ito T., Lee S., Park H., et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013;14:518–524. doi: 10.1016/j.jamda.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Kojima G., Iliffe S., Taniguchi Y., Shimada H., Rakugi H., Walters K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017;27:347–353. doi: 10.1016/j.je.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health, Labor and Welfare Summary of the Results of the 2017 National Health and Nutrition Survey. [(accessed on 11 September 2019)];2018 September 11; Available online: https://www.mhlw.go.jp/content/10904750/000351576.pdf.
- 7.Yasumura S., Abe M. Fukushima health management survey and related issues. Asia Pac. J. Public Health. 2017;29:29S–35S. doi: 10.1177/1010539516687022. [DOI] [PubMed] [Google Scholar]
- 8.Utsukushima Fukushima Future Support Center . National University Corporation Fukushima University; 2015. [(accessed on 28 October 2019)]. Report on the Survey and Research Project on the Necessary Support by Analyzing the Causes of the Increase in the Number of People Certified as Needing Long-Term Care in Fukushima Prefecture. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-12300000-Roukenkyoku/0000140299.pdf. [Google Scholar]
- 9.Shimizu S., Tamamura K. The elderly people and the Great East Japan disaster of March 11, 2011. Bull. Nara Univ. Educ. 2013;62:59–70. [Google Scholar]
- 10.Tanba F. Current Status and Issues of Evacuees in the Accident at TEPCO’s Fukushima Daiichi Nuclear Power Plant. 32nd Atomic Energy Commission: Document 1–3. [(accessed on 20 December 2019)]. Available online: http://www.aec.go.jp/jicst/NC/iinkai.teirei/siryo2013/siryo32/siryo1-3.pdf.
- 11.Mihara M., Harada M., Oka J., Tsuboyama-Kasaoka N. The effect of lunch box provision and mass feeding on energy and nutrient supply at emergency shelters after the Great East Japan Earthquake. Nihon Koshu Eisei Zasshi. 2019;66:629–637. doi: 10.11236/jph.66.10_629. [DOI] [PubMed] [Google Scholar]
- 12.Ohira T., Hosoya M., Yasumura S., Satoh H., Suzuki H., Sakai A., Ohtsuru A., Kawasaki Y., Takahashi A., Ozasa K., et al. Effect of evacuation on body weight after the Great East Japan Earthquake. Am. J. Prev. Med. 2016;50:553–560. doi: 10.1016/j.amepre.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Ohira T., Hosoya M., Yasumura S., Satoh H., Suzuki H., Sakai A., Ohtsuru A., Kawasaki Y., Takahashi A., Ozasa K., et al. Evacuation and risk of hypertension after the Great East Japan Earthquake: The Fukushima Health Management Survey. Hypertension. 2016;68:558–564. doi: 10.1161/HYPERTENSIONAHA.116.07499. [DOI] [PubMed] [Google Scholar]
- 14.Satoh H., Ohira T., Hosoya M., Sakai A., Watanabe T., Ohtsuru A., Kawasaki Y., Suzuki H., Takahashi A., Kobashi G., et al. Evacuation after the Fukushima Daiichi nuclear power plant accident is a cause of diabetes: Results from the Fukushima health management survey. J. Diabetes Res. 2015;2015:627390. doi: 10.1155/2015/627390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh H., Ohira T., Nagai M., Hosoya M., Sakai A., Watanabe T., Ohtsuru A., Kawasaki Y., Suzuki H., Takahashi A., et al. Hypo-high-density lipoprotein cholesterolemia caused by evacuation after the Fukushima Daiichi nuclear power plant accident: Results from the Fukushima Health Management Survey. Intern. Med. 2016;55:1967–1976. doi: 10.2169/internalmedicine.55.6030. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto S., Nagai M., Fukuma S., Ohira T., Hosoya M., Yasumura S., Satoh H., Suzuki H., Sakai A., Ohtsuru A., et al. Influence of Post-disaster Evacuation on Incidence of Metabolic Syndrome. J. Atheroscler. Thromb. 2017;24:327–337. doi: 10.5551/jat.35824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi A., Ohira T., Hosoya M., Yasumura S., Nagai M., Ohira H., Hashimoto S., Satoh H., Sakai A., Ohtsuru A., et al. Effect of evacuation on liver function after the Fukushima Daiichi Nuclear Power Plant accident: The Fukushima Health Management Survey. J. Epidemiol. 2017;27:180–185. doi: 10.1016/j.je.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Ohira T., Abe M., Kamiya K., Yamashita S., Yasumura S., Ohtsuru A., Masaharu M., Harigane M., Horikoshi N., et al. Evacuation after the Great East Japan Earthquake was associated with poor dietary intake: The Fukushima Health Management Survey. J. Epidemiol. 2017;27:14–23. doi: 10.1016/j.je.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsubota-Utsugi M., Yonekura Y., Tanno K., Nozue M., Shimoda H., Nishi N., Sakata K., Kobayashi S., RIAS Study Association between health risks and frailty in relation to the degree of housing damage among elderly survivors of the great East Japan earthquake. BMC Geriatr. 2018;18:133. doi: 10.1186/s12877-018-0828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa-Silva M.C. Subjective and objective nutritional assessment methods: What do they really assess? Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:248–254. doi: 10.1097/MCO.0b013e3282fba5d7. [DOI] [PubMed] [Google Scholar]
- 21.Sasazuki S., Inoue M., Tsuji I., Sugawara Y., Tamakoshi A., Matsuo K., Wakai K., Nagata C., Tanaka K., Mizoue T., et al. Body Mass Index and Mortality from All Causes and Major Causes in Japanese: Results of a Pooled Analysis of 7 Large-Scale Cohort studies. J. Epidemiol. 2011;21:417–430. doi: 10.2188/jea.JE20100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoyama Y., Sasaki M., Sato K. Nutrition intake among the Japanese elderly: An intergenerational comparison based on national health and nutrition survey scores. Ann. Hum. Biol. 2019;46:311–322. doi: 10.1080/03014460.2019.1662943. [DOI] [PubMed] [Google Scholar]
- 23.Cook Z., Kirk S., Lawrenson S., Sandford S. Use of BMI in the assessment of undernutrition in older subjects: Reflecting on practice. Proc. Nutr. Soc. 2005;64:313–317. doi: 10.1079/PNS2005437. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama Y., Kitamura A., Kawano Y., Shinkai S. Dietary intake and nutritional status among Japanese elderly participants in the National health and nutritional survey Japan 2003–2011. J. Jpn. Soc. Shokuiku. 2018;12:33–40. [Google Scholar]
- 25.Japan Geriatrics Society Statement of the Japan Geriatrics Society on Frailty. May 13, 2014. [(accessed on 3 December 2019)]. Available online: https://www.jpn-geriat-soc.or.jp/info/topics/pdf/20140513_01_01.pdf.
