Abstract
The incidence of dementia is steadily increasing worldwide. The risk factors for dementia are diverse, and include genetic background, environmental factors, sex differences, and vascular abnormalities. Among the subtypes of dementia, diabetes-related dementia is emerging as a complex type of dementia related to metabolic imbalance, due to the increase in the number of patients with metabolic syndrome and dementia worldwide. Thyroid hormones are considered metabolic regulatory hormones and affect various diseases, such as liver failure, obesity, and dementia. Thyroid dysregulation affects various cellular mechanisms and is linked to multiple disease pathologies. In particular, hypothyroidism is considered a critical cause for various neurological problems—such as metabolic disease, depressive symptoms, and dementia—in the central nervous system. Recent studies have demonstrated the relationship between hypothyroidism and brain insulin resistance and dyslipidemia, leading to diabetes-related dementia. Therefore, we reviewed the relationship between hypothyroidism and diabetes-related dementia, with a focus on major features of diabetes-related dementia such as insulin resistance, neuronal dysfunction, and dyslipidemia.
Keywords: thyroid hormone, hypothyroidism, diabetes-related dementia, insulin resistance, dyslipidemia
1. Introduction
Thyroid hormones (THs) secreted from the thyroid gland are regulated by the thyrotropin-releasing hormone (TRH), which is released from the hypothalamus, and the thyroid-stimulating hormone (TSH), which is released from the pituitary gland [1]. Two forms of TH are commonly known as triiodothyronine (T3) and thyroxine (T4) [2]. T3 and T4 control many organs′ metabolic processes such as lipogenesis, liver function, glucose metabolism, and thermogenesis, through numerous cellular mechanisms and gene expression regulation; they do this by binding to TH receptors (TRs) [3,4,5]. In the central nervous system (CNS), THs have been known to promote neurogenesis, neuronal synaptic plasticity, synaptic transmission, modulation of neurotransmitters, and brain tissue repair systems [6,7].
TH dysregulation is directly associated with endocrine disorders and metabolic syndromes such as diabetes and obesity [8,9,10,11,12]. Hypothyroidism is referred to as decreased TH levels [13], characterized by impaired glucose metabolism such as reduction in glucose uptake and impaired energy metabolism, including decreased liver gluconeogenesis and reduced muscle gluconeogenesis and glycogenolysis [8]; therefore, it is associated with the development of diabetes [14,15]. In the CNS, TH dysfunction is strongly linked to the development of neurodegenerative diseases such as dementia [16,17]. On the other hand, some clinical studies mention that hypothyroidism is not directly related to dementia neuropathology, and that levels of TSH are not directly related to cognitive impairment [18,19]. Recent cohort studies have reported a negative correlation between TSH level and cognitive deficit caused by subcortical ischemic vascular dementia [20], as well as in the relationship between subclinical hypothyroidism and cognitive dysfunction in old people [21].
Subclinical and overt hypothyroidism contribute to mood dysregulation, anxiety, attention, psychomotor function, and cognitive impairment [22,23,24,25,26] and are highly linked to the development of neurological diseases such as Alzheimer’s disease and depression [27,28]. Patients with hypothyroidism showed hippocampal atrophy, leading to memory loss, cerebral blood flow impairment, and a reduction in working memory [29].
Clinical studies reported that subclinical hypothyroidism and overt hypothyroidism patients showed memory deficit due to hippocampus damage [23,30].
One functional MRI clinical study showed working memory loss in subclinical hypothyroidism patients, and subsequently, working memory loss was improved by thyroxine treatment [31]. Another clinical study suggested a reduction in verbal memory processing and hippocampal function in hypothyroidism patients [24,32].
Dementia is a global epidemic neurodegenerative disease. It affected approximately 47 million in 2015, and is increasing gradually worldwide [33]. In a recent global report, metabolic syndrome patients, such as those with type 2 diabetes, also suffer from dementia globally [34]. Diabetes patients showed neuropathological features such as cognitive impairment, amyloid beta accumulation, and reduced attention, which are involved in dementia pathologies [35]. Many researchers are investigating various risk factors and related mechanisms in diabetes-related dementia, because there are several common risk factors in type 2 diabetes (T2DM) and dementia [36,37,38].
Even though there are many risk factors related with the onset of type 2 diabetes and dementia, many researchers have suggested that diabetes patients are considerably involved in an increased risk for dementia [39,40]. Some epidemiological studies have mentioned that the risk of dementia and cognitive impairment is increased in patients with diabetes compared to subjects without [41,42]. The brain of a T2DM patient is found to exhibit brain atrophy, reduced brain volume, neuronal cell myelin loss and white matter vacuoles, leading to memory deficit [43,44,45].
Clinical studies suggest that patients with diabetes have more risk for dementia at RR 1.73 [46], for AD at RR 1.53 [41], and for vascular dementia at RR 2.27 [46] compared to patients without diabetes. Many researchers report that the relationship between diabetes and dementia are involved in vascular alteration [47,48] and impaired cerebral insulin signaling [49], leading to cognition [49,50].
A recent study mentioned the association between thyroid dysfunction and dementia and suggested related metabolic mechanisms [51]. Some studies report that the deficit of T3 aggravates mitochondrial dysfunction, poor glucose metabolism and impaired brain metabolic mechanisms, leading to cognitive impairment [52,53]. Other studies present evidence that the thyroid hormone modulates brain glucose metabolism, and subsequently contributes to memory formation in the brain hippocampus [54,55]. As such, thyroid dysfunction is strongly related to dementia and diabetic pathologies, such as insulin resistance and impaired glucose metabolism.
