Abstract
The Zika virus (ZIKV) was first isolated from a rhesus macaque in the Zika forest of Uganda in 1947. Isolated cases were reported until 2007, when the first major outbreaks of Zika infection were reported from the Island of Yap in Micronesia and from French Polynesia in 2013. In 2015, ZIKV started to circulate in Latin America, and in 2016, ZIKV was considered by WHO to be a Public Health Emergency of International Concern due to cases of Congenital Zika Syndrome (CZS), a ZIKV-associated complication never observed before. After a peak of cases in 2016, the infection incidence dropped dramatically but still causes concern because of the associated microcephaly cases, especially in regions where the dengue virus (DENV) is endemic and co-circulates with ZIKV. A vaccine could be an important tool to mitigate CZS in endemic countries. However, the immunological relationship between ZIKV and other flaviviruses, especially DENV, and the low numbers of ZIKV infections are potential challenges for developing and testing a vaccine against ZIKV. Here, we discuss ZIKV vaccine development with the perspective of the immunological concerns implicated by DENV-ZIKV cross-reactivity and the use of a controlled human infection model (CHIM) as a tool to accelerate vaccine development.
Keywords: Zika, dengue, cross-reactivity, vaccine, pathogenesis, CHIM
1. Introduction
Zika virus (ZIKV) was initially isolated from a rhesus macaque in the Zika forest of Uganda in 1947 [1]. It is an arbovirus from the Flaviviridae family and, together with other Flaviviruses like dengue (DENV) and yellow fever (YFV), poses as one of the major public health problems in Latin America. Other important flaviviruses of public health concerns include West Nile virus (WNV) and Japanese encephalitis virus (JEV), which together with DENV and ZIKV, are considered emerging tropical viruses. ZIKV is transmitted by the female Aedes aegypti or Aedes Albopictus mosquito, both being widely distributed in Latin America [2]. However, other routes of transmission are also described including blood transfusion, sexual transmission, and transmission via breast milk [3,4,5].
Sporadic reports of natural Zika and/or serologic evidence of ZIKV infection have been reported since its discovery [6]. The first major outbreak of ZIKV was reported from the Island of Yap in Micronesia in 2007 where it was estimated that 72.6% of the population ≥3 years of age was infected, demonstrating the rapid transmission of ZIKV in a naïve population [7]. A second major outbreak occurred in French Polynesia from October of 2013 through early 2014, when it was estimated that 28,000 ZIKV infections occurred (~11% of the population) [8].
ZIKV began to circulate in Latin America between 2013 and 2014. In March of 2014, Chilean public health authorities confirmed that ZIKV infection was detected in cases reported in February, concurrent with the circulation of the virus in French Polynesia [9]. Indeed, the strain of ZIKV circulating in Latin America from 2014 to 2016 is related to the French Polynesia strain, which is estimated to have arrived in Latin America in 2013 [10]. Clinical cases of ZIKV started to be reported in Brazil in October 2014 after cases of disease presenting with low-grade fever, exanthema, pruritus, arthralgia, and limb edema tested negative for dengue, yellow fever, measles, rubella, enterovirus and chikungunya in Rio Grande do Norte state. After cases were also reported in Bahia state, the identification of ZIKV as the aetiological agent of the new disease was confirmed in May of 2015 [11]. In response, PAHO issued an epidemiological alert of ZIKV infection with recommendations for clinical management and prevention and control measures. The Brazilian Ministry of Health started to receive notification of increased frequencies of microcephaly in areas where ZIKV was circulating and an epidemiological investigation was started [12]. In December of 2015, PAHO, together with the Brazilian Ministry of Health, recognized the epidemiological association between ZIKV infection in pregnant women and microcephaly in newborns and released another epidemiological alert [13]. After confirmation of ZIKV-induced microcephaly, the WHO issued a Public Health Emergency of International Concern (PHEIC) on 1 February 2016, attracting greater attention and scientific resources for this epidemic. Anecdotal evidence of ZIKV and microcephaly began appearing in the literature in January of 2016 [14], and was confirmed by subsequent stronger epidemiological and virological evidence [15,16].
Several seroprevalence studies have shown that Zika incidence may have reached up to 70–80% of the population in Latin American countries and that its introduction was silent, especially when introduced in dengue-endemic regions. Recently, a new immunological survey of undergraduate students using humoral and cellular tests has identified ZIKV-positivity in more than 80% of samples that could at least partially differentiate DENV and ZIKV infections [17]. These numbers are not far from those reported in Brazil, where a serological survey estimated that ZIKV seroprevalence exceeded 60% in Salvador (state of Bahia) [18]. Likewise, the prevalence of flavivirus infections was estimated to be around 92% in the state of Ceará in 2018, of which only 37% were considered to be associated with DENV [19].
ZIKV has been reported in 87 countries with autochthonous transmission of the virus in the Americas, the Caribbean, Asia and Africa [20]. However, since the Latin American peak of infections in 2016, the number of reported cases has decreased dramatically. While in 2016, Brazil alone reported over 273,000 Zika cases to PAHO, in 2017 the number of cases dropped to 31,000. Since, it has varied from 18,000 to 31,000 cases per year, showing signs of stabilization [21]. Unfortunately, this means that ZIKV is still circulating and is probably under-reported. Nevertheless, Zika is still causing the devastating effects of Congenital Zika Syndrome (CZS). A serological survey in Recife (Pernambuco, Brazil), the epicenter of CZS, performed in 2018, estimated that while the prevalence of anti-ZIKV IgM in pregnant women in the general population was around 1.6%, therefore higher than expected for official case numbers, ZIKV-associated complicated pregnancies was around 7% [22], suggesting that health policies to address congenital Zika are still very relevant.
2. Clinical Presentation
The clinical presentation of ZIKV infection is mostly asymptomatic or oligosymptomatic. It is estimated that 80% of the cases do not seek health care [23]. In symptomatic cases, ZIKV generally causes a mild infection characterized by rash, low-grade fever, non-purulent conjunctivitis and myalgia [7,16]. Nearly all symptomatic patients from the Yap Island outbreak, for example, presented with rash (90%), arthritis/arthralgia (65%), and fever (65%). Unlike dengue or yellow fever, Zika does not cause hemorrhagic manifestations, vascular leak syndrome, or liver function abnormalities. Only approximately 19% of subjects found to be seropositive to ZIKV in a serosurvey from Yap Island recounted being symptomatic with a Zika-like illness [7]. In October of 2013, the largest outbreak of ZIKV recorded up to that time began in French Polynesia [8,24]. It was estimated that 28,000 ZIKV symptomatic infections occurred (~11% of the island population) with most infections presenting with low-grade fever, rash, arthralgia and conjunctivitis [24]. However, household surveys and serological investigations estimated that 73% of French Polynesia residents have been infected [7].
The French Polynesia outbreak revealed that ZIKV infection was also associated with Guillain–Barré Syndrome (GBS) with an estimated incidence of 1 up to 2.4 GBS cases per 10,000 ZIKV infections [8,24,25,26]. Similar numbers were estimated in the Latin American outbreak, which found 2.0 GBS cases per 10,000 ZIKV infections. It was calculated that ZIKV infection is associated with up to 10× higher incidence than those observed in the general population [27]. ZIKV-associated GBS was related to increased frequencies of facial weakness and paresthesia, dysphagia, shortness of breath, admission to intensive care unit and required mechanical ventilation when compared to non-ZIKV GBS [28].
The most devasting effect of Zika, however, is CZS. It was first noticed as an increased incidence of microcephaly in newborns from the Brazilian states where ZIKV was circulating [29]. The historical prevalence of microcephaly was around 0.6 cases per 10,000 live births and, in 2015, reached 2.8 cases per 10,000 [29]. Soon, it became clear that in addition to decreased head circumference and decreased birth weight, newborns from mothers who had gestational Zika developed several neurological, osteoskeletal, ophthalmic and many other abnormalities, including fetal death [30].
