Abstract
The pharmacokinetics of a 2-g bolus of cefepime were measured in critically ill patients with normal renal function. Variable and low trough plasma drug concentrations were found, and 8 of 10 patients had levels below the MIC at which 50% of the isolates are inhibited for Pseudomonas aeruginosa. Computer simulations predicted that continuous infusion and shorter dosing intervals would increase trough levels.
Cefepime (2, 17), a β-lactam antibiotic, covers most organisms recovered from patients in intensive care (10, 18). As killing of gram-negative bacilli by β-lactams is almost entirely related to the time that levels in tissue and plasma exceed a certain threshold (22), it is important that the dosing regimen maintains adequate plasma drug levels for as long as possible during the dosing interval. It is not surprising then that dosing regimens of β-lactam antibiotics are being reevaluated to sustain plasma drug levels (3, 6, 12, 15, 19).
Drug dosage regimens used for critically ill patients are often based on data for healthy patients, who do not typically share the characteristics of the critically ill, such as abnormal fluid balances, different volumes of distribution, altered protein metabolism, and low albumin levels (4, 7, 8, 14, 16, 20, 21). However, there is only sparse documentation on the pharmacokinetics of cefepime in critically ill patients (9).
The aim of the study was to document levels of cefepime in plasma from critically ill patients with normal renal function. This data was then used to develop a pharmacokinetic model, allowing a variety of dosing regimens to be simulated to identify doses that predict sustained levels.
This study was approved by the hospital ethics committee. Critically ill patients ranging in age from 18 to 75 years for whom the staff intensive care specialist deemed cefepime to be appropriate therapy were enrollable if they had an infected site as defined by clinical suspicion with or without positive culture results, systemic inflammatory response syndrome, and serum creatinine level of <0.1 mmol/liter. Enrolled patients were considered nonevaluable if they developed renal dysfunction (creatinine clearance, <75 ml/min).
After informed consent had been obtained, cefepime (2 g, diluted into 20 ml of sterile water for injection and infused over 3 min) was administered twice daily at precise 12-h intervals via an intravenous line. Two sets of blood samples were taken over two 12-h dosing intervals: those used to generate profile A were collected after the first dose had been administered, and those used to generate profile B were collected after multiple doses (day 3, 4, 5, or 6). Samples were taken immediately prior to dose administration (time [T] = 0 at the start of the 3-min infusion) and at 5, 10, 20, 30, 60, 90, 120, 240, 360, 480, and 600 min postdosing and immediately prior to the next dose (T = 720). Blood (10 ml) was drawn into heparinized Vacutainers from an in-situ arterial line and centrifuged at 4°C, and the plasma was frozen at −20°C until it was stored at −80°C.
Patient plasma samples were assayed by an in-house modification of existing high-performance liquid chromatography methods (1, 5). Briefly, sample preparation involved precipitation of proteins with acetonitrile and trichloroacetic acid containing cefadroxil (internal standard), followed by washing with dichloromethane. Separations were performed on a reverse-phase C18 column with a pH 4.9 acetonitrile:20 mM ammonium acetate mobile phase (ratio, 8:92). The assay was linear from 1 to 200 μg/ml. The intraday and interday imprecision values were all under 6%, and the inaccuracy values were under 5% at the test concentrations of 4.18, 16.7 and 83.5 μg/ml.
The trough levels C0 and C720 were those at 0 and 720 min, respectively. Elimination half-life, area under the curve, total body cefepime clearance, mean residence time, and volume of distribution at steady state were determined by fitting the data for plasma drug concentration over time for profile A to a two-compartment pharmacokinetic model by using WinNonlin (Scientific Consulting, Inc.). Slopes and intercepts of the biexponential declines were estimated with iterative reweighting to the inverse of the square of the predicted concentration (1/y2), and the fit was evaluated from the standard errors of the parametric estimates. The model parameters for each of the 10 evaluable patients were used to stimulate various cefepime dosing regimens.