Therefore, TH is a major metabolic regulating hormone, and TH dysfunction leads to both systemic metabolic disorders, as well as neurological problems. However, its functions in diabetes-related dementia have not been fully understood until now. Thus, we review recent evidence on the relationship and related mechanisms between hypothyroidism and diabetes-related dementia.
2. TH Dysfunction in the CNS
THs are produced by the thyroid gland. They are secreted into the blood and contribute to the cellular mechanisms in many organs through blood circulation [56]. Thyroid dysfunctions are classified based on the serum levels of TH and TSH. Overt hypothyroidism manifests as decreased T3 and T4 levels and increased TSH; overt hyperthyroidism manifests as increased T3 and T4 levels and decreased TSH; subclinical hypothyroidism shows normal T3 and T4 levels with increased TSH and subclinical hyperthyroidism shows normal T3 and T4 levels and decreased TSH levels [57,58,59].
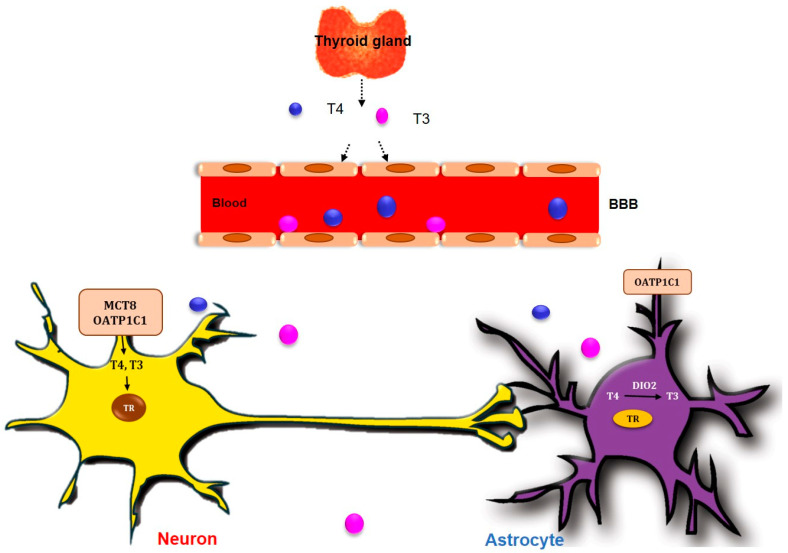
The concentration of T4 and T3 in the CNS is known to be approximately 20% that of blood serum [60]. TH is important for nervous system development and the maintenance of brain function, including the cognitive function process and affective mood process [57,61]. For the TH to enter into the brain, T4 and T3 cross over the blood brain barrier (BBB) of the choroid plexus through an MCT8 TH transporter or OATP1C1 TH transporter [62] (Figure 1). T4, as a prohormone, is taken up into astrocytes through OATP1C1, and it should be converted into T3 through an enzymatic reaction of deiodinase enzymes to work in the cells [63]. Deiodinase 2 (DIO2) is known to be the main enzyme responsible for the conversion of T4 into functional T3 (fT3) in the brain [64] (Figure 1). Subsequently, T3 generated from astrocytes enters the neuronal cells through an MCT8 transporter [65] (Figure 1). T3 binds to the nuclear receptor TRs and leads to a nucleus transcriptional change in CNS cells [62,66] (Figure 1). There are three TR isoforms: alpha and two forms of beta [67,68]. TRα1 is commonly expressed in the brain and skeletal muscle, and is existed over 80% of all TR expression in the brain [69,70]. T3 contributed to the progression of neural stem cells for memory formation, neuronal plasticity, and brain development in the subventricular zone and hippocampus dentate gyrus by binding TRα in a hypothyroid animal model [71,72]. TRβ1 is more prevalent and is expressed in the brain, heart, liver, and kidney, and TRβ2 is concentrated in pituitary and hypothalamic tissues [73].
Figure 1.
TH crosses blood brain barrier into the brain. T3 and T4, secreted from thyroid gland, cross the blood–brain barrier (BBB) into the brain through MCT8 and OATP1C1 TH transporters in neuron and OATP1C1 TH transporters in astrocytes. T4 is converted to T3 by type II 5′-deiodinase enzyme (DIO2) in astrocyte.
The imbalanced secretion of neurotransmitters such as acetylcholine, and noradrenaline in the hippocampus and prefrontal cortex, is routinely observed in hypothyroid and hyperthyroid animal models [74,75]. Furthermore, thyroid dysfunction leads to the imbalanced secretion of neurotransmitters, such as γ-aminobutyric acid (GABA), serotonin, catecholamine, and neurite growth factors in the brain, ultimately leading to cognitive impairment and depression [57,74,76].
Several clinical studies have shown that there is a strong, positive correlation between hypothyroidism and memory loss, attention error, poor verbal ability, abnormal motor function, deafness, and spasticity [23,24,77,78]. Patients with hypothyroidism showed typical behavioral symptoms similar to those of depressive and anxiety disorders [79,80,81,82]. The treatment of levothyroxine (L-T4) in patients with hypothyroidism improves cognition, mood disorders, and behavioral problems [83,84,85]. Subclinical and overt hyperthyroidism leads to apathy, lethargy, and depressive mood disorders [86,87].
One clinical study revealed that higher levels of T3 are found in the brain hippocampus and cerebrospinal fluid of Alzheimer’s disease patients [88]. Another study showed the negative correlation between T3 level and the development of Alzheimer’s disease [89]. One study reported that the administration of T3 offers protection to the prefrontal cortex, hippocampus, and amygdala—which are related to cognition and emotion—against amyloid beta toxicity in Alzheimer’s disease [90].
In this review, we summarized the common mechanisms and specific noteworthy risk factors between hypothyroidism and diabetes-related dementia pathologies.
