Abstract
Environmental adversity increases child susceptibility to disrupted developmental outcomes, but the mechanisms by which adversity can shape development remain unclear. A translational cross-species approach was used to examine stress-mediated pathways by which poverty-related adversity can influence infant social development. Findings from a longitudinal sample of low-income mother–infant dyads indicated that infant cortisol (CORT) on its own did not mediate relations between early-life scarcity-adversity exposure and later infant behavior in a mother-child interaction task. However, maternal CORT through infant CORT served as a mediating pathway, even when controlling for parenting behavior. Findings using a rodent “scarcity-adversity” model indicated that pharmacologically blocking pup corticosterone (CORT, rodent equivalent to cortisol) in the presence of a stressed mother causally prevented social transmission of scarcity-adversity effects on pup social behavior. Furthermore, pharmacologically increasing pup CORT without the mother present was not sufficient to disrupt pup social behavior. Integration of our cross-species results suggests that elevated infant CORT may be necessary, but without elevated caregiver CORT, may not be sufficient in mediating the effects of environmental adversity on development. These findings underscore the importance of considering infant stress physiology in relation to the broader social context, including caregiver stress physiology, in research and interventional efforts.
Keywords: cortisol, corticosterone, early-life adversity, early-life stress, mother–infant, social transmission
Introduction
Decades of research have converged on findings that environmental adversity, such as low socioeconomic status (SES) and poverty, can “get under the skin” in early life to shape the development of infant behavior and physiology (Blair & Raver, 2016; Finegood, Rarick, & Blair, 2017; Frankenhuis & Nettle, 2019; Hackman & Farah, 2009; Herzberg & Gunnar, 2019; Lipina & Posner, 2012). Early development appears to be particularly vulnerable to the effects of adversity, as evidenced by sensitive period research involving both human studies (Davidson & McEwen, 2012; Feldman, 2015; Masten & Cicchetti, 2010) and animal experiments explicating underlying causal mechanisms (Cameron, 2001; Curley & Champagne, 2016; Opendak & Sullivan, 2016; Roth & Sweatt, 2011; Upton & Sullivan, 2010). However, the specific mechanisms underlying transmission of risk from poverty-related adversity exposure to the infant’s developmental processes remain poorly understood and are not well validated.
Chronic elevation of glucocorticoids (cortisol in humans, corticosterone in rodents, i.e. CORT) is an indicator of hypothalamic–pituitary–adrenal (HPA) axis system hyper-functioning and is one likely mechanism by which poverty-related risk can shape developmental trajectories starting in infancy. Across human and animal research, ample evidence illustrates that chronic elevation of stress hormones shapes the brain across levels of analyses – from gene activity to structural plasticity – to influence developmental outcomes (reviewed in McEwen & Gianaros, 2011; Perry & Sullivan, 2014). A consistent message across decades of research and from myriad multispecies models of early-life adversity is that chronic or frequently repeated stress exposure can lead to dysregulation of the HPA axis, mediating the effects of adversity on brain and behavioral development (Badanes, Watamura, & Hankin, 2011; Callaghan, Sullivan, Howell, & Tottenham, 2014; Dallman, 2007; Koss & Gunnar, 2018; Loman & Gunnar, 2010; McEwen, 1998).
Yet, very few studies (reviewed herein) have examined the mediating role of CORT as it relates to the transmission of poverty-related adversity to subsequent developmental outcomes. A psychophysiological measure of stress, which included CORT, has been shown to mediate relations between poverty status and socioemotional adjustment using cross-sectional data of 8- to 10-year-old children (Evans & English, 2002). Similarly, chronic physiological stress during childhood has been shown to longitudinally mediate the inverse relation between childhood poverty and working memory in adulthood (Evans & Schamberg, 2009). Similar longitudinal research has demonstrated that factors of early-life environmental risk predict elevated cortisol in early childhood which in turn is associated with decreased IQ (Suor, Sturge-Apple, Davies, Cicchetti, & Manning, 2015), decreased executive functions (Blair et al., 2011b), and increased risk for adjustment and social competence problems (Lengua et al., 2019).
Animal models of scarcity-adversity have also been used to experimentally demonstrate that environmental scarcity causally produces elevated CORT in mother rats and infant pups (Ivy, Brunson, Sandman, & Baram, 2008; Perry, Finegood, et al., 2019b; Raineki, Moriceau, & Sullivan, 2010; Raineki, Morgan, Ellis, & Weinberg, 2019), with subsequent disruption of neurobehavioral developmental outcomes (Avishai-Eliner, Gilles, Eghbal-Ahmadi, Bar-El, & Baram, 2001; Bale et al., 2010; Baram et al., 2012; Doherty, Blaze, Keller, & Roth, 2017; Junod, Opendak, LeDoux, & Sullivan, 2019; Perry et al., 2019a; Perry et al., 2019b; Perry et al., 2019c; Raineki, Rincón-Cortés, Belnoue, & Sullivan, 2012; Rincón-Cortés et al., 2015; Rincón-Cortés & Sullivan, 2016; Robinson-Drummer et al., 2019; Sevelinges et al., 2007; Walker et al., 2017). Taken together, these studies support the idea that chronically elevated CORT in infants and children may be one mechanism by which poverty-related adversity “gets under the skin” to influence child development. However, much remains unknown about how the effects of poverty-related risk are transmitted from the environment to the child to influence their stress physiology.
Of the few studies that have examined stress-mediated pathways for the transmission of poverty-related risk to child development, none that we are aware of have considered infant stress physiology together with caregiver stress physiology as a potential mediating pathway. However, the role of the caregiver has been increasingly examined as a potential mechanism of the social transmission of environmental risk to child outcomes. Decades of research have converged on findings that early-life stress, including poverty-related adversity, can impact child development through the quality of parenting behaviors (e.g., Blair & Raver, 2012; Brooks-Gunn & Duncan, 1997; Granero, Louwaars, & Ezpeleta, 2015; Hackman, Gallop, Evans, & Farah, 2015; Holochwost et al., 2016; Luby et al., 2013; McLoyd, 1998; Perry, Blair, & Sullivan, 2017; Perry et al., 2019b). Negative caregiving behaviors have also been modeled in nonhuman primates and rodents since the 1950s, with animal findings converging with human findings, ultimately demonstrating that child development is shaped by the quality of care received in infancy (reviewed in Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015; Perry et al., 2017). However, while research has predominantly focused on the behavioral components of caregiver-infant interactions as they pertain to the regulation and scaffolding of child development, far less has considered physiological components of caregiver-infant interactions as they relate to the social transmission of risk between caregiver and infant.
Those studies that have considered physiological mechanisms linking adversity to child development have typically only focused on the child’s physiology. For instance, it has been demonstrated that elevated salivary CORT across infancy partially mediates the effects of poverty and parenting on executive functions in early childhood (Blair et al., 2011b). Yet, several studies have also found associations between caregiver and child physiology over and above observed global measures of caregiver behavior (Braren, Perry, Ursache, & Blair, 2019; Halevi et al., 2017; Waters, West, & Mendes, 2014; Zelenko et al., 2005). In other words, the joint contribution of caregiver and infant stress physiology may explain variability in developmental outcomes that cannot be explained by caregiver behavior or infant stress physiology alone. Along these lines, it stands to reason that elevated child CORT may be necessary, but without elevated caregiver CORT, may not be sufficient in mediating the effects of environmental adversity on development. Rather, the relation between the caregiver’s and infant’s CORT may be a sufficient mediator of adversity on child development. This interpersonal association may be indicative of a route of social transmission of environmental risk from caregiver to child. Thus, in the present cross-species study, we evaluated not only infant CORT on its own, but also the association of maternal CORT with infant CORT and caregiving behavior (i.e., a social transmission pathway), as potential mediators of poverty-related risk and subsequent child behavior. Specifically, by integrating human and rodent research, we were better able to determine whether these potential mediators were necessary and/or sufficient for the transmission of environmental risk to developing offspring.
In examining physiological mechanisms by which poverty-related adversity might “get under the skin” to influence child development, human researchers face technical and research design restrictions that limit the ability to make causal inferences (Perry, 2019; Perry et al., 2019b). Specifically, in correlational studies, human researchers often rely on statistical tests of mediation that probe hypothetical causal relations between variables. However, as this is merely a statistical approach, in the absence of randomization and experimental manipulation of variables, there is no logically valid means by which to formally determine causality. Understandably, this is a normal limitation of correlational research. As such, experimental studies are needed to validly infer causal relations. However, as human research cannot experimentally control conditions of adversity, we often look to animal models for the discernment of causality and directionality of relations between adversity exposure and developmental outcomes, and to explore intermediary physiological mechanisms. To this end, in the present manuscript, we capitalize on both the ecological validity of human research and the experimental control of animal research to examine relations between early-life poverty-related adversity, mother and infant stress, and infant behavioral development. To do so, we utilized a well-validated model of early-life scarcity-adversity, whereby mother rats are provided with insufficient materials for nest-building, which produces aberrant maternal behavior, as well as an increase in both mother rats’ and infant rat pups’ basal CORT levels (Ivy et al., 2008; Perry et al., 2019b; Raineki et al., 2010; Raineki et al., 2019; Walker et al., 2017). Furthermore, this animal model of scarcity-adversity has previously been successfully integrated alongside human developmental research related to poverty, demonstrating translational validity of rodent findings as it relates to scarcity’s impact on caregiving behaviors and subsequent infant developmental outcomes (Perry et al., 2019a; Perry et al., 2019b). When carefully designed and considered within the context of human research findings, rodent models provide powerful means for efficient assessment of theory-based mechanisms of change. Moreover, integrative cross-species research enables the assessment of potential cause-effect mechanisms across multiple levels of analyses, while allowing for direct assessment of the translational relevance of mechanistic findings.
Thus, the purpose of the current study was to utilize a translational cross-species research approach to examine stress-mediated pathways by which poverty-related risk can influence infant development. To this end, we drew from the Family Life Project (FLP; n = 1,292), a prospective longitudinal study examining the impact of poverty on child development, as well as the rodent scarcity-adversity model. Our overarching hypothesis, across both species, was that elevated infant CORT levels would be a necessary component linking environments of risk to developmental outcomes. We first tested this hypothesis in humans using data from the FLP. Specifically, we first examined if infant chronic CORT levels across multiple time points, when considered on their own, were sufficient in mediating the relation between scarcity-adversity exposure and infant behavioral development. Second, to probe for evidence of social transmission of risk between the mother and child, we assessed if caregiver maternal CORT levels through infant CORT levels serially mediated the relation between poverty-related exposure and infant behavior. We then utilized the rodent model to challenge the causal nature of scarcity-adversity, mother–infant CORT, and child behavior relations indicated in our human data. Specifically, using a pharmacological manipulation, we tested if elevated pup CORT, independent of maternal influences, was causally sufficient for the disruption of pup social behavior. Furthermore, we tested the causal, necessary mediating role of pup CORT in the presence of a stressed mother for the social transmission of scarcity-adversity effects on pup social behavior. By using a cross-species research approach, we aim to scientifically advance mechanistic research related to poverty and child development.
Method
Human
Participants
Data came from the FLP, a prospective longitudinal, population-based, observational study of 1,292 children and their families living in predominantly low-income, nonurban communities in central Pennsylvania (PA) and eastern North Carolina (NC). Families were recruited locally in hospitals after the birth of the target child, with consent from the primary caregiver, most of whom were biological mothers. Low-income households were oversampled in both states and for African American families in NC. Further details regarding the recruitment process and sampling plan have been previously published elsewhere (Vernon-Feagans & Cox, 2013). The data presented here were collected during a series of home visits to the families when the target child was approximately 6, 15, 24, and 36 months old. Home visits were conducted by two trained research assistants, with each visit lasting 2–3 hr., including semi-structured interviews and self-reported measures assessing household characteristics and family demographics, as well as assessment of infant and caregiver behavior in a mother-child interaction task, and primary caregivers’ and infants’ CORT levels. For the current analysis, only data from children and their primary caregivers who were biological mothers were used. In addition, participants were included in the analyses if the mother and child both had at least one CORT sample and were not missing data on the main dependent variable (child engagement during the mother-child interaction at 36 months). Table 1 shows descriptive statistics of the sample used in the present analysis.
Table 1.
Demographics and descriptive statistics for observed study variables
| Variable | N | Mean or % | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Race (% African American) | 977 | 44% | |||
| Sex (% male) | 977 | 49% | |||
| State of residence (% NC) | 977 | 59% | |||
| Child age at 36 months (in years) | 977 | 3.08 | 0.14 | 2.87 | 3.94 |
| Scarcity-adversity (6 months) | 961 | .007 | 0.63 | −2.51 | 2.17 |
| Income-to-needs ratio (6 months) | 876 | 1.90 | 1.67 | 0.00 | 16.47 |
| Economic strain (6 months) | 951 | 11.57 | 3.66 | 1.00 | 22.00 |
| Maternal education (6 months) | 961 | 14.48 | 2.82 | 6.00 | 22.00 |
| Consistent partner (6 months) | 961 | 62% | |||
| Neighborhood safety (6 months) | 961 | 2.99 | 0.58 | 1.00 | 4.00 |
| Household density (6 months) | 961 | 0.88 | 0.36 | 0.36 | 3.33 |
| Child CORT (6 months, ln μg/dL) | 899 | −1.88 | 0.70 | −3.91 | 0.22 |
| Child CORT (15 months, ln μg/dL) | 828 | −1.99 | 0.75 | −4.27 | 0.48 |
| Child CORT (24 months, ln μg/dL) | 818 | −2.06 | 0.73 | −4.27 | 0.25 |
| Maternal CORT (6 months, ln μg/dL) | 920 | −1.78 | 0.67 | −3.61 | 0.44 |
| Maternal CORT (15 months, ln μg/dL) | 773 | −2.23 | 0.69 | −4.71 | 0.60 |
| Maternal CORT (24 months, ln μg/dL) | 752 | −2.08 | 0.70 | −3.99 | 0.62 |
| Time of day for CORT Collection (6 months, hh:mm) | 950 | 13:31 | 2:53 | 8:12 | 20:05 |
| Time of day for CORT Collection (15 months, hh:mm) | 953 | 13:57 | 2:55 | 8:50 | 21:03 |
| Time of day for CORT collection (24 months, hh:mm) | 932 | 13:52 | 3:13 | 8:20 | 20:46 |
| Negative parenting (6 months) | 918 | 2.42 | 0.77 | 1.00 | 5.00 |
| Negative parenting (15 months) | 921 | 2.27 | 0.70 | 1.00 | 5.00 |
| Negative parenting (24 months) | 909 | 2.43 | 0.87 | 1.00 | 5.00 |
| Child persistence (36 months) | 977 | 3.29 | 0.89 | 1.00 | 5.00 |
| Child enthusiasm (36 months) | 977 | 3.13 | 0.87 | 1.00 | 5.00 |
| Child compliance (36 months) | 977 | 3.90 | 0.90 | 1.00 | 5.00 |
Scarcity-adversity exposure
Early-life scarcity-adversity exposure was assessed through a poverty-related risk index, as used in prior analyses of the FLP (Perry et al., 2019b; Vernon-Feagans & Cox, 2013). This cumulative risk index was computed as a composite of six indicators measured at 6 months (family income-to-needs ratio, neighborhood safety, maternal education, economic strain, household density, and consistent partnership of a spouse/partner living within the home). Specifically, the continuous cumulative risk index was generated by reverse-scoring positively-framed indicators, standardizing each measure, and then averaging the standardized variables. Correlation coefficients between the six indicators included in the cumulative risk index ranged from r = .13 to .53, p < .001.
