Abstract
Background
The purpose of this study was to determine the safety and efficacy of enteral nutrition in combination with microbial preparations for bowel preparation in elderly patients with colorectal cancer.
Material/Methods
Were divided 160 patients diagnosed with colorectal cancer into a control group (n=80) and an experimental group (n=80) by random number table method. The control group took the traditional intestinal preparation, and the experimental group took oral enteral nutrition combined with microbial preparations. Both groups were treated by the same medical team. The postoperative recovery, complications, nutritional status, inflammation, and other indicators of the 2 groups were compared.
Results
The nutritional status of the experimental group was significantly better than that of the control group, the incidence of tissue inflammation and postoperative complications was significantly lower than that of the control group, and the stool test results of patients with postoperative diarrhea were better than those of the control group, and the difference between groups was statistically significant.
Conclusions
The intestinal preparation using enteral nutrition combined with microbial preparations can alleviate the systemic inflammatory response in elderly patients, improve the nutritional status, reduce the occurrence of postoperative complications, and facilitate rapid postoperative recovery.
Keywords: Aged, Gastrointestinal Agents, Gastrointestinal Neoplasms, Rehabilitation
Background
According to the latest statistics provided by the WHO International Centre for Research on Cancer (IARC) in 2020, the incidence of colorectal cancer is increasing in China; it is the second most prevalent malignant tumor and the fifth leading cause of death [1–3]. It generally occurs in adults aged 60–70 years old and gravely endangers health. Preoperative intestinal preparation is a crucial step to enhance postoperative recovery. The classic intestinal preparation approach must be switched from semi-fluid diet to fluid diet, with repeated enemas until fasting and intravenous fluids [4,5]. These methods not only affect preoperative relaxation, but also lower the intake of calories and minerals, alter the intestinal mucosal barrier, and may lead to intestinal bacterial translocations and enterogenic infections [6].
Intestinal nutrition preparation is easy to absorb, does not form stool, keeps the intestines empty and clean, can meet the nutritional needs of patients, and can stimulate the gastrointestinal tract, activate the intestinal endocrine system, accelerate intestinal hormone synthesis and release, regulating the pancreas, gastrointestinal, biliary tract, such as the secretion of digestive juice, and is conducive to maintaining immunity, biology, and mechanical barrier function [7,8]. It has been observed that the combined use of intestinal nutrition and microbial agents in preoperative intestinal preparation for gastric cancer has a favorable therapeutic impact, but there is little information available on preoperative intestinal preparation in elderly patients with colorectal cancer. In this research, older patients with colorectal cancer were preoperatively prepped with enteral nutrition mixed with probiotics, and generally excellent clinical outcomes were attained.
Material and Methods
Study Population
From November 2020 to July 2021, 160 patients with colorectal cancer were admitted to the Gastrointestinal Surgery Department at Huai’an Hospital Affiliated to Xuzhou Medical University. The inclusion criteria were: 1) Electronic colonoscopy and pathological diagnosis of rectal or colon cancer; 2) No neoadjuvant radiation or chemotherapy was administered; 3) cTNM stages I–III; 4) There was no gastrointestinal obstruction; (5) American Society of Anesthesiologists (ASA) rating of I through III; 6) Active participation and completion of numerous indicator tests and participants and their family members must provide informed consent and sign the informed consent; 8) Age 60–80 years. The exclusion criteria were: 1) Preoperative cognitive impairment and a history of mental illness; 2) Severe cardiopulmonary insufficiency; 3) Emergencies such as intestinal perforation or blockage; 4) Inability to participate with follow-up visits or refusal to sign informed permission. We randomly assigned 160 patients to the experimental or control group, with 80 patients in each group. The Medical Ethics Committee at Huai’an Hospital, which is connected with Xuzhou Medical University, authorized this research (HEYLL-202028).
Covariates
Leukocyte count, neutrophil percentage, C-reactive protein, and IL-6 levels were determined on the day of admission (before intestinal preparation) and postoperatively (1, 3, and 7 days after surgery) to assess the inflammatory response in all recruited patients. Serum total protein, serum albumin, serum prealbumin, and transferrin levels were determined on the admission day (before intestinal preparation) and postoperatively (only before and 7 days after intestinal preparation). During intestinal preparation, we recorded adverse effects such as dizziness, palpitations, fatigue, and nausea, as well as the general postoperative circumstances, including postoperative initial exhaust time, peristalsis recovery time, hospital stay, postoperative delirium, postoperative diarrhea, incision infection, and anastomotic fistula. The fuzzy awareness exam was used by trained caregivers (CAM) to screen for postoperative delirium. For patients with postoperative diarrhea, a stool test was performed and the stool test results were observed. Major objectives were reduction of intestinal bacteria, inversion of rod-to-ball ratio, and reduced fungal infection.
