Abstract
Background
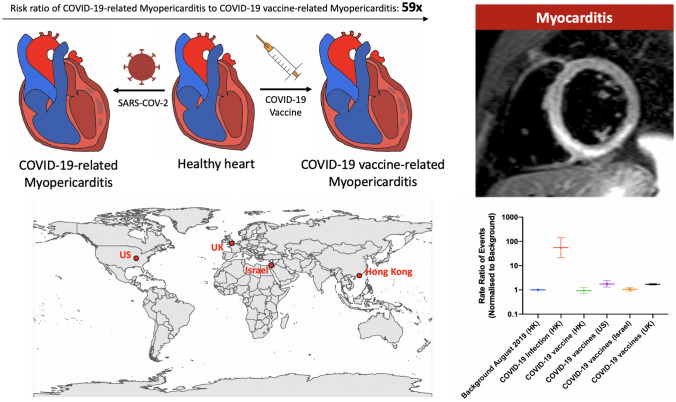
Both COVID-19 infection and COVID-19 vaccines have been associated with the development of myopericarditis. The objective of this study is to (1) analyse the rates of myopericarditis after COVID-19 infection and COVID-19 vaccination in Hong Kong, (2) compared to the background rates, and (3) compare the rates of myopericarditis after COVID-19 vaccination to those reported in other countries.
Methods
This was a population-based cohort study from Hong Kong, China. Patients with positive RT-PCR test for COVID-19 between 1st January 2020 and 30th June 2021 or individuals who received COVID-19 vaccination until 31st August were included. The main exposures were COVID-19 positivity or COVID-19 vaccination. The primary outcome was myopericarditis.
Results
This study included 11,441 COVID-19 patients from Hong Kong, four of whom suffered from myopericarditis (rate per million: 326; 95% confidence interval [CI] 127–838). The rate was higher than the pre-COVID-19 background rate in 2019 (rate per million: 5.5, 95% CI 4.1–7.4) with a rate ratio of 55.0 (95% CI 21.4–141). Compared to the background rate, the rate of myopericarditis among vaccinated subjects in Hong Kong was similar (rate per million: 5.5; 95% CI 4.1–7.4) with a rate ratio of 0.93 (95% CI 0.69–1.26). The rates of myocarditis after vaccination in Hong Kong were comparable to those vaccinated in the United States, Israel, and the United Kingdom.
Conclusions
COVID-19 infection was associated with significantly higher rate of myopericarditis compared to the vaccine-associated myopericarditis.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-022-02007-0.
Keywords: COVID-19, Vaccine, Myopericarditis, Myocarditis, Pericarditis
Introduction
Since the beginning of the COVID-19 pandemic, cases of myocarditis and pericarditis related to the infection have been described [1, 2]. Recent studies have also reported possible associations between COVID-19 vaccines and the risk of myo-pericardial inflammation, raising concerns about vaccination uptake especially in teenagers. Previously, a preprint study published went viral as it miscalculated the denominator, such that that 1 in 1000 would develop heart inflammation upon COVID-19 vaccination. In this study, we conducted a population-based study using data from Hong Kong, China to determine the rates of myopericarditis after COVID-19 infection and COVID-19 vaccination, comparing them to background rates and addressing the rate ratio of vaccine versus COVID-19 myopericarditis.
Methods
This population-based retrospective cohort study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 20–250). The need for informed consent was waived by the Ethics Committee owing to its observational retrospective nature. Patients who tested positive for COVID-19 by real-time polymerase chain reaction (RT-PCR) at any of the Hong Kong public hospitals or outpatient clinics between 1st January 2020 and 30th June 2021 were included. The background number of myopericarditis in 2018 and 2019 in Hong Kong were obtained using the ICD codes 420.9, 422.x, 423.9, and 429.0 suggested by a previously published paper [3]. The data were obtained from the local electronic healthcare database, Clinical Data Analysis and Reporting System as reported previously [4]. The primary outcome was myopericarditis. The vaccination data of different countries were extracted using keywords labelled “Myocarditis” and “Pericarditis” upon searching PubMed and the official reports of Hong Kong and the United Kingdom. The data in Hong Kong and the United Kingdom were up to 31st August 2021 and 29th September 2021, respectively.