Child behavior in a mother-child interaction task
Child behaviors during play interactions with their mothers were videotaped during the 36-month home visits. During a 10-minute task, mothers and children were asked to complete a set of three puzzles of increasing difficulty selected to appropriately match cognitive and motor skills at 36 months of age. The task was video recorded, and child behavior was coded for multiple subscales on an adapted parent-child interaction coding system developed for use by the Study of Early Child Care and Youth Development (Cox, 1997; NICHD ECCRN, 1999), including enthusiasm, persistence, and compliance subscales. The enthusiasm subscale refers to the child’s eagerness and agency regarding the task. The persistence subscale refers the child’s concentration on and perseverance in the puzzle task. The compliance subscale refers to the child’s willingness to comply with and follow out the parent’s instructions during the task. The child’s behavior was coded by highly trained raters on each subscale and rated between 1–5, with 1 indicating “not at all characteristic” and 5 indicating “highly characteristic.” Coders were trained by a master coder until acceptable reliability was achieved, as determined by intra-class correlation coefficients greater than 0.80. After establishing reliability, coders worked in pairs to complete a minimum of 30% of the same videos as their master coder. Coding pairs met biweekly to resolve scoring differences, and consensus codes were used for analysis.
Mother and infant cortisol
Mother and infant cortisol levels were assayed from saliva samples collected near the end of the 6-, 15-, and 24-month home visits. Samples were collected after data collectors had been in the home for a minimum of one hour, allowing the caregivers’ and infants’ cortisol levels more than sufficient time to return to baseline following arrival of the data collectors. Baseline saliva samples were collected immediately preceding administration of emotion induction tasks as an index of resting or basal levels of CORT. Unstimulated whole saliva was collected using cotton or hydrocellulose absorbent material and stored in 2 mL cryogenic vials using a needleless syringe (from cotton) or by centrifugation (from hydrocellulose). Samples were then immediately placed on ice and stored at −20°C. Samples of 25 μL of saliva were assayed in duplicate using a highly sensitive immunoassay (Salimetrics, State College, PA). The test had a range of sensitivity from 0.007 to 3.0 μg/dl, and average intra- and inter-assay coefficients of variation less than 10% and 15%, respectively. The average of duplicates was used in all analyses. Natural log transformations were applied to the cortisol values to correct for positive skew, and cortisol values greater than ±3 SD after transformation were excluded from analyses. Because home visits were scheduled at times most convenient for the families, time of day for saliva collection varied between families, although the majority of families were seen in the afternoon (Table 1). Prior research demonstrates that afternoon cortisol is more related to environmental (vs. genetic) factors and thus may serve as a more reliable bio-marker of environmental stress (Schreiber et al., 2006; Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012). Time of day of saliva collection was controlled for in all analyses.
Covariates
Observed variables for state of residence (PA = 0, NC = 1), race of infant (not African American = 0, African American = 1), sex of infant (male = 0, female = 1), age of infant, and time of day for each saliva collection timepoint were included as covariates in all models. For models incorporating maternal CORT levels, observed variables for body mass index (BMI) of the mother, duration of breastfeeding, tobacco usage, and pregnancy status, and negative parenting behavior were also included as covariates. Negative parenting behavior at 6-, 15-, and 24-months was captured during a mother–child play task. Specifically, highly trained coders scored mother-child interactions from video recordings, scoring mothers’ intrusiveness, detachment, and negative regard, with each dimension of behavior coded from 1 (“not at all characteristic”) to 5 (“highly characteristic”) (Cox, 1997; NICHD ECCRN, 1999). Negative parenting was calculated as the average of intrusiveness, detachment, and negative regard at each timepoint. A latent variable of negative caregiving behavior at 6, 15, and 24 months was included as the final analysis covariate.
Statistical analyses
Descriptive analyses and correlations were conducted using IBM SPSS Statistics software (Version 21.0). Structural equation modeling was used to test direct effects, mediation, and serial mediation, using the bootstrapping procedure (5,000 bootstraps) (Shrout & Bolger, 2002) in Mplus 7 software (Muthén & Muthén, 1998–2012). Specifically, structural equation modeling was used to examine relations between early-life scarcity-adversity exposure, measures of infant and maternal CORT, and child behavior in the mother-child interaction task in the FLP. To capture a more chronic index of CORT levels, and to improve measurement reliability, latent variables of infant and caregiver CORT were created from observed basal levels of CORT at the 6-, 15-, and 24-month home visits. To ensure CORT levels at each time point were given an equal weight, factor loadings were constrained to equality when creating the latent variables. Furthermore, child engagement during the mother-child interaction task at 36 months was modeled as a latent variable using infant enthusiasm, persistence, and compliance as indicators (Skinner, Kindermann, Connell, & Wellborn, 2009). Parameter estimates are reported as standardized effects (Preacher & Hayes, 2008). The comparative fit index (CFI), standardized root square mean residual (SRMR), and root mean squared error of approximation (RMSEA) fit indices were used to evaluate model fit, with CFI ≥ 0.95, SRMR < 0.08, and RMSEA < 0.05 indicating good model fit (Kline, 2015). Raw data of this manuscript will be made available by the authors without reservation to any qualified researcher.
Rodent
Subjects
Long-Evans hooded rat pups were generated through breeding in a temperature (20°C), humidity, and light (12-hour light/dark cycle) controlled animal room within the Sullivan Lab, where they continued to live throughout the experiment with ad lib food and water. The pregnant dams were placed alone in their own polypropylene cages (34 cm × 29 cm × 17 cm) with wood chips approximately one week before giving birth. Litters were culled on PN (postnatal day) 1 (day of birth is PN 0) to 12 pups per litter with an approximately equal number of male and female pups. Cages were cleaned twice a week; however, the nest was retained and placed into the cleaned cage. All procedures pertaining to the use of live rats were approved by the Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
Scarcity-adversity model
Litters were randomly assigned into control or scarcity-adversity conditions on PN 8. In control-reared litters, mothers were given ample bedding materials (4500 mL of wood shavings), which permitted construction of a nest for her pups. In scarcity-adversity conditions, bedding materials were reduced to 100 mL, producing a thin 1 cm layer of bedding. Depriving the mother of nest-making materials is stressful to both the mother (Ivy et al., 2008) and pups (Raineki et al., 2019), as evidenced by heightened CORT production. As such, as previously reported, the impoverished cage environment produced aberrant maternal care, including frequent unsuccessful attempts to build a nest and increased incidental rough treatment of the pups (Raineki et al., 2010; Raineki et al., 2019; Rincón-Cortés & Sullivan, 2016), as well as disruption of neurobehavioral development in pups (Perry et al., 2019b, Roth & Sullivan, 2005; Walker et al., 2017). Furthermore, we have previously demonstrated that our rodent model of scarcity-adversity has translational validity pertaining to at least some aspects of the human condition of poverty, including scarcity-adversity exposure, parenting behaviors, and infant development (Perry et al., 2019b). Scarcity-adversity exposure occurred for 1 hour a day from PN 8–12, which has been validated to induce stressed maternal behavior and disrupt pup neural and behavioral development (Asok, Bernard, Roth, Rosen, & Dozier, 2013; Blaze & Roth, 2013). Videos were recorded and hand-scored by highly trained raters to validate that the adversity-rearing treatment induced rough handling by mothers (Figure 1).
Figure 1.
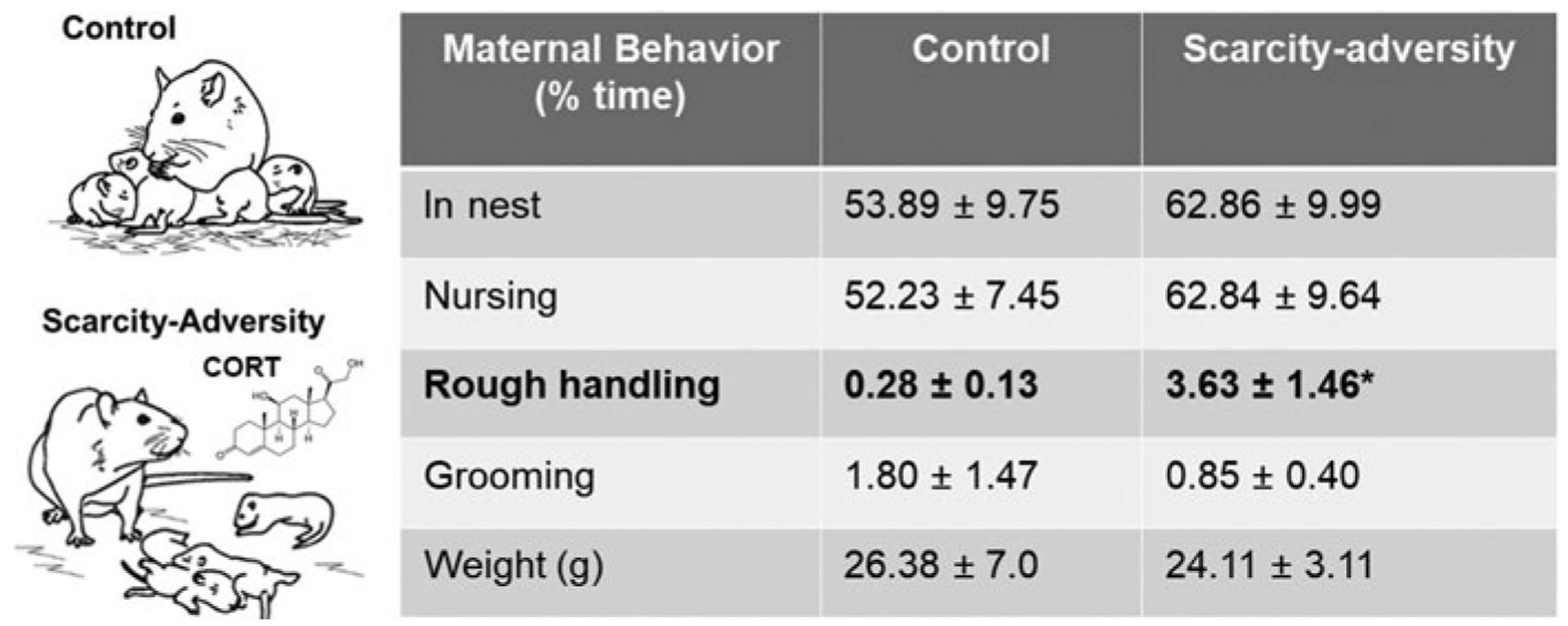
Depiction of rodent model of scarcity-adversity. Rodents are randomly assigned into control or low bedding (scarcity-adversity) conditions. Limiting the mother’s access to bedding needed for nest-building increases maternal and pup corticosterone (CORT) levels, and rough handling of pups (t(6) = −3.34, p = .02).
Drug manipulations
To pharmacologically elevate pup CORT levels independent of the mother rat’s stress levels, pups received daily (from PN 8–12) administration of corticosterone (CORT) 2-hydroxypropyl-β-cyclodextrin complex (3 mg/kg; Sigma) or an equal volume of saline 30 minutes before being placed (without the mother) in a chamber with a polyethylene tube for 90 minutes per day. To block CORT changes associated with the scarcity-adversity rearing, pups received intraperitoneal injections of the corticosterone inhibitor metyrapone HCL (50 mg/kg, Sigma) or an equal volume of 0.9% saline. Pups received daily metyrapone or saline injections 90 minutes before scarcity-adversity exposure and were returned to their home cage 90 minutes after the injection. Timing of this procedure limited CORT reduction effects to within the daily window of scarcity-adversity exposure. Prior work by Sullivan lab and others has shown that metyrapone reliably reduces pup corticosterone levels by 55%–75% in this age range, and that the drug effect is gone after 90 minutes (Faturi et al., 2010; Raineki et al., 2019; Rosenfeld et al.,1992; Suchecki, Nelson, Van Oers, & Levine, 1995; Upton & Sullivan, 2010).
Infant behavior test with mother after 5 days of treatment
Pup behavior was tested 24 hours after the last drug injection. Specifically, after 5 days of scarcity-adversity rearing paired with CORT blockade, or 5 days of CORT increase without the mother present, pup behavior with the mother was tested using a semi-structured social behavior test with an anesthetized mother. The use of an anesthetized mother facilitates the analysis of pups’ behavior without interference from the mother’s scaffolding of pups’ behavior. The mother was placed on her side in a polypropylene cage (34 cm × 29 cm × 17 cm; 2,000 mL bedding), so that the pups had access to her nipples. The pup was then placed on the opposite side of the cage and allowed to freely behave during the 5-minute test. Video recordings of the interactions were scored offline by highly trained raters blind to rearing conditions or drug manipulations. The pup behaviors measured were categorized as pup engagement with the mother (i.e. crawl to mother’s ventrum, nipple attachment) or disengagement (i.e. pup sleeping/laying alone, pup behind the mother).
Statistical analysis
Rodent data were analyzed by two-way analysis of variance (ANOVA), with Sidak’s multiple comparisons tests. Data used for figure-making are expressed as mean ± standard error of the mean (SEM) in all cases. Group differences were considered statistically significant when p < .05.
