Abstract
Chemotherapy of soft tissue sarcomas (STS) is restricted by low chemosensitivity and multiple drug resistance (MDR). The purpose of our study was the analysis of MDR mechanism in different types of STS. We assessed the expression of ABC-transporters, MVP, YB-1, and analyzed their correlation with chemosensitivity of cancer cells. STS specimens were obtained from 70 patients without metastatic disease (2018–2020). Expression level of MDR-associated genes was estimated by qRT-PCR and cytofluorimetry. Mutations in ABC-transporter genes were captured by exome sequencing. Chemosensitivity (SI) of STS to doxorubicin (Dox), ifosfamide (Ifo), gemcitabine (Gem), and docetaxel (Doc) was analyzed in vitro. We found strong correlation in ABCB1, ABCC1, and ABCG2 expression. We demonstrated strong negative correlations in ABCB1 and ABCG2 expression with SI (Doc) and SI (Doc + Gem), and positive correlation of MVP expression with SI (Doc) and SI (Doc + Gem) in undifferentiated pleomorphic sarcoma. Pgp expression was shown in 5 out of 44 STS samples with prevalence of synovial sarcoma relapses and it is strongly correlated with SI (Gem). Mutations in MDR-associated genes were rarely found. Overall, STS demonstrated high heterogeneity in chemosensitivity that makes reasonable in vitro chemosensitivity testing to improve personalized STS therapy, and classic ABC-transporters are not obviously involved in MDR appearance.
Keywords: soft tissue sarcoma, chemosensitivity test, ABCB1, ABCC1, ABCG2, MVP, YB-1, Pgp, undifferentiated pleomorphic sarcoma, synovial sarcoma
1. Introduction
Success in chemotherapy of soft tissue sarcomas (STS) is still difficult to achieve due to multiple obstacles. STS are heterogeneous cancers with more than 100 histological subtypes, different in molecular abnormality profiles [1] that make investigations in the field of STS personalized therapy very complex [2]. In spite of the multiple ongoing clinical trials for novel targeted drugs [3], no one of them was introduced in clinical practice at the first line of treatment, and combination of doxorubicin and ifosfamide remain the gold standards chemotherapy [4,5], as well as gemcitabine and docetaxel are often used in second line. Unfortunately, chemotherapy is efficient for not more than 50% of patients and it is followed by fast development of MDR. In different other cancer types these drugs usually promote the development of MDR through the activation of ABC-transporters [6].
ABC-transporters play a key role in cell viability by transporting essential molecules, toxins, xenobiotics and metabolites inside and outside the cell [7]. One of the most important ABC-transporters for MDR development is P-glycoprotein (Pgp, ABCB1/MDR1). Both doxorubicin and docetaxel represent the substrates for Pgp [8]. ABCC1 (MRP1) and ABCG2 (BCRP) are other members from ABC-transporters family [7]. In breast cancer high level of ABCG2 expression is observed in stem cancer cells [9]. The role of mutations in ABC-transporter genes on the protein function has become a new object of interest, which is under the intensive investigation [10].
Drug reflux-associated MVP/LRP (major vault protein/lung resistance protein) also plays an important role in MDR. MVP/LRP is the principal component of vaults, which are the largest ribonucleoprotein particles with barrel-like structures. These vaults may mediate MDR via drug sequestration and exocytosis [11].
Activation of several ABC-transporters is induced by transcription factors from a number of signaling pathways. One of these transcription factors is DNA/RNA-binding protein YB-1 [12], which regulate the expression of Pgp [13], MVP [14], and possibly MRP1. Overexpression of YB-1 in tumors is often associated with highly malignant phenotype and poor prognosis [15].
Contribution of ABC-transporters to MDR in STS was studied earlier. In particular, ABCB1 and ABCC3 activation and an increase in corresponding protein levels associated with pour prognosis were shown in malignant peripheral nerve sheath tumor (MPNST) [16]. Moreover, Pgp expression was shown to correlate significantly with large tumor size and high AJCC stages (III and IV) [16]. Other study of STS prognostic factors revealed the correlation of Pgp expression with poor outcome [17]. For other types of STS the results were controversial.
The main goal of our study was the analysis of MDR mechanism in different types of STS: undifferentiated pleomorphic sarcoma, liposarcoma, synovial sarcoma, leiomyosarcoma, and others.
2. Results
2.1. Evaluation of the Chemosensitivity of Primary Cultured STS Cells to Anticancer Drugs
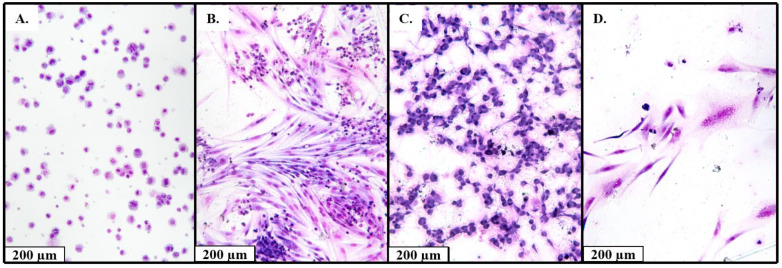
We obtained 70 primary cell cultures from STS samples. At passages 0 and 2–3 we evaluated the percentage of tumor cells in the cultures with cytological staining. In further analysis we used the cultures with ≥90% of tumor cells (Figure 1).
Figure 1.
Examples of cytological studies of the derived cell cultures (Leishman stain; 80×). (A) Cell culture obtained from the patient with synovial sarcoma (99% malignant cells), (B) cell culture obtained from the patient with malignant schwannoma (20% malignant cells, 80% lymphocytes), (C) cell culture obtained from the patient with undifferentiated pleomorphic sarcoma (99% malignant cells), (D) cell culture obtained from the patient with liposarcoma (99% of malignant cells).
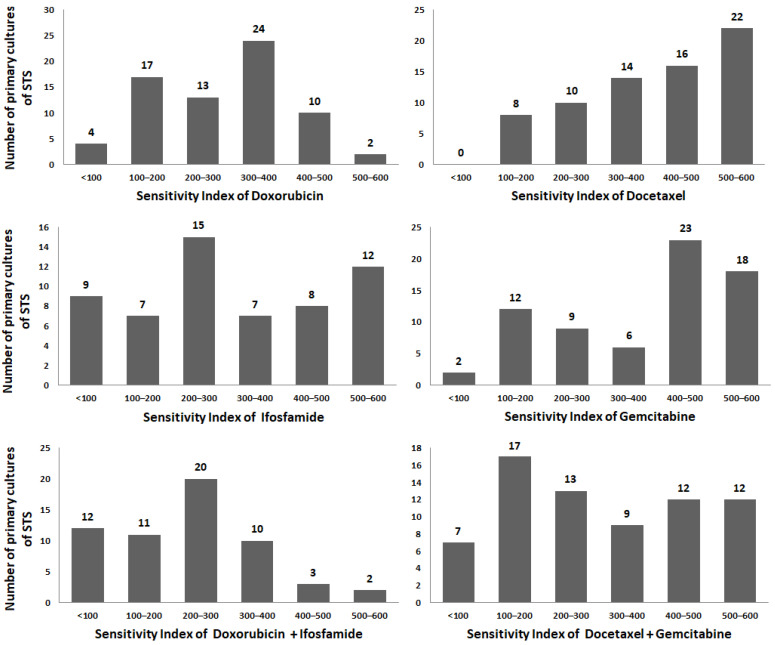
We evaluated the resistance of cancer cells from STS primary cultures to doxorubicin (Dox), ifosfamide (Ifo), docetaxel (Doc), gemcitabine (Gem), and the combinations: (Dox + Ifo) and (Doc + Gem). If the sensitivity index (SI) was less than 300, cells were accounted as sensitive to the drug or drug combination. Figure 2 demonstrates the histograms of SI frequencies for all drugs individually and for drug combinations. It is noteworthy that primary cells revealed the high heterogeneity; therefore, the SI frequencies did not match to normal distribution. Resistant STS are more frequently occurred in case of Doc and Gem: SI > 300 in 74.3% and 67.1% of samples after individual treatment and 47.1% after combination treatment. In case of Dox and Ifo these indexes were 51.4% and 46.7%, respectively, and 25.9% for combination.
Figure 2.
Frequency histograms of the sensitivity index for each single drug (Dox, Ifo, Doc, Gem) and two combinations (Dox + Ifo, Doc + Gem).
