Abstract
Biofilm growth is thought to be a significant obstacle to the successful treatment of Mycobacterium abscessus infections. A search for agents capable of inhibiting M. abscessus biofilms led to our interest in 2-aminoimidazoles and related scaffolds, which have proven to display antibiofilm properties against a number of Gram-negative and Gram-positive bacteria, including Mycobacterium tuberculosis and Mycobacterium smegmatis. The screening of a library of 30 compounds led to the identification of a compound, AB-2-29, which inhibits the formation of M. abscessus biofilms with an IC50 (the concentration required to inhibit 50% of biofilm formation) in the range of 12.5 to 25 μM. Interestingly, AB-2-29 appears to chelate zinc, and its antibiofilm activity is potentiated by the addition of zinc to the culture medium. Preliminary mechanistic studies indicate that AB-2-29 acts through a distinct mechanism from those reported to date for 2-aminoimidazole compounds.
Keywords: Mycobacterium abscessus, nontuberculous mycobacteria, biofilm, 2-aminoimidazoles, zinc
1. Introduction
Mycobacterium abscessus subspecies abscessus (Mabs), massiliense (Mmas) and bolletii (Mbol) form a group of opportunistic, rapidly growing mycobacteria that can cause an array of clinical diseases in humans, including lung, skin and soft tissue, central nervous system and disseminated infections. In recent years, the prevalence of pulmonary infections caused by M. abscessus, particularly in susceptible individuals with structural or functional lung conditions such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and bronchiectasis, has been increasing at an alarming rate [1,2,3]. Treatment for M. abscessus pulmonary disease as recommended by the American Thoracic Society and the British Thoracic Society is largely empirical [4,5] and consists of 12–24 months of chemotherapy with a minimum of three antibiotics that lack bactericidal activity and are associated with significant adverse effects [6,7,8]. Despite aggressive chemotherapy, treatment outcomes remain poor. M. abscessus bacteria are indeed the most antibiotic-resistant and antibiotic-tolerant nontuberculous mycobacteria (NTM) owing to their highly impermeable cell envelope and the variety of efflux pumps and drug- and drug-target-modifying enzymes encoded within their genomes [8,9,10]. Further compounding this problem is the propensity of M. abscessus to form biofilms [11] and the clinical evidence for M. abscessus biofilm formation within the airways and lung cavity of human patients [12,13,14]. The presence of biofilms, where bacilli are not only shielded from the effect of antibiotics but may also persist in a drug-tolerant state, may help explain why M. abscessus lung infections are usually incurable with antibiotic therapy alone and why adjunctive surgical resection of cavities may improve treatment outcome [4].
With the premise that agents capable of inhibiting M. abscessus biofilm formation and/or of dispersing established M. abscessus biofilms may potentiate the activity of antibiotics used in combination, our attention turned to 2-aminoimidazoles (2-AI). The decision to study 2-AIs was based upon previous studies by the Melander laboratory and others that showed that the 2-AI class of small molecules and related scaffolds (2-aminopyrimidines (2-AP), 2-aminobenzimidazoles (2-ABI), 2-aminoquinazolines and 2-AI-containing meridianin analogs) display broad-spectrum biofilm inhibition and dispersion activity against Gram-negative and Gram-positive bacteria, including Mycobacterium tuberculosis and the nontuberculous Mycobacterium species, M. smegmatis [15,16,17,18,19,20,21,22,23,24]. Importantly, some 2-AI and 2-ABI compounds demonstrated the ability to sensitize M. tuberculosis and M. smegmatis to isoniazid, rifampicin and β-lactams [15,19,23,25].
We here identify a series of 2-AI compounds with the ability to inhibit the formation of M. abscessus biofilms with IC50s (the concentration required to inhibit 50% of biofilm formation) in the range of 12.5 to 25 μM. Interestingly, the lead compound of this series, AB-2-29, is a zinc chelator whose antibiofilm activity is enhanced in the presence of zinc.
2. Results
2.1. Inhibition of M. abscessus Biofilm Formation by 2-AI Compounds
To determine whether 2-AI-based small-molecule compounds and related scaffolds inhibited the formation of M. abscessus biofilms, we screened a library of 30 compounds, including twenty-six 2-AI analogs, one 2-AP compound (EL-05-047) and three meridianin analogs (7.079; 7.025 and 8.001), of which two contained the 2-AI moiety (Table 1). The screening was conducted using the Mmas reference strain CIP 108297 grown as submerged biofilms on poly-D-lysine-coated plates in chemically defined synthetic CF medium (SCFM) as previously described [11]. Recent studies from our laboratory have indeed shown that SCFM closely mimics the nutritional conditions encountered and metabolic adaptation undergone by M. abscessus grown in actual CF sputum [26], making this model more relevant to infection than other models based on laboratory media.
Table 1.
IC50 values for the inhibition of M. abscessus biofilms. Biofilm assays and MIC determinations were repeated at least two times. n.d., not determined. MICs were determined against all isolates for which biofilm assays were run. MIC values were the same for all isolates unless otherwise indicated.
