Abstract
Background
Pulmonary neuroendocrine neoplasms can be divided into typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma, and small cell (lung) carcinoma. According to the World Health Organization, these four neoplasms have different characteristics and morphological traits, mitotic counts, and necrotic status. Importantly, “a grey-zone” neoplasm with an atypical carcinoid-like morphology, where the mitotic rate exceeds the criterion of 10 mitoses per 2 mm2, have still not been well classified. In clinical practice, the most controversial area is the limit of 11 mitoses to distinguish between atypical carcinoids and large cell neuroendocrine carcinomas.
Methods
Basic and clinical information was obtained from patient medical records. A series of grey-zone patients (n = 8) were selected for exploring their clinicopathological features. In addition, patients with atypical carcinoids (n = 9) and classical large cell neuroendocrine carcinomas (n = 14) were also included to compare their similarity to these neoplasms with respect to tumour morphology and immunohistochemical staining.
Results
We found that these grey-zone tumour sizes varied and affected mainly middle-aged and older men who smoked. Furthermore, similar gene mutations were found in the grey-zone neoplasms and large cell neuroendocrine carcinomas, for the mutated genes of these two are mainly involved in PI3K-Akt signal pathways and Pathways in cancer, including a biallelic alteration of TP53/RB1 and KEAP1.
Conclusions
Our findings indicate that neuroendocrine neoplasm with atypical carcinoid morphology and elevated mitotic counts is more similar to large cell neuroendocrine carcinoma than atypical carcinoid. Furthermore, this study may help improve diagnosing these special cases in clinical practice to avoid misdiagnosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09391-w.
Keywords: Atypical carcinoid morphology, Elevated mitotic count, Atypical carcinoid, Large cell neuroendocrine carcinoma
Background
The World Health Organization (WHO) has added large cell neuroendocrine carcinoma (LCNEC) to the classification of pulmonary neuroendocrine neoplasms (pNENs) for the first time [1]. The 2017 consensus conference of the International Agency for Research on Cancer suggests that neuroendocrine neoplasms (NENs) are subdivided into well-differentiated neuroendocrine tumours (NETs) and poorly differentiated neuroendocrine carcinomas (NECs) [2–12]. Thus, in their newest edition, WHO divides pNENs into two groups (i) NETs, comprising typical carcinoid (TC) and atypical carcinoid (AC); (ii) and NECs, comprising LCNEC and small cell (lung) carcinoma (SCLC) [13].
NENs are relatively rare, and 20–30% develop in the lung [14]. Within the lung, 95% of NENs are NECs, with NETs accounting for only a small proportion [13]. The diagnostic criteria of pNENs are clearly defined based on their morphological traits, mitotic counts, and necrotic status [13]. Moreover, NETs characteristically do not occur in combination with LCNEC or SCLC [13], and differences are indeed exhibited in the biological behaviour, therapeutic consideration, the clinical prognosis of NET and NEC [5, 15, 16].
However, a grey-zone does exist as some pNENs have an AC-like morphology with elevated mitotic counts over 10 per 2 mm2 (AC-h), although they have only been investigated in a few studies [8, 17–19]. Due to their rare prevalence and the lack of specific classification, it is still difficult to characterise these tumours. Therefore, updates should be made to the existing classification system. To that end, additional studies to classify the characteristics of AC-h need to be carried out. As such, we conducted this retrospective study to explore the similarities and differences among AC-h, AC, and LCNEC.
Methods
Sample selection
Forty-four samples of surgical resected primary untreated ACs and LCNECs diagnosed between January 1, 2016 and January 1, 2021 were collected from the specimen bank of the Department of Pathology, West China Hospital, Sichuan University, with the approval of the Institutional Ethics Committee (NO: 2020 (120)). All specimens were reviewed by two experienced pathologists, and a multi-head microscope was used for joint judgment with the participation of a third professional pathologist if the results were inconsistent, based on the new 5th edition WHO.
After reviewing all the slides of 44, eight AC-h were selected, meanwhile, considering the preservation time of the wax block, this study only included AC samples after January 1, 2018. Finally, 31 samples, consisting of nigh ACs, eight AC-hs and 14 LCNECs (the data had been previously collected which could be found in doi: 10.1186/s13000-022-01204-9.), were enrolled from 31 independent patients. Overall survival (OS), identified from the resection date to the cutoff date of follow-up (June 1, 2021), was identified as the primary survival outcome in this study, due to case 25 whose tumour could not be completely removed.
Immunohistochemical analysis
Antibodies against CD56 (clone UMAB83 and BIO), synaptophysin (Syn, polyclonal, MXB), chromogranin A (CgA, clone EP38, and BIO), TTF-1 (clone 8G7G3/1, and ZECA), and Ki67 (clone MIB-1) were used for immunohistochemical (IHC) staining of all samples. Blinded to all patients’ information, two experienced pathologists assessed IHC expression independently. Controversial cases were revaluated under a multi-head microscope for joint judgment with the participation of a third professional respiratory diagnostic pathologist.
DNA extraction and next-generation sequencing
According to the manufacturer’s instructions, DNA was extracted by a QIAamp DNA FFPE Tissue Kit (Qiagen, Carlsbad, CA, USA) after twice of de-paraffinized by xylene. Extracted DNA was purified and qualified employing the Nanodrop2000 (Thermo), and then using Qubit3.0 (Life Technology) with a dsDNA HS Assay Kit (Life Technology) to quantify DNA.
Amplified and purified DNA Libraries by PCR and then pooled together 1-2 μg of different libraries for targeted enrichment. Hybridization-based target enrichment was carried out with NimbleGen SeqCap EZ Hybridization and Wash Kit (Roche). Captured libraries by Dynabeads M-270 (Life Technologies) were amplified in KAPA HiFi HotStart ReadyMix (KAPA Biosystems), followed by purification by Agencourt AMPure XP beads. Customized xGen lockdown probes panel (Integrated DNA Technologies) were used to targeted enrich for 425 predefined genes. The enriched libraries were sequenced on Hiseq 4000 NGS platforms (Illumina) to coverage depths of at least 100 × and 300 × after removing PCR duplicates for tumour and normal tissue, respectively.