Thirteen patients (11 males) ranging from 34 to 75 years old (mean, 55 years old) were enrolled. APACHE II scores (11) ranged from 8 to 24 at study entry (Table 1). There was an identifiable source of sepsis in 11 patients. In eight patients cefepime produced a clinical cure, and in six there was a bacteriological cure. There was one clinical and bacteriological failure in which Pseudomonas aeruginosa was isolated (MIC of cefepime, 6 μg/ml). Clinical and bacteriological assessments were indeterminate (there was no change) in the other patients. Three patients were nonevaluable as they had abnormal creatinine clearances on enrollment, even though their serum creatinine levels were within the normal laboratory range.
TABLE 1.
Patient demographics, cefepime levels, and pharmacokinetic parameters following a 3-min infusion of 2 g of cefepime to intensive care unit patientsa
| Patient | APACHE scorec | CLCR (ml/min) | C12h (μg/ml) | t1/2β (h) | AUC (μg · h/ml) | CL (ml/min) | MRT (h) | VSS (liter) |
|---|---|---|---|---|---|---|---|---|
| 1b | 24 | 54.0 | 19.7 | 5.6 (0.3) | 690 (22) | 48 (2) | 7.6 (0.4) | 22.0 (1.0) |
| 2 | 10 | 133.8 | 2.6 | 1.8 (0.1) | 239 (12) | 140 (7) | 2.0 (0.1) | 17.0 (1.4) |
| 3 | 16 | 103.2 | 5.7 | 2.9 (0.1) | 423 (10) | 79 (2) | 3.9 (0.1) | 18.4 (0.6) |
| 4b | 15 | 67.2 | 9.6 | 4.7 (0.3) | 396 (13) | 84 (3) | 6.2 (0.4) | 31.4 (1.6) |
| 5 | 11 | 157.2 | 1.1 | 1.5 (0.1) | 226 (9) | 148 (6) | 1.7 (0.1) | 15.0 (0.9) |
| 6 | 9 | 153.6 | 1.6 | 2.3 (0.1) | 199 (12) | 167 (10) | 2.6 (0.1) | 26.4 (2.4) |
| 7 | 9 | 135.6 | 2.1 | 2.5 (0.1) | 241 (9) | 138 (5) | 3.0 (0.1) | 24.6 (1.3) |
| 8b | 14 | 63.6 | 10.0 | 4.0 (0.2) | 467 (14) | 71 (2) | 5.3 (0.2) | 22.8 (1.1) |
| 9 | 15 | 187.2 | 1.6 | 2.2 (0.1) | 257 (8) | 130 (4) | 2.6 (0.1) | 20.2 (0.9) |
| 10 | 11 | 117.6 | 1.1 | 1.9 (0.1) | 285 (11) | 117 (4) | 2.3 (0.1) | 16.2 (0.8) |
| 11 | 8 | 97.2 | 6.3 | 3.5 (0.2) | 337 (12) | 99 (4) | 4.4 (0.2) | 26.3 (1.7) |
| 12 | 8 | 141.0 | 1.6 | 2.3 (0.1) | 194 (9) | 172 (8) | 2.9 (0.1) | 29.8 (2.0) |
| 13 | 13 | 79.8 | 8.5 | 3.8 (0.1) | 424 (10) | 79 (2) | 5.1 (0.2) | 24.0 (0.9) |
| Mediand | 10.5 | 134.7 | 1.9 | 2.5 | 249 | 134 | 2.8 | 22.1 |
| Meand | 11.0 | 130.6 | 3.2 | 3.0 | 283 | 127 | 3.1 | 21.8 |
| SDd | 2.8 | 32.0 | 2.6 | 1.2 | 85 | 33 | 1.1 | 5.1 |
CLCR, creatinine clearance; C12h, cefepime concentration at 12 h postdose; t1/2β, elimination half-life; AUC, area under the plasma cefepime concentration-time curve; CL, cefepime clearance; MRT, mean residence time; VSS, apparent volume at steady state. Values shown are estimates, and their standard errors are shown in parentheses.