Results
Human
Descriptive statistics and correlations
Descriptive statistics and bivariate correlations among all analysis variables are presented in Tables 1 and 2, respectively. Early-life scarcity-adversity exposure was positively associated with both child and maternal CORT levels across the infant’s first 2 years of life. Scarcity-adversity at 6 months was also negatively associated with measures of child engagement in the mother-child interaction task at 36 months, including infant persistence and enthusiasm. Furthermore, infant CORT measures at 6, 15, and 24 months, as well as maternal CORT measures at 6, 15, and 24 months, were negatively associated with measures of child engagement in the mother-child interaction task. Finally, child and maternal CORT levels were positively correlated with one another at the 6-, 15-, and 24-month timepoints. This pattern of significant associations supported testing of the mediating models presented in Figures 2 and 4.
Table 2.
Correlations among analysis variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Scarcity-Adversity (6 mos) | 1 | ||||||||||||||||
| 2. Child CORT (6 mos) | 0.11** | 1 | |||||||||||||||
| 3. Child CORT (15 mos) | 0.15** | 0.02 | 1 | ||||||||||||||
| 4. Child CORT (24 mos) | 0.14** | 0.13** | 0.10* | 1 | |||||||||||||
| 5. Maternal CORT (6 mos) | 0.11** | 0.29** | 0.08* | 0.14** | 1 | ||||||||||||
| 6. Maternal CORT (15 mos) | 0.12** | 0.03 | 0.24** | 0.11** | 0.23** | 1 | |||||||||||
| 7. Maternal CORT (24 mos) | 0.15** | 0.06 | 0.09* | 0.33** | 0.25** | 0.19** | 1 | ||||||||||
| 8. Time of Day (6 mos) | −0.13** | −0.27** | −0.03 | −0.07 | −0.48** | −0.06 | −0.06 | 1 | |||||||||
| 9. Time of Day (15 mos) | −0.12** | −0.13** | −0.20** | −0.09* | −0.19** | −0.46** | −0.10** | 0.24** | 1 | ||||||||
| 10. Time of Day (24 mos) | −0.18** | −0.13** | −0.06 | −0.34** | −0.15** | −0.11** | −0.51** | 0.23** | 0.23** | 1 | |||||||
| 11. Persistence (36 mos) | −0.24** | −0.10** | −0.09* | −0.13** | −0.08* | −0.11** | −0.09* | 0.08* | 0.06 | 0.14** | 1 | ||||||
| 12. Enthusiasm (36 mos) | −0.18** | −0.08* | −0.03 | −0.07* | −0.11** | −0.08* | −0.06 | 0.07* | 0.06 | 0.08* | 0.54** | 1 | |||||
| 13. Compliance (36 mos) | −0.03 | −0.02 | 0.01 | −0.03 | −0.04 | −0.01 | −0.02 | −0.01 | −0.01 | 0.03 | 0.43** | 0.34** | 1 | ||||
| 14. Negative Parenting (6 mos) | 0.34** | 0.06 | 0.04 | 0.10** | 0.03 | 0.03 | 0.03 | −0.04 | −0.01 | −0.06 | −0.06 | −0.01 | −0.01 | 1 | |||
| 15. Negative Parenting (15 mos) | 0.32** | 0.04 | 0.05 | 0.05 | 0.01 | 0.05 | 0.04 | 0.01 | −0.02 | −0.10** | −0.15** | −0.11** | −0.12** | 0.40** | 1 | ||
| 16. Negative Parenting (24 mos) | 0.38** | 0.06 | 0.12** | 0.14** | 0.04 | 0.12** | 0.01 | −0.06 | −0.07* | −0.10** | −0.28** | −0.17** | −0.19** | 0.37** | 0.41** | 1 | |
| 17. Child Age (36 mos) | −0.03 | −0.03 | −0.01 | −0.05 | −0.02 | 0.01 | −0.01 | −0.01 | 0.06 | 0.10** | 0.11** | 0.02 | 0.03 | 0.04 | −0.01 | 0.03 | 1 |
Note: mos, months;
p < 0.01,
p < 0.05
Figure 2.
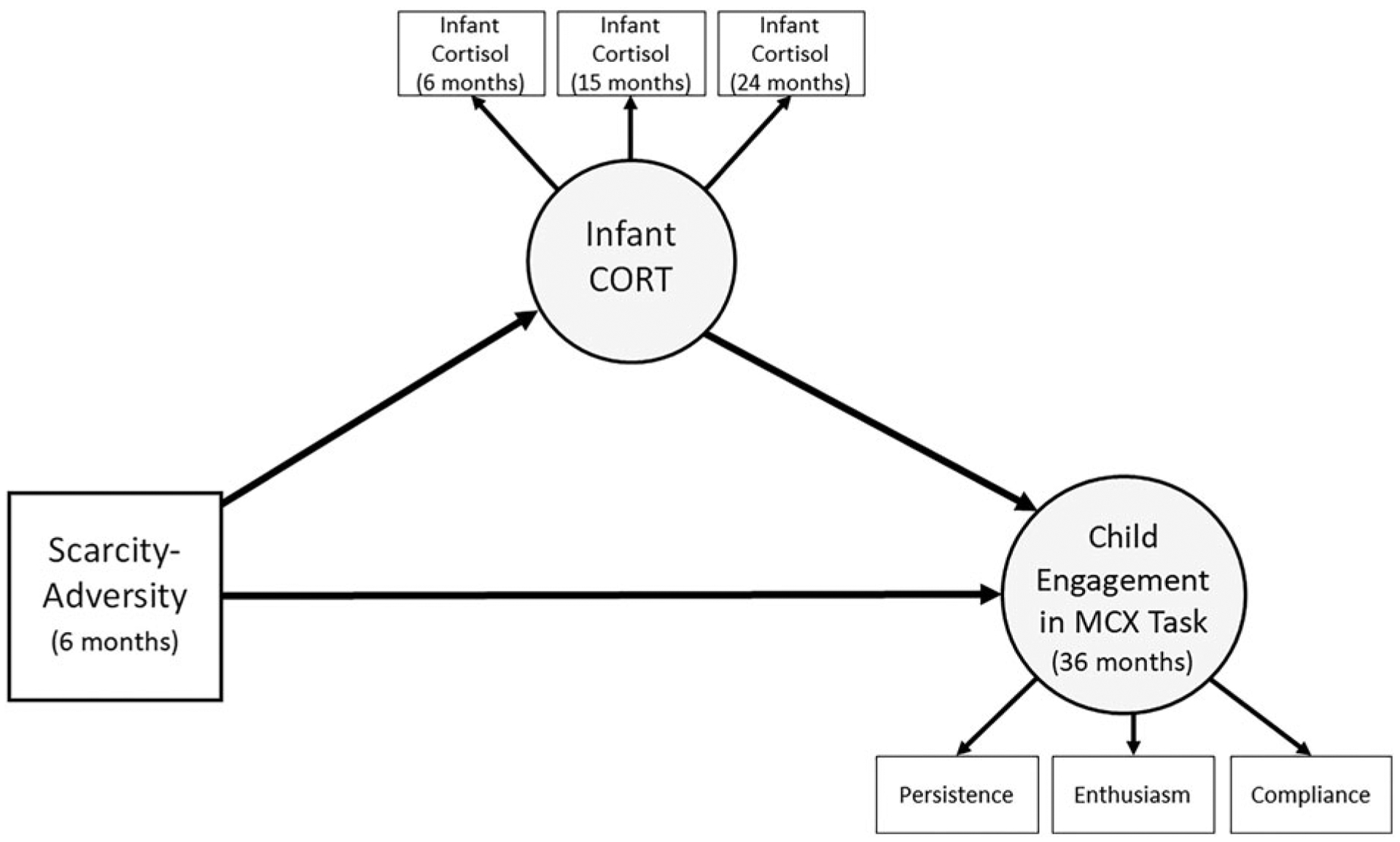
Proposed structural mediation model relating scarcity-adversity (6 months), infant cortisol (CORT; 6, 15, and 24 months), and child engagement in a mother-child interaction task. MCX = mother-child interaction task. Covariates are not shown.
Figure 4.
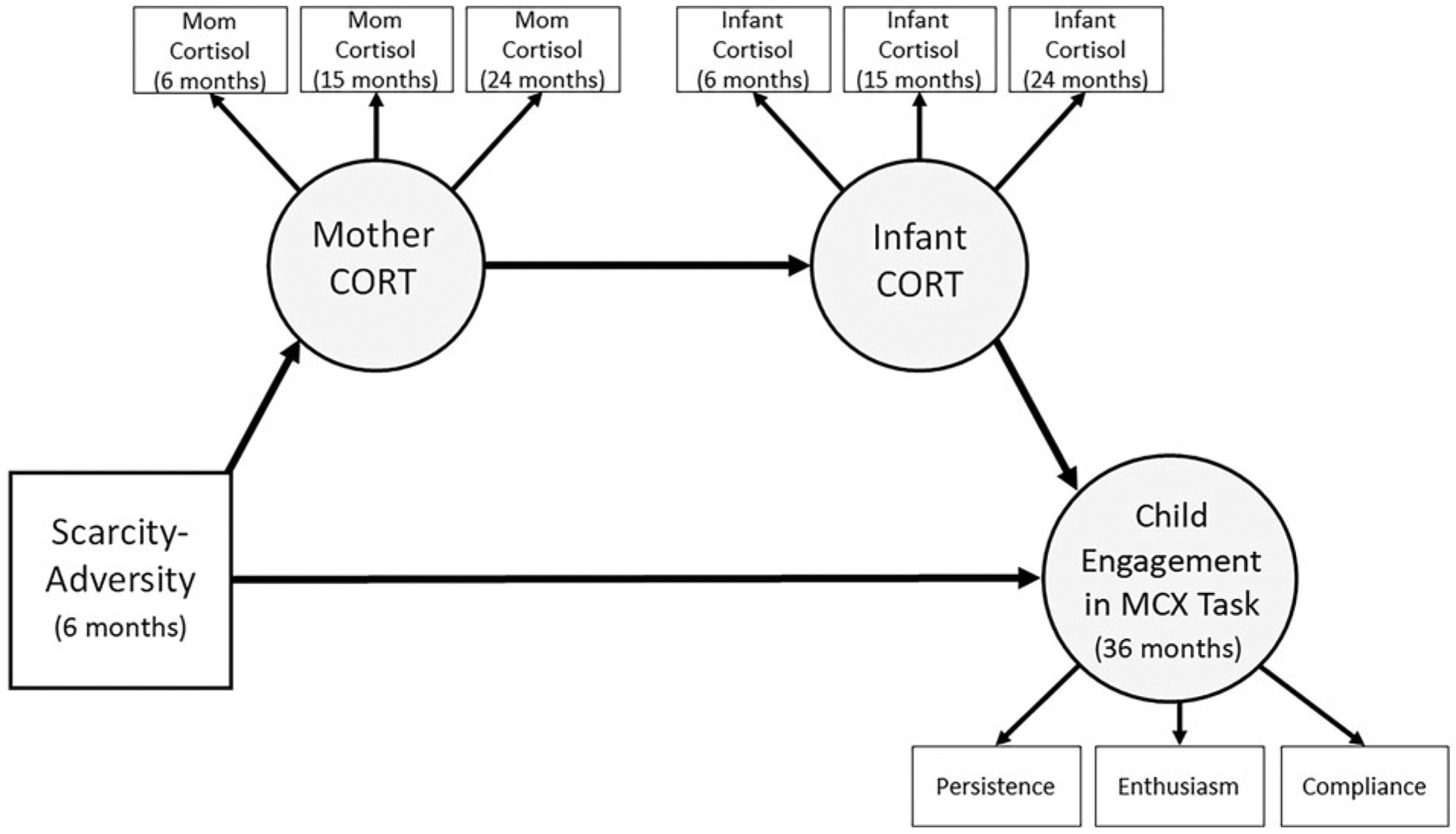
Proposed structural serial mediation model relating scarcity-adversity (6 months), maternal cortisol (CORT; 6, 15, 24 months), infant CORT (6, 15, 24 months), and child engagement in a mother-child interaction task (36 months). MCX = mother-child interaction task. Covariates are not shown. Circles represent latent variables.
Measurement model
Prior to addressing our main research questions, we evaluated our measurement model, which included infant CORT, maternal CORT, and negative parenting at infant ages 6, 15, and 24 months, as well as child engagement in the mother-child interaction task at 36 months as latent variables. The measurement model showed good fit to the data: CFI = 0.95; RMSEA = 0.03 (90% confidence interval [CI] [0.027, 0.041]); SRMR = 0.04. Factor loadings of observed indicators on latent variables are reported in Table 3. Parameter estimates indicated that loadings were statistically significant and in the anticipated direction, and that all latent variances were statistically significant.
Table 3.
Loadings of observed indicators on latent variables
| Latent Variable | Indicators | β |
|---|---|---|
| Infant CORT | Infant basal CORT (6 months) | 0.29 |
| Infant basal CORT (15 months) | 0.27 | |
| Infant basal CORT (24 months) | 0.28 | |
| Maternal CORT | Maternal basal CORT (6 months) | 0.42 |
| Maternal basal CORT (15 months) | 0.41 | |
| Maternal basal CORT (24 months) | 0.40 | |
| Negative parenting | Negative parenting (6 months) | 0.54 |
| Negative parenting (15 months) | 0.62 | |
| Negative parenting (24 months) | 0.69 | |
| Child engagement in MCX | Persistence (36 months) | 0.85 |
| Enthusiasm (36 months) | 0.64 | |
| Compliance (36 months) | 0.52 |
Note. All coefficients are standardized and significant at p < .0001. CORT, cortisol; MCX = Mother×Child interaction task.
Prior to testing mediation, in three separate models, we also evaluated independent, direct associations with early-life scarcity-adversity predicting infant CORT across 6, 15, and 24 months, maternal CORT across 6, 15, 24 months, and child engagement at 36 months. As expected, scarcity-adversity negatively predicted child engagement in the mother–child task at 36 months (β = −0.17, SE = 0.05, p = .001). Scarcity-adversity also positively predicted infant CORT (β = 0.28, SE = 0.09, p = .002) and maternal CORT (β = 0.07, SE = 0.02, p = .002). Finally, both infant CORT (β = −0.22, SE = 0.11, p = .04) and caregiver CORT (β = −0.22, SE = 0.11, p = .04) negatively predicted child engagement in the mother–child interaction task.
Is infant CORT on its own sufficient to mediate the impact of scarcity-adversity exposure on behavioral development?
Figure 2 displays our proposed structural model of infant CORT mediation (without covariates). In this model we estimated a mediation model that included scarcity-adversity exposure as assessed when the infant was 6 months of age, infant CORT (across 6, 15, and 24 months), and child engagement in the mother-child interaction task at 36 months. Specifically, we examined direct effects of scarcity-adversity and infant CORT on child engagement in the mother-child interaction task. Furthermore, we examined the indirect effect of scarcity-adversity on child engagement through infant CORT to determine if infant CORT mediated the effect of poverty-related scarcity-adversity on child engagement in the mother-child interaction task.