Clinical Intervention
The control group received the following treatments: the standard intestinal preparation procedure was used, with semi-fluid diet on the third day prior to surgery, and a whole-fluid diet on the first day before to surgery. Gentamicin 80 000 U and tinidazole 0.5 g were administered 3 times daily on the last 3 days before surgery. Polyethylene glycol, an oral laxative, was administered 16 h before surgery. The experimental group received the following treatment: Three days before surgery, patients received bifidobacteria triplex viable capsules (Inner Mongolia Shuangqi Pharmaceutical Co., LTD, Approval No.: National Drug Approval 19980004, specification: 0.5 g/tablet) 2.0 g/time, 3 times per day; and oral enteral nutrition preparation (Huarui Pharmaceutical Company, Approval No.: National Drug Approval H20040723, specification: 500 ml) 30~40 mL/kg/d. The specific ingredients of enteral nutrition preparations are shown in Table 1.
Table 1.
Nutritional composition of the enteral nutrition.
| Protein | 29.3 g | Chromium | 33 μg |
| RNA | 8.9 | Molybdenum | 50 μg |
| Fat | 36 g | Selenium | 33.5 μg |
| Saturated fatty acid | 14.5 g | Vitamin A | 1 mg |
| Essential fatty acids | 4.5 g | Vitamin D | 2.3 μg |
| omega-3 fatty acids | 1.5 g | Vitamin E | 13.5 mg |
| Medium chain triglycerides | 11.5 g | Vitamin K | 133 μg |
| Carbohydrates | 52 g | Vitamin B1 | 0.65 mg |
| Sugar | 2.5 g | Vitamin B2 | 0.85 mg |
| Lactose | <0.5 g | Niacinamide | 6 mg |
| Dietary fiber | 6.5 g | Vitamin B6 | 0.8 mg |
| Sodium | 800 mg | Vitamin B12 | 1.3 μg |
| Potassium | 1.2 g | Pantothenic acid | 2.3 mg |
| Chloride | 800 mg | Biotin | 65 μg |
| Calcium | 335 mg | Folic acid | 65 μg |
| Phosphorus | 315 mg | Vitamin C | 40 mg |
| Magnesium | 135 mg | Choline | 133 mg |
| Iron | 6.5 mg | Osmotic pressure | 390 mosm/L |
| Zinc | 5 mg | Energy | 2730 kJ (650 kcal) |
| Copper | 0.65 mg | Energy source | |
| Manganese | 1.35 mg | Protein | 18% |
| iodide | 66.5 μg | Fat | 50% |
| Fluoride | 0.65 mg | Carbohydrates | 32% |
For patients in both groups, the same group of doctors performed laparoscopic-aided radical resection of colorectal cancer (CME/TME). Apart from the various preoperative intestinal preparation techniques, the remaining treatment strategies for colorectal surgery were based on the Chinese expert consensus and the Path Management Guide of Accelerated Rehabilitation Surgery (2018 edition). Throughout the entire process, standardized management was used, including preoperative education, preoperative pre-rehabilitation, preoperative medication, preventive antithrombotic therapy, anesthesia program and intraoperative management, prevention and treatment of postoperative nausea and vomiting, perioperative fluid management, pelvic or abdominal drainage tube management, and postoperative analgesia [9,10].
Statistical Analysis
All graphics were drawn and assembled using Adobe Illustrator 2020 (Adobe, Inc, USA), GraphPad Prism 9 (San Diego, California, USA). All data were analyzed using SPSS version 22.0 (SPSS, Inc. Chicago, IL, USA) statistical software. The mean and standard deviation of normally distributed measurement data were calculated, and test was used for comparison between groups, and repeated measures analysis of variance was used for repeated measurement data. Statistics are reported as instance counts or percentages (percentages) and compared using a two-tailed test. All statistical tests were two-tailed and a significance level of P<0.05 was considered statistically significant.
Results
Baseline Characteristics
There were no significant differences in age, sex, body mass index, tumor site, or pathological type between the 2 groups (P<0.05), as shown in Table 2.
Table 2.
Comparison of general data between the 2 groups.