Patient nonevaluable.
APACHE score at time of initial cefepime dose.
Only data for evaluable patients are included in these statistical results.
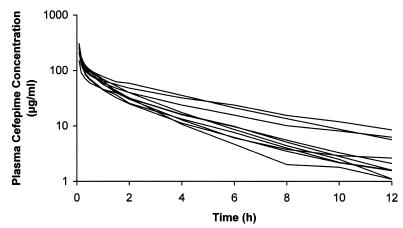
The plasma cefepime concentrations of the 10 evaluable patients after the first dose (profile A) are shown in Fig. 1. There was a large variation in plasma drug concentrations among patients, and a number of patients had very low plasma drug levels (median level, 1.9 μg/ml) toward the end of the dosing interval (Table 1). Trough levels after multiple doses were particularly low (median levels, 1.7 and 1.8 μg/ml), with 4 of 10 evaluable patients having levels under 1 μg/ml and another 4 having trough levels lower than 2.83 μg/ml, the MIC at which 50% of the isolates are inhibited for P. aeruginosa (Table 2) (17). The consequences of these low levels could be important if they involve inadequate bacterial killing or the development of resistance, as has been documented to occur in vitro (6).
FIG. 1.
Plasma cefepime concentrations for 10 intensive care unit patients following administration of an initial dose of 2 g intravenously (over 3 min) (profile A).
TABLE 2.
Trough cefepime levels and creatinine clearance measured after multiple 2-g doses of cefepime over 12 h (profile B), and trough plasma cefepime concentrations at 48 h predicted by simulated dosing regimens
| Patient | CLCR (ml/min) | C0h (μg/ml) | C12h (μg/ml) |
C48h trougha (μg/ml)
|
|
|---|---|---|---|---|---|
| 6 g/dayb | 1 g/4 hc | ||||
| 2 | 128.4 | 2.6 | 2.1 | 29.8 | 9.6 |
| 3 | 87.6 | 5.4 | 4.0 | 52.9 | 29.5 |
| 5 | 127.2 | <1.0 | <1.0 | 28.2 | 7.4 |
| 6 | 195.6 | 1.4 | <1.0 | 24.9 | 10.0 |
| 7 | 137.4 | 3.0 | 2.8 | 30.1 | 13.5 |
| 9 | 146.4 | <1.0 | <1.0 | 32.1 | 13.0 |
| 10 | 115.8 | 2.0 | 2.2 | 35.7 | 13.2 |
| 11 | 118.8 | <1.0 | <1.0 | 42.2 | 24.1 |
| 12 | 150.6 | 1.4 | 1.4 | 24.3 | 10.9 |
| 13 | 74.4 | 6.2 | 7.7 | 53.0 | 33.6 |
| Median | 127.8 | 1.7 | 1.8 | 31.1 | 13.1 |
| Mean | 128.2 | 35.3 | 16.5 | ||
| SD | 30.5 | 9.7 | 8.3 | ||
Predicted minimum cefepime concentration after 48 h.
Simulated continuous infusion of 6 g of cefepime per 24 h with an initial 0.5-g loading dose.
Simulated 3-min infusion of 1 g of cefepime every 4 h.
Pharmacokinetic parameters for the first dose are displayed in Table 1. Generally, cefepime was cleared in the patients than in healthy volunteers, but overall the kinetics are similar to those reported in the published literature (2). The large scatter in trough levels in our study may be partly accounted for by the variance in kidney function of our patients. The three patients that were nonevaluable due to poor kidney function (creatinine clearances, <75 ml/min) had high cefepime levels, and after multiple doses the highest trough levels were found in those with creatinine clearances of <100 ml/min. This is not surprising, as cefepime is largely excreted unchanged by the kidney (2), and there was a strong relationship between creatinine clearance and cefepime clearance revealed by our data (r2 = 0.74).