Bioinformatics analysis
Base calling analysis was used to transfer original image data into raw sequence data, which contained sequence information and corresponding sequencing quality information. Single nucleotide variants (SNVs) and short insertions or deletions (indels) were identified by VarScan2. In-house-developed software was used to detect Copy number variations (CNVs).
Statistical analysis
Statistical Package for the Social Sciences version 25.0 statistical software (SPSS Inc., Chicago, IL, USA) and the Kyoto Encyclopaedia of Genes and Genomes website (KEGG web) were used to conduct statistical analysis and query the gene mutation pathways, respectively. Continuous data were evaluated by were assessed by one-way analysis of variance (ANOVA). Categorical data were assessed by Pearson’s chi-squared test or Fisher’s exact test. The Kaplan–Meier method was used for survival analysis. P < 0.05 was considered statistically significant.
Results
Clinical information
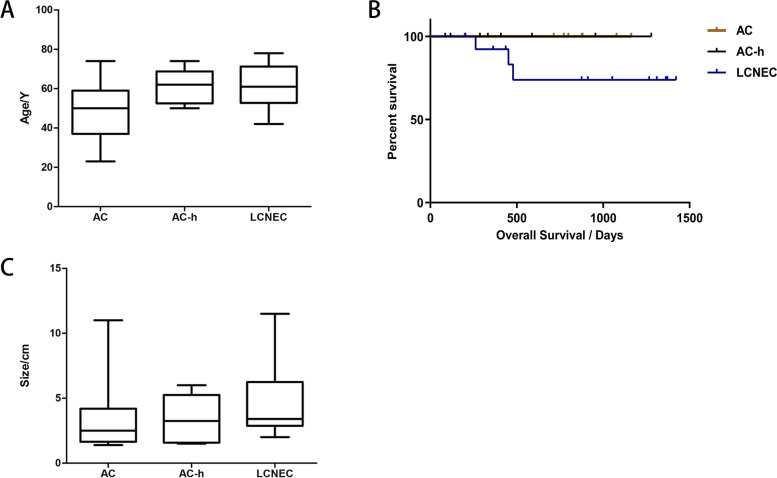
Basic information of the patients in the cohort is presented in Table 1 and Fig. 1A. There was a significant difference in average age at diagnosis between the three groups (P = 0.048) and smoking status (P = 0.028). In all 31 patients, asymptomatic patients were most commonly seen in the AC group. More than half the patients with AC-h or LCNEC were symptomatic; coughing was the most common symptom, followed by expectoration. In 77.8% of patients, the ACs were clinically staged I or II, far greater than that of the other two groups of tumours (Table 2). Moreover, the follow-up analyses of 28 patients showed no recurrence, metastasis, or death among the AC group (Fig. 1B). Patient’s postoperative treatment and prognosis are shown in Table 3.
Table 1.
The demographic characteristics and smoking status of 31 samples
| Characeristics | AC | AC-h | LCNEC | P-value |
|---|---|---|---|---|
| Age (years | 0.048 | |||
| < 40 | 2 | 0 | 0 | |
| 40–49 | 2 | 0 | 1 | |
| 50–59 | 3 | 3 | 6 | |
| 60–69 | 1 | 4 | 3 | |
| > 70 | 1 | 1 | 4 | |
| Range | 23–74 | 50–74 | 42–78 | |
| Mean | 49 | 61 | 61 | |
| M:F | 5:4 | 7:1 | 13:1 | 0.074 |
| Smoking | 0.028 | |||
| Never | 6 | 2 | 2 | |
| Has/Had | 3 | 6 | 12 |
Abbreviations: AC Atypical carcinoid, AC-h Atypical carcinoid morphology with increased mitotic counts, LCNEC Large cell neuroendocrine carcinoid, P-value The associations of age was assessed by One-Way ANOVA, meanwhile, other information were assessed by Pearson’s chi-squared test or Fisher’s exact test and P < 0.05 was considered statistically significant for all results; Age: at diagnosed; Range: the range of diagnosed age; M:F: male: female; Smoking: smoking status; Never: never smoker; Has/Had: still smoking/previous smoker
Fig. 1.
Abbreviations: AC Atypical carcinoid, AC-h Atypical carcinoid morphology with increased mitotic counts, LCNEC Large cell neuroendocrine carcinoid, A) the age-specific box diagram of the three groups of cases; B) the overall survival in 31 patients with AC, AC-h and LCNEC; C) the tumour size-specific box diagram of the three groups of cases
Table 2.
The clinical information and preoperative imaging data of 31 samples
| Variable | AC | AC-h | LCNEC | P-value |
|---|---|---|---|---|
| Lung lobe | ||||
| Left lung | 5 (55.6%) | 4 (50.0%) | 6 (42.9%) | 0.833 |
| Right lung | 4 (44.4%) | 4 (50.0%) | 8 (57.1%) | |
| Upper lobe | 2 | 6 | 7 | 0.030 |
| Others | 7 | 2 | 7 | |
| Type | 0.183 | |||
| Central | 3 (33.3%) | 2 (25.0%) | 4 (28.6%) | |
| Peripheral | 6 (66.7%) | 4 (50.0%) | 10 (71.4%) | |
| Unknown | 0 | 2 | 0 | |
| Tumor size (cm) | 0.503 | |||
| ≤ 5 | 8 | 5 | 10 | |
| > 5 | 1 | 1 | 4 | |
| Unknown | 0 | 2 | 0 | |
| Stage | 0.056 | |||
| I,II | 7 | 2 | 7 | |
| III,IV | 2 | 4 | 7 | |
| Unknown | 0 | 2 | 0 | |
| Symptom | ||||
| Asymptomatic | 6 (66.7%) | 3 (37.5%) | 6 (42.9%) | |
| Cough | 2 | 3 | 7 | |
| Expectoration | 2 | 2 | 5 | |
| Hemoptysis | 0 | 1 | 2 | |
| Chest pain | 0 | 0 | 3 | |
| Expiratory dyspnea | 1 | 2 | 0 | |
Abbreviations: Central Central type of lung cancer, Peripheral Peripheral type of lung cancer, Tumour size The value took from the surgical records, Stage Evaluated basing on the Eighth Edition of the American Joint Committee on Cancer (AJCC) guidelines, Symptom When they first found the mass on lung, Asymptomatic Asymptomatic cases, for the size of operation of case 8 was not queried, the value from preoperative imaging was took to indicate the size; the patient of umber 12 who underwent lung transplantation due to severe chronic obstructive pulmonary disease and pathological examination of the diseased lung showed tumours, but no tumour evidence was found in preoperative imaging thus the tumour location, tumour size and stage could not be judged; P-value: the associations of tumour size was assessed by One-Way ANOVA, meanwhile, other information were assessed by Pearson’s chi-squared test or Fisher’s exact test and 0.05 was considered as statistically significant results
Table 3.