| Test group (n=80) | Control group (n=80) | t/χ2 | P-value | |
|---|---|---|---|---|
| Age | 66.85±4.05 | 64.95±4.347 | 1.429 | 0.609 |
| Gender | 0.312 | 0.555 | ||
| Male | 21 | 22 | ||
| Female | 19 | 18 | ||
| BMI | 25.665±1.59 | 25.304±0.96 | 0.865 | 0.249 |
| Tumor site | 1.623 | 0.379 | ||
| The colon | 20 | 22 | ||
| The rectum | 20 | 18 | ||
| The pathologic types | 2.208 | 0.757 | ||
| Moderately | 25 | 24 | ||
| Differentiated adenocarcinoma | ||||
| Poorly | 9 | 11 | ||
| Differentiated adenocarcinoma | ||||
| Moderately – poorly | 6 | 5 | ||
| differentiated adenocarcinoma |
The Inflammatory Response
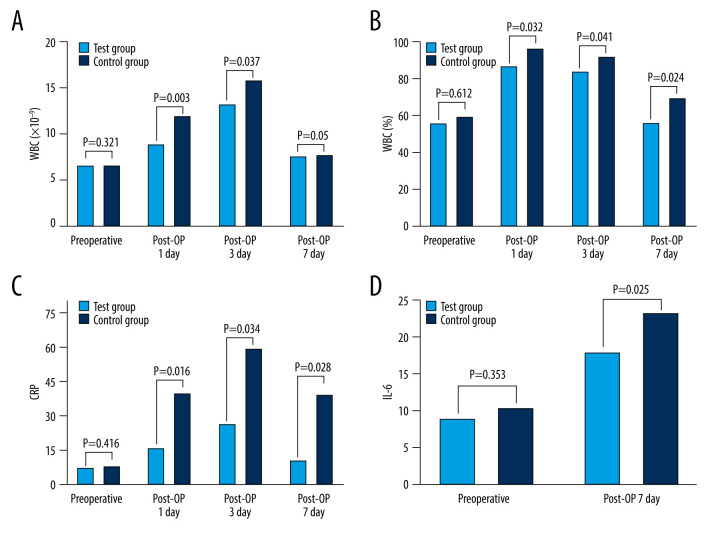
As shown in Table 3 and Figure 1, there were no significant differences in preoperative inflammatory markers such as total white blood cell count, neutrophil ratio, C-reactive protein, and IL-6 between the experimental and control groups (C-reactive protein: P=0.416, total white blood cell count: P=0.321, NBC/percent: P=0.612, IL-6: P=0.353). On the first and third days after surgery, the experimental group’s total white blood cell count and neutrophil ratio were significantly lower than those in the control group (P<0.05). C-reactive protein levels rose considerably in both groups on the first, third, and seventh days after surgery, with statistically significant differences (first day after surgery: P=0.016, third day after surgery: P=0.034, and seventh day after surgery: P=0.028). As shown in Figure 1D, both groups had a substantial rise in IL-6 levels 7 days after surgery as compared to before surgery, but the degree of increase was less in the experimental group than in the control group (P=0.025).
Table 3.
Comparison of inflammatory indexes between 2 groups.
| Test group (n=80) | Control group (n=80) | t | P-value | |
|---|---|---|---|---|
| WBC, ×109/L | ||||
| Preintestinal preparation | 6.59±1.32 | 6.60±1.69 | 0.009 | 0.321 |
| Post-OP 1 day | 8.87±2.88 | 11.94±1.35 | 4.327 | 0.003 |
| Post-OP 3 day | 13.20±2.98 | 15.83±1.28 | 3.625 | 0.037 |
| Post-OP 7 day | 7.58±1.86 | 7.67±1.26 | 1.169 | 0.05 |
| Neuter cell ratio, % | ||||
| Preintestinal preparation | 56.49±3.52 | 59.42±2.38 | 11.51 | 0.612 |
| Post-OP 1 day | 86.96±3.30 | 96.87±2.70 | 1.959 | 0.032 |
| Post-OP 3 day | 84.10±3.18 | 92.41±1.74 | 10.259 | 0.041 |
| Post-OP 7 day | 56.19±4.71 | 69.76±4.32 | 9.499 | 0.024 |
| C-reactive protein, mg/L | ||||
| Preintestinal preparation | 7.07±1.23 | 7.72±2.43 | 1.078 | 0.416 |
| Post-OP 1 day | 15.79±3.15 | 39.82±14.13 | 7.42 | 0.016 |
| Post-OP 3 day | 26.08±2.36 | 59.22±19.91 | 9.621 | 0.034 |
| Post-OP 7 day | 10.07±1.67 | 39.13±14.04 | 9.188 | 0.028 |
| IL-6, pg/ml | ||||
| Preintestinal preparation | 8.84±1.93 | 10.43±2.37 | 2.344 | 0.353 |
| Post-OP 7 day | 17.98±2.23 | 23.42±3.24 | 6.197 | 0.025 |
Post-OP 1 day – the first day after surgery; Post-OP day 3 – the third day after surgery; Post-OP day 7 – the seventh day after surgery.
Figure 1.
Comparison of inflammatory indexes between 2 groups. (A) WBC. (B) NBC. (C) CRP. (D) IL-6. WBC – white blood cell; NBC – neutrophil ratio; CRP – C-reactive protein; IL-6 – interleukin-6; Post-OP day 1 – the first day after surgery; Post-OP day 3 – the third day after surgery; Post-OP day 7 – the seventh day after surgery.