SIs of studied drugs demonstrated moderate positive correlation among themselves (r interval was 0.44–0.72, p < 0.0001). Strong correlation was shown for SI (Doc) and SI (Gem) with SI (Doc + Gem), r = 0.85 и r = 0.84, p < 0.0001, respectively, as well as between SI (Ifo) and SI (Dox + Ifo), r = 0.83, p < 0.0001.
STS cells demonstrated higher resistance to Doc and Gem, than to Dox and Ifo (p < 0.001). Drug combinations were more effective than individual treatments (р < 0.01), and combination Dox + Ifo showed higher cytotoxicity than Doc + Gem (р = 0.03) (Table 1). Forty-one percent of primary cell cultures were sensitive to both drug combinations, and 22% of them showed resistant phenotype.
Table 1.
Sensitivity index of STS primary cultures to studied drugs and its comparison to each other (Mann–Whitney test).
| Drugs | Dox | Ifo | Dox + Ifo | Doc | Gem | Doc + Gem |
| Sensitivity index (SI), mean ± SD | ||||||
| 293 ± 121 | 315 ± 172 | 230 ± 134 | 402 ± 141 | 379 ± 162 | 304 ± 171 | |
| p- value means | ||||||
| Ifo | 0.51 | - | - | - | - | - |
| Dox + Ifo | 0.0038 | 0.0065 | - | - | - | - |
| Doc | <0.0001 | 0.0036 | <0.0001 | - | - | - |
| Gem | 0.0006 | 0.04 | <0.0001 | 0.45 | - | - |
| Doc + Gem | 0.93 | 0.68 | 0.03 | 0.0005 | 0.01 | - |
Furthermore, we observed higher resistance to Dox in tumor cells from the patients of the age above 40 years old (р = 0.01). The SI of other drugs were not associated with age, sex, tumor size and previously applied chemotherapy. We compared the SI level in undifferentiated pleomorphic sarcoma, liposarcoma, and synovial sarcoma as these groups contained the largest number of samples. Undifferentiated pleomorphic sarcoma cells were more resistance to Dox then synovial sarcoma (р = 0.01 for pairwise comparison and р = 0.04 for group comparison). We did not observe any correlation between SI of other drugs and tumor histology type. However, we showed an interesting association between SI (Doc) and tumor regression grade: groups of patients, who received adjuvant or neoadjuvant chemotherapy and demonstrated tumor regression grade of 2–3 level, have Doc-resistant tumor cells more frequently: SI (Doc) 304 ± 122 in group with 0–1 tumor regression grade vs. SI (Doc) 487 ± 74 in group with 2–3 tumor regression grade (р = 0.0014). Moreover, comparing the patient groups with neoadjuvant therapy only, SI (Doc), and SI (Doc + Gem) were higher in primary cell cultures from the patients with better response to the therapy (р = 0.008 and р = 0.03, respectively) (Table 2).
Table 2.
Association of the SI to drugs and clinical characteristics of patient and parameters of STS tumors (Mann–Whitney test), р-value.
| Characteristics | n, % | Dox | Ifo | Dox + Ifo | Doc | Gem | Doс + Gem |
|---|---|---|---|---|---|---|---|
| SI, mean ± SD | 70 | 293 ± 120 | 328 ± 167 | 238 ± 130 | 401 ± 145 | 374 ± 164 | 297 ± 172 |
| Age | |||||||
| <40 >40 |
23 (33%) 46 (67%) |
0.01 * (higher resistance in >40 group) |
0.41 | 0.10 | 0.08 | 0.08 | 0.17 |
| Gender | |||||||
| Female Male |
33 (47%) 37 (53%) |
0.49 | 0.54 | 0.82 | 0.70 | 0.89 | 0.16 |
| Tumor size | |||||||
| T1–T2 Т3–Т4 |
38 (54%) 32 (46%) |
0.89 | 0.30 | 0.46 | 0.78 | 0.39 | 0.62 |
| Histology types | |||||||
| Undifferentiated pleomorphic sarcoma Liposarcoma Synovial sarcoma Others |
24 (34%) 16 (23%) 11 (16%) 19 (27%) |
0.04 * (Undifferentiated pleomorphic sarcoma more resistance) |
0.74 | 0.62 | 0.12 | 0.34 | 0.13 |
| Tumor grade | |||||||
| G1–G2 G3 |
10 (15%) 56 (85%) |
0.19 | 0.10 | 0.08 | 0.27 | 0.26 | 0.15 |
| Newly diagnosed (without NeoCT) vs. Recurrent |
22 (39%) 35 (61%) |
0.11 | 0.59 | 0.18 | 0.63 | 0.92 | 0.86 |
| Treatment characteristics | |||||||
| Chemotherapy (all patient) | |||||||
| No Yes |
34 (49%) 36 (51%) |
0.23 | 0.21 | 0.72 | 0.89 | 0.35 | 0.64 |
| Newly diagnosed vs. Newly diagnosed with NeoCT |
22 (59%) 15 (41%) |
0.32 | 0.92 | 0.98 | 0.21 | 0.16 | 0.06 |
| Tumor regression grade | |||||||
| 0–1 2–3 |
17 (73%) 6 (27%) |
0.11 | 0.31 | 0.49 |
0.0014 ** (higher resistance in 2–3 grade group) |
0.25 | 0.06 |
| Tumor regression grade after NeoCT | |||||||
| 0–1 2–3 |
10 (71%) 4 (29%) |
0.053 | 0.29 | 0.55 |
0.008 ** (higher resistance in 2–3 grade group) |
0.053 |
0.03 * (higher resistance in 2–3 grade group) |
NeoCT—neoadjuvant chemotherapy; * p-value < 0.05; ** p-value < 0.01.
2.2. Expression of MDR-Associated Genes in STS
We analyzed the relative expression of ABC-transporters’ genes ABCB1, ABCC1, and ABCG2, transcription factor YB-1 and MVP gene associated with the distribution of xenobiotics in cytoplasm, in 70 tumor samples. We revealed the strong positive correlation between ABCB1/ABCC1 and ABCB1/ABCG2 expression and between ABCC1/ABCG2 expression. MVP expression demonstrated weak positive correlation with YB-1 and weak negative correlation with ABCB1. YB-1 expression did not correlate with the expression of ABC-transporters (Table 3).
Table 3.
Correlation of expression levels of MDR-associated genes in STS samples (Spearman correlation): correlation coefficient (r)—gray background; p-value—white background.
| YB-1 | ABCB1 | ABCC1 | ABCG2 | MVP | |
|---|---|---|---|---|---|
| YB-1 | - | 0.13 | 0.08 | 0.19 | 0.36 |
| ABCB1 | 0.30 | - | 0.67 | 0.88 | −0.27 |
| ABCC1 | 0.55 | <0.0001 | - | 0.68 | 0.07 |
| ABCG2 | 0.10 | <0.0001 | <0.0001 | - | −0.17 |
| MVP | 0.001 | 0.02 | 0.60 | 0.14 | - |
We observed higher ABCG2 expression level (р = 0.007) in patients at the age above 40 years old. Expression of other studied genes were not associated with age, sex, tumor size, degree of malignancies and tumor regression grade (Table 4). In case of undifferentiated pleomorphic sarcoma YB-1 expression was higher than in liposarcoma samples (р = 0.009 for pairwise comparison, р = 0.03 for group comparison). In relapsed patients we observed the decreased expression of MVP (р = 0.04).
Table 4.