3. Diabetes-Related Dementia and THs
Dementia, as a progressive metabolic disease, impairs memory function gradually [91]. Several studies have reported that type 2 diabetes and hyperlipidemia showed an increased risk of dementia such as vascular dementia, Alzheimer’s disease, and cognitive dysfunction [42,92,93,94,95,96]. In patients with Alzheimer’s disease, metabolic imbalance, such as central and peripheral insulin resistance and impaired insulin signaling, were observed [39,97,98].
Poor insulin signaling, insulin resistance, and oxidative stress condition caused by diabetes contribute to neuronal degeneration, leading to memory loss [99]. Previous studies have shown that insulin resistance aggravates tau hyperphosphorylation and the formation of neurofibrillary tangles, involving memory loss [100,101]. Neuroinflammation and impaired cerebral vasculature, leading to cognitive decline, are a common feature in dementia and the diabetic brain [102,103]. Furthermore, dyslipidemia related to apolipoprotein E (APOE) e4 alleles, caused by diabetes, leads to lower cognitive performance, lower attention and lower motor function [104]. Some studies have reported that diabetes animal models showed reduced neurogenesis [105], mitochondrial dysfunction in neuronal cells [106], and synaptic failure in the hippocampus [107], which is involved in learning and memory [107]. Furthermore, insulin resistance in metabolic syndromes leads to an imbalanced secretion of neurotransmitters such as acetylcholine [101,108], dopamine [109,110], glutamate [111], and 5-HT serotonin [112], leading to cognitive dysfunction [113].
Several studies have reported that elevated TSH level are strongly associated with insulin resistance [114] and hyperlipidemia [115]. Subclinical hypothyroidism is easily observed in patients with type 2 diabetes compared with normal subjects [13,116,117].
TSH level is related with diabetic peripheral neuropathy [118], and one study has shown that TSH and TSH receptor administration aggravates diabetic peripheral neuropathy by inducing apoptotic responses in Schwann cells [119].
TH controls the function and cellular homeostasis of many organs, such as the liver, heart, adipose tissue, liver, and brain, and modulates energy metabolism, free fatty acid oxidation, lipid metabolism, and the thermogenesis process [67,120,121]. T3 promotes metabolic enzymes such as acetyl-CoA carboxylase, and influence fatty acid synthesis and fatty acid oxidation [68,122]. Previous studies have shown that T3 decreases the circulation of free fatty acids and triglycerides in metabolic imbalance animal models [123,124]. T3 promotes several transcription factors related with lipogenesis, such as carbohydrate-responsive element-binding protein [125], and TSH promotes lipogenesis-related transcription factors such as the peroxisome proliferator-activated receptor-gamma (PPAR-γ) and the sterol regulatory element-binding transcription factor 1 [126]. Moreover, TSH decreases phosphorylation of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase [127].
Considering the associated previous consequences of diabetes-related dementia pathologies and thyroid dysfunction, we need to conduct further studies to understand the mechanism of TH in the CNS. Collectively, we summarize each point regarding the relationship between hypothyroidism and diabetes-related dementia.
4. Neuronal Cell Damage and Imbalanced Neurotransmitters in Hypothyroidism
T3 prevents the cell death process of primary cortical neurons in hypoxia apoptosis conditions by increasing anti-apoptotic gene expression and phosphorylation of CaMK/4/CREB signaling [128,129,130,131,132,133]. Additionally, T3 could control neuronal outgrowth and synaptic plasticity through the regulation of the neuronal-specific gene Nrgn [134,135]. The neuronal-specific gene Nrgn is markedly expressed in hippocampal neuronal dendritic spines, and regulates spatial memory formation and anxiety-like behaviors [136]. Other studies have shown that T3 administration contributes to brain development by increasing Reln gene expression [137,138]. The Reln gene induces neuronal migration in the neocortex and promotes neurogenesis and synapse formation by binding with apolipoprotein E receptors [139,140] (Figure 2).
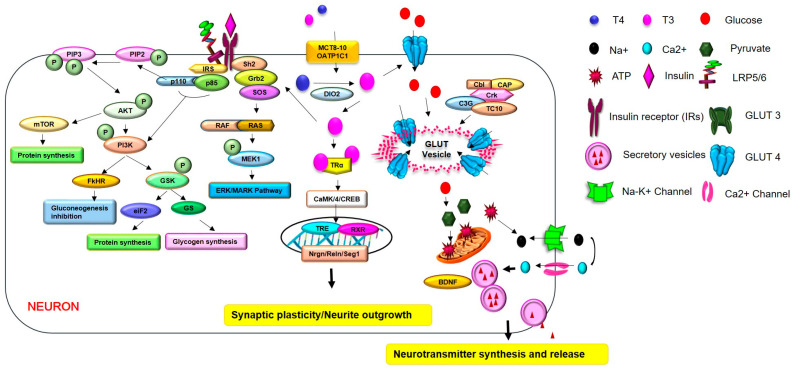
Figure 2.
TH regulates glucose metabolism and the secretion of neurotransmitters in hypothyroidism. TH controls glucose uptake and insulin sensitivity by maintaining the expression of glucose transporters such as glucose transporter (GLUT) in CNS cells. TH prevents the cell death process and promotes neurite outgrowth and synaptic plasticity through several genes, such as the Nrgn, Reln and Srg1 genes, and through the CaMK/4/CREB signaling in neuron. TH modulates the expression of neurotransmitters, including dopamine, glutamate, GABA, and BDNF, through several kinds of signaling, and can ultimately control brain functions. Additionally, TH increases the activation of PI3K/Akt signaling, and GSK3β signaling is related to the enhancement of insulin sensitivity. TH boosts the expression of BDNF, leading to the activation of PI3K/Akt signaling, which is involved in cognitive function. Finally, the improvement of insulin sensitivity leads to the enhancement of cognitive function. In hypothyroidism, reduced levels of TH lead to impaired synaptic plasticity, cognitive deficit, abnormal neurotransmitter release, impaired neurite outgrowth, and insulin resistance.
