Abstract
Schistosomiasis japonica caused by the trematode flukes of Schistosoma japonicum was one of the most grievous infectious diseases in China in the mid-20th century, while its elimination has been placed on the agenda of the national strategic plan of healthy China 2030 after 70 years of continuous control campaigns. Diagnostic tools play a pivotal role in warfare against schistosomiasis but must adapt to the endemic status and objectives of activities. With the decrease of prevalence and infection intensity of schistosomiasis in human beings and livestock, optimal methodologies with high sensitivity and absolute specificity are needed for the detection of asymptomatic cases or light infections, as well as disease surveillance to verify elimination. In comparison with the parasitological methods with relatively low sensitivity and serological techniques lacking specificity, which both had been widely used in previous control stages, the molecular detection methods based on the amplification of promising genes of the schistosome genome may pick up the baton to assist the eventual aim of elimination. In this article, we reviewed the developed molecular methods for detecting S. japonicum infection and their application in schistosomiasis japonica diagnosis. Concurrently, we also analyzed the chances and challenges of molecular tools to the field application process in China.
Keywords: schistosomiasis japonica, elimination, diagnostic tools, molecular techniques
1. Introduction
The worldwide pandemic of COVID-19 made administrators of government and all residents aware of the enormous threat from infectious disease to public health safety and social–economic growth [1,2]. Being a widespread tropical disease, schistosomiasis is endemic in 78 countries, with at least 236.6 million people required preventive treatment in 2019 according to the data from WHO. There are three major types of schistosomiasis affecting human beings: schistosomiasis japonica, schistosomiasis mansoni, and schistsomiasis hamatobium. Schistosomiasis japonica caused by Schistosoma japonicum (S. japonicum) is distributed in China, the Philippines, and small pockets of Indonesia in Asia, whereas the other two kinds of schistosomiasis, caused by S. haematobium and S. mansoni, are mainly distributed in countries belonging to Africa and South America [3,4]. Schistosomes are remarkable parasites for being exquisitely adapted to their two-host life cycle, which involves a short period of substantive population booms in the molluscan intermediate host and a long sheltered life, including prodigious egg laying in definitive mammalian hosts [5]. Based on the insight and experience that blocking any stage of the life cycle of S. japonicum would interrupt the transmission, the detection of individual or worm carriers and infected snails with the intention of identifying sources of infection and risk environments is extremely vital in control activities [6,7].
Applying optimal methodologies for diagnosis adapted to the changing control situation is crucial to every aspect of schistosomiasis control. Parasitological tests, such as the miracidia hatching technique (MHT) and Kato–Katz method (KK), are recommended as the “gold” standard for schistosomiasis diagnosis, especially in situations with high prevalence, but they are highly likely to miss infection with light intensity from low prevalence areas [8,9]. That is to say, the insensitivity of etiological detection will lead to an underestimation of disease burden, even threatening the success of the national program and the ultimate goal of elimination [10]. Serological tests based on the immunological response of antigens and antibodies have been widely applied in preliminary screening of patients and disease surveillance in many endemic countries with schistosomiasis, owing to advantages such as low cost, ease of operation, independence of complicated and expensive equipment, etc. [11,12]. However, serological methods have often been criticized for being relatively non-specific, prone to cross-reaction with other parasitic diseases, and unable to distinguish past from current infections [13,14]. With the flourishing of gene and genome research, nucleic acid detection provides a new idea for the diagnosis of schistosomiasis, presenting advantages of higher sensitivity and overwhelming specificity over immunological tests [13,15]. They are also superior in identifying infection by different species of Schistosoma in cases of low-grade infection [16,17]. Better yet, nucleic acid detection-based methods could complete detection based on various samples, including snail tissue [18,19], environmental samples [20], sera, and stool [21,22]. Although most molecular techniques still require expensive infrastructure and highly accurate pretreatment of samples, such as DNA extraction, they are the most potential and valuable methods used for disease surveillance and verifying elimination in many countries with very low endemicity of schistosomiasis, especially with the development of isothermal amplification techniques [23,24].
In this paper, we reviewed the existing diagnostic methods specially focused on schistosomiasis japonica, emphatically introducing the progress of nucleic acid detection-based methods. We also analyzed the potential of the molecular techniques applied in the national control program and the challenges that exist to provide reference for related experts of diagnosis and policymakers.
2. Schistosomiasis Japonica and the Agenda of China
Schistosomiasis japonica is a zoonotic disease, with human beings and more than 40 mammalian definitive hosts identified thus far [3,4]. Although it is endemic in China, the Philippines and Indonesia, the strain of S. japonicum in China is more virulent, with a wide range of epidemic areas and a heavier disease burden than the parasite in the other two countries [25]. Schistosomiasis japonica was a great public health threat in the 1950s with around 12 million infected people mainly distributed in 12 provinces of China along the Yangtze River [26,27,28], where the climate and environment are highly suitable for the propagation of oncomelanade snails. Slightly different from the WHO NTD roadmap’s three time-bound goals for the control or elimination of schistosomiasis, the control process was divided into four stages or three criteria, in order: infection control (prevalence < 5% in humans and animals), transmission control (prevalence < 1% in humans and animals), transmission interruption (zero infection in local residents, domestic animals and snails in five consecutive years), and elimination (zero infection in local residents, domestic animals and snails in another five consecutive years after transmission interruption) [29,30,31].
Nowadays, schistosomiasis japonica has been eliminated in many previous endemic regions due to the high priority over schistosomiasis control at the political level, nearly 70 years of continuous national control program and the effort of multi-sectoral experts and local residents, as well as the flourishing development of economy and technology in China [10,32,33,34]. Five provinces, Shanghai, Guangdong, Guangxi, Fujian, and Zhejiang, have been pioneers in schistosomiasis elimination [35,36]. In 2019, only 5 cases (5 out of 327,475) and 7 domestic animals (7 out of 134,978) were found to be etiologically positive nationwide [37]. By 2020, only 15 of 450 previous endemic counties were maintained in the stage of transmission control due to their complicated environment and relatively retarded economy. However, interrupting the transmission and eventually eliminating schistosomiasis completely nationwide, which set the national strategic plan of Healthy 2030, has turned out to be difficult [33,38]. To accelerate the process of schistosomiasis elimination, optimal methodologies with high sensitivity and absolute specificity are needed for the detection of asymptomatic cases or light infections, as well as disease surveillance to verify elimination.
3. Traditional Diagnostic Tools Applied in Schistosomiasis Control in China
It cannot be exaggerated that diagnosis is the essential basis of schistosomiasis control for case identification and treatment, assessment of morbidity, and evaluation of control strategies, which are all dependent on the performance of diagnostic tests [27,39]. Two kinds of diagnostic methodologies, namely parasitological techniques mainly including KK and MHT [40,41], and immunologic approaches based on detection of specific antibodies, were widely used in the national control program in China, accelerating the process of schistosomiasis control significantly [11,42].
3.1. Parasitological Methods
The KK method, which was originally developed in the mid-1950s, and MHT, based on the positive phototactic behavior of miracidia, are the most broadly used techniques in epidemiological surveys pertaining to schistosomiasis in China [43]. At the stage of morbidity control, especially in the 1950–1980 period, the parasitological methods were the most applicable to the field, featured by high prevalence and high infection intensity. In 1989, with the first national survey of schistosomiasis japonica, direct stool examination of the KK method or MHT was still the recommended method to evaluate the prevalence of schistosomiasis [6,43]. Until now, the KK method and MHT have been the most accepted “gold” standard methods for identifying whether people or animals are infected [6,44]. Additionally, some studies indicated that multiple KK tests per sample, or increasing the collection frequency of stool samples, would increase the diagnostic sensitivity [45,46,47], and the MHT technique possessed higher sensitivity than the KK method due to the larger volume of stool tests received. Additionally, the combination of MHT and microscopic examination of filtered stool sediment would increase the detection rate [48].
3.2. Immunologic Tests
The immunological tests for schistosomiasis diagnosis also have a long history in China. The earliest immunologic test in China was the intradermal text (ID) recorded in 1936, and it was adapted for screening prior to further parasitological examination in the national general survey in the 1950s. Subsequently, a variety of immunologic techniques were applied to the national schistosomiasis control program, such as the circumoval precipitin test (COPT), indirect hemagglutination assay (IHA), the enzyme-linked immunosorbent assay (ELISA), and some rapid diagnostic tests (RDTs). Nowadays, ID and COPT have been out of use due to their low specificity, while IHA is still widely used in most endemic areas of China. Currently, there are two IHA kits with high quality control accredited by the China Food and Drug Administration. In the 10-year World Bank Loan Project (WBLP), aiming to control the morbidity of schistosomiasis, immunological tests were used directly to determine the target of chemotherapy in areas of medium endemicity (15% > prevalence > 3%) and low endemicity (prevalence < 3%) [43]. In the second national survey of schistosomiasis in 2004, in China, the ELISA method was adopted as a screening tool followed by stool examination to understand the real infection status of schistosomiasis in human beings. With the achievement of infection control reached in 2008 and transmission control reached in 2015, a diagnostic strategy with primary immunodiagnostic screening followed by KK or MHT for antibody-positive individuals was widely used in the Chinese national control program and routine surveillance activities in sentinel sites [27].
4. Molecular Methods Developed to Detect the Pathogen of Schistosomiasis
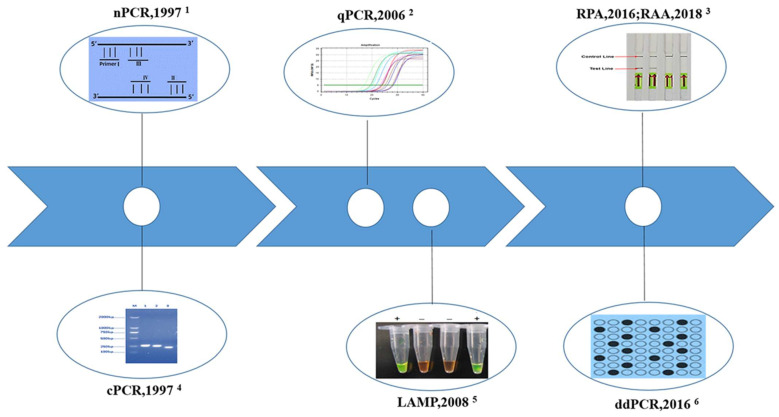
With the goal of the national program shifting from the control of schistosomiasis to elimination, extremely sensitive and specific diagnostic tools are needed emergently to explore asymptomatic cases with light infection and verify transmission interruption or elimination [34,49]. With the development of genomics and genome data for parasites and the urgent demands, molecular diagnostic techniques based on nucleic acid detection have emerged as new hot spots [50]. Various polymerase chain reaction (PCR)-based parasite DNA detection assays, including conventional polymerase chain reaction (cPCR), nested PCR (nPCR), real-time quantitative PCR (qPCR), and droplet digital PCR (ddPCR), have stimulated much interest as alternative options due to their proven diagnostic accuracy and the ability to detect early pre-patient infections [51,52]. The emergence of isothermal amplification methods, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), solves the dilemma of costly instrument dependence on PCR-based methods. The molecular diagnostic techniques of schistosomiasis japonica and the year first reported in China are shown in Figure 1. Regrettably, we have not seen any product based on molecular techniques applied in the field on a large scale.