Nutritional Status
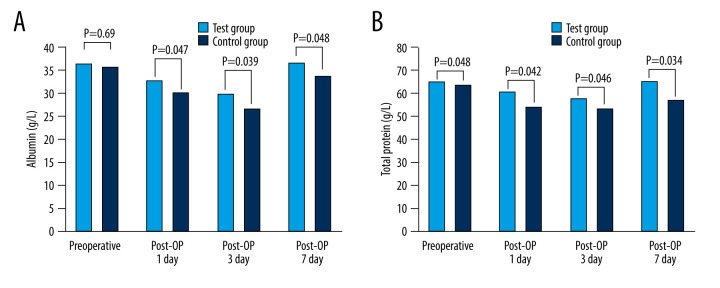
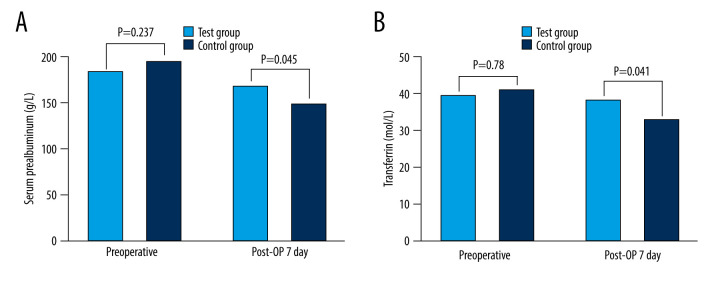
As demonstrated in Table 4 and Figure 2, there was no statistically significant difference in serum total protein or serum albumin levels prior to surgery between the 2 groups (total serum protein: P=0.48, serum albumin: P=0.69). Hypoproteinemia occurred in both groups after surgery, but the serum albumin level in the experimental group was significantly higher than that in the control group (P=0.047 on the first postoperative day, P=0.039 on the third postoperative day, P=0.048 on the seventh postoperative day). There were no significant variations in serum prealbumin and transferrin levels between the 2 groups prior to surgery (serum prealbumin: P=0.237; serum transferrin: P=0.780), as shown in Figure 3. The 2 indices in the experimental group were better to those in the control group on the seventh day after surgery (P=0.045).
Table 4.
Comparison of nutritional indexes between the 2 groups.
| Test group (n=80) | Control group (n=80) | t | P-value | |
|---|---|---|---|---|
| Total serum protein, g/L | ||||
| Preintestinal preparation | 65.46±2.03 | 63.95±0.89 | 3.03 | 0.48 |
| Post-OP 1 day | 60.91±2.22 | 54.27±1.40 | 11.303 | 0.042 |
| Post-OP 3 day | 58.04±1.80 | 53.48±2.84 | 4.761 | 0.046 |
| Post-OP 7 day | 65.64±1.27 | 57.44±1.04 | 22.245 | 0.034 |
| Serum albumin, g/L | ||||
| Preintestinal preparation | 36.32±1.04 | 35.66±0.72 | 2.305 | 0.69 |
| Post-OP 1 day | 31.76±1.17 | 29.90±0.82 | 5.823 | 0.047 |
| Post-OP 3 day | 29.82±1.37 | 26.47±0.88 | 9.213 | 0.039 |
| Post-OP 7 day | 36.50±1.15 | 33.58±0.94 | 8.758 | 0.048 |
| Serum prealbumin, g/L | ||||
| Preintestinal preparation | 183.78±23.25 | 194.87±29.26 | 1.328 | 0.237 |
| Post-OP 7 day | 167.88±11.18 | 149.39±8.56 | 5.868 | 0.045 |
| Transferrin, mol/L | ||||
| Preintestinal preparation | 39.62±5.90 | 41.03±5.76 | 0.767 | 0.78 |
| Post-OP 7 day | 38.17±3.30 | 32.77±2.30 | 5.987 | 0.041 |
Post-OP day 1 – the first day after surgery; Post-OP day 3 – the third day after surgery; Post-OP day 7 – the seventh day after surgery.
Figure 2.
Comparison of nutritional indexes between 2 groups. (A) Albumin. (B) Total protein. Post-OP day 1 – the first day after surgery; Post-OP day 3 – the third day after surgery; Post-OP day 7 – the seventh day after surgery.
Figure 3.
Comparison of nutritional indexes between the 2 groups. (A) Serum prealbumin. (B) Transferrin. Post-OP day 1 – the first day after surgery; Post-OP day 3 – the third day after surgery; Post-OP day 7 – the seventh day after surgery.
The General Situation
The experimental group’s recovery time for intestinal peristalsis was substantially less than the control group’s (P=0.025), and the experimental group’s first exhaust time was 50.43±3.47 h after surgery, compared to 58.8±5.16 h in the control group; there were no clear signs of abdominal distention, and the difference was statistically significant (P=0.043). As demonstrated in Table 5, the experimental group’s hospital stay was shorter (19.65±2.621 days) than that of the control group (21.8±2.821 days) (P=0.048).
Table 5.