During brain development, TH induces synaptic plasticity by increasing spine cytoskeleton formation and microtubule reorganization through synaptotagmin-related gene 1 (Srg1) gene expression [141,142]. TH controls microtubule-related proteins and Tau proteins in neurons during brain development [143,144]. Additionally, TH controls neuronal filaments in neurons and glial fibrillary acidic protein filament in astrocytes [130]. TH promotes neuronal migration, neuronal differentiation, and glia maturations [145,146,147]. Based on this evidence, hypothyroidism leads to neuronal cell damage and reduced neurogenesis and glia activation.
Hypothyroidism leads to dopaminergic neuronal loss [148], because T3 and T4 could maintain dopaminergic neurons by increasing Nurr1 expression, and protect neuronal damage by regulating dopamine levels in the CNS [149] (Figure 2).
T3 could regulate neurotransmitter glutamate uptake in astrocytes [150], reduces N-methyl-d-aspartate-evoked currents in the hippocampal neuron, and inhibits glutamate-induced neuronal cell damage in hippocampal neurons [151] (Figure 2).
TH controls the maturation of GABAergic neurons [152]. It could regulate the release and uptake of GABA in neuronal synaptosomes from the cerebral cortex [153] and affects GABA A receptors in the cerebral cortex [154]. Therefore, if hypothyroidism occurs during brain development, neuronal cell differentiation and neuronal cell proliferation in the cortex could be impaired [155,156] (Figure 2). Other studies have found that hypothyroidism leads to the decreased expression of brain-derived neurotrophic factor (BDNF) as a neurotrophic factor in the hippocampal brain regions [138,157] (Figure 2).
Hypothyroidism could damage neuronal cell death and aggravate synaptic plasticity, as well as promote the abnormal release and uptake of neurotransmitters such as dopamine and GABA in the brain, related to neuropathologies.
5. Brain Insulin Resistance in Hypothyroidism
Insulin, a peptide hormone produced by the pancreas, regulates glucose levels and affects various cellular mechanisms [158]. In the CNS, insulin increases brain energy metabolism and induces hippocampal neurons for memory formation [159,160]. Brain insulin resistance is considered the impairment of insulin activity and aggravates neuronal cell death, leading to memory loss and cognitive impairment [159,161,162,163].
Insulin regulates amyloid beta accumulation and tau hyperphosphorylation and glycogen synthase kinase 3 (GSK3) activity, leading to neuronal cell damage and memory dysfunction [164]. GSK3β signaling, the downstream pathway of insulin signaling, contributes to tau hyperphosphorylation. It is related with microtubule destabilization of neurons, and leads to cognitive decline [165,166]. The activity of GSK3β signaling is regulated by TH activity [167] (Figure 2). Further, BDNF as a neurotrophic factor boosts the activation of PI3K/Akt signaling and insulin signaling in CNS cells [168]. Insulin promotes the secretion of BDNF, which promotes neurite length, synaptic formation, and neuronal cell survival, and ultimately improves depressive mood disorder and Alzheimer’s disease pathologies [169,170]. Several studies have reported that T3 was shown to increase the expression of BDNF in the hippocampus region [138,157] (Figure 2). The reduction in BDNF expression leads to the decreased expression of synaptic plasticity, related genes such as calcineurin and cAMP response element-binding protein (CREB), after thyroid gland removal surgery [171].
One study mentioned that the T3/reverse T3 ration is increased in insulin resistance status, and thyroid hormone function affects insulin resistance condition [172,173]. Insulin upregulates DIO1 and DIO2 activity and contributes to the conversion of T4 into functional T3 [174,175].
Another study indicated that high levels of TSH are associated with more obese status and increased metabolic profiles such as that of insulin resistance [176].
A recent meta-analysis reported that abnormal levels of functional T3 and T4 are related to the onset and development of type 2 diabetes (T2DM) [177].
Patients with diabetes showed a higher risk of memory loss, neuropathological problems, and prevalence of dementia [178,179]. Moreover, Alzheimer’s disease dementia patients showed impaired insulin signaling in the brain, which is considered an early stage of cognitive deficit [180].
TH strongly contributes to glucose metabolism and insulin resistance [114,168,181,182,183,184,185]. A recent study showed that a diabetes animal model exhibits hypothyroidism, increased inflammatory responses, and reduced insulin sensitivity [186]. Additionally, in clinical studies, patients with hypothyroidism displayed increased metabolic syndrome, and poor glycemic control and insulin resistance [14,187,188].
Considering a previous study, T3 and T4 exhibit genomic effects by regulating Ca++ entry into cells, as well as the activation of some kinases [189]. Phosphoinositide 3-kinase (PI3K) is the one of TH-activated kinases, and activated PI3K signaling contributes to insulin signaling [190]. Some studies have demonstrated that TH increases Akt phosphorylation related to cognitive function in hippocampal neurons, and subsequently enhances insulin sensitivity [191,192,193] (Figure 2). TH activity is associated with glucose metabolism and insulin signaling through the regulation of glucose transporter expression [168,185,194]. One in vitro study mentioned that TH controls glucose uptake into cells through Glucose transporter 1 (GluT1), and modulates insulin secretion [195] (Figure 2).