The observed structural model fit the data well: CFI = 0.97; RMSEA = 0.03 (90% CI [0.014, 0.035]); SRMR = 0.03 (Figure 3). All coefficients are presented as standardized estimates, reflecting changes in standard deviations (SDs) (e.g., for every 1 SD increase in scarcity-adversity levels, it is estimated that infant CORT increases by 0.28 SD). Scarcity-adversity was negatively associated with infant CORT across 6, 15, and 24 months. Furthermore, scarcity-adversity was negatively associated with child engagement in the mother-child interaction task. However, infant CORT was not significantly associated with child engagement.
Figure 3.
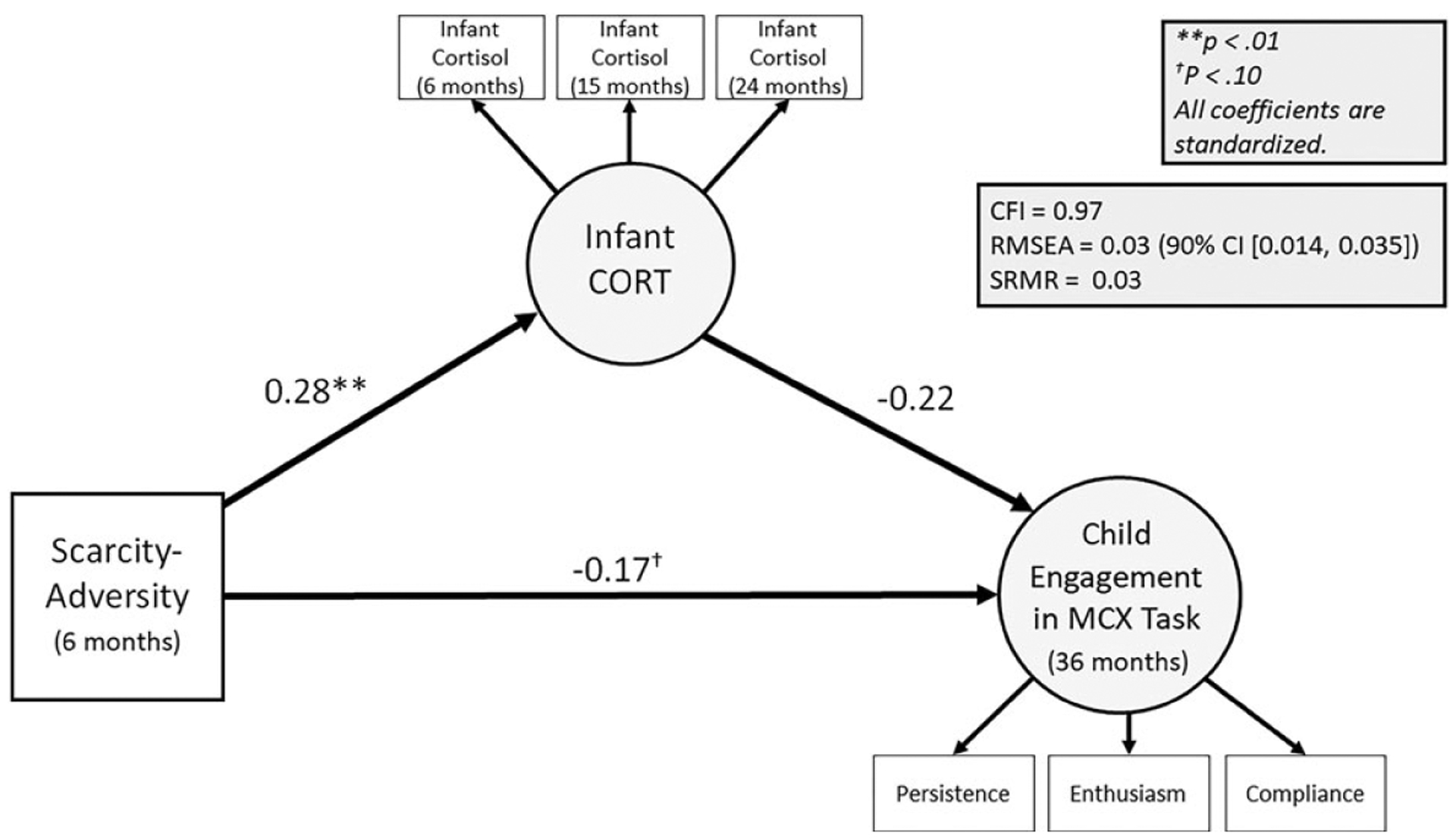
Results of mediation model relating scarcity-adversity (6 months), infant cortisol (CORT; 6, 15, 24 months), and child engagement in a mother-child interaction task (36 months). MCX = mother-child interaction task; CFI = comparative fit index; SRMR = standardized root square mean residual; RMSEA = root mean squared error of approximation. Covariates are not shown. Circles represent latent variables.
We hypothesized that infant CORT, when considered independently of maternal factors, would not be sufficient to mediate the relation between early-life scarcity-adversity and child engagement in the mother-child interaction task at 36 months. To evaluate this hypothesis, we tested the indirect effect of scarcity-adversity (6 months) on child engagement (36 months) via infant CORT (6, 15, 24 months). Table 4 displays the mediation results and indicates that infant CORT did not mediate the effects of scarcity-adversity on child engagement in the mother-Child interaction task.
Table 4.
Mediation analysis: direct and indirect effects of poverty-related scarcity-adversity on child engagement in mother–child interaction task
| Dependent Variables | ||||||
|---|---|---|---|---|---|---|
| Infant CORT | Child engagement in MCX | |||||
| Direct Effects | β | SE | p | β | SE | p |
| Scarcity-adversity | 0.28** | 0.10 | 0.01 | −0.17† | 0.09 | 0.07 |
| Infant CORT | – | – | – | −0.22 | 0.17 | 0.19 |
| Indirect Effect | β | SE | 95% CI | |||
| Scarcity-adversity → Infant CORT | – | – | – | −0.06 | 0.09 | (−0.31, 0.00) |
p < .01,
p < .10;
CORT, cortisol; MCX = mother-child interaction task; SE = standard error; CI = confidence interval. All coefficients are standardized.
Does social transmission of risk from mother to child mediate the impact of scarcity-adversity exposure on behavioral development?
Figure 4 displays our proposed structural model of social transmission (without covariates). We estimated a serial mediation model that included scarcity-adversity (6 months), maternal CORT (6, 15, 24 months), infant CORT (6, 15, 24 months), and child engagement in the mother×child interaction task (36 months). We examined direct effects of scarcity-adversity and infant CORT on child engagement in the mother-child interaction task, the direct effect of scarcity-adversity on maternal CORT, and the direct effect of maternal CORT on infant CORT. Furthermore, we examined the indirect effect of scarcity-adversity on child engagement in the mother-child interaction task via caregiver CORT through infant CORT.
The observed structural model fit was adequate: CFI = 0.93; RMSEA = 0.03 (90% CI [0.024, 0.033]); SRMR = 0.03 (Figure 5). Scarcity-adversity was negatively associated with maternal CORT across 6, 15, and 24 months. Furthermore, scarcity-adversity was negatively associated with child engagement in the mother-child interaction task. Maternal CORT was positively associated with infant CORT. Finally, infant CORT was negatively associated with child engagement in the mother-child interaction task.
Figure 5.
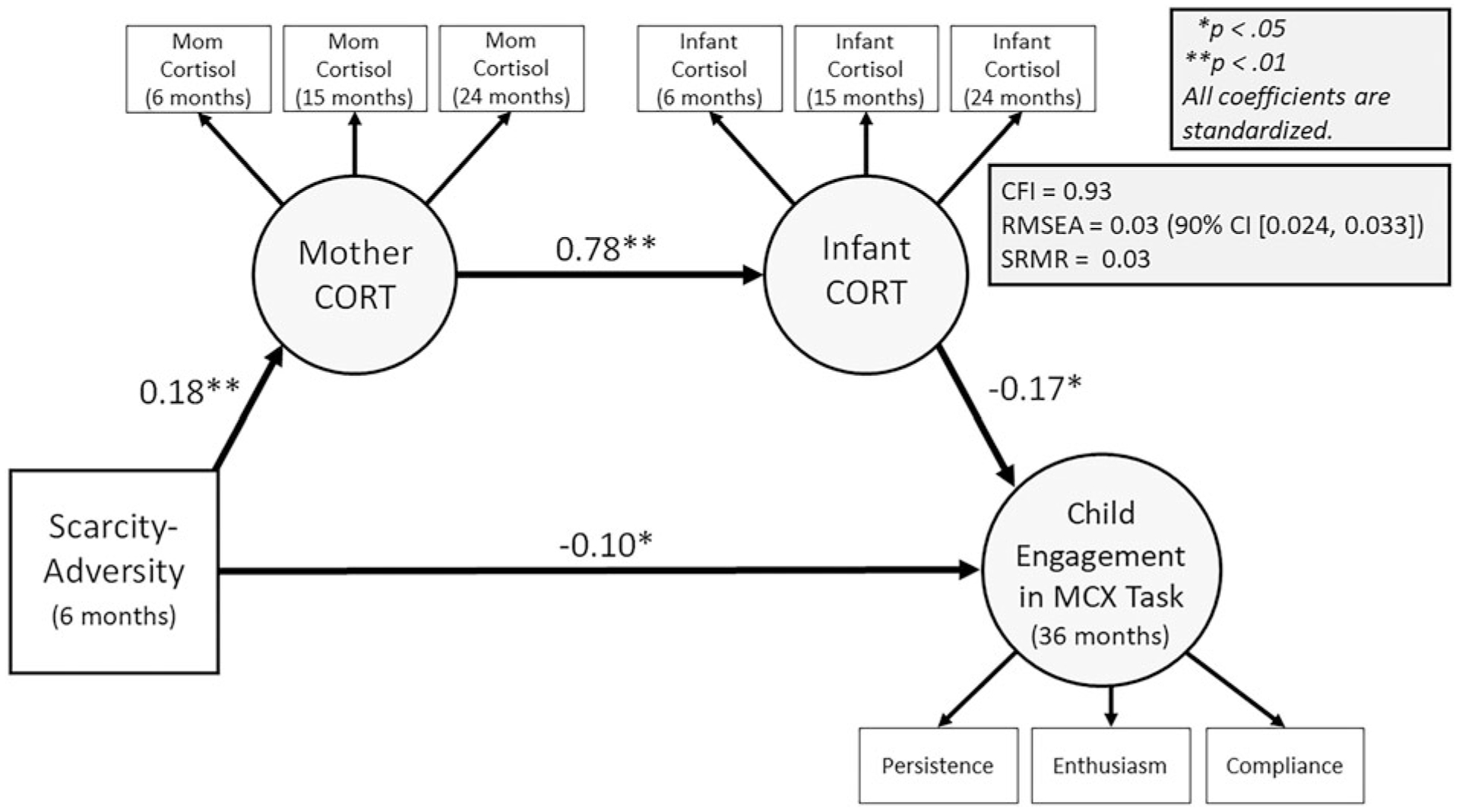
Results of serial mediation model relating scarcity-adversity (6 months), maternal cortisol (CORT; 6, 15, 24 months), infant CORT (6, 15, 24 months), and child engagement in a mother-child interaction task. MCX = mother-child interaction task; CFI = comparative fit index; SRMR = standardized root square mean residual; RMSEA = root mean squared error of approximation. Covariates are not shown. Circles represent latent variables.
We hypothesized that maternal CORT through infant CORT would serially mediate the relation between early-life scarcity-adversity and child engagement in the mother-child interaction task at 36 months. To evaluate this hypothesis, we tested the indirect effect of scarcity-adversity (6 months) on child engagement (36 months) via a serial pathway involving maternal CORT and infant CORT (6, 15, 24 months). Table 5 displays the serial mediation results and indicates that maternal CORT through infant CORT significantly mediated the effects of scarcity-adversity on child engagement in the mother-child interaction task, with this pathway mediating 26% of the total effect.
Table 5.
Serial mediation analysis: direct and indirect effects of poverty-related scarcity-adversity on child engagement in mother-child interaction task
| Dependent Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Infant CORT | Maternal CORT | Child engagement in MCX | |||||||
| Direct Effects | β | SE | p | β | SE | p | β | SE | p |
| Scarcity-adversity | – | – | – | 0.18** | 0.05 | <0.01 | −0.10* | 0.04 | 0.04 |
| Maternal CORT | 0.78** | 0.18 | <.01 | – | – | – | – | – | – |
| Infant CORT | – | – | – | – | – | – | −0.17* | 0.07 | <0.01 |
| Indirect Effect | β | SE | 95% CI | ||||||
| SA → Maternal CORT → Infant CORT | – | – | – | – | – | – | −0.03* | 0.01 | (−0.05, −0.01) |
Indicates that p < .05 or that confidence interval does not contain zero;
p < .01;
CORT, cortisol; MCX = mother-child interaction task; SE = standard error; CI = confidence interval; SA = scarcity-adversity. All coefficients are standardized.
Rodent
We next turned to the rodent model to evaluate causation in the relations between early-life scarcity-adversity, maternal and infant CORT, and child behavior indicated by the FLP data findings. Across a 5-day treatment, we tested if pharmacologically increasing pup CORT levels in the absence of the mother was causally sufficient for the disruption of pup social behavior with the mother (Figure 6a). Furthermore, we tested if causally disrupting social transmission from a stressed mother to pups – by pharmacologically blocking pups’ CORT in the presence of a stressed mother – would prevent negative effects on pup behavioral development despite scarcity-adversity exposure (Figure 7a). Results demonstrated that the 5-day CORT injection treatment during a brief separation from the mother was not causally sufficient to disrupt subsequent social behavior with the mother. Specifically, results of a t test did not reveal a significant difference in social engagement with the mother for pups pharmacologically injected with CORT versus pups injected with saline (Figure 6; t(8) = 0.04, p = .97). Furthermore, findings indicated that pharmacologically blocking pup CORT (via metyrapone [CORT synthesis inhibitor] injections) in the presence of a stressed mother prevented social transmission of scarcity-adversity effects on pup social behavior. Specifically, results of a 2 × 2 ANOVA indicated a significant interaction of drug (metyrapone vs. saline) and rearing experience (scarcity-adversity vs. control) on time spent engaged with the mother during the social behavior test (Figure 7; F(1,28) = 10.45, p = .003). Post hoc tests indicated that saline injected pups exposed to a stressed mother in scarcity-adversity conditions displayed decreased engagement with a mother, while the intraperitoneal injections of metyrapone (CORT blocker) during scarcity-adversity conditions normalized pup behavior in the social behavior test (post hoc tests, p < .05). Metyrapone injections did not significantly affect social behavior levels in control-reared pups (post hoc tests, p < .05).