Comparison of postoperative recovery time between the 2 groups.
| Test group (n=80) | Control group (n=80) | t | P-value | |
|---|---|---|---|---|
| First exhaust time (hours) | 50.43±3.47 | 58.8±5.16 | 1.964 | 0.043 |
| Days in hospital (days) | 19.65±2.621 | 21.8±2.821 | 2.497 | 0.048 |
| Postoperative peristalsis recovery time (hours) | 42.88±3.98 | 51.85±5.15 | 2.168 | 0.025 |
Postoperative Complications
Postoperative delirium occurred in none of the 80 patients in the experimental group, incision infection occurred in 2, and postoperative diarrhea occurred in 4, for a complication rate of 7.5% (6/80). Postoperative delirium occurred in 4 patients in the control group, incision infection occurred in 4 patients, and postoperative diarrhea occurred in 10 patients, for a complication rate of 22.5% (18/80). The experimental group had a much lower rate of postoperative complications than the control group, a difference that was statistically significant (P<0.05). As demonstrated in Table 6, anastomotic fistula did not develop in either group.
Table 6.
Comparison of the number of complications between the 2 groups.
| Test group (n=80) | Control group (n=80) | P-value | |
|---|---|---|---|
| Postoperative delirium | 0 | 4 | 0.0038 |
| Postoperative anastomotic fistula | 0 | 0 | — |
| Infection of incision | 2 | 4 | 0.0047 |
| Postoperative diarrhea | 4 | 10 | 0.0027 |
| Total | 7.5% (6/80) | 22.5% (18/80) | — |
Stool Examination Results
As shown in Table 7, the stool examination results of patients with postoperative diarrhea showed that the experimental group had 2 cases of decreased intestinal bacteria, 1 case of rod-ball ratio inversion, and 1 case of fungal infection, all of which were less than those of the control group, and the difference was statistically significant (P<0.05).
Table 7.
Comparison and analysis of stool examination results after different bowel preparation methods between the 2 groups.
| Reduction of intestinal bacteria | Inversion of rod-to-ball ratio | Fungal infection | |
|---|---|---|---|
| Test group (n=80) | 2 | 1 | 1 |
| Control group (n=80) | 9 | 7 | 8 |
| χ2 | 8.8613 | 6.7314 | 10.2139 |
| P-value | 0.0029 | 0.0090 | 0.0012 |
Discussion
Preoperative intestinal preparation has always been an important step in the perioperative period of colorectal cancer patients. With continuous exploration and evidence-based scientific research, various systematic preparation schemes have been formed [11–13]. In the past, mechanical enema was mostly used, but this method caused great pain for patients, and repeated enemas could easily lead to mucosal damage and other problems, thus increasing the occurrence of postoperative complications [14,15]. This method has been gradually abandoned, but it is still a necessary preoperative preparation for some patients undergoing emergency surgery [16]. For patients with non-obstructive colorectal cancer, oral laxatives and antibiotics are the most commonly used methods in clinical practice. In this regimen, patients need to take oral antibiotics for 3 consecutive days. It is believed that oral antibiotics for several consecutive days not only fail to achieve the best effect, but also tend to cause the over-propagation of intestinal fungi and increase complications [17,18].
With the gradual development and spread of rehabilitation surgery, a growing number of clinical physicians and researchers have used intestinal nutrition and microbial preparation for preoperative intestinal preparation for colorectal cancer surgery. A study on the comparison of enteral nutrition emulsion with traditional bowel preparation reported that the number of preoperative enemas in the enteral nutrition emulsion group was significantly less than that in the traditional bowel preparation group, the pain of repeated enemas was reduced, and the bowel cleaning rate was significantly higher. The results of the above study showed that patients with colorectal cancer were prepared by the preoperative intestinal tract, which ensured good intestinal cleanliness, and the nutritional status of the patients was improved, and inflammation was significantly reduced [19–21]. A recent study on preparation for intestinal bowel surgery compared the patients who were given oral probiotics before bowel preparation and those who did not take probiotics before bowel preparation, and the results showed that the intestinal cleaning efficiency of the probiotic group was significantly higher than that of the control group, there was less postoperative stress in the control group, and the immune function was better than in the control group. The results showed that oral probiotics could improve the intestinal clarity of patients with colorectal cancer, reduce postoperative stress, and enhance the immune function of patients, but these patients were prone to lack of nutritional intake, and the postoperative nutritional status was not significantly improved [22–24].
Therefore, research on an effective method that can not only improve intestinal cleanliness, but also provide sufficient calories and nutrients, prevent intestinal flora disorder, and improve immune function has become an important research topic [25,26]. In this study, enteral nutrition combined with microbial preparation was used as preoperative intestinal preparation for colorectal cancer surgery, in the expectation of improving the nutritional status of patients, reducing inflammatory response, reducing postoperative complications, and preventing and controlling bacterial imbalance.