The postoperative treatment and prognosis of 31 samples
| Group | Samples | Postoperative treatment | Prognosis | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | Loss | Death | Alive | ||
| AC | 9 | 9 | 0 | 0 | 9 | |||
| AC-h | 8 | 6 | 2 | 2* | 4 | |||
| LCNEC | 14 | 5 | 1 | 2 | 5 | 1 | 4 | 9 |
Abbreviations: Loss The contact information left was empty or out of service, Death Died of tumour recurrence or metastasis, *: death after lung transplantation
Imaging data
Preoperative chest CT scans were reviewed to determine tumour location (Table 2). Tumours involving the carina or a main segmental bronchus were defined as central, while the others were defined as peripheral. The primary tumour mass occurred preferentially in the periphery in these three groups. In addition, AC-h showed a clearer tendency than AC to occur in the upper lobe (P = 0.030) and a more stable range of tumour size fluctuations (Fig. 1C).
Pathological findings
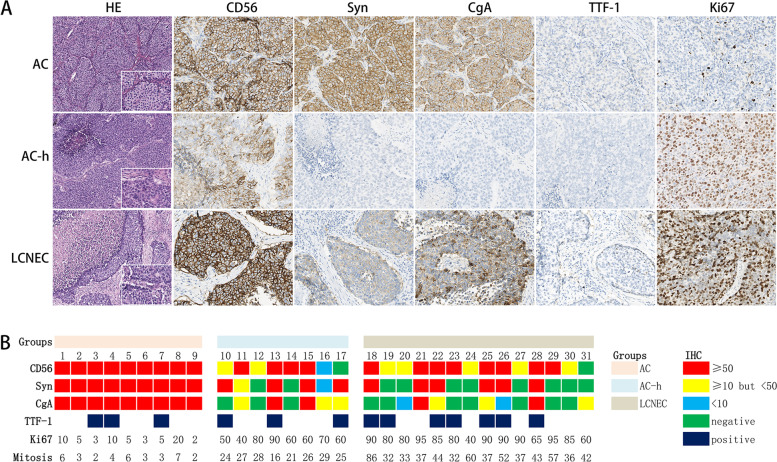
Histopathological analysis of AC-h revealed classical features of NET (tumour cells were relatively uniform, featuring moderate to abundant cytoplasm and finely nuclear chromatin) and NEC (focal necrosis, and even extensive necrosis in four cases) (Fig. 2). IHC for neuroendocrine (NE) markers (CD56, Syn, and CgA), TTF-1 and Ki67 was performed on 31 samples. The most sensitive neuroendocrine marker was CD56 (93.5%), followed by Syn and CgA (both were 67.7%). All ACs exhibited strong positivity for the three NE markers, while the expression mode was more variable in AC-hs and LCNECs. After reviewing all the slices, mitosis in AC-hs (average, 25 per 2 mm2) was found to differ significantly from that of ACs (average, 4 per 2 mm2) and LCNEC (average, 45 per 2 mm2) (P < 0.001). Similar results could be observed using Ki67 (P < 0.001).
Fig. 2.
A Representative HE and IHC imagines of AC, AC-h and LCNEC under light microscope at × 100 magnification (inset × 400) for HE and at × 200 magnification for IHC; B IHC and mitosis results of the all 31 patients. Case: case number; Ki67: calculated on the hot spot area under the field of view × 400; Mitosis: counted on the 5th edition WHO diagnostic criteria and for these samples which the mitoses near the threshold of two or ten per 2mm2, the average of counts in at least three hot sets of per 2mm2 were token as the result
Genomic features
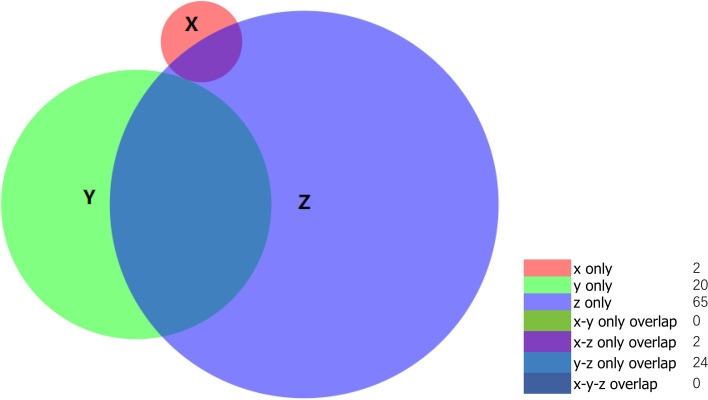
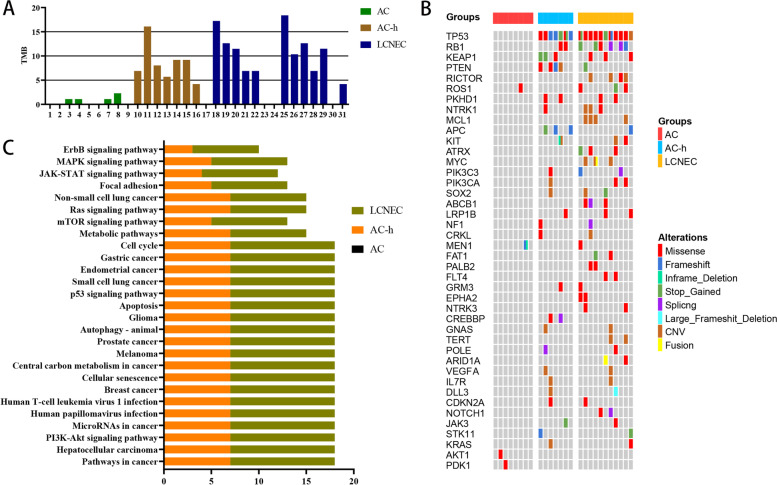
The 425-exon sequencing was performed by unsupervised clustering in 28 pure primary tumour samples (Nanjing Geneseeq Technology Inc.), revealing 113 altered genes (Fig. 3). Tumour mutational burden (TMB) is defined as the number of somatic cells, coding number, base subsets, and index of each detected genome. Our analysis revealed that the TMB value in ACs (average, 0.7 mutations/MB) contrasted with those of AC-hs (average, 8.5 mutations/MB) and LCNECs (average, 10.8 mutations/MB) (P < 0.001) (Fig. 4A).