3. Immune Responses
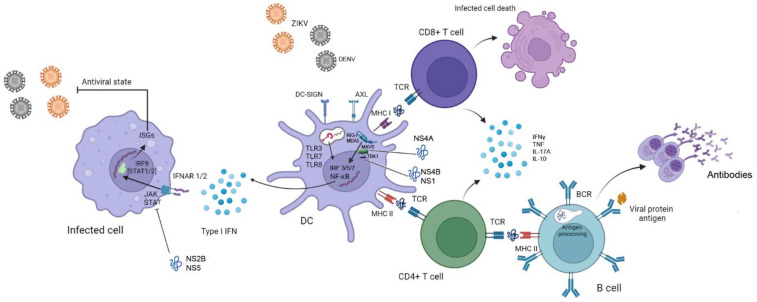
The interaction between the cells and the viruses occurs through pattern recognition receptors (PRRs), which recognize conserved and shared structures by pathogens, called pathogen-associated molecular patterns (PAMPs), which include lipids, proteins, carbohydrates, and nucleic acids [31]. In flavivirus infection, especially ZIKV, the most prominent PRRs involved in innate immunity activation are the cytoplasmic RLRs, RIG-I and MDA5, and endosomal TLRs such as TLR3, TLR7 and TLR8, involved in sensing ZIKV RNA [31,32,33,34,35,36]. These pathways activate the production of type I IFNs in viral infections, important mediators of a variety of effector mechanisms that contribute to the antiviral response (Figure 1). For example, mice which are deficient in IFNa receptor 1 (IFNAR1) are highly susceptible to ZIKV infection [37]. Lazear et al. showed that IFNaR1 was a key factor in resistance against ZIKV infection, but dependent on the downstream interferon-related factors (IRF) 3, 5 and 7. Although IRF3, 5 and 7 seem to be redundant in IFNa transduction, i.e., the depletion of each of these transduction factors individually did not impact mouse survival following ZIKV infection, the absence of the three transduction factors caused animals to succumb with high virus loads in the brain [37], showing that type I IFNs signaling is essential for immunity against ZIKV.
Figure 1.
Immune response to DENV and ZIKV. ZIKV attaches to the target cell using DC-SIGN Axl receptors, which are used to mediate membrane fusion. Viral RNA is detected by PRRs, such as RIG-I and MDA-5 in the cytoplasm, and endosomal TLRs. The viral sensing mechanism induces the activation of the transcription factors NF-κB and IRFs, which mediate the production and secretion of interferons. Binding of type I IFNs to receptors (IFNAR1/2), especially in a bystander cell, initiates signaling cascades via JAK/STAT and the formation of a STAT/IRF9 trimer, which culminates in the production of multiple ISGs and induction of the cellular anti-viral state. Adaptive immunity is initiated after recognition of viral antigens presented via MHC class I or II, by CD8+ and CD4+ T cells, respectively, that produce cytokines to promote inflammation and exert other effector mechanisms, like killing of infected cells by CD8+ T cells. CD4+ T cells can also promote better antibody responses inducing class switch (inducing the production of IgG), affinity maturation and the differentiation of B cells into plasma cells. In contrast, non-structural proteins of the virus inhibit I IFN response by binding to MAVS (NS4a), inhibiting of TBK1 (NS1 and NS4b) or JAK (NS2b), and promoting the degradation of STAT2 (NS5). Abbreviations: PRRs—pattern recognition receptors, TLRs—Toll-like receptors, IRFs—interferon-related factors, ISGs—IFN-stimulated genes. Images from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License (http://smart.servier.com/; accessed on 10 Februrary 2022).
Type I IFN activates its receptor, triggering the activation of JAK1 and phosphorylation of STAT2. Two STAT2 molecules form a trimer with one IRF9 and migrate to the nucleus to identify and activate the production of the IFN-stimulated genes (ISGs), which induce the anti-viral state (Figure 1). However, ZIKV, as well as other flaviviruses, possess several mechanisms to evade the type I IFN pathway. For example, ZIKV NS4a binds to MAVS [38], an adaptive molecule that interacts with RIG-I to promote activation of TBK1 and downstream anti-viral signaling [39], inhibiting the production of type I IFN. The viral NS1 and NS4b proteins can also inhibit type I IFN production by inhibiting the phosphorylation of TBK1, a molecule downstream of the RIG-I-MAVS complex [40]. In addition, several mechanisms may also inhibit the type I and III IFN downstream signaling which are highly dependent on JAK-STAT signaling and IRF activation. The virus can inhibit JAK activation by NS2B [40], while NS5 possesses the ability to degrade STAT2 [41,42], impairing the expression of the interferon-stimulated genes (ISGs). Indeed, animals deficient in IFNAR1 downstream signaling, like animals deficient in STAT2 display important virus proliferation [43,44] (Figure 1).
The humoral and cellular adaptive immunity has also been shown to be activated during ZIKV infection. Adaptive immunity against ZIKV is very similar to that of DENV infection, including the ability of pathogen-specific antibodies to neutralize ZIKV. For flaviviruses, neutralizing antibodies target the envelope (E) protein, which has approximately 55% amino acid identity between ZIKV and DENV [45]. Neutralizing antibodies prevent virus attachment to the host cell and/or membrane fusion after virus uptake by endocytosis [46,47]. Neutralizing antibodies are considered the main mechanism of host resistance to flavivirus infections; however, antibodies have also been implicated in pathogenesis. For example, while high levels of neutralizing antibodies are able to confer protection against a specific virus, non-neutralizing antibodies may cause antibody-dependent enhancement (ADE) of infection of a related virus, either a different serotype of the same virus or of different viruses that show cross-reactivity. ADE is a phenomenon well described in dengue and is considered the main mechanism to trigger the severe form of the disease. Usually, in a secondary DENV infection caused by a different serotype from the primary infection, cross-reactive non-neutralizing antibodies may bind to the heterotypic virus and instead of impairing the virus entry, may help the virus to gain access to the cells through FcγRs, expressed in high levels in cells like monocytes and dendritic cells [48]. As a consequence, virus particles gain facilitated access to permissible cells, while decreasing effector mechanisms like IFNg production by T cells and increasing IL-10 production by macrophages and dendritic cells [48,49], The consequence is an increasing viremia triggering hyperinflammatory responses in the host [50]. Indeed, we could observe that individuals with dengue with warning signs or severe dengue displayed lower activation of T cells [51] associated with increased innate immunity cytokine production, including IFNg production by ILC1 [52]. Although ZIKV-related ADE has been demonstrated to happen in highly controlled experimental settings [53,54,55], its relevance for the clinical presentation of ZIKV infection is still uncertain [56]. On the other hand, previous ZIKV infection was recently demonstrated to be a risk factor for more severe infection caused by dengue serotype 2 in Nicaragua [57] (more details below). Previous ZIKV infection did not appear to affect the severity of the disease caused by other DENV serotypes [57].
On the other hand, T cell-mediated immunity is also shown to play a role in ZIKV infection. T cell activity is key for optimal antibody production [58] and also to eliminating infected cells. CD8+ T cells were found necessary to control ZIKV infection in type I IFN-deficient animals [59,60] and also to protect against CZS in experimental pregnancy models [61]. Although the profile of the immune response associated with virus infections is dominated by IFNγ production, we and others have found mixed profiles associated with ZIKV-infection. For example, IFNγ is found in the peripheral blood of ZIKV-infected patients associated with high levels of IL-10, IL-17A and TNF [62]. Experimental models suggest that an effective immune response against ZIKV is driven by multifunctional CD4+ and CD8+ T cells [59,63]. We have found that ZIKV-specific T cells display a dominant multifunctional profile secreting multiple cytokines, especially IFNg, IL-17A, TNF and IL-10, simultaneously (quadruple-producing T cells) or its combinations [64] (Figure 1). Interestingly, we observed that CD8+ T cells displayed higher frequencies of multifunctional lymphocytes when compared to CD4+ T cells [64]. Indeed, multifunctional T cell responses and cellular immunity have been considered important mechanisms for host resistance against other flaviviruses like DENV [51,65] and immunity to YFV [66,67,68].
Another important factor to consider in immunity against ZIKV and other flavivirus infections is the cross-reactivity of the immune response. Cross-reactivity is well appreciated in DENV infection, since there are four serotypes of DENV (DENV1, DENV2, DENV3 and DENV4) between which are moderate levels of amino acid conservation (around 60–75% at the amino acid level of E protein) [69], making the emergence of broadly neutralizing antibodies and pan-T cell epitopes possible [70,71,72]. This level of genetic proximity causes important cross-reactivity at antibody and T cell levels between different DENV serotypes. The non-neutralizing antibody cross-reactivity between the different serotypes of DENV is considered the main cause of ADE in secondary dengue. Although the levels of similarity between ZIKV and DENV is lower than those observed between DENV serotypes, around 55% of molecular identity at the amino acid level [45], it is still sufficient to cause important cross-reactivity in both, humoral and cellular immunity [45,73,74].