TH could control cognitive function by modulating insulin activity and type 3 deiodinase activity [196]. Additionally, a recent study reported that polymorphism of TH-activation-related type 2 deiodinase enzyme is related with insulin resistance [197]. Furthermore, some studies have mentioned that TRs and the type II 5′-deiodinase enzyme (DIO2) were high in several brain regions such as the hippocampus and cortex, which are related to memory function [64], and decreased TRs were observed in the brains of Alzheimer’s disease patients [198].
Considering that brain insulin resistance is a critical factor in diabetes-related dementia, the relationship between hypothyroidism and diabetes-related dementia is an important issue.
6. Dyslipidemia in Hypothyroidism
A previous study shows that dyslipidemia is linked to both type 2 diabetes and Alzheimer’s disease [199]. Blood–brain barrier (BBB) disruption is easily observed in dementia, such as in Alzheimer’s disease [200] and diabetes [201]. The increased release of cholesterol and the impaired cholesterol transport in neurons were observed in dementia brains [202,203].
Lipid contributes to BBB function and BBB integrity, and has a role in the development of Alzheimer’s disease pathology [204]. Some studies have reported that elevated triglycerides are strongly related to dementia development [205,206].
In the brain, high cholesterol leads to increased amyloid beta production from the amyloid beta protein precursor [207], and reduced soluble amyloid precursor protein production [208]. Some studies have demonstrated that cholesterol intake accelerates amyloid beta deposition in the brain [204,209]. Other studies have reported that amyloid beta 42 formation and tau hyperphosphorylation were modulated by cholesterol [210].
ApoE, as a major constituent of chylomicrons in blood circulation, is the most abundant apolipoprotein in the brain, and is associated with cholesterol transfer, amyloid beta accumulation, higher plasma concentrations of low-density lipoprotein (LDL) cholesterol, and higher risk of Alzheimer’s disease dementia [211]. One study reported that many patients with dementia have at least one ApoE ε4 allele, compared to healthy subjects [212].
Several studies have identified that patients with diabetes showed severe hyperlipidemia [63] and dementia neuropathology [213,214]. One cohort study reported that diabetes leads to hyperlipidemia and high risk of dementia [215].
Dyslipidemia (such as hypercholesterolemia), low high-density-lipoprotein (HDL) cholesterol, high prevalence of small low-density-lipoprotein (LDL) cholesterol particles, high LDL cholesterol, and hypertriglyceridemia contribute to cognitive impairment [216,217], and lead to an increased risk for Alzheimer’s disease dementia [218,219,220,221,222,223].
TSH could modulate the secretion of serum cholesterol, the expression of HMG-CoA reductase, and the synthesis of LCL-cholesterol [224] and HDL-cholesterol [225] (Figure 3). T3 regulates the expression of the acetyl-CoA carboxylase and fatty acid synthase (ACC/FAS) ACC/FAS gene involved in lipid metabolism [226] (Figure 3). TSH regulates HMG-CoA by stimulating the expression of the SREBP-2 gene [227].
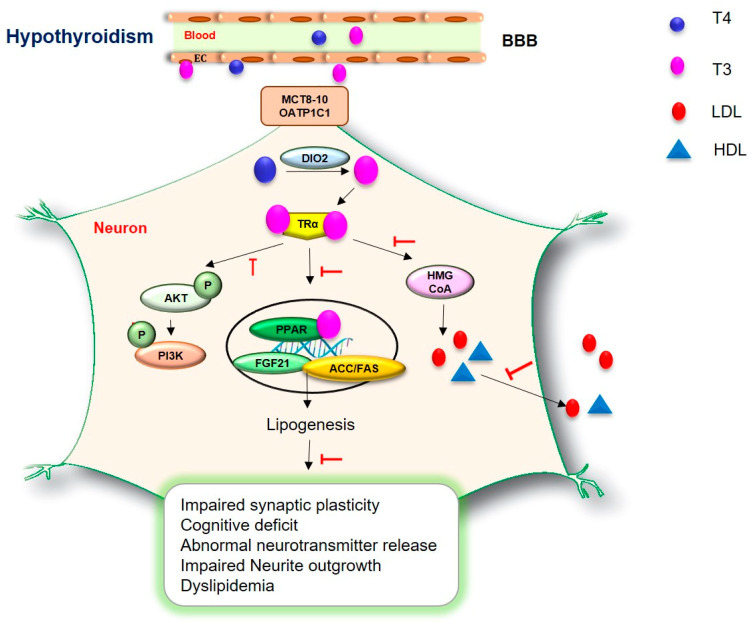
Figure 3.
TH regulates dyslipidemia in hypothyroidism. TH modulates the secretion of cholesterol, the expression of HMG-CoA reductase, and the synthesis of LCL-cholesterol and HDL-cholesterol. Additionally, TH controls the expression of the acetyl-CoA carboxylase and fatty acid synthase (ACC/FAS) ACC/FAS gene involved in lipid metabolism. TH promotes the expression of the FGF21 gene and activates proliferator-activated receptor α (PPARα) signaling, which is related to triglycerides. In hypothyroidism, reduced levels of TH lead to impaired synaptic plasticity, cognitive deficit, abnormal neurotransmitter release, impaired neurite outgrowth, and dyslipidemia.
Hypothyroidism contributes to various functions in the regulation of serum lipid profiles [228]. A previous study mentioned that hypothyroidism could decrease liver uptake of free fatty acid, reduce cholesterol secretion, and decrease plasma triglyceride clearance, accompanied with reduced lipoprotein levels [229,230,231]. Hypothyroidism leads to lower energy expenditure and high lipid storage [232,233]. Moreover, hypothyroidism has revealed lower plasma cholesteryl ester transfer proteins and decreased very-low-density lipoproteins [234].