Fig. 3.
The 425-exon sequencing in 28 pure primary tumour samples revealing 113 altered genes. Abbreviations: X: AC; Y: AC-h; Z: LCNEC; x only: AKT1, PDK1; y only: CREBBP, SMARCA4, NKX2-1, KMT2B, IKBKE, TEK, PIK3R1, RET, SETBP1, EPHA3, PRDM1, TERC, AKT2, TOP2A, CCNE1, CBL, NOTCH2, IDH1, CTCF, AXL; z only: RICTOR, MCL1, ATRX, MYC, ABCB1, FAT1, PALB2, FLT4, TERT, ARID1A, EPHA2, NTRK3, NOTCH1, PDGFRA, BRAF, JAK1, EPHA5, DAXX, ZNF217, ERBB4, TSC2, GATA2, PPARD, SDHC, SDHA, FLCN, TUBB4A, PALLD, SRC, SMAD3, EZH2, BARD1, ATM, AKT3, TPMT, GRIN2A, MAP2K2, BTK, GATA4, MET, RUNX1, BIRC3, CHEK1, DENND1A, PTK2, AXIN2, TGFBR2, PMS2, ARID1B, EP300, POLH, DUSP2, MYCN, IGF1R, DPYD, PREX2, CYSLTR2, CHEK2, EGFR, DDR2, RAD54L, WRN, LZTR1, LHCGR, BRCA1; x–z only overlap: ROS1, MEN1; y–z only overlap: TP53, PTEN, KEAP1, APC, RB1, PKHD1, NTRK1, KIT, PIK3C3, PIK3CA, SOX2, GRM3, LRP1B, GNAS, KRAS, IL7R, POLE, NF1, CRKL, VEGFA, DLL3, CDKN2A, JAK3, STK11
Fig. 4.
Abbreviations: (A) TMB Tumour mutation burden (mutations / MB); Case 1 and 17: do not acquire sufficient sequencing depth due to DNA degradation; Case 5, 6 and 9: do not detect genetic mutations; Case 23, 24 and 30: except owing to diagnosed as combined LCNEC; (B) The 42 genes consisted by the selected 26 tumour samples (due to DNA degradation, case 1 and 17 did not acquire a sufficient sequencing depth, meanwhile, for case 2, 5, 6 and 9 we did not detect any genetic mutations) involved: 40 genes which occurred more than one time and 2 genes which the atypical carcinoid samples involved; (C)The abscissa represents the number of cases involved in; The ordinate represents the involved mutation paths; The pathways which involved at least there of the top ten genes: Pathways in cancer; Hepatocellular carcinoma; PI3K-Akt signaling pathway; MicroRNAs in cancer; Human papillomavirus infection; Human T-cell leukemia virus 1 infection; Breast cancer; Cellular senescence; Central carbon metabolism in cancer; Melanoma; Prostate cancer; Autophagy-animal; Glioma; Apoptosis; p53 signaling pathway; Small cell lung cancer; mTOR signaling pathway; Metabolic pathways; Endometrial cancer; Gastric cancer and Cell cycle; The other 6 paths reported usually been seen in LCNEC: Ras signaling pathway, Non-small cell lung cancer, Focal adhesion, JAK-STAT signaling pathway, MAPK signaling pathway, ErbB signaling pathway
At the single gene level, among the commonly mutated genes, differences in P-values were found between these three groups. The most commonly mutated genes were TP53 (P < 0.001) and RB1 (P = 0.039), with a significant bi-alteration rate (P = 0.039), followed by PTEN (P = 0.011), RICTOR and MCL1 (P = 0.040), APC (P = 0.054), KEAP1 (P = 0.132), NTRK1 (P = 0.265), ROS1 (P = 0.284) and PKHD1 (P = 0.293) (Fig. 4B). Furthermore, we matched the gene mutation sites with the corresponding protein locations of the top ten genes, which showed that case 10 and 20 had the same mutation in TP53 (824G > T), corresponding to the protein location C275F. Moreover, several highly similar mutated sites existed between the AC-h and LCNEC groups (Table S1).
The KEGG web was used to search the pathways in which the top ten mutated genes are involved. As a result, the pathways which involved at least three of the top ten genes (n = 21) were collected (Table S2), within the six other common mutation pathways in LCNEC listed in Fig. 4C [20–23].