Figure 6.
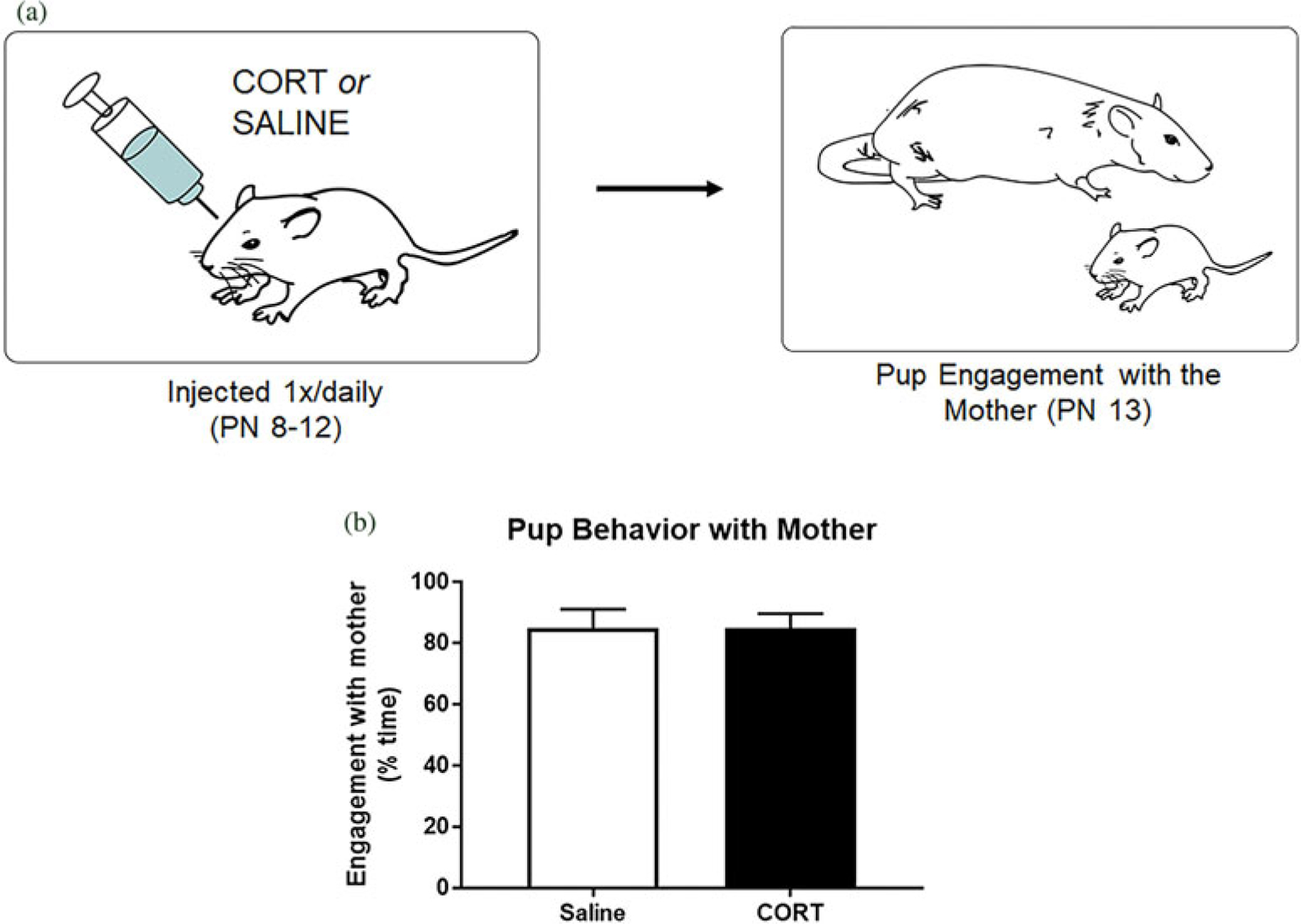
Pharmacologically elevating infant corticosterone (CORT) in the absence of the mother is not sufficient to disrupt subsequent social behavior with the mother. (a) Depiction of experimental procedure. (b) Pup engagement with mother in a social behavior test as a function of pharmacological manipulation (saline vs. CORT injection) during a brief separation from the mother. Data are displayed as mean (± SEM) percent time the pup spent socially engaged with the mother, as calculated from total time pups spent crawling to the mother, probing the mother, sleeping/laying on the mother, and nipple attached to the mother (n = 5/group).
Figure 7.
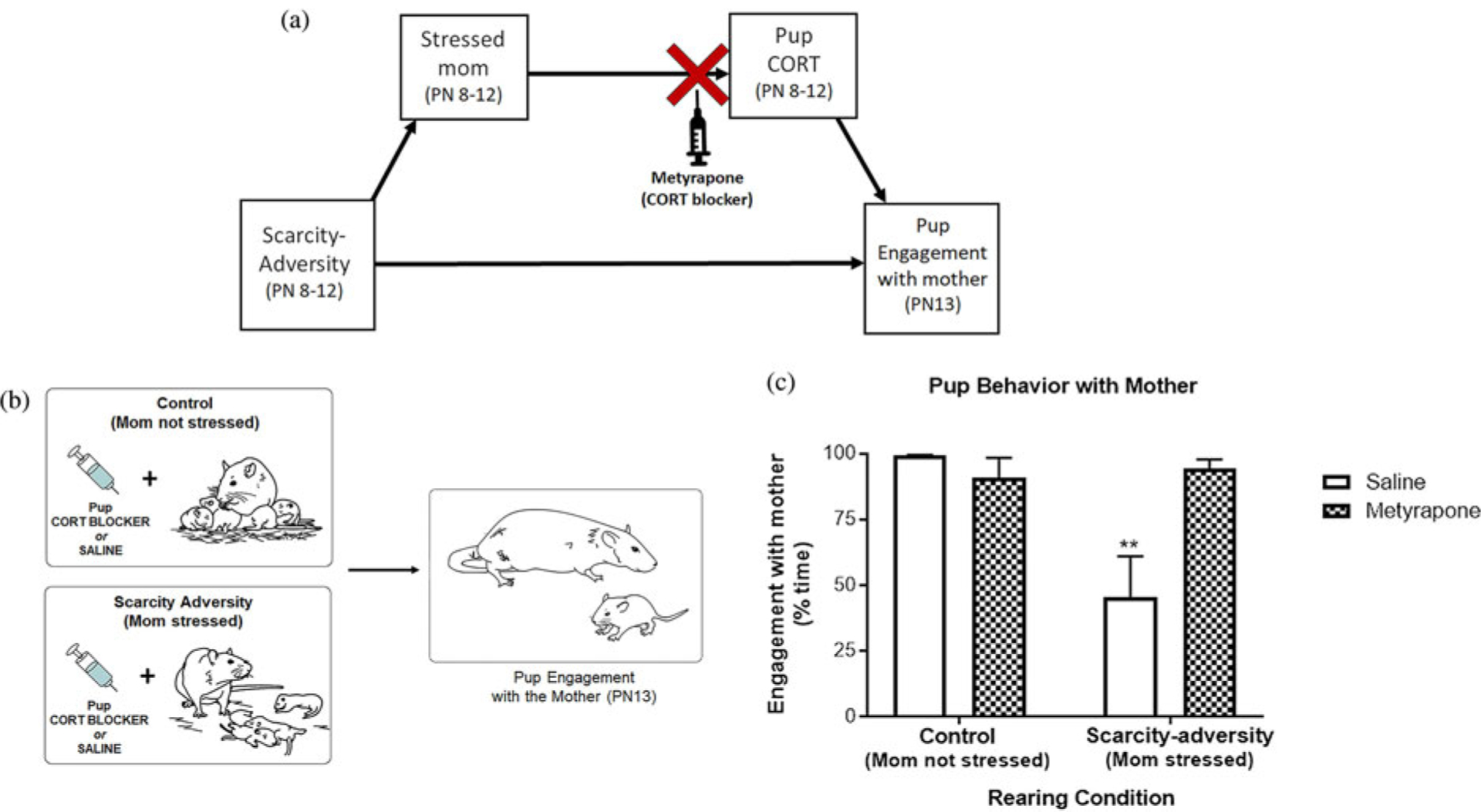
Corticosterone (CORT) blocker injection (metyrapone) prevents social transmission of scarcity-adversity effects to pup engagement with a mother in a social behavior test. (a) Depiction of model of disruption of social transmission via CORT blockade (metyrapone). (b) Depiction of experimental procedure. (c) Pup engagement with mother in a social behavior test as a function of early-life rearing condition (control vs. scarcity-adversity) and pharmacological manipulation (saline vs. metyrapone injection). Data are displayed as mean (± SEM) percent time the pup spent socially engaged with the mother, as calculated from total time pups spent crawling to the mother, probing the mother, sleeping/laying on the mother, and nipple attached to the mother (** indicates p < .01; n = 8/group).
Discussion
Children raised in poverty are at increased risk for altered health and developmental outcomes (Blair & Raver, 2016; Duncan, Ziol-Guest, & Kalil, 2010; Hackman & Farah, 2009; Lipina & Posner, 2012; Yoshikawa, Aber, & Beardslee, 2012), with altered stress physiology increasingly recognized as a likely, primary mechanism by which early-life poverty-related adversity is embedded biologically to produce disparities (Blair, 2010). Yet, the specific mechanisms by which this occurs remain unclear and have been relatively under-explored. This research gap likely arises in part from limitations faced by both human and animal researchers alike, as well as challenges associated with translating across the theoretical silos of each research domain. While most research investigating mechanisms linking the early environment with alterations to the stress response and neurobehavioral development has been conducted using animal models, the implications of such findings cannot be easily extrapolated to real world contexts, such as the complex condition of human poverty. Conversely, human research cannot control the assignment process into adversity nor manipulate physiological processes for the determination of cause–effect mechanisms, as can be done with animal models. Fortunately, there has been a rise in translational cross-species studies to balance the mechanistic and ecological utility of findings related to the effects of early adversity on child development (Cohen et al., 2013; Opendak et al., 2020; Loman & Gunnar, 2010; Perry, 2019; Perry et al., 2019a; Perry et al., 2019b; Tottenham, Shapiro, Flannery, Caldera, & Sullivan, 2019).
In the present study, we utilized a cross-species translational approach to provide evidence that elevated infant CORT is necessary, but not sufficient without elevated caregiver CORT in mediating the effects of environmental scarcity-adversity on child development. Specifically, human findings indicated that infant CORT levels alone did not significantly mediate the relation between early-life scarcity-adversity exposure and later child engagement in a mother-hild interaction task. However, maternal CORT through infant CORT serially mediated the relation between scarcity-adversity and child engagement. This indirect effect was observed when controlling for multiple relevant covariates, including race, gender, age, and parenting.
Capitalizing on an animal model, these findings were further, and causally, supported by rodent data through manipulation of both environmental adversity and CORT levels. Specifically, our rodent findings revealed that repeated pharmacological increases of pup CORT during brief separations from the mother did not disrupt subsequent pup engagement with the mother in a social behavior test. Thus, similar to our human results, elevated infant CORT alone was not sufficient in altering infant social behavior. However, repeated pharmacological blockade of pup CORT in the presence of a stressed mother prevented social transmission of scarcity-adversity effects on pup social behavior, providing causal evidence for stress-mediated social transmission of risk from the caregiver to pup. These casual findings broadly support our interpretation of the human data: that the interpersonal association of elevated CORT between the primary caregiver and child uniquely contributes to the transmission of risk to children exposed to environmental scarcity. Thus, our closely aligned cross-species research efforts provide translational implications for future research and interventional efforts for the promotion of optimal child development. Namely, they suggest potential benefits in going beyond directly targeting reduction of infant stress to targeting reduction of caregiver stress and/or the caregiver’s environmental adversity for the benefit of the child (Figure 8).
Figure 8.
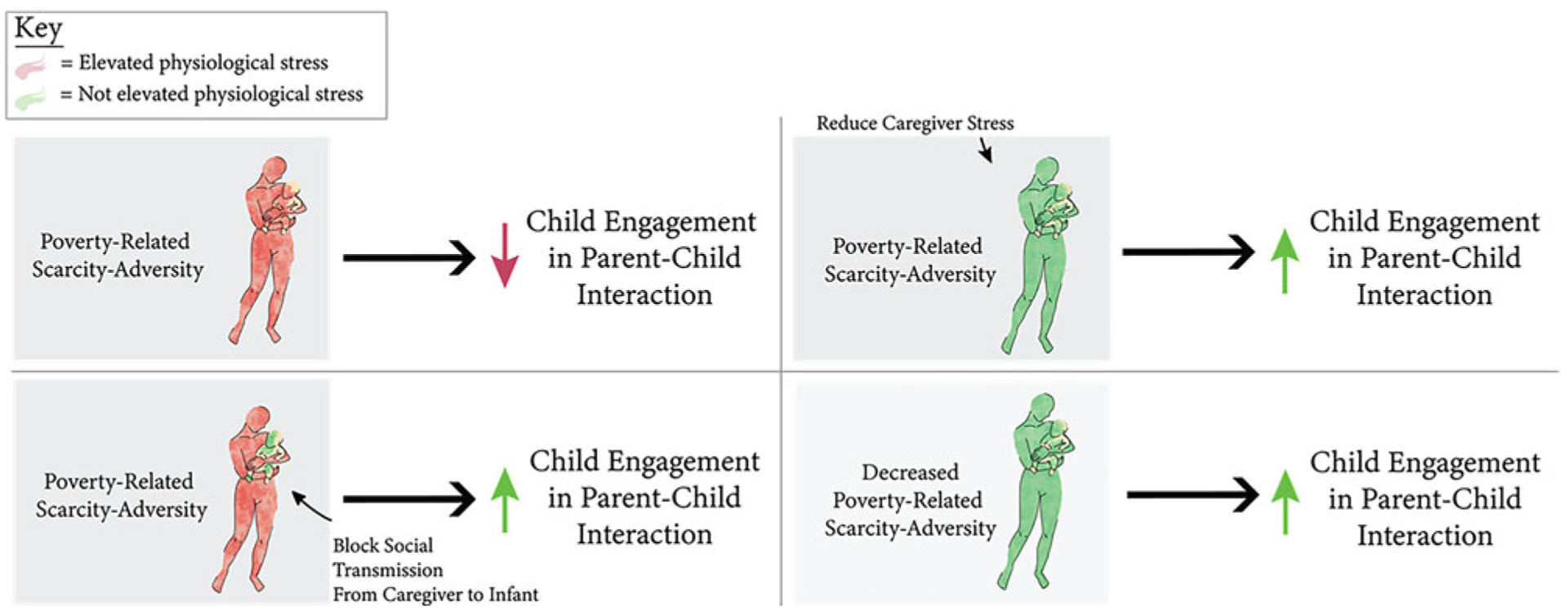
Summary schematic of the current study’s findings and their implications for future research and interventional efforts. The present study’s findings provide novel, mechanistic support that poverty-related scarcity-adversity “gets under the skin” to raise infant physiological stress levels and disrupt child behavioral development by way of elevated caregiver physiological stress levels (top left). Blocking physiological social transmission from the primary caregiver to the infant might serve as one way to prevent negative impacts of environmental risk on child development (bottom left); however, future research is needed to determine the appropriateness and/or means by which to achieve this in real-world settings. Targeting upstream mechanisms of child outcomes via reduction of primary caregiver physiological stress (top right) and/or reduction of poverty-related scarcity-adversity exposure (bottom right) are additional, more readily translatable means by which to prevent or reduce the negative impacts of environmental risk on child behavioral development.
