Abstract
The increase in the world population has generated an important need for both quality and quantity agricultural products, which has led to a significant surge in the use of chemical pesticides to fight crop diseases. Consumers, however, have become very concerned in recent years over the side effects of chemical fungicides on human health and the environment. As a result, research into alternative solutions to protect crops has been imposed and attracted wide attention from researchers worldwide. Among these alternatives, biological controls through beneficial microorganisms have gained considerable importance, whilst several biological control agents (BCAs) have been screened, among them Bacillus, Pantoea, Streptomyces, Trichoderma, Clonostachys, Pseudomonas, Burkholderia, and certain yeasts. At present, biopesticide products have been developed and marketed either to fight leaf diseases, root diseases, or fruit storage diseases. However, no positive correlation has been observed between the number of screened BCAs and available marketed products. Therefore, this review emphasizes the development of biofungicides products from screening to marketing and the problems that hinder their development. Finally, particular attention was given to the gaps observed in this sector and factors that hamper its development, particularly in terms of efficacy and legislation procedures.
Keywords: biological control, biological control agents, crop diseases, antagonist
1. Introduction
Modern agriculture is continuously developing and evolving. After the generalization of the use of chemical fertilizers and pesticide products, which allowed a considerable increase in yield in the twentieth century, the rise of biotechnologies and new cultivation techniques is underway [1]. The next challenge to be met is to feed around 9 billion people by 2050 [2,3]. In this context, one of the major concerns is to expand food production capacities, including those derived from plants, while preserving the environment [4]. Nowadays, countries are striving to expand their food production to meet their needs [5]. The increase in the production of a given crop is often linked to the improvement of cultivation techniques, particularly the use of more productive cultivars with resistance to main diseases [6].
Crop protection, which is still largely achieved by applying chemical products, is also in a transitional phase [7]. Therefore, the gradual integration of new practices, taking into account the agricultural production system, requires not only the environmental dimension but also the socio-economic dimension [8]. Nevertheless, these cultures are often subjected to parasitic attacks that farmers are still forced to control below the threshold of harmfulness to survive and be efficient [9]. In addition, ongoing growth in productivity and international trade boosts the incidence of certain diseases, thus requiring the application of more pesticides. Subsequently, these pesticides increase environmental pollution and build up chemical residues in the treated ecosystem [10]. Other alternatives such as genetic pathways offer interesting control methods from a practical point of view, but also strengthen the risks of the emergence of resistant genes in the pathogen [11]. Other alternatives such as biological controls using microorganisms are a possible way to minimize the pollution and nuisances associated with the use of synthetic chemicals and greatly reduce their negative impact on the environment [12,13].
The concept of biocontrol has caused an important technological, economic, and political debate aiming to develop sustainable agriculture at a lower ecological cost [14,15]. Accordingly, different countries have implemented a protective plan that can reduce around 50% of used pesticides [16]. These measures unequivocally illustrate a major awareness of the accumulation of toxic residues in the environment and the various links within the food chain. They also indicate the lack of alternatives to reduce the reliance of the agricultural sector on pesticides. In this context, it appears crucial to deepen our knowledge about biocontrols to improve their use and efficiency [15]. For all of these reasons, research is progressing well towards a perspective of biological control, based on the application of microbial inoculum, that could be added to the other aforementioned ways to develop a strong strategy to fight plant diseases.
Promising achievements in terms of biological control have emerged, especially after the successful use of certain antagonistic biocontrol agents (BCAs), in particular Pseudomonas spp., Bacillus spp., Burkholderia spp., and Trichoderma sp. against pathogens causing foliar and soilborne diseases like Agrobacterium radiobacter var radiobacter, Erwinia spp., Fusarium spp., Rhizoctonia solani, Phytophthora spp. and Pythium spp. diseases [13,17]. Additional BCAs have showed an antagonistic effect against a wide spectrum of diseases, namely bacterial species such as Burkholderia spp., Paenibacillus spp., Pantoea spp., Serratia spp., Streptomyces spp., and fungal species such as Aspergillus spp., Beauveria spp., Fusarium spp., Penicillium spp., and Phoma spp. Furthermore, in addition to halting the pathogen proliferation, many of these BCAs can also directly promote plant growth [9,12,13,17,18]. However, in most cases, the efficacy of BCAs has always been found to be lower than that of synthetic fungicides. This might be due to the complexity of the rhizosphere and the need to apply a high amount of BCAs to cover the entire rhizosphere [19]. This can only happen if the BCA is applied continuously and consecutively. In addition, the way BCAs are formulated and applied can also affect their efficacies [20].
Even though the research for new microorganisms as potential biopesticides has increased, biopesticides have been produced in limited numbers. However, small and medium companies are starting to adapt to this new emerging market. The North American region has the largest amount of the biopesticide market, reaching USD 539 million in 2015 and predicted to attain USD 1.67 billion by 2022 [21]. Research on biological control using microorganisms is experiencing and gaining remarkable momentum, although applications in the field are still limited. The factors limiting the use of BCAs in the field include the inconsistent efficiency in protecting plants under field conditions, decreased availability in the market, and the wide unacceptance of BCAs by farmers.
In this paper, we discuss the progress made on the prospecting of biocontrol agents, their development, and their mechanisms of action, with a special emphasis on legislative procedures and factors affecting their application and marketing development.
2. Rhizosphere as a Potential Reservoir of Biopesticides
The rhizosphere is the soil area that surrounds living roots and is influenced by plant root exudates. It is one of the most important microbial hotspots in the soil, with much greater process rates and more intensive interactions [22,23]. The rhizosphere contains a complex microbial community called the rhizosphere microbiome. This microbial consortium lives around plant roots and is formed up of many microorganisms, some of which are beneficial such as bacteria, algae, and fungi [24]. Because it is a source of utilizable carbon, the rhizosphere provides a supportive environment for crucial and intensive interactions between plants, soil, microorganisms, and soil microfauna [25]. Plants can recruit a unique beneficial rhizosphere microorganisms, which can help them to attenuate the disease activity and make them more resistant to environmental stressors [26].
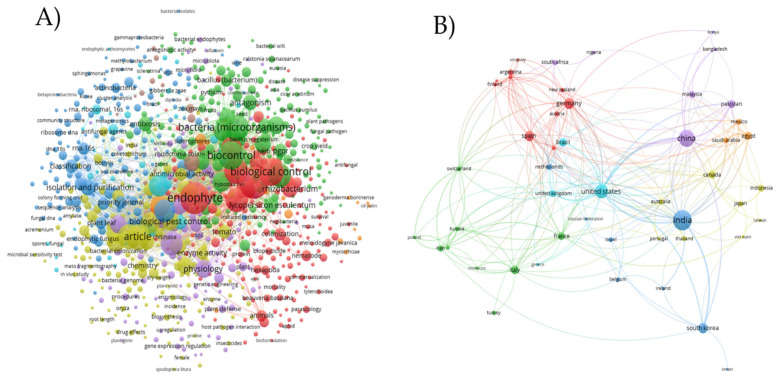
Understanding the role of rhizosphere microorganisms in pest and disease control seems to be a rising research field, as evidenced by the high increase in studies conducted between 2000 and 2019. When the term “rhizosphere” was added to a Google Scholar search with the keywords “microorganisms”, “control”, “pest”, and “diseases”, the number of records retrieved increased from roughly 5000 (2000–2005) to 8500 and >20,000 (2006–2010 and 2011–2019, respectively). In the absence of this term, records dropped from roughly 17,000 to 15,000 in the most recent period [27]. In the present review, we have extracted bibliometric data to perform a deep analysis and establish a network of worldwide distribution-related articles on biocontrol agents. The bibliometric data were extracted from the SCOPUS database (https://www.scopus.com/, accessed on 22 December 2021) using the specific keywords ‘Rhizobacteria’ OR ‘Endophytes’ OR ‘Biocontrol agents’, from which 1150 documents were obtained. The bibliometric analysis was performed using different bibliometric indices, including the most popular used keywords, countries, and the top journals, and was constructed using the VOSviewer processing software (v1.6.9., Leiden University, Leiden, The Netherlands) (Figure 1A). The network analysis showed the worldwide distribution of related articles to biocontrol agents, which revealed the relationship between keywords found and allows the obtainment of a comprehensive perspective of the current research of this area (Figure 1B).
Figure 1.
Bibliometric analysis of 1150 articles published on biological control according to the Scopus database using specific keywords such as “Rhizobacteria” OR “Endophytes” OR ”Biocontrol” (A) and the network analysis of their worldwide distribution (B); the larger the circle, the more intense the scientific activity.
Since the use of pesticides to control pests and diseases has been linked to environmental, ecological, and human health risks, it has become necessary to seek out eco-friendly biological agents known as biopesticides [28,29] to ensure biological control. The latter, often known as “biocontrol”, is a method of reducing or eliminating the impact or damage produced by a specific pest or weed by releasing a biocontrol agent, such as a predator, herbivore, or pathogen [30]. To handle plant diseases, biological control is considered a promising and reliable alternative to the use of synthetic fungicides [27,31]. Several billions of dollars are being spent on biocontrol research, and the number of biocontrol drugs that are available for various plant crops is rapidly expanding [32]. Biopesticides are microbial or products generated from microbes, plants, and other biological organisms aiming to control plant pests [29,33].
Biopesticides have long-term potential in improving sustainable agriculture [29]. There are currently many (more than 440 species) control agents available for various pests [34]. BCAs are used to control plant diseases in crops, and they involve a variety of mechanisms [35]. Van Lenteren et al. [34] presented the first list of BCAs registered worldwide, which includes bacteria, fungi, mycoviruses, and bacteriophages [34]. Certain antagonistic microorganisms have been identified from the rhizosphere of numerous agricultural plants to inhibit some plant diseases and hence reduce the need for agrochemical pesticides.
In the last two decades, researchers have been studying the use of these specialized antagonistic microorganisms in the biological control of soil-borne diseases. Biopesticides are available inside the rhizosphere to combat pests and microbial diseases due to the close connection of root-colonizing probiotic microorganisms with plant host cells [36]. Several bacteria, fungi, protozoa, and nematodes have recently demonstrated antagonistic activity that could be employed in the biocontrol of root and foliar diseases in a variety of crops, and also insect pests [37,38]. Antibiotics, bacteriocins, siderophores, hydrolytic enzymes, and other secondary metabolites produced by these beneficial rhizosphere microorganisms inhibit pathogenic bacteria and fungi [28,32].
Direct antagonism of potential pests through the production of biopesticides is one of the features of plant-probiotic microorganisms that contribute to plant health. Antagonistic fungi such as Trichoderma spp., as well as bacteria from the genera Pseudomonas, Bacillus, and Streptomyces, account for the majority of rhizosphere microorganisms commonly used in biocontrols [27].
2.1. Beneficial Bacteria
Most plants grown in the field are colonized by various rhizosphere bacteria [39]. Some plant-associated bacteria are classified as beneficial microorganisms, based on their effects on plant performance. Among these free-living bacteria, plant-growth-promoting rhizobacteria (PGPR) thrive freely in the rhizosphere soil [25,33] and produce a variety of antifungal metabolites and plant growth-promoting features [25]. Based on the ability or behavior of the crops and biocontrol agents, PGPRs also act as biopesticides [33]. PGPRs are bacterial species including Alcaligenes, Azospirillum, Arthrobacter, Acinetobacter, Bradyrhizobium, Bacillus, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Pseudomonas, Rhizobium, Azorhizobium, Bradyrhizobium, Allorhizobium, Sinorhizobium, Frankia, Mesorhizob, Azoarcus, Exiguobacterium, Methylobacterium, Paenibacillus, and Pantoea [29,40,41,42,43,44,45,46,47,48]. In reaction to diverse chemicals found in root exudates, beneficial bacterial communities in the soil are chemoattracted towards plant roots. These beneficial associations boost plant health and crop productivity by increasing nutrient availability, releasing plant growth-stimulating hormones, decreasing pathogen/pest-caused diseases, or improving the environmental stress resistance [39]. Antibiotics, endotoxins, bacteriocins, siderophores, hydrolytic enzymes, hydrogen cyanide (HCN), phenazine-1-carboxylic acid (PCA), 2,4-diacetylphloroglucinol (DAPG), and other secondary metabolites produced by certain rhizosphere bacteria kill pathogens, thus preventing disease development [39,43,44,47,49,50,51,52,53,54]. Some PGPRs utilized in the control of plant pests and diseases are discussed below.
Bacillus species have a long history in biocontrol and crop growth-promoting applications [55]. Bacillus thuringiensis (Bt) is the most commercially successful biopesticide on the market [29,56]. Bt produces endotoxins [51,52], which are toxic and can be exploited as biopesticides as well as a source of genes for the development of insect-resistant transgenic plants [52]. During their stationary phase of growth, Bt strains produce crystalline proteins (δ-endotoxins) that are toxic to lepidopterous, coleopterous, and dipterous insects as well as mites, nematodes, protozoa, and flukes [51]. Insect bioassays were performed on the spore crystal mixtures of 12 Bt isolates, and it was found that the isolate F8.IIPR has the maximum toxicity against Spilosoma obliqua Walker (100%), Olepa ricini Fabricius (92%), and Helicoverpa armigera Hubner (100%) larvae [57]. Bacillus subtilis (BCB-19) and Bacillus megaterium (SB-9) induced considerable larval death and growth inhibition in both H. armigera and Spodoptera litura [58]. Bacillus amyloliquefaciens LMR2, Bacillus halotolerans (SF3 and SF4), and Bacillus mojarvensis SF16 were recently isolated from soil of fire blight host plants in different Moroccan regions for their higher efficacy in reducing apple fire blight disease [48]. Monilinia fructigena and Monilinia laxa, which cause brown rot disease of fruits, are controlled by B. amyloliquefaciens SF14 and B. amyloliquefaciens SP10, respectively. In a semi-commercial large-scale study, the efficacy of these strains was found to be comparable to that of two commercial BCAs, but slightly lower than that of a commercial synthetic fungicides [47,59]. The considerable biological control activity of a Bacillus velezensis strain named ZW10, as well as its ability to boost host defenses, make it a potential biopesticide for rice blast biocontrol caused by the fungus Magnaporthe oryzae [60]. The commercially available B. amyloliquefaciens FZB42 product has considerably decreased lettuce bottom rot caused by R. solani. This reduction was due to the secretion of surfactin and other FZB42-non-ribosomally synthesized secondary metabolites in the lettuce rhizosphere. Subsequently, plant regulation genes are triggered and expressed to protect plants against the pathogen R. solani [53].
In a dual culture bioassay, endophytic Bacillus strains obtained from cotton roots have demonstrated an inhibitory efficacy against Verticillium dahliae strain VD-080. Scanning electron microscopy examination underlined mycelial disintegration, curling, and shrinkage of V. dahliae hyphae after treatment with methanolic extracts of isolated endophytes. Furthermore, when compared to control treatments, cotton plants treated with two Bacillus strains (HNH7 and HNH9) showed a considerable reduction in verticillium wilt severity. Moreover, the expression of some defense-related genes was significantly higher in plants treated with Bacillus strains and inoculated with VD-080 [54].
Pseudomonas chlororaphis isolates are used as biopesticides in agriculture as they protect plants from various microbial diseases, insects, and nematodes. These isolates directly suppress microbial pathogens, insects, and nematodes by producing a variety of metabolites [36]. P. chlororaphis PcO6, isolated from the roots of dryland, enhanced plant health and was used as a biofertilizer and BCA in agriculture. Plant growth is stimulated by an array of metabolites generated by this bacterium through direct pathogen antagonism and induction of systemic resistance in the plant. The mechanisms by which specific bacterial metabolites create protection against pathogenic microorganisms, insects, and nematodes have been identified in studies on PcO6. The role of a global regulatory system, the Gac/Rsm regulon, in conferring protection against plant pathogens has been highlighted [61]. The Gac/Rsm system network in PcO6 communicates the capacities required to adapt to various stressors and enhance survival while also maintaining plant health by priming stress responses. When the bacterium reaches a critical cell density, the Gac/Rsm network is activated, causing the microbe to switch its nutrition consumption and create protectants instead of adding cell mass. Other systems influencing gene expression are linked to the Gac/Rsm system. Antimicrobials are produced when the Gac/Rsm network in PcO6 is activated, which may help in sustaining plant root colonization and protecting the plant against microbial diseases. PcO6 impacts microbiomes and plant health in the rhizosphere by secreting antimicrobials and volatiles. The bacterium forms a protective biofilm on the root surface, which acts as a physical shield and a water-holding gel [61]. Antifungal compounds such as bacteriocin, HCN, and siderophore were produced by Pseudomonas aeruginosa isolated from the banana field rhizosphere. Phytopathogens such as Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, and Alternaria alternata were suppressed by the isolate’s bacteriocinogenic, siderophoregenic, and HCN rich broth. Bacteriocin has a toxic effect on bacteria, whereas siderophore and HCN inhibit fungal phytopathogens. It is worth mentioning that none of the helpful rhizobia were inhibited by these compounds. In comparison to copper-based systemic chemical fungicide, the P. aeruginosa isolate showed higher antifungal activity and a lower minimum inhibitory concentration [43]. The ability of Pseudomonas sp. LBUM 223 to control common scab potato caused by Streptomyces scabies was proven via PCA synthesis. This PCA synthesis is critical for LBUM 223’s capacity to control common scabs of potato, limit pathogen growth, and inhibit the expression of important pathogenicity genes [50]. Similarly, Lanteigne et al. [49] have identified DAPG and HCN as the chemicals responsible for Pseudomonas sp. LBUM300’s capacity to inhibit the growth of Clavibacter michiganensis subsp. michiganensis in vitro and regulate the development of bacterial canker in tomato under soil conditions. In both H. armigera and S. litura, Pseudomonas spp. (SB-21) was reported to induce considerable larval death and growth inhibition [58].