Previous immunity to other flaviviruses can dramatically influence the immune response to ZIKV infection [75,76,77,78]. It has been shown that DENV-experienced individuals displayed consistently higher ZIKV-specific neutralizing antibody titers with low ADE activity compared with DENV-naïve individuals [75]. Interestingly, better antibody protective responses were directly associated with higher levels of ZIKV-specific CD4+ T cells producing IFNγ [75]. Animal data suggest that CD4+ T cells present high levels of cross-reactivity between ZIKV, DENV, WNV and YFV [79]. In addition, CD4+ follicular T cells, which are responsible for the activation of germinal centers and the emergence of high-affinity antibodies, improve ZIKV-specific antibody responses and are important for host resistance during rechallenge [80]. This implies that flavivirus-experienced individuals may display better antibody responses possibly due to faster and improved responses of follicular T cells in providing help to germinal center B cells, when compared to flavivirus-naïve individuals [81].
The clinical implications of such relatedness and cross-reactivity are still the subject of major debate in the literature. For example, it has been suggested that antibodies to any flaviviruses can enhance infection in vitro to almost any other flavivirus at a low enough dilution [82]. Therefore, we can speculate that finding DENV-induced ADE against ZIKV, or vice versa, in vitro, is not surprising because of the cross-reactivity. However, as mentioned, the ADE implication to ZIKV infection is the subject of much debate. Some authors suggest that pre-formed DENV-specific antibodies can cause ADE during a ZIKV infection [53,55,83,84,85], enhancing vertical transmission and triggering microcephaly in pups during pregnancy [86]. In contrast, data show that neutralizing anti-DENV cross-reactive antibodies can also neutralize ZIKV [87,88] and some data suggest a relationship between anti-DENV antibodies and protection from ZIKV infection in humans [89]. Meanwhile, some have suggested that a previous DENV infection in mice can prevent a lethal ZIKV challenge, not because of antibodies, but due to CD8+ T cells [90]. Interestingly, an epidemiological study in Brazil found that ZIKV-related microcephaly was more common in Brazilian regions with the least coverage of YFV vaccination [91] suggesting a role for cross-reactivity between YFV immunity. In agreement, Vicente et al. demonstrated that immunization against YFV using 17DD vaccine can decrease cerebral virus load, prevent neurological manifestations, weight loss and mortality in a fatal murine model of ZIKV infection by mechanisms possibly associated with cross-reactive cell-mediated immunity [92]. Indeed, YFV vaccination leads to the emergence of ZIKV-specific CD8 T cells [93]. Finally, other authors sustain that pre-formed immunity against DENV or YFV will not affect a subsequent ZIKV infection as found in non-human primates [94,95] or in human subjects [96]. On the other hand, there are data emerging showing that a primary ZIKV exposure may have a larger impact on subsequent DENV infection due to ADE [57].
4. Implications of Flaviviruses Immunity on ZIKV Vaccine Development
The interaction of ZIKV immunity with other flaviviruses, and vice versa, may have important implications for vaccine development. The challenges for DENV vaccine development are examples of how difficult it might be to develop a vaccine for ZIKV. A vaccine for DENV must concomitantly induce homotypic neutralizing antibodies to each of the serotypes. If not, partial immunity against one of the serotypes with the presence of non-neutralizing antibodies may increase the risk of ADE and severe dengue. This happened to CYD (Denvaxia©), the dengue vaccine of Sanofi Pasteur, which induced antibodies that were not balanced between the four serotypes in individuals who were not previously exposed to natural DENV infection [97].
CYD tetravalent vaccine is a vaccine combination of four recombinant viral vector vaccines using yellow fever 17DD structure to express the structural proteins (prM and E proteins) of each DENV serotype [98]. This vaccine was able to induce neutralizing antibodies against the four DENV serotypes in more than 90% of vaccinees [99]; however, homotypic neutralizing antibody against DENV-4 component of the vaccine was dominant [97]. The DENV-1 and DENV-2 components were poorly infectious and the majority of DENV-1 and DENV-2 antibodies induced by the vaccine were heterotypic [97,100]. The vaccine induced some levels of IFNγ-producing DENV-specific CD4+ T cells directed to DENV E protein [98] and possibly to boost, by cross-reactivity between YFV and DENV antigens, some preexisting levels of DENV NS3 T cells [101]. Despite the capacity to induce neutralizing antibodies and IFNγ against DENV [102,103], CYD did not perform well in in phase III clinical efficacy trials. CYD displayed a general efficacy of 50%, 35–42%, 74–78% and 75–77% against DENV 1, 2, 3 and 4, respectively [99,104,105]. Its protection was better in the dengue-experienced population, in which it reached 70% efficacy, but only 35% in dengue-naïve individuals [104]. Unfortunately, an excess number of dengue hospitalizations occurred in year 3 of the trial in those volunteers who received the CYD vaccine compared to those who received a placebo in the first year of the trial. This was observed more frequently in 9-year-old children and below [106]. Detailed analysis of CYD immunogenicity data demonstrated that: (1) homotypic anti-DENV4 antibodies were dominant, while the other serotypes were neutralized mostly by cross-reactive heterotypic antibodies [97]; (2) individuals with higher titers of neutralizing antibodies were more protected than individuals with lower titers [107]; and (3) most of the CD4+ and CD8+ T cells induced by the vaccine were directed against the yellow fever component [107,108,109]. Some important conclusions can be drawn from these data: (a) the mere presence of neutralizing antibodies is not a sufficient correlate of protection for DENV, and perhaps for other flaviviruses like ZIKV, as unbalanced homotypic versus heterotypic antibody titers lead to higher titers of heterotypic, non-neutralizing, potentially enhancing antibodies; and (b) T cell mediated immunity seems to play an important role as a mechanism of protection, since this was a major missing mechanism of CYD.
Considering immunization against ZIKV, the scenario is not less complex. If the implications of DENV-induced ZIKV ADE are uncertain, there is evidence to suggest that cross-reactivity of anti-ZIKV antibodies may cause ADE against DENV, and this could be a problem in vaccination strategies (Figure 2). It has been demonstrated that ZIKV immunity can precipitate ADE against DENV in vitro [110,111] and in the pediatric population [57]. Katzelnick et al. observed in a careful Nicaraguan pediatric cohort that previous exposure to DENV or ZIKV infection in naïve children, or one ZIKV infection in one DENV infection-experience children, increased the risk of severe disease in a subsequent DENV2 infection. Their findings also found that while high titers of anti-DENV antibodies (developed after multiple DENV infections) have protective effects, intermediate anti-DENV titers precipitate ADE in subsequent DENV orZIKV infections [57]. The implication of this finding for vaccine development is clear: immunization against DENV must be addressed in the context of ZIKV immunization. DENV/ZIKV immunization can be achieved by sequential immunization, i.e., vaccination against DENV and then against ZIKV [112], or preferentially by the combination of dengue and Zika vaccines.
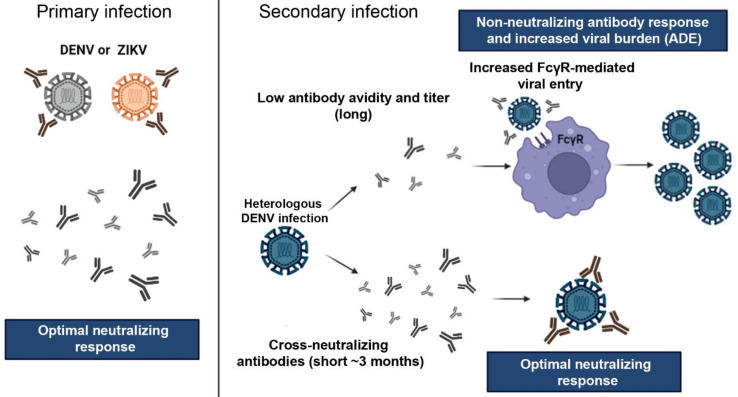
Figure 2.
Antibody response and cross-reaction effect between DENV and ZIKV. Primary infection by one of the viruses can promote the production of long-lasting neutralizing antibodies the homologous virus and short-lived cross-reactive neutralizing antibodies, i.e., in the first few months (usually 3 months) after infection, neutralizing antibodies also neutralizes heterologous related viruses. However, after 3 few months, cross-neutralization is lost and cross-reactive non-neutralizing antibodies may enhance the infection during a secondary exposure by a heterologous related virus, for example, a second distinct DENV serotype, or a DENV infection following a primary ZIKV infection. Virus particles opsonized with non-neutralizing antibodies have facilitated access to permissive cells via FcγR which causes enhanced virus proliferation and increased viral load. This phenomenon is known as antibody-dependent enhancement (ADE). On the other hand, heterologous infections after short periods between the primary and secondary infections can induce virus neutralization by cross-reactive antibodies. Abbreviations: ADE—antibody-dependent enhancement. Images from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License (http://smart.servier.com/; accessed on 10 Februrary 2022).