- 26.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 27.Gaddey H.L., Holder K. Unintentional weight loss in older adults. Am. Fam. Physician. 2014;89:718–722. [PubMed] [Google Scholar]
- 28.Ministry of Health, Labor and Welfare Standard health screening and health Guidance Program. Final Version. Part 2 Medical Examination Attachment 3 Standard Questionnaire. [(accessed on 4 January 2020)];2007 :45–46. Available online: https://www.mhlw.go.jp/bunya/kenkou/seikatsu/pdf/02.pdf.
- 29.Ministry of Health, Labor and Welfare Physical Activity Reference for Health Promotion 2013. [(accessed on 10 January 2020)];2013 Available online: https://www.mhlw.go.jp/content/000306883.pdf.
- 30.Hubner S., Boron J.B., Koehler K. The effects of exercise on appetite in older adults: A systematic review and meta-analysis. Front. Nutr. 2021;8:734267. doi: 10.3389/fnut.2021.734267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianfredi V., Blandi L., Cacitti S., Minelli M., Signorelli C., Amerio A., Odone A. Depression and objectively measured physical activity: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2020;17:3738. doi: 10.3390/ijerph17103738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T., Tsumura K., Suematsu C., Okada K., Fujii S., Endo G. Walking to work and the risk for hypertension in men: The Osaka Health Survey. Ann. Intern. Med. 1999;131:21–26. doi: 10.7326/0003-4819-131-1-199907060-00005. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S., Kojima M., Tokudome S., Mori M., Sakauchi F., Fujino Y., Wakai K., Lin Y., Kikuchi S., Tamakoshi K., et al. Effect of physical activity on breast cancer risk: Findings of the Japan collaborative cohort study. Cancer Epidemiol. Biomark. Prev. 2008;17:3396–3401. doi: 10.1158/1055-9965.EPI-08-0497. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi H., Kuriyama S., Tsubono Y., Nakaya N., Fujita K., Nishino Y., Shibuya D., Tsuji I. Time spent walking and risk of colorectal cancer in Japan: The Miyagi Cohort study. Eur. J. Cancer Prev. 2007;16:403–408. doi: 10.1097/01.cej.0000236249.63489.05. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum S., Sherrington C. Is exercise effective in promoting mental well-being in older age? A systematic review. Br. J. Sports Med. 2011;45:1079–1080. doi: 10.1136/bjsports-2011-090466. [DOI] [PubMed] [Google Scholar]
- 36.Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exerc. Sport Sci. Rev. 2009;37:157–164. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron I.D., Fairhall N., Langron C., Lockwood K., Monaghan N., Aggar C., Sherrington C., Lord S.R., Kurrle S.E. A multifactorial interdisciplinary intervention reduces frailty in older people: Randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazuhiro N. Current status of diets and nutritional issues in disaster areas: Report on the areas affected by the Great East Japan Earthquake (Kesennuma City, Miyagi Prefecture) J. Jpn. Diabetes Soc. 2011;54:724–726. [Google Scholar]
- 39.Ishida Y., Yoshida D., Honda T., Hirakawa Y., Shibata M., Sakata S., Furuta Y., Oishi E., Hata J., Kitazono T., et al. Influence of the accumulation of unhealthy eating habits on obesity in a general japanese population: The Hisayama study. Nutrients. 2020;12:3160. doi: 10.3390/nu12103160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubota K., Okubo T., Satoh Y., Hirose T., Imai Y. Reasons for not receiving the specific health examination and health attitudes of those who have not received the examination in Hanamaki City, Iwate Prefecture. J. Health Welf. Stat. 2021;57:1–6. [Google Scholar]
- 41.Goto M., Takeda M., Kainuma Y., Suijo Y. Factors and Countermeasures for undiagnosed patients based on questionnaire survey of undiagnosed patients for specific health examination. Indic. Health Welf. 2022;58:34–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the present study are not publicly available because the data from the Fukushima Health Management Survey belongs to the government of Fukushima Prefecture and can only be used within the organization.