The rates after COVID-19 infection were calculated by dividing the number of myocarditis and pericarditis patients by the number of RT-PCR positive COVID-19 patients and adjusted to 14 days. The number of doses was defined as the sum of the number of people who received the first and second doses. The hybrid Wilson/Brown method was used to calculate 95% confidence intervals for the rates (Supplementary Table 1). The analysis was conducted using PRISM (Version: 9.0.0) and RStudio (Version: 1.4.1103).
Results
A total of 11,441 COVID-19 patients from Hong Kong were included, of which four (mean age: 71.2; 50% male) suffered from myopericarditis (rate per million: 326; 95% confidence interval [CI] 127–838). The rate was higher than the pre-pandemic background rate in 2019 (rate per million: 5.9, 95% CI 5.4–6.5) with a rate ratio of 55.0 (95% CI 21.4–141.; Table 1). As of 31st August 2021, 2,811,500 doses of CoronaVac (37.05%) and 4,776,700 doses of BioNTech (62.95%) have been administered [5]. The mean age of the individuals vaccinated with CoronaVac was 61.58 (SD: 11.08) and with Comirnaty was 56.81 (SD: 13.43) [6]. The proportion of male in individuals vaccinated with CoronaVac was 48.7% and with BioNTech was 47.4% [6]. 42 patients developed myopericarditis within 14 days after vaccination, 16 of which belonged to the 12–15 age group. Out of the 42 patients, 41 patients were vaccinated with BioNTech vaccine, while one patient was vaccinated with CoronaVac vaccine. Compared to the background rates, the rate of myopericarditis among the vaccinated subjects in Hong Kong was similar (rate per million: 5.5; 95% CI 4.1–7.4) with a rate ratio of 0.93 (95% CI 0.69–1.26). The rates of myocarditis after vaccination in Hong Kong were comparable to those vaccinated in the United States, Israel, and the United Kingdom (Fig. 1).
Table 1.
Incidence rate and rate ratio of myocarditis and pericarditis after COVID-19 infection and vaccination
| Outcomes | Background 2019 (Hong Kong, China) |
Covid-19 infection (Hong Kong, China) |
COVID-19 vaccine (Hong Kong, China)* [5] |
COVID-19 vaccines (US) [12] |
COVID-19 vaccines (Israel) [2] |
COVID-19 vaccines (UK) [13]** |
|---|---|---|---|---|---|---|
| Diagnosis | Myopericarditis | Myopericarditis | Myopericarditis | Myopericarditis | Myocarditis | Myopericarditis |
| Type of vaccines | NA | NA |
CoronaVac BNT162b2 |
BNT162b2 mRNA-1273 Ad26.COV2.S |
BNT162b2 |
BNT162b2 mRNA-1273 ChAdOx1 |
| Cases | 529 | 4 | 42 | 57 | 136 | 947 |
| Unit | Persons | Persons | Doses | Doses | Doses | Doses |
| Time interval | 183 days | 15 days | ≤ 14 days |
3.5 days (myocarditis) 20 days (pericarditis) |
≤ 21 days (1st dose) ≤ 30 days (2nd dose) |
NA |
| Persons or doses received | 6,819,672 | 11,441 | 7,588,200 | 3,530,507 | 10,568,331 | 93,600,000 |
| Rate per million persons or doses per 14 days (95% CI) |
5.9 (5.4, 6.5) |
326 (127, 838) |
5.5 (4.1, 7.4) |
10.3 (7.7, 14.7) |
6.27 (5.3, 7.4) |
10.1 9.5, 10.8 |
| Rate ratio (95% CI) | 1 (baseline) | 55.0 (21.4, 141) | 0.93 (0.69 1.26) | 1.74 (1.30, 2.47) | 1.06 (0.89, 1.25) | 1.70 (1.60, 1.82) |
*Cases for ≥ 12 years old only
**The rate and rate ratio of the COVID-19 vaccines (UK) were not adjusted to 14 days as the time period was not available
Fig. 1.