A major rationale for the combination of vaccines, when possible, is the simplification of the immunization schedule [113]. The number of shots necessary to cover immunization programs in developed or even Low–Middle Income Countries (LMIC) can decrease compliance and increase costs. On the other hand, the combination of vaccines has been a mechanism to reach adequate levels of immunization to as many as 14 diseases in infants below two years old. For instance, the combination of vaccines into a single shot such as pentavalent DtaP-HepB-IPV and trivalent MMR has contributed significantly to simplification of immunization schedules, increased compliance and cost reduction because of delivery efforts and vaccination campaigns are optimized to many vaccines at once [113].
DENV and ZIKV often co-circulate in endemic countries because the mosquito vector is the same, A. Aegypti or A. albopictus [2], and the most affected populations live in developing countries where financial resources for public health are limited. There are strong epidemiological, compliance and cost-bases for the combination of DENV and ZIKV vaccines into one single shot, especially targeting the possibility of a robust immune response to such a combination due to cross-reactivity between ZIKV and DENV. If the immunization against ZIKV poses a threat for severe dengue because of cross-reactive non-neutralizing antibodies, the combination of ZIKV and DENV vaccines may provide further protection against both diseases. Again, a great lesson comes from dengue. The live attenuated dengue vaccine (LADV) TV003 induces antibodies that are mostly homotypic, with little cross-reactivity, for each of the four DENV serotypes [114] and induces T cell responses to highly conserved CD8 epitopes [115]. The addition of a ZIKV vaccine in the mix, or a concomitant immunization, has the potential to induce ZIKV-specific antibodies and to also add ZIKV T cell epitopes to the mix, further selecting highly conserved epitopes and improving both DENV and ZIKV immune responses.
5. Current Challenges and Solutions: A Role for Controlled Human Infection Model of ZIKV
The need for a Zika vaccine is still a public health necessity. Although ZIKV circulation has shown a dramatic drop since 2016 (Figure 3), the numbers of pregnancy complications associated with Zika are still a concern [22,116,117] and a vaccine aiming to protect pregnancies in Zika endemic countries is of great interest. Several vaccines are under development, mostly in the preclinical phase (88 vaccines), a fair number in phase I (20 vaccines) and only one vaccine in phase II clinical trials [118]. Although there is a reasonable pipeline of ZIKV vaccines, the slow pace of development and the low numbers of ZIKV infections might make it difficult to perform a traditional Phase III efficacy trial.
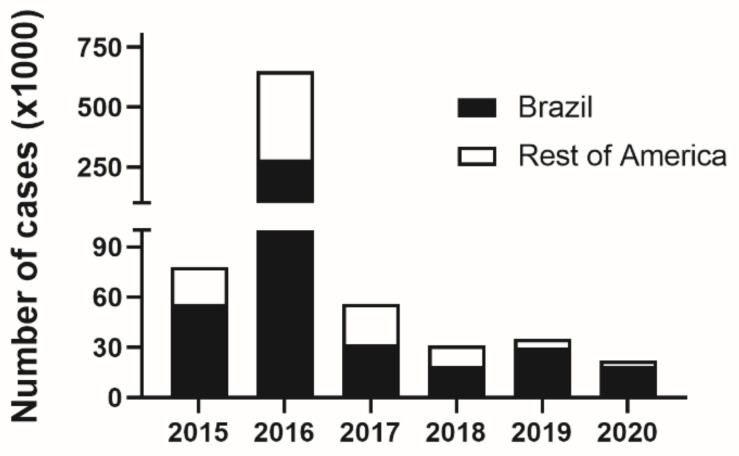
Figure 3.
Number of zika case notifications in Latin America and Brazil in the years between 2015 and 2020. Number of ZIKV infections were accessed in PAHO website (https://www3.paho.org/data/index.php/es/temas/indicadores-zika.html; accessed on 20 December 2021) and Brazilian DataSUS (https://datasus.saude.gov.br/informacoes-de-saude-tabnet/; accessed on 20 December 2021).
After the peak of the Zika outbreak in 2016, the commercial interest in a Zika vaccine considerably dimmed. Despite its relevance for public health, especially in women of child-bearing age, lower investment decreased the pace of a Zika vaccine development. In addition, Brazil reports only between 15,000 and 31,000 Zika cases per year since 2018. The estimated attack rate for the population of Brazil is too low for a Phase III vaccine efficacy trial and the probability of individuals participating in a ZIKV vaccine trial being actually exposed to ZIKV is too low [119].
Controlled human infection “models” (CHIM) are great tools for vaccine development. CHIM refers to the use of iatrogenic infection of humans for study purposes. Models of controlled infection are usually developed to study the clinical aspects and physiopathology of infection and to develop drugs or vaccines for specific pathogens [120]. For instance, variolation was a “primitive” procedure of controlled human infection to induce active immunization of a subject against the natural infection with variola. On the other hand, the same procedure was used by Jenner as a model to test the efficacy of immunization with bovine variola, therefore, creating the first use of CHIM which gave the world the first vaccine.
Estimates account that more than 20,000 volunteers have participated in CHIM studies since World War II and that these studies have helped to characterize aspects of the clinical presentation and evolution, immune responses, microbiology and pathogenesis of many infections. Furthermore, CHIM has contributed to the development of a number of vaccines, making a tremendous impact on public health. Vaccines that have utilized CHIMs include those for influenza, shigella, enteric fever, malaria, campylobacter, cholera and RSV, among others [121].
The use of CHIM can benefit vaccine development as they can down-select candidates so that only the most promising candidates are further evaluated in Phase II and III clinical trials. While formal efficacy trials (Phase III) need to recruit tens of thousands of individuals and follow them until the efficacy endpoint is met, which may vary according to the incidence of the infection, CHIMs are designed to ensure a high attack rate allowing for a rapid assessment of efficacy. For example, malaria-controlled infection models are now a standard step in malaria vaccine development to assess if a vaccine should advance to larger Phase II and Phase III clinical trials. The malaria-controlled human infection model was utilized to select the antigen RTS,S (a Plasmodium falciparum circumsporozoite protein (S) fused with hepatitis B antigen (RTS)) for the Mosquirix vaccine [122,123]. The efficacy endpoint for the malaria CHIM is the prevention of infection. A malaria CHIM may recruit 10–20 volunteers in each group (vaccinated and placebo), proceed with vaccination and infect all the groups. Volunteers are evaluated daily (or more frequently) for parasitemia (i.e., the appearance of the sporozoites in the blood). Once parasitemia occurs, the volunteer is treated with anti-malaria medication, even before they become symptomatic, making the procedure highly informative for science and safe for the participants. If the vaccine were to work, the placebo group should become positive while the vaccinated group would be negative at the same timepoint. It is important to note that even if a vaccine demonstrates efficacy in a CHIM study, it still must be evaluated in thousands of volunteers to ensure safety.
The use of CHIM for vaccine development, although very common in developed countries like USA and England, faces many barriers in developing countries. For example, Vietnam has laws that forbid the intentional infection of a subject, as stated in constitutional amendment No. 51/2001/QH10, Article 8.1. Other developing countries may see the use of CHIM as detrimental to their own dignity, or still other countries may even face the lack of regulatory expertise which would enable CHIMs to be performed ethically. Nevertheless, the use of CHIM can bring many benefits to developing countries as a tool that can speed up the development of vaccines and therapeutics for public health problems important to them, including Zika.
So far, only one vaccine, Vaxchora, has been approved by the FDA based on CHIM efficacy data. Vaxchora is a cholera vaccine that was approved for travelers going to cholera endemic areas. Since it demonstrated good efficacy against cholera challenge in naïve non-endemic individuals [124,125,126,127], the FDA has approved its use by travelers, despite poor performance in endemic settings [128]. The case of Vaxchora also illustrates the importance of performing accelerated efficacy studies, using CHIM, in endemic settings since genetic and exposition history may change the immunogenicity to the vaccine and host response to the pathogen.
The U.S. National Institutes of Health are developing a CHIM for Zika to aid in vaccine development and evaluation. The proposal for a Zika CHIM underwent ethical review in late 2016. The committee determined that a Zika CHIM was not needed at that time for vaccine development as the Zika outbreak was causing large numbers of cases in Latin America. However, after Zika circulation essentially came to a halt in late 2017, the need for a Zika CHIM was reconsidered and determined to be a useful tool for vaccine development [129]. A Zika CHIM study is underway and will evaluate two strains of ZIKV recovered from persons who developed uncomplicated ZIKV infection (clinicaltrials.gov: NCT05123222). The study population will be composed of healthy non-pregnant, non-breastfeeding women (18–40 years old), who will be housed in an inpatient unit during the viremic period. The study will evaluate up to three different doses of the ZIKV and follow the study participants for up to 6 months following inoculation for assessment of safety (including GBS) and immunogenicity. During this period, participants must agree to use acceptable effective birth control measures. The aim of the study is to identify a suitable challenge virus to evaluate vaccine efficacy early in development. This ZIKV CHIM will also be used to evaluate the protective efficacy of the tetravalent DENV vaccine TV003 against subsequent ZIKV infection as well as evaluating the effects of ZIKV antibody on subsequent DENV infection and vice versa.