Association of expression of MDR-associated genes and clinical characteristics of patient and parameters of STS tumors (Mann–Whitney test), p-value.
| Characteristics | n, % | mRNA ABCB1 |
mRNA ABCC1 |
mRNA ABCG2 |
mRNA YB-1 |
mRNA MVP |
|---|---|---|---|---|---|---|
| Age | ||||||
| <40 >40 |
23 (33%) 46 (67%) |
0.06 | 0.22 |
0.007 ** (high in >40) |
0.48 | 0.54 |
| Gender | ||||||
| Female Male |
33 (47%) 37 (53%) |
0.10 | 0.16 | 0.34 | 0.20 | 0.52 |
| Tumor size | ||||||
| T1–T2 Т3–Т4 |
38 (54%) 32 (46%) |
0.71 | 0.80 | 0.94 | 0.13 | 0.19 |
| Histology types | ||||||
| Undifferentiated pleomorphic sarcoma Liposarcoma Synovial sarcoma Others |
24 (34%) 16 (23%) 11 (16%) 19 (27%) |
0.07 | 0.12 | 0.23 |
0.03 * (high in Undifferentiated pleomorphic sarcoma) |
0.39 |
| Tumor grade | ||||||
| G1–G2 G3 |
10 (16%) 56 (84%) |
0.26 | 0.58 | 0.32 | 0.53 | 0.73 |
| Newly diagnosed (without NeoCT) vs. Recurrent |
22 (40%) 33 (60%) |
0.47 | 0.74 | 0.21 | 0.94 |
0.04 * (low in recurrent) |
| Treatment characteristics | ||||||
| Chemotherapy (all patient) | ||||||
| No Yes |
34 (49%) 36 (51%) |
0.17 | 0.52 | 0.32 | 0.19 | 0.1 |
| Newly diagnosed vs. Newly diagnosed with NeoCT |
22 (59%) 15 (41%) |
0.21 | 0.19 | 0.52 | 0.1 | 0.17 |
| Tumor regression grade | ||||||
| 0–1 2–3 |
17 (73%) 6 (27%) |
0.60 | 0.32 | 0.80 | 0.91 | 0.07 |
| Tumor regression grade after NeoCT | ||||||
| 0–1 2–3 |
10 (71%) 4 (29%) |
0.94 | 1 | 0.9 | 0.94 | 0.054 |
NeoCT—neoadjuvant chemotherapy; * p-value < 0.05; ** p-value < 0.01.
Generally, expression of studied genes did not correlate with the drug resistance (SI), except for weak negative correlation for ABCB1 expression and SI (Doc), r = −0.26; p = 0.02. MVP expression demonstrated weak positive correlation with SI (Doc), r = 0.24; p = 0.04.
Then we analyzed the correlation between expression of MDR-associated genes and SIs of chemotherapeutics in three groups of STS of different histological subtypes: undifferentiated pleomorphic sarcoma (24 samples), liposarcomas (16 samples), synovial sarcomas (11 samples). In liposarcomas we did not show any correlations in SIs of studied drugs. In undifferentiated pleomorphic sarcoma we demonstrated the medium negative correlation of ABCB1 and ABCG2 expression with SI (Doc) and SI (Doc + Gem). In further analysis we showed that these correlations were more pronounced in the group of undifferentiated pleomorphic sarcoma relapses. Moreover, we found the positive correlation of MVP expression with SI (Doc) and SI (Doc + Gem) in undifferentiated pleomorphic sarcoma samples from patients treated with neoadjuvant chemotherapy (Table 5). In synovial sarcomas we demonstrated positive correlation of ABCC1 expression with SI (Ifo) and SI (Dox + Ifo) as well as ABCG2 expression gene with SI (Doс). We did not analyze the groups of primary and relapsed patients because of insufficient groups size.
Table 5.
Correlation of MDR-associated gene expression and SI of primary STS cell cultures in undifferentiated pleomorphic and synovial sarcomas (Spearman correlation).
| Gene | n | Dox | Ifo | Dox + Ifo | Doc | Gem | Doс + Gem |
|---|---|---|---|---|---|---|---|
| Undifferentiated pleomorphic sarcoma | |||||||
| ABCB1 (all patients) | 24 | ns | ns | ns | r = −0.51 p = 0.009 |
ns | r = −0.55 p = 0.005 |
| ABCB1 (recurrent patients) | 6 | ns | r = −0.90 p = 0.03 |
ns | r = −0.94 p = 0.005 |
ns | r = −0.77 p = 0.07 |
| ABCG2 (all patients) | 23 | ns | ns | ns | r = −0.48 p = 0.02 |
ns | r = −0.55 p = 0.006 |
| ABCG2 (recurrent patients) | 5 | r = −0.90 p = 0.03 |
ns | ns | r = −0.80 p = 0.10 |
ns | r = −0.90 p = 0.03 |
| MVP (all patients) | 22 | ns | ns | ns | r = 0.49 p = 0.02 |
ns | r = 0.49 p = 0.02 |
| MVP (patients with NeoCT) | 8 | ns | ns | ns | r = 0.69 p = 0.054 |
ns | r = 0.76 p = 0.03 |
| Synovial sarcoma | |||||||
| ABCC1 (all patients) | 6 | ns | r = 0.90 p = 0.04 |
r = 1.0 p < 0.0001 |
ns | ns | ns |
| ABCG2 (all patients) | 11 | ns | ns | ns | r = 0.60 p = 0.04 |
ns | ns |
NeoCT—neoadjuvant chemotherapy; ns—no significant.
2.3. Expression of P-Glycoprotein and ABCG2 in Primary Cultures of STS
We analyzed the protein Pgp and ABCG2 expression in primary STS cultures of 1–2 passages by flow cytometry. We used cell cultures K562/i-S9 with Pgp overexpression and HBL-100/Dox with ABCG2 overexpression as positive controls.
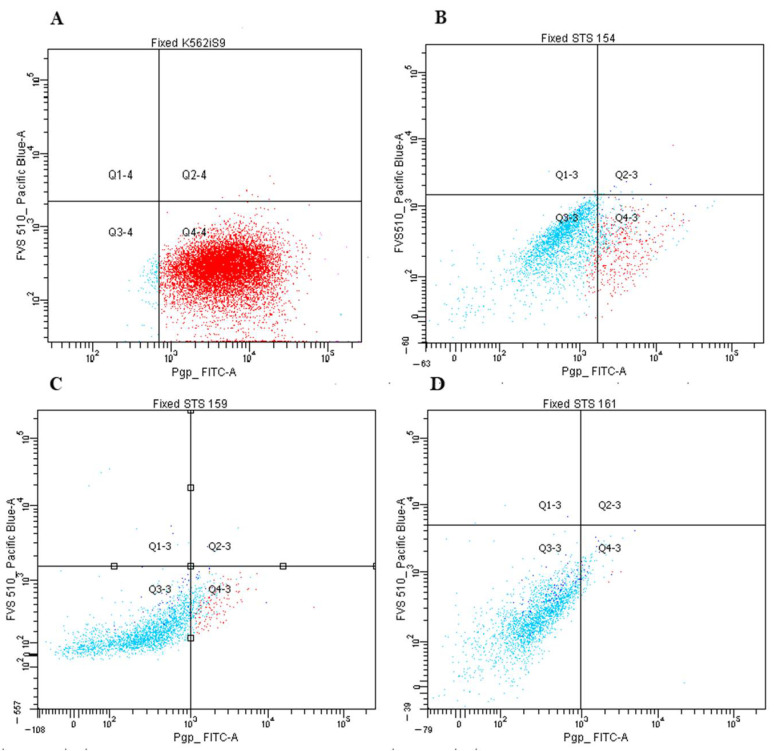
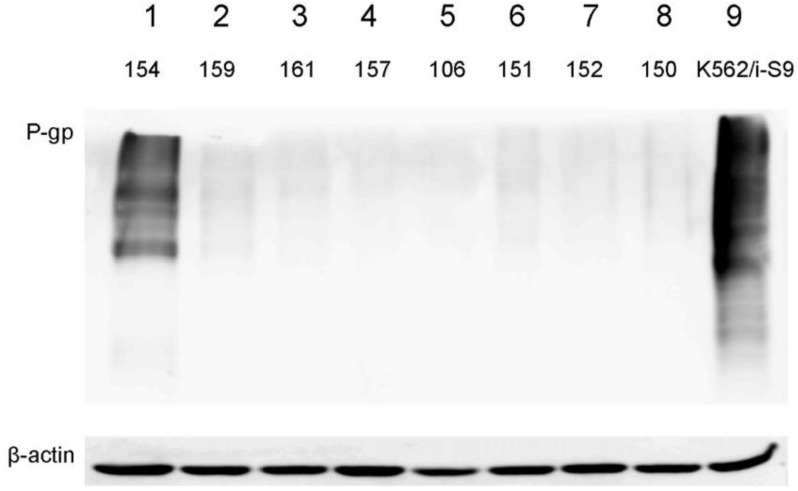
Pgp expression was evaluated in 44 samples. The threshold value was 5% of cells population, which expressed Pgp. We supposed, it was the minimal level of protein expression with significant contribution in the resistance of tumor population and with the effect on clonal selection under the drug exposure. In 5 samples (11%) the Pgp expression was above this value with mean expression level 19.4% of STS cell population in positive group (Figure 3). As in this sample series the amount of medium and high Pgp expression were lower than expected, we selected 8 STS samples with different Pgp expression and analyzed the protein amount by Western blotting (Figure 4). The results of flow cytometry were confirmed by Western blot analysis that supported the observation on rare Pgp expression in studied sample series.