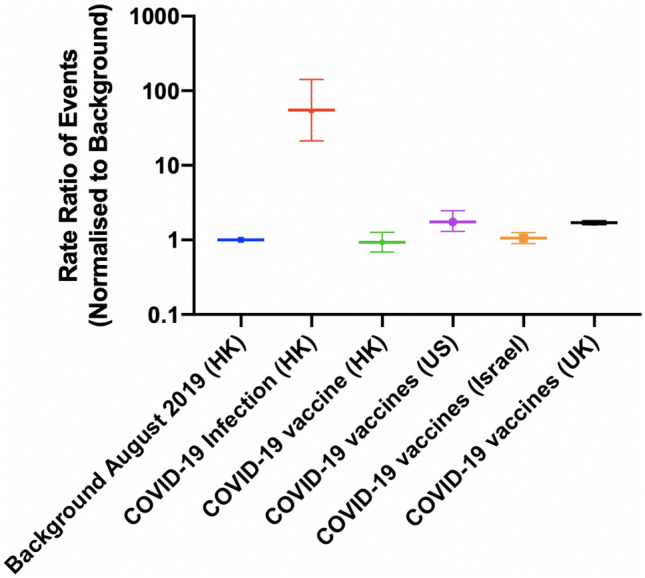
The rate ratio of the events after COVID-19 infection and COVID-19 vaccination
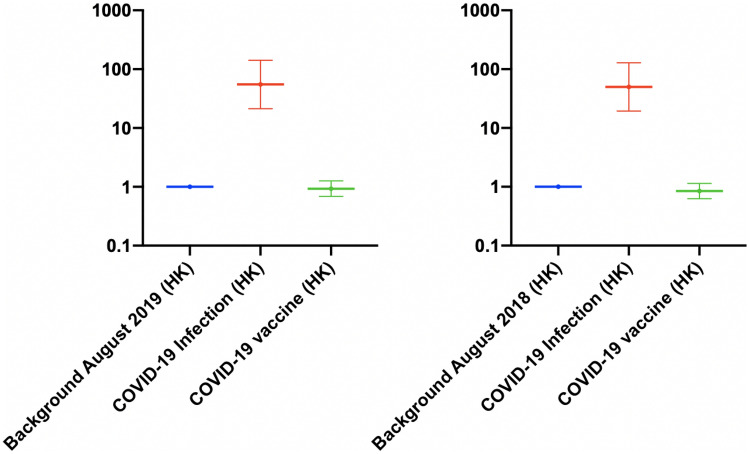
The rate ratio of the myopericarditis were stratified according to the vaccine types (Fig. 2, Supplementary Table 2). Compared to the background, the rates of myopericarditis were higher among individuals vaccinated with BioNTech (Rate ratio: 1.45; 95% CI 1.07, 1.96) and lower among those vaccinated with CoronaVac vaccine (rate ratio: 0.06; 95% CI 0.003, 0.34). Among the individuals vaccinated with BioNTech, the rate of myopericarditis was similar to the background among individuals aged > 15 years (rate ratio: 0.92; 95% CI: 0.62, 1.36). Meanwhile, for individuals aged 12–15 years, the rate was higher than the background (Rate ratio: 13.5; 95% CI 8.30, 21.9) (Fig. 3, Supplementary Table 3). Sensitivity analysis was conducted to verify the trend of our data. The trend of myopericarditis after COVID-19 infection and vaccination in Hong Kong remained consistent while comparing the background to March-August in 2018 (Fig. 4).
Fig. 2.