Hypothyroidism has shown severe hyperlipidemia, such as the reduction in cholesterol synthesis, by reducing the expression of β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase in the liver [63] (Figure 3).
A current study suggested that patients with subclinical hypothyroidism showed high levels of total cholesterol and abnormal lipid profiles [235]. One clinical study demonstrated that more than 30% and more than 90% of patients with hypothyroidism showed increased total cholesterol and increased LDL cholesterol levels and dyslipidemia, respectively [63,236,237]. Other recent studies have shown that patients with hypothyroidism manifested with hypertriglyceridemia and exhibited elevated triglycerides and triglyceride-rich lipoproteins in the serum [238,239]. A previous study showed that patients with hypothyroidism exhibited higher levels of LDL cholesterol and homocysteine [240].
A recent study reported that fibroblast growth factor 21 (FGF21), which is a cytokine increased in dyslipidemia [241], was reduced in hypothyroidism patients compared to normal subjects [242]. T3 induces the expression of the FGF21 gene and activates proliferator-activated receptor α (PPARα) signaling, which is related to triglycerides [243] (Figure 3). The present study reported that patients with hypothyroidism exhibit low HDL-cholesterol, elevated homocysteine, and increased apolipoprotein ApoA 1 concentration [244]. Given that dyslipidemia is a crucial issue in diabetes-related dementia, the association between dyslipidemia and hypothyroidism warrants further investigation for finding specific treatment for diabetes-related dementia.
7. Neurological Problems in Hypothyroidism
TH dysfunction causes numerous neurological problems, including anxiety, depression, and cognitive impairment [55]. In dementia patients, the positive relationship with TH dysfunction has been observed in some studies [245,246]. Several studies have identified that hypothyroidism changes neuronal function and is involved in cognition processes [247,248]. The study suggested that the hippocampus, as the central brain region of cognitive performance [249], was damaged, and apoptotic processes were found in the brains of patients with hypothyroidism [250]. Other recent studies reported that hypothyroidism exhibits an increased inflammatory response in hippocampal brain regions and induces spatial memory loss [251,252]. The subclinical hypothyroidism animal model exhibits spatial memory loss through BDNF TrkA/p75NTR signaling pathway [253]. One animal study reported that hypothyroidism damages learning and memory function, aggravates long- and short- term memory, and impairs synaptic plasticity [254]. Another study showed that hypothyroidism was noted in elderly people with mild cognitive impairment [255]. Previous studies reported that hypothyroidism brains showed disruption of synaptic plasticity, which impaired long-term potentiation in the hippocampal CA1 region [256,257,258,259]. Based on previous studies regarding the effect of THs on cognitive impairment and depressive disorders, the neurological features of both hypothyroidism and diabetes-related dementia warrant further study to understand the cognitive decline in both hypothyroidism and diabetes-related dementia.
8. Conclusions
Thyroid dysregulation is an important issue both in metabolic syndromes and neurological disorders, because TH is involved in the regulation of cell death response, the modulation of neuronal function, the production of neurotransmitters, the regulation of glucose metabolism, and the regulation of lipid metabolism. Here, we summarized the mechanisms of neuronal dysfunction, insulin resistance, and dyslipidemia in both hypothyroidism and diabetes-related dementia. We suggest that further studies should be conducted on the correlation and mechanism between hypothyroidism and diabetes-related dementia, due to the association with the multiple metabolic processes of THs.
Thus, we assume that the modulation of insulin action and triglyceride levels will be beneficial for the treatment of both hypothyroidism and diabetes-related dementia.
Given the existing clinical approach to thyroid medication use in Alzheimer’s disease patients [260], appropriate monitoring of TH levels would be a good approach to predicting neuropathological problems caused by diabetes-related dementia in advance; modulation of TH levels would be another approach to improve neuropathogenesis in diabetes-related dementia.
Acknowledgments
We thank Archana Arjunan for helping with the creation of figures.
Abbreviations
Thyroid hormone, TH; thyroid stimulating hormone, TSH; triiodothyronine, T3; tetraiodothyronine, T4; thyroid hormone receptor, TR; central nervous system, CNS; carbohydrate-responsive element-binding protein, ChREBP; peroxisome proliferator-activated receptor-gamma, PPAR-γ; sterol regulatory element-binding transcription factor 1, SREBP-1c; N-methyl-d-aspartate, NMDA; apolipoprotein E, APOE; free thyroxine, FT4; free triiodothyronine, FT3; glial fibrillary acidic protein, GFAP; synaptotagmin-related gene 1, Srg1; glycogen synthase kinase 3, GSK3; brain-derived neurotrophic factor, BDNF; blood–brain barrier, BBB; cholesteryl ester transfer proteins, CETPs; very-low-density lipoproteins, VLDL; low-density lipoprotein, LDL; high-density lipoprotein, HDL; β-hydroxy β-methylglutaryl-CoA, HMG-CoA; fibroblast growth factor 21, FGF21; triglyceride, TG.