Discussion
Lung neuroendocrine tumours represent a group of heterogeneous malignancies, and according to the 5th edition of WHO, apparent differences exist between NET and NEC for NETs lack mutations in TP53, RB1, KRAS, and STK11/KEAP1, while, in 40% of cases they have mutations in chromatin-remodelling genes [13]. Genetic screening of 45 surgically resected pure-LCNEC by Rekhtman et al. found two cases of carcinoid-like molecular profiles. Moreover, these two cases displayed apparent carcinoid-like morphology, although the elevated proliferation rate above the cut-off value accepted of NET had led them to be classified as LCNEC [17]. Similar results were found in other studies [8, 24]. This type of neoplasm in the pancreas is classified as NET G3, which has a common mutation lineage with NET G1 and NET G2 and can evolve from G1/G2, and has nothing to do with the progress of NEC [15]. Although some researchers believe these tumours generally correspond to those regarded as NETs in the pancreas, however, in the lung, the WHO still classifies these neoplasms as LCNEC, although their prognosis has been suggested to be different from traditional LCNECs, which means more clinical, pathological, and genetic studies are needed to determine how to fit these rare tumours into the classification [1, 13]. Thus, it is essential to recognise these grey-zone AC-hs to improve disease classification and avoid incorrect clinical treatment choices.
Previous studies have pointed out that AC occurred in younger patients than LCNEC [15, 25–27], and comparison with the age is mentioned by WHO, the patients with AC or LCNEC in this study tended to be younger [13]. Unlike AC, which does not show a strong association with cigarette smoking [6, 15, 25, 28, 29], and is slightly more common in women [9, 11, 26, 27], AC-h primarily affects middle-aged men with a smoking history. Caplin ME et al. reported that well-differentiated lung NETs are usually located centrally in the main or lobar bronchi (up to 80% of tumours) [30–32], although some reports have opposed this view [4, 27, 33]. Results indicated all three groups had a trend of occurring in the periphery.
The WHO (4th edition) diagnostic criteria recommends the use of NE markers to confirm a diagnosis of neuroendocrine differentiation [1] the guidelines for the diagnosis and management of pNEN (2020) support this point [34], and this is reiterated in the 5th edition [13]. Our results suggest that compared with HE, IHC is more accurate in diagnosing lung carcinoids, in particular, Syn and CgA can be used to distinguish NET from NEC [35]. TTF-1, a putative regulator of neurogenesis expressed in pNEC at various sites [36, 37] was found to be mostly positive in peripheral NETs. However, for LCNEC, the positive expression rate of TTF-1 (50%) was slightly lower than that described by WHO (70%) [13].
The Ki67 antigen can identify proliferating cells and is important for distinguishing NETs and NECs, especially in small squeezed biopsy samples [38], and the value was now increased to 30% for AC [13]. Based on this change, some studies have used Ki67 to identify the proper cut-off value of these four pNENs, however, there was no conclusive result even in resected samples [6, 10, 38–40]. As shown in Fig. 2, malignant divergences existed in both the proliferation level and mitotic counts in these three neoplasms.
Global genomic studies have demonstrated that AC has a low mutation rate (0.3–0.4 mutations/Mb) [29, 41] and very few genetic changes [42]. High-frequency mutations include KIT, ERBB4, and MET [35, 43]. Unlike NECs, mutations in chromatin-remodelling genes are observed in approximately 40–50% of NET cases [3, 10, 41, 44]. For example, MEN1 (11–22%) is the most frequently mutated gene with somatic mutations in lung carcinoids [2, 41, 45]. Other statistically significant commonly mutated genes include EIF1AX and ARID1A [41]. However, except for the MEN1 mutation in case 8, NGS testing indicated no other commonly mutated genes in AC. Conversely, three other genes were found to be mutated in this cohort—ROS1 (a common driver gene), PDK1, and AKT1. These have never previously been reported for AC and should be investigated further.
Sazonova et al. recently applied IHC to surgical samples from 18 lung cancer patients, four of whom had defined borderline tumours (LCNEC with a low mitotic count and carcinoid-like morphology). They found that all AC and borderline tumours had preserved P53/RB expression [8]. Indeed, Meder et al. and Nakamura et al. reported that TP53 and RB1 gene inactivation are among the hallmarks of SCLC, existing in approximately 39.3% of SCLC cases. At the same time, for LCNEC, the rate was approximately 36.8% [41, 46]. Biallelic alterations of TP53 and RB1 are strikingly correlated with high-grade NECs, although uncommon in pNETs [2, 3, 21, 47]. The co-mutation of TP53/RB1, and common mutations in LCNECs like TP53, RB1, MEN1, STK11, KEAP1, and KRAS were commonly seen in AC-hs in our study. Furthermore, LCNEC is a heterogeneous tumour, which can be divided into three genotypes: (i) an SCLC-like subtype with biallelic inactivation of TP53 and RB1, (ii) a non-small-cell-like subtype with mutations in TP53 and STK11/KEAP1, (iii) and a carcinoid-like subtype sharing the low TMB and MEN1 changes seen in lung carcinoids [2, 13, 17, 18, 21, 37, 48, 49]. These results, taken together, suggests a way to classify the AC-hs effectively. The extensive TMB fluctuation range of AC-hs also supports this. In addition, according to the summary results of the three neoplasms gene mutations provided in Fig. 3, there is no shared mutation gene type between AC-h and AC. This result reiterated that according to our data, in the lung, there exist a big difference between AC-h and AC, which is completely different from the existing research of NET G3 in pancreatic NENs.
Subsequently, the 27 pathways considered in this study showed a high degree of similarity in the level of involvement of AC-h and LCNEC, suggesting that differences do exist between these tumours and ACs.
Tumorigenesis results from multiple factors, and abnormal activation of the PI3K-Akt-mTOR pathway is a frequent event in the non-small cell lung cancer development [20, 22, 23, 50]. The mammalian target of rapamycin (mTOR) serves as a signal amplifier in this pathway [22, 51]. It is generally believed that mutated genes in the PI3K/AKT/mTOR pathway are significantly related to the occurrence of NEC [2, 3, 17, 52]. However, inconsistent findings have been reported in pNEN, with some reporting that most mutated genes in NETs are located in this pathway [53] or that these mutated genes exhibit a high degree of participation [3, 52]. Our data support the conclusion that the mutated genes in AC are involved in these pathways. In addition, alterations in this pathway were far more common in LCNEC patients than previously reported [3, 17, 54].