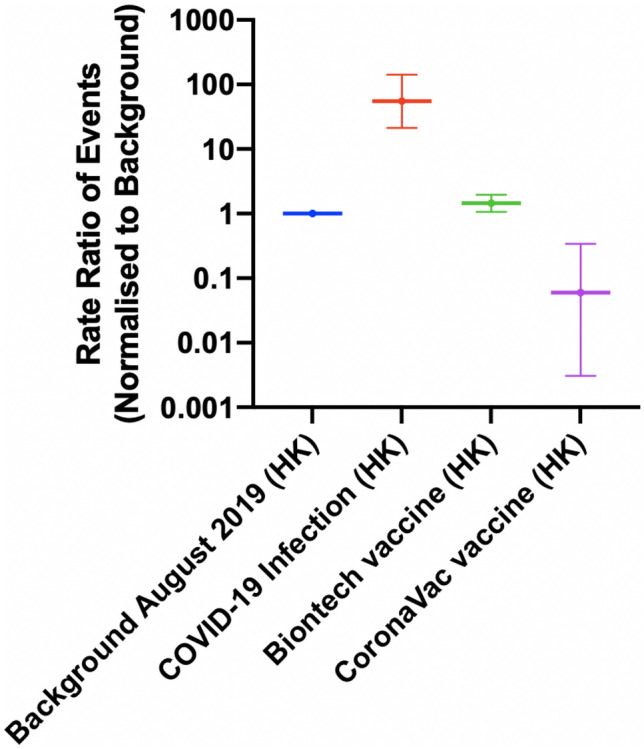
The rate ratio of the events after COVID-19 infection and COVID-19 vaccination in Hong Kong stratified by vaccine type
Fig. 3.
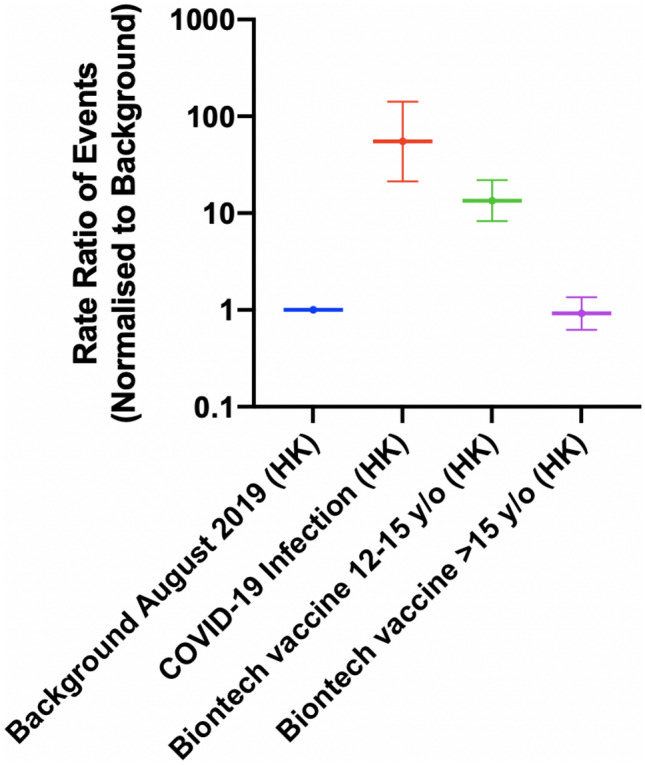
The rate ratio of the events after COVID-19 infection and BioNTech vaccination in Hong Kong stratified by age
Fig. 4.
Sensitivity testing 1: the rate ratio of the events after COVID-19 infection and COVID-19 vaccination in Hong Kong with different background years [5]. The background period was between March and August in 2018–2019
Discussion
The main finding from our study is that COVID-19 patients had a higher rate of myopericarditis compared to the pre-COVID-19 era background. With COVID-19 vaccination, the rate of myopericarditis was significantly lower than the COVID-19 positive patients.
Our team recently developed a predictive model to identify COVID-19 patients at risk of severe disease [7]. Using an updated dataset, we found that 0.035% of the COVID-19-infected patients developed myopericarditis. This is significantly higher than the baseline, reflecting the association between COVID-19 infection and myopericarditis. Previously, it was described that among 718,365 patients with COVID-19, approximately 6.5% developed new-onset myopericarditis [8]. This higher rate might be an overestimate, since most of the patients presented with mild COVID-19 symptoms may not get admitted and diagnosed, thus, not registered with the electronic medical records. Our cases represented population-based data locally in Hong Kong, which has practised meticulous contact tracing since the pandemic; the number of total COVID-19 patients in our cohort would, therefore, likely reflect most of the infected patients in the community [9].