Figure 1.
The molecular diagnostic techniques of schistosomiasis japonica and the year of first report in China. 1 nPCR is characterized by two pairs of primers, inner primer and outer primer; 2 qPCR can quantitatively and qualitatively analyze the initial template of samples by detecting the fluorescence signal corresponding to each cyclic amplification product in real time; 3 The combination of RPA and lateral flow dipstick (LFD) for visual detection. Generally, visualization of control line and the test line is positive, and only the control line is negative; 4 cPCR determined positive or negative results by the size of the gel electrophoresis band; 5 The combination of LAMP reaction with chemical dyes for visual detection. + positive reaction, − negative reaction; 6 Dilute sample or samples DNA to the single molecule level and collect the fluorescence signal of a single reaction unit to achieve the absolute quantitative detection. Black spots: positive reaction units, blank spots: negative reaction units.
4.1. Conventional Polymerase Chain Reaction (cPCR)
cPCR emerged in the 1980s with a specific ability to amplify a small amount of target DNA and was the first nucleic acid amplification test used in schistosomiasis japonica diagnosis [53]. The character of amplification of microscale DNA of different samples greatly improves the analytical ability, simplifies the diagnostic process, and increases the sensitivity [14,16,51]. Another obvious advantage of this technology is that the amplified products can be visualized by gel electrophoresis and verified by sequencing. So far, many cPCR methods for the diagnosis of schistosomiasis japonica targeting various genes from chromosomes and mitochondria extracted from different samples have been established (Table 1). One of the most important factors impacting the sensitivity of cPCR or other types of molecular detection assays is the abundance of the target sequences or biomarkers in the chromosome or mitochondrial genome [16,51]. The earliest reported PCR test for schistosomiasis diagnosis in China was based on the gene coding miracidium antigen named Sj5D [54,55,56]. Nowadays, the highly repetitive and conserved subunits of 18S rRNA [57,58,59,60], 28S rRNA [18,61,62,63], cytochrome c oxidase subunit 1 (COX I) [64,65], and the repetitive sequences SjR2 (an RTE-like, non-long terminal repeat retrotransposon from S. japonicum) [18,21,66,67,68,69] were the most common detection biomarkers. To evaluate the efficiency of established cPCR methods, several kinds of specimens, including genomic DNA (gDNA), pooled samples of snail and cercaria, and a mixture of eggs and feces, were employed. The lowest detection limit of cPCR established targeting a 607 base pair (bp) region of COX I can reach 10 fg of gDNA, which is less than that of 1 egg or 1 cercariae [64].
Table 1.
The target genes and sample types of established cPCR methods for schistosomiasis japonica.
| Target Sequence | No. of GenBank Accession | Fragment Size (bp) | Detection Limit | Specimen | Year of Publication | |
|---|---|---|---|---|---|---|
| 1 | Sj5D | N/D 1 | 262 | 1 cercarial; 1 egg |
Animal tissue and blood | 1997,1998 [54,55] |
| 2 | Sj5D | N/D | 262 | 10 fold diluted single cercarial DNA | Cercarial DNA | 2004 [56] |
| 3 | 18S rRNA | DQ442999 | 469 | 40 pg | gDNA | 2006 [57] |
| 4 | 18S rRNA | DQ442999 | 469 | 1 cercaria | Cercaria | 2008 [58] |
| 5 | 18S rRNA | DQ442999 | 463 | 62.5 pg | gDNA | 2010 [59] |
| 6 | 18S rRNA | FJ176682 | 157 | 1 cercaria in pooled 10 non-infected snails; 2 eggs in 100 mg of non-infected fecal sample |
Snail; Fecal of mice |
2013 [60] |
| 7 | 28S rRNA | Z46504 | 405 | 100 fg | gDNA | 2010 [18] |
| 8 | 28S rRNA | Z46504 | 607 | 15 pg | gDNA | 2006 [61] |
| 9 | 28S rRNA | Z46504 | 200 | N/D | Cattle fecal | 2017 [62] |
| 10 | 28S rRNA | EU835689.1 | 330 | N/D | Snail | 2018 [63] |
| 11 | COX1 | AF215860 | 614 | 10fg | gDNA | 2010 [64] |
| 12 | COX1 | AF215860 | 254 | N/D | Serum and urine | 2015 [65] |
| 13 | SjR2 | AF412221 | 230 | 1 pg | gDNA | 2010 [18] |
| 14 | SjR2 | AF412221 | 230 | 0.8 pg | gDNA | 2009 [21] |
| 15 | SjR2 | N/D | 176 | 1 cercariae | Cercariae | 2005 [66] |
| 16 | SjR2 | AF412221 | 230 | 0.021 eggs | Egg DNA | 2007 [67] |
| 17 | SjR2 | AF412221 | 230 | 0.5 eggs/g of feces | Human feces | 2012 [68] |
| 18 | SjR2 | N/D | 408 | 1 egg | Colon tissue | 2019 [69] |
| 19 | Mitochondrial DNA gene | N/A 2 | 668/242 | 0.3 eggs | Fecal of mice | 2005 [72] |
1 N/D: Non-disclosed; 2 N/A: Non-applicable.
cPCR also provided a potential tool for the early detection and therapy evaluation of S. japonicum infection. The cPCR assay using a 230-bp sequence of SjR2 established by Xia could detect S. japonicum DNA in sera at the first week post-infection, and it became negative at 10 weeks post-treatment in a rabbit model infected by S. japonicum [21]. Similarly, schistosome DNA can be detected from one day post infection using pooled urine samples of mice by COX I-cPCR [65]. Moreover, the cPCR assay was always used as a reference method to assess the efficacy of other established methods, not only molecular methods but also serological methods [60,70,71]. The cPCR assay targeting 254-bp size of COX I gene was used in the detection of human samples in highly endemic areas of the Philippines, and the results showed that schistosome DNA in the serum and urine of KK-positive subjects could be detected by COX I -cPCR with 100% sensitivity [65]. However, few studies have reported on detecting field samples by the cPCR method, which may largely be due to its dependence on specific equipment and relatively complicated producers.
4.2. Nested PCR (nPCR)
The assay of nPCR, which can be considered a variant of cPCR, requires two rounds of PCR amplification using two sets of primers, commonly called the outer primers and the inner primers [17]. nPCR is more sensitive and specific than cPCR because the probability is extremely low if the first round amplification produces an erroneous fragment; primer pairing and amplification will occur in the second round amplification using the wrong fragment [51]. The repetitive sequences of SjR2 and SjCHGCS19 (a new 303-bp sequence from non-long terminal repeat (LTR) retrotransposon) are the common biomarkers in nPCR assays [73,74]. The published literature showed that the detection limit of SjR2-nPCR stabilized at fg level with 10fg of minimum limit [75]. Validated by the schistosome-infected mice model [76], rabbit model [74,77], and domestic animals (goat and buffalo) [78], the SjR2-nPCR or SjCHGCS19-nPCR could all be used for early diagnosis of schistosomiasis, even light infection, showing positive results at 3 days post-infection through testing sera samples. For samples from humans with chronic schistosomiasis, the detection rate of 230-bp SjR2-nPCR assay was 88.79% (95/107), significantly higher than that of the KK method (69.16%, 74/107) [79]. The sensitivity of the SjR2-nPCR method established by Zhang et al. using 14 and 28 days post-infection buffalo samples was 92.30% (36/39) and 100% (39/39), while the specificity was 97.60% (41/42) [78]. Moreover, the SjCHGCS19-nPCR assay demonstrated 97.67% sensitivity for 43 patient serum samples and 96.07% specificity for 51 serum samples from healthy individuals [74]. In addition, an nPCR assay targeting the 420-bp fragment of the Sjα1 gene, which is a short dispersal element retrotransposon gene with high copy and expression throughout the life cycle of schistosomes, could detect 0.1 fg of gDNA and distinguish the infection status of snail 4h post-infection [80]. However, the impressive performance of the detection efficacy of nPCR needed more verification.
4.3. Real-Time Quantitative PCR (qPCR)
The technique of the qPCR assay uses fluorescent labeled probes or double-stranded DNA-specific fluorescence dye to enable the continuous monitoring of amplicon (PCR product) formation throughout the reaction, thus allowing the quantification of PCR products by measuring fluorescence [81,82]. Compared with cPCR, qPCR has the following advantages: the amount of DNA in a sample can be measured using a standard curve, which can be determined by either spiking samples with a known amount of template or serial DNA dilutions; the qPCR procedures are streamlined with no need for an additional electrophoresis step to detect end-products of PCR, and the results can be preserved for a long time; and qPCR can utilize multiplex assays to detect multiple infections within a single clinical sample using specific probes and is preferred over cPCR in multiplex assays for having improved specificity by the use of probes [51,83].
The first reported qPCR assay for the detection of S. japonicum appeared in 2006, with mitochondrial NADH I as the target gene, and its detection limit could reach 1 egg per gram (EPG) fecal [84]. In the detection of 1,727 persons in field settings of Anhui Province, China, the prevalence (no. positive/no. examined) determined by NADH I-qPCR was 5.3%, significantly higher than those of the hatching test (3.2%) and Kato-Katz thick smear (3.0%) [85]. In a field evaluation of qPCR assay conducted in Hunan, Anhui, Hubei, and Jiangxi provinces of China, the qPCR assay exhibited a high level of sensitivity (100% for humans, 96.83% for bovines) and specificity (100%), and obtained a significantly higher prevalence in both the human (11.06% for qPCR, 0.93% for MHT) and bovine samples (24.73% for qPCR, 7.69% for MHT) [86]. The research conducted in the Philippines also showed similar trends, demonstrating that traditional copro-parasitological techniques underestimate the infection rate, signifying the advantages of qPCR for case finding and disease surveillance and monitoring [87,88,89,90,91]. Besides the gene of NADH I [85,86,87,88,89,90,91,92,93,94,95], several qPCR methods have been established targeting other genes, including COX I [96], 18S rRNA [97,98,99,100,101], ITS 2 [102], SjR2 [99,103,104], SjCHGCS20 [22], SjCHGC08270 [20,105], and Sjrh1.0 [99,106] (Table 2). Those established qPCR methods have mostly completed the laboratory evaluation, but further validation should be conducted in field settings. Notably, the TaqMan qPCR assay targeting the COX I gene was developed to detect the environmental DNA (water samples) of S. japonicum, and its potential utility to schistosomiasis japonica surveillance in the Philippines was assessed. The results showed that the qPCR method could complement malacological surveys for monitoring schistosomes in endemic areas, especially those with a high risk of human infection [96].