2.2. Fungi and Yeasts
Several fungal species have been identified as insect pest entomopathogens, as well as plant endophytic fungi [62]. Trichoderma is a ubiquitous fungus genus found as soil inhabitants, plant symbionts, saprotrophs, and mycoparasites [63]. This genus contains filamentous fungi that have been extensively investigated and utilized in agriculture as a biocontrol agent against phytopathogens due to its capacity to compete and parasitize them (mycoparasitism), among other mechanisms of action [62,63,64], as well as mitigating unfavorable growth conditions [63]. Trichoderma is by far the most studied fungal biocontrol agent, with some species having previously been commercialized as biopesticides or biofertilizers [63,64]. The use of Trichoderma as a BCA for insect pests has been discussed in recent years, both directly and indirectly [62]. Trichoderma has been shown to directly suppress insect pests by parasitism and the generation of secondary insecticidal metabolites [62,65], antifeedant chemicals, and repelling metabolites. Indirectly, by activating systemic plant defense systems, attracting natural enemies, and parasitizing insect-symbiotic microorganisms. As a result, Trichoderma use in agriculture is effective not only against plant pathogens but also against insect pests, making it a promising future alternative for the development of sustainable agriculture [62]. Trichoderma aggressivum isolates were collected from numerous Agaricus bisporus growing substrates from farms in Castilla-La Mancha (Spain). This fungus had high antagonistic activity against a variety of phytopathogens, greater than 80%. In a detached leaves assay, the most effective isolate, T. aggressivum f. europaeum TAET1, has completely suppressed the mycelial growth of fungal pathogens Botrytis cinerea, Sclerotinia sclerotiorum, and Mycosphaerella melonis and inhibited S. sclerotiorum sclerotia germination. This excellent compatibility with chemical fungicides may allow this isolate to be used in combination with various pest management strategies [66]. Trichoderma asperellum TaspHu1, an isolate obtained from Juglans mandshurica Maxim. rhizosphere soils in China improved tomato seedling resistance to A. alternata leaf spot disease [67]. Trichoderma spp. from olive rhizosphere soils in northern Algeria had great biocontrol potential against V. dahliae, the causal agent of wilting on olive trees. These isolates showed an effective potential in reducing the in vitro mycelial growth of this pathogenic fungus. By using the confrontation assay method, Trichoderma isolate T12 inhibited V. dahliae isolates at a rate of 69% [68]. Trichoderma spp. has been proven to be effective in suppressing Sclerospora graminicola, the causal agent of pearl millet downy mildew disease. Their prominent role indirectly suppressing the pathogen in the rhizosphere and establishing systemic resistance has been well demonstrated [69].
Furthermore, yeasts are often used in commercial formulations because of their biocontrol capacity. They are already available for postharvest use on a variety of crops, including pome and citrus fruits. Because of their great ability for quick colonization and inhibition of pathogens, as well as their potential for competition for nutrients, physical interaction, and the generation of lytic enzymes, these microorganisms have been shown to have a significant BCA activity [70].
Millan et al. [71] investigated the in vivo and in vitro effects of 69 yeast strains isolated from Spanish vineyards against Alternaria alternata, Penicillium expansum, and B. cinerea, as well as soil-borne diseases V. dahliae and Fusarium oxysporum, to find a potential yeast with high biocontrol capabilities against postharvest diseases and wilt diseases. As a result, Wickerhamomyces anomalus Wa-32 reduced the severity of disease caused by V. dahliae up to 40%, and that due to F. oxysporum up to 50%. Furthermore, the postharvest assays revealed a high biocontrol performance against P. expansum and B. cinerea (up to 86 and 97% reduction in disease severity, respectively). Furthermore, W. anomalus Wa-32 and two Metschnikowia pulcherrima strains showed significant action against B. cinerea (Mp-22 and Mp-30). However, according to the same study, Candida lusitaniae Cl-28, Candida oleophila Co-13, Debaryomyces hansenii Dh-67, and Hypopichia pseudoburtonii Hp-54 were the most efficient against P. expansum, according to the study. This was also observed with grapes and apples [71].
3. BCAs Modes of Action
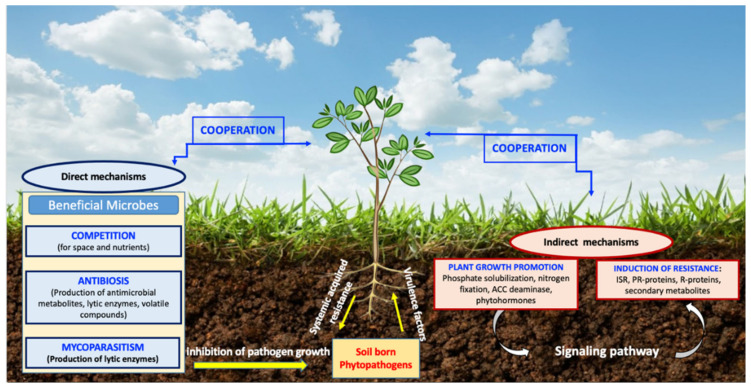
BCAs are applied in the disease management of plant pathogens, where they act via a variety of different modes of action to control plant pathogens. Understanding the mechanisms behind the protective effects of BCAs will facilitate the optimization of control and will allow the use of more efficient strains in the correct environment [72,73]. These mechanisms might be used alone or in combination by the BCA to control plant disease directly and indirectly (Figure 2).
Figure 2.
Possible modes of action of biological control agents.
3.1. Direct Mode of Action
In the direct way of disease control, BCAs act through a direct antagonistic effect on the pathogen, encompassing (i) antibiosis, (ii) parasitism, and (iii) reducing pathogen virulence, and (iv) infection pressure by competition [9,74]. BCAs act through antibiosis that relies on BCAs secretion of allelochemicals which could either be diffusible metabolites such as lipopeptides, bacteriocins, antibiotics, biosurfactants, and cell-wall-degrading enzymes or microbial volatile compounds, which interfere with the metabolism of phytopathogen and thereby inhibit the pathogen development [9,75,76]. There are two types of parasitism: necrotrophic parasitism and biotrophic parasitism. In the first type, the BCA kills the host cells before or just after the invasion of the target pathogen and uses its released nutrients [77]. Whilst in the second type of parasitism, the development of the BCA is favored by a living rather than a dead host structure [78]. Depending on the BCA, we can distinguish the parasitism of viruses on bacteria (bacteriophages), bacteria on fungi (mycophagy), and fungi on fungi (mycoparasitism) [9,75,76]. BCAs may also reduce pathogen virulence by secreting enzymes that interfere with pathogenicity factors, including pectinases and chitinases, which reduce pathogen virulence [76]. Finally, BCAs can reduce pathogen infection pressure through the competition for nutrients and space where both BCAs and pathogens compete with each other. This will help BCAs to establish themselves in the environment through the physical occupation of the site, biofilm formation resulting in reduced colonization of roots by the pathogen, and secretion of the essential micronutrients chelating such as siderophores as well as the characteristics of BCAs, which have more efficient uptake system for micronutrients in the case of the pests [72,79].
3.1.1. Involvement of Lytic Enzymes
Plants are probably primed for SAR by some Trichoderma spp., but the full pathway remains inactivated until a subsequent pathogen/parasite attack [80]. That is the case for example of Trichoderma asperellum (T203) which was used to prime cucumber against angular leaf spot caused by Pseudomonas syringae pv. Lachrymans. Disease symptoms decreased by up to 80%, equating to a two-orders-of-magnitude drop in bacterial cell densities in leaves of T. asperellum-treated plants. Bacterial cell multiplication in these plants was inhibited. The accumulation of mRNA of two defense genes, the phenylpropanoid pathway gene expressing phenylalanine ammonia-lyase (PAL) and the lipoxygenase pathway gene encoding hydroxyperoxide lyase (HPL), was linked to BCAs protection. This was further corroborated by the accumulation of secondary phenolic compounds, which demonstrated a six-fold increase in the capacity of inhibition of bacterial growth in vitro [81].
Trichoderma asperellum, which was isolated from banana wilt-affected plantations, has anti-pathogenic properties based on a variety of mechanisms. One of them includes antibiosis, which is used by a variety of pathogenic fungi, belonging to Fusarium genus. T. asperellum has been proven to decrease phytopathogen growth by 65–74% and impede spore germination by 30–75%. Furthermore, T. asperellum releases mycolytic enzymes (chitinase and β-1,3, glucanase), which may be able to destroy phytopathogen cell walls. In pathogen-induced cultures, the accumulation of respective transcripts and enzymatic activity of both chitinase and β-1,3, glucanase were significantly higher [82]. In the stimulation of the plant immune system by biocontrol fungi against RKNs, ET- and SA-responsive, genes were upregulated by glucanase and chitinase activity, whilst downregulated by genes encoding antioxidant enzymes [80].
3.1.2. Antimicrobial Molecules
Antimicrobial molecules are secondary metabolites that belong to a diverse category of organic, low-molecular-weight compounds produced by microorganisms and are toxic to the growth of other microorganisms or metabolic processes [83]. Antibiotics and related natural compounds can be produced by nearly all living organisms as they are secondary metabolites. They are produced by both prokaryotic and eukaryotic (plant and animal kingdom) species, within unicellular bacteria and eukaryotic fungus. Generally, filamentous actinomyces are the most common and varied producers of antibiotics. Furthermore, amongst prokaryotic and unicellular bacteria, Bacillus and Pseudomonas species are the most common producers [84].
3.1.3. Biofilm Formation
Understanding how to use BCAs effectively is critically important in sustainable agriculture. The exact mechanisms, chemical or physical, involved when crops are attacked by infectious pathogens are therefore the first steps to determine the factors influencing the efficacity of biocontrol against plant diseases. Physical mechanisms of BCAs, for example, include techniques triggered at the molecular level to physically protect the plant because of antagonistic interactions with host plants. Understanding these mechanisms of action of BCAs would help develop biofungicides products with superior biocontrol properties. BCAs from either the same species or different species can physically interact with surfaces to form complex multicellular assemblies or aggregates of microorganisms also called, biofilms. These biofilms provide various advantages including increased resistance to certain biotic stresses [85]. The microbial cells adhere to each other’s surface through a complex matrix medium comprising a variety of extracellular polymeric substances (EPS) including exopolysaccharides, proteins, and DNA, known as signaling molecules. Microorganisms, including bacteria, cyanobacteria, and fungi, specifically interact with plant tissues through quorum sensing (QS). The latter exists widely in all kinds of microorganisms and is a communication channel for microorganisms [86] and allows individual microbe within colonies to coordinate with each other. In one experiment on Arabidopsis, B. subtilis 6051 formed a stable, extensive biofilm to protect plants from Pseudomonas syringae attacks [87]. Furthermore, both the bacterium Azotobacter chroococcum and the fungus Trichoderma viride [88] can form biofilms to protect various crops against pathogens [89]. Strains of B. velezensis QST713 can also develop a biofilm on inert surfaces to inhibit the growth of T. aggressivum, which causes green mold disease [90]. The mechanism of biofilm formation is a powerful tool that helps plants to protect themselves from pathogenic attacks. However, previous knowledge of BCAs and plant species interactions is required before utilization, as BCAs interact specifically with plant species, and thus, the adopted techniques should be species-specific.
3.1.4. Competition for Nutrients and Space
Within BCAs species, competition is defined as the niche overlap of BCAs and pathogens, resulting in simultaneous demand for the same resource [91]. Competition for nutrients (carbohydrates, nitrogen, and oxygen) and space is often suggested as a potential mechanism of action in biological control systems [92]. Fungi, in particular, use the competitive saprophytic ability (CSA), which was originally used to describe the ability of the fungus to colonize dead organic substrates through competition [93]. A fungus with high CSA also exhibits adaptation abilities including the production of antimicrobial substances which are inhibitory to the pathogen growth through both mycoparasitism and the rapid adaptation to environmental triggers (temperature and water availability) [93,94]. Many plant fungal pathogens, including B. cinerea, and S. sclerotiorum, can be effectively managed through the mechanism of CSA [95]. Both B. cinerea and S. sclerotiorum are necrotrophic fungal pathogens [96,97]. B. cinerea conidia and S. sclerotiorum ascospores require external nutrients from plant wounds, senescent plant organs, and/or pollen grains for germination, hyphal development, creation of infection cushions, and infection of plant tissues. Numerous studies highlighted that fungal saprophytes such as Candida oleophila, Clolostachys rosea, Epicoccum purpurascens, Metschnikowia fructicola, Pichia guilliermondii, Rhodotorula glutinis, Trichoderma harzianum, and Ulocladium atrum are excellent competitors and are therefore effective BCAs of B. cinerea and S. sclerotiorum [98,99,100,101,102,103,104]. Competition for both nutrients and space is the key physical mechanism used by some BCAs to prevent the development of pathogens.
BCAs decrease the population level of pathogens by depleting food sources as, generally, BCAs are more efficient in nutrient uptake than pathogens [105]. BCAs also use competition for space. Certain microorganisms such as yeasts and bacteria form extracellular polysaccharide capsules that facilitate the adhesion to the fruit surface [106]. Various BCAs limit the amount of nutrients available and reduce the pathogen spore germination percentage [107,108], which subsequently reduces germ-tube growth, infection, and necrosis, and then prevents the expansion of the pathogen [108].
Successful biological controls require colonization by the added biocontrol agents under nutrient deficiency. These BCAs reduced the available nutrients in the wound site and make nutrients inaccessible for the pathogens to germinate, grow, and infect their hosts [109,110]. Iron, for example, is an essential micronutrient that is present in a high percentage of soils. This element, however, has low solubility in soil with a pH > 6, making its acquisition hard for microorganisms [111]. Iron bioavailability has thus become a limiting factor that may lead to competition among organisms. In these conditions, BCAs produce siderophores which are iron-chelating compounds that bind to iron, thus making it inaccessible to pathogens. This iron sequestering mechanism causes inhibition of both growth and metabolic activity of soil-borne pathogens. One siderophore compound in particular, rhodotorulic acid produced by Rhodotorula glutinis, improved the control of apple blue rot caused by P. expansum [112]. In vitro experiments showed that Pseudomonas aeruginosa produced siderophores, in addition to several metabolites with a broad spectrum of antagonistic activities against Fusarium oxysporium f. sp. ciceri, F. udum, and Aspergillus niger [113]. Additionally, Bacillus species including B. subtilis and fluorescent pseudomonads have been effective in rhizosphere colonization [110,114]. B. subtilis produced siderophores that played an important role in the control of F. oxysporum [115]. In avocado flowers, for example, B. subtilis B246 effectively adhered to conidia and hyphae of stem-end rot pathogens and caused cell degradation, thus preventing pathogens from colonizing the flowers [116,117]. This competition mechanism is described in various biocontrol studies with various antagonists such as Pantoea agglomerans [118], Serratia plymuthica [118], and Aureobasidium pullulans [119]. These BCAs were proven to be effective against post-harvest pathogens of fruits [120]. Especially for the control of post-harvest diseases, the use of microorganisms that compete with pathogens for nutrients may be a preferable option to antibiotic-producing microorganisms to avoid food contamination and a build-up of antibiotic resistance within the pathogen population. Generally, for a successful control through competing mechanisms, both the pathogen and the antagonist must have the same requirement for a specific nutrient or resource. The effectiveness of these controls entails that BCAs are present in sufficient quantities at the correct time and location. Overall, the key effects of competition include (i) a reduction of pathogen potential by nutrient competition, (ii) an increase of saprotrophic competition for initial resources in substrate colonization, and (iii) a reduction of the actual amount of the pathogen in either the dormant survival or pathogenic growth phases. Those mechanisms involved in BCA controls can physically protect key crops from devastating pathogens while respecting the environment. Effective use of the correct BCAs is, therefore, an important component of a successful disease management program.