| Compound | MIC (M) | IC50 (M) | |||||
|---|---|---|---|---|---|---|---|
| Mmas CIP 108297 | Mabs ATCC 19977 |
Mabs NJH12 |
Mmas 1239 |
Mabs NJH9 |
Mmas NJH18 |
||
EL-05-047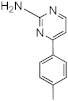
|
>100 | >100 | n.d. | 50–100 | n.d. | 50–100 | 100 |
2B8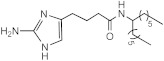
|
25 | 12.5–25 | n.d. | 25 | n.d. | 12.5–25 | 25 |
SEM-002-004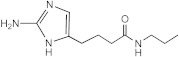
|
>50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-075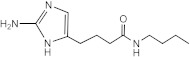
|
>50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-073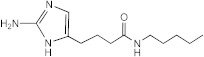
|
>50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-078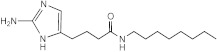
|
100–200 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-002-003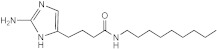
|
50–100 (Mmas CIP 108297 and Mabs ATCC 19977) 50 (Mabs NJH9, Mabs NJH12 and Mmas NJH18) |
25 | n.d. | 12.5–25 | n.d. | 12.5–25 | 50 |
RA10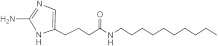
|
>50 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
RA12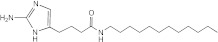
|
50 | >50 | n.d. | n.d. | n.d. | n.d. | n.d. |
RA13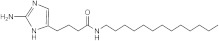
|
>50 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-034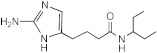
|
>50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-044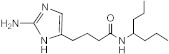
|
>50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-046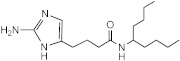
|
100–200 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-056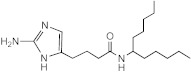
|
50 | 12.5–25 | 12.5–25 | 25 | 25 | 12.5–25 | 25 |
SEM-001-049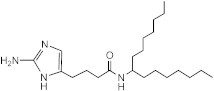
|
12.5–25 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-050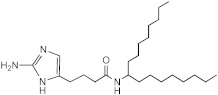
|
50 (Mabs NJH9, Mabs NJH12, Mmas NJH18, Mmas CIP108297) 100 (Mabs ATCC 19977) |
12.5–25 | n.d. | 12.5–25 | n.d. | 12.5–25 | 12.5–25 |
SEM-001-057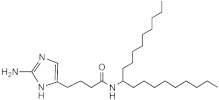
|
50–100 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-049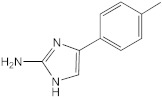
|
50–100 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-063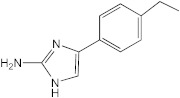
|
25–50 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-074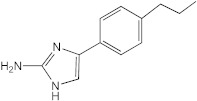
|
50–100 | 25–50 | n.d. | n.d. | n.d. | n.d. | n.d. |
4C3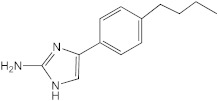
|
25–50 | 12.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
4B10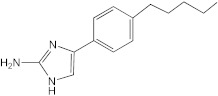
|
12.5–25 | 12.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
4C2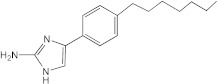
|
50 | 12.5–25 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-064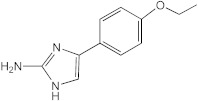
|
25 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
AB-2-29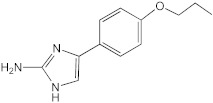
|
>100 | 25 | 25 | 12.5–25 | 50 | 12.5 | 25 |
AB-2-24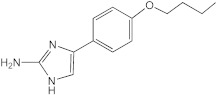
|
64 | 12.5 | 25–50 | 12.5 | 25 | 12.5–25 | 12.5–25 |
AB-2-26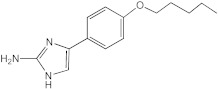
|
64 | 12.5–25 | 25–50 | 12.5–25 | 25 | 6.25–12.5 | 12.5–25 |
7.079 (meridianin)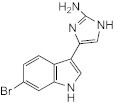
|
>100 | 100 | 100 | 50 | n.d. | 50–100 | 100 |
7.025 (meridianin)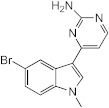
|
>100 | >100 | 100 | 50–100 | n.d. | >100 | 50–100 |
8.001 (meridianin)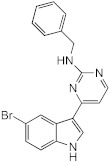
|
>100 | >100 | >100 | >100 | n.d. | >100 | >100 |
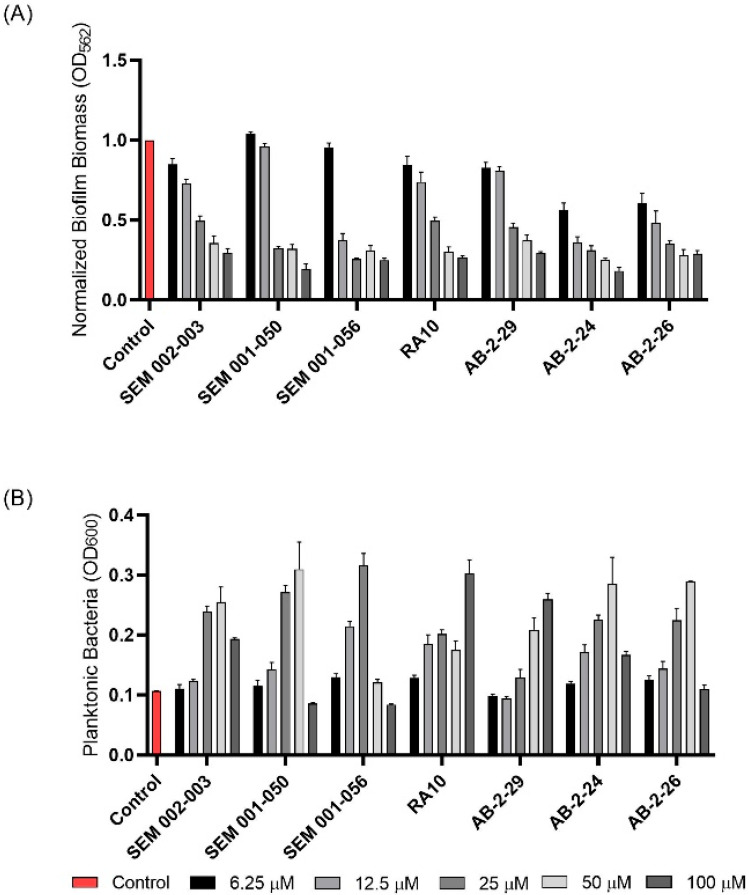
Whereas the 2-AP compound and the meridianin analogs failed to show any activity against Mmas CIP 108297 biofilms, twelve 2-AI compounds were found to inhibit biofilm formation in a dose-dependent manner and with IC50 values at least 2- to 4-fold below their measured MIC in the same medium (Table 1; Figure 1). A subset of these compounds (SEM-002-003; SEM-001-056; SEM-001-050; AB-2-24; AB-2-26, AB-2-29) was retested against other reference and clinical M. abscessus isolates encompassing the subspecies Mabs and Mmas with similar results (Table 1). Subsequent analyses focused on compound AB-2-29, which displayed an IC50 less than 4-fold its MIC value. Within the same range of concentrations (6.25 to 50 μM), however, AB-2-29 was not able to disperse pre-established biofilms (Figure S1).
Figure 1.
Effect of 2-aminoimidazoles on M. abscessus biofilm formation. (A) Biofilm formation of 2-AI-treated Mmas CIP 108297 cultures after 5 days of growth in SCFM medium in poly-D-lysine-coated microplates as determined by crystal violet staining. The compounds were added to the culture medium at the indicated concentrations on the first day and maintained throughout the duration of the experiment. The control corresponds to DMSO diluent (0.2% final concentration) without any added 2-AI compound. (B) In parallel, the turbidity of planktonic bacteria released in the medium was assessed spectrophotometrically at 600 nm. Inhibition of biofilm formation correlates with an increase in planktonically growing bacteria in the wells. Decreases in both biofilm and planktonic growth are indicative of the inhibitors having reached their MIC values. The results presented are the means (±SD) of quadruplicate wells and are representative of at least two independent experiments.
2.2. Preliminary Investigations into the Mechanism of Biofilm Inhibition by AB-2-29 in M. abscessus
Three main mechanisms of action have thus far been associated with 2-AI compounds and related scaffolds in Gram-positive and Gram-negative bacteria. First is their ability to interfere with two-component signaling systems, resulting in the inhibition and dispersion of Acinetobacter baumannii, Pseudomonas aeruginosa and Staphylococcus aureus biofilms and resensitization of these and other multidrug-resistant bacteria to antibiotics [16,21,27,28,29,30,31,32,33]. A second mechanism highlighted by our recent studies in M. tuberculosis and M. smegmatis relates to the effect of certain 2-AIs on membrane bioenergetics and the proton motive force (PMF) [19,34]. Given the known importance of membrane-mediated anaerobic metabolism in the maintenance of bacterial biofilms [35], this effect of 2-AIs is thought to be the primary driver of their antibiofilm activity in these mycobacterial species. Another report in A. baumannii also highlighted the ability of certain 2-AI derivatives to permeabilize bacterial membranes [36].