Table 2.
The target genes and application performance of qPCR assay for schistosomiasis japonica.
| Target Sequence | No. of GenBank Accession | Fragment Size (bp) | Detection Limit | Sensitivity (%) | Specificity (%) | Prevalence 1 | Specimen | Year of Publication | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NADH I | N/D 2 | 82 | 1 EPG | N/A 3 | N/A | N/A | Human feces | 2006 [84] |
| 2 | NADH I | N/D | 75 | 1 EPG | N/A | N/A | 5.3% | Human feces | 2009 [85] |
| 3 | NADH I | N/D | 82 | 5 EPG | 100/96.83 | 100 | 11.06% of human; 24.73% of bovines | Buffalo and human feces | 2018 [86] |
| 4 | NADH I | N/D | 82 | 1 EPG | 100 | 100 | 51.5% | Buffalo feces | 2010 [87] |
| 5 | NADH I | N/D | 82 | one egg; 14 pg | 95.25/94.0 | 100 | 95.45% | buffalo and Human feces; gDNA |
2012 [88] |
| 6 | NADH I | N/D | 82 | N/A | N/A | N/A | 87.50% | Bovines feces | 2015 [89] |
| 7 | NADH I | N/D | 82 | N/A | N/A | N/A | 90.2% | Human feces | 2015 [90,91] |
| 8 | NADH I | N/D | 82 | N/A | N/A | N/A | N/A | Serum, urine and fecal of pig model | 2008 [92] |
| 9 | NADH I | N/D | 82 | 5 EPG | N/A | N/A | N/A | Buffalo feces | 2009 [93] |
| 10 | NADH I | N/D | 82 | N/A | N/A | N/A | 9.21% of rodents; 18.37% of dogs; 6.9% of goats | Rodents, dogs and goats feces | 2017 [94] |
| 11 | NADH I | AF215860 | 75 | N/A | N/A | N/A | N/A | Organs | 2018 [95] |
| 12 | COX I | N/D | 119 | N/A | N/A | N/A | N/A | Water samples | 2019 [96] |
| 13 | 18S rRNA | AYl57226 | 81 | 6.15 pg | N/A | N/A | 48.0% | gDNA | 2008 [97] |
| 14 | 18S rRNA | AY157226 | 81 | 10 fg | N/A | N/A | N/A | gDNA | 2011 [98] |
| 15 | 18S rRNA | AY157226.1 | N/D | 20 fg | N/A | N/A | N/A | gDNA | 2011 [99] |
| 16 | 18S rRNA | FJ176682 | 156 | 4.3 × 102 copies plasmid; 0.4 fg gDNA; 10 EPG; one cercaria in 10 pooled snails; | N/A | N/A | N/A | Plasmids; gDNA; mice feces; snail | 2013 [100] |
| 17 | 18S rRNA | AY157226 | 280 | 10 fg | N/A | N/A | N/A | gDNA | 2015 [101] |
| 18 | ITS 2 | U22167 | 192 | 1.42 × 102 copies plasmid; 10 pg gDNA; 10 EPG; | 100 | 100 | N/A | Snail and mice feces | 2011 [102] |
| 19 | SjR2 | AF412221.1 | N/D | 2 pg | N/A | N/A | N/A | gDNA | 2011 [99] |
| 20 | SjR2 | AF412221 | N/D | 44.7 copies plasmid | N/A | N/A | N/A | Plasmids and sera of rabbit | 2014 [103] |
| 21 | SjR2 | N/D | N/D | N/A | N/A | N/A | N/A | Water samples | 2021 [104] |
| 22 | SjCHGCS20 | FN356222.1 | N/D | N/D | 98.74 | 100 | 8.33 | Plasma of goat | 2020 [22] |
| 23 | SjCHGC08270 | AY812553 | 85 | half of one cercaria | 93.75 | N/D | N/A | N/A | 2008 [20] |
| 24 | SjCHGC08270 | AY812553 | 85 | one cercaria | N/A | N/A | 6.48 | Water samples | 2011 [105] |
| 25 | Sjrh1.0 | U92488.1 | N/D | 2 fg | N/A | N/A | N/A | gDNA and sera of mice | 2011 [99] |
| 26 | Sjrh1.0 | U92488.1 | N/D | 2 fg | N/A | N/A | N/A | gDNA and water samples | 2011 [106] |
1 Prevalence: no. positive/no. examined; 2 N/D: Non-disclosed; 3 N/A: Non-applicable.
4.4. Droplet Digital PCR (ddPCR)
The technique of ddPCR can still be considered a ‘new’ technology in parasitology, including schistosomiasis. Owing to its sensitivity and absolute quantitative characteristics, ddPCR is a potential candidate to become an appealing new method for parasite detection and quantitative analysis in the future [107]. Weerakoon et al. developed a ddPCR duplex assay targeting SjR2 and NADH I for the detection of S. japonicum, which provides improved detection sensitivity and specificity. The assay was able to detect as little as 0.05 fg of template DNA, and exhibited a high sensitivity for the detection of low levels of parasite DNA in stool, serum, urine, and saliva of mice model [108,109]. The ddPCR assay was also validated using clinical samples collected from 412 residents in a moderate-endemic area of schistosomiasis in the Philippines, proving its higher level of sensitivity obtained for human stool, serum, urine and saliva samples compared with the microscopy-based KK test [110,111]. Moreover, the capacity of ddPCR to quantify infection intensity has important public health implications for schistosomiasis control. Van Dorssen et al. determined the infection prevalence of S. japonicum in fecal samples of goats using the gene NADH I (46.4% ddPCR vs. 6.9% qPCR), showing that ddPCR was more sensitive than qPCR [93]. In general, the ddPCR technique with high sensitivity and specificity attracts increasing interest in its potential for clinical diagnosis and screening, and has the potential to be considered in schistosomiasis diagnosis as a complement to routine assays in schistosomiasis elimination programs.
4.5. Loop-Mediated Isothermal Amplification (LAMP)
The LAMP technique, which uses isothermal conditions to amplify DNA, is relatively simple, cost-effective, rapid, and more field-friendly compared with commonly used PCR-based methods [51,112]. Isothermal amplification does not require specific equipment, such as a thermocycler, electrophoresis apparatus, UV transilluminator, etc., while only a heating block or hot water bath is required for the reaction to progress [83,113,114]. The amplification results can be judged by precipitation turbidity of magnesium pyrophosphate or color reaction with the naked eye [115]. Hence, it is more suitable in resource-poor settings and grass-roots units. In addition, the four specific primers designed for six regions of target genes make the assay highly specific [113,114]. Of course, the LAMP technique has some shortcomings that need to be improved. It is complicated and time-consuming in the process of initial optimization with the use of multiple primers. Sometimes, the false-positive reaction is the fatal defect of the LAMP assay because of its high sensitivity [116,117]. Overall, the LAMP method is an extraordinary innovation trying to break through the restriction of equipment, and it has tremendous potential to apply in schistosomiasis control program for rapid screening, identification of transmission foci and environmental risk assessment.
A series of LAMP assays have been designed for schistosome-infected snail detection, schistosomiasis japonica diagnosis, and chemotherapy efficacy evaluation. The genes of CaBP (calcium-binding protein) [118,119], 28S rRNA [18,19,60], and SjR2 [120,121,122,123,124,125] were selected as the target sequence in the LAMP assay. Research has shown that the LAMP assay usually displays a higher detection rate than the conventional microscopy method for snail at different stages from 1 to 10 weeks post-infection [126]. 28S rRNA-LAMP was able to amplify the target band using DNA of a 1 day post-infection snail infected with one miracidium [18]. Nowadays, the LAMP assay has been applied to the surveillance of schistosoma infection of O. hupensis snail in national schistosomiasis sentinel sites in China [60,127]. In addition, a few studies have been conducted to evaluate the detection efficacy of LAMP for definitive hosts of S. japonicum. The SjR2-LAMP assay developed by Xu et al. was able to detect S. japonicum DNA in rabbit sera on the 3rd day post-infection. When LAMP was used to detect S. japonicum DNA in clinical serum samples (n = 152) from S. japonicum-infected patients and healthy persons, the sensitivity and specificity were 95.5% and 100%, respectively [71]. Moreover, for 47 patients after treatment 3 months, 6 months, and 9 months, the negative conversion rate of S. japonicum DNA in patient sera increased from 23.4% to 61.7% to 83.0%, respectively [123]. The above study demonstrated that the SjR2-LAMP method provides a useful and practical tool for the routine diagnosis and therapeutic evaluation of animals and human schistosomiasis.
4.6. Recombinase Polymerase Amplification (RPA)
Another isothermal amplification technology named RPA is a relatively new method that has experienced exponential growth in terms of publications, popularity, and applications since its first report in 2006 [128]. The central components of RPA mainly include DNA polymerase, DNA binding proteins, and recombinase. It is reported that RPA can operate at 37~42 °C and amplify as low as 1~10 copies of target DNA to detectable levels in less than 20 min. Therefore, the novel method is remarkable for its high sensitivity, simplicity, and extremely rapid amplification, as well as its operation at a low and constant temperature [129,130]. The RPA technique has been successfully integrated with different detection strategies, from end-point lateral flow strips to real-time fluorescent detection, among others, making this technique more user friendly, equipment-free, and facilitating the quantification of DNA [130]. In a meta-analysis of the diagnostic value of nucleic acid detection in schistosomiasis japonica, the isothermal amplification technique showed a relatively higher accuracy than the PCR-based amplification technique, and the sensitivity and specificity of the RPA method was higher than the LAMP assay [131]. However, RPA also has some disadvantages, such as the higher cost, carry-over contamination, and complicated optimization process [129,130]. Furthermore, due to the single source of RPA reagents or commercial kits, alternative products of the recombinase-aided isothermal amplification technique (RAA) have been developed in China [132,133,134,135,136,137].