3.1.5. Parasitism
Parasitism is an important biological control mechanism exhibited by antagonists to directly target pathogens that cause soil-borne and foliar diseases [121]. Parasitism or predation occurs when an antagonist feeds on or within the pathogen. It causes the immediate destruction of affected pathogens [122]. Some BCAs use parasitism mechanisms by producing propagules which are aggregates of BCA around or inside the pathogen. The density and the distance between a pathogen and its nearest BCA propagule are important components in effectively controlling pathogens. The denser BCAs are to each other, the more effective they are at controlling pathogens. Many pathogens share the same BCA propagule as their nearest BCA neighbor, and this interaction between BCAs and pathogens helps tremendously in the parasitism process [123]. Some pathogens are resistant to BCAs if optimum environmental conditions are not fulfilled such as adequate temperature and space. BCAs are also influenced by their quality and quantity when introduced to a targeted ecosystem. Pathogen resistance can be determined by measuring the proportion of pathogen propagules that remain infective as a function of the amount of BCAs introduced to the system [124]. Consequently, efforts should be devoted to improving the parasitism process for successful controls. Some of these measures include the use of mixtures of BCAs, optimal timing of antagonist application, integrated biological and chemical controls, and optimization of environmental conditions [124]. The consumption of one fungus by another is called mycoparasitism [125]. Mycoparasitism specifically utilizes fungal cell-wall-degrading enzymes to access the cells [126]. Trichoderma, for example, can effectively eliminate phytopathogenic fungi through the suppression of other microorganisms at the same site, making Trichoderma the dominant organism in the infested site [127,128,129]. Parasitism between yeasts and fungus was also studied. Cytological damage and protuberances in the cell wall and degeneration of the cytoplasm were observed in vivo culture of both Candida saitoana yeast cells with B. cinerea mycelium [125]. In 1991, Wisniewski et al. [125] observed a strong in vitro adhesion of the antagonistic yeast Pichia guilliermondii cells to B. cinerea mycelium [130]. Mechanisms of action determining the success of biocontrol are complex, which, to some extent, may explain the limited effectiveness of biocontrol against plant diseases in field crops. In this case, the screening of these BCAs can be achieved by phenotype-based screenings by evaluating pathogen growth by dual culture assay or by volatile antifungal compounds (VOCs) or by marker-based screening: secretion of antimicrobial metabolites (antibiosis via bacterial supernatant), the secretion of cell-wall-degrading or screening bacteria with specific inhibitory mechanisms [76,131].
3.2. Indirect Mode of Action
Indirect mechanisms include the induction of resistance by stimulating plant defense reactions and stimulating plant growth and soil fertilization [74,132]. BCAs can initiate plant systemic resistance, which results in an accumulation of structural barriers and elicitation of many biochemical and molecular defense responses in the host. This action requires a signalization of the pathway of phytohormones, phytoalexins, and defense enzymes such as phenylalanine ammonia-lyase, chitinase, PR-proteins, and phenolic compounds (Figure 3) [74,133]. Plant defense signaling molecules include salicylic acid involved in defense against biotrophic pathogens and systemic acquired resistance, as well as jasmonic acid and ethylene, both of which are generally considered necessary for defense against necrotrophic pathogens and are beneficial in plant–microbe interactions [134,135]. Inoculation of the plants with specific PGPRs elicits a phenomenon referred to as induced systemic resistance (ISR; Van Loon et al. [136]). ISR provides plants with the ability to withstand attacks by pathogens, which, without bacterial pre-inoculation, would be potentially lethal. Salicylic acid, in response to BCA treatment, activates a hypersensitive response leading to localized programmed cell death that limits pathogen spread, together with the expression of pathogenesis-related genes, which exhibit antimicrobial properties and provide protection against stress [75]. Ethylene is produced when plants are exposed to abiotic or biotic stressors. The 1-aminocyclopropane-1-carboxylate (ACC) synthase is the immediate precursor of ethylene [137]. An increase in the levels of this phytohormone in plants leads to senescence, chlorosis, and abscission [138]. BCAs with ACC deaminase activity can prevent the increase of phytohormone levels in the plant through ACC hydrolysis into α-ketobutyrate and ammonia products, which can be easily assimilated by plants [138]. The application of BCAs may provide tolerance to stress conditions such as (flooding, drought, temperature, and various contaminants), thus allowing plant survival, even under critical conditions [137]. BCAs could also act by stimulating plant growth through the production of plant growth regulators such as gibberellic acid (GA3), indole-3-acetic acid (IAA), cytokinins, and abscisic acid that have a close relation with plant nutrient availability [133]. The contribution to improving plant nutrition could be manifested in a variety of ways, such as the availability of iron (Fe), siderophore production, phosphate solubilization (P), nitrogen (N) fixation, etc. [132].
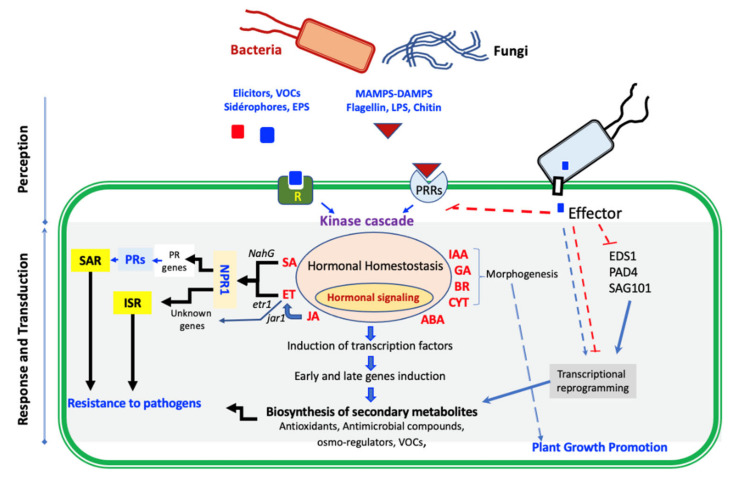
Figure 3.
Schematic model of signal transduction events triggered by microbes. Microbes may produce microbe-associated molecular patterns (MAMPs) or damage-associated molecular patterns (DAMPs), such as flagellin or chitin, which are perceived by pattern-recognition receptors (PRRs), or other elicitors, such as volatile organic compounds (VOCs), or siderophores, which are perceived by receptors. The activated receptors may then trigger different signaling cascades, acting as a precursor for the biosynthesis of phytohormones that trigger defensive pathways. The kinase cascade may also phosphorylate transcription factors that modulate the expression of early and late response genes. Abbreviations: 3-indole acetic acid (IAA); abscisic acid (ABA); brassinosteroid (BR); cytokinin (CYT); enhanced disease susceptibility (EDS); ethylene (ET); exopolysaccharides (EPS); gibberellic acid (GA); induced systemic resistance (ISR), jasmonic acid (JA); lipopolysaccharides (LPS); nonexpressor of pathogenesis-related genes (NPR); pathogenesis-related protein (PR); peptidyl arginine deiminase (PAD), salicylic acid (SA); senescence-associated gene (SAG), systemic acquired resistance (SAR).
3.2.1. Induced Resistance and Priming
Plant defense against biotic threats is known to be activated by beneficial microorganisms [80], through a variety of mechanisms, giving plants resistance to diseases [139]. However, the molecular processes through which plants with an activated defense respond to biotic stresses more quickly are still unknown [80]. The defense network of plants is made up of various components that are triggered in response to pathogen attacks. To perform efficiently, this network necessitates a significant commitment at the cellular level, including genetic reprogramming. This includes the stimulation of defense-related genes in plants, such as pathogen resistance genes (PR proteins), as well as the expression of genes encoding specific metabolites or proteins that are involved in the defense setup of the plant system (Figure 3) [140]. For example, the activation of multiple defense responses in the biocontrol activity of several examined BCAs to combat grapevine bunch rot caused by B. cinerea and Aspergillus carbonarius is indicated by transcriptomic analysis of genes encoding pathogenesis-related proteins PR2, PR3, PR4, and PR5 [141]. Beneficial microbes cause early plant ISR events such as increased expression of pathogenesis-related PR genes, increased activity of defense-related substances such as phenylalanine ammonia-lyase, polyphenol oxidase, peroxidase, β-1,3, glucanase, chitinase, and the accumulation of reactive oxygen species [142,143].
Induced resistance offers the possibility of long-term and broad-spectrum disease control using the natural resistance of plants. The resulting resistance is usually broad and long-lasting but rarely complete, with most inducing agents only lowering the disease prevalence by 20% to 85% [144]. Systemic acquired resistance (SAR) and ISR are two forms of induced resistance that have been characterized based on distinctions in signaling pathways and efficacy spectra [144]. ISR and SAR are two types of plant systemic resistance induced by non-pathogenic and pathogenic microorganisms, respectively [139,145,146]. They frequently overlap with interaction occurring between the two pathways [147]. ISR has emerged as a major method by which bacteria and fungi in the rhizosphere prepare the entire plant body for increased defense against a variety of diseases and insect herbivores [147]. Plants use long-distance systemic signaling to protect distal tissue after ISR activation, inducing quick and powerful immune responses against pathogen invasions [139]. BCAs can therefore suppress pests and diseases by activating the plant immune system [148]. Various BCAs have proven the ability to produce ISR in the past. Beneficial bacteria such as Bacillus spp. and Pseudomonas spp. can help plants to develop broad-spectrum disease resistance by stimulating defense responses [149,150]. Bacillus amyloliquefaciens, Bacillus atrophaeus, Bacillus cereus, Pseudomonas fluorescens, and other bacteria are efficient against fungal, bacterial, and viral invasion by ISR [139]. Trichoderma spp. and arbuscular mycorrhizal fungi (AMFs) have long been considered to be widespread potential BCAs [151,152]. A meta-analysis was conducted on papers published between 2010 and 2021 that looked at cross-talk in the tomato–Trichoderma–B. cinerea system. The analysis was carried out on 15 publications, starting with a collection of 40 papers. Trichoderma’s role in the control of grey mold in tomato leaves (decrease in disease intensity, severity, and occurrence, as well as modulation of resistance genes in the host) was highlighted in the research [151]. Defense priming, or AMF-induced resistance, is becoming more widely recognized as AMF’s ability to induce systemic resistance to insect herbivores and diseases [152].
The induction of a distinct physiological state known as “priming” occurs when plants are infected with necrotizing pathogens or when helpful microorganisms colonize the roots of plants. The different cellular defense mechanisms that are triggered during the attack by pathogens or insects, or in reaction to abiotic stress, are activated faster, stronger, or both in primed plants [153]. Plants often switch to a primed state of heightened defense when they detect prospective opponents, invading pathogens, wound signals, or abiotic stress, and some natural or manufactured compounds. The communication appears to vary depending on the considered beneficial microorganism and the elicited plant species [154]. Here, we presented the molecular mechanisms by which some BCAs, especially Trichoderma, confer plant protection [63]. Trichoderma is a genus of filamentous fungus that colonizes the root surface and play an important role in stimulating plant growth. However, the main proteins and chemical pathways that control this stimulation are still unknown [155]. Several Trichoderma spp. can interfere with signaling networks in their host plants to improve disease resistance and stress tolerance, in addition to their ability to directly antagonize plant pathogens and boost plant growth [63]. Trichoderma isolates use different strategies to boost the defense pathways of the plant host, depending on their origin and application place. Whilst the phyllosphere Trichoderma isolate (BHUF4) used the SAR channel to elicit the defense response in the host plant under Colletotrichum truncatum challenge, the rhizospheric Trichoderma strain (T16A) used the ISR pathway [140]. Trichoderma adapts to different interactions including inter- and cross-kingdom interactions [63]. For example, during the mycoparasitism between Trichoderma species and the phytopathogenic fungal infections, several signaling cascades are activated [156]. Important processes in Trichoderma mycoparasitism are adapted such as the development of infection structures called appressoria during mycoparasitism, the generation of hydrolytic enzymes, antimicrobial metabolites, and the induction of systemic resistance in plants. All these processes rely on signaling pathways that are activated by the binding of host-derived ligands to receptors [157].
3.2.2. Implication of Phytohormones
Several low-molecular-weight compounds known as phytohormones, such as SA, JA, and ET, influence the immune response in plants. To trigger the plant immune response against pathogen and parasite attacks, BCAs use a variety of sophisticated molecular processes [80]. SAR is one of the most well-studied mechanisms, which provides long-term protection against a wide range of microorganisms [158]. SAR confers long-term resistance to (hemi) biotrophic pathogens and pests and is mediated by SA. It is linked to the activation of pathogenesis-related (PR-) genes. Rhizobacteria ISR is largely effective against necrotrophic diseases and herbivorous insects and is regulated by JA and ET. It is not related to changes in PR-gene expression [147,159]. The transmission of ISR signaling was formerly thought to be dependent on JA and ET, but not on SA. The involvement of both the SA and JA/ET signaling pathways, as well as the regulatory roles of small RNA in ISR, has been revised in the previous decade [139]. T. harzianum T22 improves plant direct resistance against stink bug (Nezara viridula) feeding attack by increasing JA marker gene transcript levels early. Tomato plants were shown to respond to N. viridula at the molecular level. N. viridula feeding activates the JA defensive signaling pathway, as evidenced by an increase in ToLOX D expression after 8 h of stink bug feeding. ToPIN2 was also highly elevated 8 h after herbivore feeding, most likely because of the JA-cascade being activated. Upregulation of ToPIN2 may play a role in the lowered growth rate of stink bug nymphs [160]. Beneficial root endophytes, such as Trichoderma and Glomus spp., have been proven in numerous studies to minimize endoparasitic nematode infections by eliciting the plant immune system [80,161,162]. When attacked by root-knot nematodes (RKNs), tomato plants treated with T. harzianum had their SA-signaling pathway and ET biosynthesis activated, which helped in controlling the infection. Monitoring the expression of the genes PR-1/PR-5 and JERF3/ACO, which are indicators of the SA- and JA/ET-dependent signaling pathways, respectively, revealed this effect. Five days after nematode inoculation, roots of plants pre-treated with T. harzianum-strains showed an over-expression of PR-1, PR-5, and ACO genes. In T. harzianum-colonized plants challenged with nematodes, JERF3 gene expression remained unchanged [161]. Plants are primed against RKNs through BCA contact with roots. BCA-mediated immunity appears to be dependent on SA-mediated SAR and is linked to both the activation and inhibition of chitinase and glucanase enzyme activities, as well as the inhibition of the plant antioxidant enzyme system [80].
Hence, the screening of such mechanisms can be based on phenotype-based screenings by measuring plant growth and root colonization traits, a reduction in disease severity, and alleviating abiotic stress. It can also be based on marker-based screening, either by using specific medium culture for BCAs screening or by searching the presence of genes responsible for these mechanisms [76]. Some other techniques require a high-throughput tool, such as induced systemic response markers, expression of pathogenesis-related) proteins at the transcriptomic level, and the production of reactive oxygen species (ROS) [76,79]. BCAs with multiple modes of action are highly demanded. The nature of the mode of action of selected BCA is among the data requirements for the registration of BCA-based products.
4. Biological Control: Facing Reality
The modern application of biological control was introduced at the end of the nineteenth century; however, related techniques were in use for at least 2000 years [34]. Biological control is composed of four different types, including conservation, natural, classical, and augmentative controls [163]. The method is widely considered an attractive, eco-friendly alternative for pest management [164].
Although the strategy offers a promising alternative to synthetic pesticides in the control of pests and plant disease, the method faces many challenges. Serious ecological consequences such as outbreaks could be associated with the introduction of non-native living species that could become invasive and cause significant deleterious impacts on the environment [164]. Moreover, the use of BCAs has not always been successful, probably due to changes in environmental conditions. Trichoderma sp., for example, showed its predatory behavior only under limited nutrient conditions. It has been reported that the Trichoderma spp. do not attack R. solani in the presence of compost, which gives the availability of cellulose as nutrients for the agent [164].
Even if the evidence about the effectiveness of biopesticide is reported to be satisfactory, the availability of such products is still not well established in the market. Additionally, the commercialization of plant-based biopesticide highly depends on the availability of plant sources in large quantities and cultivation. Moreover, a plant could have many active substances, which makes biopesticide formulation a very challenging task [165]. Several other issues are related to the use of BCAs, including the shelf life of biological pesticides which are known to have a high rate of biodegradability. Furthermore, pesticide-based microbes may not be efficient on all pests in the field; they only control a small portion of pests [165].