Regarding survival and prognosis, several studies have suggested a similar prognosis between LCNEC and SCLC [55–57], which is significantly poorer than that of AC [55, 58]. The five-year survival rate of LCNEC patients is 15–57% [25, 59–63], while that of AC patients is 44–87% [6, 26, 59–61]. We attempted to enrol as many cases as possible but limited by the rare incidence and short DNA storage period, significantly different OS outcomes among these three tumour types were not obtained (P = 0.123). However, a trend from the available information suggested that AC-hs seemed to have a better prognosis than LCNECs.
Conclusion
We present basic information on the clinical features and genomic changes in 31 tumour samples. Despite limitations in the number of cases and the lack of effective differential data for OS, we can still clearly see that AC-h and LCNEC patients are more similar to each other with respect to demographic characteristics, tumour size and location, clinical presentation, pathological data, and genomic changes. Thus, we believe that carcinoid morphology with increased mitotic index is more similar to LCNEC, but has a better survival prognosis. However, to test this hypothesis, a larger cohort study is needed and until these neoplasms are better classified, we endorse that it is necessary to add a diagnostic note stating the histological morphology, mitotic count and Ki67 index of this type of tumour in the clinical diagnosis process.
Supplementary Information
Additional file 1. Gene mutation sites and affected protein changes.
Additional file 2. The pathways in which the top ten mutated genes are involved.
Abbreviations
- WHO
World health organization
- LCNEC
Large cell neuroendocrine carcinoma
- pNENs
Pulmonary neuroendocrine neoplasms
- NENs
Neuroendocrine neoplasms
- NETs
Neuroendocrine tumours
- NECs
Neuroendocrine carcinomas
- TC
Typical carcinoid
- AC
Atypical carcinoid
- SCLC
Small cell (lung) carcinoma
- AC-h
PNENs have an AC-like morphology with elevated mitotic counts over 10 per 2 mm2
- OS
Overall survival
- Syn
Synaptophysin
- CgA
Chromogranin A
- IHC
Immunohistochemica
- KEGG web
The Kyoto Encyclopedia of Genes and Genomes website
- NE
Neuroendocrine
- TMB
Tumour mutational burden
Authors’ contributions
Y.Z. and W.W. analyzed the data and prepared the manuscript. Q.H, Z.L. and P.Z. performed the histopathological examinations. Y.T helped to carry out the NGS studies. Y.Z. and Z.L carried out the IHC studies. L.J. was responsible for the diagnosis and revised the manuscript. Y.Z was responsible for submitting the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the Sichuan Science and Technology Program (No. 2020YFS0275) and 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2019HXFH002).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.The KEGG datasets were obtained from:- Kanehisa, M. and Goto, S.; KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000). [PMID:10592173]- Kanehisa, M; Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019). [PMID:31441146]- Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M., and Tanabe, M.; KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545-D551 (2021). [PMID:33125081].
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with institutional ethics regulations. All pathological tissues used were obtained from the database of the Department of Pathology, West China Hospital of Sichuan University and were approved by the Ethics Committee of West China Hospital of Sichuan University (NO: 2020 (120)).
Consent for publication
N/A.
Competing interests
The authors declare that no conflicts of interest exist.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Zhang, Weiya Wang, Zuoyu Liang, Ping Zhou, Yuan Tang and Lili Jiang contributed equally to this work.
References
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol. 2015;10(9):1240–1242. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 2.Derks JL, Leblay N, Lantuejoul S, Dingemans AC, Speel EM, Fernandez-Cuesta L. New Insights into the Molecular Characteristics of Pulmonary Carcinoids and Large Cell Neuroendocrine Carcinomas, and the Impact on Their Clinical Management. J Thorac Oncol. 2018;13(6):752–766. doi: 10.1016/j.jtho.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol. 2017;241(4):488–500. doi: 10.1002/path.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herde RF, Kokeny KE, Reddy CB, Akerley WL, Hu N, Boltax JP, et al. Primary Pulmonary Carcinoid Tumor: A Long-term Single Institution Experience. Am J Clin Oncol. 2018;41(1):24–29. doi: 10.1097/COC.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velinovic M, Jankovic R, Jovanovic D, Skodric Trifunovic V, Gavrilovic D, Stojsic J, et al. Tumor characteristics, expressions of ERCC1, Bax, p53, IGF1R, Bcl2, Bcl2/Bax and prognostic factors for overall survival in patients with lung carcinoid. J BUON. 2019;24(1):256–266. [PubMed] [Google Scholar]
- 6.Pericleous M, Karpathakis A, Toumpanakis C, Lumgair H, Reiner J, Marelli L, et al. Well-differentiated bronchial neuroendocrine tumors: Clinical management and outcomes in 105 patients. Clin Respir J. 2018;12(3):904–914. doi: 10.1111/crj.12603. [DOI] [PubMed] [Google Scholar]
- 7.Derks JL, Hendriks LE, Buikhuisen WA, Groen HJ, Thunnissen E, van Suylen RJ, et al. Clinical features of large cell neuroendocrine carcinoma: a population-based overview. Eur Respir J. 2016;47(2):615–624. doi: 10.1183/13993003.00618-2015. [DOI] [PubMed] [Google Scholar]
- 8.Sazonova O, Manem V, Orain M, Khoshkrood-Mansoori B, Gaudreault N, Desmeules P, et al. Transcriptomic data helps refining classification of pulmonary carcinoid tumors with increased mitotic counts. Modern Pathol. 2020;33(9):1712–21. doi: 10.1038/s41379-020-0538-8. [DOI] [PubMed] [Google Scholar]
- 9.Yoon JY, Sigel K, Martin J, Jordan R, Beasley MB, Smith C, et al. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J Thorac Oncol. 2019;14(2):184–192. doi: 10.1016/j.jtho.2018.10.166. [DOI] [PubMed] [Google Scholar]
- 10.Pelosi G, Sonzogni A, Harari S, Albini A, Bresaola E, Marchiò C, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513–529. doi: 10.21037/tlcr.2017.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clay V, Papaxoinis G, Sanderson B, Valle JW, Howell M, Lamarca A, et al. Evaluation of diagnostic and prognostic significance of Ki-67 index in pulmonary carcinoid tumours. Clin Transl Oncol. 2017;19(5):579–586. doi: 10.1007/s12094-016-1568-z. [DOI] [PubMed] [Google Scholar]
- 12.Baine MK, Rekhtman N. Multiple faces of pulmonary large cell neuroendocrine carcinoma: update with a focus on practical approach to diagnosis. Transl Lung Cancer Res. 2020;9(3):860–878. doi: 10.21037/tlcr.2020.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis WD, Al-Dayel FH, Bubendorf L, Chung JH. Rekhtman N and Scagliotti G. Thoracic Tumours: WHO Classification of Tumours; 2021.