Figure 3.
P-gp expression analysis by cytofluorometry. (A) K562/i-S9 cells were stained with FITC-labeled antibodies against Pgp (Pgp expressed by 98.2% cells); K562/i-S9 cell subline were obtained from chronic myeloid leukemia cell line K562 by transduction of MDR1 (ABCB1). K562/i-S9 cells were used as positive control for the determination of Pgp staining. (B) Expression of Pgp in 33.8% of cells in STS 154 (synovial sarcoma). (C) Expression of Pgp in 8% of cells in STS 159 (leiomyosarcoma). (D) Expression of Pgp in 0.7% of cells in STS 161 (synovial sarcoma).
Figure 4.
Validation of Pgp protein expression in STS samples by Western blotting. Lines 1–8: analyzed STS samples. Line 9: positive control of anti-Pgp antibodies activity (K562/i-S9 cells).
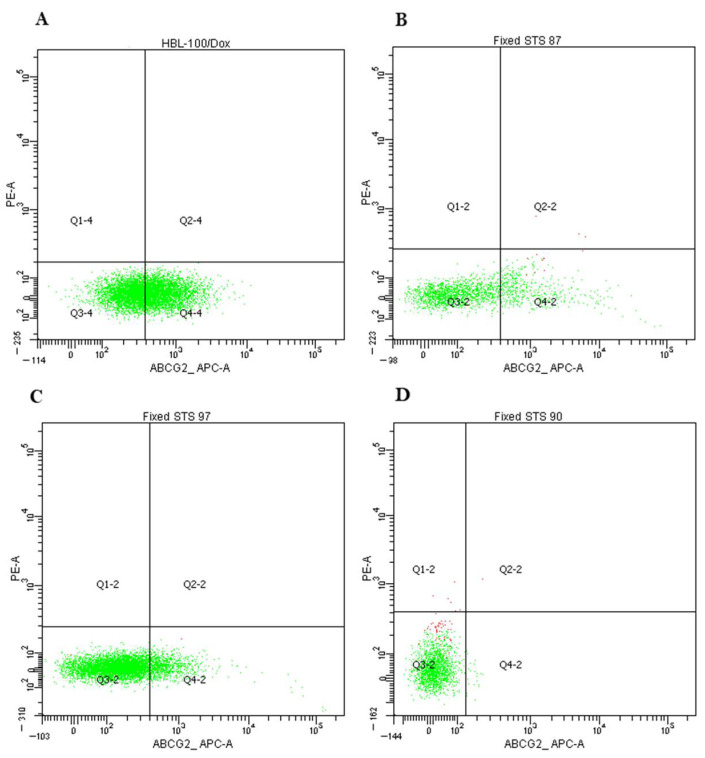
ABCG2 expression was analyzed in 39 samples, the threshold value was 1% as ABCG2 expresses in cancer stem cells being the minor population. We found 17 samples (44%) with the expression level above this threshold with mean expression level 3,75% of STS cell population in positive group (Figure 5). Thus, we did not find correlations in ABCB1 and ABCG2 mRNA expression and Pgp and ABCG2 protein expression, respectively.
Figure 5.
ABCG2 expression analysis by cytofluorometry. (A) HBL-100/Dox cells were stained with APC-labelled anti-ABCG2 antibodies (ABCG2 expressed by 50% cells). Dox-resistant cell subline HBL-100/Dox with overexpressed ABCG2 (BCRP) was obtained from the HBL-100 cells after the selection with Dox. HBL-100/Dox cells were used as positive control for the determination of ABCG2 staining. (B) Expression of ABCG2 in 17.1% of cells in STS 87 (liposarcoma). (C) Expression of ABCG2 in 8.6% of cells in STS 97 (undifferentiated pleomorphic sarcoma). (D) Expression of ABCG2 in 0.4% of cells in STS 90 (synovial sarcoma).
Pgp and ABCG2 expressions were not associated with such clinical patient characteristics as sex, age, disease stage, degree of malignancy, chemo/radiotherapy delivery and the treatment response (Table 6). ABCG2 expression did not vary in different histological STS subtypes. Within the subgroups we did not find the association of ABCG2 with SI of primary STS cultures.
Table 6.
Association of Pgp and ABCG2 protein expression in STS primary cultures and clinical characteristics of patient and parameters of STS tumors (Mann–Whitney test).
| Characteristics | n = 44, (%) | Pgp-Positive (n, %) | p-Value | n = 36, (%) | ABCG2-Positive (n, %) | p-Value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female Male |
22 (50%) 22 (50%) |
3 (14%) 2 (9%) |
0.67 | 13 (36%) 23 (64%) |
2 (15%) 13 (57%) |
0.07 |
| Age | ||||||
| >40 <40 |
31 (70%) 13 (30%) |
3 (10%) 2 (15%) |
0.41 | 11 (36%) 25 (64%) |
4 (36%) 11 (44%) |
0.56 |
| Tumor size | ||||||
| Т1–Т2 Т3–Т4 |
28 (64%) 16 (36%) |
3 (11%) 2 (13%) |
0.83 | 20 (56%) 16 (44%) |
9 (45%) 6 (38%) |
0.43 |
| Tumor grade | ||||||
| G1–G2 G3 |
5 (13%) 34 (87%) |
0 (0%) 5 (15%) |
0.48 | 2 (6%) 34 (94%) |
1 (50%) 13 (41%) |
1.00 |
| Histology types | ||||||
| Undifferentiated pleomorphic sarcoma | 16 (36%) | 0 (0%) |
0.04 (higher in synovial sarcoma) |
13 (36%) | 5 (38%) | 0.30 |
| Liposarcoma | 9 (20%) | 1 (11%) | 11 (31%) | 7 (64%) | ||
| Synovial sarcoma | 6 (14%) | 2 (33%) | 8 (22%) | 2 (25%) | ||
| Others | 13 (30%) | 2 (15%) | 4 (11%) | 1 (25%) | ||
| Treatment characteristics | ||||||
| Chemotherapy | ||||||
| No Yes |
28 (68%) 14 (32%) |
3 (14%) 2 (20%) |
0.84 | 23 (64%) 12 (36%) |
5 (22%) 9 (75%) |
0.74 |
| Tumor regression grade | ||||||
| 0–1 2–3 |
8 (89%) 1 (11%) |
1 (13%) 0 (0%) |
- | 7 (47%) 8 (53%) |
3 (43%) 3 (38%) |
0.48 |
We demonstrated that Pgp expression frequency in synovial sarcomas was significantly higher than in undifferentiated pleomorphic sarcomas (p = 0.04). In synovial sarcomas 5 out of 6 samples were obtained from the relapsed patient, and in this subgroup, we showed strong positive correlation of Pgp expression with SI (Gem) (r = 0.97, p = 0.005). However, it should be mentioned that Gem is not Pgp substrate.
2.4. Identification of Mutations in Several Genes of ABC-Transporter Family
We analyzed the mutations in several genes from ABC-transporter family in the group of 39 STS samples (synovial and pleomorphic histology subtypes). We selected 36 genes associated with tumor progression out of total 300 genes from ABC-transporters family. We found mutations in 9 out of 39 samples, in 3 samples (STS 29, 104, 119) we observed 2–3 mutations. In the Table 7, A3, A13, C1, C6, C11, B1, and G2 genes are associated with drug elimination from cell, whereas A1 and B11 genes play a role in transport of lipids and biliary acids. Mutations were found in the most significant MDR-associated genes B1, С1, G2 in 1, 2, and 1 cases, respectively (10% of total number of samples).
Table 7.