6. Conclusions
ZIKV caused a major epidemic in 2015 in Latin America, but its incidence decreased and has stabilized to around 15,000–30,000 infections yearly. Those numbers are likely underestimated since around 5–10% of microcephaly cases in Brazil seem to be associated with CZS [22,116,117]. Immunological data suggest broad cross-reactive immunity of ZIKV with other flaviviruses, especially DENV. This may have important implications for flavivirus immunization programs. The development of a ZIKV vaccine should consider the immunological cross-reactivity between ZIKV and other flaviviruses, especially the possibility of enhancing the severity of subsequent DENV infection. Additionally, the currently low yearly ZIKV incidence will make it difficult to conduct formal Phase III clinical trials. For these reasons, there is a strong rationale for developing a combined DENV and ZIKV vaccine and using the ZIKV CHIM to assess the efficacy of a Zika vaccine. The development and use of a Zika CHIM to evaluate the protective efficacy of potential ZIKV vaccines is of great interest to countries where Zika is endemic and is still causing CZS, like Brazil.
Author Contributions
Conceptualization, H.C.S.; Writing—Original Draft Preparation, H.C.S. and A.P.D.; Data curation, Writing—Review and Editing, T.A.P.-N., M.H.G.-P. and A.C.B.T. All authors have read and agreed to the published version of the manuscript.
Funding
HCS is CNPq fellow and supported by CNPq grant #303442/2018-2. APD is supported by NIH grant #75N93019D00031.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer M.U.G., Reiner R.C., Jr., Brady O.J., Messina J.P., Gilbert M., Pigott D.M., Yi D., Johnson K., Earl L., Marczak L.B., et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desgraupes S., Hubert M., Gessain A., Ceccaldi P.E., Vidy A. Mother-to-child transmission of Arboviruses during breastfeeding: From epidemiology to cellular mechanisms. Viruses. 2021;13:1312. doi: 10.3390/v13071312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimenez-Richarte A., de Salazar M.O., Arbona C., Gimenez-Richarte M.P., Collado M., Fernandez P.L., Quiles F., Clavijo C., Marco P., Ramos-Rincon J.M. Prevalence of chikungunya, dengue and Zika viruses in blood donors: A systematic literature review and meta-analysis. Blood Transfus. 2021. online ahead of print . [DOI] [PMC free article] [PubMed]
- 5.Yuan X., Lou Y., He D., Wang J., Gao D. A Zika endemic model for the contribution of multiple transmission routes. Bull. Math. Biol. 2021;83:111. doi: 10.1007/s11538-021-00945-w. [DOI] [PubMed] [Google Scholar]
- 6.Hayes E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009;15:1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy M.R., Chen T.H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., Dubray C., et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 8.Cao-Lormeau V.M., Roche C., Teissier A., Robin E., Berry A.L., Mallet H.P., Sall A.A., Musso D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PAHO/WHO, PAHO Timeline of Emergence of Zika Virus in the Americas. [(accessed on 27 November 2021)]. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=11959:timeline-of-emergence-of-zika-virus-in-the-americas&Itemid=41711&lang=en.
- 10.Faria N.R., Azevedo R., Kraemer M.U.G., Souza R., Cunha M.S., Hill S.C., Theze J., Bonsall M.B., Bowden T.A., Rissanen I., et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovanetti M., Faria N.R., Nunes M.R.T., de Vasconcelos J.M., Lourenco J., Rodrigues S.G., Vianez J.L., Jr., da Silva S.P., Lemos P.S., Tavares F.N., et al. Zika virus complete genome from Salvador, Bahia, Brazil. Infect. Genet. Evol. 2016;41:142–145. doi: 10.1016/j.meegid.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Schuler-Faccini L., Ribeiro E.M., Feitosa I.M., Horovitz D.D., Cavalcanti D.P., Pessoa A., Doriqui M.J., Neri J.I., Neto J.M., Wanderley H.Y., et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 13.PAHO Epidemiological Alert: Neurological Syndrome, Congenital Malformations, and Zika Virus Infection. Implications for Public Health in the America. 2015. [(accessed on 20 December 2021)]. Available online: https://iris.paho.org/handle/10665.2/50697?show=full.
- 14.Oliveira Melo A.S., Malinger G., Ximenes R., Szejnfeld P.O., Alves Sampaio S., Bispo de Filippis A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 15.Brasil P., Pereira J.P., Jr., Moreira M.E., Ribeiro Nogueira R.M., Damasceno L., Wakimoto M., Rabello R.S., Valderramos S.G., Halai U.A., Salles T.S., et al. Zika Virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvet G., Aguiar R.S., Melo A.S., Sampaio S.A., de Filippis I., Fabri A., Araujo E.S., de Sequeira P.C., de Mendonca M.C., de Oliveira L., et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016;16:653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 17.Cardenas D.M., Jaimes M.A., Vega L.D., Oliveros N.L., Soto J.A., Chia C.R., Osorio J.E., Ciuoderis K.A. Immunological memory to Zika virus in a University Community in Colombia, South America. Acad. Bras. Cienc. 2020;92:e20190883. doi: 10.1590/0001-3765202020190883. [DOI] [PubMed] [Google Scholar]
- 18.Netto E.M., Moreira-Soto A., Pedroso C., Hoser C., Funk S., Kucharski A.J., Rockstroh A., Kummerer B.M., Sampaio G.S., Luz E., et al. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. mBio. 2017;8:e01390-17. doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreto F.K.A., Alencar C.H., Araujo F.M.C., Oliveira R., Cavalcante J.W., Lemos D.R.Q., Farias L., Boriz I.L.F., Medeiros L.Q., Melo M.N.P., et al. Seroprevalence, spatial dispersion and factors associated with flavivirus and chikungunha infection in a risk area: A population-based seroprevalence study in Brazil. BMC Infect. Dis. 2020;20:881. doi: 10.1186/s12879-020-05611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Zika Epidemiology Update. Jul, 2019. [(accessed on 20 December 2021)]. Available online: https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1.
- 21.PAHO/WHO Cases of Zika Virus Disease by Country or Territory. Weekly Report. 2021. [(accessed on 20 December 2021)]. Available online: https://www3.paho.org/data/index.php/en/?option=com_content&view=article&id=524:zika-weekly-en&Itemid=352.