The link between COVID-19 vaccination and myopericarditis was raised in Israel in May 2021. The results demonstrated that COVID-19 vaccinations are associated with a lower myopericarditis rate than the COVID-19-infected patients. This indicated that the COVID-19 vaccination might protect the vaccinated people from the myocardial injury caused by SARS-CoV-2 infection [10]. The differences in the outcome suggested that the myopericarditis upon vaccination is unlikely due to the mimicry between the spike proteins [11]. While the rate of myopericarditis was similar to the background in our data; this does not rule out the causation between the COVID-19 vaccine and myopericarditis. Indeed, the majority of myopericarditis developed after the second dose of the vaccine, in particular among the younger male (< 19 years old) [2]. While the rate of myopericarditis following COVID-19 infection is relatively low; further studies are needed to characterise the mechanism of myopericarditis after COVID-19 vaccination. Nonetheless, with the current increasing numbers in COVID-19 globally, it appears that every person will come in contact with SARS-CoV-2, and this is alarming given the overall rate of myopericarditis is much higher.
Strengths and limitations
This study has several strengths. First, COVID-19 cases were identified by RT-PCR testing across the public sector. Therefore, missing cases are likely to be few. Second, possible cases of vaccine-related myopericarditis were reviewed by an expert panel, which examined the medical records independently. This adjudication has permitted the accurate classification of cases according to established international guidelines. Nevertheless, had we included possible cases rather than cases definitely linked to vaccinations, the rate ratios of myopericarditis cases in infected patients to cases occurring after COVID-19 vaccination would be even lower, and therefore does not alter our conclusion.
However, several limitations should be noted. First, the cohort included patients recruited from a single region and as such, is unable to account for any geographical heterogeneity that may exist. Second, the vaccination data reported in the local Department of Health did not provide the number of myopericarditis after the first and the second dose of vaccination, and therefore, the results were not stratified. Third, we might have missed COVID-19 myopericarditis in patients who might have died from acute heart failure complications due to myopericarditis but recorded as heart failure deaths. This, however, would have increased the difference between COVID-19 and vaccine-related myopericarditis, further strengthening the outcome of vaccination. Furthermore, the COVID-19-infected patients that were undetected by PCR were not included in this study. However, as Hong Kong has imposed a relatively strict policy in contact tracing and quarantine, we believe that this number is relatively low [9]. Finally, given the retrospective nature of this study, the incidence of myopericarditis can be underestimated.
Conclusion
COVID-19 infection is associated with significantly higher rate of myopericarditis compared to the vaccine-associated myopericarditis.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Vassilios S. Vassiliou, Email: v.vassiliou@uea.ac.uk
Bernard Man Yung Cheung, Email: mycheung@hku.hk.
Gary Tse, Email: garytse86@gmail.com.
References
- 1.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai FTT, Li X, Peng K, et al (2022) Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. Annals Intern Med. 175 (3):362–370 [DOI] [PMC free article] [PubMed]
- 4.Tse G, Zhou J, Lee S, et al. Relationship between angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and COVID-19 incidence or severe disease. J Hypertens. 2021;39(8):1717–1724. doi: 10.1097/HJH.0000000000002866. [DOI] [PubMed] [Google Scholar]
- 5.Drug Office of the Department of Health HKS, China Adverse Drug Reactions (Reporting) 2021. https://www.drugoffice.gov.hk/eps/do/en/healthcare_providers/adr_reporting/index.html accessed 10 Oct 2021
- 6.Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13(1):411. doi: 10.1038/s41467-022-28068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Lee S, Wang X, et al. Development of a multivariable prediction model for severe COVID-19 disease: a population-based study from Hong Kong. NPJ Digit Med. 2021;4(1):66. doi: 10.1038/s41746-021-00433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Lane DA, Lip GYH. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Investig n/a(n/a): e13679. [DOI] [PMC free article] [PubMed]
- 9.Yuan HY, Blakemore C. The impact of contact tracing and testing on controlling COVID-19 outbreak without lockdown in Hong Kong: an observational study. Lancet Reg Health West Pac. 2022;20:100374. doi: 10.1016/j.lanwpc.2021.100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106(15):1127–1131. doi: 10.1136/heartjnl-2020-317186. [DOI] [PubMed] [Google Scholar]
- 11.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GOV.UK. Coronavirus vaccine—weekly summary of Yellow Card reporting 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting accessed 10 Oct 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.