List of mutations in ABC-transporters family.
| Patient ID | Gender | Age | Histology Types | Gene | Reference Allele (Position) | Sequenced Allele | Mutations |
|---|---|---|---|---|---|---|---|
| STS 8 | Male | 37 | Synovial sarcoma | ABCA13 | T (chr7:48271819) |
A | p.M718K |
| A (chr7:48644634) | T | p.L4987F | |||||
| STS 22 | Male | 37 | Synovial sarcoma | ABCC6 | T (chr16:16188976) | G | Intron mutation |
| STS 24 | Male | 62 | Undifferentiated pleomorphic sarcoma | ABCC6 | T (chr16:16188949) |
G | p.H554P |
| T (chr16:16188976) |
G | Intron mutation | |||||
| STS 29 | Female | 70 | Undifferentiated pleomorphic sarcoma | ABCB11 | C (chr2:168944742) |
T | p.E825K |
| ABCC1 | G (chr16:16090474) |
A | p.G844S | ||||
| ABCC11 | C (chr16:48176983) |
T | p.G1160D | ||||
| STS 80 | Female | 43 | Synovial sarcoma | ABCA3 | C (chr16:2323599) |
T | Nonsense Mutation |
| STS 93 | Female | 32 | Undifferentiated pleomorphic sarcoma | ABCG2 | C (chr4:88113437) |
T | p.G354R |
| STS 104 | Male | 66 | Undifferentiated pleomorphic sarcoma | ABCA1 | C (chr9:104831048) |
A | p.W590L |
| ABCA3 | G (chr16:2288133) |
A | p.T908I | ||||
| STS 106 | Female | 60 | Undifferentiated pleomorphic sarcoma | ABCC11 | T (chr16:48187226) |
C | p.M970V |
| STS 119 | Male | 67 | Undifferentiated pleomorphic sarcoma | ABCC1 | C (chr16:16131855) |
T | p.R1296W |
| ABCB1 | G (chr7:87536512) |
T | p.N809K |
3. Discussion
We hypothesized that expression of ABC-transporters is the important mechanism of MDR in STS as it was shown for malignant peripheral nerve sheath tumor and sarcomas of the pulmonary artery [16,18]. Further, it was described earlier that ABCC1/MRP1 expression may have a prognostic significance for patients with STS of high risk, who received anthracycline-based treatment [19]. In cases of embryonal rhabdomyosarcoma ABCC1 expression correlated with poor survival [20]. More frequently this disease is associated with ABCG2 expression [21].
However, in our study we found only 11% of samples with Pgp expression, and only in 1 case we observed 30% Pgp-positive cells. The similar results were obtained for ABCG2 expression: This index reached 16% in rare cases and generally did not exceed 2–5%. As the low level of Pgp and ABCG2 expression in cancer cells resistant to the studied drugs was observed, we propose that other mechanisms of MDR may be more important in STS. Possibly, the rare Pgp expression is associated with the histological tumor subtypes in our specific sample series as in literature high Pgp expression was described in rhabdomyosarcomas [17] and low expression of this gene was shown to be associated with Ewing sarcoma [22]. Interesting, results were obtained for the subgroup of 6 samples of synovial sarcoma, 5 of which belonged to relapsed patients. We demonstrated the increased Pgp expression strongly positively correlated with the resistance to Gem, nevertheless, Gem is not the Pgp substrate. However, one study described the increased sensitivity to Gem in Pgp or MRP1-overexpressing cell lines and their counterparts without MDR phenotype [23]. In our study, we suppose, that Pgp expression does not lead to Gem resistance, but Gem may activate Pgp expressions [24].
In primary analysis of the whole sample series, we did not observe any strong correlations of MDR-associated genes expression and resistance of primary cell cultures. Moreover, in the analysis of undifferentiated pleomorphic sarcoma subgroup we demonstrated the negative correlation for the ABCB1 and ABCG2 expression and SI (Doc) or SI (Gem + Doc). As corresponding proteins were not expressed on the sufficient level in STS cells in our sample series, we guess that decreased expression of ABCB1 and ABCG2 in resistant cells is the indicator of the activation of the specific signaling resulting in STS resistance. If we consider other MDR mechanisms in undifferentiated pleomorphic sarcoma, then it may be the activation of LAPTM4A and LAPTM4B, which are responsible for the transport of small molecules through endosomal and lysosomal membranes. Its increased expression correlated with lower chemosensitivity, especially to the anthracycline-based therapy [25].
We found that studied ABC-transporter genes correlated with each other by mRNA expression level; however, we did not observe sufficient protein amount in spite of high level of mRNA expression in most samples. We believe that it could be associated with the alternative regulation on translation level. It is known that small non-coding RNA (miRNA) participate in posttranscriptional regulation of gene expression either via activation of mRNA degradation or via inhibition of translation repression [26,27]. Thus, canonic mRNA binding with complementary seed regions in 3′UTR mRNA results in translation repression, and, similarly, regulatory RNA-binding proteins change its stability or further translation process [28] 38. In one study it was shown that the three-component complex of HuR, miR-19b, and UTR inhibits the expression of ABCB1/Pgp [29]. In another study it was demonstrated that interactions of ABCB1-3′-UTR—miR-485-3p and ABCC2-3′-UTR—miR-26a-5p induce the translation repression of Pgp and MRP1, which was resulted in the low expression on protein level in spite of the high mRNA expression observed [30].
In our study we demonstrated that resistance to Doc and its combination with Gem was higher in patients with good response to the therapy Dox + Ifo. It could prove the hypothesis that tumor cells survived the neoadjuvant chemotherapy and undergone the clonal selection, obtain higher resistance to Doc. In addition, it could be in accordance with the data that viability of tumor cells with low-grade therapy-related pathomorphism is more dependent from the tumor microenvironment [31]. After culturing in vitro this protective property was withdrawn, and cells demonstrated the chemosensitivity in MTT-test.
We found that the expression of MVP (LRP) gene on mRNA level is lower in tumor samples from the relapsed patients than in primary tumors. It could be associated with the differentiation grade in primary and relapsed tumors. In one study it was demonstrated that MVP protein was predominantly expressed in differentiated cells in rhabdomyosarcoma samples before and after chemotherapy, possibly MVP expression allow rhabdomyosarcoma cells to survive the chemotherapy [32]. In analysis of undifferentiated pleomorphic sarcoma subgroup, we found the positive correlation in MVP expression and resistance to Doc and Doc + Gem combination, and this correlation become stronger in the cells obtained from patients after neoadjuvant chemotherapy. That was in agreement with our previous study on glioblastoma samples. We showed that increased MVP expression on mRNA level was associated with the low proliferation and high resistance to Temozolomide [33]. In the undifferentiated pleomorphic sarcoma subgroup, we proposed direct action of MVP as an MDR protein, but this conclusion requires further verification on a larger group of patients and study of MVP at the protein level.
Our study had a number of limitations: Some of the analyzed groups had very few samples. Almost the entire group of synovial sarcomas with increased Pgp expression came from relapsed patients, and we cannot establish whether high Pgp expression is characteristic only of relapse samples or of all synovial sarcomas. The next limitation is that at this stage of investigation, we studied the expression of YB-1, ABCC1, and MVP only at the mRNA level, but not at the protein level, which could provide new information concerning their involvement in the MDR formation of STS.
4. Materials and Methods
4.1. Methodological Approaches
We assessed the expression of ABC-transporters, MVP and YB-1 and analyzed their correlation with chemosensitivity of corresponding STS cells to the above-mentioned chemotherapeutic agents. Drug resistance was estimated using in vitro chemosensitivity assay proposed by Kurbacher et al. [34], which, currently, are widely used in many modifications [35,36,37,38], based on different methods for estimation of cell viability [39,40,41].
4.2. Tumor Specimens
A total of 70 fresh tumor specimens were obtained from patients who had soft tissue sarcoma and underwent surgery at the N.N. Blokhin National Medical Research Center of Oncology in 2018–2020 years. The prospective study included patients over 18 years old with diagnosed soft tissue sarcoma confirmed by histology, stages I–III. Patients with metastatic disease were excluded from the study. The average age of patients was 46.2 ± 16.2 years and there were 37 males and 33 females. Tumor localization was as follows: lower extremity—42 (60.0%), upper extremity—10 (14.3%), trunk—13 (18.6%), head and neck—5 (7.1%). The STS stages were distributed as follows: I—1 (1.4%), II—14 (20.0%), III—54 (77.2%), IV—1 (1.4%). STS histology types included 24 undifferentiated pleomorphic sarcoma (34.2%), 16 liposarcoma (22.9%), 11 synovial sarcoma (15.7%), 6 leiomyosarcoma (8.6%), 4 malignant schwannoma (5.7%), epithelioid sarcoma (5.7%), dermatofibrosarcoma (4.3%) and Ewing sarcoma (2.9%). Liposarcoma was represented by the following types: 9 myxoid liposarcoma (56.3%), 6 dedifferentiated liposarcoma (37.5%), and 1 pleomorphic liposarcoma (6.2%). The studied STS cohort included 37 newly diagnosed patients (52.9%) and 33 patients (47.1%) with recurrent disease. Chemotherapy in the neoadjuvant setting was administered to 13 patients (18.6%), all patients received 2–4 courses of doxorubicin and ifosfamide. Signed written informed consents were obtained from all participants before the study.