Our findings suggest that primary caregivers are uniquely positioned to shape child developmental trajectories of risk versus resilience. Bi-directional, translational human and animal research has provided ample support for the powerful role social context plays during early development. Cross-species research has revealed that in early-life the caregiver mediates “a stress hyporesponsive period” (SHRP) in the infant, marked by an absence or reduced presence of HPA axis activation in the infant’s response to external stress, which occurs via maternal sensory stimulation (Gunnar & Fisher, 2006; Hofer, 1994; Rosenfeld, Suchecki, & Levine, 1992; Sapolsky & Meaney, 1986). Furthermore, following the SHRP, rodent (Stanton & Levine, 1988; Stanton & Levine, 1990), nonhuman primate (Coe, Franklin, Smith, & Levine, 1982; Hennessy, Kaiser, & Sachser, 2009; Stanton, Patterson, & Levine, 1985; Wiener, Johnson, & Levine, 1987), and human research (Gunnar & Donzella, 2002; Hostinar, Johnson, & Gunnar, 2015; Seltzer, Ziegler, & Pollak, 2010) have demonstrated that maternal presence can diminish the infant’s response to threat, via attenuation of CORT levels during threat exposure. Experimental rodent research has demonstrated that the mother’s presence during threat blocks pups’ CORT release at the level of the hypothalamus, by inhibiting norepinephrine release into the paraventricular nucleus (Shionoya, Moriceau, Bradstock, & Sullivan, 2007). This CORT blockade switches off the amygdala and the pup’s fear response to threat, as well as the pup’s fear learning: the infant rat pup’s amygdala is uniquely dependent upon CORT to function (Barr et al., 2009; Moriceau, Wilson, Levine, & Sullivan, 2006). This buffering process wanes as pups mature and prepare for independence (Opendak et al., 2019; Robinson-Drummer et al., 2019). In humans, maternal presence during an aversive conditioning procedure has similarly been shown to block threat learning in children, with the strongest effects noted among children with the lowest CORT levels (Tottenham et al., 2019).
The presence of the scent of the mother alone has also been demonstrated to decrease the threat response in children (Jessen, 2020). In children, as found in rodent research, the presence of maternal stimuli suppresses amygdala reactivity, with this effect waning with maturation (Gee et al., 2014). Social buffering of threat-induced CORT is known to be phylogenetically conserved and widespread across social species (Culbert, Gilmour, & Balshine, 2019; Edgar et al., 2015; Faustino et al., 2017; Hennessy, Zate, & Maken, 2008; Kikusui, Winslow, & Mori, 2006; Sullivan & Perry, 2015). In addition, cross-species research is providing evidence that the quality of the mother–infant relationship might influence how well the mother can socially buffer the stress response of her young (Ahnert, Gunnar, Lamb, & Barthel, 2004; Gunnar et al., 2015; Gunnar & Sullivan, 2017; Hostinar, Sullivan, & Gunnar, 2014). Since the mother communicates with the offspring via sensory systems, these results suggest that the ability of maternal cues to reduce CORT appears to be diminished with adversity. This idea is supported by emerging neuroscience in rats (Perry et al., 2019b) and humans (Callaghan et al., 2019) suggesting that maternal cues, such as maternal odor, have reduced value in the infant brain following scarcity-adversity rearing.
The present study’s findings dovetail with an increasing body of literature demonstrating that maternal physiology uniquely contributes to the modulation of child physiology. The infant and caregiver are oftentimes attuned on a biobehavioral level, regulating child development, for better or worse (Barrett & Fleming, 2011; Davis, West, Bilms, Morelen, & Suveg, 2018, Dozier, Stoval, Albus, & Bates, 2001; Feldman, 2017; Gottlieb, 1996; Gunnar & Quevedo, 2007). During caregiver-infant interactions, the caregiver and child engage in dynamic, bidirectional, reciprocal exchanges of conscious and nonconscious behavioral, affective, and physiological cues (Beebe, 2017; Feldman, 2007, 2012; Feldman, Greenbaum, Yirmiya, & Mayes, 1996; Field, 2012; Papousek, 2007; Welch et al., 2015;). This behavioral and physiological linkage is thought to serve as a foundation for social affiliation, bonding, and attachment, and prepares one for the transmission and reception of information within dyads (Bornstein, 2013; Fleming & Li, 2002; Feldman, 2017; Harrist & Waugh, 2002; Papoušek & Papoušek, 2002).
Thus, the caregiver is well positioned to serve as a powerful regulatory presence, particularly before children fully develop the mechanisms needed for self-regulation and homeostasis (Bernier et al., 2010; Hofer, 1984; Hofer, 1994). However, just as the caregiver may buffer stress, the caregiver is also well positioned to exacerbate or transmit stress to the infant in contexts of increased adversity. Rodent research has demonstrated that caregivers can communicate information about threat via social exchanges, including from mothers to her pups, via CORT-mediated mechanisms (Carew et al., 2018; Debiec & Sullivan, 2014; Keum & Shin, 2019; Monfils & Agee, 2019; Rickenbacher, Perry, Sullivan, & Moita, 2017). Similar human research has documented social transmission of stress from mother to infants (Halevi et al., 2017; Hibel, Trumbell, Valentino, & Buhler-Wassmann, 2018; Hibel, Mercado, & Valentino, 2019; Waters et al., 2014). Collectively, these findings demonstrate that social influences can operate independently of overt caregiving behaviors and physical contact for the transmission of risk, as a function of CORT-mediated covert cues between mother and infant. This is in line with the present study’s findings that maternal CORT through infant CORT serially mediates the effects of scarcity-adversity on child behavior even when controlling for negative parenting behaviors. However, far more research is needed to disentangle the unique contributions of parenting behaviors versus caregiver physiology, especially within the context of chronic early-life adversity.
The present study’s findings support the idea that in contexts of increased stress, relations between caregiver-child physiology may serve as a pathway of stress transmission between a parent and child. Attuning one’s stress physiology to another may be adaptive under well-resourced and supportive circumstances in which one’s stress is well regulated, but not in environments of chronic adversity and high stress. For instance, a child who is physiologically linked to their chronically stressed caregiver may be at risk for compromised neurodevelopmental via chronic up-regulation of HPA axis or autonomic nervous system (ANS) activity. Prior research with the FLP data set revealed relations between maternal baseline CORT levels and infant CORT reactivity to an emotion induction task at child ages 7, 15, and 24 months of age. However, by 24 months of age, the association between mother and infant CORT was moderated by poverty-related risk, such that maternal baseline CORT and infant CORT reactivity remained associated only in dyads characterized by low poverty-related risk (Braren et al., 2019). These findings might indicate a loss of maternal regulation of infant CORT responses following chronic exposure to poverty-related adversity, as has been evidenced in human and rodent studies that report decreased social buffering of children/pups by high-stress mothers (Gunnar & Herrera, 2013; Raineki et al., 2010; Sullivan & Perry, 2015; Tottenham et al., 2019). However, decoupling of infant CORT activity from that of the mother in environments of chronic stress might serve as an adaptive response, to protect the child from continued exposure to the mother’s high level of physiological stress. Far more research is needed to understand how linkage of caregiver–infant stress physiology functions in contexts of risk. Investigating how linkage operates in at-risk populations will be critical and especially important for understanding pathways of social and intergenerational transmission of risk.
The present study is an important contribution to a growing body of literature regarding the effects of poverty-related adversity on infant CORT activity (Blair et al., 2008; Blair et al., 2011a; Blair et al., 2011b; Blair, Berry, Mills-Koonce, Granger, & Investigators, 2013; Braren et al., 2019; Clearfield, Carter-Rodriguez, Merali, & Shober, 2014; Finegood et al., 2017; Hill-Soderlund et al., 2015). Namely, we report evidence that early-life poverty-related adversity is associated with elevated CORT levels across infancy. This finding is in line with prior reports of hypercortisolism in contexts of high socioeconomic risk (Blair et al., 2011a; Blair et al., 2011b; Blair et al., 2013; Evans & English, 2002; Finegood et al., 2017; Lupien, King, Meaney, & McEwen, 2000; Lupien, King, Meaney, & McEwen, 2001). However, other studies have reported evidence of hypocortisolism following exposure to poverty-related adversity (Badanes et al., 2011; Bernard, Zwerling, & Dozier, 2015; Chen, Cohen, & Miller, 2010; Chen & Paterson, 2006; Fernald, Burke, & Gunnar, 2008; Karb, Elliott, Dowd, & Morenoff, 2012; Kliewer, Reid-Quiñones, Shields, & Foutz, 2009; Zalewski, Lengua, Kiff, & Fisher, 2012). These seemingly contradictory results are also found in the animal literature, with findings of both elevated CORT levels (Ivy et al., 2008; Raineki et al., 2010) and blunted CORT levels following infant adversity (Perry et al., 2019c).
The disparate results regarding the directionality of CORT levels following poverty exposure may stem from differences in the age of CORT assessment across studies, as findings of hypercortisolism tend to occur in samples of infants exposed to poverty-related adversity (Blair et al., 2011a; Blair et al., 2011b; Finegood et al., 2017; Ivy et al., 2008; Raineki et al., 2010), while hypocortisolism oftentimes occurs in samples of older children and adolescents (e.g., Chen & Paterson, 2006; Karb, Elliott, Dowd, & Morenoff, 2012; Kliewer et al., 2009; Perry et al., 2019c). Moreover, studies examining age effects have reported that poverty exposure relates to higher CORT among younger children, but not older children (Lupien et al., 2001; Ursache, Noble, & Blair, 2015). Furthermore, using the rodent scarcity-adversity model, it has been experimentally shown that pharmacologically blocking CORT rescues scarcity-adversity effects on neurobehavioral development in early life (Raineki et al., 2019), whereas pharmacologically increasing CORT rescues scarcity-adversity effects on neurobehavioral development in later life (Perry et al., 2019c). These findings converge with results of a meta-analysis of human studies, supporting the idea that the time elapsed following adversity onset is negatively associated with CORT production such that as more time passes the more an individual’s CORT secretion decreases (Miller, Chen, & Zhou, 2007). In other words, following hyper-activation of the HPA axis earlier in development, a process of down-regulation may occur to produce patterns of blunted CORT secretion (Miller et al., 2007). However, more longitudinal and mechanistic research is needed to disentangle the effects of poverty-related adversity on stress physiology across development.
Limitations
While the present study provides novel evidence regarding stress-mediated pathways for the transmission of environmental risk to infant development, they should be interpreted with the following limitations in mind. First, our rodent findings should be interpreted within a domain-specific framework of poverty-related adversity. That is, while the human condition of poverty is complex and involves a multitude of risks factors (e.g., greater family instability, more crowded homes, elevated stress levels, increased exposure to violence, less cognitive stimulation, greater exposure to environmental toxins, poorer diets, scarcity of material resources, less parental nurturance), our rodent model mirrors conditions of scarcity in terms of material resources only, with subsequent negative effects on caregiving behaviors (Darmon & Drewnowski, 2008; Evans, 2004; Perry et al., 2019b). Furthermore, while both rodents and humans are altricial species where caregiver-infant interactions are fundamental to development and early-life programming of the brain for later-life adaptive behavior (Perry et al., 2017), the rodent data should be interpreted with the understanding that the complexity of human behaviors and conditions cannot be fully modeled by rodents. In addition, while our human mediational analyses use prospective longitudinal data to imply a causal role of caregiver and infant CORT for the transmission of environmental risk to infant development, they are ultimately based upon correlational data only. Collectively, these limitations bolster our rationale for utilizing human and rodent research approaches in tandem. Finally, the present study’s findings are explored in relation to only one aspect of infant development (social behavior with the primary caregiver), as well as only one indicator of physiological stress (CORT). Future experiments should assess if infant and/or caregiver CORT levels, as well as other markers of HPA axis and ANS activity, similarly mediate the effects of scarcity-adversity on additional aspects of infant development.
Conclusion
For the present study, we capitalized on a cross-species research approach to provide novel, mechanistic evidence that scarcity-related environmental adversity “gets under the skin” to disrupt child behavioral development via physiological social transmission between the mother and child. Our cross-species findings revealed that on its own, elevated infant stress hormones (cortisol in humans, corticosterone in rats) are not sufficient in mediating the effects of adversity on development. Rather, only when considering both maternal and infant stress hormone levels did we observe the mediating effects of environmental adversity on child behavioral development, suggesting an interpersonal, physiological route of social transmission. These findings support a growing literature indicating that there are covert pathways by which the caregiver regulates infant development, including increasing evidence that infant and caregiver physiology are oftentimes attuned for the regulation of child development, for better or worse. Furthermore, these findings underscore the importance of considering infant stress physiology within the context of caregiver stress physiology when devising interventions for at-risk families. Continued cross-species research will enable further testing and discernment of causal, yet ecologically relevant interpersonal pathways of social transmission of risk or resilience.
Acknowledgments.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF. We thank the families and research assistants for making this study possible.