The ability of AB-2-29 to permeabilize the plasma membrane of Mabs subsp. abscessus ATCC 19977 was first tested using the LIVE/DEAD BacLight assay, which is based on the nucleic-acid-specific viability dyes propidium iodide and SYTO9. While 0.2 and 0.5% SDS had a dramatic permeabilization effect on the bacilli after one hour of incubation at 37 °C, AB-2-29 failed to show any such effect at its IC50 value (20 μM) (Figure S2).
The ability of AB-2-29 to dissipate the transmembrane potential (ΔΨ), the transmembrane electrochemical proton gradient (ΔpH) or both components of the PMF in M. abscessus was next tested using whole-cell-based and cell-free assays. Impact on the ΔΨ and ΔpH of intact Mabs ATCC 19977 bacilli grown in SCFM was determined by labeling with 3,3′-diethyloxacarbocyanine iodide (DiOC2(3)) and 5-chloromethyl-fluorescein diacetate (CMFDA), respectively. As shown in Figure S3, treating the bacilli with 5 or 20 μM of AB-2-29 for 4 h had no significant impact on either the intracellular pH or the ΔΨ of M. abscessus. A slight but significant effect on ΔΨ (but not on intracellular pH) only manifested at the highest concentration of AB-2-29 tested (100 μM; i.e., 5 times its antibiofilm IC50 value). Consistent with these results, AB-2-29 also failed to dissipate the ΔpH of Mabs inverted membrane vesicles in a succinate-driven proton translocation assay with the fluorescent substrate ACMA (Figure S4). In conclusion, at concentrations where biofilm formation was inhibited, AB-2-29 did not dissipate the PMF of M. abscessus.
2.3. Alteration of M. abscessus Response to Zinc Starvation by AB-2-29
As an unbiased approach to gain insight into the mechanism of action of AB-2-29, we next turned to RNA sequencing (RNAseq) to determine the changes undergone by the transcriptional profile of Mabs upon exposure to AB-2-29. Duplicate samples of exponentially growing Mabs ATCC 19977 cells in SCFM were exposed to 20 μM of AB-2-29 or 0.2% DMSO control for 3 or 24 h at 37 °C with shaking. A comparison of the transcriptional profiles of DMSO- and AB-2-29-treated bacilli at the 3 and 24 h time points was next conducted, and the list of differentially expressed (DE) genes (log2 fold-change > 2 with a false discovery rate adjusted p < 0.05) is presented in Table S1.
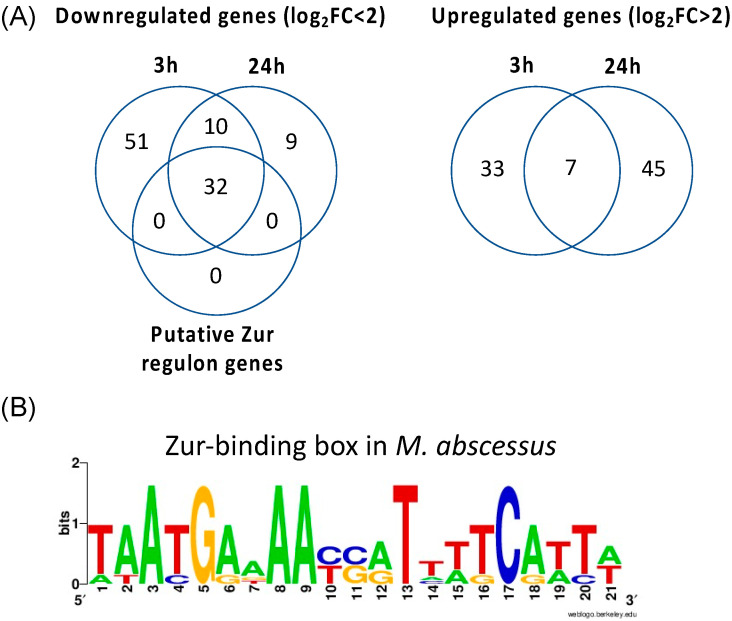
This analysis revealed 40 and 52 upregulated genes and 93 and 51 downregulated genes when comparing DMSO- versus AB-2-29-treated cells after 3 h and 24 h, respectively. A very clear pattern that emerged was a strong (>2.4 to 10.5 log2-fold) downregulation of genes required for adaptation to zinc starvation in the AB-2-29 treatment groups, both at the 3 and 24 h time points. Indeed, at both time points, all 32 predicted Zur regulon genes of M. abscessus were expressed at a significantly lower level in the AB-2-29-treated bacilli (Table 2; Figure 2). Other DE genes at both time points were few and essentially encompassed genes involved in lysine and cobalamin biosynthesis, glyoxylase/bleomycin resistance, a β-lactamase gene and genes encoding a number of hypothetical proteins of unknown function. RNAseq analyses otherwise failed to reveal any two-component system regulators among the DE genes, suggesting that AB-2-29 may act differently from other prototypical 2-AIs in inhibiting M. abscessus biofilm formation.
Table 2.
List of Zur regulon genes that were expressed at higher or lower levels upon treatment with AB-2-29 compared to DMSO control. Differentially expressed genes upon treatment with 20 μM AB-2-29 for 3 or 24 h were defined as ≥ 2 log2 fold-change in expression compared to cells treated with 0.2% DMSO for the same amount of time, with a false discovery rate adjusted p-value (padj) < 0.05. Genes harboring a zur-box in their promoter (see Figure 2B) are marked with an asterisk. Similarly colored genes denote gene clusters likely to be cotranscribed. MAB_0331c, MAB_0332c, MAB_0333c, MAB_0334c and MAB_0336 are Zn-independent alternative ribosomal proteins. MAB_0335 is likely to be involved in cobalamin biosynthesis. MAB_0575c-MAB_0576c-MAB_0577c encode a putative zinc importer of the ABC-transporter family. The operon encompassing genes MAB_1680 to MAB_1701 encodes putative Zn-siderophore biosynthesis and transport proteins, including a putative ABC-transporter and an MCE family transporter.