4.3. Chemotheraputic Drugs
In our study we used doxorubicin (Dox, RONC, Moscow, Russia), docetaxel (Doc, NATIVA, Moscow region, Russia), and gemcitabine (Gem, BIOCAD, Moscow, Russia). Because ifosfamide (Ifo) is a prodrug and it requires in vivo hepatic activation, we used the active metabolite 4-hydroperoxy-Ifosfamide (4-OH-Ifo, NIOMECH, Bielefeld, Germany).
4.4. Primary Cancer Cell Cultures and Chemosensitivity Assay
Tumor chemosensitivity assay was performed as a routine procedure immediately following surgery. Solid tumors were obtained during surgery and cut into smaller fragments (1 mm3), which were then dissociated by incubation in 5–10 mL sterile collagenase mix for 2–3 h at 37 °C on a shaker to prepare suspensions of single cells. One part of the cells was used for the chemosensitivity test, and the other one for the analysis of multi-drug resistance. Аfter adjusting the concentration of cells in the suspension to 1–2 × 105 cells/mL, 100-μL cell suspensions were added to each well of a 96-well microplate. Single agents were tested at six different concentrations of a standard test drug concentration (TDC), in particular, 6.25, 12.5, 25, 50, 100, and 200% of the peak plasma concentration of the drug (Table 8), as it was proposed by Andriotti et al. [42].
Table 8.
Drugs tested and their 100% TDC as used in the ex vivo tests.
| Drug/Combination | 100% TDC (mg/mL) |
|---|---|
| Doxorubicin | 3.0 |
| Ifosfamide (4-hydroxy-ifosfamide) | 3.0 |
| Doxorubicin + ifosfamide | 3.0 + 3.0 |
| Docetaxel | 11.3 |
| Gemcitabine | 25.0 |
| Docetaxel + gemcitabine | 11.3 + 25.0 |
The TDCs were based on pharmacokinetic data for standard doses of the agents, adjusted to give good discrimination [42]. Plates were incubated for 5–6 days at 37 °C with 95% humidity in a 5% CO2 incubator. Cell viability was measurement using resazurin-based assay described previously [25]. The results in vitro chemosensitivity tests were interpreted and compared using the sensitivity index SI (SI = 600—sum of % inhibition at 200, 100, 50, 25, 12.5, and 6.25% TDC) [42,43,44].
4.5. Cytology for Isolated Cell Culture
In order to determine the percent of malignant cells for each isolated primary culture, cytological study was performed. Derived cells were used to thin-layer slides preparation through a cytocentrifugation process by the Thermo Shandon Cytospin 3. Morphological assay to determine the percent of malignant cells in Leishman-stained slides were performed on a microscope «Nikon Eclipse Ci-S» (Nikon Corporation, Tokyo, Japan) at 1000× magnification in 3 fields of view.
4.6. Quantitative PCR (Q-PCR)
Total RNA was isolated with PureZOL RNA Isolation Reagent (BIO-RAD, Hercules, CA, USA) according to the manufacturer’s protocol. The RNA quality was checked by electrophoresis in 1% agarose gel containing 0.01% ethidium bromide. Samples with clearly visible 18S and 28S RNA bands were used for further analysis. For the synthesis of cDNA, we used a set of reagents for reverse transcription with primers Random6 (Syntol, Moscow, Russia). The real-time PCR reaction was performed using the intercalating fluorescent agent Eva Green (Synthol, Moscow, Russia) and Taq DNA polymerase from ThermoScientific (EP0402), the CFX Connect Real-Time PCR Detection System (BIO-RAD, USA) was used. Amplification steps: 95 °C—3:00 min, (95 °C—0:10 min, 60 °C—00:10 min, 72 °C—00:30 min)—39 cycles, melt curve 65 °C–95 °C. The primer sequences were selected in PrimerBank (PrimerBank. Available online: https://pga.mgh.harvard.edu/primerbank/ (accessed on 20 November 2018). The results were normalized using housekeeping RPL27 gene. The following pairs of primers were used: YBX-1 forward CCCCAGGAAGTACCTTCGC, reverse AGCGTCTATAATGGTTACGGTCT; ABCB1 forward GGGATGGTCAGTGTTGATGGA, reverse GCTATCGTGGTGGCAAACAATA; ABCC1 forward GTGAATCGTGGCATCGACATA, reverse GCTTGGGACGGAAGGGAATC; MVP forward TACATCCGGCAGGACAATGAG, reverse CTGTGCAGTAGTGACGTGGG; ABCG2 forward TGAGCCTACAACTGGCTTAGA, reverse CCCTGCTTAGACATCCTTTTCAG; RPL27 forward ACCGCTACCCCCGCAAAGTG, reverse CCCGTCGGGCCTTGCGTTTA.
4.7. Flow Cytometry
The expression of Pgp and ABCG2 proteins was assessed in primary cultures of STS by flow cytometry on a BD FACSCanto II flow cytometer with BD FACSDiVa software v6 1.3 (BD Biosciences, Franklin Lakes, NJ, USA). Cells were fixed by 4% paraformaldehyde solution in PBS for 10 min, when cells washed twice in PBS. After suspension of 500 thousand cells per point was incubated in 100 μL of PBS with antibodies in the ratio specified in the manufacturer’s protocol for 40 min in the dark at room temperature, then washed twice in PBS. Fixable Viability Stain 510 (FVS510) was used as a marker of cell viability (Biosciences, Cat. No. 564406). The K562/i-S9 cell line with Pgp overexpression (obtained from K562 by transfection with the MDR1 (ABCB1) gene) was used as a positive control. K562/i-S9 cell line was kindly provided by Mechetner [45]. In our laboratory of tumor cell genetics, we were derived a doxorubicin-resistant cell subline of Pgp and ABCG2 (BCRP) overexpressing HBL-100/Dox cell line from the HBL-100 cells (received from Institute of Cytology RAS) after the continuous cell selection in the presence of Dox [46]. HBL-100/Dox cell was used as a positive control for anti-ABCG2 antibody performance. The range of autofluorescence for the samples varied from 0.1 to 10%. Antibodies were used in the work: FITC-Pgp (BD Biosciences, USA, clone 17F9, Cat. No. 557002), APC anti-human CD338 (ABCG2) (BioLegend, San Diego, CA, USA, clone 5D3, Cat. No. 332020).
4.8. Western Blotting
Two million cells were suspended in 300 µL of RIPA buffer x1 (Thermo Scientific, Waltham, MA, USA) for 20–30 min at +4 °С. Lysates were centrifuged at 13,400 rpm for 30 min, supernatants were placed in 4× sample buffer (1 M Tris-HCl, pH 6.8, 10% SDS, 50% glycerol, 10% β-mercaptoethanol, and bromophenol blue) to 1x-dilution. Samples were placed in the water bath for 10 min at 96 °С. Proteins were resolved in 10% PAAG with 10% SDS and transferred on nitrocellulose membrane (Amersham, Chicago, IL, USA). Membranes were blocked in 5% BSA in 1× TBST (Tris buffer pH 7.5 with 0.005% Tween-20) for 1 h at room temperature. Then membranes were incubated with specific primary monoclonal antibodies against Pgp (MDR1/ABCB1 (D3H1Q) Rabbit mAb Cat. No.12683, Cell Signaling, Danvers, MA, USA) at 1:800 dilutions overnight at 4 °С. For the normalization membranes were incubated with anti-actin antibodies (β-Actin Antibody (C4) HRPsc-47778 HRP, Santa Cruz Biotechnology, Dallas, TX, USA), at 1:500 dilutions. Membranes were washed in TBST three times for 10 min and incubated with secondary peroxidase-conjugated antibodies at 1:10,000 dilutions (Jachson ImmunoResearch, Dallas, TX, USA). Then membranes were washed for three times and 10 min in TBST and visualized with ECL reagent (Thermo Fisher, Waltham, MA, USA) and ImageQuant Las 4000 (GE Healthcare, Chicago, IL, USA).
4.9. Exome Capture, Alignments and Base-Calling
Exomes for the 39 patients were captured with Agilent SureSelect Focused Exome. Libraries were indexed, pooled, and sequenced on Illumina HiSeq2000 machines (paired-end, 250-bp reads). Reads were mapped to GRCh38/hg38 build using BWA 0.7.15 [47] followed by marking duplicate reads with Picard-tools 2.20 (Picard-tools 2.20. Available online http://broadinstitute.github.io/picard/ (accessed on 14 September 2020)). GATK4 [48] base quality score recalibration pre-processing step was performed to detect systematic errors made by the sequencing machine. Tumor-only variant calling was performed on tumor samples with no paired normal using advantages of normal cell contamination implemented in GATK4 Mutect2 (tumor-only mode) probabilistic models for genotyping and filtering.