- 22.Jacques I., Katz L., Sena M.A., Guimaraes A.B.G., Silva Y.L., Albuquerque G.D.M., Pereira R.O., de Albuquerque C., Silva M.A.L., Oliveira P.A.S., et al. High Incidence of Zika or Chikungunya Infection among pregnant women hospitalized due to obstetrical complications in Northeastern Brazil-Implications for laboratory screening in Arbovirus endemic area. Viruses. 2021;13:744. doi: 10.3390/v13050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen L.R., Jamieson D.J., Powers A.M., Honein M.A. Zika virus. N. Engl. J. Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 24.Musso D., Nilles E.J., Cao-Lormeau V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014;20:O595–O596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 25.Bautista L.E., Sethi A.K. Association between Guillain-Barre syndrome and Zika virus infection. Lancet. 2016;387:2599–2600. doi: 10.1016/S0140-6736(16)30844-3. [DOI] [PubMed] [Google Scholar]
- 26.Oehler E., Watrin L., Larre P., Leparc-Goffart I., Lastere S., Valour F., Baudouin L., Mallet H., Musso D., Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. Eur. Commun. Dis. Bull. 2014;19:20720–20722. doi: 10.2807/1560-7917.ES2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 27.Mier Y.T.-R.L., Delorey M.J., Sejvar J.J., Johansson M.A. Guillain-Barre syndrome risk among individuals infected with Zika virus: A multi-country assessment. BMC Med. 2018;16:67. doi: 10.1186/s12916-018-1052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dirlikov E., Major C.G., Medina N.A., Lugo-Robles R., Matos D., Munoz-Jordan J.L., Colon-Sanchez C., Garcia M., Olivero-Segarra M., Malave G., et al. Clinical features of Guillain-Barre Syndrome with vs without Zika virus infection, Puerto Rico, 2016. JAMA Neurol. 2018;75:1089–1097. doi: 10.1001/jamaneurol.2018.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleber de Oliveira W., Cortez-Escalante J., De Oliveira W.T., do Carmo G.M., Henriques C.M., Coelho G.E., Araujo de Franca G.V. Increase in Reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy-Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 30.Freitas D.A., Souza-Santos R., Carvalho L.M.A., Barros W.B., Neves L.M., Brasil P., Wakimoto M.D. Congenital Zika syndrome: A systematic review. PLoS ONE. 2020;15:e0242367. doi: 10.1371/journal.pone.0242367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D., Wu M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H.Y., Zhang X.C., Jia R.Y. Toll-Like Receptors and RIG-I-Like Receptors play important roles in resisting Flavivirus. J. Immunol. Res. 2018;2018:6106582. doi: 10.1155/2018/6106582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamel R., Dejarnac O., Wichit S., Ekchariyawat P., Neyret A., Luplertlop N., Perera-Lecoin M., Surasombatpattana P., Talignani L., Thomas F., et al. Biology of Zika virus infection in human skin cells. J. Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hertzog J., Dias Junior A.G., Rigby R.E., Donald C.L., Mayer A., Sezgin E., Song C., Jin B., Hublitz P., Eggeling C., et al. Infection with a Brazilian isolate of Zika virus generates RIG-I stimulatory RNA and the viral NS5 protein blocks type I IFN induction and signaling. Eur. J. Immunol. 2018;48:1120–1136. doi: 10.1002/eji.201847483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chazal M., Beauclair G., Gracias S., Najburg V., Simon-Loriere E., Tangy F., Komarova A.V., Jouvenet N. RIG-I Recognizes the 5′ region of dengue and Zika virus genomes. Cell Rep. 2018;24:320–328. doi: 10.1016/j.celrep.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 36.Schilling M., Bridgeman A., Gray N., Hertzog J., Hublitz P., Kohl A., Rehwinkel J. RIG-I plays a dominant role in the induction of transcriptional changes in Zika virus-infected cells, which protect from virus-induced cell death. Cells. 2020;9:1476. doi: 10.3390/cells9061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazear H.M., Govero J., Smith A.M., Platt D.J., Fernandez E., Miner J.J., Diamond M.S. A Mouse Model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y., Dong X., He Z., Wu Y., Zhang S., Lin J., Yang Y., Chen J., An S., Yin Y., et al. Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains. Cell Biosci. 2019;9:46. doi: 10.1186/s13578-019-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu B., Hur S. How RIG-I like receptors activate MAVS. Curr. Opin. Virol. 2015;12:91–98. doi: 10.1016/j.coviro.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y., Liu Q., Zhou J., Xie W., Chen C., Wang Z., Yang H., Cui J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017;3:17006. doi: 10.1038/celldisc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant A., Ponia S.S., Tripathi S., Balasubramaniam V., Miorin L., Sourisseau M., Schwarz M.C., Sanchez-Seco M.P., Evans M.J., Best S.M., et al. Zika Virus Targets Human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A., Hou S., Airo A.M., Limonta D., Mancinelli V., Branton W., Power C., Hobman T.C. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016;17:1766–1775. doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashour J., Morrison J., Laurent-Rolle M., Belicha-Villanueva A., Plumlee C.R., Bernal-Rubio D., Williams K.L., Harris E., Fernandez-Sesma A., Schindler C., et al. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe. 2010;8:410–421. doi: 10.1016/j.chom.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry S.T., Buck M.D., Lada S.M., Schindler C., Shresta S. STAT2 mediates innate immunity to Dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 2011;7:e1001297. doi: 10.1371/journal.ppat.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Priyamvada L., Quicke K.M., Hudson W.H., Onlamoon N., Sewatanon J., Edupuganti S., Pattanapanyasat K., Chokephaibulkit K., Mulligan M.J., Wilson P.C., et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA. 2016;113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowd K.A., Pierson T.C. Antibody-mediated neutralization of flaviviruses: A reductionist view. Virology. 2011;411:306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diamond M.S., Pierson T.C. Molecular insight into Dengue Virus pathogenesis and its implications for disease control. Cell. 2015;162:488–492. doi: 10.1016/j.cell.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halstead S.B. Dengue Antibody-dependent enhancement: Knowns and unknowns. Microbiol. Spectr. 2014;2:249–271. doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 49.Halstead S.B., Mahalingam S., Marovich M.A., Ubol S., Mosser D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010;10:712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayala-Nunez N.V., Hoornweg T.E., van de Pol D.P., Sjollema K.A., Flipse J., van der Schaar H.M., Smit J.M. How antibodies alter the cell entry pathway of dengue virus particles in macrophages. Sci. Rep. 2016;6:28768. doi: 10.1038/srep28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goncalves Pereira M.H., Figueiredo M.M., Queiroz C.P., Magalhaes T.V.B., Mafra A., Diniz L.M.O., da Costa U.L., Gollob K.J., Antonelli L., Santiago H.D.C. T-cells producing multiple combinations of IFNgamma, TNF and IL10 are associated with mild forms of dengue infection. Immunology. 2020;160:90–102. doi: 10.1111/imm.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintino-de-Carvalho I.L., Goncalves-Pereira M.H., Faria Ramos M., de Aguiar Milhim B.H.G., Da Costa U.L., Santos E.G., Nogueira M.L., Da Costa Santiago H. Type 1 Innate Lymphoid Cell and Natural Killer Cells Are Sources of Interferon-gamma and Other Inflammatory Cytokines Associated With Distinct Clinical Presentation in Early Dengue Infection. J. Infect. Dis. 2022;225:84–93. doi: 10.1093/infdis/jiab312. [DOI] [PubMed] [Google Scholar]
- 53.Castanha P.M.S., Nascimento E.J.M., Braga C., Cordeiro M.T., de Carvalho O.V., de Mendonca L.R., Azevedo E.A.N., Franca R.F.O., Dhalia R., Marques E.T.A. Dengue Virus-Specific Antibodies Enhance Brazilian Zika Virus Infection. J. Infect. Dis. 2017;215:781–785. doi: 10.1093/infdis/jiw638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chareonsirisuthigul T., Kalayanarooj S., Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J. Gen. Virol. 2007;88:365–375. doi: 10.1099/vir.0.82537-0. [DOI] [PubMed] [Google Scholar]