| Gene | Description | Putative Function | Log2 Fold-Change | |
|---|---|---|---|---|
| AB-2-29 vs. Ctrl 3 h | AB-2-29 vs. Ctrl 24 h | |||
| MAB_0331c | 30S ribosomal protein S18 RpsR2 | Zn-independent ribosomal proteins | −8.04 | −9.31 |
| MAB_0332c | 30S ribosomal protein S14 RpsN2 | −7.76 | −8.93 | |
| MAB_0333c | 50S ribosomal protein L33 RpmG1 | −9.72 | −6.26 | |
| MAB_0334c * | 50S ribosomal protein L28 RpmB2 | −9.30 | −7.83 | |
| MAB_0335 * | Probable cobalamin synthesis protein | Cobalamin biosynthesis | −7.95 | −10.46 |
| MAB_0336 | 50S ribosomal protein L31 type B | Zn-independent ribosomal protein | −8.52 | −9.38 |
| MAB_0575c | Putative ABC-transporter transmembrane protein | ZnuABC transporter (Zn import) | −2.91 | −2.39 |
| MAB_0576c | Putative ABC-transporter ATP-binding protein | −4.05 | −2.89 | |
| MAB_0577c * | Putative ABC-transporter solute binding protein | −5.54 | −5.40 | |
| MAB_0809c * | Conserved hypothetical PPE family protein | Unknown | −5.77 | −4.66 |
| MAB_1680 * | Hypothetical protein | Zn-siderophore biosynthesis and transport | −9.76 | −8.14 |
| MAB_1681 | Hypothetical protein | −9.84 | −7.80 | |
| MAB_1682 | Probable NAD-dependent epimerase/dehydratase | −9.04 | −8.69 | |
| MAB_1683 | Putative fatty acid desaturase | −10.30 | −7.96 | |
| MAB_1684 | Diaminobutyrate-−2-oxoglutarate aminotransferase | −9.48 | −8.31 | |
| MAB_1685 | Putative decarboxylase | −7.35 | −7.01 | |
| MAB_1686 | Hypothetical protein | −8.48 | −7.31 | |
| MAB_1687 | Hypothetical protein | −8.09 | −8.20 | |
| MAB_1688 | Hypothetical protein | −7.85 | −7.90 | |
| MAB_1689 | Probable ABC-transporter ATP-binding subunit DrrA | −7.99 | −7.48 | |
| MAB_1690 | Putative ABC-transporter transmembrane protein | −8.82 | −6.46 | |
| MAB_1691 | Hypothetical protein | −9.86 | −8.31 | |
| MAB_1692 | Putative polyketide synthase Pks16/acyl-CoA synthetase | −7.95 | −7.05 | |
| MAB_1693 | Conserved hypothetical protein (YrbE family?) | −8.46 | −7.52 | |
| MAB_1694 | Putative YrbE family protein | −8.65 | −6.58 | |
| MAB_1695 | Putative Mce family protein | −8.60 | −5.87 | |
| MAB_1696 | Putative Mce family protein | −7.56 | −6.09 | |
| MAB_1697 | Putative Mce family protein | −7.06 | −6.29 | |
| MAB_1698 | Putative Mce family protein | −7.13 | −5.57 | |
| MAB_1699 | Putative Mce family protein | −7.33 | −4.90 | |
| MAB_1700 | Putative Mce family protein | −6.82 | −5.02 | |
| MAB_1701 | Hypothetical protein | −5.82 | −3.61 | |
Figure 2.
Transcriptional response of M. abscessus to AB-2-29 exposure. (A) Venn diagrams showing the number of genes expressed at a significantly higher or lower level in Mabs ATCC19977 upon exposure to 20 μM AB-2-29 for 3 and 24 h compared to DMSO-treated bacilli, and the number of genes among these that are predicted to belong to the M. abscessus Zur regulon. The complete list of these genes is provided in Table S1. (B) Consensus sequence logo for predicted M. abscessus Zur-binding sites. The Mabs ATCC 19977 genome was scanned using the Pattern Locator online software [40] for the presence of putative Mycobacteriaceae Zur-binding sites, as defined by Mikhaylina et al. [37] (TRWYGRNAAYSRTNNNCRWYW), in intergenic regions and allowing for up to one mismatch. The search retrieved six binding sites potentially regulating 32 genes, which we defined as Zur regulon genes. The sequence logo for the consensus Zur-binding motif in Mabs was constructed using WebLogo [41].
The “zinc uptake regulator” Zur is the most widespread zinc-responsive transcriptional factor in prokaryotes [37]. In mycobacteria as in most other prokaryotes, Zur transcriptional regulators act as repressors when Zn2+ is not limiting in the culture medium. Under these conditions, Zn2+ ions become bound to Zur, enabling the protein to bind a zur-box in the promoter region of a number of genes, which results in the blockage of the binding site for the RNA polymerase transcription initiation complex. Under low-zinc conditions, Zur dissociates from the zur-box derepressing the transcription of a variety of genes involved in Zn2+ uptake and the production of zinc-independent enzymes (including zinc-independent ribosomal proteins), among others [37,38,39]. In our study, the strong expression of Zur-regulated genes in DMSO-treated cells was not unexpected given the absence of added zinc in the SCFM medium used to culture Mabs ATCC 19977 (Table S1; Table 2; Figure 2). The fact that the level of expression of these genes considerably decreased in the AB-2-29-treated bacilli was thus indicative of either the presence of zinc brought into the medium by the inhibitor itself or of the ability of the inhibitor to block the Zur repressor in a DNA-binding (i.e., repressing) conformation, even in the absence of zinc in the medium.
2.4. AB-2-29 Binds Zinc
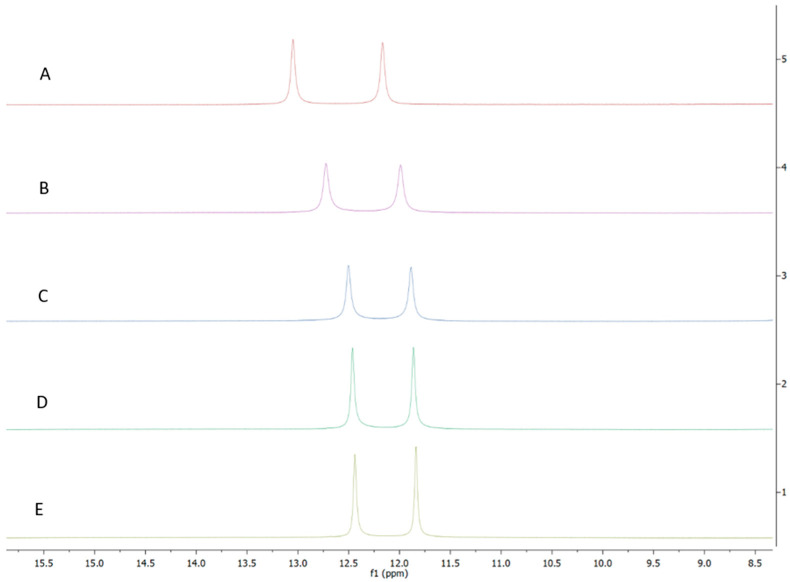
To differentiate between these two hypotheses, we first sought to determine whether AB-2-29 bound zinc, especially since there was precedence for compounds based on the related 2-aminobenzimidazole scaffold inhibiting Staphylococcus aureus biofilms through their ability to chelate zinc from the medium [42,43]. Atomic absorption spectroscopy and NMR-based [44] analyses both converged to indicate that AB-2-29 binds zinc (Figure 3). In contrast, the inhibitor did not bind Fe2+ (Figure S5). Per atomic absorption spectroscopy analysis, 0.126 moles of Zn2+ came with every one mole of the AB-2-29 batch used in our experiments.