4.10. Statistical Analysis
Data were presented as M ± S.D. The Mann–Whitney test was carried out to analyze the differences between groups and the Spearman’s correlation coefficient was calculated to quantitate the degree of correlation between parameters. The Kruskal–Wallis test with post test (compare all pairs of columns) was used to analyze groups with different histology types. GraphPad Prism 6.0 was used (GraphPad Software, San Diego, CA, USA). The difference was considered statistically significant at p-value < 0.05 (two-tailed).
5. Conclusions
Overall, we used STS primary cultures as a new approach for the study of MDR mechanisms in STS of different types. Pgp protein expression is a very rare event and probably does not have clinical significance, with the exception of synovial sarcoma. Our results afford us to conclude that MVP expression may play role in MDR of undifferentiated pleomorphic STS. Mechanisms of resistance in STS require further study and search for new targets.
Author Contributions
Conceptualization, G.A.B., K.I.K. and M.G.Y.; methodology, N.I.M., L.A.L., T.I.F., B.Y.B., A.M.S. and E.M.K.; validation, N.I.M., L.A.L., T.I.F., A.S.V., L.V.M., N.A.K., A.M.S., E.M.K., M.G.Y. and K.I.K.; formal analysis, N.I.M., L.A.L., T.I.F., L.F.M. and E.M.K.; investigation, N.I.M., L.A.L., T.I.F., L.F.M., A.E.M., L.Y.F., D.A.B., B.Y.B., V.Y.Z., A.S.V., L.V.M., N.A.K., A.M.S., E.M.K., A.M.S. and K.I.K.; resources, A.E.M., L.Y.F., D.A.B., B.Y.B., V.Y.Z., A.S.V., L.V.M. and N.A.K., data curation, N.I.M., L.A.L., T.I.F., E.M.K. and K.I.K.; writing—original draft preparation, N.I.M., L.A.L., T.I.F. and E.M.K., writing—review and editing, N.I.M., L.A.L., E.A.L., G.A.B., K.I.K. and M.G.Y.; visualization, N.I.M., L.A.L., T.I.F., L.F.M., L.Y.F., A.M.S. and E.M.K., software, N.I.M., L.A.L., T.I.F. and E.M.K.; supervision, E.A.L., G.A.B., K.I.K. and M.G.Y.; project administration, N.I.M., B.Y.B., A.M.S., K.I.K. and M.G.Y.; funding acquisition, K.I.K. and M.G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Work is supported by Russian Foundation of Basic Research, grant No MK 18-29-09095.
Institutional Review Board Statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of N.N. Blokhin NMRCO (protocol code 18-29-09095 and date of approval 28 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gamboa A.C., Gronchi A., Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 2020;70:200–229. doi: 10.3322/caac.21605. [DOI] [PubMed] [Google Scholar]
- 2.Oda Y., Yamamoto H., Kohashi K., Yamada Y., Iura K., Ishii T., Maekawa A., Bekki H. Soft tissue sarcomas: From a morphological to a molecular biological approach. Pathol. Int. 2017;67:435–446. doi: 10.1111/pin.12565. [DOI] [PubMed] [Google Scholar]
- 3.Grünwald V., Karch A., Schuler M., Schöffski P., Kopp H.G., Bauer S., Kasper B., Lindner L.H., Chemnitz J.M., Crysandt M., et al. Randomized Comparison of Pazopanib and Doxorubicin as First-Line Treatment in Patients with Metastatic Soft Tissue Sarcoma Age 60 Years or Older: Results of a German Intergroup Study. J. Clin. Oncol. 2020;38:3555–3564. doi: 10.1200/JCO.20.00714. [DOI] [PubMed] [Google Scholar]
- 4.Liu W., Jiang Q., Zhou Y. Advances of systemic treatment for adult soft-tissue sarcoma. Chin. Clin. Oncol. 2018;7:42. doi: 10.21037/cco.2018.08.02. [DOI] [PubMed] [Google Scholar]
- 5.Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., Eesa M., Fischer U., Hausegger K., Hirsch J.A., et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke. 2018;13:612–632. doi: 10.1016/j.jvir.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Muriithi W., Macharia L.W., Heming C.P., Echevarria A.L., Nyachieo A., Filho P.N., Neto V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020;17:253–269. doi: 10.20892/j.issn.2095-3941.2019.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005;5:30. doi: 10.1186/1475-2867-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safa A.R. Identification and characterization of the binding sites of P-glycoprotein for multidrug resistance-related drugs and modulators. Curr. Med. Chem. Anticancer Agents. 2004;4:1–17. doi: 10.2174/1568011043482142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britton K.M., Eyre R., Harvey I.J., Stemke-Hale K., Browell D., Lennard T.W.J., Meeson. A.P. Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012;323:97–105. doi: 10.1016/j.canlet.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadioglu O., Saeed M.E.M., Munder M., Spuller A., Greten H.J., Efferth T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020;131:110718. doi: 10.1016/j.biopha.2020.110718. [DOI] [PubMed] [Google Scholar]
- 11.Lara P.C., Pruschy M., Zimmermann M., Henríquez-Hernández L.A. MVP and vaults: A role in the radiation response. Radiat. Oncol. 2011;6:148. doi: 10.1186/1748-717X-6-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliseeva I.A., Kim E.R., Guryanov S.G., Ovchinnikov L.P., Lyabin D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry. 2011;76:1402–1433. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- 13.Bargou R.C., Jurchott K., Wagener C., Bergmann S., Metzner S., Bommert K., Mapara M.Y., Winzer K.J., Dietel M., Dorken B., et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 1997;3:447–450. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- 14.Stein U., Bergmann S., Scheffer G.L., Scheper R.J., Royer H.D., Schlag P.M., Walther W. YB-1 facilitates basal and 5-fluorouracil-inducible expression of the human major vault protein (MVP) gene. Oncogene. 2012;24:3606–3618. doi: 10.1038/sj.onc.1208386. [DOI] [PubMed] [Google Scholar]
- 15.Kuwano M., Uchiumi T., Hayakawa H., Ono M., Wada M., Izumi H., Kohno K. The basic and clinical implications of ABC transporters, Y-box-binding protein-1 (YB-1) and angiogenesis-related factors in human malignancies. Cancer Sci. 2003;94:9–14. doi: 10.1111/j.1349-7006.2003.tb01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oda Y., Saito T., Tateishi N., Ohishi Y., Tamiya S., Yamamoto H., Yokoyama R., Uchiumi T., Iwamoto K., Kuwano M., et al. ATP-binding cassette superfamily transporter gene expression in human soft tissue sarcomas. Int. J. Cancer. 2005;114:854–862. doi: 10.1002/ijc.20589. [DOI] [PubMed] [Google Scholar]
- 17.Levine E.A., Holzmayer T., Bacus S., Mechetner E., Mera R., Bolliger C., Roninson I.B., Das Gupta T.K. Evaluation of newer prognostic markers for adult soft tissue sarcomas. J. Clin. Oncol. 1997;15:3249–3257. doi: 10.1200/JCO.1997.15.10.3249. [DOI] [PubMed] [Google Scholar]
- 18.Gaumann A., Tews D.S., Mentzel T., Petrow P.K., Mayer E., Otto M., Kirkpatrick C.J., Kriegsmann J. Expression of drug resistance related proteins in sarcomas of the pulmonary artery and poorly differentiated leiomyosarcomas of other origin. Virchows Arch. 2003;442:529–537. doi: 10.1007/s00428-003-0815-1. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Broto J., Gutierrez A.M., Ramos R.F., Lopez-Guerrero J.A., Ferrari S., Stacchiotti S., Picci P., Calabuig S., Collini P., Gambarotti M., et al. MRP1 Overexpression Determines Poor Prognosis in Prospectively Treated Patients with Localized High-Risk Soft Tissue Sarcoma of Limbs and Trunk Wall: An ISG/GEIS Study. Mol. Cancer Ther. 2013;13:249–259. doi: 10.1158/1535-7163.MCT-13-0406. [DOI] [PubMed] [Google Scholar]
- 20.Gallego S., Llort A., Parareda A., Sanchez De Toledo J. Expression of multidrug resistance-1 and multidrug resistance-associated protein genes in pediatric rhabdomyosarcoma. Oncol. Rep. 2004;11:179–183. doi: 10.3892/or.11.1.179. [DOI] [PubMed] [Google Scholar]