Author Contributions
Writing, H.K.K. and J.S.; figure, J.S.; manuscript revision, J.S.; manuscript finalization, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by grant NRF-2022R1A2C1006125 (J.S.) from the National Research Foundation of Korea (NRF) and by grant no. HCRI 22019 from the Chonnam National University Hwasun Hospital Institute for Biomedical Science, South Korea (H.K.K., J.S.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eerdekens A., Verhaeghe J., Darras V., Naulaers G., Van den Berghe G., Langouche L., Vanhole C. The placenta in fetal thyroid hormone delivery: From normal physiology to adaptive mechanisms in complicated pregnancies. J. Matern. Fetal Neonatal Med. 2020;33:3857–3866. doi: 10.1080/14767058.2019.1586875. [DOI] [PubMed] [Google Scholar]
- 2.Raymaekers S.R., Darras V.M. Thyroid hormones and learning-associated neuroplasticity. Gen. Comp. Endocrinol. 2017;247:26–33. doi: 10.1016/j.ygcen.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Barez-Lopez S., Guadano-Ferraz A. Thyroid Hormone Availability and Action during Brain Development in Rodents. Front. Cell. Neurosci. 2017;11:240. doi: 10.3389/fncel.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis P.J., Goglia F., Leonard J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016;12:111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 5.Sun X., Sun Y., Li W.C., Chen C.Y., Chiu Y.H., Chien H.Y., Wang Y. Association of thyroid-stimulating hormone and cardiovascular risk factors. Intern. Med. 2015;54:2537–2544. doi: 10.2169/internalmedicine.54.4514. [DOI] [PubMed] [Google Scholar]
- 6.Remaud S., Gothie J.D., Morvan-Dubois G., Demeneix B.A. Thyroid hormone signaling and adult neurogenesis in mammals. Front. Endocrinol. 2014;5:62. doi: 10.3389/fendo.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostol M., Keeran M., Klempf N., McCoskey V., Ernst A.A., Weiss S.J., Sarangarm D. Thyroid stimulating hormone testing in ED evaluation of patients with atrial fibrillation and various psychiatric diagnoses. Am. J. Emerg. Med. 2019;37:1114–1117. doi: 10.1016/j.ajem.2018.08.076. [DOI] [PubMed] [Google Scholar]
- 8.Biondi B., Kahaly G.J., Robertson R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019;40:789–824. doi: 10.1210/er.2018-00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwen K.A., Schroder E., Brabant G. Thyroid hormones and the metabolic syndrome. Eur. Thyroid J. 2013;2:83–92. doi: 10.1159/000351249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor P.N., Razvi S., Pearce S.H., Dayan C.M. Clinical review: A review of the clinical consequences of variation in thyroid function within the reference range. J. Clin. Endocrinol. Metab. 2013;98:3562–3571. doi: 10.1210/jc.2013-1315. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima Y., Yamada M., Akuzawa M., Ishii S., Masamura Y., Satoh T., Hashimoto K., Negishi M., Shimomura Y., Kobayashi I., et al. Subclinical hypothyroidism and indices for metabolic syndrome in Japanese women: One-year follow-up study. J. Clin. Endocrinol. Metab. 2013;98:3280–3287. doi: 10.1210/jc.2013-1353. [DOI] [PubMed] [Google Scholar]
- 12.Oh J.Y., Sung Y.A., Lee H.J. Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in euthyroid young women. Korean J. Intern. Med. 2013;28:180–186. doi: 10.3904/kjim.2013.28.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaker L., Ligthart S., Korevaar T.I., Hofman A., Franco O.H., Peeters R.P., Dehghan A. Thyroid function and risk of type 2 diabetes: A population-based prospective cohort study. BMC Med. 2016;14:150. doi: 10.1186/s12916-016-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra S., Aggarwal S., Khandelwal D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther. 2019;10:2035–2044. doi: 10.1007/s13300-019-00700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronich N., Deftereos S.N., Lavi I., Persidis A.S., Abernethy D.R., Rennert G. Hypothyroidism is a Risk Factor for New-Onset Diabetes: A Cohort Study. Diabetes Care. 2015;38:1657–1664. doi: 10.2337/dc14-2515. [DOI] [PubMed] [Google Scholar]
- 16.De Jong F.J., Masaki K., Chen H., Remaley A.T., Breteler M.M., Petrovitch H., White L.R., Launer L.J. Thyroid function, the risk of dementia and neuropathologic changes: The Honolulu-Asia aging study. Neurobiol. Aging. 2009;30:600–606. doi: 10.1016/j.neurobiolaging.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalmijn S., Mehta K.M., Pols H.A., Hofman A., Drexhage H.A., Breteler M.M. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin. Endocrinol. 2000;53:733–737. doi: 10.1046/j.1365-2265.2000.01146.x. [DOI] [PubMed] [Google Scholar]
- 18.Rieben C., Segna D., da Costa B.R., Collet T.H., Chaker L., Aubert C.E., Baumgartner C., Almeida O.P., Hogervorst E., Trompet S., et al. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: A Meta-Analysis of Prospective Cohort Studies. J. Clin. Endocrinol. Metab. 2016;101:4945–4954. doi: 10.1210/jc.2016-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasqualetti G., Pagano G., Rengo G., Ferrara N., Monzani F. Subclinical Hypothyroidism and Cognitive Impairment: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015;100:4240–4248. doi: 10.1210/jc.2015-2046. [DOI] [PubMed] [Google Scholar]
- 20.George K.M., Lutsey P.L., Selvin E., Palta P., Windham B.G., Folsom A.R. Association Between Thyroid Dysfunction and Incident Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. J. Endocrinol. Metab. 2019;9:82–89. doi: 10.14740/jem588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akintola A.A., Jansen S.W., van Bodegom D., van der Grond J., Westendorp R.G., de Craen A.J., van Heemst D. Subclinical hypothyroidism and cognitive function in people over 60 years: A systematic review and meta-analysis. Front. Aging Neurosci. 2015;7:150. doi: 10.3389/fnagi.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer M., Goetz T., Glenn T., Whybrow P.C. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008;20:1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 23.Correia N., Mullally S., Cooke G., Tun T.K., Phelan N., Feeney J., Fitzgibbon M., Boran G., O’Mara S., Gibney J. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2009;94:3789–3797. doi: 10.1210/jc.2008-2702. [DOI] [PubMed] [Google Scholar]