- 14.Pusceddu S, Lo Russo G, Macerelli M, Proto C, Vitali M, Signorelli D, et al. Diagnosis and management of typical and atypical lung carcinoids. Crit Rev Oncol Hematol. 2016;100:167–176. doi: 10.1016/j.critrevonc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Rindi G, Klimstra Ds, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern Pathol. 2018;31(12):1770–86. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol. 2017;12(3):425–436. doi: 10.1016/j.jtho.2016.11.2222. [DOI] [PubMed] [Google Scholar]
- 17.Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Can Res. 2016;22(14):3618–3629. doi: 10.1158/1078-0432.CCR-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, et al. Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes with Specific Genomic Alterations. J Thorac Oncol. 2019;14(9):1651–1661. doi: 10.1016/j.jtho.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Alcala N, Leblay N, Gabriel AAG, Mangiante L, Hervas D, Giffon T, et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat Commun. 2019;10(1):3407. doi: 10.1038/s41467-019-11276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z, Zhu L, Niu X, Shen S, Zhao Y, Zhang J, et al. Comparison of genomic landscapes of large cell neuroendocrine carcinoma, small cell lung carcinoma, and large cell carcinoma. Thorac Cancer. 2019;10(4):839–847. doi: 10.1111/1759-7714.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi: 10.1038/s41467-018-03099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J Thorac Oncol. 2012;7(8):1315–1326. doi: 10.1097/JTO.0b013e31825493eb. [DOI] [PubMed] [Google Scholar]
- 23.Yip PY. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR) signaling pathway in non-small cell lung cancer. Transl Lung Cancer Res. 2015;4(2):165–176. doi: 10.3978/j.issn.2218-6751.2015.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn AM, Chaturvedi A, Nonaka D. High-grade Neuroendocrine Carcinoma of the Lung With Carcinoid Morphology: A Study of 12 Cases. Am J Surg Pathol. 2017;41(2):263–270. doi: 10.1097/PAS.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 25.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 26.Petursdottir A, Sigurdardottir J, Fridriksson BM, Johnsen A, Isaksson HJ, Hardardottir H, et al. Pulmonary carcinoid tumours: incidence, histology, and surgical outcome. A population-based study. Gen Thorac Cardiovasc Surg. 2020;68(5):523–9. doi: 10.1007/s11748-019-01261-w. [DOI] [PubMed] [Google Scholar]
- 27.Grøndahl V, Binderup T, Langer SW, Petersen RH, Nielsen K, Kjaer A, et al. Characteristics of 252 patients with bronchopulmonary neuroendocrine tumours treated at the Copenhagen NET Centre of Excellence. Lung Cancer (Amsterdam, Netherlands) 2019;132:141–149. doi: 10.1016/j.lungcan.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 29.Miyanaga A, Masuda M, Motoi N, Tsuta K, Nakamura Y, Nishijima N, et al. Whole-exome and RNA sequencing of pulmonary carcinoid reveals chromosomal rearrangements associated with recurrence. Lung Cancer (Amsterdam, Netherlands) 2020;145:85–94. doi: 10.1016/j.lungcan.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604–1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 31.Phan AT, Oberg K, Choi J, Harrison LH, Jr, Hassan MM, Strosberg JR, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus) Pancreas. 2010;39(6):784–798. doi: 10.1097/MPA.0b013e3181ec1380. [DOI] [PubMed] [Google Scholar]
- 32.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134(11):1628–1638. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 33.Gridelli C, Rossi A, Airoma G, Bianco R, Costanzo R, Daniele B, et al. Treatment of pulmonary neuroendocrine tumours: state of the art and future developments. Cancer Treat Rev. 2013;39(5):466–472. doi: 10.1016/j.ctrv.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Bergsland EK, Card CM, Hope TA, Kunz PL, Laidley DT, et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J Thorac Oncol. 2020;15(10):1577–1598. doi: 10.1016/j.jtho.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Hou Y, Shi T, He Y, Ren D, Song Z, et al. Clinicopathological characteristics and genetic analysis of pulmonary carcinoid tumors: A single-center retrospective cohort study and literature review. Oncol Lett. 2020;19(3):2446–2456. doi: 10.3892/ol.2020.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folpe AL, Gown AM, Lamps LW, Garcia R, Dail DH, Zarbo RJ, et al. Thyroid transcription factor-1: immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol. 1999;12(1):5–8. [PubMed]
- 37.Rekhtman N, Pietanza CM, Sabari J, Montecalvo J, Wang H, Habeeb O, et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod Pathol. 2018;31(1):111–21. [DOI] [PMC free article] [PubMed]
- 38.Garg R, Bal A, Das A, Singh N, Singh H. Proliferation Marker (Ki67) in Sub-Categorization of Neuroendocrine Tumours of the Lung. Turk Patol Derg. 2019;35(1):15–21. doi: 10.5146/tjpath.2018.01436. [DOI] [PubMed] [Google Scholar]
- 39.Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29(2):179–187. doi: 10.1097/01.pas.0000149690.75462.29. [DOI] [PubMed] [Google Scholar]