The diagnostic method of RPA established for schistosomiasis japonica are concentrated after 2015, and the first retrievable literature was published in 2016 [23]. The visual detection method LFD-RPA (combination of RPA and lateral flow dipstick (LFD)) targeting SjR2 could detect 5 fg of S. japonicum DNA and showed no cross-reaction with other parasites. The reaction could be finished within 15~20 min at a wide temperature range (25–45 °C). Furthermore, the LFD-RPA assay performed 92.86% sensitivity (13/14), 100% of specificity (31/31) and excellent diagnostic agreement with the KK method (k = 0.947, Z = 6.36, p < 0.001), indicating that the LFD-RPA assay has a great potency in field application [35]. The real-time RPA (RT-RPA) targeting SjR2 gene performed 0.9 fg S. japonicum DNA detection limit, 100% sensitivity and specificity in detection of S. japonicum in stool samples from 30 infected patients and 30 healthy persons. The reaction could distinguish S. japonicum from other worms by measuring fluorescence using the TwistaTM incubator block [24]. Deng et al. tried to establish a detection method for S. japonicum using the SjR2 gene by RPA combined with electrochemical (EC) DNA biosensor. The RPA-EC combinational detection method also exhibited high sensitivity (0.01 fg detection limit), good specificity, and the ability to complete reaction within 30 min at 37 °C [138]. Afterwards, the RPA or LFD-RPA assay for different biomarkers of 28S rRNA and SjCHGCS19 was developed, which also proved that the technology was sensitive, specific, fast, and convenient [139,140].
5. Prospect of Molecular Detection Methods in Schistosomiasis Diagnosis
Unlike laboratory research, the costs for large-scale screening using the molecular detection method certainly constitute a major constraint. This is why the KK method is widely used for epidemiological surveys and recommended by WHO for surveillance and monitoring of schistosomiasis control programs [29,141]. However, in the current era featured by low endemicity of schistosomiasis, the asymptomatic cases or light infections would be missed by the KK method and MHT, resulting in significant underestimation of prevalence. There is also a consensus that diagnostic tools should be adapted when moving from morbidity control to elimination of infection [29]. It emphasizes that the accuracy of a given diagnostic technique may vary significantly with different schistosomiasis transmission levels. Antibody detection methods are indeed the most widely used nationwide in China, but this pattern of invasive sample collection may not be accepted in the future. Additionally, the detection results are prone to cause confusion and equivocal answers due to their low specificity. Therefore, in the case of reducing the cost, the molecular detection methods are greatly promising, at least in China. In fact, China is also promoting molecular detection methods in surveillance activities, such as LAMP for snail infection detection.
Among the available molecular detection methods, the methods of directly displaying results may be more suitable for the elimination stage, such as qPCR, LAMP, RPA, or RAA. Less procedures, especially casting off an additional electrophoresis step to detect PCR end-products, can be less time consuming and less labor intensive. The qPCR methods can also provide a measurement of infection intensity. The isothermal amplification methods, including LAMP and LFD-RPA, are more field-friendly for visualizing the results directly using the naked eye. The technique of RPA or RAA further reduces the reaction temperature and shortens the reaction time, which is more in line with the requirements of POCT (point of care test). However, the reagents of RPA are much more expensive than the two other methods, and the purchase of its reagents is harder than others for a source of monopoly. Overall, the above molecular detection methods should also be optimized and verified before large-scale application. For other molecular detection methods, they can be used as reference methods for laboratory testing, and are not suitable for field use. The key advantages, limitations, and relative costs of involved molecular methods are summarized in Table 3.
Table 3.
Advantages, limitations, and prospects of large-scale application of different DNA diagnostics in China [51]. (The copyright permission of the Table 3 in the cited reference with modified form has been obtained from corresponding author of Professor Don McManus).
| Method Type | Advantages | Limitation | Instrument Cost * | Reagents Cost * | Prospect of Large-Scale Application ** |
|---|---|---|---|---|---|
| cPCR | Low cost and simple among the molecular detection methods; Can be multiplexed based on different size domains of the gene. | Requires post-PCR processing causing it to be more time consuming and labor intensive; | $ | $ | ∆ |
| nPCR | Improved the sensitivity and specificity for using two sets of primers | Relatively complicated initial optimization process; More time consuming and labor intensive than two rounds of cPCR amplifications; Prone to contamination with amplified PCR products | $ | $ | ∆ |
| qPCR | Higher sensitivity and specificity when probes are used; No post PCR processing and less time consuming and less labor intensive compared to cPCR and nPCR; Can quantify the amount of amplicons; Lower potential laboratory contamination | Relatively complicated initial optimization process; Requires triplicate reactions to improve the accuracy of final calculations | $$ | $ | ∆∆ |
| ddPCR | Higher sensitivity, specificity, specifically when probes are used; Can quantify the amount of amplicons (absolute quantification); Lower potential laboratory contamination | Requires specific and expensive machinery for the initial establishment; Relatively time consuming and complicated initial optimization process | $$$ | $$ | ∆ |
| LAMP | Less equipment required; Can visualize the end products directly using naked eye | Relatively time consuming and complicated initial optimization process; Prone to carryover contamination | - | $ | ∆∆∆ |
| RPA | Less equipment required; End products can be visualized on a chip/lateral flow device; Has great potential to be developed as a point of care diagnostic tool | Relatively complicated initial optimization process; Prone to contamination | - | $$$ | ∆∆ |
* The cost of the instrument and reagents is given as a relative scale to each other. Although the same conformity is used, the price of the instrument and reagents is not the same. $:—low, $$—moderate, $$$—high. ** It represents the possibility that it can be used as a tool for on-site screening and surveillance in the future: ∆—low, ∆∆—moderate, ∆∆∆—high.
6. Chance, Challenges, and the Way Forward
The pandemic of COVID-19 is really a catastrophe for human beings; however, this control progress has promoted the improvement of detection ability for disease control units and personnel, including those engaged in schistosomiasis control, especially for primary-level staff. In a questionnaire survey conducted in 36 countries or districts among the 12 schistosomiasis-endemic provinces in China, all CDC (Center for Disease Control and Prevention) or schistosomiasis control stations participated in the local campaign against COVID-19, and the participation rate of professionals previously engaged in schistosomiasis was 84.32% (936/1110) [38]. A few schistosomiasis control agencies have become a fixed point of coronavirus detection. Moreover, 9.51% of professionals from schistosomiasis control stations participated in virus detection, and the average working time reached 53 days, remarkably promoting the practice ability of molecular detection technologies [38]. Nowadays, most schistosomiasis control stations can independently carry out molecular detection assays, including PCR, qPCR, and isothermal amplification methods. Besides, the recognition for nucleic acid detection of residents and employees is getting higher with the widespread use of nucleic acid detection methods in COVID-19 control, providing an inside track for the field application of molecular detection methods of schistosomiasis, as long as methods with high sensitivity and accuracy are developed.
Although DNA amplification-based molecular diagnostic techniques for schistosomiasis japonica truly have made gratifying progress in recent 20 years, continuous efforts are still needed to establish accurate, field-deployable diagnostics, meeting the demands of national control programs and adapting to resource-limited and low prevalence endemic settings. It should also be noted that the current variable temperature and isothermal DNA amplification techniques present several important disadvantages in real application, including complicated DNA extraction, inseparable cryogenic storage, instability, carry-over contamination, and the unfriendliness of large-scale screening [51,113,116,117]. Nucleic acid extraction is a pivotal procedure, and it may be a bottleneck in DNA detection assays since the yield and quality of DNA directly affect the outcome of the amplification procedure. Meanwhile, nucleic acid purification is considered one of the important challenges preventing molecular diagnostics adoption from reaching the field, and the extraction procedure is also often the most expensive part of DNA-based diagnosis, particularly when using commercially available extraction kits [142,143]. Hence, simplifying available DNA extraction procedures to make them more convenient and less expensive is an urgent challenge to be solved. Currently, most molecular diagnostic reagents need cold chain transportation, and even some enzymes require more stringent temperature conditions [143,144]. Although the developed logistics can partly solve the transportation problem, long-distance transportation will undoubtedly increase the cost and waste of reagents, especially in resource-limited areas and remote areas. Nowadays, there are studies focused on ready-to-use reaction mixes stored at room temperature or at 4 °C [145]. Stability is also a crucial factor in evaluating a method, especially in different conditions of carriage, storage, and experimental environments. Unfortunately, contrasting results occurred in some studies [18,71,146], suggesting that the developed approaches should be evaluated in a multicenter manner. Carry-over contamination is another nerve-wracking problem, especially for isothermal amplification techniques with higher sensitivity [51,112]. Moreover, once the contamination appeared, it was very difficult to remove it in the same lab room. Hence, it is necessary to optimize the operation procedure to adapt to the resource-limit lab. However, this optimization process is also time-consuming, complicated, and costly [116,117].
There are a set of criteria that the diagnostic methods should fulfill to be considered an ideal POCT or large-scale screening test. The criteria can be established by the acronym ASSURED, namely affordable, sensitive, specific, user-friendly, rapid, and robust, equipment free, and deliverable [147]. The isothermal amplification techniques, especially LAMP, already fulfill most of the requirements of the criteria of ASSURED. However, there is a very low probability that the isothermal amplification techniques (LAMP and RPA) can be developed to be completely equipment-free technologies; equipment dependence can be reduced and simplified to the fullest by combining with lateral flow dipsticks, microchips, and other lab-on-chip displays [148,149,150]. Actually, we have to admit that there are no significant changes in current molecular diagnostic protocols for schistosomiasis japonica, including the isothermal amplification method, although LAMP has been available since 2000.
7. Conclusions
Development and implementation of optimal methodologies for diagnosis is crucial in all aspects of schistosomiasis japonica control. Diagnostic tools with high sensitivity and specificity are needed as programs shift their goals from control to elimination in China [6,7]. Diagnostic technologies based on nucleic acid amplification can offset the deficiency of traditional parasitic methods. Meanwhile, the isothermal amplification techniques have made significant breakthroughs in breaking traditional laboratory boundaries by providing nucleic acid replication at constant temperatures [129]. It is gratifying that some glorious progress has been achieved, including the discovery of various biomarkers and the establishment of multiple kinds of detection techniques. Moreover, we have also tried to carry out the integration of LAMP in routine surveillance of schistosomiasis [127]. In the stage of schistosomiasis elimination, not only PCR-based detective methods but also isothermal amplification assays can be used as a vital supplement to traditional diagnostic methods of etiological and serological techniques. Certainly, simplifying and standardizing the existing molecular diagnostic methods and adjusting them to field application, especially the isothermal amplification method, are aspects that require continuous effort.
Author Contributions
Conceptualization, J.X. and X.Z.; writing—original draft preparation, C.L.; writing—review and editing, W.D., L.W. and Z.Q. All authors have read and agreed to the published version of the manuscript.