Figure 3.
1H NMR spectra of AB-2-29 with (A) 0.0 (B) 0.5 (C) 1.0 (D) 2.0 (E) 4.0 equivalents of zinc. The shifting of the NH2 peaks (between 11.5 and 13.0 ppm) with increased equivalents of zinc implies complexation between AB-2-29 and zinc.
Since Zn2+ ions are naturally absent from SCFM, where M. abscessus forms abundant biofilms, one can exclude that AB-2-29 prevents biofilm formation by acting as a zinc chelator. Likewise, we exclude that the amount of zinc that comes with AB-2-29 (2.52 μM of zinc at its IC50 value of 20 μM) is responsible for the observed antibiofilm activity of this compound, since we previously established that much higher concentrations of zinc were required to inhibit M. abscessus biofilm formation in SCFM (IC50 of zinc~150 μM against Mabs NJH12) [11]. The fact that AB-2-29 comes with small quantities of zinc, however, explains the repression of zur regulon genes in AB-2-29-treated cultures.
2.5. Potentiation of the Biofilm Inhibitory Properties of AB-2-29 by Zinc
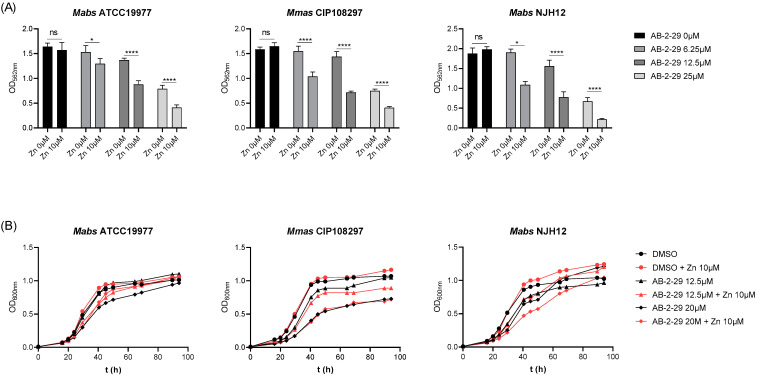
Given the zinc-binding properties of AB-2-29, we next sought to determine how the presence of zinc in the medium affected the activity of the inhibitor. To this end, biofilm assays were repeated in the absence or presence of different concentrations of ZnSO4 in SCFM. The results, which are presented in Figure 4A, showed a striking potentiation of the antibiofilm activity of AB-2-29 against three different Mabs and Mmas strains in the presence of zinc. Since the same treatments did not noticeably impact bacterial growth (with the exception of Mmas CIP108297 whose growth rate was slightly reduced in the presence of 20 μM AB-2-29), one can exclude that the reduction in biofilm formation observed in the AB-2-29 +/− Zn2+-treated groups was in fact the result of growth inhibition (Figure 4B).
Figure 4.
Potentiation of the antibiofilm activity of AB-2-29 by zinc. (A) Biofilm formation by Mabs ATCC 19977, Mabs NJH12 and Mmas CIP108297 in SCFM after 5 days of incubation in the presence or absence of AB-2-29 (0, 6.25, 12.5 or 20 μM) and zinc (0 or 10 μM). The results presented are the means (±SD) of sextuplicate wells and are representative of at least two independent experiments. Asterisks denote statistically significant differences between biofilm inhibitor treatment with and without zinc (* p < 0.05 and **** p < 0.00005); ns: not significant. (B) Growth of Mabs ATCC 19977, Mabs NJH12 and Mmas CIP108297 in SCFM in the presence or absence of AB-2-29 (0, 6.25, 12.5 or 20 μM) and zinc (0 or 10 μM). The results presented are representative of at least two independent experiments.
3. Discussion
A novel inhibitor of M. abscessus biofilm formation has been identified that not only binds to zinc but whose activity also increases in the presence of zinc. The precise mechanism(s) underlying the antibiofilm activity of AB-2-29 and its potentiation by zinc remain(s) to be determined. Unlike previously reported 2-AI compounds and related scaffolds with antibiofilm activity against other Gram-negative or Gram-positive bacteria [16,19,21,27,28,29,30,31,32,33,34,36], AB-2-29 does not appear to act by dissipating the proton motive force or by permeabilizing the plasma membrane of M. abscessus. Likewise, no clear evidence could be derived from our RNAseq studies that AB-2-29 interfered with a particular two-component regulatory system in Mabs ATCC 19977, although one cannot exclude that such a mechanism is at play. Although the amount of zinc brought by AB-2-29 to the culture medium is not sufficient to explain, by itself, the antibiofilm activity of this compound, one may speculate that the complexation of zinc by AB-2-29 facilitates the penetration of the inhibitor inside the cells. Alternatively, the repression of Zur regulon genes caused by AB-2-29 may contribute, at least in part, to its activity. Indeed, the zinc-independent ribosomal proteins S18, S14, L33 and L28 have been involved in biofilm formation in M. smegmatis [45]. Moreover, we note that a number of genes that are downregulated upon exposure to AB-2-29 (MAB_1044c, MAB_1046c, MAB_2204, MAB_2706c, MAB_3438 and the Zur-regulated Mce operon encoded by the gene cluster encompassing MAB_1680 to MAB_1701) were found to be upregulated in M. abscessus during biofilm development [11]. Clearly, further studies are needed to assess the relative contribution of these genes, either individually or combined, to the biofilm-forming capacity of M. abscessus. The importance of such studies resides in their potential to lead to better targeted strategies to inhibit M. abscessus biofilm formation during infection.
Interestingly, AB-2-29 is not the first example of a metal complex displaying antibiofilm activity against M. abscessus. Indeed, metal complexes made of gold, silver, copper or cadmium-containing sulfonamides have recently been shown to display similar properties against a variety of NTM [46,47]. Given the relatively high concentration of zinc, magnesium, calcium and iron found in the sputum of persons with CF and non-CF bronchiectasis [48], this observation provides a basis for the development of innovative therapeutics and adjunct therapeutics directed against difficult-to-cure NTM pulmonary infections.
4. Materials and Methods
4.1. Bacterial Strains and Culture Media
Reference strains Mabs ATCC 19977 and Mmas CIP 108297 were obtained from the ATCC and CIP collections, respectively. M. abscessus subsp. abscessus and M. abscessus subsp. massiliense clinical isolates, NJH9, NJH12 and NJH18 were from persons with CF at National Jewish Health (Denver, CO, USA) [11]. M. abscessus subsp. massiliense clinical isolate 1239 was from a person with CF at the Royal Papworth Hospital, Cambridge, UK [26]. M. abscessus strains were grown under agitation at 37 °C in Middlebrook 7H9 medium supplemented with 10% albumin-dextrose-catalase (ADC) (BD Sciences) and 0.05% Tween 80, in SCFM [11] or on Middlebrook 7H11 agar supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) (BD Sciences). To assess the impact of zinc on growth and biofilm formation, ZnSO4 was added at different concentrations to SCFM.