Funding Statement.
Research was supported by NSF 1810208 (REP), NSF DGE1342536 (SHB), BBRF NARSAD (MO), R25NS080686 (JW), NIH R37-HD083217 (RMS) and NIH P01 HD039667 to The Family Life Project with Phase I Key Investigators: Lynne Vernon-Feagans, University of North Carolina; Martha Cox, University of North Carolina at Chapel Hill; Clancy Blair, New York University; Margaret Burchinal and Patricia Garrett-Peters University of North Carolina; Mark Greenberg, The Pennsylvania State University; Roger Mills-Koonce, The University of North Carolina; and Michael Willoughby, RTI International.
Footnotes
Conflicts of Interest. None.
References
- Ahnert L, Gunnar MR, Lamb ME, & Barthel M (2004). Transition to child care: Associations with infant–mother attachment, infant negative emotion, and cortisol elevations. Child Development, 75, 639–650. doi: 10.1111/j.1467-8624.2004.00698.x [DOI] [PubMed] [Google Scholar]
- Asok A, Bernard K, Roth TL, Rosen JB, & Dozier M (2013). Parental responsiveness moderates the association between early-life stress and reduced telomere length. Development and Psychopathology, 25, 577–585. doi: 10.1017/S0954579413000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles E, Eghbal-Ahmadi M, Bar-El Y, & Baram T (2001). Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. Journal of Neuroendocrinology, 13, 799–807. doi: 10.1046/j.1365-2826.2001.00698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, & Hankin BL (2011). Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology, 23, 881–896. doi: 10.1017/S095457941100037X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, … Nestler EJ (2010). Early life programming and neurodevelopmental disorders. Biological Psychiatry, 68, 314–319. doi: 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, & Stern H (2012). Fragmentation and unpredictability of early-life experience in mental disorders. American Journal of Psychiatry, 169, 907–915. doi: 10.1176/appi.ajp.2012.11091347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, & Sullivan RM (2009). Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience, 12, 1367. doi: 10.1038/nn.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, & Fleming AS (2011). Annual research review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52, 368–397. doi: 10.1111/j.1469-7610.2010.02306.x [DOI] [PubMed] [Google Scholar]
- Beebe B (2017). My journey in infant research and psychoanalysis: Microanalysis, a social microscope. Moments of meeting in psychoanalysis (pp. 13–44). New York: Routledge. [Google Scholar]
- Bernard K, Zwerling J, & Dozier M (2015). Effects of early adversity on young children’s diurnal cortisol rhythms and externalizing behavior. Developmental Psychobiology, 57, 935–947. doi: 10.1002/dev.21324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81, 326–339. doi: 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Blair C (2010). Stress and the development of self-regulation in context. Child Development Perspectives, 4, 181–188. doi: 10.1111/j.1750-8606.2010.00145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Berry D, Mills-Koonce R, Granger D, & Investigators F. L. P. (2013). Cumulative effects of early poverty on cortisol in young children: moderation by autonomic nervous system activity. Psychoneuroendocrinology, 38, 2666–2675. doi: 10.1016/j.psyneuen.2013.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, … Fortunato CK (2008). Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology, 44, 1095. doi: 10.1037/0012-1649.44.4.1095 [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, … FLP Investigators (2011b). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82, 1970–1984. doi: 10.1111/j.1467-8624.2011.01643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2012). Child development in the context of adversity: experiential canalization of brain and behavior. American Psychologist, 67, 309–318. doi: 10.1037/a0027493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2016). Poverty, stress, and brain development: New directions for prevention and intervention. Academic Pediatrics, 16, S30–S36. doi: 10.1016/j.acap.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L, & Investigators F. L. P. (2011a). Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology, 23, 845–857. doi: 10.1017/S0954579411000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, & Roth TL (2013). Exposure to caregiver maltreatment alters expression levels of epigenetic regulators in the medial prefrontal cortex. International Journal of Developmental Neuroscience, 31, 804–810. doi: 10.1016/j.ijdevneu.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH (2013). Mother–Infant Attunement: A Multilevel Approach. In Haley MHB (Ed.), The infant mind: Origins of the social brain (pp. 266–300). New York, NY: The Guilford Press. [Google Scholar]
- Braren SH, Perry RE, Ursache A, & Blair C (2019). Socioeconomic risk moderates the association between caregiver cortisol levels and infant cortisol reactivity to emotion induction at 24 months. Developmental Psychobiology, 61, 573–591. doi: 10.1002/dev.21832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, & Duncan GJ (1997). The effects of poverty on children. Future Child, 7, 55–71. doi: 10.2307/1602387 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, … Caldera C (2019). Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: An examination across 3 years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4, 664–671. doi: 10.1016/j.bpsc.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, & Tottenham N (2014). The international society for developmental psychobiology Sackler symposium: Early adversity and the maturation of emotion circuits—A cross-species analysis. Developmental Psychobiology, 56, 1635–1650. doi: 10.1002/dev.21260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL (2001). Critical periods for social attachment: Deprivation and neural systems in rhesus monkeys. Social Research in Child Development Abstr, 2, 054. [Google Scholar]
- Carew SJ, Mukherjee B, MacIntyre IT, Ghosh A, Li S, Kirouac GJ, … Yuan Q (2018). Pheromone-induced odor associative fear learning in rats. Scientific Reports, 8, 1–10. doi: 10.1038/s41598-018-36023-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Cohen S, & Miller GE (2010). How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science, 21, 31–37. doi: 10.1177/0956797609355566 [DOI] [PubMed] [Google Scholar]
- Chen E, & Paterson LQ (2006). Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology, 25, 704. doi: 10.1037/0278-6133.25.6.704 [DOI] [PubMed] [Google Scholar]
- Clearfield MW, Carter-Rodriguez A, Merali AR, & Shober R (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behavior and Development, 37, 298–304. doi: 10.1016/j.infbeh.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, & Levine S (1982). Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiology & Behavior, 29, 1051–1057. doi: 10.1016/0031-9384(82)90297-9 [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jing D, Yang RR, Tottenham N, Lee FS, & Casey BJ (2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences, 110, 18274–18278. doi: 10.1073/pnas.1310163110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M (1997). Qualitative ratings: Parent/child interaction at 24–36 months of age. Unpublished coding manual. [Google Scholar]
- Culbert BM, Gilmour KM, & Balshine S (2019). Social buffering of stress in a group-living fish. Proceedings of the Royal Society B, 286, 20191626. doi: doi.org/ 10.1098/rspb.2019.1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, & Champagne FA (2016). Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Frontiers in Neuroendocrinology, 40, 52–66. doi: 10.1016/j.yfrne.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF (2007). Modulation of stress responses: how we cope with excess glucocorticoids. Experimental Neurology, 206, 179. doi: 10.1016/j.expneurol.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon N, & Drewnowski A (2008). Does social class predict diet quality? American Journal of Clinical Nutrition, 87, 1107–1117. doi: 10.1093/ajcn/87.5.1107 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & McEwen BS (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience, 15, 689–695. doi: 10.1038/nn.3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, West K, Bilms J, Morelen D, & Suveg C (2018). A systematic review of parent-child synchrony: It is more than skin deep. Developmental Psychobiology, 60, 674–691. doi: 10.1002/dev.21743 [DOI] [PubMed] [Google Scholar]
- Debiec J, & Sullivan RM (2014). Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proceedings of the National Academy of Sciences, 111, 12222–12227. doi: 10.1073/pnas.1316740111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TS, Blaze J, Keller SM, & Roth TL (2017). Phenotypic outcomes in adolescence and adulthood in the scarcity-adversity model of low nesting resources outside the home cage. Developmental Psychobiology, 59, 703–714. doi: 10.1002/dev.21547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Stoval KC, Albus KE, & Bates B (2001). Attachment for infants in foster care: The role of caregiver state of mind. Child Development, 72, 1467–1477. doi: 10.1111/1467-8624.00360 [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Ziol-Guest KM, & Kalil A (2010). Early-childhood poverty and adult attainment, behavior, and health. Child Development, 81, 306–325. doi: 10.1111/j.1467-8624.2009.01396.x [DOI] [PubMed] [Google Scholar]
- Edgar J, Held S, Paul E, Pettersson I, Price RIA, & Nicol C (2015). Social buffering in a bird. Animal Behaviour, 105, 11–19. doi: 10.1016/j.anbehav.2015.04.007 [DOI] [Google Scholar]
- Evans GW (2004). The environment of childhood poverty. American Psychologist, 59, 77–92. doi: 10.1037/0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development, 73, 1238–1248. doi: 10.1111/1467-8624.00469 [DOI] [PubMed] [Google Scholar]
- Evans GW, & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, 106, 6545–6549. doi: 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faturi CB, Tiba PA, Kawakami SE, Catallani B, Kerstens M, & Suchecki D (2010). Disruptions of the mother–infant relationship and stress-related behaviours: altered corticosterone secretion does not explain everything. Neuroscience & Biobehavioral Reviews, 34, 821–834. doi: 10.1016/j.neubiorev.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Faustino AI, Tacão-Monteiro A, & Oliveira RF (2017). Mechanisms of social buffering of fear in zebrafish. Scientific Reports, 7, 44329. doi: 10.1038/srep44329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48, 329–354. doi: 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting, 12, 154–164. doi: 10.1080/15295192.2012.683342 [DOI] [Google Scholar]
- Feldman R (2015). Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high-risk parenting. Development and Psychopathology, 27, 369–395. doi: 10.1017/S0954579415000048 [DOI] [PubMed] [Google Scholar]
- Feldman R (2017). The Neurobiology of human attachments. Trends in Cognitive Sciences, 21, 80–99. doi: 10.1016/j.tics.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N, & Mayes LC (1996). Relations between cyclicity and regulation in mother-infant interaction at 3 and 9 months and cognition at 2 years. Journal of Applied Developmental Psychology, 17, 347–365. doi: 10.1016/S0193-3973(96)90031-3 [DOI] [Google Scholar]
- Fernald LC, Burke HM, & Gunnar MR (2008). Salivary cortisol levels in children of low-income women with high depressive symptomatology. Development and Psychopathology, 20, 423–436. doi: 10.1017/S0954579408000205 [DOI] [PubMed] [Google Scholar]
- Field T (2012). Relationships as Regulators. Psychology, 3, 467. doi: 10.4236/psych.2012.36066 [DOI] [Google Scholar]
- Finegood ED, Rarick JR, Blair C, & Family Life Project Investigators (2017). Exploring longitudinal associations between neighborhood disadvantage and cortisol levels in early childhood. Development and Psychopathology, 29, 1649–1662. doi: 10.1017/S09554579417001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, & Li M (2002). Psychobiology of maternal behavior and its early determinants in nonhuman mammals. In Bornstein MH (Ed.), Handbook of parenting: Biology and ecology of parenting (pp. 61–97). Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Frankenhuis WE, & Nettle D (2019). The Strengths of people in poverty. Current Directions in Psychological Science, 29, 16–2. doi: 10.1177/0963721419881154 [DOI] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, … Tottenham N (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25, 2067–2078. doi: 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G (1996). Developmental psychobiological theory. In Cairns RB, Elder JH, & Costello EJ (Eds.), Developmental science (pp. 63–77). New York, NY: Cambridge University Press. [Google Scholar]
- Granero R, Louwaars L, & Ezpeleta L (2015). Socioeconomic status and oppositional defiant disorder in preschoolers: parenting practices and executive functioning as mediating variables. Frontiers in Psychology, 6, 1412. doi: 10.3389/fpsyg.2015.01412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27, 199–220. doi: 10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Fisher PA (2006). Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology, 18, 651–677. doi: 10.1017/S0954579406060330 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Herrera AM (2013). The development of stress reactivity: A neurobiological perspective. In Zelazo PD (Ed.), The Oxford handbook of developmental psychology: Vol.2. Self and other (pp. 45–80). New York, NY: Oxford University Press. [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, & Sullivan RM (2015). Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience, 10, 474–47. doi: 10.1080/17470919.2015.1070198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. doi: 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Sullivan RM (2017). The neurodevelopment of social buffering and fear learning: integration and crosstalk. Social Neuroscience, 12, 1–7. doi: 10.1080/17470919.2016.1151824 [DOI] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13, 65–73. doi: 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Gallop R, Evans GW, & Farah MJ (2015). Socioeconomic status and executive function: developmental trajectories and mediation. Developmental Science, 18, 686–702. doi: 10.1111/desc.12246 [DOI] [PubMed] [Google Scholar]
- Halevi G, Djalovski A, Kanat-Maymon Y, Yirmiya K, Zagoory-Sharon O, Koren L, & Feldman R (2017). The social transmission of risk: Maternal stress physiology, synchronous parenting, and well-being mediate the effects of war exposure on child psychopathology. Journal of Abnormal Psychology, 126, 1087–1103. doi: 10.1037/abn0000307 [DOI] [PubMed] [Google Scholar]
- Harrist AW, & Waugh RM (2002). Dyadic synchrony: Its structure and function in children’s development. Developmental Review, 22, 555–592. doi: 10.1016/S0273-2297(02)00500-2 [DOI] [Google Scholar]
- Hennessy MB, Kaiser S, & Sachser N (2009). Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology, 30, 470–482. doi: 10.1016/j.yfrne.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Zate R, & Maken DS (2008). Social buffering of the cortisol response of adult female guinea pigs. Physiology & Behavior, 93, 883–888. doi: 10.1016/j.physbeh.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Herzberg MP, & Gunnar MR (2019). Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. NeuroImage, 209, 116493. doi: 10.1016/j.neuroimage.2019.116493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, Mercado E, & Valentino K (2019). Child maltreatment and mother–child transmission of stress physiology. Child Maltreatment, 24, 340–352. doi: 10.1016/j.physbeh.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, Trumbell JM, Valentino K, & Buhler-Wassmann AC (2018). Ecologically salient stressors and supports and the coordination of cortisol and salivary alpha-amylase in mothers and infants. Physiology & Behavior, 195, 48–57. doi: 10.1016/j.physbeh.2018.07.024 [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Holochwost SJ, Willoughby MT, Granger DA, Gariépy JL, Mills-Koonce WR, & Cox MJ (2015). The developmental course of salivary alpha-amylase and cortisol from 12 to 36 months: Relations with early poverty and later behavior problems. Psychoneuroendocrinology, 52, 311–323. doi: 10.1016/j.psyneuen.2014.08.011 [DOI] [PubMed] [Google Scholar]
- Hofer MA (1984). Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine, 46, 183–197. doi: 10.1097/00006842-198405000-00001 [DOI] [PubMed] [Google Scholar]
- Hofer MA (1994). Hidden regulators in attachment, separation, and loss. Monographs of the Society for Research in Child Development, 59, 192–207. doi: 10.2307/1166146 [DOI] [PubMed] [Google Scholar]