Funding
Major national R&D projects (2018ZX10101-002-002); Scientific research project of Shanghai Health Commission (20204Y0050).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A novel coronavirus from patients with pneumonia in china, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Case J.B., Winkler E.S., Errico J.M., Diamond M.S. On the road to ending the covid-19 pandemic: Are we there yet? Virology. 2021;557:70–85. doi: 10.1016/j.virol.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett R. Schistosomiasis. Lancet. 2018;392:2431. doi: 10.1016/S0140-6736(18)33008-3. [DOI] [PubMed] [Google Scholar]
- 5.Nelwan M.L. Schistosomiasis: Life cycle, diagnosis, and control. Curr. Ther. Res. Clin. Exp. 2019;91:5–9. doi: 10.1016/j.curtheres.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergquist R., Yang G.J., Knopp S., Utzinger J., Tanner M. Surveillance and response: Tools and approaches for the elimination stage of neglected tropical diseases. Acta Trop. 2015;141:229–234. doi: 10.1016/j.actatropica.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist R., Zhou X.N., Rollinson D., Reinhard-Rupp J., Klohe K. Elimination of schistosomiasis: The tools required. Infect. Dis. Poverty. 2017;6:158. doi: 10.1186/s40249-017-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergquist R., Johansen M.V., Utzinger J. Diagnostic dilemmas in helminthology: What tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Knopp S., Speich B., Hattendorf J., Rinaldi L., Mohammed K.A., Khamis I.S., Mohammed A.S., Albonico M., Rollinson D., Marti H., et al. Diagnostic accuracy of kato-katz and flotac for assessing anthelmintic drug efficacy. PLoS Negl. Trop. Dis. 2011;5:e1036. doi: 10.1371/journal.pntd.0001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollinson D., Knopp S., Levitz S., Stothard J.R., Tchuem Tchuenté L.A., Garba A., Mohammed K.A., Schur N., Person B., Colley D.G., et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Hinz R., Schwarz N.G., Hahn A., Frickmann H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell Probes. 2017;31:2–21. doi: 10.1016/j.mcp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Chen N.G., Lin D.D., Xie S.Y., Wang Q.Z., Tang L., Liu Y.M., Zeng X.J., Liu H.Y., Huang M.J., Chen H.G. Diagnostic efficiency of indirect hemagglutination assay kit for antibody detection of schistosomiasis japonica. Chin. J. Schistosomiasis Control. 2011;23:377–380. (In Chinese) [PubMed] [Google Scholar]
- 13.Hawkins K.R., Cantera J.L., Storey H.L., Leader B.T., de Los Santos T. Diagnostic tests to support late-stage control programs for schistosomiasis and soil-transmitted helminthiases. PLoS Negl. Trop. Dis. 2016;10:e0004985. doi: 10.1371/journal.pntd.0004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weerakoon K.G., Gobert G.N., Cai P., McManus D.P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 2015;28:939–967. doi: 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv Z., Wu Z., Zhang L., Ji P., Cai Y., Luo S., Wang H., Li H. Genome mining offers a new starting point for parasitology research. Parasitol. Res. 2015;114:399–409. doi: 10.1007/s00436-014-4299-5. [DOI] [PubMed] [Google Scholar]
- 16.He P., Song L.G., Xie H., Liang J.Y., Yuan D.Y., Wu Z.D., Lv Z.Y. Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infect. Dis. Poverty. 2016;5:25. doi: 10.1186/s40249-016-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C., Guo Q., Fu Z., Liu J., Lin J., Xiao K., Sun P., Cong X., Liu R., Hong Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2021;213:105743. doi: 10.1016/j.actatropica.2020.105743. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai T., Furushima-Shimogawara R., Ohmae H., Wang T.P., Lu S., Chen R., Wen L., Ohta N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am. J. Trop. Med. Hyg. 2010;83:542–548. doi: 10.4269/ajtmh.2010.10-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong Q.B., Chen R., Zhang Y., Yang G.J., Kumagai T., Furushima-Shimogawara R., Lou D., Yang K., Wen L.Y., Lu S.H., et al. A new surveillance and response tool: Risk map of infected Oncomelania hupensis detected by loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop. 2015;141:170–177. doi: 10.1016/j.actatropica.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Hung Y.W., Remais J. Quantitative detection of Schistosoma japonicum cercariae in water by real-time PCR. PLoS Negl. Trop. Dis. 2008;2:e337. doi: 10.1371/journal.pntd.0000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia C.M., Rong R., Lu Z.X., Shi C.J., Xu J., Zhang H.Q., Gong W., Luo W. Schistosoma japonicum: A PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp. Parasitol. 2009;121:175–179. doi: 10.1016/j.exppara.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q., Chen C., Zhou K., Li Y., Tong L., Yue Y., Zhou K., Liu J., Fu Z., Lin J., et al. Evaluation of a real-time pcr assay for diagnosis of schistosomiasis japonica in the domestic goat. Parasites Vectors. 2020;13:535. doi: 10.1186/s13071-020-04420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun K., Xing W., Yu X., Fu W., Wang Y., Zou M., Luo Z., Xu D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasites Vectors. 2016;9:476. doi: 10.1186/s13071-016-1745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing W., Yu X., Feng J., Sun K., Fu W., Wang Y., Zou M., Xia W., Luo Z., He H., et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in hunan province of China. BMC Infect. Dis. 2017;17:164. doi: 10.1186/s12879-017-2182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudge J.W., Webster J.P., Lu D.B., Wang T.P., Fang G.R., Basáñez M.G. Identifying host species driving transmission of schistosomiasis japonica, a multihost parasite system, in China. Proc. Natl. Acad. Sci. USA. 2013;110:11457–11462. doi: 10.1073/pnas.1221509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utzinger J., Zhou X.N., Chen M.G., Bergquist R. Conquering schistosomiasis in china: The long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Steinman P., Maybe D., Zhou X.N., Lv S., Li S.Z., Peeling R. Evolution of the national schistosomiasis control programmes in the people’s republic of china. Adv Parasitol. 2016;92:1–38. doi: 10.1016/bs.apar.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X.N., Wang L.Y., Chen M.G., Wu X.H., Jiang Q.W., Chen X.Y., Zheng J., Utzinger J. The public health significance and control of schistosomiasis in china—Then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Tchuem Tchuenté L.A., Rollinson D., Stothard J.R., Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: Time to change and adapt strategies. Infect. Dis. Poverty. 2017;6:42. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Xu J.F., Li S.Z., Zhang L.J., Wang Q., Zhu H.H., Zhou X.N. Integrated control programmes for schistosomiasis and other helminth infections in P.R. China. Acta Trop. 2015;141:332–341. doi: 10.1016/j.actatropica.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X.N., Xu J., Lv S., Li S.Z. Progress of the national programme and achievements of scientific researches on schistosomiasis elimination in china. Chin. J. Dis. Control Prev. 2019;23:749–753. doi: 10.16462/j.cnki.zhjbkz.2019.07.002. (In Chinese) [DOI] [Google Scholar]
- 32.Zhang L.J., Dai S.M., Xue J.B., Li Y.L., Zhou X.N. The epidemiological status of schistosomiasis in p. R. China after the world bank loan project, 2002–2017. Acta Trop. 2019;195:135–141. doi: 10.1016/j.actatropica.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Xu J., Lv S., Cao C.L., Li S.Z., Zhou X.N. Progress and challenges of schistosomiasis elimination in china. Chin. J. Schistosomiasis Control. 2018;30:605–609. doi: 10.16250/j.32.1374.2018249. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 34.Lei Z.L., Zhou X.N. Eradication of schistosomiasis: A new target and a new task for the national schistosomiasis control porgramme in the People’s Republic of China. Chin. J. Schistosomiasis Control. 2015;27:1–4. (In Chinese) [PubMed] [Google Scholar]
- 35.Zhang L.J., Li S.Z., Wen L.Y., Lin D.D., Abe E.M., Zhu R., Du Y., Lv S., Xu J., Webster B.L., et al. The establishment and function of schistosomiasis surveillance system towards elimination in the People’s Republic of China. Adv. Parasitol. 2016;92:117–141. doi: 10.1016/bs.apar.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Li-Juan Z., Zhi-Min X., Ying-Jun Q., Hui D., Shan L., Jing X., Shi-Zhu L., Xiao-Nong Z. endemic status of schistosomiasis in people’s republic of china in 2016. Chin. J. Schistosomiasis Control. 2017;29:669–677. doi: 10.16250/j.32.1374.2017204. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 37.Zhang L.J., Xu Z.M., Dang H., Li Y.L., Lü S., Xu J., Li S.Z., Zhou X.N. Endemic status of schistosomiasis in People’s Republic of China in 2019. Chin. J. Schistosomiasis Control. 2020;32:551–558. doi: 10.16250/j.32.1374.2020263. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 38.Guo J.Y., Zhang L.J., Cao C.L., Lü S., Xu J., Li S.Z., Zhou X.N. Challenges of schistosomiasis control in china during the coronavirus disease 2019 (Covid-19) epidemic. Chin. J. Schistosomiasis Control. 2020;32:511–516. doi: 10.16250/j.32.1374.2020198. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 39.Collins C., Xu J., Tang S. Schistosomiasis control and the health system in P.R. China. Infect. Dis. Poverty. 2012;1:8. doi: 10.1186/2049-9957-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leuenberger A., Nassoro T., Said K., Fenner L., Sikalengo G., Letang E., Montresor A., Zhou X.N., Steinmann P., Marti H., et al. Assessing stool quantities generated by three specific kato-katz thick smear templates employed in different settings. Infect. Dis. Poverty. 2016;5:58. doi: 10.1186/s40249-016-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin D.D., Liu J.X., Liu Y.M., Hu F., Zhang Y.Y., Xu J.M., Li J.Y., Ji M.J., Bergquist R., Wu G.L., et al. Routine kato-katz technique underestimates the prevalence of Schistosoma japonicum: A case study in an endemic area of the people’s republic of China. Parasitol. Int. 2008;57:281–286. doi: 10.1016/j.parint.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Xu J., Peeling R.W., Chen J.X., Wu X.H., Wu Z.D., Wang S.P., Feng T., Chen S.H., Li H., Guo J.G., et al. Evaluation of immunoassays for the diagnosis of Schistosoma japonicum infection using archived sera. PLoS Negl. Trop. Dis. 2011;5:e949. doi: 10.1371/journal.pntd.0000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J.F., Xu J., Bergquist R., Yu L.L., Yan X.L., Zhu H.Q., Wen L.Y. Development and application of diagnostics in the national schistosomiasis control programme in the people’s republic of China. Adv. Parasitol. 2016;92:409–434. doi: 10.1016/bs.apar.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Xu J., Li S.Z., Zhang L.J., Bergquist R., Dang H., Wang Q., Lv S., Wang T.P., Lin D.D., Liu J.B., et al. Surveillance-based evidence: Elimination of schistosomiasis as a public health problem in the peoples’ republic of China. Infect. Dis. Poverty. 2020;9:63. doi: 10.1186/s40249-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu B., Feng Z., Xu X.J., Hu W. Evaluation of kato-katz technique combined with stool hatching test in diagnosis of schistosomiasis japonica. Chin. J. Schistosomiasis Control. 2011;23:321–323. (In Chinese) [PubMed] [Google Scholar]