4.2. Biofilm Assay
M. abscessus submerged biofilms were formed in 96-well (polystyrene, flat bottom) poly-D-lysine-coated plates in 200 μL of SCFM and monitored by crystal violet staining as described [11].
4.3. Drug-Susceptibility Testing
The minimum inhibitory concentrations (MICs) of 2-AI-based small-molecule compounds against Mabs ATCC 19977 and Mmas CIP108297 grown planktonically were determined in SCFM in a total volume of 200 μL in 96-well microtiter plates. Cultures grown to early log phase were diluted to a final concentration of 106 CFU mL−1 and incubated in the presence of serial dilutions of the compounds for 5 days at 37 °C. MICs were determined as the lowest concentration of 2-AI-based compound, showing no visible growth.
4.4. Membrane Permeabilization Assay
Plasma membrane permeabilization by AB-2-29 and SDS was measured with the LIVE/DEAD BacLight kit (Thermo Fisher, Waltham, MA, USA). The fluorescence of Mabs ATCC 19977 bacilli, either treated with DMSO (0.2% final concentration), SDS (0.2 and 0.5%) or AB-2-29 (5 and 20 μM in 0.2% DMSO final concentration) for 1 h at 37 °C, followed by incubation with LIVE/DEAD BacLight solution for 15 min at room temperature, was measured using an excitation wavelength of 485 nm and emission wavelengths of 535 nm (green) and 615 nm (red).
4.5. Membrane Potential and Electrochemical Proton Gradient Measurements in Intact M. abscessus Bacilli
The effects of AB-2-29 on the transmembrane potential (ΔΨ) and transmembrane electrochemical proton gradient (ΔpH) of intact Mabs ATCC 19977 cells were determined by fluorescence quenching of the membrane potential-sensitive probe 3-3′ Diethyloxacarbocyanine iodide (DiOC2(3)) (Thermo Fisher, Waltham, MA, USA) and the pH-sensitive probe 5-chloromethyl-fluorescein diacetate (CMFDA) (Thermo Fisher, Waltham, MA, USA), respectively, as described [49].
4.6. Assay for Succinate-Driven Proton Translocation into M. abscessus Inverted Membrane Vesicles (IMVs)
Succinate-driven proton translocation assays with the fluorescent substrate ACMA were conducted as previously described [49] to determine the effect of AB-2-29 on the electrochemical proton gradient of Mabs ATCC 19977 IMVs. IMVs (0.2 mg mL−1 membrane proteins) were preincubated at 37 °C in 10 mM HEPES-KOH pH 7.5, 100 mM KCl, 5 mM MgCl2 containing 20 μM ACMA, and the baseline was monitored for 10 min with a Victor X5 fluorescence spectrophotometer (PerkinElmer, Waltham, MA, USA). The reaction was then initiated by adding 5 mM succinate. Upon stabilization of the signal, control compounds (nigericin), AB-2-29 or diluent (0.2% DMSO) were added, and proton translocation was monitored fluorometrically. The excitation and emission wavelengths were 419 nm and 483 nm, respectively.
4.7. RNA Extraction, Reverse Transcription and RNAseq
Two independent cultures of DMSO (0.2%)- or AB-2-29 (20 μM)-treated Mabs ATCC 19977 were used for transcriptomics analyses. RNA extraction, reverse transcription reactions, RNAseq library preparation and RNAseq data analysis were conducted as described previously [11].
4.8. Atomic Absorption Spectroscopy
AB-2-29 was resuspended in DMSO at a final concentration of 10 mM, and 90 μL of sample was mixed with 200 μL HNO3 and incubated for 1 h at 80 °C and overnight at 20 °C. Digestions were concluded after addition of 60 μL of 30% H2O2 and dilution to 2 mL with water. Samples were dried overnight at 65 °C and weighed on a 5-place scale. Then, samples were dry-washed overnight at 600 °C, dissolved in 3.6 N HNO3, diluted and analyzed using an atomic absorption spectrophotometer Model 240AA (Agilent) with reference standards for each metal. DMSO alone was included as control.
4.9. Chemical Synthesis
4-(4-propoxyphenyl)-1H-imidazol-2-amine hydrochloride (AB-2-29) was synthesized as previously reported [22].
4.10. Zinc and Iron Binding Studies
DMSO-d6 was purchased from Sigma Aldrich. Fe(II)sulfate-heptahydrate and Zn(II)Cl2 were purchased from Acros Organics. For zinc binding studies, 4-(4-propoxyphenyl)-1H-imidazol-2-amine hydrochloride was dissolved in 500 μL of DMSO-d6 dosed with the appropriate equivalents of anhydrous Zn(II)Cl2. 1H NMR spectra were obtained on a Bruker Avance spectrometer (400 MHz) at ambient temperature. For iron binding studies, 5 μL of a 10 mM stock of 4-(4-propoxyphenyl)-1H-imidazol-2-amine hydrochloride in DMSO was pipetted into 1 mL of the appropriate aqueous solution of Fe(II)SO4 heptahydrate. The samples were vortexed thoroughly and left at ambient temperature for 20 min. The UV spectra were obtained on a SynergyHTX (BioTek, Winooski, VT, USA) multimode plate reader using a Take3 plate and BioCell (BioTek), scanning from 200 to 800 nM.
Acknowledgments
We thank the Colorado State University Next Generation Sequencing Core facility for assistance with Illumina sequencing.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23062950/s1.