The compartmental variables determined by the model for the 10 evaluable patients were used to simulate alternative dosing regimens in an attempt to identify regimens that would maintain high trough levels. Dosing regimens were simulated, and their predicted median trough values after 48 h were as follows: for continuous infusions (with a 0.5-g loading dose) of 4 and 6 g/day, 20.8 and 31.1 μg/ml, respectively; and for intermittent bolus dosing of 2 g 8 hourly, 5.4 μg/ml; 1.5 g 6 hourly, 8.5 μg/ml; 1 g 4 hourly, 13.1 μg/ml. The 48-h trough values predicted for the 10 evaluable patients for the 1-g, 4 hourly bolus and for the 6-g/day continuous infusion regimens are shown in Table 2. The lowest 48-h trough obtained with 4 hourly boluses, 7.4 μg/ml, is almost three times the MIC at which 50% of the isolates are inhibited for P. aeruginosa for the entire dosing interval, whereas the lowest steady-state concentration obtained with a 6-g/day continuous infusion is 24 μg/ml.
Previous modeling studies on ceftazidime given as a continuous infusion to critically ill patients (23) have predicted levels in plasma that were subsequently shown to be achievable clinically (13). In spite of the interpatient variability, we propose that the regimens of 1-g, 4 hourly bolus dosing and 6-g/day continuous infusions would help eliminate the unpredictably low trough cefepime levels obtained in this study.
Cefepime has a broad spectrum of activity against gram-negative organisms (2, 10, 17) and can be used for both proven and suspected resistant gram-negative bacterial infections (2, 18), including those with P. aeruginosa. However, the variable and low trough levels reported here in critically ill patients may decrease efficacy in a situation where optimal dosing is essential. Pharmacokinetic modeling suggests that shorter dosing intervals would maintain sustained levels with higher troughs in more patients. Our data suggests that the daily dose of cefepime in critically ill patients with normal renal function should be increased to 6 g/day, given preferably as 1-g, 4 hourly doses or, alternatively, as a continuous infusion.
Acknowledgments
We thank Sue Parry-Jones and Bristol-Myers Squibb Australia Pty. Ltd. for their financial support, for the supply of cefepime, and for the supply of cefadroxil for the high-performance liquid chromatography standard.
REFERENCES
- 1.Barbhaiya R H, Forgue S T, Shyu W C, Papp E A, Pittman K A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987;31:55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barradell L B, Bryson H M. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1994;47:471–505. doi: 10.2165/00003495-199447030-00007. [DOI] [PubMed] [Google Scholar]
- 3.Benko A S, Cappelletty D M, Kruse J A, Rybak M J. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected gram-negative infections. Antimicrob Agents Chemother. 1996;40:691–695. doi: 10.1128/aac.40.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.du Souich P, Verges J, Erill S. Plasma protein binding and pharmacological response. Clin Pharmacokinet. 1993;24:435–440. doi: 10.2165/00003088-199324060-00001. [DOI] [PubMed] [Google Scholar]
- 5.Elkhaïli H, Linger L, Monteil H, Jehl F. High-performance liquid chromatographic assay for cefepime in serum. J Chromatogr B Biomed Appl. 1997;690:181–188. doi: 10.1016/s0378-4347(96)00406-9. [DOI] [PubMed] [Google Scholar]
- 6.Fantin B, Farinotti R, Thabaut A, Carbon C. Conditions for the emergence of resistance to cefpirome and ceftazidime in experimental endocarditis due to Pseudomonas aeruginosa. J Antimicrob Chemother. 1994;33:563–569. doi: 10.1093/jac/33.3.563. [DOI] [PubMed] [Google Scholar]