- 46.Lamberton P.H., Kabatereine N.B., Oguttu D.W., Fenwick A., Webster J.P. Sensitivity and specificity of multiple kato-katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl. Trop. Dis. 2014;8:e3139. doi: 10.1371/journal.pntd.0003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian M.B., Zhuang S.F., Zhu S.Q., Deng X.M., Li Z.X., Zhou X.N. Improving diagnostic performance of the kato-katz method for clonorchis sinensis infection through multiple samples. Parasites Vectors. 2019;12:336. doi: 10.1186/s13071-019-3594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H.Q., Xu J., Zhu R., Cao C.-L., Bao Z.-P., Yu Q., Zhang L.J., Xu X.L., Feng Z., Guo J.G. Comparison of the miracidium hatching test and modified kato-katz method for detecting Schistosoma japonicum in low prevalence areas of China. Southeast Asian J. Trop. Med. Public Health. 2014;45:20–25. [PubMed] [Google Scholar]
- 49.Chen J., Xu J., Bergquist R., Li S.Z., Zhou X.N. “Farewell to the god of plague”: The importance of political commitment towards the elimination of schistosomiasis. Trop. Med. Infect. Dis. 2018;3:108. doi: 10.3390/tropicalmed3040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weerakoon G.K., Gordon C.A., McManus D.P. DNA diagnostics for schistosomiasis control. Trop. Med. Infect. Dis. 2018;3:81. doi: 10.3390/tropicalmed3030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utzinger J., Becker S.L., van Lieshout L., van Dam G.J., Knopp S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015;21:529–542. doi: 10.1016/j.cmi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 1986;51:263–273. doi: 10.1101/SQB.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y.P., Weng X.H. Detection DNA of Schistosoma japonicum using PCR assay. Chin. J. Infect. Dis. 1997;15:203–206. (In Chinese) [Google Scholar]
- 55.Chen Y.P., Weng X.H. The research of utilizing PCR to detect 5D gene of Schistosoma japonicum. Chin. J. Parasitol. Parasit. Dis. 1998;26:13–61. (In Chinese) [Google Scholar]
- 56.Zhou J., Tao K.H., Li Y.X., Qian W.H., Zhang J.H., Wang Y., Zhang Z.S. Detection of Schistosomia japonicum 5D gene by polymerase chain reaction and genechip technique. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:154–157. (In Chinese) [PubMed] [Google Scholar]
- 57.Chen J.H., Wen L.Y., Zhang X.Z., Zhang J.F., Yu L.L., Hong L.D. Development of a PCR assay for detecting Schistosoma japonicum-infected Oncomelania hupensis. Chin. J. Parasitol. Parasit. Dis. 2006;24:204–207. (In Chinese) [PubMed] [Google Scholar]
- 58.Li H.J., Liang Y.S., Dai J.R., Tao Y.H., Wang W., Qu G.L., Wei J.Y. Homology of 18s small subunit ribosomal RNA gene among species and strains of schistosoma and sensitivity of PCR assay to detect single cercaria. Chin. J. Schistosomiasis Control. 2008;20:418–422. (In Chinese) [Google Scholar]
- 59.Wang T.A., Shen H.Y., Shen Y.X., Wang W.B., Wang B.J., Liang Y.S., Hong-Xiang Z. Establishment of PCR assay for detection of Schistosoma japonicum miracidium in water. Chin. J. Zoonoses. 2010;26:562–564. (In Chinese) [Google Scholar]
- 60.Thanchomnang T., Tantrawatpan C., Intapan P.M., Sri-Aroon P., Limpanont Y., Limpanont Y., Lulitanond V., Janwan P., Sanpool O., Tourtip S., et al. Pyrosequencing for rapid molecular identification of Schistosoma japonicum and S. Mekongi eggs and cercariae. Exp. Parasitol. 2013;135:148–152. doi: 10.1016/j.exppara.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Sandoval N., Siles-Lucas M., Pérez-Arellano J.L., Carranza C., Puente S., López-Abán J., Muro A. A new PCR-based approach for the specific amplification of DNA from different schistosoma species applicable to human urine samples. Parasitology. 2006;133:581–587. doi: 10.1017/S0031182006000898. [DOI] [PubMed] [Google Scholar]
- 62.Feng T., Qin Z.Q., Jing X.U., Zhou J., Qian Y.J., Zhu H.Q., Lv S., Cao C.L., Li S.Z. Efficacy evaluation of a loop mediated isothermal amplification technique in detection of DNA of Schistosoma japonicum eggs in fecal samples. Chin. J. Parasitol. Parasit. Dis. 2017;35:230–234. (In Chinese) [Google Scholar]
- 63.Qin Z.Q., Xu J., Feng T., Lv S., Qian Y.J., Zhang L.J., Li Y.L., Lv C., Bergquist R., Li S.Z., et al. Field evaluation of a loop-mediated isothermal amplification (lamp) platform for the detection of Schistosoma japonicum infection in Oncomelania hupensis snails. Trop. Med. Infect. Dis. 2018;3:124. doi: 10.3390/tropicalmed3040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kato-Hayashi N., Kirinoki M., Iwamura Y., Kanazawa T., Kitikoon V., Matsuda H., Chigusa Y. Identification and differentiation of human schistosomes by polymerase chain reaction. Exp. Parasitol. 2010;124:325–329. doi: 10.1016/j.exppara.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 65.Kato-Hayashi N., Leonardo L.R., Arevalo N.L., Tagum M.N., Apin J., Agsolid L.M., Chua J.C., Villacorte E.A., Kirinoki M., Kikuchi M., et al. Detection of active schistosome infection by cell-free circulating DNA of Schistosoma japonicum in highly endemic areas in Sorsogon Province, the Philippines. Acta Trop. 2015;141:178–183. doi: 10.1016/j.actatropica.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Driscoll A.J., Kyle J.L., Remais J. Development of a novel PCR assay capable of detecting a single Schistosoma japonicum cercaria recovered from Oncomelania hupensis. Parasitology. 2005;131:497–500. doi: 10.1017/S0031182005007961. [DOI] [PubMed] [Google Scholar]
- 67.Lu Z.X., Xu J., Gong W., Luo W., Zhang H.Q., Rong R., Shi C.J., Xia C.M. Detection of Schistosoma japonicum DNA by polymerase chain reaction. Chin. J. Zoonoses. 2007;23:479–483. (In Chinese) [Google Scholar]
- 68.Fung M.S., Xiao N., Wang S., Carlton E.J. Field evaluation of a PCR test for Schistosoma japonicum egg detection in low-prevalence regions of China. Am. J. Trop. Med. Hyg. 2012;87:1053–1058. doi: 10.4269/ajtmh.2012.12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X., Gu K., Zeng Q., Gao L., Cheng D. Diagnostic value of SjR2 gene in colonic tissue from Schistosoma japonicum infected hosts. Med. Sci. Monit. 2019;25:427–435. doi: 10.12659/MSM.912997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu J., Rong R., Zhang H.Q., Shi C.J., Zhu X.Q., Xia C.M. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP) Int. J. Parasitol. 2010;40:327–331. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Xu X., Zhang Y., Lin D., Zhang J., Xu J., Liu Y.M., Hu F., Qing X., Xia C., Pan W. Serodiagnosis of Schistosoma japonicum infection: Genome-wide identification of a protein marker, and assessment of its diagnostic validity in a field study in China. Lancet Infect. Dis. 2014;14:489–497. doi: 10.1016/S1473-3099(14)70067-2. [DOI] [PubMed] [Google Scholar]
- 72.Gobert G.N., Chai M., Duke M., McManus D.P. Copro-PCR based detection of schistosoma eggs using mitochondrial DNA markers. Mol. Cell Probes. 2005;19:250–254. doi: 10.1016/j.mcp.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Laha T., Brindley P.J., Smout M.J., Verity C.K., McManus D.P., Loukas A. Reverse transcriptase activity and untranslated region sharing of a new RTE-like, non-long terminal repeat retrotransposon from the human blood fluke, Schistosoma japonicum. Int. J. Parasitol. 2002;32:1163–1174. doi: 10.1016/S0020-7519(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 74.Guo J.J., Zheng H.J., Xu J., Zhu X.Q., Wang S.Y., Xia C.M. Sensitive and specific target sequences selected from retrotransposons of Schistosoma japonicum for the diagnosis of schistosomiasis. PLoS Negl. Trop. Dis. 2012;6:e1579. doi: 10.1371/journal.pntd.0001579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang F., Hou M., Chuan-Xin Y.U., Yang K., Yang B.Y., Zhang W.Y., Luo X.F., Chen L., Min-Jun J.I. The use of nested PCR to monitor sentinel mice in aquatic areas infested with schistosomiasis japonica. J. Pathog. Biol. 2015;10:325–328. (In Chinese) [Google Scholar]
- 76.Xu J., Duan Z.L., Guan Z.X., Wang Y.Y., Lin C., Zhang T.T., Zhang H.Q., Qian X., Xia C.M. Early detection of circulating DNA of Schistosoma japonicum in sentinel mice models. Exp. Parasitol. 2017;176:82–88. doi: 10.1016/j.exppara.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Liu A.P., Yang Q.L., Guo J.J., Jing X.U., Xia C.M. DNA detection of low-intensity Schistosoma japonicum infection by nested-PCR assay in serum of host. Suzhou Univ. J. Med. Sci. 2010;30:915–917. (In Chinese) [Google Scholar]
- 78.Zhang X., He C.C., Liu J.M., Li H., Lu K., Fu Z.Q., Zhu C.G., Liu Y.P., Tong L.B., Zhou D.B., et al. Nested-PCR assay for detection of Schistosoma japonicum infection in domestic animals. Infect. Dis. Poverty. 2017;6:86. doi: 10.1186/s40249-017-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng F.S., He L., He X.M., Yang J., Qin Z.Q. Nested-PCR assay for detection of Schistosoma japonicum. J. Trop. Dis. Parasitol. 2017;15:136–138. (In Chinese) [Google Scholar]
