Abstract
The purpose of this study was to determine the distribution profile of a novel endotoxin antagonist, [14C]E5531, at 1 μg/ml in plasma samples obtained from fasted human subjects with various lipid and protein concentrations. Our findings suggest that the majority of E5531 binds with high-density lipoproteins (HDLs) independently of plasma lipid and protein levels tested. Furthermore, it appears that an increase in triglyceride-rich lipoprotein (TRL) lipid and protein levels and an increase in low-density lipoprotein (LDL) lipid levels significantly increase TRL plus LDL binding of E5531. However, only an increase in HDL protein levels significantly increases HDL binding of E5531.
Recent work by Rose et al. (7a) suggests that E5531, a novel endotoxin antagonist, binds primarily to high-density lipoproteins (HDLs) upon incubation in human serum at 37°C. Furthermore, they observed that loss of drug activity occurred rapidly upon binding to HDLs (7). However, studies have not been done to determine if changes in plasma lipid and lipoprotein concentrations, often observed in septic patients (1) who would be receiving this drug, would modify the plasma lipoprotein binding of E5531. In addition, no studies have been done to elucidate which components of plasma lipoproteins (i.e., cholesterol, triglycerides [TGs], and protein) are responsible for the binding of E5531 to HDL. Thus, the objective of the proposed study was to determine the profile of distribution of E5531 in human plasma obtained from patients with various total and plasma lipoprotein lipid and protein concentrations by using density gradient ultracentrifugation and affinity chromatography.
Radiolabeled E5531 ([14C]E5531; specific activity, 105 mCi/mmol) was solubilized with sodium hydroxide in lactose-phosphate buffer as described previously (7). The volume of vehicle required to reconstitute 1 μg of E5531 per ml did not modify the lipoprotein concentration or composition (data not shown). Randomly selected human plasma, obtained from the Red Cross (Vancouver, British Columbia, Canada), was provided freshly drawn (i.e., removed from the donor within the previous 48 h) and frozen. Initial screening of individual patient plasma samples for differences in the lipoprotein lipid composition and a patient medication profile were completed. Plasma from patients that were receiving medication that could modify lipoprotein metabolism (i.e., lipid-lowering agents or cyclosporine) was not used. Patient plasma samples were selected based on significant differences in the total cholesterol (TC) and/or the total TG of the separated lipoprotein fractions. For all E5531 incubation studies, plasma samples were separated into their different lipoprotein and lipoprotein-deficient subfractions by step-gradient ultracentrifugation as previously described (2, 10). To ensure that the distribution of E5531 found in each of these fractions was a result of its association with each lipoprotein or lipoprotein-deficient (LPDP) fraction and not a result of the density of the formulation, the density of the E5531 formulation incubated in the LPDP fraction for 60 min at 37°C was determined by ultracentrifugation. The concentration of E5531 in each lipoprotein and LPDP sample was determined by radioactivity and compared to an external calibration curve to correct for quenching within each fraction. Total plasma and lipoprotein cholesterol, TG, and protein concentrations were determined by enzymatic assays purchased from Sigma Chemical Co. (2).
To assess the influence of modified plasma and lipoprotein cholesterol profiles on the distribution of [14C]E5531 in plasma, the following protocol was used. [14C]E5531 at 1.0 μg/ml (0.64 μM; physiological concentrations in plasma likely to be obtained following infusion into patients [unpublished observations]) was incubated in plasma samples from six human subjects with various total plasma cholesterol levels (70 to 229 mg/dl) for 5 min, 1 h, 3 h, and 6 h at 37°C. Following incubation at each time point, plasma fractions were separated into their plasma lipoprotein and LPDP fractions, and each of these fractions was analyzed for E5531 content by radioactivity. The plasma and lipoprotein cholesterol, TG, and protein concentrations were determined in each fraction and correlated to the percentage of E5531 recovered in each fraction.
To confirm that the distribution of E5531 found in each of these fractions was a result of its association with each lipoprotein or LPDP fraction and not a function of the lipoprotein separation technique, E5531 lipoprotein distribution was determined by affinity chromatography (LDL-Direct; Isolab) (2) to separate the plasma into its different lipoprotein fractions.
Correlation coefficients between the percentage of E5531 recovered within the plasma triglyceride-rich lipoprotein (TRL), low-density lipoprotein (LDL), and HDL fractions and the concentration of cholesterol, TG, and protein within these fractions were determined by using Pearson’s test. The interday coefficient of variation (%CV) for E5531 assay detection within each of the lipoprotein and LPDP fractions of each patient’s plasma was also determined. A difference was considered significant if the probability of chance explaining the results was reduced to less than 5% (P < 0.05). All data were expressed as a mean ± standard deviation.