4.1. Biopesticide from Lab to the Field
The screening of potential strains is part of specialized laboratories’ mission to develop biopesticide products that are highly efficient against plant pathogens in agriculture [166]. These strains are generally selected based on their effectiveness against pathogens, host range, availability, ease for mass production, formulation, and application by farmers. The strains that had the lowest LC50 and LT50 are tested under greenhouse and later in controlled field experiments. The formulation and field efficacy trials are conducted by partners from the private sector and accredited laboratories, who conduct the required eco and mammalian toxicity tests and quality assurance for later commercialization of biopesticide [167]. However, field bioassays are required to confirm the efficacy of selected products.
The biopesticides are classified by governments so that the authorities can regulate their use through authorizations. The main focus of this regulation is the environment, human safety, and the reliability of the product. The efficacy and labeling of the biopesticide also should meet the requirement set up by the authorities and EU for their safe handling [168]. The regulation portfolio of biopesticide registration is normally a modified version of the conventional chemical pesticides, with risk assessment. That includes toxicological and ecotoxicological evaluations, their mode of action, and host range. These requirements can be challenging for regulators. One of the challenges is identifying the appropriate biopesticides while at the same time ensuring safety and consistency standards that are acceptable for commercialization [168].
4.2. Limited Number of Registered Products
An increase in agricultural productivity has occurred in the latest decades due to population expansion and, as a result, the demand for food [169]. To date, the consumption of chemical fertilizers and pesticides has led to ecological imbalance and the contamination of natural resources [170]. Sustainable agriculture is the solution to problems arising from decades of uncontrolled use of chemical-based agronomic techniques to boost crop yields. As a result, an eco-friendly substitute for chemical fertilizers and pesticides is a necessity. With their diverse and unique capabilities, microbial agents represent an appealing and realistic option to substitute chemicals [171].
BCAs influence the interactions between plants, pathogens, and their environments, resulting in biological and physical pathways that affect pathogen fitness, plant health, and ecological function. These interconnections create a panorama of compromises between natural and social functions of biological control, therefore requiring a full assessment of its benefits and costs from societal and farmer perspectives for their long-term development and deployment [15]. BCAs have shown significant results against a wide range of phytopathogenic fungi, oomycetes, bacteria, and weeds. They constitute a promising alternative to synthetic pesticides, although their application in agriculture remains remarkably scarce.
Commercially accessible biocontrol solutions that control plant disease, unlike insect biocontrol, are a novel potential. The first bacterium, A. radiobacter strain K 84, was enlisted by the United States Environmental Protection Agency (EPA) in 1979 to combat crown gall disease in plants. A decade later, the EPA approved the first fungus, T. harzianum ATCC 20476, to manage plant diseases. Currently, 14 bacteria and 12 fungi strains have been recorded by EPA that aid in treating plant disease [172]. The plurality of these BCAs was commercialized as one or more products on the market. Commercialization technology is still in its beginning phases (Figure 4). Most of the EPA-registered species (64%) were recorded in the last decade, while the remaining 36% were documented within the last five years. For these products to reach the market, several technological challenges were surmounted. Table 1 lists some commercially available biocontrol products on the market [173,174].
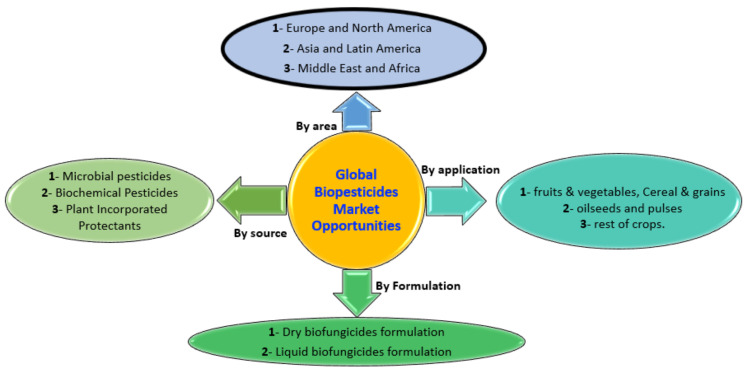
Figure 4.
The potential market of biopesticide.
Table 1.
| Biopesticides Active Agents | Trade Name | Target Disease and/or Target Organism Pathogen |
Crop | Manufacturer/Distributor |
|---|---|---|---|---|
| ||||
| Agrobacterium radiobacter strain k84 | Galltrol | Agrobacterium tumeifaciens | Ornamentals, Fruits, Nuts | AgBioChem, Los Molinos, CA, USA |
| Bacillus subtilis QST 713 | Serenade | Foliar pathogens, rots, Fire blight, and blights | Cherries, cucurbits, grapes, leafy vegetables, peppers, potatoes, tomatoes, and walnuts | AgraQuest, Davis, CA, USA |
| Bacillus firmus NCIM 2637 | Bionemagon | Root-knot nematode, Remiform nematode Cyst nematode, Burrowing nematode, Lesion nematode | Cereals, millets, pulses, oilseeds, fibre crops, sugar crops, forage crops, plantation crops, vegetables, fruits, etc. | ---- |
| Bacillus subtilis GB03 | Companion, Kodiak | Fusarium, Pythium, Rhizoctonia, Aspergillus, and others | Crop seeds, including seeds for cotton, peanuts, soybeans, wheat, barley, peas, and beans | Growth products, White Plains, NY, USA |
| Bacillus subtilis MBI 600 | Subtilex; Histick N/T | Damping-off Fusarium, Rhizoctonia, Alternaria, and Aspergillus |
Cotton, beans, barley, wheat, corn, peas, peanuts, and soybeans | Becker Underwood, Ames, Iowa, USA; Premier Horticulture, Quakertown, PA, USA |
| Bacillus subtilis var. amyloliquefaciens strain FZB24 | Taegro | Rhizoctonia and Fusarium | Shade and forest tree seedlings, ornamentals, and shrubs | Earth BioSciences, Salem, OR, USA |
| Bacillus licheniformis strain SB3086 | Ecoguard; Novozymes Biofungicide GreenRelief | Foliar pathogens and blights | Ornamental plants and ornamental turf | Novozymes Biologicals, Davis, CA, USA |
| Bacillus pumilus strain GB34 | GB34 Concentrate Biological Fungicide | Rhizoctonia, Fusarium | Soybean | Gustafson, Inc, Plano, TX, USA |
| Bacillus thuringiensis | Bio-Dart, Biolep, Halt, Taciobio-Btk, Tacibio, Technar | Lepidopteran pests | Stored grains, fiber, and food crops | ---- |
| Bacillus thuringiensis tenebrionis | Novodor, Trident | Colorado potato beetle | Potato | ---- |
| Burkholderia spp. strain A396 | Venerate | Aphids, leafhopper, lygus, stink bug, thrips | Almonds, blueberry, citrus crops, cucurbits, fruiting, vegetables, grapes | ---- |
| Chromobacterium subtsugae | Grandevo WDG | Armyworms, Aphids, Asian Citrus Psyllid, Mites, Spotted Wing Drosophila, Thrips, Whiteflies. | Blueberry, citrus crops, cucurbits, fruiting vegetables, grapes, leafy greens | ---- |
| Pseudomonas chlororaphis strain 63–28 | AtEze | Pythium, Rhizoctonia solani, Fusarium oxysporum | Vegetables and ornamentals in greenhouses | EcoSoil Systems, San Diego, CA, USA |
|
Psuedomonas fluoroscens strain A506 |
Frostban | Fire blight, bunch rot | Fruit crop, tomato, potato |
Plant Health Technologies, Burlington, CO, USA |
| Pseudomonas aureofaciens strain TX-1 | Bio–Ject, Spot Less | Rhizoctonia solani, Sclerotinia homeocarpa, Colletotrichum graminicola, Pythium aphanadermatum, Michrodochium nivale | Vegetables and ornamentals in greenhouses, golf course turf | EcoSoil system, Canyon Lake, TX, USA |
| Pseudomonas fluorescens A506 | BlighBan A506; Frostban | Frost damage, fire blight, bunch rot | Fruit crops, almond, potato, and tomato crops | Frost Technology Corporation, St Croix Falls, WI, USA; Plant Health Technologies, Burlington, CO, USA |
| ||||
|
Ampelomyces quisqualis isolate M-10 |
AQ10 BioFungicide | Powdery mildew | Fruit, vegetable, and ornamental crops | Ecogen, Grand Junction, CO, USA |
| Aspergillus flavus AF36 | Aspergillus flavus AF36 | Aspergillus flavus | Cotton | Arizona Cotton Research and Protection Council, Phoenix AZ, USA |
| Aspergillus flavus NRRL21, 882 | Afla-guard | Aspergillus flavus | Peanut | Circle One Global, Cuthbert, GA, USA |
| Beauveria spp. | Biosoft, ATEC Beauveria, Larvo-Guard, Biorin, Biolarvex, Biogrubex, Biowonder, Veera, Phalada 101B, Bioguard, Bio-power, Myco-Jaal | Coffeeberry borer, diamondback moth, thrips, grasshoppers, whiteflies, aphids, codling moth | Coffee berries, canola, mustard, cruciferous vegetables, and others | ---- |
| Chaetomium globosum | Ketomium | Rice blast, durian, and black Pepper rot, citrus rot, strawberry rot, anthracnose, and others | Rice, black pepper, citrus, strawberry, tomato, corn, and others | ---- |
| Coniothyrium minitans CON/M/91-08 | Contans WG; Intercept | Sclerotinia sclerotiorum and Sclerotinia minor | Agricultural soil | PROPHYTA Biologischer Pflanzenschutz GmbH, Wismar, Germany; Technology Sciences Group, Sacramento, CA, USA |
| Gliocladium catenulatum strain JI446 | Prima stop soil guard | Soil-borne pathogens | Vegetables, herbs, spices, turf, ornamentals, tree, and shrub seedlings | Kemira Agro Oy, Helsinki, Finland; RegWest Co., Holland, MI, USA |
| Gliocladium virens GL-21 | SoilGard | Soil-borne pathogens | Ornamentals, vegetables, cotton | Thermo Trilogy Corporation, WALTHAM, MA, USA |
| Gliocladium virens | SoilGard 12G | Clubroot Plasmodiophora brassicae |
Canola and crucifer vegetable crops | Certis USA L.L.C., Columbia, MD, USA |
| Metarhizium anisopliae | Meta-Guard, Biomet, Biomagic, Meta, Biomet, Sun Agro Meta, BioMagic, ABTEC, Verticillium | Coleoptera and Lepidoptera, termites, mosquitoes, leafhoppers, beetles, grubs | Cotton, vegetable, field crops, and others | ---- |
| Pseudozyma flocculosa strain PF-A22UL | Sporodex L. | Powdery mildew | Roses and cucumbers in greenhouses | Plant Products Co., Leamington, ON, Canada; Technology Sciences Group, Washington DC, USA |
| Streptomyces lydicus | Actinovate AG | Clubroot Plasmodiophora brassicae |
Canola and crucifer vegetable crops | Natural Industries Inc., Houston, TX, USA |
| Streptomyces griseoviridis strainK61 | Mycostop | Soil-borne pathogens | Ornamentals, tree seedlings | Kemira Oy, Helsinki, Finland |
| Trichoderma harzianum ATCC 20, 476 | Binab T | Tree wound pathogens | Wounds in ornamental, shade, and forest trees | BINAB Bio-Innovation AB, Helsingborg, Sweden |
| Trichoderma album 2.5% | Bio Zeid | Tomato wilt Fusarium oxysporum |
Tomato | ---- |
| Trichoderma harzianum T39 | Trichodex | Botrytis cinerea | Most of the food crops | Bio works, Victor, NY, USA |
| Trichoderma harzianum T22 | Root shield, plant shield | Soil-borne pathogens | Greenhouse nurseries | Bio works, Victor, NY, USA |
| Verticillium lecanii | Verisoft, ABTEC, Verticillium, Vert-Guard, Bioline, Biosappex, Versitile, Ecocil, Phalada 107 V, Biovert Rich, ROM Verlac, ROM Gurbkill, Sun Agro Verti, Bio-Catch | Whitefly, coffee green bug, homopteran pests | Coffee crops and others | ---- |
| ||||
| Candida oleophila | Aspire | Botrytis, Penicillium, Monilinia | Pome fruit, citrus, strawberry, stone fruit | Ecogen, Grand Junction, CO, USA |
| Candida oleophila isolate I-182 | Aspire | Postharvest diseases | Various fruits, vegetables, flowers, ornamentals, other plants | Ecogen, Grand Junction, CO, USA |
| Cryptococcus albidus | YieldPlus | Botrytis, Penicillium, Mucor | Pome fruit, citrus | Lallemand, Bellville, South Africa |
| Candida sake | Candifruit | Penicillium, Botrytis, Rhizopus | Pome fruit | IRTA/Sipcam-Inagra, Valencia Spain |
| Pseudomonas syringae | Biosave | Penicillium, Botrytis, Mucor | Pome fruit, citrus, strawberry, cherry, potato | Jet Harvest Solutions, Longwood, FL, USA |
|
Bacillus subtilis Candida oleophila |
Avogreen Nexy |
Cercospora, Colletotrichum Botrytis, Penicillium |
Avocado Pome fruit |
South Africa Lesaffre Belgium |
| Aureobasisium pullulans | BoniProtect | Botrytis, Penicillium, Monilinia | Pome fruit | Bio-ferm, Herzogenburg, Austria |
| Pantoea agglomerans | Pantovital | Botrytis, Penicillium, Monilinia | Citrus, pome fruit | IRTA/Sipcam-Inagra, Valencia Spain |
| Metschnikowia fructicola | Shemer | Botrytis, Penicillium, Rhizopus, Aspergillus | Table grape, pome fruit, strawberry, stone fruit, sweet potato | Bayer/Koppert, The Netherlands |
To date, BCAs have a commercial value of less than 5% of the whole crop protection industry [175], despite their well-known documentation. The limited number of licensed products for biocontrol of plant diseases is significantly linked to the low technology transfer, implying that the agricultural sector, mainly in developing countries, has yet to recognize its economic potential. Besides that, for promising microbial agent candidates, sufficient knowledge of organism-specific research methods for mass manufacturing and conceptualization is often lacking, making mass production of the entire microbe in in vivo conditions an expensive and time-consuming task. The cost of this approach is a major constraint, as its production and licensing are too expensive when compared to chemical agents [174,176]. Furthermore, ensuring BCAs reach right area at the right time and with sufficient density to be effective, as well as keeping them permanently there, represent one of the most challenging aspects of their use. Since biocontrol involves the introduction of non-native living organisms, serious ecological impacts may be associated with them. Non-native species, for instance, may become invasive and have harmful environmental consequences if they spread beyond the area where they were introduced.
Moreover, some BCAs exhibit efficacy under in vivo circumstances in the laboratory, but ecological restrictions hamper their performance under real full-scale conditions [177,178,179], making them economically non-viable with chemical pesticides. Given the increasing number of microorganisms used for biological control, few are recorded against plant diseases in the EU, according to the European project ENDURE (European Network for Durable Exploitation of Crop Protection Strategies). Several studies found that when certain BCAs were introduced under commercial field circumstances, they were less effective or altogether ineffective, despite their high performance in controlled conditions [45,180,181,182]. For example, many Pseudomonas BCAs showed a good performance in trials but cannot be translated into consistent, efficient disease control in various field settings [182]. For the antagonist, Candida oleophila strains, a significant difference in enzymatic activity existed between in vivo and in vitro applications [15,183].
Despite the fact that BCAs are genetically stable, their use has not been very successful due to ongoing climate change. As a result, it is critical to choose agents that are effective across a variety of environmental situations (soil texture, temperature, humidity, radiation). A small change in temperature amends the microbial concentration. The virulence of the agents is decreased when they are exposed to UV rays from the sun. Some BCAs only exhibit predatory behavior when nutrients are scarce rather than in normal growth conditions. For example, Trichoderma spp. does not directly attack the R. solani when fresh bark compost is introduced. The availability of cellulose itself is a reason for this. When lower amounts of cellulose, genes governing chitinase synthesis, in Trichoderma spp. are activated, the enzyme for parasitic activity is then produced [164,184].
Since BCAs have a limited shelf life, their viability must be managed. For instance, Trichoderma viride is viable for four months, while Pseudomonas fluorescens can only survive for three months [164,185]. Bacillus is thought to be a microbiological factory that generates a large set of antimicrobial substances and is found in about 85% of commercially available BCAs [175,186]. Although basic fermentation and formulation processes can create this type of formula, the commercial applicability of such procedures is limited. This is due to a lack of acceptable methods for large-scale manufacturing and appropriate technology to address the problem of short product shelf life. Maintaining a high spore density is important to achieve optimal sporulation efficiency, which is a viable solution to this problem. As a result, the entire production process must be streamlined in generating an efficient and high-quality product [187,188].