- 21.Oda Y., Kohashi K., Yamamoto H., Tamiya S., Kohno K., Kuwano M., Iwamoto Y., Tajiri T., Taguchi T., Tsuneyoshi M. Different expression profiles of Y-box-binding protein-1 and multidrug resistance-associated proteins between alveolar and embryonal rhabdomyosarcoma. Cancer Sci. 2004;99:726–732. doi: 10.1111/j.1349-7006.2008.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roundhill E., Burchill S. Membrane expression of MRP-1, but not MRP-1 splicing or Pgp expression, predicts survival in patients with ESFT. Br. J. Cancer. 2013;109:195–206. doi: 10.1038/bjc.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergman A.M., Pinedo H.M., Talianidis I., Veerman G., Loves W.J.P., van der Wilt C.L., Peters G.J. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br. J. Cancer. 2003;88:1963–1970. doi: 10.1038/sj.bjc.6601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X., Xu X., Chen Y., Guan R., Cheng T., Wang Y., Jin R., Song M., Hang T. Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein. Molecules. 2019;24:2011. doi: 10.3390/molecules24102011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vita A., Recine F., Mercatali L., Miserocchi G., Spadazzi C., Liverani C., Bongiovanni A., Pieri F., Casadei R., Riva N., et al. Primary Culture of Undifferentiated Pleomorphic Sarcoma: Molecular Characterization and Response to Anticancer Agents. Int. J. Mol. Sci. 2017;18:2662. doi: 10.3390/ijms18122662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasquinelli A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 27.Baek D., Villén J., Shin C., Camargo F.D., Gygi S.P., Bartel D.P. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimson A., Farh K.K., Johnston W.K., Garrett-Engele P., Lim L.P., Bartel D.P. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol. Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorne J.L., Battaglia S., Baxter D.E., Hayes J.L., Hutchinson S.A., Jana S., Millican-Slater R.A., Smith L., Teske M.C., Wastall L.M., et al. MiR-19b non-canonical binding is directed by HuR and confers chemosensitivity through regulation of P-glycoprotein in breast cancer. BBA Gene Regul. Mech. Bbagrm. 2018;1861:996–1006. doi: 10.1016/j.bbagrm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Brueck S., Bruckmueller H., Wegner D., Busch D., Martin P., Oswald S., Cascorbi I., Siegmund W. Transcriptional and Post-Transcriptional Regulation of Duodenal P-Glycoprotein and MRP2 in Healthy Human Subjects after Chronic Treatment with Rifampin and Carbamazepine. Mol. Pharm. 2019;16:3823–3830. doi: 10.1021/acs.molpharmaceut.9b00458. [DOI] [PubMed] [Google Scholar]
- 31.Lin Z., Fan Z., Zhang X., Wan J., Liu T. Cellular plasticity and drug resistance in sarcoma. Life Sci. 2020;263:118589. doi: 10.1016/j.lfs.2020.118589. [DOI] [PubMed] [Google Scholar]
- 32.Klunder J.W., Komdeur R., Van Der Graaf W.T.A., De Bont E.J.S.M., Hoekstra H.J., Van Den Berg E., Molenaar W.M. Expression of multidrug resistance-associated proteins in rhabdomyosarcomas before and after chemotherapy: The relationship between lung resistance-related protein (LRP) and differentiation. Hum. Pathol. 2003;34:150–155. doi: 10.1053/hupa.2003.10. [DOI] [PubMed] [Google Scholar]
- 33.Moiseeva N.I., Susova O.Y., Mitrofanov A.A., Panteleev D.Y., Pavlova G.V., Pustogarov N.A., Stavrovskaya A.A., Rybalkina E.Y. Connection between Proliferation Rate and Temozolomide Sensitivity of Primary Glioblastoma Cell Culture and Expression of YB-1 and LRP/MVP. Biochemistry. 2016;81:628–635. doi: 10.1134/S0006297916060109. [DOI] [PubMed] [Google Scholar]
- 34.Kurbacher C.M., Cree I.A., Brenne U., Bruckner H.W., Kurbacher J.A., Mallmann P., Andreotti P.E., Krebs D. Heterogeneity of in vitro chemosensitivity in perioperative breast cancer cells to mitoxantrone versus doxorubicin evaluated by a microplate ATP bioluminescence assay. Breast Cancer Res. Treat. 1996;41:161–170. doi: 10.1007/BF01807161. [DOI] [PubMed] [Google Scholar]
- 35.Morand du Puch C.B., Vanderstraete M., Giraud S., Lautrette C., Christou N., Mathonnet M. Benefits of functional assays in personalized cancer medicine: More than just a proof-of-concept. Theranostics. 2021;11:9538–9556. doi: 10.7150/thno.55954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia F., Ma S., Bian Y., Yu D., Ma W.X., Miao M., Huang C., Miao L. A retrospective study of the correlation of in vitro chemosensitivity using ATP-TCA with patient clinical outcomes in acute myeloid leukemia. Cancer Chemother. Pharm. 2020;85:509–515. doi: 10.1007/s00280-019-03973-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Li H. Heterogeneity of tumor chemosensitivity in ovarian epithelial cancer revealed using the adenosine triphosphate-tumor chemosensitivity assay. Oncol. Lett. 2015;9:2374–2380. doi: 10.3892/ol.2015.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z., Zhang S., Ma S., Li C., Xu C., Shen Y., Zhao J., Miao L. Evaluation of the in vitro Chemosensitivity and Correlation with Clinical Outcomes in Lung Cancer using the ATP-TCA. Anticancer Agents Med. Chem. 2018;18:139–145. doi: 10.2174/1871520617666170419123713. [DOI] [PubMed] [Google Scholar]
- 39.Sargent J.M., Taylor C.G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br. J. Cancer. 1989;60:206–210. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosserman L., Prendergast F., Herbst R., Fleisher M., Salom E., Strickland S., Raptis A., Hallquist A., Perree M., Rajurkar S., et al. The microculture-kinetic (MiCK) assay: The role of a druginduced apoptosis assay in drug development and clinical care. Cancer Res. 2012;72:3901–3905. doi: 10.1158/0008-5472.CAN-12-0681. [DOI] [PubMed] [Google Scholar]
- 41.Tapias L.F., Gilpin S.E., Ren X., Wei L., Fuchs B.C., Tanabe K.K., Lanuti M., Ott H.C. Assessment of Proliferation and Cytotoxicity in a Biomimetic Three-Dimensional Model of Lung Cancer. Ann. Thorac. Surg. 2015;100:414–421. doi: 10.1016/j.athoracsur.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Andreotti P.E., Cree I.A., Kurbacher C.M., Hartmann D.M., Linder D., Harel G., Gleiberman I., Caruso P.A., Ricks S.H., Untch M. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: Clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995;55:5276–5282. [PubMed] [Google Scholar]
- 43.Rodríguez-Corrales J.Á., Josan J.S. Resazurin Live Cell Assay: Setup and Fine-Tuning for Reliable Cytotoxicity Results. In: Lazar I.M., Kontoyianni M., Lazar A.C., editors. Proteomics for Drug Discovery: Methods and Protocols. Springer; New York, NY, USA: 2017. pp. 207–219. [DOI] [PubMed] [Google Scholar]
- 44.Qi C.J., Ning Y.L., Zhu Y.L., Min H.Y., Ye H., Qian K.Q. In vitro chemosensitivity in breast cancer using ATP-tumor chemosensitivity assay. Arch. Pharm. Res. 2009;32:1737–1742. doi: 10.1007/s12272-009-2211-0. [DOI] [PubMed] [Google Scholar]
- 45.Homolya L., Hollo Z., Muller M., Mechetner E., Sarkadi B. A new method for a quantitative assessment of P-glycoprotein-related multidrug resistance in tumour cells. Br. J. Cancer. 1996;73:849–855. doi: 10.1038/bjc.1996.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rybalkina E.Y., Moiseeva N.I., Karamysheva A.F., Eroshenko D.V., Konysheva A.V., Nazarov A.V., Grishko V.V. Triterpenoids with modified A-ring as modulators of P-gp-dependent drug-resistance in cancer cells. Chem.-Biol. Interact. 2021;348:109645. doi: 10.1016/j.cbi.2021.109645. [DOI] [PubMed] [Google Scholar]
- 47.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. 20131303.3997 [Google Scholar]
- 48.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., DePristo M.A. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.