- 40.Wang HY, Li ZW, Sun W, Yang X, Zhou LX, Huang XZ, et al. Automated quantification of Ki-67 index associates with pathologic grade of pulmonary neuroendocrine tumors. Chin Med J. 2019;132(5):551–561. doi: 10.1097/CM9.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Cuesta L, Peifer M, Lu X, Sun R, Ozretić L, Seidal D, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun. 2014;5:3518. doi: 10.1038/ncomms4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical Lung Cancer Genome Project (CLCGP) Network Genomic Medicine (NGM) A genomics-based classification of human lung tumors. Sci Transl Med. 2013;5(209):209ra153. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou G, Yu X, Song Z. Molecular Profiling and Survival of Completely Resected Primary Pulmonary Neuroendocrine Carcinoma. Clin Lung Cancer. 2017;18(3):e197–e201. doi: 10.1016/j.cllc.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Debelenko LV, Brambilla E, Agarwal SK, Swalwell JI, Kester MB, Lubensky IA, et al. Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Hum Mol Genet. 1997;6(13):2285–2290. doi: 10.1093/hmg/6.13.2285. [DOI] [PubMed] [Google Scholar]
- 45.Swarts DR, Scarpa A, Corbo V, Van Criekinge W, van Engeland M, Gatti G, et al. MEN1 gene mutation and reduced expression are associated with poor prognosis in pulmonary carcinoids. J Clin Endocrinol Metab. 2014;99(2):E374–E378. doi: 10.1210/jc.2013-2782. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura H, Fujii K, Gupta V, Hata H, Koizumu H, Hoshikawa M, et al. Identification of key modules and hub genes for small-cell lung carcinoma and large-cell neuroendocrine lung carcinoma by weighted gene co-expression network analysis of clinical tissue-proteomes. PloS One. 2019;14(6):e0217105. doi: 10.1371/journal.pone.0217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beasley MB, Lantuejoul S, Abbondanzo S, Chu WS, Hasleton PS, Travis WD, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol. 2003;34(2):136–142. doi: 10.1053/hupa.2003.8. [DOI] [PubMed] [Google Scholar]
- 48.Peng WX, Sano T, Oyama T, Kawashima O, Nakajima T. Large cell neuroendocrine carcinoma of the lung: a comparison with large cell carcinoma with neuroendocrine morphology and small cell carcinoma. Lung Cancer (Amsterdam, Netherlands) 2005;47(2):225–233. doi: 10.1016/j.lungcan.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Karlsson A, Brunnström H, Micke P, Veerla S, Mattsson J, La Fleur L, et al. Gene Expression Profiling of Large Cell Lung Cancer Links Transcriptional Phenotypes to the New Histological WHO 2015 Classification. J Thorac Oncol. 2017;12(8):1257–1267. doi: 10.1016/j.jtho.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25(11):2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 51.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 52.Bago-Horvath Z, Sieghart W, Grusch M, Lackner A, Hayden H, Pirker C, et al. Synergistic effects of erlotinib and everolimus on bronchial carcinoids and large-cell neuroendocrine carcinomas with activated EGFR/AKT/mTOR pathway. Neuroendocrinology. 2012;96(3):228–237. doi: 10.1159/000337257. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, Wang M. PI3K/AKT/mTOR pathway in pulmonary carcinoid tumours. Oncol Lett. 2017;14(2):1373–1378. doi: 10.3892/ol.2017.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyoshi T, Umemura S, Matsumura Y, Mimaki S, Tada S, Makinoshima H, et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin Cancer Res. 2017;23(3):757–765. doi: 10.1158/1078-0432.CCR-16-0355. [DOI] [PubMed] [Google Scholar]
- 55.Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol. 2006;24(1):70–76. doi: 10.1200/JCO.2005.04.1202. [DOI] [PubMed] [Google Scholar]
- 56.Mochizuki E, Matsuura S, Oishi K, Miyashita K, Ichijyo K, Furukawa S, et al. Surgical resection for clinical stage I high-grade neuroendocrine carcinoma of the lung. World J Surg Oncol. 2018;16(1):33. doi: 10.1186/s12957-018-1337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinoshita T, Yoshida J, Ishii G, Aokage K, Hishida T, Nagai K. The differences of biological behavior based on the clinicopathological data between resectable large-cell neuroendocrine carcinoma and small-cell lung carcinoma. Clin Lung Cancer. 2013;14(5):535–540. doi: 10.1016/j.cllc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72. [DOI] [PubMed]
- 59.Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22(8):934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Skuladottir H, Hirsch FR, Hansen HH, Olsen JH. Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung cancer (Amsterdam, Netherlands). 2002;37(2):127–35. [DOI] [PubMed]
- 61.Cooper WA, Thourani VH, Gal AA, Lee RB, Mansour KA, Miller JI. The surgical spectrum of pulmonary neuroendocrine neoplasms. Chest. 2001;119(1):14–18. doi: 10.1378/chest.119.1.14. [DOI] [PubMed] [Google Scholar]
- 62.Beasley MB, Thunnissen FB, Brambilla E, Hasleton P, Steele R, Hammar SP, et al. Pulmonary atypical carcinoid: predictors of survival in 106 cases. Hum Pathol. 2000;31(10):1255–1265. doi: 10.1053/hupa.2000.19294. [DOI] [PubMed] [Google Scholar]
- 63.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21 Suppl 7:vii65–71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Gene mutation sites and affected protein changes.
Additional file 2. The pathways in which the top ten mutated genes are involved.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.The KEGG datasets were obtained from:- Kanehisa, M. and Goto, S.; KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000). [PMID:10592173]- Kanehisa, M; Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019). [PMID:31441146]- Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M., and Tanabe, M.; KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545-D551 (2021). [PMID:33125081].