The evolvability of target pathogens is negatively correlated with the endurance of BCAs [189]. BCAs targeting pathogens with higher genetic variation from sexual reproduction, large effective population size, and long dispersal ability are expected to have a shorter durability [190]. Continuous commercial use of the same BCAs might lead to a strong selection of infections, resulting in the emergence of new pathogen populations that can evade or attenuate the negative effects of the BCAs [15,45]. Moreover, because it is difficult to estimate the specificity of the age and stage of targeted pathogens, the time of application of BCAs must be established [174,185].
Another aspect limiting the amount of BCAs registered is growing skepticism regarding their efficacy. Many farmers, particularly in underdeveloped countries, are hesitant to adopt the practice due to technical difficulties, environmental concerns, or economic appeal [45,75,191]. They choose which plant disease management technologies to implement, and their viewpoints on biological control are influenced by economic, technological, and practical considerations. Examples of the latter include effectiveness, profit, availability, and convenience. To entice farmers to use biological control, technology must be simple to assess and prepare, whilst providing economic advantages over alternative options in terms of cost, demand, and supply efficiency.
Some farmers prefer formulations in granules that can be stored and easily applied by machines over those that require more expensive equipment or cold storage. Farmers are discouraged from using the biological control strategy due to a lack of awareness of technological features and a limited number of successful technologies. In this scenario, training and field demonstrations are crucial for exchanging information between technology developers and end-users. Biological management has a direct impact on farmers’ expenses and revenue, but it also has an indirect impact on their economic benefits through its effect on the ecological function and sustainability of farmland [15]. On the other side, it became evident that current regulation and registration procedures for microorganisms, which are mostly based on those for chemical pesticides, are not well suited. This has contributed to the slow implementation of BCAs [176]. To summarize, the full potential of BCAs was not realized, mostly because research is only confined to the laboratory, and thus, little emphasis has been dedicated to developing commercial formulations of BCAs. Furthermore, because of a lack of information on how to use what has been commercially produced, farmers have not used it effectively. As a result, there is a need to strengthen the notion of biological control extension to popularize the concept.
4.3. Legislative Procedures
Although the number of biocontrol products used in plant disease management is growing, they still account for just 1% of agricultural control measures, while synthetic fungicides account for 15% of total chemicals used in agriculture [172].
The commercialization of biofungicides is a multi-step process with several restrictions. BCAs, like synthetic pesticides, are submitted to risk assessments before being approved for commercialization. The European Regulation (EC) No 1107/2009 specifies rules for the marketing of plant protection products based on a risk assessment. Regulation (EC) No 540/2011 establishes a list of authorized microorganisms for biocontrol usage in Europe. However, current risk assessment approaches are not entirely adapted to the extremely difficult task of assessing the safety and dangers of such living substances. Eventually, instructions on how to conduct BCA risk assessments are required [198].
Regulation EC1107/2009 took effect in June 2011 to prevent the approval of substances that present unacceptable risks to human/animal health and the environment. As a result of the revised registration standards, some active compounds will not be reapproved due to their classification (cut-off criteria). When a substance meets one of the following environmental requirements, it cannot be registered: persistent organic pollutant (POP); persistent, bioaccumulative, and toxic (PBT); and/or highly persistent, very bioaccumulative (vPvB). These toxicological criteria will also be used to eliminate substances classified as carcinogens (C1A and C1B), mutagens (M1A and M1B), or reproductive toxins (R1A and R1B) [199].
Regulation EC 1107/2009 (2011)-Article 4 (3) claims that a plant protection product shall meet the following requirements: (a) it shall be sufficiently effective; (b) it shall have no immediate or delayed harmful effect on human health or animal health, directly or through drinking water, food, feed or air, or consequences in the workplace or through other indirect effects or on groundwater; (c) it shall not have any unacceptable effects on plants or plant products; (d) it shall not cause unnecessary suffering and pain to vertebrates; and (e) it shall have no unacceptable effects on the environment, having particular regard to the following considerations: (i) its fate and distribution in the environment; (ii) its impact on non-target species; (iii) its impact on biodiversity and the ecosystem [199].
Finally, commercial authorizations for biopesticides (and synthetic pesticides) are awarded after a laborious process [9,199] requiring a set of tests, such as toxicological and environmental investigations, as well as efficiency. In many circumstances, toxicological studies do not exist or are too expensive and time-consuming for local manufacturers [168,197]. Nonetheless, the present trend toward lessening the usage of synthetic pesticides and easing the regulatory process for low-risk compounds may allow BCAs to be produced and utilized globally [200].
5. Factors Affecting the Success/Failure of Biological Control of Plant Pathogens
Despite decades of research, the importance of biological control in plant health management has remained relatively minor in the face of rising demand for alternatives to chemical control. In laboratory conditions (in vitro or planta), reports of a substantial effect were significantly more common than in field trials. In comparison to chemicals, it is widely understood that moving from the controlled conditions of a laboratory experiment to the harsh conditions encountered in the field has proven more difficult for biopesticides. Whilst the BCA field efficacy can match or exceed that of chemical pesticides, this can vary over time and from one area to another [201]. In other words, the antagonist that controls or suppresses pathogens in the laboratory is rarely effective in the field. This is due to the antagonist, the pathogen, the host, the environment, and all the intricate interactions that occur between them. The host, for example, passes through a series of evolutions or mutations, changing its physical, biological, and chemical features. The antagonist’s efficacy is also determined by pathogenic features. The antagonist may change in response to changes in environmental conditions, population, and the presence of microbial colonizers in the biological system [32]. For example, although Trichoderma is by far the most widely studied fungal BCA, its widespread use has been impeded by its unpredictability in the field. Understanding how Trichoderma interacts with plants, other microorganisms, and the environment is critical for developing and implementing Trichoderma-based agricultural production and protection strategies [63].
5.1. Effect of the Plant on Biocontrol Activity
The outcome of biological control can be heavily influenced by plant species and genotypes [202]. Umer et al. [32] widely documented the plant effect on biocontrol activity. The plant itself serves a dual purpose in biocontrol [32]. The degree of rhizosphere colonization and antibiotic production by antagonists, as well as the development of induced resistance by plants, can all be influenced by plant genotype. The plant susceptibility to some nematode species, for example, can influence the effectiveness of control; good hosts will require more suppression than bad hosts [202]. The plant also serves as a place of action for pathogens and antagonists, where they interact. Pathogen and antagonist interactions are influenced by the host exudate excretion, ion, and water absorption, gaseous exchange, and surface temperature [32]. When plant genes are expressed, they have an impact on the microbial community on the surface of the plant and its surrounding space.
5.2. Effect of the Pathogen on Biocontrol Activity
Pathogen behavior is one of the most significant factors to consider when choosing BCAs; due to genetic variability and ecological fitness diversity, each pathogen has a unique behavior interaction with the host. It is important to highlight that pathogen behavior differs from that of the antagonist due to both its pathogenicity and susceptibility to antagonist action [32]. The persistence of a plant protection control efficacy in space and time is referred to as its durability. It depends on two factors: (i) the selection pressure exerted on plant-pathogen populations and (ii) the pathogen’s ability to adapt to the control strategy. In other words, regardless of the complexity of their mode of action, plant pathogens can show a wide range of sensitivity to BCAs (including very low sensitivity). Certain pathogens can adapt themselves to the selection pressure exerted by BCAs in a few generations [203]. Biological control is frequently thought to have a longer lifespan than chemical control. This assumption may not always be justified, according to research on pest management in agricultural systems. Resistance of several pests to one or more Bt toxins, as well as the emergence of resistance of the codling moth Cydia pomonella to the C. pomonella granulovirus, have been documented.
In contrast to pest control, the persistence of biological control of plant diseases has received little attention, and no scientific papers demonstrating the loss of efficacy of BCAs against plant pathogens in practice have been reported yet. The success of BCAs against plant diseases may not be durable, as pathogen populations may develop resistance similar to that seen with single-mode chemical fungicides. Variation in susceptibility to the mode of action within the pathogen population, leading to changes in pathogen populations toward less sensitive strains due to selection pressure, and the fitness of the selected strains in the environment under conditions without selection pressure are all important factors in the deterioration of effectiveness [203]. For successful biocontrol, a weak link in the pathogen life cycle needs to be identified as an opportunity window. The antagonist should penetrate the opportunity window and disrupt the disease cycle. For some unspecialized necrotrophic pathogens, for example, the window of opportunity is to prevent the uptake of nutrients necessary for growth. When the antagonist competes for nutrients and prevents the saprophytic phase from establishing, this phase will be suppressed. The loss of endogenous nutrients from the pathogen spore may limit or prevent germination when antagonistic organisms are present in sufficient quantities in the area surrounding the pathogen spores. The synthesis of enzymes or antibiotics by the antagonist may also be effective against these diseases [32].
5.3. Effect of the Biocontrol Agent on Biocontrol Activity
The ability of BCAs to adapt to local biotic and abiotic environmental conditions is the main reason behind their diminished efficiency in the field. Therefore, the spatial distribution pattern of BCAs in the rhizosphere should be studied to fully understand this phenomenon [69]. Additionally, more suitable native strains of BCAs should be collected and screened to achieve relevant biocontrol results [67]. The efficiency of BCAs is closely linked to their modes of action [204] and frequently involves tradeoffs with other natural qualities of the agents, such as specificity and environmental persistence [31]. The most commonly observed hurdles in biocontrol include the microbial strain intrinsic biological properties (such as its “ecological competence”), as well as the quality of the formulated preparations utilized in the field or inadequate application time or method [201]. When an ideal relationship prevails, the biocontrol agent will be more effective. Therefore, for successful biocontrols, the application of the antagonist at the right time is critical. When the antagonist is applied before the pathogen’s establishment, the biocontrol will accomplish its goals [32]. Furthermore, to achieve optimal disease control, it is critical to understand how BCAs work. Understanding the mode of action is also necessary for identifying potential risks to humans or the environment, as well as risks of resistance development against the BCA [35]. Fluorescent pseudomonads have many features that make them ideal BCAs. These characteristics include (i) efficient colonization of roots, tubers, hypocotyls, and other parts of the plant; (ii) the ability to use a variety of organic substrates commonly found in root and seed exudates; (iii) ease of cultivation in the laboratory, (iv) production of a variety of secondary metabolites; and (v) compatibility with commonly used pesticides and other biocontrol agents [28].
5.4. Effect of the Environment on Biocontrol Activity
Soil biological and environmental factors have a significant impact on the success of biocontrol programs. Subsequently, soil biology research should identify the numerous significant characteristics of various organisms, particularly in the plant rhizosphere, by recognizing their relative contribution to the biocontrol process. Similarly, ecological research should look into all biotic and abiotic elements that influence the BCAs as vital components of plant health [205]. Biological control of postharvest diseases (causing losses up to 55%) through BCAs is a potentially sustainable control method. However, in comparison to synthetic chemical pesticides, there is a significant lack of reliability in field conditions. The biocontrol activity of BCAs has been improved by combining BCAs application to nutrient additives, salts, edible coatings, or physical treatments, but with only limited success. Complex microbial populations inhabit the fruit surface, which is frequently resilient. It may be hard to build a BCA in such an environment. Integrating the role of microbial communities in the formation of a BCA which is compatible with other soil microbiome is a viable option for managing BCAs dependability in realistic conditions. A microbial BCA is a complex metabolic phenotype that can be broken down into many processes, all of which are maintained by a complex network function in the soil. Combining BCAs application with a suitable complex biocontrol mix that includes beneficial helper strains, essential macro, micronutrients, and biocontrol prebiotics may aid the establishment of BCAs in the epiphytic microbial network. At the same time, it could be able to attain biocontrol efficacy and consistency on par with synthetic chemical pesticides. In addition, based on the literature, the time of beneficial microbial application has been examined [206]. Sare et al. [206] proposed, for example, that shifting treatment at the blooming stage (to establish a “path dependency”) be investigated for future fruit and vegetable postharvest disease management. Other plant organs, such as seeds, could benefit from this application moment shift [206]. In many cases, although plant-parasitic nematodes (PPNs) had demonstrated potential susceptibility in laboratory or field plots, satisfactory success was not attained [207]. Econem, a bionematicide containing in vitro produced Pasteuria sp., was found to be ineffective in the management of a PPN, Belonolaimus longicaudatus Rau, on golf course grass. In one of eight field experiments, the product only reduced B. longicaudatus populations on a single sample [208]. Timper et al. [202] highlighted examples of how agricultural practices can help or hinder the biological control of PPNs and other soil-borne pests. The effectiveness of nematode antagonists can be improved by supplying additional food sources, such as when organic amendments are provided to the soil. Some organic additions, such as manures and plants that contain allelopathic substances, can, however, be harmful to nematode antagonists. Crop rotation, fallow periods, tillage, and pesticide sprays are all examples of production practices that can disrupt antagonistic organism populations directly. By diminishing antagonists’ primary nematode host, these measures can have an indirect effect [202]. BCAs are being more widely utilized to combat a variety of PPN pests, and they are a safer alternative to toxic chemical nematicides. Despite this, due to their lack of efficacy, uneven field performance, and/or negative economic circumstances, they have been confined to a limited sector of the pesticide industry. A holistic understanding of soil biological and ecological components can improve the efficacy and biocontrol success including improved sampling, a better understanding of BCAs interactions with soil biota and ecology, cost-effective BCAs use, genetic manipulation for better PPNs control, grower acceptance and awareness of BCAs techniques, and commercial application [205].
6. Conclusions
The available data suggest that the assumption that biological control is always more sustainable than chemical control is not always valid. However, concluding the existence of unique features related to the plant pathogen or the BCA that could explain the BCA’s lack of effectiveness in practice is insufficient. Briefly, to ensure BCAs’ long-term efficacy against target plant diseases, it is critical to understand how their efficacy may be compromised. This knowledge will lead to the identification of risk factors that will promote the selection of plant pathogen strains that are resistant to BCAs. It will also lead to the identification of BCAs with a lower threat of failure efficacy, i.e., BCAs with modes of action that do not favor the selection of resistant isolates in natural plant pathogen populations [203]. Manipulation of the environment, the use of mixtures of beneficial organisms, physiological and genetic enhancement of the biocontrol mechanisms, formulation manipulation, and the integration of biocontrol with other alternative methods that provide additive effects can all help to improve the efficiency of these biocontrol products. These BCAs could be used effectively in sustainable agriculture to boost plant growth [28]. To ensure effective biocontrol, it is critical to choose agents that are effective in a variety of situations, including soil texture, wetness, temperature extremes, and competition [31]. In addition to the aforementioned factors, the need for scientists to publish negative data, as well as their ability to do so, appears to be critical to the success of biological control. The researchers will be able to determine biopesticides weaknesses such as lack of efficacy, uneven field performance, and/or unfavorable economic considerations to tackling them in the future [209].
Acknowledgments
This research was financially supported by Department of Plant Protection, Ecole Nationale d’Agriculture de Meknes, and Unité de Recherche Résistance Induite et Bio-Protection des Plantes-EA 4707, Université de Reims Cham-pagne-Ardenne.