Initial studies were designed to determine if changes in incubation time would alter the distribution profile of the compound in plasma. E5531 at 1 μg/ml was incubated for 5 min, 1 h, 3 h, and 6 h in one normocholesterolemic plasma sample (TC = 161 mg/dl) and one hypercholesterolemic plasma sample (TC = 280 mg/dl), and the distribution profile in plasma was determined. In both plasma samples, increases in incubation time from 5 min to up to 6 h did not alter the distribution profile for E5531 (data not shown). Based on these observations, all future studies reported in this article were done with an incubation time of 5 min.
To determine if the use of different lipoprotein separation techniques would modify the distribution profile of the compound in plasma, E5531 at 1 μg/ml was incubated for 5 min in a normolipidemic human plasma sample (TC = 161 mg/dl). Although significant differences in the percentage of E5531 recovered in the different plasma fractions were observed, the pattern of drug distribution was similar (data not shown). Similar findings were observed when the analysis of E5531 plasma lipoprotein distribution by using different lipoprotein separation techniques was tested in other human plasmas (data not shown). Based on these observations, all studies reported in this article used density gradient ultracentrifugation to partition plasma into its different lipoprotein and LPDP components.
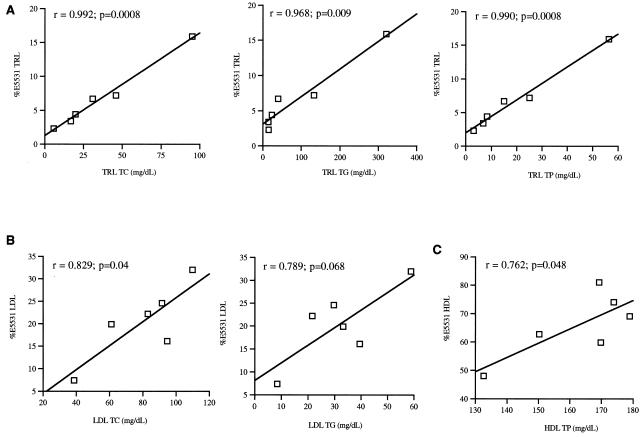
When E5531 at 1.0 μg/ml was incubated within plasma obtained from six human subjects with various TC levels, the majority of drug was recovered in the HDL fraction for all plasmas tested (Table 1). In addition, when an E5531 concentration range of 0.25 to 1.5 μg/ml was incubated in these plasmas, similar distribution profiles in plasma to 1 μg/ml were observed (data not shown). When the correlations between the percentage of E5531 recovered within the TRL, LDL, and HDL fractions versus the concentration of cholesterol, TG, and protein in these fractions, respectively, were determined, the following results were found. Positive correlations were observed for TRL cholesterol, TG, and protein concentrations (Fig. 1A); LDL cholesterol and TG concentrations (Fig. 1B); and HDL protein concentrations (Fig. 1C). A negative correlation was observed for the percentage of E5531 recovered in the LDL fraction versus HDL protein concentrations (r = −0.84; P < 0.04; data not shown). No significant correlations were observed for the percentage of E5531 recovered within these lipoprotein fractions versus the TC/TG, TC/total protein (TP), and TG/TP ratios within each lipoprotein fraction.
TABLE 1.
Distribution of 1 μg of [14C]E5531 per ml within plasma from six human subjects
| Patient | Concn of TC (mg/dl) | % [14C]E5531 distributed in fractiona
|
|||
|---|---|---|---|---|---|
| HDLb | LDLc | TRL | LPDP | ||
| I | 70 | 74.0 ± 3.0 | 7.4 ± 0.2 | 2.3 ± 0.1 | 14.7 ± 1.1 |
| II | 121 | 81.0 ± 0.7 | 19.9 ± 0.3 | 4.4 ± 0.2 | 9.4 ± 1.0 |
| III | 137 | 59.8 ± 2.3 | 22.2 ± 0.4 | 3.4 ± 0.2 | 6.5 ± 1.4 |
| IV | 165 | 62.7 ± 1.9 | 24.6 ± 0.9 | 6.7 ± 0.3 | 11.6 ± 1.8 |
| V | 175 | 69.0 ± 1.6 | 16.1 ± 2.1 | 7.2 ± 0.3 | 9.4 ± 0.9 |
| VI | 229 | 48.0 ± 0.9 | 32.0 ± 1.4 | 15.9 ± 2.2 | 8.3 ± 0.8 |
Data are expressed as means ± standard deviations for five individual replicates. Following incubation for 5 min at 37°C, plasma samples were assayed by radioactivity for E5531 in each of the plasma lipoprotein and LPDP fractions. The LPDP fraction contains albumin and α-1-glycoprotein. The TRL fraction includes very-low-density lipoproteins and chylomicrons. Plasma was separated into its lipoprotein and LPDP fractions by single-spin density gradient ultracentrifugation. Total recovery was between 90 and 98.4%. The %CV for the E5531 detection assay was between 1 and 13%.
Percentage of initial E5531 concentration.