Most colorectal cancer patients have varying degrees of malnutrition before surgery, and patients eat less, resulting in an imbalance of intestinal flora [27–29]. Therefore, nutritional support and appropriate probiotics supplementation are very important to correct malnutrition and improve the intestinal flora [30]. Inthe present study, hypoproteinemia occurred in both groups after surgery, but serum albumin in the experimental group was significantly higher than that in the control group (P=0.047 on the first postoperative day, P=0.039 on the third postoperative day, and P=0.048 on the seventh postoperative day). Due to surgical shock, all patients showed an increase in inflammatory indicators after surgery, but the inflammatory response of the experimental group was significantly less than in the control group, in which the change of C-reactive protein (CRP) was the most significant. CRP is an acute-stage protein that rises rapidly after tissue lesions, malignant tumors, surgical trauma, and various acute or chronic infections, and quickly returns to normal with improvement of the condition. C-reactive protein is positively correlated with the degree of tissue damage. It is not affected by other factors in the acute stage, such as blood pressure, respiration, and heart rate, and can accurately reflect the degree of tissue damage and inflammatory response of patients in the early stage [31–33]. In the present study, C-reactive protein increased in both groups on the first, third, and seventh days after surgery compared with that before surgery, and the increase was more obvious in the control group, with statistically significant differences (the first day after surgery: P=0.016, the third day after surgery: P=0.028, and the seventh day after surgery: P=0.034). The above results indicated that the postoperative nutritional status of patients in the experimental group was better than that in the control group, and the inflammatory response was lower than that in the control group, which improved the postoperative recovery of patients. It has been reported that inflammatory factor C-reactive protein was significantly increased in peripheral blood of patients with colorectal cancer on the third day after surgery, which can be used as a blood marker to evaluate the occurrence of postoperative delirium [34–36]. However, postoperative delirium may be associated with inflammatory disorders caused by external stimuli and central nervous system inflammation. A number of recent studies have reported a correlation between intestinal microbes and mental state. Intestinal probiotics can improve patients’ mental state to a certain extent, and even play an important role in severe mental diseases such as depression and schizophrenia [37,38]. Although the occurrence of postoperative delirium is affected by many factors, the number of cases of postoperative delirium in the experimental group was significantly lower than that in the control group, suggesting that enteral nutrition combined with microbial agents instead of traditional intestinal preparation can reduce the occurrence of postoperative delirium by reducing the stress response of patients and improving the intestinal flora. The intestinal nutrient solution matched with microbial preparation is easy to digest, provides comprehensive nutrition, has high bioavailability, improves intestinal flora, and is easy to use.
Simple general anesthesia or epidural anesthesia and postoperative opioid analgesics as needed were often the main anesthesia methods in the past. Now general anesthesia combined with epidural block anesthesia and postoperative indwelling epidural catheter for postoperative pain relief are widely used in clinical practice. The use of mid-thoracic cannulae is emphasized in epidural block anesthesia, thereby inhibiting the afferent of adrenal sympathetic nerve excitation, reducing the patient’s stress response, in order to allow patients to receive surgical treatment in a state closer to their own physiological state. Optimizing the preoperative medication regimen, speeding post-anesthesia recovery, avoiding early postoperative complications, reducing stress response, and adequate and effective analgesia are the main manifestations of the optimization of anesthesia methods required by ERAS. To enable patients to move early and reduce postoperative complications after anesthesia, fast- and short-acting anesthetics are recommended by ERAS; for better postoperative analgesia and relief of respiratory depression and early recovery of postoperative intestinal paralysis, ERAS recommends the use of epidural anesthesia as an alternative to general anesthesia. The latest research shows that in terms of anti-stress, continuous infusion of dexmedetomidine under general anesthesia has the same effect as general anesthesia combined with mid-thoracic epidural block and can be used instead. Total intravenous anesthesia can also manage the traumatic stress of abdominal surgery, especially laparoscopic surgery with minimally invasive characteristics [26].
The safety of enteral nutrition therapy as a method of intestinal preparation for patients with colorectal cancer has been confirmed in many previous studies, but the subjects of previous studies were young patients. Because elderly patients may have other basic diseases and poor general conditions while their physiological metabolism is gradually slow and organ function is declining, the safety and effectiveness in elderly patients have not been confirmed. In this study, after excluding cases of severe cardiopulmonary diseases, enteral nutrition combined with microbial agents was used as preoperative intestinal preparation for elderly patients with colorectal cancer, which improved their nutritional status. At the same time, due to better nutritional preparation, the tolerance of elderly patients was also improved during the operation [15,24]. Compared with the previous application of enteral nutrition therapy in young patients, our results suggest that use in elderly patients can have a direct impact on the treatment effect and prognosis of postoperative diseases, and can significantly improve the short-term prognosis.
In conclusion, in this study, the basic principles of Enhanced Recovery After Surgery (ERAS) were followed in advanced laparoscopic radical colorectal cancer surgery. The nutritional status, inflammatory response, general conditions, and postoperative complications of patients were compared between enteral nutrition combined with microbial preparation as preoperative intestinal preparation and traditional intestinal preparation. Our results suggest that the intestinal nutrition combination probiotics as bowel preparation is superior to the traditional bowel preparation, does not increase the postoperative complications, and improves the nutritional status of patients to reduce inflammation, reduce the incidence of postoperative delirium, and speed rehabilitation, while oral enteral nutrient solution with microbial preparation does not increase the patient’s discomfort, is easy to use, and is associated with good patient compliance. As a routine preoperative intestinal preparation method for patients with colorectal cancer, it has a positive impact on clinical treatment and short-term prognosis. The sample size included in this study was small, and the long-term prognosis was not evaluated. The results of this study need to be confirmed by large-sample, multi-center, randomized controlled studies. In addition, the present study was insufficient for fecal detection of patients after bowel preparation. A weakness of our study was that we did not conduct detailed comparisons of the specific intestinal bacteria between the experimental group and the control group. More detailed research is needed to further compare the results of intestinal flora detection.