Author Contributions
Conceptualization, M.J., B.R.B. and C.M.; methodology, J.M.B., W.L., M.J.Z., K.H.M., E.L., C.J.W., V.C.N.d.M., C.A. and V.J.; formal analysis, J.M.B., C.J.W., W.L., M.J.Z., M.J., B.R.B. and C.M.; investigation, J.M.B., W.L., M.J.Z., K.H.M., E.L., C.J.W., V.C.N.d.M., C.A., B.A., T.V.N. and V.J.; writing—original draft preparation, M.J.; writing—review and editing, J.M.B., C.M. and M.J.; supervision, C.M. and M.J.; project administration, M.J. and C.M.; funding acquisition, M.J., C.A. and J.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a Strategic Research Centre Award (2017-SRC 010) from the UK Cystic Fibrosis Trust (to M.J.), a Research Grant from the Cystic Fibrosis Foundation (to M.J.), the National Institute of Allergy and Infectious Diseases/National Institutes of Health grants AI147326 (to M.J.) and 1 S10 RR023735-01 (Zeiss LSM 510 Laser Scanning Microscope), and the Colorado State University Libraries Open Access Research and Scholarship Fund. J.M.B. is the recipient of a Vertex Research Innovation Award. C.A. is supported by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant No. 845479. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequencing data described in this publication have been submitted to the NCBI gene expression omnibus (GEO) under BioProject No. PRJNA658400.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Floto R.A., Haworth C.S. The growing threat of nontuberculous mycobacteria in CF. J. Cyst. Fibros. 2015;14:1–2. doi: 10.1016/j.jcf.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Martiniano S.L., Nick J.A., Daley C.L. Nontuberculous mycobacterial infections in cystic fibrosis. Thorac. Surg. Clin. 2019;29:95–108. doi: 10.1016/j.thorsurg.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Park I.K., Olivier K.N. Nontuberculous mycobacteria in cystic fibrosis and non–cystic fibrosis bronchiectasis. Semin. Respir. Crit. Care Med. 2015;36:217–224. doi: 10.1055/s-0035-1546751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daley C.L., Iaccarino J.M., Lange C., Cambau E., Wallace R.J., Andrejak C., Böttger E.C., Brozek J., Griffith D.E., Guglielmetti L., et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020;71:905–913. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haworth C.S., Banks J., Capstick T., Fisher A.J., Gorsuch T., Laurenson I.F., Leitch A., Loebinger M.R., Milburn H.J., Nightingale M., et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72:ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 6.Egorova A., Jackson M., Gavrilyuk V., Makarov V. Pipeline of anti-Mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities. Med. Res. Rev. 2021;41:2350–2387. doi: 10.1002/med.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer F.P., Bruderer V.L., Ritter C., Castelberg C., Bloemberg G.V., Böttger E.C. Lack of antimicrobial bactericidal activity in mycobacterium abscessus. Antimicrob. Agents Chemother. 2014;58:3828–3836. doi: 10.1128/AAC.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu M.-L., Aziz D.B., Dartois V., Dick T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today. 2018;23:1502–1519. doi: 10.1016/j.drudis.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Elliott B.A., Nash K.A., Wallace R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012;25:545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luthra S., Rominski A., Sander P. The Role of Antibiotic-Target-Modifying and Antibiotic-Modifying Enzymes in Mycobacterium abscessus Drug Resistance. Front. Microbiol. 2018;9:2179. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belardinelli J.M., Li W., Avanzi C., Angala S.K., Lian E., Wiersma C.J., Palčeková Z., Martin K.H., Angala B., de Moura V.C.N., et al. Unique Features of Mycobacterium abscessus Biofilms Formed in Synthetic Cystic Fibrosis Medium. Front. Microbiol. 2021;12:743126. doi: 10.3389/fmicb.2021.743126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fennelly K.P., Ojano-Dirain C., Yang Q., Liu L., Lu L., Progulske-Fox A., Wang G.P., Antonelli P., Schultz G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016;193:692–693. doi: 10.1164/rccm.201508-1586IM. [DOI] [PubMed] [Google Scholar]
- 13.Høiby N. A short history of microbial biofilms and biofilm infections. APMIS. 2017;125:272–275. doi: 10.1111/apm.12686. [DOI] [PubMed] [Google Scholar]
- 14.Qvist T., Eickhardt S., Kragh K.N., Andersen C.B., Iversen M., Høiby N., Bjarnsholt T. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 2015;46:1823–1826. doi: 10.1183/13993003.01102-2015. [DOI] [PubMed] [Google Scholar]
- 15.Ackart D.F., Lindsey E.A., Podell B.K., Melander R.J., Basaraba R.J., Melander C. Reversal of Mycobacterium tuberculosis phenotypic drug resistance by 2-aminoimidazole-based small molecules. Pathog. Dis. 2014;70:370–378. doi: 10.1111/2049-632X.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackledge M.S., Worthington R.J., Melander C. Biologically inspired strategies for combating bacterial biofilms. Curr. Opin. Pharmacol. 2013;13:699–706. doi: 10.1016/j.coph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brackett S.M., Cox K.E., Barlock S.L., Huggins W.M., Ackart D.F., Bassaraba R.J., Melander R.J., Melander C. Meridianin D analogues possess antibiofilm activity against Mycobacterium smegmatis. RSC Med. Chem. 2020;11:92–97. doi: 10.1039/C9MD00466A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox K.E., Melander C. Anti-biofilm activity of quinazoline derivatives against Mycobacterium smegmatis. MedChemComm. 2019;10:1177–1179. doi: 10.1039/C9MD00156E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon A.B., Obregón-Henao A., Ackart D.F., Podell B.K., Belardinelli J.M., Jackson M., Nguyen T.V., Blackledge M., Melander R.J., Melander C., et al. 2-aminoimidazoles potentiate ß-lactam antimicrobial activity against Mycobacterium tuberculosis by reducing ß-lactamase secretion and increasing cell envelope permeability. PLoS ONE. 2017;12:e0180925. doi: 10.1371/journal.pone.0180925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin S.E., Nguyen C.M., Basaraba R.J., Melander C. Analogue synthesis reveals decoupling of antibiofilm and beta-lactam potentiation activities of a lead 2-aminoimidazole adjuvant against Mycobacterium smegmatis. Chem. Biol. Drug Des. 2018;92:1403–1408. doi: 10.1111/cbdd.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melander R.J., Melander C. Innovative Strategies for Combating Biofilm-Based Infections. Adv. Exp. Med. Biol. 2015;831:69–91. doi: 10.1007/978-3-319-09782-4_6. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen T.V., Minrovic B.M., Melander R.J., Melander C. Identification of Anti-Mycobacterial Biofilm Agents Based on the 2-Aminoimidazole Scaffold. ChemMedChem. 2019;14:927–937. doi: 10.1002/cmdc.201900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen T.V., Peszko M., Melander R.J., Melander C., Worthington R. Using 2-aminobenzimidazole derivatives to inhibit Mycobacterium smegmatis biofilm formation. MedChemComm. 2019;10:456–459. doi: 10.1039/C9MD00025A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeiler M.J., Melander R.J., Melander C.C. Second-Generation Meridianin Analogues Inhibit the Formation of Mycobacterium smegmatis Biofilms and Sensitize Polymyxin-Resistant Gram-Negative Bacteria to Colistin. ChemMedChem. 2020;15:1672–1679. doi: 10.1002/cmdc.202000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T.V., Blackledge M.S., Lindsey E.A., Minrovic B.M., Ackart D.F., Jeon A.B., Obregón-Henao A., Melander R.J., Basaraba R.J., Melander C. The Discovery of 2-Aminobenzimidazoles That Sensitize Mycobacterium smegmatis and M. tuberculosis to beta-Lactam Antibiotics in a Pattern Distinct from beta-Lactamase Inhibitors. Angew. Chem. Int. Ed. Engl. 2017;56:3940–3944. doi: 10.1002/anie.201612006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiersma C.J., Belardinelli J.M., Avanzi C., Angala S.K., Everall I., Angala B., Kendall E., De Moura V.C.N., Verma D., Benoit J., et al. Cell Surface Remodeling of Mycobacterium abscessus under Cystic Fibrosis Airway Growth Conditions. ACS Infect. Dis. 2020;6:2143–2154. doi: 10.1021/acsinfecdis.0c00214. [DOI] [PubMed] [Google Scholar]