Percentage of initial drug incubated.
FIG. 1.
Correlation coefficients between the percentage of E5531 recovered in each lipoprotein fraction with the concentration (milligrams per deciliter) in plasma of TRL TC, TG, and TP (A); LDL TC, TG, and TP (B); and HDL TC and TP (C) from six different patients following the incubation of [14C]E5531. Results are expressed as r (Pearson correlation coefficient) values with significance (P values).
Preliminary studies by Rose and coworkers have supported the importance of plasma lipoprotein binding in influencing the long-term effectiveness of E5531 (7). They reported that E5531 slowly inactivated when bound to HDL, while little or no loss in activity occurred when associated with LDL in in vitro assays (7). In the present investigation, we observed that regardless of total plasma and lipoprotein lipid and protein levels, the majority of E5531 incubated in plasma from different human subjects was recovered in the HDL fraction (Table 1). Since HDL and LDL cholesterol are not usually found in equimolar ratios in human plasma, but are found at an LDL/HDL ratio of 4:1 to 6:1 (11), these findings suggest that some mechanism besides random probability or specific characteristics of HDL must dictate E5531’s preferential association with HDL rather than LDL. A possibility may be the protein component of HDL. This is supported by the fact that nearly 50% of HDL by weight is composed of protein (3, 4) and our observations that increases in HDL protein levels resulted in an increasing percentage of E5531 recovered in this fraction (Fig. 1).
We have further observed that various plasma lipoprotein levels and specific increases in TRL lipid and protein and LDL lipid levels resulted in an increasing percentage of E5531 recovered in these fractions (Fig. 1). However, changes in lipoprotein composition did not modify the E5531 lipoprotein distribution profile (Fig. 1). These findings suggest that the E5531 lipoprotein distribution may be partially dictated by LDL and TRL lipid mass and lipoprotein particle number. The findings further suggest that the redistribution of E5531 from one lipoprotein class (HDL) to another (LDL or TRL) could be influenced by different disease states (5, 6, 8, 9) and adjunct therapies, such as Intralipid infusion (12), in which plasma lipoprotein concentrations and composition are altered.
Acknowledgments
Funding for this work was provided by Eisai to K.M.W.
REFERENCES
- 1.Alvares C, Ramos A. Lipids, lipoproteins, and apoproteins in serum during infection. Clin Chem. 1986;32:142–145. [PubMed] [Google Scholar]
- 2.Cassidy S M, Strobel F W, Wasan K M. Plasma lipoprotein distribution of liposomal nystatin is influenced by protein content of high-density lipoproteins. Antimicrob Agents Chemother. 1998;42:1878–1888. doi: 10.1128/aac.42.8.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis R A, Vance J E. Structure, assembly and secretion of lipoproteins. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1996. pp. 473–493. [Google Scholar]
- 4.Davis R A. Lipoprotein structure and secretion. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1991. pp. 403–426. [Google Scholar]
- 5.Feingold K R, Krauss R M, Pang M, et al. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low-density lipoprotein subclass pattern. J Clin Endocrinol Metab. 1993;76:1423–1431. doi: 10.1210/jcem.76.6.8501146. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:2045–2051. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 7.Rose, J., M. Mullarkey, W. J. Christ, L. D. Hawkins, M. Lynn, Y. Kishi, K. M. Wasan, K. Peteherych, and D. P. Rossignol. Unpublished data. [DOI] [PMC free article] [PubMed]
- 7a.Rose, J., et al. Unpublished observations.
- 8.Umeki S. Decreases in serum cholesterol levels in advanced lung cancer. Respiration. 1993;60:178–181. doi: 10.1159/000196195. [DOI] [PubMed] [Google Scholar]
- 9.Vitols S, Gahrton G, Bjorkholm M, Peterson C. Hypocholesterolemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumor cells: evidence from studies in patients with leukemia. Lancet. 1985;iv:1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- 10.Wasan K M, Cassidy S M, Ramaswamy M, Kennedy A, Strobel F W, Ng S P, Lee T P. A comparison of step-gradient and sequential density ultracentrifugation and the use of lipoprotein deficient plasma controls in determining the plasma lipoprotein distribution of lipid-associated nystatin and cyclosporine. Pharm Res. 1999;16:165–169. doi: 10.1023/a:1011951602894. [DOI] [PubMed] [Google Scholar]
- 11.Wasan K M, Lopez-Berestein G. Targeted liposomes in fungi: modifying the therapeutic index of amphotericin B by its incorporation into negatively charged liposomes. J Liposome Res. 1995;5:883–903. [Google Scholar]
- 12.Wasan K M, Grossie V B, Jr, Lopez-Berestein G. Concentrations in serum and tissue distribution of free and liposomal amphotericin B in rats during continuous Intralipid infusion. Antimicrob Agents Chemother. 1994;38:2224–2226. doi: 10.1128/aac.38.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]