Conclusions
After surgery, older people may benefit from intestinal preparation using enteral nutrition mixed with microbial preparations to minimize the inflammatory response, enhance nutritional status, and decrease the occurrence of postoperative complications.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by grants from the 15th Batch of High-level Talent Selection and Training Program of “Six Talent Peaks” in Jiangsu Province to Xiaoyu Zhang [grant number: WSW-291]
References
- 1.Silano V, Barat Baviera JM, Bolognesi C, et al. Assessment of the impact of the IARC Monograph Vol. 121 on the safety of the substance styrene (FCM No 193) for its use in plastic food contact materials. EFSA J. 2020;18:e06247. doi: 10.2903/j.efsa.2020.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Li L, Tian Y, et al. Analysis of KRAS mutations in circulating tumor DNA and colorectal cancer tissue. Biotech Histochem. 2021;96:376–83. doi: 10.1080/10520295.2020.1810775. [DOI] [PubMed] [Google Scholar]
- 3.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46:77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Koller SE, Bauer KW, Egleston BL, et al. Comparative effectiveness and risks of bowel preparation before elective colorectal surgery. Ann Surg. 2018;267:734–42. doi: 10.1097/SLA.0000000000002159. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Yang Z, Chen HQ, Qin HL. [Effect of bowel preparation with probiotics on intestinal barrier after surgery for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:528–31. [in Chinese] [PubMed] [Google Scholar]
- 6.Liu Y, Tao KX, Wang GB, et al. [Effect of enteral nutrition as replacement of traditional bowel preparation on the intraperitoneal and intraluminal disseminated tumor cells, recurrence and metastasis in patients with colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:350–53. [in Chinese] [PubMed] [Google Scholar]
- 7.Pittet O, Nocito A, Balke H, et al. Rectal enema is an alternative to full mechanical bowel preparation for primary rectal cancer surgery. Colorectal Dis. 2015;17:1007–10. doi: 10.1111/codi.12974. [DOI] [PubMed] [Google Scholar]
- 8.Lei P, Ruan Y, Yang X, et al. Preoperative mechanical bowel preparation with oral antibiotics reduces surgical site infection after elective colorectal surgery for malignancies: Results of a propensity matching analysis. World J Surg Oncol. 2020;18:35. doi: 10.1186/s12957-020-1804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldas JR, Panerai RB, Bor-Seng-Shu E, et al. Dynamic cerebral autoregulation: A marker of post-operative delirium? Clin Neurophysiol. 2019;130:101–8. doi: 10.1016/j.clinph.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janjua MS, Spurling BC, Arthur ME. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Postoperative delirium. [PubMed] [Google Scholar]
- 11.Mangas-Sanjuan C, Santana E, Cubiella J, et al. Variation in colonoscopy performance measures according to procedure indication. Clin Gastroenterol Hepatol. 2020;18:1216–23e2. doi: 10.1016/j.cgh.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Luceri C, Femia AP, Tortora K, et al. Supplementation with phytoestrogens and insoluble fibers reduces intestinal carcinogenesis and restores ER-β expression in Apc-driven colorectal carcinogenesis. Eur J Cancer Prev. 2020;29:27–35. doi: 10.1097/CEJ.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 13.Maida M, Sinagra E, Morreale GC, et al. Effectiveness of very low-volume preparation for colonoscopy: A prospective, multicenter observational study. World J Gastroenterol. 2020;26:1950–61. doi: 10.3748/wjg.v26.i16.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niedermaier T, Amitay EL, Gies A, et al. Impact of inadequate bowel cleansing on colonoscopic findings in routine screening practice. Clin Transl Gastroenterol. 2020;11:e00169. doi: 10.14309/ctg.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovász BD, Szalai M, Oczella L, et al. Improved adenoma detection with linked color imaging technology compared to white-light colonoscopy. Scand J Gastroenterol. 2020;55:877–83. doi: 10.1080/00365521.2020.1786850. [DOI] [PubMed] [Google Scholar]
- 16.Su H, Lao Y, Wu J, et al. Personal instruction for patients before colonoscopies could improve bowel preparation quality and increase detection of colorectal adenomas. Ann Palliat Med. 2020;9:420–27. doi: 10.21037/apm.2020.03.24. [DOI] [PubMed] [Google Scholar]