- 27.Frei R., Breitbach A.S., Blackwell H.E. 2-Aminobenzimidazole Derivatives Strongly Inhibit and Disperse Pseudomonas aeruginosa Biofilms. Angew. Chem. Int. Ed. 2012;51:5226–5229. doi: 10.1002/anie.201109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris T.L., Worthington R.J., Hittle L.E., Zurawski D.V., Ernst R., Melander C. Small Molecule Downregulation of PmrAB Reverses Lipid A Modification and Breaks Colistin Resistance. ACS Chem. Biol. 2014;9:122–127. doi: 10.1021/cb400490k. [DOI] [PubMed] [Google Scholar]
- 29.Harris T.L., Worthington R.J., Melander C. Potent Small-Molecule Suppression of Oxacillin Resistance in Methicillin-Resistant Staphylococcus aureus. Angew. Chem. Int. Ed. 2012;51:11254–11257. doi: 10.1002/anie.201206911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milton M.E., Allen C.L., Feldmann E.A., Bobay B., Jung D.K., Stephens M.D., Melander R.J., Theisen K.E., Zeng D., Thompson R.J., et al. Structure of the Francisella response regulator QseB receiver domain, and characterization of QseB inhibition by antibiofilm 2-aminoimidazole-based compounds. Mol. Microbiol. 2017;106:223–235. doi: 10.1111/mmi.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milton M.E., Minrovic B.M., Harris D.L., Kang B., Jung D., Lewis C.P., Thompson R.J., Melander R.J., Zeng D., Melander C., et al. Re-sensitizing Multidrug Resistant Bacteria to Antibiotics by Targeting Bacterial Response Regulators: Characterization and Comparison of Interactions between 2-Aminoimidazoles and the Response Regulators BfmR from Acinetobacter baumannii and QseB from Francisella spp. Front. Mol. Biosci. 2018;5:15. doi: 10.3389/fmolb.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson R.J., Bobay B.G., Stowe S.D., Olson A.L., Peng L., Su Z., Actis L.A., Melander C., Cavanagh J. Identification of BfmR, a Response Regulator Involved in Biofilm Development, as a Target for a 2-Aminoimidazole-Based Antibiofilm Agent. Biochemistry. 2012;51:9776–9778. doi: 10.1021/bi3015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worthington R.J., Blackledge M.S., Melander C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Futur. Med. Chem. 2013;5:1265–1284. doi: 10.4155/fmc.13.58. [DOI] [PubMed] [Google Scholar]
- 34.Jeon A.B., Ackart D.F., Li W., Jackson M., Melander R.J., Melander C., Abramovitch R.B., Chicco A.J., Basaraba R.J., Obregón-Henao A. 2-aminoimidazoles collapse mycobacterial proton motive force and block the electron transport chain. Sci. Rep. 2019;9:1513. doi: 10.1038/s41598-018-38064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurdle J.G., O’neill A.J., Chopra I., Lee R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011;9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stowe S.D., Thompson R.J., Peng L., Su Z., Blackledge M.S., Draughn G.L., Coe W.H., Johannes E., Lapham V.K., MacKenzie J., et al. Membrane-permeabilizing activity of reverse-amide 2-aminoimidazole antibiofilm agents against Acinetobacter baumannii. Curr. Drug Deliv. 2015;12:223–230. doi: 10.2174/1567201811666140924125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikhaylina A., Ksibe A.Z., Scanlan D., Blindauer C.A. Bacterial zinc uptake regulator proteins and their regulons. Biochem. Soc. Trans. 2018;46:983–1001. doi: 10.1042/BST20170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goethe E., Laarmann K., Lührs J., Jarek M., Meens J., Lewin A., Goethe R. Critical Role of Zur and SmtB in Zinc Homeostasis of Mycobacterium smegmatis. mSystems. 2020;5:e00880-19. doi: 10.1128/mSystems.00880-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maciag A., Dainese E., Rodriguez G.M., Milano A., Provvedi R., Pasca M.R., Smith I., Palu G., Riccardi G., Manganelli R. Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J. Bacteriol. 2007;189:730–740. doi: 10.1128/JB.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mrázek J., Xie S. Pattern locator: A new tool for finding local sequence patterns in genomic DNA sequences. Bioinformatics. 2006;22:3099–3100. doi: 10.1093/bioinformatics/btl551. [DOI] [PubMed] [Google Scholar]
- 41.Crooks G.E., Hon G., Chandonia J.-M., Brenner S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey E.A., Brackett C.M., Mullikin T., Alcaraz C., Melander C. The discovery of N-1 substituted 2-aminobenzimidazoles as zinc-dependent S. aureus biofilm inhibitors. MedChemComm. 2012;3:1462–1465. doi: 10.1039/c2md20244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers S.A., Huigens R.W., III, Melander C. A 2-aminobenzimidazole that inhibits and disperses gram-positive biofilms through a zinc-dependent mechanism. J. Am. Chem. Soc. 2009;131:9868–9869. doi: 10.1021/ja9024676. [DOI] [PubMed] [Google Scholar]
- 44.Mathuthu E., van Rensburg A.J., Du Plessis D., Mason S. EDTA as a chelating agent in quantitative 1H-NMR of biologically important ions. Biochem. Cell Biol. 2021;99:465–475. doi: 10.1139/bcb-2020-0543. [DOI] [PubMed] [Google Scholar]
- 45.Ojha A., Hatfull G.F. The role of iron in Mycobacterium smegmatis biofilm formation: The exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol. Microbiol. 2007;66:468–483. doi: 10.1111/j.1365-2958.2007.05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agertt V.A., Bonez P.C., Rossi G.G., Flores V.D.C., Siqueira F.D.S., Mizdal C.R., Marques L.L., De Oliveira G.N.M., De Campos M.M.A. Identification of antimicrobial activity among new sulfonamide metal complexes for combating rapidly growing mycobacteria. BioMetals. 2016;29:807–816. doi: 10.1007/s10534-016-9951-3. [DOI] [PubMed] [Google Scholar]
- 47.Bonez P.C., Agertt V.A., Rossi G.G., Siqueira F.D.S., Siqueira J.D., Marques L.L., de Oliveira G.N.M., Santos R.C.V., de Campos M.M.A. Sulfonamides complexed with metals as mycobacterial biofilms inhibitors. J. Clin. Tuberc. Other Mycobact. Dis. 2021;23:100217. doi: 10.1016/j.jctube.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith D.J., Anderson G., Bell S., Reid D. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J. Cyst. Fibros. 2014;13:289–295. doi: 10.1016/j.jcf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Li W., Stevens C.M., Pandya A.N., Darzynkiewicz Z.M., Bhattarai P., Tong W., Gonzalez-Juarrero M., North E.J., Zgurskaya H.I., Jackson M.C. Direct Inhibition of MmpL3 by Novel Antitubercular Compounds. ACS Infect. Dis. 2019;5:1001–1012. doi: 10.1021/acsinfecdis.9b00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data described in this publication have been submitted to the NCBI gene expression omnibus (GEO) under BioProject No. PRJNA658400.