Author Contributions
Conceptualization, R.L. and E.A.B.; methodology, S.E., N.R., H.E.H., J.K., Z.B., R.L. and E.A.B.; software, E.A.B., S.E., N.R., Q.E., H.E.H. and Z.B.; validation, R.L. and E.A.B.; data curation, S.E., N.R., J.K., H.E.H., Z.B. and R.L., resources, Z.B. and R.L.; writing—original draft preparation, S.E., N.R., J.K., H.E.H., Z.B. and R.L.; writing—review and editing, R.L., Z.B. and E.A.B.; supervision, R.L. and E.A.B.; project administration, E.A.B. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This Research was financially supported by MESRSI under the SIRAM project of PRIMA Call Section 2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used for the analyses in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Montagu M. The future of plant biotechnology in a globalized and environmentally endangered world. Genet. Mol. Biol. 2020;43:e20190040. doi: 10.1590/1678-4685-gmb-2019-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfray H.C.J. The challenge of feeding 9–10 billion people equitably and sustainably. J. Agric. Sci. 2014;152:2–8. doi: 10.1017/S0021859613000774. [DOI] [Google Scholar]
- 3.Guillou M., Matheron G. The World’s Challenge. Springer; Dordrecht, The Netherlands: 2014. [Google Scholar]
- 4.Dwivedi S.L., Lammerts van Bueren E.T., Ceccarelli S., Grando S., Upadhyaya H.D., Ortiz R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017;22:842–856. doi: 10.1016/j.tplants.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs P.R., Sayre K., Gupta R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007;363:543–555. doi: 10.1098/rstb.2007.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngoune Liliane T., Shelton Charles M. Agronomy—Climate Change and Food Security. Volume 32. InTech Open; London, UK: 2020. Factors Affecting Yield of Crops; pp. 137–144. [Google Scholar]
- 7.Hazell P., Wood S. Drivers of change in global agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007;363:495–515. doi: 10.1098/rstb.2007.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purvis B., Mao Y., Robinson D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019;14:681–695. doi: 10.1007/s11625-018-0627-5. [DOI] [Google Scholar]
- 9.Köhl J., Kolnaar R., Ravensberg W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019;10:845. doi: 10.3389/fpls.2019.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauscher C.I.E.D.E.M., Rauscher M. International Trade, Factor Movements, and the Environment. Clarendon Press; Oxford, UK: 1997. [Google Scholar]
- 11.Pilet-Nayel M.L., Moury B., Caffier V., Montarry J., Kerlan M.C., Fournet S., Durel C.E., Delourme R. Quantitative resistance to plant pathogens in pyramiding strategies for durable crop protection. Front. Plant Sci. 2017;8:1838. doi: 10.3389/fpls.2017.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usta C. Current Progress in Biological Research. Volume 32. InTech Open; London, UK: 2013. Microorganisms in Biological Pest Control—A Review (Bacterial Toxin Application and Effect of Environmental Factors) pp. 137–144. [Google Scholar]
- 13.Compant S., Duffy B., Nowak J., Clément C., Ait Barka E. Biocontrol of plant diseases using plant growth-promoting bacteria (PGPB): Principles, mechanisms of action and future prospects. Appl. Environ. Microbiol. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017;46:293–304. doi: 10.1007/s13313-017-0481-4. [DOI] [Google Scholar]
- 15.Barratt B.I.P., Moran V.C., Bigler F., Van Lenteren J.C. The status of biological control and recommendations for improving uptake for the future. BioControl. 2018;63:155–167. doi: 10.1007/s10526-017-9831-y. [DOI] [Google Scholar]
- 16.Macfadyen S., Hardie D.C., Fagan L., Stefanova K., Perry K.D., DeGraaf H.E., Holloway J., Spafford H., Umina P.A. Reducing Insecticide Use in Broad-Acre Grains Production: An Australian Study. PLoS ONE. 2014;9:e89119. doi: 10.1371/journal.pone.0089119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-García A., Romero D., De Vicente A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011;22:187–193. doi: 10.1016/j.copbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Ruiu L. Microbial Biopesticides in Agroecosystems. Agronomy. 2018;8:235. doi: 10.3390/agronomy8110235. [DOI] [Google Scholar]
- 19.Angelopoulou D.J., Naska E.J., Paplomatas E.J., Tjamos S.E. Biological control agents (BCAs) of verticillium wilt: Influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol. 2014;63:1062–1069. doi: 10.1111/ppa.12198. [DOI] [Google Scholar]
- 20.Abd-Elgawad M.M.M., Askary T.H. Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt. J. Biol. Pest Control. 2020;30:17. doi: 10.1186/s41938-020-00215-2. [DOI] [Google Scholar]
- 21.Arthurs S., Dara S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019;165:13–21. doi: 10.1016/j.jip.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Kuzyakov Y., Blagodatskaya E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015;83:184–199. [Google Scholar]
- 23.Lahlali R., Ibrahim D.S.S., Belabess Z., Kadir Roni M.Z., Radouane N., Vicente C.S.L., Menéndez E., Mokrini F., Barka E.A., Galvão de Melo e Mota M., et al. High-throughput molecular technologies for unraveling the mystery of soil microbial community: Challenges and future prospects. Heliyon. 2021;7:e08142. doi: 10.1016/j.heliyon.2021.e08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohanram S., Kumar P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019;69:307–320. doi: 10.1007/s13213-019-01448-9. [DOI] [Google Scholar]
- 25.Shaikh S.S., Wani S.J., Sayyed R.Z. Impact of Interactions between Rhizosphere and Rhizobacteria: A Review. J. Bacteriol. Mycol. 2018;5:1058. [Google Scholar]
- 26.Tena G. Recruiting microbial bodyguards. Nat. Plants. 2018;4:857. doi: 10.1038/s41477-018-0308-5. [DOI] [PubMed] [Google Scholar]
- 27.Ciancio A., Pieterse C.M.J., Mercado-blanco J. Editorial: Harnessing Useful Rhizosphere Microorganisms for Pathogen and Pest Biocontrol—Second Edition. Front. Microbiol. 2019;10:1935. doi: 10.3389/fmicb.2019.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindhu S.S., Sehrawat A., Sharma R., Dahiya A. Biopesticides: Use of Rhizosphere Bacteria for Biological Control of Plant Pathogens. Def. Life Sci. J. 2016;1:135–148. doi: 10.14429/dlsj.1.10747. [DOI] [Google Scholar]
- 29.Seenivasagan R., Babalola O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology. 2021;10:1111. doi: 10.3390/biology10111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheppard A.W., Paynter Q., Mason P., Murphy S., Stoett P., Cowan P., Brodeur J., Warner K., Villegas C., Shaw R., et al. The Application of Classical Biological Control for the Management of Established Invasive Alien Species Causing Environmental Impacts. Secretariat of the Convention on Biological Diversity; Montreal, QC, Canada: 2019. pp. 1–94. IUCN SSC Invasive Species Specialist Group. [Google Scholar]
- 31.He D.C., He M.H., Amalin D.M., Liu W., Alvindia D.G., Zhan J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens. 2021;10:1311. doi: 10.3390/pathogens10101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umer M., Mubeen M., Iftikhar Y., Shad M.A., Usman H.M., Sohail M.A., Atiq M.N., Abbas A., Ateeq M. Role of Rhizobacteria on Plants Growth and Biological Control of Plant Diseases: A Review. Plant Prot. 2021;5:59–73. doi: 10.33804/pp.005.01.3565. [DOI] [Google Scholar]
- 33.Labuschagne N., Pretorius T., Idris A.H. Plant Growth Promoting Rhizobacteria as Biocontrol Agents Against Soil-Borne Plant Diseases. Microbiol. Monogr. 2010;18:211–230. doi: 10.1007/978-3-642-13612-2. [DOI] [Google Scholar]
- 34.Van Lenteren J.C., Bolckmans K., Köhl J., Ravensberg W.J., Urbaneja A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl. 2018;63:39–59. doi: 10.1007/s10526-017-9801-4. [DOI] [Google Scholar]
- 35.Esmaeel Q., Jacquard C., Sanchez L., Clément C., Ait Barka E. The mode of action of plant associated Burkholderia against grey mould disease in grapevine revealed through traits and genomic analyses. Sci. Rep. 2020;10:19393. doi: 10.1038/s41598-020-76483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson A.J., Kim Y.C. Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Prot. 2018;105:62–69. doi: 10.1016/j.cropro.2017.11.009. [DOI] [Google Scholar]
- 37.Mendes R., Kruijt M., de Bruijn I., Dekkers E., van der Voort M., Schneider J.H.M., Piceno Y.M., DeSantis T.Z., Andersen G.L., Bakker P.A.H.M., et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science. 2011;332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 38.Sehrawat A., Sindhu S.S. Potential of Biocontrol Agents in Plant Disease Control for Improving Food Safety. Def. Life Sci. J. 2019;4:220–225. doi: 10.14429/dlsj.4.14966. [DOI] [Google Scholar]
- 39.Sindhu S.S., Parmar P., Phour M., Kumari K. Rhizosphere microorganisms for improvement in soil fertility and plant growth. In: Nagpal R., Kumar A., editors. Microbes in the Service of Mankind. JBC Press; New Delhi, India: 2014. pp. 32–94. [Google Scholar]
- 40.Sharma P., Kumawat K.C., Kaur S. Plant Growth Promoting Rhizobacteria in Nutrient Enrichment: Current Perspectives. In: Singh U., Praharaj C.S., Singh S.S., Singh N.P., editors. Biofortification of Food Crops. Springer; New Delhi, India: 2016. pp. 263–289. [Google Scholar]
- 41.Patel S., Sayyed R.Z., Saraf M. Bacterial Determinants and Plant Defense Induction: Their Role as Biocontrol Agents in Sustainable Agriculture. In: Hakeem K.R., Akhtar M.S., editors. Plant, Soil and Microbes Volume 2: Mechanisms and Molecular Interactions. Springer; Cham, Switzerland: 2016. pp. 187–204. [Google Scholar]
- 42.Shaikh S.S., Sayyed R.Z., Reddy M.S. Plant Growth Promoting Rhizobacteria: A Sustainable Approach to Agro- Plant Growth-Promoting Rhizobacteria: An Eco-friendly Approach for Sustainable Agroecosystem. In: Hakeem K.R., Akhtar M.S., editors. Plant, Soil and Microbes Volume 2: Mechanisms and Molecular Interactions. Springer; Cham, Switzerland: 2016. pp. 181–201. [Google Scholar]
- 43.Shaikh S.S., Patel P.R., Patel S.S., Nikam S.D., Rane T.U., Sayyed R.Z. Production of biocontrol traits by banana field fluorescent Pseudomonads and comparison with chemical fungicide. Indian J. Exp. Biol. 2014;52:917–920. [PubMed] [Google Scholar]
- 44.Chauhana H., Bagyaraja D.J., Selvakumarb G., Sundaramc S.P. Novel plant growth promoting rhizobacteria—Prospects and potential. Appl. Soil Ecol. 2015;95:38–53. doi: 10.1016/j.apsoil.2015.05.011. [DOI] [Google Scholar]
- 45.Backer R., Rokem J.S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., Subramanian S., Smith D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018;9:1473. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyousfi N., Lahlali R., Letrib C., Belabess Z., Ouaabou R., Ennahli S., Blenzar A., Ait Barka E. Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy. 2021;11:209. doi: 10.3390/agronomy11020209. [DOI] [Google Scholar]
- 47.Lahlali R., Mchachti O., Radouane N., Ezrari S., Belabess Z., Khayi S., Mentag R., Tahiri A., Ait Barka E. The Potential of Novel Bacterial Isolates from Natural Soil for the Control of Brown Rot Disease (Monilinia fructigena) on Apple Fruits. Agronomy. 2020;10:1814. doi: 10.3390/agronomy10111814. [DOI] [Google Scholar]
- 48.Ait Bahadou S., Ouijja A., Karfach A., Tahiri A., Lahlali R. New potential bacterial antagonists for the biocontrol of fire blight disease (Erwinia amylovora) in Morocco. Microb. Pathog. 2018;117:7–15. doi: 10.1016/j.micpath.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Lanteigne C., Gadkar V.J., Wallon T., Novinscak A., Filion M. Production of DAPG and HCN by Pseudomonas sp. LBUM300 Contributes to the Biological Control of Bacterial Canker of Tomato. Biol. Control. 2012;102:967–973. doi: 10.1094/PHYTO-11-11-0312. [DOI] [PubMed] [Google Scholar]
- 50.St-Onge R., Gadkar V.J., Arseneault T., Goyer C., Filion M. The ability of Pseudomonassp. LBUM223 to produce phenazine-1- carboxylic acid affects the growth of Streptomyces scabies, the expression of thaxtomin biosynthesis genes and the biological control potential against common scabof potato. FEMS Microbiol. Ecol. 2011;75:173–183. doi: 10.1111/j.1574-6941.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 51.Jisha V.N., Smitha R.B., Benjamin S. An Overview on the Crystal Toxins from Bacillus thuringiensis. Adv. Microbiol. 2013;3:462–472. doi: 10.4236/aim.2013.35062. [DOI] [Google Scholar]
- 52.Schünemann R., Knaak N., Fiuza L.M. Mode of Action and Specificity of Bacillus thuringiensis Toxins in the Control of Caterpillars and Stink Bugs in Soybean Culture. ISRN Microbiol. 2014;2014:e135675. doi: 10.1155/2014/135675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chowdhury S.P., Uhl J., Grosch R., Alquéres S., Pittroff S., Dietel K., Schmitt-kopplin P., Borriss R., Hartmann A. Cyclic Lipopeptides of Bacillus amyloliquefaciens subsp. plantarum Colonizing the Lettuce Rhizosphere Enhance Plant Defense Responses Toward the Bottom Rot Pathogen Rhizoctonia solani. Mol. Plant-Microbe Interact. 2015;28:984–995. doi: 10.1094/MPMI-03-15-0066-R. [DOI] [PubMed] [Google Scholar]
- 54.Hasan N., Farzand A., Heng Z., Khan I.U., Moosa A., Zubair M., Na Y., Ying S., Canming T. Antagonistic Potential of Novel Endophytic Bacillus Strains and Mediation of Plant Defense against Verticillium Wilt in Upland Cotton. Plants. 2020;9:1438. doi: 10.3390/plants9111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L., Wang Y., Lu J., Liu S., Min Y., Liu X., Qiu Y., Hu H., Zhou R. Complete Genome Sequence of Bacillus badius NBPM-293, a Plant Growth-Promoting Strain Isolated from Rhizosphere Soil. Am. Soc. Microbiol. 2021;10:e00977-21. doi: 10.1128/MRA.00977-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anckaert A., Arias A.A., Hoff G. The use of Bacillus spp. as bacterial biocontrol agents to control plant diseases. In: Köhl J., Ravensberg W., editors. Microbial Bioprotectants for Plant Disease Management. Burleigh Dodds Science Publishing; Cambridge, UK: 2022. pp. 1–54. [Google Scholar]
- 57.Sujayanand G.K., Akram M., Konda A., Nigam A., Bhat S., Dubey J., Kumar K., Muthusamy S.K. Distribution and toxicity of Bacillus thuringiensis (Berliner) strains from different crop rhizosphere in Indo-Gangetic plains against polyphagous lepidopteran pests. Int. J. Trop. Insect Sci. 2021;41:2713–2731. doi: 10.1007/s42690-021-00451-5. [DOI] [Google Scholar]
- 58.Gopalakrishnan S., Rao G.V.R., Humayun P., Rao V.R., Alekhya G., Jacob S., Deepthi K., Vidya M.S., Srinivas V., Mamatha L., et al. Efficacy of botanical extracts and entomopathogens on control of Helicoverpa armigera and Spodoptera litura. Afr. J. Biotechnol. 2011;10:16667–16673. doi: 10.5897/AJB11.2475. [DOI] [Google Scholar]
- 59.De Curtis F., Ianiri G., Raiola A., Ritieni A., Succi M., Tremonte P., Castoria R. Integration of biological and chemical control of brown rot of stone fruits to reduce disease incidence on fruits and minimize fungicide residues in juice. Crop Prot. 2019;119:158–165. doi: 10.1016/j.cropro.2019.01.020. [DOI] [Google Scholar]
- 60.Chen Z., Zhao L., Dong Y., Chen W., Li C., Gao X., Chen R., Li L., Xu Z. The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: Evaluation of ZW10 as a potential biopesticide. PLoS ONE. 2021;16:e0256807. doi: 10.1371/journal.pone.0256807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson A.J., Kang B.R., Kim Y.C. The Gac/Rsm Signaling Pathway of a Biocontrol Bacterium, Pseudomonas chlororaphis O6. Res. Plant Dis. 2017;23:212–227. doi: 10.5423/RPD.2017.23.3.212. [DOI] [Google Scholar]
- 62.Poveda J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control. 2021;159:e104634. doi: 10.1016/j.biocontrol.2021.104634. [DOI] [Google Scholar]