- 80.Tong Q.B., Lu S.H., Wang T.P., Chen R., Lou D., Zhuo M.M. Utilization nested PCR to detect the infection of Schistosoma japonicum. Acta Parasitol. Med. Entomol. Sin. 2009;16:203–207. (In Chinese) [Google Scholar]
- 81.Bell A., Ranford-Cartwright L. Real-time quantitative PCR in parasitology. Trends Parasitol. 2002;18:338. doi: 10.1016/S1471-4922(02)02331-0. [DOI] [PubMed] [Google Scholar]
- 82.Williams P.M., Giles T., Tucker A., Winer J., Heid C. Development and application of real-time quantitative PCR. Birkhäuser Boston. 1998;1:313–325. doi: 10.1007/978-1-4612-4164-5_18. [DOI] [Google Scholar]
- 83.Gordon C.A., Gray D.J., Gobert G.N., Mcmanus D.P. DNA amplification approaches for the diagnosis of key parasitic helminth infections of humans. Mol. Cell Probes. 2011;25:143–152. doi: 10.1016/j.mcp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Lier T., Simonsen G.S., Haaheim H., Hjelmevoll S.O., Vennervald B.J., Johansen M.V. Novel real-time PCR for detection of Schistosoma japonicum in stool. Southeast Asian J. Trop. Med. Public Health. 2006;37:257–264. [PubMed] [Google Scholar]
- 85.Lier T., Simonsen G.S., Wang T., Lu D., Haukland H.H., Vennervald B.J., Hegstad J., Johansen M.V. Real-time polymerase chain reaction for detection of low-intensity Schistosoma japonicum infections in China. Am. J. Trop. Med. Hyg. 2009;81:428–432. doi: 10.4269/ajtmh.2009.81.428. [DOI] [PubMed] [Google Scholar]
- 86.He P., Gordon C.A., Williams G.M., Li Y., Wang Y., Hu J., Gray D.J., Ross A.G., Harn D., McManus D.P. Real-time PCR diagnosis of Schistosoma japonicum in low transmission areas of china. Infect. Dis. Poverty. 2018;7:8. doi: 10.1186/s40249-018-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu H.W., Qin Y.F., Chu K., Meng R., Liu Y., McGarvey S.T., Olveda R., Acosta L., Ji M.J., Fernandez T., et al. High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real-time polymerase chain reaction. Am. J. Trop. Med. Hyg. 2010;82:646–652. doi: 10.4269/ajtmh.2010.09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon C.A., Acosta L.P., Gray D.J., Olveda R.M., Jarilla B., Gobert G.N., Ross A.G., McManus D.P. High prevalence of Schistosoma japonicum infection in carabao from samar province, the philippines: Implications for transmission and control. PLoS Negl. Trop. Dis. 2012;6:e1778. doi: 10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gordon C.A., Acosta L.P., Gobert G.N., Jiz M., Olveda R.M., Ross A.G., Gray D.J., Williams G.M., Harn D., Li Y., et al. High prevalence of Schistosoma japonicum and fasciola gigantica in bovines from northern samar, the philippines. PLoS Negl. Trop. Dis. 2015;9:e0003108. doi: 10.1371/journal.pntd.0003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon C.A., Acosta L.P., Gobert G.N., Olveda R.M., Ross A.G., Williams G.M., Gray D.J., Harn D., Li Y., McManus D.P. Real-time PCR demonstrates high prevalence of Schistosoma japonicum in the philippines: Implications for surveillance and control. PLoS Negl. Trop. Dis. 2015;9:e0003483. doi: 10.1371/journal.pntd.0003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gordon C.A., McManus D.P., Acosta L.P., Olveda R.M., Williams G.M., Ross A.G., Gray D.J., Gobert G.N. Multiplex real-time PCR monitoring of intestinal helminths in humans reveals widespread polyparasitism in northern samar, the Philippines. Int. J. Parasitol. 2015;45:477–483. doi: 10.1016/j.ijpara.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Lier T., Johansen M.V., Hjelmevoll S.O., Vennervald B.J., Simonsen G.S. Real-time PCR for detection of low intensity Schistosoma japonicum infections in a pig model. Acta Trop. 2008;105:74–80. doi: 10.1016/j.actatropica.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Qin Y.F., Liu Y., Xue-Li D.U., Meng R., Chu K., Min-Jun J.I., Kurtis J.D., Hai-Wei W.U. Sensitivity of SYBR green real-time copro-PCR on detection of Schistosoma japonicum infection. J. Pathog. Biol. 2009;4:432–435. (In Chinese) [Google Scholar]
- 94.Van Dorssen C.F., Gordon C.A., Li Y., Williams G.M., Wang Y., Luo Z., Gobert G.N., You H., McManus D.P., Gray D.J. Rodents, goats and dogs—Their potential roles in the transmission of schistosomiasis in China. Parasitology. 2017;144:1633–1642. doi: 10.1017/S0031182017000907. [DOI] [PubMed] [Google Scholar]
- 95.Dang-Trinh M.A., Angeles J.M.M., Moendeg K.J., Macalanda A.M.C., Higuchi L., Oto C., Kirinoki M., Chigusa Y., Kawazu S.I. Utilization of real time PCR for the assessment of egg burden in the organs of Schistosoma japonicum experimentally infected mice. Exp. Parasitol. 2018;189:61–65. doi: 10.1016/j.exppara.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 96.Fornillos R.J.C., Sato M.A.O., Tabios I.K.B., Sato M., Leonardo L.R., Chigusa Y., Minamoto T.A.O., Kikuchi M., Legaspi E.R., Fontanilla I.K.C. Detection of Schistosoma japonicum and Oncomelania hupensis quadrasi environmental DNA and its potential utility to schistosomiasis japonica surveillance in the Philippines. PLoS ONE. 2019;14:e0224617. doi: 10.1371/journal.pone.0224617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z., Liang B., Zhao Y.Y., Huang L., Wang Y.F. Fluorescent quantitative real-time PCR for detection of Schistosoma japonicum. Chin. J. Parasitol. Parasit. Dis. 2008;26:299–303. (In Chinese) [PubMed] [Google Scholar]
- 98.Zhou L., Tang J., Zhao Y., Gong R., Lu X., Gong L., Wang Y. A highly sensitive Taqman real-time PCR assay for early detection of schistosoma species. Acta Trop. 2011;120:88–94. doi: 10.1016/j.actatropica.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Wang B.J., Wang W.B., Zhou X., Chen Y.Q., Zhang J., Liu C.C., Liang Y.S., Hong-Xiang Z. Fluorescent quantitative real-time PCR for quantitative detection of Schistosoma japonicum cercariae in water. Chin. J. Zoonoses. 2011;27:1075–1081. (In Chinese) [Google Scholar]
- 100.Kongklieng A., Kaewkong W., Intapan P.M., Sanpool O., Janwan P., Thanchomnang T., Lulitanond V., Sri-Aroon P., Limpanont Y., Maleewong W. Molecular differentiation of Schistosoma japonicum and Schistosoma mekongi by real-time PCR with high resolution melting analysis. Korean J. Parasitol. 2013;51:651–656. doi: 10.3347/kjp.2013.51.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J., Zhao G.H., Lin R., Blair D., Sugiyama H., Zhu X.Q. Rapid detection and identification of four major schistosoma species by high-resolution melt (HRM) analysis. Parasitol. Res. 2015;114:4225–4232. doi: 10.1007/s00436-015-4660-3. [DOI] [PubMed] [Google Scholar]
- 102.Thanchomnang T., Intapan P., Sri-Aroon P., Lulitanond V., Janwan P., Sanpool O., Maleewong W. Molecular detection of Schistosoma japonicum in infected snails and mouse faeces using a real-time PCR assay with fret hybridisation probes. Mem. Inst. Oswaldo Cruz. 2011;106:831–836. doi: 10.1590/S0074-02762011000700008. [DOI] [PubMed] [Google Scholar]
- 103.Guan W., Jing X., Liang S., Sun H., Dong L.L., Xia C.M. Quantificational detection of Schistosoma japonicum DNA in serum of the host and assessment of infectiosity by real-time pcr. Chin. J. Zoonoses. 2014;30:263–267. doi: 10.3969/cjz.j.issn.1002-2694.2014.03.009. (In Chinese) [DOI] [Google Scholar]
- 104.Xu M.Z., Wen L., Shen X.J., Liao Y., Tian B. RT-PCR was used to monitor Schistosoma japonicum cercariae on the water surface in the reservoir area of Xiangjiang Changsha comprehensive hub project from 2016 to 2017. Pract. Prev. Med. 2021;28:837–839. (In Chinese) [Google Scholar]
- 105.Worrell C., Xiao N., Vidal J.E., Chen L., Zhong B., Remais J. Field detection of Schistosoma japonicum cercariae in environmental water samples by quantitative PCR. Appl. Environ. Microbiol. 2011;77:2192–2195. doi: 10.1128/AEM.01561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ben-Jing W., Wen-Bo W., Xia Z., You-Sheng L., Hong-Xiang Z. Establishment eraly diganostic method for Schistosoma japonicum infetion based on mice model. Beijing Forum Trop. Med. Parasitol. 2011 (In Chinese) [Google Scholar]
- 107.Pomari E., Piubelli C., Perandin F., Bisoffi Z. Digital PCR: A new technology for diagnosis of parasitic infections. Clin. Microbiol. Infect. 2019;25:1510–1516. doi: 10.1016/j.cmi.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 108.Weerakoon K.G., Gordon C.A., Gobert G.N., Cai P., McManus D.P. Optimisation of a droplet digital PCR assay for the diagnosis of Schistosoma japonicum infection: A duplex approach with DNA binding dye chemistry. J. Microbiol. Methods. 2016;125:19–27. doi: 10.1016/j.mimet.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 109.Weerakoon K.G., Gordon C.A., Cai P., Gobert G.N., Duke M., Williams G.M., McManus D.P. A novel duplex ddPCR assay for the diagnosis of schistosomiasis japonica: Proof of concept in an experimental mouse model. Parasitology. 2017;144:1005–1015. doi: 10.1017/S003118201700021X. [DOI] [PubMed] [Google Scholar]
- 110.Weerakoon K.G., Gordon C.A., Williams G.M., Cai P., Gobert G.N., Olveda R.M., Ross A.G., Olveda D.U., McManus D.P. Droplet digital PCR diagnosis of human schistosomiasis: Parasite cell-free DNA detection in diverse clinical samples. J. Infect. Dis. 2017;216:1611–1622. doi: 10.1093/infdis/jix521. [DOI] [PubMed] [Google Scholar]
- 111.Cai P., Weerakoon K.G., Mu Y., Olveda R.M., Ross A.G., Olveda D.U., McManus D.P. Comparison of kato katz, antibody-based elisa and droplet digital PCR diagnosis of schistosomiasis japonica: Lessons learnt from a setting of low infection intensity. PLoS Negl. Trop. Dis. 2019;13:e0007228. doi: 10.1371/journal.pntd.0007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.García-Bernalt J.D., Fernández-Soto P.A., Febrer-Sendra B., Crego-Vicente B., Muro A.A. Loop-mediated isothermal amplification in schistosomiasis. J. Clin. Med. 2021;10:511. doi: 10.3390/jcm10030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Avendaño C.A., Patarroyo M.A. Loop-mediated isothermal amplification as point-of-care diagnosis for neglected parasitic infections. Int. J. Mol. Sci. 2020;21:7981. doi: 10.3390/ijms21217981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomita N., Mori Y., Fau-Kanda H., Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 116.Hsieh K., Mage P.L., Csordas A.T., Eisenstein M., Soh H.T. Simultaneous elimination of carryover contamination and detection of DNA with uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification (UDG-LAMP) Chem. Commun. 2014;50:3747–3749. doi: 10.1039/c4cc00540f. [DOI] [PubMed] [Google Scholar]