- 7.Gosling P, Sanghera K, Dickson G. Generalized vascular permeability and pulmonary function in patients following serious trauma. J Trauma. 1994;36:477–481. doi: 10.1097/00005373-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gous A G, Dance M D, Lipman J, Luyt D K, Mathivha R, Scribante J. Changes in vancomycin pharmacokinetics in critically ill infants. Anaesth Intensive Care. 1995;23:678–682. doi: 10.1177/0310057X9502300603. [DOI] [PubMed] [Google Scholar]
- 9.Kieft H, Hoepelman A I, Knupp C A, van Dijk A, Branger J M, Struyvenberg A, Verhoef J. Pharmacokinetics of cefepime in patients with the sepsis syndrome. J Antimicrob Chemother. 1993;32(Suppl. B):117–122. doi: 10.1093/jac/32.suppl_b.117. [DOI] [PubMed] [Google Scholar]
- 10.Klugman K P, Saunders J, Khoosal M. In-vitro activity of cefepime against bacterial pathogens from hospitalized patients. J Antimicrob Chemother. 1993;32:164–166. doi: 10.1093/jac/32.1.164. [DOI] [PubMed] [Google Scholar]
- 11.Knaus W A, Draper E A, Wagner D P, Zimmerman J E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 12.Lipman J, Crewe Brown H H, Saunders G L, Gous A G. Subtleties of antibiotic dosages—do doses and intervals make a difference in the critically ill? S Afr J Surg. 1996;34:160–162. [PubMed] [Google Scholar]
- 13.Lipman J, Gomersall C, Gin T, Joynt G, Young R. Continuous infusion ceftazidime in intensive care: a randomised controlled trial. J Antimicrob Chemother. 1999;43:309–311. doi: 10.1093/jac/43.2.309. [DOI] [PubMed] [Google Scholar]
- 14.Marik P E, Havlik I, Monteagudo F S, Lipman J. The pharmacokinetics of amikacin in critically ill adult and paediatric patients: comparison of once- versus twice-daily dosing regimens. J Antimicrob Chemother. 1991;27(Suppl. C):81–89. doi: 10.1093/jac/27.suppl_c.81. [DOI] [PubMed] [Google Scholar]
- 15.Mouton J W, den Hollander J G. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1994;38:931–936. doi: 10.1128/aac.38.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudy A, Brater D. Pharmacokinetics. In: Chernow B, editor. The pharmacologic approach to the critically ill patient. Baltimore, Md: Williams and Wilkins; 1994. pp. 3–17. [Google Scholar]
- 17.Sanders C C. Cefepime: the next generation? Clin Infect Dis. 1993;17:369–379. [PubMed] [Google Scholar]
- 18.Sanders W E, Jr, Tenney J H, Kessler R E. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin Infect Dis. 1996;23:454–461. doi: 10.1093/clinids/23.3.454. [DOI] [PubMed] [Google Scholar]
- 19.Turnidge J D. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998;27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 20.van Dalen R, Vree T B. Pharmacokinetics of antibiotics in critically ill patients. Intensive Care Med. 1990;16(Suppl. 3):S235–S238. doi: 10.1007/BF01709707. [DOI] [PubMed] [Google Scholar]
- 21.van Dalen R, Vree T B, Baars I M. Influence of protein binding and severity of illness on renal elimination of four cephalosporin drugs in intensive-care patients. Pharm Weekbl. 1987;9:98–103. doi: 10.1007/BF01960743. [DOI] [PubMed] [Google Scholar]
- 22.Vogelman B, Craig W A. Kinetics of antimicrobial activity. J Pediatr. 1986;108:835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- 23.Young R J, Lipman J, Gin T, Gomersall C D, Joynt G M, Oh T E. Intermittent bolus dosing of ceftazidime in critically ill patients. J Antimicrob Chemother. 1997;40:269–273. doi: 10.1093/jac/40.2.269. [DOI] [PubMed] [Google Scholar]