- 63.Alfiky A., Weisskopf L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi. 2021;7:61. doi: 10.3390/jof7010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfordt A., Schiwek S., Karlovsky P., von Tiedemann A. Trichoderma Afroharzianum Ear Rot—A New Disease on Maize in Europe. Front. Agron. 2020;2:e547758. doi: 10.3389/fagro.2020.547758. [DOI] [Google Scholar]
- 65.Degani O., Dor S. Trichoderma Biological Control to Protect Sensitive Maize Hybrids Against Late Wilt Disease in the Field. J. Fungi. 2021;7:315. doi: 10.3390/jof7040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sánchez-Montesinos B., Santos M., Moreno-Gavíra A., Marín-Rodulfo T., Gea F.J., Diánez F. Biological Control of Fungal Diseases by Trichoderma aggressivum f. europaeum and Its Compatibility with Fungicides. J. Fungi. 2021;7:598. doi: 10.3390/jof7080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu Z., Wang Z., Zhang Y., Wang Y., Liu Z. Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol. Res. 2021;242:e126596. doi: 10.1016/j.micres.2020.126596. [DOI] [PubMed] [Google Scholar]
- 68.Reghmit A., Benzina-tihar F., Escudero F.J.L., Halouane-Sahir F., Oukali Z., Bensmail S., Ghozali N. Trichoderma spp. isolates from the rhizosphere of healthy olive trees in northern Algeria and their biocontrol potentials against the olive wilt pathogen, Verticillium dahliae. Org. Agric. 2021;11:639–657. doi: 10.1007/s13165-021-00371-1. [DOI] [Google Scholar]
- 69.Nandini B., Puttaswamy H., Saini R.K., Prakash H.S., Geetha N. Trichovariability in rhizosphere soil samples and their biocontrol potential against downy mildew pathogen in pearl millet. Sci. Rep. 2021;11:9517. doi: 10.1038/s41598-021-89061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lima G., Arru S., De Curtis F., Arras G. Influence of antagonist, host fruit and pathogen on the biological control of postharvest fungal diseases by yeasts. J. Ind. Microbiol. Biotechnol. 1999;23:223–229. doi: 10.1038/sj.jim.2900727. [DOI] [Google Scholar]
- 71.Fernandez-San Millan A., Larraya L., Farran I., Ancin M., Veramendi J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control. 2021;160:104683. doi: 10.1016/j.biocontrol.2021.104683. [DOI] [Google Scholar]
- 72.Mishra R.K., Bohra A., Kamaal N., Kumar K., Gandhi K., Sujayanand G.K., Saabale P.R., Satheesh Naik S.J., Sarma B.K., Kumar D., et al. Utilization of biopesticides as sustainable solutions for management of pests in legume crops: Achievements and prospects. Egypt. J. Biol. Pest Control. 2018;28:3. doi: 10.1186/s41938-017-0004-1. [DOI] [Google Scholar]
- 73.Ghorbanpour M., Omidvari M., Abbaszadeh-Dahaji P., Omidvar R., Kariman K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control. 2018;117:147–157. doi: 10.1016/j.biocontrol.2017.11.006. [DOI] [Google Scholar]
- 74.Dukare A.S., Paul S., Nambi V.E., Gupta R.K., Singh R., Sharma K., Vishwakarma R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019;59:1498–1513. doi: 10.1080/10408398.2017.1417235. [DOI] [PubMed] [Google Scholar]
- 75.Farhana S., Ab S., Singh E., Pieterse C.M.J., Schenk P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018;267:102–111. doi: 10.1016/j.plantsci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Raymaekers K., Ponet L., Holtappels D., Berckmans B., Cammue B.P.A. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control. 2020;144:104240. doi: 10.1016/j.biocontrol.2020.104240. [DOI] [Google Scholar]
- 77.Karlsson M., Atanasova L., Jensen D.F., Zeilinger S. Necrotrophic mycoparasites and their genomes. Microbiol. Spectr. 2017;5:2–5. doi: 10.1128/microbiolspec.FUNK-0016-2016. [DOI] [PubMed] [Google Scholar]
- 78.Jadhav H.P., Shaikh S.S., Sayyed R.Z. Rhizotrophs Plant Growth Promotion to Bioremediation. Springer; Singapore: 2017. Role of hydrolytic enzymes of rhizoflora in biocontrol of fungal phytopathogens: An overview; pp. 183–203. [Google Scholar]
- 79.Bubici G., Kaushal M., Prigigallo M.I., Cabanás C.G.L., Mercado-Blanco J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019;10:616. doi: 10.3389/fmicb.2019.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molinari S., Leonetti P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE. 2019;14:e0213230. doi: 10.1371/journal.pone.0213230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yedidia I., Shoresh M., Kerem Z., Benhamou N., Kapulnik Y., Chet I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003;69:7343–7353. doi: 10.1128/AEM.69.12.7343-7353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Win T.T., Bo B., Malec P., Khan S., Fu P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021;103:549–561. doi: 10.1007/s42161-021-00780-x. [DOI] [Google Scholar]
- 83.Nakatsuji T., Gallo R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012;132:887–895. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berdy J. Bioactive microbial metabolites. J. Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 85.Flemming H.-C., Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019;17:247–260. doi: 10.1038/s41579-019-0158-9. [DOI] [PubMed] [Google Scholar]
- 86.Li J., Zhao X. Effects of quorum sensing on the biofilm formation and viable but non-culturable state. Food Res. Int. 2020;137:109742. doi: 10.1016/j.foodres.2020.109742. [DOI] [PubMed] [Google Scholar]
- 87.Bais H.P., Fall R., Vivanco J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004;134:307–319. doi: 10.1104/pp.103.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Triveni S., Prasanna R., Shukla L., Saxena A.K. Evaluating the biochemical traits of novel Trichoderma-based biofilms for use as plant growth-promoting inoculants. Ann. Microbiol. 2013;63:1147–1156. doi: 10.1007/s13213-012-0573-x. [DOI] [Google Scholar]
- 89.Velmourougane K., Prasanna R., Supriya P., Ramakrishnan B., Thapa S., Saxena A.K. Transcriptome profiling provides insights into regulatory factors involved in Trichoderma viride–Azotobacter chroococcum biofilm formation. Microbiol. Res. 2019;227:126292. doi: 10.1016/j.micres.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Pandin C., Darsonval M., Mayeur C., Le Coq D., Aymerich S., Briandet R. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 2019;85:e00327-19. doi: 10.1128/AEM.00327-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janisiewicz W.J., Tworkoski T.J., Sharer C. Characterizing the mechanism of biological control of postharvest diseases on fruits with a simple method to study competition for nutrients. Phytopathology. 2000;90:1196–1200. doi: 10.1094/PHYTO.2000.90.11.1196. [DOI] [PubMed] [Google Scholar]
- 92.Spadaro D., Ciavorella A., Dianpeng Z., Garibaldi A., Gullino M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010;56:128–137. doi: 10.1139/W09-117. [DOI] [PubMed] [Google Scholar]
- 93.Garrett S.D. Cellulolysis rate and competitive saprophytic colonization of wheat straw by foot-rot fungi. Soil Biol. Biochem. 1975;7:323–327. doi: 10.1016/0038-0717(75)90075-9. [DOI] [Google Scholar]
- 94.Shearer C.A. Fungal competition. Can. J. Bot. 1995;73:1259–1264. doi: 10.1139/b95-386. [DOI] [Google Scholar]
- 95.Fokkema N.J. Opportunities and problems of control of foliar pathogens with micro-organisms. Pestic. Sci. 1993;37:411–416. doi: 10.1002/ps.2780370416. [DOI] [Google Scholar]
- 96.Bolton M.D., Thomma B.P.H.J., Nelson B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006;7:1–16. doi: 10.1111/j.1364-3703.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 97.Williamson B., Tudzynski B., Tudzynski P., Van Kan J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhou T. Application of Epicoccum purpurascens Spores to Control White Mold of Snap Bean. Plant Dis. 1989;73:639. doi: 10.1094/PD-73-0639. [DOI] [Google Scholar]
- 99.Lima G., Ippolito A., Nigro F., Salerno M. Effectiveness of Aureobasidium pullulans and Candida oleophila against postharvest strawberry rots. Postharvest Biol. Technol. 1997;10:169–178. doi: 10.1016/S0925-5214(96)01302-6. [DOI] [Google Scholar]
- 100.Köhl J., Gerlagh M., De Haas B.H., Krijger M.C. Biological control of Botrytis cinerea in cyclamen with Ulocladium atrum and Gliocladium roseum under commercial growing conditions. Phytopathology. 1998;88:568–575. doi: 10.1094/PHYTO.1998.88.6.568. [DOI] [PubMed] [Google Scholar]
- 101.Li G.Q., Huang H.C., Acharya S.N. Antagonism and biocontrol potential of Ulocladium atrum on Sclerotinia sclerotiorum. Biol. Control. 2003;28:11–18. doi: 10.1016/S1049-9644(03)00050-1. [DOI] [Google Scholar]
- 102.Wszelaki A.L., Mitcham E.J. Effect of combinations of hot water dips, biological control and controlled atmospheres for control of gray mold on harvested strawberries. Postharvest Biol. Technol. 2003;27:255–264. doi: 10.1016/S0925-5214(02)00095-9. [DOI] [Google Scholar]
- 103.Karabulut O.A., Tezcan H., Daus A., Cohen L., Wiess B., Droby S. Control of preharvest and postharvest fruit rot in Strawberry by Metschnikowia fructicola. Biocontrol Sci. Technol. 2004;14:513–521. doi: 10.1080/09583150410001682287. [DOI] [Google Scholar]
- 104.Zhang H., Zheng X., Yu T. Biological control of postharvest diseases of peach with Cryptococcus laurentii. Food Control. 2007;18:287–291. doi: 10.1016/j.foodcont.2005.10.007. [DOI] [Google Scholar]
- 105.Handelsman J., Parke J.L. Mechanisms of biocontrol of soilborne plant pathogens. Plant Soil. 1989;129:85–92. [Google Scholar]
- 106.Spadaro D., Gullino M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004;91:185–194. doi: 10.1016/S0168-1605(03)00380-5. [DOI] [PubMed] [Google Scholar]
- 107.Heydari A., Pessarakli M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010;10:273–290. doi: 10.3923/jbs.2010.273.290. [DOI] [Google Scholar]
- 108.Roca-Couso R., Flores-Félix J.D., Rivas R. Mechanisms of Action of Microbial Biocontrol Agents against Botrytis cinerea. J. Fungi. 2021;7:1045. doi: 10.3390/jof7121045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Q., Shen Q., Ran W., Xiao T., Xu D., Xu Y. Inoculation of soil by Bacillus subtilis Y-IVI improves plant growth and colonization of the rhizosphere and interior tissues of muskmelon (Cucumis melo L.) Biol. Fertil. Soils. 2011;47:507–514. doi: 10.1007/s00374-011-0558-0. [DOI] [Google Scholar]
- 110.Li S., Zhang N., Zhang Z., Luo J., Shen B., Zhang R., Shen Q. Antagonist Bacillus subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation. Biol. Fertil. Soils. 2013;49:295–303. doi: 10.1007/s00374-012-0718-x. [DOI] [Google Scholar]
- 111.Vellasamy S., Hariharan H. Controlling Strategies (Diagnosis/Quarantine/Eradication) of Plant Pathogenic Bacteria. Sustain. Approaches Control Plant Pathog. Bact. 2015;2015:98–127. [Google Scholar]
- 112.Calvente V., De Orellano M.E., Sansone G., Benuzzi D., Sanz de Tosetti M.I. Effect of nitrogen source and pH on siderophore production by Rhodotorula strains and their application to biocontrol of phytopathogenic moulds. J. Ind. Microbiol. Biotechnol. 2001;26:226–229. doi: 10.1038/sj.jim.7000117. [DOI] [PubMed] [Google Scholar]
- 113.Sulochana M.B., Jayachandra S.Y., Anil Kumar S., Dayanand A. Antifungal attributes of siderophore produced by the Pseudomonas aeruginosa JAS-25. J. Basic Microbiol. 2014;54:418–424. doi: 10.1002/jobm.201200770. [DOI] [PubMed] [Google Scholar]
- 114.Weller D.M., Raaijmakers J.M., Gardener B.B.M., Thomashow L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- 115.Yu X., Ai C., Xin L., Zhou G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011;47:138–145. doi: 10.1016/j.ejsobi.2010.11.001. [DOI] [Google Scholar]
- 116.Demoz B.T., Korsten L. Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stem-end rot pathogens. Biol. Control. 2006;37:68–74. doi: 10.1016/j.biocontrol.2005.11.010. [DOI] [Google Scholar]
- 117.Wang X.Q., Zhao D.L., Shen L.L., Jing C.L., Zhang C.S. Application and Mechanisms of Bacillus subtilis in Biological Control of Plant Disease. In: Meena V.S., editor. Role of Rhizospheric Microbes in Soil. Springer; Singapore: 2018. pp. 225–250. [Google Scholar]
- 118.Poppe L., Vanhoutte S., Höfte M. Modes of action of Pantoea agglomerans CPA-2, an antagonist of postharvest pathogens on fruits. Eur. J. Plant Pathol. 2003;109:963–973. doi: 10.1023/B:EJPP.0000003747.41051.9f. [DOI] [Google Scholar]
- 119.Bencheqroun S.K., Bajji M., Massart S., Labhilili M., El Jaafari S., Jijakli M.H. In vitro and in situ study of postharvest apple blue mold biocontrol by Aureobasidium pullulans: Evidence for the involvement of competition for nutrients. Postharvest Biol. Technol. 2007;46:128–135. doi: 10.1016/j.postharvbio.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 120.Spadaro D., Droby S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016;47:39–49. doi: 10.1016/j.tifs.2015.11.003. [DOI] [Google Scholar]
- 121.Bonaterra A., Mari M., Casalini L., Montesinos E. Biological control of Monilinia laxa and Rhizopus stolonifer in postharvest of stone fruit by Pantoea agglomerans EPS125 and putative mechanisms of antagonism. Int. J. Food Microbiol. 2003;84:93–104. doi: 10.1016/S0168-1605(02)00403-8. [DOI] [PubMed] [Google Scholar]
- 122.Kerry B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000;38:423–441. doi: 10.1146/annurev.phyto.38.1.423. [DOI] [PubMed] [Google Scholar]
- 123.Xu X., Hu X. The effect of aggregation of pathogen and biocontrol microbe propagules on biocontrol potential: A simple modelling study. Phytopathol. Res. 2020;2:5. doi: 10.1186/s42483-020-0047-1. [DOI] [Google Scholar]
- 124.Johnson K.B. Pathogen refuge: A key to understanding biological control. Annu. Rev. Phytopathol. 2010;48:141–160. doi: 10.1146/annurev.phyto.112408.132643. [DOI] [PubMed] [Google Scholar]
- 125.Freimoser F.M., Rueda-Mejia M.P., Tilocca B., Migheli Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019;35:154. doi: 10.1007/s11274-019-2728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elad Y., Chet I., Boyle P., Henis Y. Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii-scanning electron microscopy and fluorescence microscopy. Phytopathology. 1983;73:85–88. doi: 10.1094/Phyto-73-85. [DOI] [Google Scholar]
- 127.Harman G., Khadka R., Doni F., Uphoff N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021;11:610065. doi: 10.3389/fpls.2020.610065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Howell C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003;87:4–10. doi: 10.1094/PDIS.2003.87.1.4. [DOI] [PubMed] [Google Scholar]
- 129.Abdullah N.S., Doni F., Mispan M.S., Saiman M.Z., Yusuf Y.M., Oke M.A., Suhaimi N.S.M. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy. 2021;11:2559. doi: 10.3390/agronomy11122559. [DOI] [Google Scholar]
- 130.Wisniewski M., Biles C., Droby S., McLaughlin R., Wilson C., Chalutz E. Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. I. Characterization of attachment to Botrytis cinerea. Physiol. Mol. Plant Pathol. 1991;39:245–258. doi: 10.1016/0885-5765(91)90033-E. [DOI] [Google Scholar]
- 131.Ezrari S., Mhidra O., Radouane N., Tahiri A., Polizzi G., Lazraq A., Lahlali R. Potential role of rhizobacteria isolated from citrus rhizosphere for biological control of citrus dry root rot. Plants. 2021;10:872. doi: 10.3390/plants10050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shafi J., Tian H., Ji M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017;31:446–459. doi: 10.1080/13102818.2017.1286950. [DOI] [Google Scholar]
- 134.Li N., Han X., Feng D., Yuan D., Huang L.-J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019;20:671. doi: 10.3390/ijms20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghozlan M.H., Eman E.-A., Tokgöz S., Lakshman D.K., Mitra A. Plant Defense against Necrotrophic Pathogens. J. Plant Sci. 2020;11:2122–2138. doi: 10.4236/ajps.2020.1112149. [DOI] [Google Scholar]