- 117.Ma C., Wang F., Wang X., Han L., Jing H., Zhang H., Shi C. A novel method to control carryover contamination in isothermal nucleic acid amplification. Chem. Commun. 2017;53:10696–10699. doi: 10.1039/C7CC06469A. [DOI] [PubMed] [Google Scholar]
- 118.Deng P.C., Zhang R.J., Liang Y., Zhang Y.K., Yang Q.L. Detection of Schistosoma japonicum—Infected Oncomelania hupensis by loop—Mediated isothermal amplification. Chin. J. Vet. Sci. 2009;29:1017–1018+1027. (In Chinese) [Google Scholar]
- 119.Yang Q.L., Xu L.F., Zhang Y.K., Wang K.G. Detection of Schistosoma japonicum cercaria DNA by loop-mediated isothermal amplification. Chin. J. Schistosomiasis Control. 2008;20:209–211. (In Chinese) [Google Scholar]
- 120.Yu C.X., Yin X.R., Hua W.Q., Gao Q. Establishment of loop mediated isothermal DNA amplification for identifying oncomelania snails infected with Schistosoma japonicum. J. Pathog. Biol. 2008;3:661–664. (In Chinese) [Google Scholar]
- 121.Yu C.X., Yin X.R., Wang J., Song L.J., Qian C.Y., Wu F., He W., Zhang W. Creation and use of a LAMP kit to rapidly detect snails infected with Schistosoma japonicum. J. Pathog. Biol. 2011;6:121–124. (In Chinese) [Google Scholar]
- 122.Feng J.T., Xing W.W., Sun K., Yu X.L., Luo Z.H., Mao J.W., Xu D.G. Application of visible loop-mediated isothermal amplification (LAMP) technologies in detecting oncomelania infected with Schistosoma japonicum. Mil. Med. Sci. 2016;40:133–136. (In Chinese) [Google Scholar]
- 123.Xu J., Guan Z.X., Zhao B., Wang Y.Y., Cao Y., Zhang H.Q., Zhu X.Q., He Y.K., Xia C.M. DNA detection of Schistosoma japonicum: Diagnostic validity of a lamp assay for low-intensity infection and effects of chemotherapy in humans. PLoS Negl. Trop. Dis. 2015;9:e0003668. doi: 10.1371/journal.pntd.0003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang C., Chen L., Yin X., Hua W., Hou M., Ji M., Yu C., Wu G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasites Vectors. 2011;4:164. doi: 10.1186/1756-3305-4-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang C., Chuan-Xin Y.U., Min-Jun J.I., Song L.J., Yin X.R., Qian C.Y., Wang J., Guan-Ling W.U. Use of loop-mediated isothermal amplification (LAMP) to detect whole blood samples infected with Schistosoma japonicum. J. Pathog. Biol. 2010;5:749–753. (In Chinese) [Google Scholar]
- 126.Xiong C.R., Yin X.R., Song L.J., Shen S., Wang J., Gao G., Liu Q., Yu C.X., Yang K. Comparison of the effectiveness of loop-mediated isothermal DNA amplification (LAMP) and mocroscopic dissection at detecting snails infected with Schistosoma japonicum. J. Pathog. Biol. 2014;9:1084–1087. (In Chinese) [Google Scholar]
- 127.Li Y.L., Dang H., Guo S.Y., Cao C.L., Lü S., Xu J., Li S.Z. National surveillance of Oncomelania hupensis in China. Chin. J. Schistosomiasis Control. 2021;33:127–132. doi: 10.16250/j.32.1374.2020349. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 128.Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS Biol. 2006;4:e204. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J.A., Macdonald J.A., von Stetten F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. 2018;144:31–67. doi: 10.1039/C8AN01621F. [DOI] [PubMed] [Google Scholar]
- 130.Lobato I.M., O’Sullivan C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends Analyt. Chem. 2018;98:19–35. doi: 10.1016/j.trac.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang S.L., Wang L.P., Wu L.L., Li Y.L., Zhang L.J., Lü S., Xu J. Diagnostic value of nucleic acid detection in schistosomiasis japonica: A meta-analysis. Chin. J. Schistosomiasis Control. 2020;32:15–22. doi: 10.16250/j.32.1374.2019174. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 132.Song Z., Ting L., Kun Y., Wei L., Jian-Feng Z., Li-Chuan G., Yan-Hong L., Yang D., Qing-Jie Y., Hai-Tao Y. Establishment of a recombinase-aided isothermal amplification technique to detect Schistosoma japonicum specific gene fragments. Chin. J. Schistosomiasis Control. 2018;30:273–277. doi: 10.16250/j.32.1374.2018120. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 133.Zhao S., Liu Y.H., Li T., Li W., Zhang J.F., Guo L.C., Ying Q.J., Yang H.T., Yang K. Rapid detection of Schistosoma japonicum specific gene fragment by recombinase aided isothermal amplification combined with fluorescent probe. Chin. J. Parasitol. Parasit. Dis. 2019;37:23–27. doi: 10.12140/j.issn.1000-7423.2019.01.005. (In Chinese) [DOI] [Google Scholar]
- 134.Ting L., Yan-Hong L., Song Z., Chun-Rong X., Xuan D., Jian-Feng Z., Wei L., Qing-Jie Y., Kun Y. Rapid detection of Schistosoma japonicum-infected snails with recombinase-aided isothermal amplification assay. Chin. J. Schistosomiasis Control. 2019;31:109–114. doi: 10.16250/j.32.1374.2019026. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 135.Dong X., Xiong C.R., Li T., Zhao S., Zhang J.F., Li W., Yang K. Early detection of Oncomelania snails infected eith Schistosoma japonicun by recombinase aided amplification. J. Pathog. Biol. 2019;14:1245–1249. doi: 10.13350/j.cjpb.191102. (In Chinese) [DOI] [Google Scholar]
- 136.Ye Y.Y., Zhao S., Liu Y.H., Zhang J.F., Xiong C.R., Ying Q.J., Yang K. Establishment of a nucleic acid dipstick test for detection of Schistosoma japonicum specific gene fragments based on the recombinase-aided isothermal amplification assay. Chin. J Schistosomiasis Control. 2021;33:334–338. doi: 10.16250/j.32.1374.2021016. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 137.Ye Y.Y., Zhao S., Liu Y.H., Bi N.N., Dong X., Xiong C.R., Zhu H.R., Tang F., Wang X.Y., Zhang J.F., et al. Performance of a recombinase-aided amplification assay for detection of Schistosoma japonicum infections in Oncomelania hupensis. Chin. J. Schistosomiasis Control. 2021;33:185–188. doi: 10.16250/j.32.1374.2020281. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 138.Deng W.P., Xu B., Hong Q.H., Wang S.L., Lv C., Li Y.L., Song S.P., Chen J.H., Xu J., Li S.Z. Establishment of the detection method for Schistosoma japonicum by recombinase polymerase amplification combined with electrochemical DNA biosensor. Chin. J. Parasitol. Parasit. Dis. 2020;38:42–48. doi: 10.12140/j.issn.1000-7423.2020.02.006. (In Chinese) [DOI] [Google Scholar]
- 139.Wang S.L., Deng W.P., Li Y.L., Wang L.P., Zhang L.J., Lv S., Xu J. Establishment of recombinase polymerase amplification technique for rapid detection of Schistosoma japonicum nucleic acid. Chin. J. Parasitol. Parasit. Dis. 2020;38:293–298. doi: 10.12140/j.issn.1000-7423.2020.03.006. (In Chinese) [DOI] [Google Scholar]
- 140.Deng W.P., Hong Q.H., Xu B., Wang S.L., Wang L.P., Xu J., Hu W., Zhou X.N. Development and preliminary evaluation of a rapid visualization detection method for circulating nucleic acids of Schistosoma japonicum based on RPA-LFD. Chin. J. Parasitol. Parasit. Dis. 2020;38:286–292. (In Chinese) [Google Scholar]
- 141.Stothard J.R., Stanton M.C., Bustinduy A.L., Sousa-Figueiredo J.C., Van Dam G.J., Betson M., Waterhouse D., Ward S., Allan F., Hassan A.A., et al. Diagnostics for schistosomiasis in Africa and Arabia: A review of present options in control and future needs for elimination. Parasitology. 2014;141:1947–1961. doi: 10.1017/S0031182014001152. [DOI] [PubMed] [Google Scholar]
- 142.Silva M.A., Medeiros Z., Soares C.R., Silva E.D., Miranda-Filho D.B., Melo F.L. A comparison of four DNA extraction protocols for the analysis of urine from patients with visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2014;47:193–197. doi: 10.1590/0037-8682-0233-2013. [DOI] [PubMed] [Google Scholar]
- 143.Van den Broeck F., Geldof S., Polman K., Volckaert F.A.M., Huyse T. Optimal sample storage and extraction procotols for reliable multilocus genotyping of the human parasite Schistosoma mansoni. Infect. Genet. Evol. 2011;11:1413–1418. doi: 10.1016/j.meegid.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Papaiakovou M.A., Pilotte N., Baumer B., Grant J., Asbjornsdottir K., Schaer F., Hu Y., Aroian R., Walson J., Williams S.A. A comparative analysis of preservation techniques for the optimal molecular detection of hookworm DNA in a human fecal specimen. PLoS Negl. Trop. Dis. 2018;12:e0006130. doi: 10.1371/journal.pntd.0006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.García-Bernalt Diego J., Fernández-Soto P.A., Crego-Vicente B., Alonso-Castrillejo S., Febrer-Sendra B., Gómez-Sánchez A., Vicente B., López-Abán J., Muro A. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: Towards a ready-to-use test. Sci. Rep. 2019;9:14744. doi: 10.1038/s41598-019-51342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rahman M.A., Sassa M., Parvin N., Islam M.R., Yajima A.A., Ota E. Diagnostic test accuracy for detecting Schistosoma japonicum and S. mekongi in humans: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021;15:e0009244. doi: 10.1371/journal.pntd.0009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Land K.A., Boeras D.I., Chen X.S., Ramsay A.R., Peeling R.W. Reassured diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019;4:46–54. doi: 10.1038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang H., Xu Y., Fohlerova Z., Chang H., Iliescu C., Neuzil P. Lamp-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. Trends Analyt. Chem. 2019;113:44–53. doi: 10.1016/j.trac.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shang Y., Sun J., Ye Y., Zhang J., Zhang Y., Sun X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2020;60:201–224. doi: 10.1080/10408398.2018.1518897. [DOI] [PubMed] [Google Scholar]
- 150.Ye X., Fang X., Li X., Kong J. Gold nanoparticle-mediated nucleic acid isothermal amplification with enhanced specificity. Anal. Chim. Acta. 2018;1043:150–157. doi: 10.1016/j.aca.2018.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.