- 55.Dejnirattisai W., Supasa P., Wongwiwat W., Rouvinski A., Barba-Spaeth G., Duangchinda T., Sakuntabhai A., Cao-Lormeau V.M., Malasit P., Rey F.A., et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat. Immunol. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durbin A.P. Dengue antibody and Zika: Friend or foe? Trends Immunol. 2016;37:635–636. doi: 10.1016/j.it.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Katzelnick L.C., Narvaez C., Arguello S., Lopez Mercado B., Collado D., Ampie O., Elizondo D., Miranda T., Bustos Carillo F., Mercado J.C., et al. Zika virus infection enhances future risk of severe dengue disease. Science. 2020;369:1123–1128. doi: 10.1126/science.abb6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elong Ngono A., Young M.P., Bunz M., Xu Z., Hattakam S., Vizcarra E., Regla-Nava J.A., Tang W.W., Yamabhai M., Wen J., et al. CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog. 2019;15:e1007474. doi: 10.1371/journal.ppat.1007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elong Ngono A., Vizcarra E.A., Tang W.W., Sheets N., Joo Y., Kim K., Gorman M.J., Diamond M.S., Shresta S. Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe. 2017;21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hassert M., Harris M.G., Brien J.D., Pinto A.K. Identification of protective CD8 T Cell responses in a mouse model of Zika virus infection. Front. Immunol. 2019;10:1678. doi: 10.3389/fimmu.2019.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Regla-Nava J.A., Elong Ngono A., Viramontes K.M., Huynh A.T., Wang Y.T., Nguyen A.T., Salgado R., Mamidi A., Kim K., Diamond M.S., et al. Cross-reactive Dengue virus-specific CD8(+) T cells protect against Zika virus during pregnancy. Nat. Commun. 2018;9:3042. doi: 10.1038/s41467-018-05458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naveca F.G., Pontes G.S., Chang A.Y., Silva G., Nascimento V.A.D., Monteiro D., Silva M.S.D., Abdalla L.F., Santos J.H.A., Almeida T.A.P., et al. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Do Inst. Oswaldo Cruz. 2018;113:e170542. doi: 10.1590/0074-02760170542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassert M., Wolf K.J., Schwetye K.E., DiPaolo R.J., Brien J.D., Pinto A.K. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018;14:e1007237. doi: 10.1371/journal.ppat.1007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira Neto T.A., Goncalves-Pereira M.H., de Queiroz C.P., Ramos M.F., de Oliveira F.F.S., Oliveira-Prado R., do Nascimento V.A., Abdalla L.F., Santos J.H.A., Martins-Filho O.A., et al. Multifunctional T cell response in convalescent patients two years after ZIKV infection. J. Leukoc. Biol. 2020;108:1265–1277. doi: 10.1002/JLB.4MA0520-708R. [DOI] [PubMed] [Google Scholar]
- 65.Hatch S., Endy T.P., Thomas S., Mathew A., Potts J., Pazoles P., Libraty D.H., Gibbons R., Rothman A.L. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011;203:1282–1291. doi: 10.1093/infdis/jir012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akondy R.S., Monson N.D., Miller J.D., Edupuganti S., Teuwen D., Wu H., Quyyumi F., Garg S., Altman J.D., Del Rio C., et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blom K., Braun M., Ivarsson M.A., Gonzalez V.D., Falconer K., Moll M., Ljunggren H.G., Michaelsson J., Sandberg J.K. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J. Immunol. 2013;190:2150–2158. doi: 10.4049/jimmunol.1202234. [DOI] [PubMed] [Google Scholar]
- 68.Gaucher D., Therrien R., Kettaf N., Angermann B.R., Boucher G., Filali-Mouhim A., Moser J.M., Mehta R.S., Drake D.R., 3rd, Castro E., et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmes E.C., Twiddy S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003;3:19–28. doi: 10.1016/S1567-1348(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed S.F., Quadeer A.A., Barton J.P., McKay M.R. Cross-serotypically conserved epitope recommendations for a universal T cell-based dengue vaccine. PLoS Negl. Trop. Dis. 2020;14:e0008676. doi: 10.1371/journal.pntd.0008676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durham N.D., Agrawal A., Waltari E., Croote D., Zanini F., Fouch M., Davidson E., Smith O., Carabajal E., Pak J.E., et al. Broadly neutralizing human antibodies against dengue virus identified by single B cell transcriptomics. eLife. 2019;8:e52384. doi: 10.7554/eLife.52384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu D., Zhu Z., Li S., Deng Y., Wu Y., Zhang N., Puri V., Wang C., Zou P., Lei C., et al. A broadly neutralizing germline-like human monoclonal antibody against dengue virus envelope domain III. PLoS Pathog. 2019;15:e1007836. doi: 10.1371/journal.ppat.1007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keasey S.L., Pugh C.L., Jensen S.M., Smith J.L., Hontz R.D., Durbin A.P., Dudley D.M., O’Connor D.H., Ulrich R.G. Antibody responses to Zika virus infections in environments of Flavivirus endemicity. Clin. Vaccine Immunol. CVI. 2017;24:e00036-17. doi: 10.1128/CVI.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stettler K., Beltramello M., Espinosa D.A., Graham V., Cassotta A., Bianchi S., Vanzetta F., Minola A., Jaconi S., Mele F., et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 75.Delgado F.G., Torres K.I., Castellanos J.E., Romero-Sanchez C., Simon-Loriere E., Sakuntabhai A., Roth C. Improved immune responses against Zika virus after sequential dengue and Zika virus infection in humans. Viruses. 2018;10:480. doi: 10.3390/v10090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grifoni A., Pham J., Sidney J., O’Rourke P.H., Paul S., Peters B., Martini S.R., de Silva A.D., Ricciardi M.J., Magnani D.M., et al. Prior dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol. 2017;91:e01469-17. doi: 10.1128/JVI.01469-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrera B.B., Tsai W.Y., Brites C., Luz E., Pedroso C., Drexler J.F., Wang W.K., Kanki P.J. T cell responses to nonstructural protein 3 distinguish infections by dengue and Zika viruses. mBio. 2018;9:e00755-18. doi: 10.1128/mBio.00755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrera B.B., Tsai W.Y., Chang C.A., Hamel D.J., Wang W.K., Lu Y., Mboup S., Kanki P.J. Sustained specific and cross-reactive T cell responses to Zika and dengue virus NS3 in West Africa. J. Virol. 2018;92:e01992-17. doi: 10.1128/JVI.01992-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reynolds C.J., Suleyman O.M., Ortega-Prieto A.M., Skelton J.K., Bonnesoeur P., Blohm A., Carregaro V., Silva J.S., James E.A., Maillere B., et al. T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci. Rep. 2018;8:672. doi: 10.1038/s41598-017-18781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ngono A.E., Shresta S. Immune response to dengue and Zika. Annu. Rev. Immunol. 2018;36:279–308. doi: 10.1146/annurev-immunol-042617-053142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrade P., Gimblet-Ochieng C., Modirian F., Collins M., Cardenas M., Katzelnick L.C., Montoya M., Michlmayr D., Kuan G., Balmaseda A., et al. Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika. Nat. Commun. 2019;10:938. doi: 10.1038/s41467-019-08845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peiris J.S., Porterfield J.S. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature. 1979;282:509–511. doi: 10.1038/282509a0. [DOI] [PubMed] [Google Scholar]
- 83.Bardina S.V., Bunduc P., Tripathi S., Duehr J., Frere J.J., Brown J.A., Nachbagauer R., Foster G.A., Krysztof D., Tortorella D., et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li M., Zhao L., Zhang C., Wang X., Hong W., Sun J., Liu R., Yu L., Wang J., Zhang F., et al. Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS ONE. 2018;13:e0200478. doi: 10.1371/journal.pone.0200478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paul L.M., Carlin E.R., Jenkins M.M., Tan A.L., Barcellona C.M., Nicholson C.O., Michael S.F., Isern S. Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunol. 2016;5:e117. doi: 10.1038/cti.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rathore A.P.S., Saron W.A.A., Lim T., Jahan N., St John A.L. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci. Adv. 2019;5:eaav3208. doi: 10.1126/sciadv.aav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barba-Spaeth G., Dejnirattisai W., Rouvinski A., Vaney M.C., Medits I., Sharma A., Simon-Loriere E., Sakuntabhai A., Cao-Lormeau V.M., Haouz A., et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536:7614. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 88.Kam Y.W., Lee C.Y., Teo T.H., Howland S.W., Amrun S.N., Lum F.M., See P., Kng N.Q., Huber R.G., Xu M.H., et al. Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI Insight. 2017;2:e92428. doi: 10.1172/jci.insight.92428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez-Barraquer I., Costa F., Nascimento E.J.M., Nery N.J., Castanha P.M.S., Sacramento G.A., Cruz J., Carvalho M., De Olivera D., Hagan J.E., et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science. 2019;363:607–610. doi: 10.1126/science.aav6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen J., Elong Ngono A., Regla-Nava J.A., Kim K., Gorman M.J., Diamond M.S., Shresta S. Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017;8:1459. doi: 10.1038/s41467-017-01669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Goes Cavalcanti L.P., Tauil P.L., Alencar C.H., Oliveira W., Teixeira M.M., Heukelbach J. Zika virus infection, associated microcephaly, and low yellow fever vaccination coverage in Brazil: Is there any causal link? J. Infect. Dev. Ctries. 2016;10:563–566. doi: 10.3855/jidc.8575. [DOI] [PubMed] [Google Scholar]
- 92.Vicente Santos A.C., Guedes-da-Silva F.H., Dumard C.H., Ferreira V.N.S., da Costa I.P.S., Machado R.A., Barros-Aragao F.G.Q., Neris R.L.S., Dos-Santos J.S., Assuncao-Miranda I., et al. Yellow fever vaccine protects mice against Zika virus infection. PLoS Negl. Trop. Dis. 2021;15:e0009907. doi: 10.1371/journal.pntd.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blom K., Sandberg J.T., Lore K., Ljunggren H.G. Prospects for induction of CD8 T cell-mediated immunity to Zika virus infection by yellow fever virus vaccination. J. Intern. Med. 2017;282:206–208. doi: 10.1111/joim.12638. [DOI] [PubMed] [Google Scholar]