- 136.Van Loon L.C., Bakker P., Pieterse C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 137.Numan M., Bashir S., Khan Y., Mumtaz R., Shinwari Z.K., Khan A.L., Khan A., AL-Harrasi A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018;209:21–32. doi: 10.1016/j.micres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 138.Ezrari S., Radouane N., Tahiri A., El Housni Z., Mokrini F., Özer G., Lazraq A., Belabess Z., Amiri S., Lahlali R. Dry root rot disease, an emerging threat to citrus industry worldwide under climate change: A review. Physiol. Mol. Plant Pathol. 2022;117:101753. doi: 10.1016/j.pmpp.2021.101753. [DOI] [Google Scholar]
- 139.Yu Y., Gui Y., Li Z., Jiang C., Guo J., Niu D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants. 2022;11:386. doi: 10.3390/plants11030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saxena A., Mishra S., Ray S., Raghuwanshi R., Singh H.B. Differential reprogramming of defense network in Capsicum annum L. plants against colletotrichum truncatum infection by phyllospheric and rhizospheric trichoderma strains. J. Plant Growth Regul. 2020;39:751–763. doi: 10.1007/s00344-019-10017-y. [DOI] [Google Scholar]
- 141.Gkizi D., Poulaki E.G., Tjamos S.E. Towards Biological Control of Aspergillus carbonarius and Botrytis cinerea in Grapevine Berries and Transcriptomic Changes of Genes Encoding Pathogenesis-Related (PR) Proteins. Plants. 2021;10:970. doi: 10.3390/plants10050970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo Q., Li Y., Lou Y., Shi M., Jiang Y., Zhou J., Sun Y., Xue Q., Lai H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019;137:154–166. doi: 10.1016/j.apsoil.2019.01.015. [DOI] [Google Scholar]
- 143.Wang M., Xue J., Ma J., Feng X., Ying H., Xu H. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front. Microbiol. 2020;11:942. doi: 10.3389/fmicb.2020.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walters D.R., Ratsep J., Havis N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013;64:1263–1280. doi: 10.1093/jxb/ert026. [DOI] [PubMed] [Google Scholar]
- 145.Ross A.F. Systemic acquired resistance induced by localized virus infections in plants. Virology. 1961;14:340–358. doi: 10.1016/0042-6822(61)90319-1. [DOI] [PubMed] [Google Scholar]
- 146.Kloepper J.W., Tuzun S., Kuć J.A. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992;2:349. doi: 10.1080/09583159209355251. [DOI] [Google Scholar]
- 147.Pieterse C.M.J., Zamioudis C., Berendsen R.L., Weller D.M., Van Wees S.C.M., Bakker P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 148.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 149.Van Peer R., Niemann G.J., Schippers B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS 417 r. Phytopathology. 1991;81:728–734. doi: 10.1094/Phyto-81-728. [DOI] [Google Scholar]
- 150.Ongena M., Duby F., Jourdan E., Beaudry T., Jadin V., Dommes J., Thonart P. Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl. Microbiol. Biotechnol. 2005;67:692–698. doi: 10.1007/s00253-004-1741-0. [DOI] [PubMed] [Google Scholar]
- 151.Risoli S., Cotrozzi L., Sarrocco S., Nuzzaci M., Pellegrini E., Vitti A. Trichoderma-Induced Resistance to Botrytis cinerea in Solanum Species: A Meta-Analysis. Plants. 2022;11:180. doi: 10.3390/plants11020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frew A., Antunes P.M., Cameron D.D., Hartley S.E., Johnson S.N., Rillig M.C., Bennett A.E. Plant herbivore protection by arbuscular mycorrhizas: A role for fungal diversity? New Phytol. 2022;233:1022–1031. doi: 10.1111/nph.17781. [DOI] [PubMed] [Google Scholar]
- 153.Conrath U., Beckers G.J.M., Langenbach C.J.G., Jaskiewicz M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015;53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- 154.Shoresh M., Harman G.E., Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 155.Liu Q., Tang S., Meng X., Zhu H., Zhu Y., Liu D., Shen Q. Proteomic Analysis Demonstrates a Molecular Dialog Between Trichoderma guizhouense NJAU 4742 and Cucumber (Cucumis sativus L.) Roots: Role in Promoting Plant Growth. Mol. Plant-Microbe Interact. 2021;34:631–644. doi: 10.1094/MPMI-08-20-0240-R. [DOI] [PubMed] [Google Scholar]
- 156.Sarma B.K., Yadav S.K., Patel J.S., Singh H.B. Molecular mechanisms of interactions of Trichoderma with other fungal species. Open Mycol. J. 2014;8:140–147. doi: 10.2174/1874437001408010140. [DOI] [Google Scholar]
- 157.Pandey V., Shukla A., Kumar J. Current Trends in Plant Disease Diagnostics and Management Practices. Springer; Cham, Switzerland: 2016. Physiological and molecular signalling involved in disease management through Trichoderma: An effective biocontrol paradigm; pp. 317–346. [Google Scholar]
- 158.Durrant W.E., Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 159.Pineda A., Zheng S.-J., van Loon J.J.A., Pieterse C.M.J., Dicke M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010;15:507–514. doi: 10.1016/j.tplants.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 160.Alınç T., Cusumano A., Peri E., Torta L., Colazza S. Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J. Chem. Ecol. 2021;47:455–462. doi: 10.1007/s10886-021-01260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leonetti P., Zonno M.C., Molinari S., Altomare C. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. Plant Cell Rep. 2017;36:621–631. doi: 10.1007/s00299-017-2109-0. [DOI] [PubMed] [Google Scholar]
- 162.Martínez-Medina A., Fernandez I., Lok G.B., Pozo M.J., Pieterse C.M.J., Van Wees S.C.M. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017;213:1363–1377. doi: 10.1111/nph.14251. [DOI] [PubMed] [Google Scholar]
- 163.Stenberg J.A., Sundh I., Becher P.G., Björkman C., Dubey M., Egan P.A., Friberg H., Gil J.F., Jensen D.F., Jonsson M., et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021;94:665–676. doi: 10.1007/s10340-021-01354-7. [DOI] [Google Scholar]
- 164.Singh A., Singh V.K., Dwivedy A.K., Deepika, Tiwari S., Dwivedi A., Dubey N.K. Plant Microbiome Paradigm. Springer International Publishing; Cham, Switzerland: 2020. Biological Control of Plant Diseases: Opportunities and Limitations; pp. 121–146. [Google Scholar]
- 165.Essiedu J.A., Adepoju F.O., Ivantsova M.N. Benefits and limitations in using biopesticides: A review. AIP Conf. Proc. 2020;2313:080002. [Google Scholar]
- 166.Navon A. Bacillus thuringiensis Application in Agriculture. Springer; Dordrecht, The Netherlands: 2000. [Google Scholar]
- 167.Akutse K.S., Subramanian S., Maniania N.K., Dubois T., Ekesi S. Biopesticide Research and Product Development in Africa for Sustainable Agriculture and Food Security—Experiences from the International Centre of Insect Physiology and Ecology (icipe) Front. Sustain. Food Syst. 2020;4:152. doi: 10.3389/fsufs.2020.563016. [DOI] [Google Scholar]
- 168.Chandler D., Bailey A.S., Tatchell G.M., Davidson G., Greaves J., Grant W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011;366:1987–1998. doi: 10.1098/rstb.2010.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hassen A.I., Bopape F.L., Sanger L.K. Microbial Inoculants in Sustainable Agricultural Productivity. Springer; New Deli, India: 2016. Microbial Inoculants as Agents of Growth Promotion and Abiotic Stress Tolerance in Plants; pp. 23–36. [DOI] [Google Scholar]
- 170.Stamenković S., Beškoski V., Karabegović I., Lazić M., Nikolić N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018;16:1. doi: 10.5424/sjar/2018161-12117. [DOI] [Google Scholar]
- 171.Prashar P., Kapoor N., Sachdeva S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Biotechnol. 2014;13:63–77. doi: 10.1007/s11157-013-9317-z. [DOI] [Google Scholar]
- 172.Fravel D.R. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005;43:337–359. doi: 10.1146/annurev.phyto.43.032904.092924. [DOI] [PubMed] [Google Scholar]
- 173.Junaid J.M., Dar N.A., Bhat T.A., Bhat A.H., Bhat M.A. Commercial Biocontrol Agents and Their Mechanism of Action in the Management of Plant Pathogens. Int. J. Mod. Plant Anim. Sci. 2013;1:39–57. [Google Scholar]
- 174.Gehlot J.S.P. Fungi and Their Role in Sustainable Development: Current Perspectives. Springer; Singapore: 2018. 779p. [DOI] [Google Scholar]
- 175.Bisht N., Mishra S.K., Chauhan P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2019;143:937–951. doi: 10.1016/j.ijbiomac.2019.09.154. [DOI] [PubMed] [Google Scholar]
- 176.Sundh I., Goettel M.S. Regulating biocontrol agents: A historical perspective and a critical examination comparing microbial and macrobial agents. BioControl. 2013;58:575–593. doi: 10.1007/s10526-012-9498-3. [DOI] [Google Scholar]
- 177.Taylor P., Deacon J.W. Significance of ecology in the development of biocontrol agents against soil-borne plant pathogens. Biocontrol Sci. Technol. 2008;1:37–41. doi: 10.1080/09583159109355181. [DOI] [Google Scholar]
- 178.Lacey L.A., Grzywacz D., Frutos R., Brownbridge M., Goettel M.S. JIP-15-82 Formerly with Agriculture and Agri-Food Canada, Lethbridge Research Centre, Lethbridge. J. Invertebr. Pathol. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 179.Holah J.C., Alexander H.M. Soil pathogenic fungi have the potential to affect the co- existence of two tallgrass prairie species. J. Ecol. 1999:598–608. doi: 10.1046/j.1365-2745.1999.00383.x. [DOI] [Google Scholar]
- 180.Shtienberg D., Elad Y. Incorporation of Weather Forecasting in Integrated, Biological-Chemical Management of Botrytis cinerea. Phytopathology. 1996;87:332–340. doi: 10.1094/PHYTO.1997.87.3.332. [DOI] [PubMed] [Google Scholar]
- 181.Guetsky R., Shtienberg D., Elad Y., Dinoor A. Combining Biocontrol Agents to Reduce the Variability of Biological Control. Phytopathology. 2001;91:621–627. doi: 10.1094/PHYTO.2001.91.7.621. [DOI] [PubMed] [Google Scholar]
- 182.Mark G.L., Morrissey J.P., Higgins P., Gara F.O. Molecular-based strategies to exploit Pseudomonas biocontrol strains for environmental biotechnology applications. FEMS Microbiol. Ecol. 2006;56:167–177. doi: 10.1111/j.1574-6941.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- 183.Guerrero V., Guigon C., Berlanga D., Ojeda D. Complete control of Penicillium expansum on apple fruit using a combination of antagonistic yeast Candida oleophila. Chil. J. Agric. Res. 2014;74:427–431. doi: 10.4067/S0718-58392014000400008. [DOI] [Google Scholar]
- 184.Pal K.K., Scholar V., Gardener B.M. Biological Control of Plant Pathogens. American Psychopathological Society; St. Paul, MN, USA: 2006. pp. 1–25. [DOI] [Google Scholar]
- 185.Prajapati S., Kumar N., Kumar S., Iakharan L., Maurya S. Biological control a sustainable approach for plant disease management: A review. J. Pharmacogn. Phytochem. 2020;9:1514–1523. [Google Scholar]
- 186.Tunde D., Molnár I., Rakosy-Tican E. New insights into the interaction between cultivated potato and Phytophthora infestans. Stud. Biol. UBB. 2015;1:2015–2165. [Google Scholar]
- 187.Stojanović S.S., Karabegović I., Beškoski V., Nikolić N., Lazić M. Bacillus based microbial formulations: Optimization of the production process. Hem. Ind. 2019;73:169–182. doi: 10.2298/HEMIND190214014S. [DOI] [Google Scholar]
- 188.Savary S., Willocquet L., Pethybridge S.J., Esker P., Mcroberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019. 3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 189.Berling M., Blachere-lopez C., Soubabere O., Lery X., Bonhomme A., Sauphanor B., Lopez-ferber M. Cydia pomonella granulovirus Genotypes Overcome Virus Resistance in the Codling Moth and Improve Virus Efficiency by Selection against Resistant Hosts. Appl. Environ. Microbiol. 2009;75:925–930. doi: 10.1128/AEM.01998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhan J., Mcdonald B.A. Field-based experimental evolution of three cereal pathogens using a mark—Release–recapture strategy. Plant Pathol. 2013;62:106–114. doi: 10.1111/ppa.12130. [DOI] [Google Scholar]
- 191.Sundh I., Eilenberg J. Why Has the Authorization of Microbial Biological Control Agents Been Slower in the EU than in Comparable Jurisdictions? Pest Manag. Sci. 2021;77:2170–2178. doi: 10.1002/ps.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abou-Zeid N.M., Mahmoud N.A., Eid H.T., Abbas M.S., Soliman A.S. Induction of systemic resistance against Tomato wilt disease under greenhouse conditions in Egypt. Biosci. Res. 2018;15:4559–4570. [Google Scholar]
- 193.Dhakal R. Biopesticides: A Key to Sustainable Agriculture. Int. J. Pure Appl. Biosci. 2019;7:391–396. doi: 10.18782/2320-7051.7034. [DOI] [Google Scholar]
- 194.Peng G., Mcgregor L., Lahlali R., Gossen B.D., Hwang S.F., Adhikari K.K., Strelkov S.E., Mcdonald M.R. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 2011;60:566–574. doi: 10.1111/j.1365-3059.2010.02400.x. [DOI] [Google Scholar]
- 195.Kean S., Soytong K., To-anun C. Application of biological fungicides to control citrus root rot under field condition in Cambodia. J. Agric. Technol. 2010;6:219–230. [Google Scholar]
- 196.Soytong K., Song J.J., Tongon R. Agricultural Inputs for Organic Agriculture. Proceeding of International Seminar on Promoting Local Resources for Sustainable Agriculture and Development (ISPLRSAD 2020) Adv. Biol. Sci. Res. 2021;13:523–532. doi: 10.2991/absr.k.210609.079. [DOI] [Google Scholar]
- 197.Wisniewski M., Droby S., Norelli J., Liu J., Schena L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016;122:3–10. doi: 10.1016/j.postharvbio.2016.05.012. [DOI] [Google Scholar]
- 198.Mudgal S., De Toni A., Tostivint C., Hokkanen H., Chandler D. Scientific support, literature review and data collection and analysis for risk assessment on microbial organisms used as active substance in plant protection products—Lot 1 Environmental Risk characterisation. EFSA Support. Publ. 2017;10:518E. doi: 10.2903/sp.efsa.2013.EN-518. [DOI] [Google Scholar]
- 199.Villaverde J.J., Sevilla-Morán B., Sandín-España P., López-Goti C., Alonso-Prados J.L. Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014;70:2–5. doi: 10.1002/ps.3663. [DOI] [PubMed] [Google Scholar]
- 200.Raveau R., Fontaine J., Lounès-Hadj Sahraoui A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods. 2020;9:365. doi: 10.3390/foods9030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nicot P.C., Alabouvette C., Bardin M., Blum B., Köhl J., Ruocco M. Review of factors influencing the success or failure of biocontrol: Technical, industrial and socio-economic perspectives. Biol. Control Fungal Bact. Plant Pathog. 2012;78:95–98. [Google Scholar]
- 202.Timper P. Conserving and Enhancing Biological Control of Nematodes. J. Nematol. 2014;46:75–89. [PMC free article] [PubMed] [Google Scholar]
- 203.Bardin M., Ajouz S., Comby M., Lopez-Ferber M., Graillot B., Siegwart M., Nicot P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015;6:566. doi: 10.3389/fpls.2015.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hassan M.N., Afghan S., Hafeez F.Y. Biological control of red rot in sugarcane by native pyoluteorin-producing Pseudomonas putida strain NH-50 under field conditions and its potential modes of action. Pest Manag. Sci. 2011;67:1147–1154. doi: 10.1002/ps.2165. [DOI] [PubMed] [Google Scholar]
- 205.Singh J.S., Vimal S.R. Microbial Services in Restoration Ecology. 1st ed. Elsevier; Amsterdam, The Netherlands: 2020. [DOI] [Google Scholar]
- 206.Sare A.R., Jijakli M.H., Jijakli M.H., Massart S. Microbial ecology to support integrative efficacy improvement of biocontrol agents for postharvest diseases management. Postharvest Biol. Technol. 2021;179:111572. doi: 10.1016/j.postharvbio.2021.111572. [DOI] [Google Scholar]
- 207.Askary T.H., Martinelli P.R.P. In: Biocontrol Agents of Phytonematodes. Askary T.H., Martinelli P.R.P., editors. CABI; Wallingford, UK: 2015. [Google Scholar]
- 208.Crow W.T., Luc J.E., Giblin-Davis R.M. Evaluation of EconemTM, a Formulated Pasteuria sp. Bionematicide, for Management of Belonolaimus longicaudatus on Golf Course Turf. J. Nematol. 2011;43:101–109. [PMC free article] [PubMed] [Google Scholar]
- 209.Wilson M.J., Jackson T.A. Progress in the commercialisation of bionematicides. BioControl. 2013;58:715–722. doi: 10.1007/s10526-013-9511-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the analyses in this study are available within the article.