- Holochwost SJ, Gariépy J-L, Propper CB, Gardner-Neblett N, Volpe V, Neblett E, & Mills-Koonce WR (2016). Sociodemographic risk, parenting, and executive functions in early childhood: The role of ethnicity. Early Childhood Research Quarterly, 36, 537–549. doi: 10.1016/j.ecresq.2016.02.001 [DOI] [Google Scholar]
- Hostinar CE, Johnson AE, & Gunnar MR (2015). Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science, 18, 281–297. doi: 10.1111/desc.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the HPA axis: A review of animal models and human studies across development. Psychological Bulletin, 140, 256. doi: 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, & Baram TZ (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience, 154, 1132–1142. doi: 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen S (2020). Maternal odor reduces the neural threat response in human infants. Developmental Cognitive Neuroscience, 45, 100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junod A, Opendak M, LeDoux JE, & Sullivan RM (2019). Development of threat expression following infant maltreatment: Infant and adult enhancement but adolescent attenuation. Frontiers in Behavioral Neuroscience, 13, 130. doi: 10.3389/fnbeh.2019.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karb RA, Elliott MR, Dowd JB, & Morenoff JD (2012a). Neighborhood-level stressors, social support, and diurnal patterns of cortisol: The Chicago community adult health study. Social Science & Medicine, 75, 1038–1047. doi: 10.1016/j.socscimed.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karb RA, Elliott MR, Dowd JB, & Morenoff JD (2012b). Neighborhood-level stressors, social support, and diurnal patterns of cortisol: The Chicago community adult health study. Social Science & Medicine, 75, 1038–1047. doi: 10.1016/j.socscimed.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum S, & Shin HS (2019). Neural basis of observational fear learning: A potential model of affective empathy. Neuron, 104, 78–86. doi: j.neuron.2019.09.013 [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 2215–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer W, Reid-Quiñones K, Shields BJ, & Foutz L (2009). Multiple risks, emotion regulation skill, and cortisol in low-income African American youth: A prospective study. Journal of Black Psychology, 35, 24–43. doi: 10.1177/0095798408323355 [DOI] [Google Scholar]
- Kline RB (2015). Principles and practice of structural equation modeling (4th ed.). New York, NY: Guilford Publications. [Google Scholar]
- Koss KJ, & Gunnar MR (2018). Annual Research Review: early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, 59, 327–346. doi: 10.1111/jcpp.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Thompson SF, Moran LR, Zalewski M, Ruberry EJ, Klein MR, & Kiff CJ (2019). Pathways from early adversity to later adjustment: Tests of the additive and bidirectional effects of executive control and diurnal cortisol in early childhood. Development and Psychopathology, 33, 545–558. doi: 10.1017/S0954579419000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina SJ, & Posner MI (2012). The impact of poverty on the development of brain networks. Frontiers in Human Neuroscience, 6, 238. doi: 10.3389/fnhum.2012.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, & Gunnar MR (2010). Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews, 34, 867–876. doi: 10.1016/j.neubiorev.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167, 1135–1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry, 48, 976–980. doi: 10.1016/S0006-3223(00)00965-3 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2001). Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology, 13, 653–676. doi: 10.1017/S0954579401003133 [DOI] [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Developmental Psychopathology, 22, 491–495. doi: 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress-and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. doi: 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC (1998). Socioeconomic disadvantage and child development. American Psychologist, 53, 185–204. doi: 10.1037/0003-066X.53.2.185 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Monfils MH, & Agee LA (2019). Insights from social transmission of information in rodents. Genes, Brain and Behavior, 18, e12534. doi: 10.1111/gbb.12534 [DOI] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, & Sullivan RM (2006). Dual circuitry for odor–shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. Journal of Neuroscience, 26, 6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2012). Mplus User’s Guide (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- National Institute of Child Health and Human Development Early Child Care Research Network (NICHD ECCRN). (1999). Child care and mother-child interaction in the first 3 years of life. Developmental Psychology, 35, 1399–1413. doi: 10.1016/S0163-6383(03)00035-3 [DOI] [PubMed] [Google Scholar]
- Opendak M, Robinson-Drummer P, Blomkvist A, Zanca RM, Wood K, Jacobs L, … Kirschner E (2019). Neurobiology of maternal regulation of infant fear: The role of mesolimbic dopamine and its disruption by maltreatment. Neuropsychopharmacology, 44, 1247–1257. doi: 10.1038/s41386-019-0340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, & Sullivan RM (2016). Unique neurobiology during the sensitive period for attachment produces distinctive infant trauma processing. European Journal of Psychotraumatology, 7, 31276. doi: 10.3402/ejpt.v7.31276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, Theisen E, Blomkvist A, Hollis K, Lind T, Sarro E, … Sullivan RM (2020). Adverse caregiving in infancy blunts neural processing of the mother: Translating across species. Nature Communication, 11, 1–12. doi: 10.1038/s41467-020-14801-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papousek M (2007). Communication in early infancy: an arena of intersubjective learning. Infant Behavior and Development, 30, 258–266. doi: 10.1016/j.infbeh.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Papoušek H, & Papoušek M (2002). Intuitive parenting. In Bornstein & Marc H (Eds.), Handbook of parenting: Biology and ecology of parenting Vol. 2 (2nd ed). (pp. 183–203). Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Perry RE (2019). Poverty and its impact: enhancing mechanistic research via a cross-species approach. In Lipina SJ, & Segretin MS (Eds.), Exploraciones neurocientíficas de la pobreza (pp. 230–254). Erice, Italy: International Mind, Brain and Education School (Ettore Majorana Foundation for Scientific Culture). ISBN 978-987-86-2055-8. [Google Scholar]
- Perry RE, Blair C, & Sullivan RM (2017). Neurobiology of infant attachment: Attachment despite adversity and parental programming of emotionality. Current Opinion in Psychology, 17, 1–6. doi: 10.1016/j.copsyc.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Braren SH, Rincón Cortés M, Brandes-Aitken AN, Chopra D, Sullivan RM, & Blair C (2019a). Enhancing executive functions through social interactions: Causal evidence using a cross-species model. Frontiers in Psychology, 10, 2472. doi: 10.3389/fpsyg.2019.02472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Finegood ED, Braren SH, DeJoseph ML, Putrino DF, Wilson DA, … Blair C, Family Life Project Key Investigators. (2019b). Developing a neurobehavioral animal model of poverty: Drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Development and Psychopathology, 31, 399–418. doi: 10.1017/S095457941800007X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Rincón-Cortés M, Braren SH, Brandes-Aitken AN, Opendak M, Pollonini G, … Sullivan RM (2019c). Corticosterone administration targeting a hypo-reactive HPA axis rescues a socially-avoidant phenotype in scarcity-adversity reared rats. Developmental Cognitive Neuroscience, 40, 100716. doi: 10.1016/j.dcn.2019.100716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R, & Sullivan RM (2014). Neurobiology of attachment to an abusive caregiver: Short-term benefits and long-term costs. Developmental Psychobiology, 56, 1626–1634. doi: 10.1016/j.copsyc.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Behavioral Research Methods, 40, 879–891. doi: 10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- Raineki C, Morgan EJ, Ellis L, & Weinberg J (2019a). Glucocorticoid receptor expression in the stress-limbic circuitry is differentially affected by prenatal alcohol exposure and adolescent stress. Brain Research, 1718, 242–251. doi: 10.1016/j.brainres.2019.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, & Sullivan R (2010). Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry, 67, 1137–1145. doi: 10.1016/j.biopsych.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Opendak M, Sarro E, Showler A, Bui K, McEwen BS, … Sullivan RM (2019b). During infant maltreatment, stress targets hippocampus, but stress with mother present targets amygdala and social behavior. Proceedings of the National Academy of Sciences, 116, 22821–22831. doi: 10.1073/pnas.1907170116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Rincón-Cortés M, Belnoue L, & Sullivan RM (2012). Effects of early-life abuse differacross development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. The Journal of Neuroscience, 32, 7758–7765. doi: 10.1523/jneurosci.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenbacher E, Perry RE, Sullivan RM, & Moita MA (2017). Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. Elife, 6, e24080. doi: 10.7554/eLife.24080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, Barr GA, Mouly AM, Shionoya K, Nuñez BS, & Sullivan RM (2015). Enduring good memories of infant trauma: rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proceedings of the National Academy of Sciences of the United States of America, 112, 881–886. doi: 10.1073/pnas.1416065112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, & Sullivan RM (2016). Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Translational Psychiatry, 6, e930. doi: 10.1038/tp.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Opendak M, Blomkvist A, Chan S, Tan S, Delmer C, … Chopra D (2019). Infant trauma alters social buffering of threat learning: emerging role of prefrontal cortex in preadolescence. Frontiers in Behavioral Neuroscience, 13, 132. doi: 10.3389/fnbeh.2019.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, & Levine S (1992). Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neuroscience & Biobehavioral Reviews, 16, 553–568. doi: 10.1016/S0149-7634(05)80196-4 [DOI] [PubMed] [Google Scholar]
- Roth TL, & Sullivan RM (2005). Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry, 57, 823–831. doi: 10.1016/j.biopsych.2005.01.032 [DOI] [PubMed] [Google Scholar]
- Roth TL, & Sweatt DJ (2011). Annual research review: epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. Journal of Child Psychology and Psychiatry, 52, 398–408. doi: 10.1111/j.1469-7610.2010.02282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, & Meaney MJ (1986). Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews, 11, 65–76. doi: 10.1016/0165-0173(86)90010-X [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, … Goldsmith HH (2006). Environmental influences on family similarity in afternoon cortisol levels: twin and parent-offspring designs. Psychoneuroendocrinology, 31, 1131–1137. doi: 10.1016/j.psyneuen.2006.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, & Pollak SD (2010). Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences, 277, 2661–2666. doi: 10.1098/rspb.2010.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervias R, … Sullivan RM (2007). Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biological Psychiatry, 62, 1070–1079. doi: 10.1016/j.biopsych.2007.04.025 [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, & Sullivan RM (2007). Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior, 52, 391–400. doi: 10.1016/j.yhbeh.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods, 7, 422–445. doi: 10.1037/1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Skinner EA, Kindermann TA, Connell JP, & Wellborn JG (2009). Engagement and disaffection as organizational constructs in the dynamics of motivational development. In Wentzel K & Vigfield A (Eds.), Handbook of motivation at school (pp. 223–245). New York: Routledge. [Google Scholar]
- Stanton ME, & Levine S (1988). Maternal modulation of infant glucocorticoid stress responses: Role of age and maternal deprivation. Psychobiology, 16, 223–228. doi: 10.3758/BF03327311 [DOI] [Google Scholar]
- Stanton ME, & Levine S (1990). Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 23, 411–426. doi: 10.1002/dev.420230504 [DOI] [PubMed] [Google Scholar]
- Stanton ME, Patterson JM, & Levine S (1985). Social influences on conditioned cortisol secretion in the squirrel monkey. Psychoneuroendocrinology, 10, 125–134. doi: 10.1016/0306-4530(85)90050-2 [DOI] [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, Van Oers H, & Levine S (1995). Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: effects of maternal deprivation. Psychoneuroendocrinology, 20, 169–182. doi: 10.1016/0306-4530(94)00051-B [DOI] [PubMed] [Google Scholar]
- Sullivan RM, & Perry RE (2015). Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Social Neuroscience, 10, 500–511. doi: 10.1002/dev.21260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suor JH, Sturge-Apple ML, Davies PT, Cicchetti D, & Manning LG (2015). Tracing differential pathways of risk: Associations among family adversity, cortisol, and cognitive functioning in childhood. Child Development, 86, 1142–1158. doi: 10.1111/cdev.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Flannery J, Caldera C, & Sullivan RM (2019). Parental presence switches avoidance to attraction learning in children. Nature Human Behaviour, 3, 1070–1077. doi: 10.1038/s41562-019-0656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton KJ, & Sullivan RM (2010). Defining age limits of the sensitive period for attachment learning in rat pups. Developmental Psychobiology, 52, 453–464. doi: 10.1002/dev.20448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, Noble KG, & Blair C (2015). Socioeconomic status, subjective social status, and perceived stress: Associations with stress physiology and executive functioning. Behavioral Medicine, 41, 145–154. doi: 10.1080/08964289.2015.1024604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, & Goldsmith HH (2012). Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior, 62, 36–42. doi: 10.1016/j.yhbeh.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L, & Cox M (2013). The Family Life Project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development, 78, 1–150. [DOI] [PubMed] [Google Scholar]
- Walker CD, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, … Taché Y (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 20, 421–448. doi: 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SF, West TV, & Mendes WB (2014). Stress contagion: physiological covariation between mothers and infants. Psychological Science, 25, 934–942. doi: 10.1177/0956797613518352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, Firestein MR, Austin J, Hane AA, Stark RI, Hofer MA, … Myers MM (2015). Family nurture intervention in the neonatal intensive care unit improves social-relatedness, attention, and neurodevelopment of preterm infants at 18 months in a randomized controlled trial. Journal of Child Psychology and Psychiatry, 56, 1202–1211. doi: 10.1111/jcpp.12405 [DOI] [PubMed] [Google Scholar]
- Wiener SG, Johnson DF, & Levine S (1987). Influence of postnatal rearing conditions on the response of squirrel monkey infants to brief perturbations in mother-infant relationships. Physiology & Behavior, 39, 21–26. doi: 10.1016/0031-9384(87)90339-8 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Aber JL, & Beardslee WR (2012). The effects of poverty on the mental, emotional, and behavioral health of children and youth: implications for prevention. American Psychologist, 67, 272–284. doi: 10.1037/a0028015 [DOI] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Kiff CJ, & Fisher PA (2012). Understanding the relation of low income to HPA-axis functioning in preschool children: cumulative family risk and parenting as pathways to disruptions in cortisol. Child Psychiatry & Human Development, 43, 924–942. doi: 10.1007/s10578-012-0304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenko M, Kraemer H, Huffman L, Gschwendt M, Pageler N, & Steiner H (2005). Heart rate correlates of attachment status in young mothers and their infants. Journal of the American Academy of Child & Adolescent Psychiatry, 44, 470–476. doi: 10.1097/01.chi.0000157325.10232.b1 [DOI] [PubMed] [Google Scholar]