- 17.He JD, Kong C, Gao RY, et al. [Effects of probiotics on the intestinal microecological abnormalities and colorectal cancer of mice induced by high-fat diet]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:77–85. doi: 10.3760/cma.j.cn.441530-20200417-00223. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 18.Li PP, Yan Y, Zhang HT, et al. Biological activities of siRNA-loaded lanthanum phosphate nanoparticles on colorectal cancer. J Control Release. 2020;328:45–58. doi: 10.1016/j.jconrel.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Pangeni R, Subedi L, Jha SK, et al. Improvements in the oral absorption and anticancer efficacy of an oxaliplatin-loaded solid formulation: Pharmacokinetic properties in rats and nonhuman primates and the effects of oral metronomic dosing on colorectal cancer. Int J Nanomedicine. 2020;15:7719–43. doi: 10.2147/IJN.S267424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgari-Karchekani S, Karimian M, Mazoochi T, et al. CDX2 protein expression in colorectal cancer and itscorrelation with clinical and pathological characteristics, prognosis, and survival rate of patients. J Gastrointest Cancer. 2020;51:844–49. doi: 10.1007/s12029-019-00314-w. [DOI] [PubMed] [Google Scholar]
- 21.Iwama T, Fujiya M, Konishi H, et al. Bacteria-derived ferrichrome inhibits tumor progression in sporadic colorectal neoplasms and colitis-associated cancer. Cancer Cell Int. 2021;21:21. doi: 10.1186/s12935-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ER, Chang DK. Management of complications of colorectal submucosal dissection. Clin Endosc. 2019;52:114–19. doi: 10.5946/ce.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Othman MH, Zayed GM, Ali UF, Abdellatif AAH. Colon-specific tablets containing 5-fluorouracil microsponges for colon cancer targeting. Drug Dev Ind Pharm. 2020;46:2081–88. doi: 10.1080/03639045.2020.1844730. [DOI] [PubMed] [Google Scholar]
- 24.Kang RK, Mishr N, Rai VK. Guar gum micro-particles for targeted co-delivery of doxorubicin and metformin HCL for improved specificity and efficacy against colon cancer: In vitro and in vivo studies. AAPS PharmSciTech. 2020;21:48. doi: 10.1208/s12249-019-1589-3. [DOI] [PubMed] [Google Scholar]
- 25.Jalilzadeh N, Samadi N, Salehi R, et al. Novel nano-vehicle for delivery and efficiency of anticancer auraptene against colon cancer cells. Sci Rep. 2020;10:1606. doi: 10.1038/s41598-020-58527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo R, Wang YJ, Liu M, et al. The effect of quality of segmental bowel preparation on adenoma detection rate. BMC Gastroenterol. 2019;19:119. doi: 10.1186/s12876-019-1019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson R, Burr NE, Valori R. Causes of post-colonoscopy colorectal cancers based on world endoscopy organization system of analysis. Gastroenterology. 2020;158:1287–99e2. doi: 10.1053/j.gastro.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Miacci FLC, Guetter CR, Moreira PH, et al. Predictive factors of low anterior resection syndrome following anterior resection of the rectum. Rev Col Bras Cir. 2020;46:e20192361. doi: 10.1590/0100-6991e-20192361. [DOI] [PubMed] [Google Scholar]
- 29.Cambray M, Gonzalez-Viguera J, Berenguer MA, et al. Short-course radiotherapy in locally advanced rectal cancer. Clin Transl Gastroenterol. 2020;11:e00162. doi: 10.14309/ctg.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima R, Parra RS, Feitosa MR, et al. Surgical and postoperative evaluations of rectal adenomas excised with a rigid proctoscope. Acta Cir Bras. 2020;35:e202000807. doi: 10.1590/s0102-865020200080000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi Y, Sawaya M, Oka S, et al. Efficacy of autofluorescence imaging for flat neoplasm detection: A multicenter randomized controlled trial (A-FLAT trial) Gastrointest Endosc. 2019;89:460–69. doi: 10.1016/j.gie.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Kong F. Malnutrition-related factors increased the risk of anastomotic leak for rectal cancer patients undergoing surgery. Biomed Res Int. 2020;2020:5059670. doi: 10.1155/2020/5059670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy B, Myers E, O’Shea T, et al. Correlation between adenoma detection rate and polyp detection rate at endoscopy in a non-screening population. Sci Rep. 2020;10:2295. doi: 10.1038/s41598-020-58963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16:335–45. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araki M. Blockade of IL-6 signaling in neuromyelitis optica. Neurochem Int. 2019;130:104315. doi: 10.1016/j.neuint.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Hu P, Chen Y, Pang J, Chen X. Association between IL-6 polymorphisms and sepsis. Innate immunity. 2019;25:465–72. doi: 10.1177/1753425919872818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72:4–12. doi: 10.4097/kja.d.18.00073.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korc-Grodzicki B, Root JC, Alici Y. Prevention of post-operative delirium in older patients with cancer undergoing surgery. J Geriatr Oncol. 2015;6:60–69. doi: 10.1016/j.jgo.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]