- 94.Pantoja P., Perez-Guzman E.X., Rodriguez I.V., White L.J., Gonzalez O., Serrano C., Giavedoni L., Hodara V., Cruz L., Arana T., et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 2017;8:15674. doi: 10.1038/ncomms15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCracken M.K., Gromowski G.D., Friberg H.L., Lin X., Abbink P., De La Barrera R., Eckles K.H., Garver L.S., Boyd M., Jetton D., et al. Impact of prior Flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog. 2017;13:e1006487. doi: 10.1371/journal.ppat.1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Terzian A.C.B., Schanoski A.S., Oliveira Mota M.T., Silva R.A., Estofolete C.F., Colombo T.E., Rahal P., Hanley K.A., Vasilakis N., Kalil J., et al. Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed zika virus–infected patients. Clin. Infect. Dis. 2017;65:1260. doi: 10.1093/cid/cix558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henein S., Swanstrom J., Byers A.M., Moser J.M., Shaik S.F., Bonaparte M., Jackson N., Guy B., Baric R., de Silva A.M. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J. Infect. Dis. 2017;215:351–358. doi: 10.1093/infdis/jiw576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dayan G.H., Galan-Herrera J.F., Forrat R., Zambrano B., Bouckenooghe A., Harenberg A., Guy B., Lang J. Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naive adults in Mexico. Hum. Vaccines Immunother. 2014;10:2853–2863. doi: 10.4161/21645515.2014.972131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabchareon A., Wallace D., Sirivichayakul C., Limkittikul K., Chanthavanich P., Suvannadabba S., Jiwariyavej V., Dulyachai W., Pengsaa K., Wartel T.A., et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 100.Torresi J., Richmond P.C., Heron L.G., Qiao M., Marjason J., Starr-Spires L., van der Vliet D., Jin J., Wartel T.A., Bouckenooghe A. Replication and excretion of the live attenuated tetravalent dengue vaccine CYD-TDV in a Flavivirus-naive adult population: Assessment of vaccine viremia and virus shedding. J. Infect. Dis. 2017;216:834–841. doi: 10.1093/infdis/jix314. [DOI] [PubMed] [Google Scholar]
- 101.Harenberg A., Begue S., Mamessier A., Gimenez-Fourage S., Ching Seah C., Wei Liang A., Li Ng J., Yun Toh X., Archuleta S., Wilder-Smith A., et al. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum. Vaccines Immunother. 2013;9:2317–2325. doi: 10.4161/hv.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guirakhoo F., Pugachev K., Zhang Z., Myers G., Levenbook I., Draper K., Lang J., Ocran S., Mitchell F., Parsons M., et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 2004;78:4761–4775. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guirakhoo F., Weltzin R., Chambers T.J., Zhang Z.X., Soike K., Ratterree M., Arroyo J., Georgakopoulos K., Catalan J., Monath T.P. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J. Virol. 2000;74:5477–5485. doi: 10.1128/JVI.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sridhar S., Luedtke A., Langevin E., Zhu M., Bonaparte M., Machabert T., Savarino S., Zambrano B., Moureau A., Khromava A., et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 105.Villar L., Dayan G.H., Arredondo-Garcia J.L., Rivera D.M., Cunha R., Deseda C., Reynales H., Costa M.S., Morales-Ramirez J.O., Carrasquilla G., et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 106.Hadinegoro S.R., Arredondo-Garcia J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R., Muhammad Ismail H.I., Reynales H., Limkittikul K., Rivera-Medina D.M., et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 107.Moodie Z., Juraska M., Huang Y., Zhuang Y., Fong Y., Carpp L.N., Self S.G., Chambonneau L., Small R., Jackson N., et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J. Infect. Dis. 2018;217:742–753. doi: 10.1093/infdis/jix609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guy B., Jackson N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016;14:45–54. doi: 10.1038/nrmicro.2015.2. [DOI] [PubMed] [Google Scholar]
- 109.Guy B., Nougarede N., Begue S., Sanchez V., Souag N., Carre M., Chambonneau L., Morrisson D.N., Shaw D., Qiao M., et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine. 2008;26:5712–5721. doi: 10.1016/j.vaccine.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 110.Castanha P.M.S., Erdos G., Watkins S.C., Falo L.D., Jr., Marques E.T.A., Barratt-Boyes S.M. Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI Insight. 2020;5:e133653. doi: 10.1172/jci.insight.133653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fagbami A.H., Halstead S.B., Marchette N.J., Larsen K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios. 1987;49:49–55. [PubMed] [Google Scholar]
- 112.Castanha P.M.S., Marques E.T.A. Zika vaccines: Can we solve one problem without creating another one? Lancet Infect. Dis. 2021;21:1198–1200. doi: 10.1016/S1473-3099(20)30768-4. [DOI] [PubMed] [Google Scholar]
- 113.Skibinski D.A., Baudner B.C., Singh M., O’Hagan D.T. Combination vaccines. J. Glob. Infect. Dis. 2011;3:63–72. doi: 10.4103/0974-777X.77298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nivarthi U.K., Swanstrom J., Delacruz M.J., Patel B., Durbin A.P., Whitehead S.S., Kirkpatrick B.D., Pierce K.K., Diehl S.A., Katzelnick L., et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021;12:1102. doi: 10.1038/s41467-021-21384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiskopf D., Cerpas C., Angelo M.A., Bangs D.J., Sidney J., Paul S., Peters B., Sanches F.P., Silvera C.G., Costa P.R., et al. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J. Infect. Dis. 2015;212:1743–1751. doi: 10.1093/infdis/jiv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Castanha P.M.S., Souza W.V., Braga C., Araujo T.V.B., Ximenes R.A.A., Albuquerque M., Montarroyos U.R., Miranda-Filho D.B., Cordeiro M.T., Dhalia R., et al. Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil. PLoS Negl. Trop. Dis. 2019;13:e0007246. doi: 10.1371/journal.pntd.0007246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ximenes R.A.A., Miranda-Filho D.B., Montarroyos U.R., Martelli C.M.T., Araujo T.V.B., Brickley E., Albuquerque M., Souza W.V., Ventura L.O., Ventura C.V., et al. Zika-related adverse outcomes in a cohort of pregnant women with rash in Pernambuco, Brazil. PLoS Negl. Trop. Dis. 2021;15:e0009216. doi: 10.1371/journal.pntd.0009216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castanha P.M.S., Marques E.T.A. A glimmer of hope: Recent updates and future challenges in zika vaccine development. Viruses. 2020;12:1371. doi: 10.3390/v12121371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cohen J. As massive Zika vaccine trial struggles, researchers revive plan to intentionally infect humans. Science. 2018;361:1055–1056. doi: 10.1126/science.361.6407.1055. [DOI] [PubMed] [Google Scholar]
- 120.Jamrozik E., Selgelid M.J. Ethical issues surrounding controlled human infection challenge studies in endemic low-and middle-income countries. Bioethics. 2020;34:797–808. doi: 10.1111/bioe.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gordon S.B., Rylance J., Luck A., Jambo K., Ferreira D.M., Manda-Taylor L., Bejon P., Ngwira B., Littler K., Seager Z., et al. A framework for Controlled Human Infection Model (CHIM) studies in Malawi: Report of a Wellcome Trust workshop on CHIM in Low Income Countries held in Blantyre, Malawi. Wellcome Open Res. 2017;2:70. doi: 10.12688/wellcomeopenres.12256.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Casares S., Brumeanu T.D., Richie T.L. The RTS,S malaria vaccine. Vaccine. 2010;28:4880–4894. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 123.Stanisic D.I., McCarthy J.S., Good M.F. Controlled Human Malaria Infection: Applications, advances, and challenges. Infect. Immun. 2018;86:e00479-17. doi: 10.1128/IAI.00479-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Losonsky G.A., Tacket C.O., Wasserman S.S., Kaper J.B., Levine M.M. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: Changes with time and lack of correlation with protection. Infect. Immuninty. 1993;61:729–733. doi: 10.1128/iai.61.2.729-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shirley D.A., McArthur M.A. The utility of human challenge studies in vaccine development: Lessons learned from cholera. Vaccine. 2011;2011:3–13. doi: 10.2147/VDT.S23634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tacket C.O., Cohen M.B., Wasserman S.S., Losonsky G., Livio S., Kotloff K., Edelman R., Kaper J.B., Cryz S.J., Giannella R.A., et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect. Immun. 1999;67:6341–6345. doi: 10.1128/IAI.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tacket C.O., Losonsky G., Nataro J.P., Cryz S.J., Edelman R., Kaper J.B., Levine M.M. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 1992;166:837–841. doi: 10.1093/infdis/166.4.837. [DOI] [PubMed] [Google Scholar]
- 128.Richie E.E., Punjabi N.H., Sidharta Y.Y., Peetosutan K.K., Sukandar M.M., Wasserman S.S., Lesmana M.M., Wangsasaputra F.F., Pandam S.S., Levine M.M., et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18:2399–2410. doi: 10.1016/S0264-410X(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 129.Vannice K.S., Giersing B.K., Kaslow D.C., Griffiths E., Meyer H., Barrett A., Durbin A.P., Wood D., Hombach J. Meeting Report: WHO consultation on considerations for regulatory expectations of Zika virus vaccines for use during an emergency. Vaccine. 2019;37:7443–7450. doi: 10.1016/j.vaccine.2016.10.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.