- 24.Miller K.J., Parsons T.D., Whybrow P.C., Van Herle K., Rasgon N., Van Herle A., Martinez D., Silverman D.H., Bauer M. Verbal memory retrieval deficits associated with untreated hypothyroidism. J. Neuropsychiatry Clin. Neurosci. 2007;19:132–136. doi: 10.1176/jnp.2007.19.2.132. [DOI] [PubMed] [Google Scholar]
- 25.Gibney S.M., Drexhage H.A. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. 2013;8:900–920. doi: 10.1007/s11481-013-9462-8. [DOI] [PubMed] [Google Scholar]
- 26.Davis J.D., Tremont G. Impact of frontal systems behavioral functioning in dementia on caregiver burden. J. Neuropsychiatry Clin. Neurosci. 2007;19:43–49. doi: 10.1176/jnp.2007.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bavarsad K., Hosseini M., Hadjzadeh M.A., Sahebkar A. The effects of thyroid hormones on memory impairment and Alzheimer’s disease. J. Cell. Physiol. 2019;234:14633–14640. doi: 10.1002/jcp.28198. [DOI] [PubMed] [Google Scholar]
- 28.Thvilum M., Brandt F., Almind D., Christensen K., Brix T.H., Hegedus L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: A nationwide register study. Thyroid. 2014;24:802–808. doi: 10.1089/thy.2013.0555. [DOI] [PubMed] [Google Scholar]
- 29.He X.S., Ma N., Pan Z.L., Wang Z.X., Li N., Zhang X.C., Zhou J.N., Zhu D.F., Zhang D.R. Functional magnetic resource imaging assessment of altered brain function in hypothyroidism during working memory processing. Eur. J. Endocrinol. 2011;164:951–959. doi: 10.1530/EJE-11-0046. [DOI] [PubMed] [Google Scholar]
- 30.Miller K.J., Parsons T.D., Whybrow P.C., van Herle K., Rasgon N., van Herle A., Martinez D., Silverman D.H., Bauer M. Memory improvement with treatment of hypothyroidism. Int. J. Neurosci. 2006;116:895–906. doi: 10.1080/00207450600550154. [DOI] [PubMed] [Google Scholar]
- 31.Zhu D.F., Wang Z.X., Zhang D.R., Pan Z.L., He S., Hu X.P., Chen X.C., Zhou J.N. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain. 2006;129:2923–2930. doi: 10.1093/brain/awl215. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler S.M., McLelland V.C., Sheard E., McAndrews M.P., Rovet J.F. Hippocampal Functioning and Verbal Associative Memory in Adolescents with Congenital Hypothyroidism. Front. Endocrinol. 2015;6:163. doi: 10.3389/fendo.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince M., Ali G.C., Guerchet M., Prina A.M., Albanese E., Wu Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 2016;8:23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 35.McCrimmon R.J., Ryan C.M., Frier B.M. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 36.Exalto L.G., Whitmer R.A., Kappele L.J., Biessels G.J. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp. Gerontol. 2012;47:858–864. doi: 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Hanyu H. Diabetes-Related Dementia. Adv. Exp. Med. Biol. 2019;1128:147–160. doi: 10.1007/978-981-13-3540-2_8. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia P., Singh N. Thromboxane A2 synthase inhibition ameliorates endothelial dysfunction, memory deficits, oxidative stress and neuroinflammation in rat model of streptozotocin diabetes induced dementia. Physiol. Behav. 2021;241:113592. doi: 10.1016/j.physbeh.2021.113592. [DOI] [PubMed] [Google Scholar]
- 39.Biessels G.J., Despa F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018;14:591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koekkoek P.S., Kappelle L.J., van den Berg E., Rutten G.E., Biessels G.J. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015;14:329–340. doi: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Chen C., Hua S., Liao H., Wang M., Xiong Y., Cao F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 43.Moran C., Beare R., Phan T., Starkstein S., Bruce D., Romina M., Srikanth V. Neuroimaging and its Relevance to Understanding Pathways Linking Diabetes and Cognitive Dysfunction. J. Alzheimer’s Dis. 2017;59:405–419. doi: 10.3233/JAD-161166. [DOI] [PubMed] [Google Scholar]
- 44.Biessels G.J., Reijmer Y.D. Brain changes underlying cognitive dysfunction in diabetes: What can we learn from MRI? Diabetes. 2014;63:2244–2252. doi: 10.2337/db14-0348. [DOI] [PubMed] [Google Scholar]
- 45.Ly H., Verma N., Wu F., Liu M., Saatman K.E., Nelson P.T., Slevin J.T., Goldstein L.B., Biessels G.J., Despa F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann. Neurol. 2017;82:208–222. doi: 10.1002/ana.24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gudala K., Bansal D., Schifano F., Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feinkohl I., Price J.F., Strachan M.W., Frier B.M. The impact of diabetes on cognitive decline: Potential vascular, metabolic, and psychosocial risk factors. Alzheimer’s Res. Ther. 2015;7:46. doi: 10.1186/s13195-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haroon N.N., Austin P.C., Shah B.R., Wu J., Gill S.S., Booth G.L. Risk of dementia in seniors with newly diagnosed diabetes: A population-based study. Diabetes Care. 2015;38:1868–1875. doi: 10.2337/dc15-0491. [DOI] [PubMed] [Google Scholar]
- 49.Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biessels G.J., Reagan L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 51.Van Vliet N.A., van Heemst D., Almeida O.P., Asvold B.O., Aubert C.E., Bae J.B., Barnes L.E., Bauer D.C., Blauw G.J., Brayne C., et al. Association of Thyroid Dysfunction with Cognitive Function: An Individual Participant Data Analysis. JAMA Intern. Med. 2021;181:1440–1450. doi: 10.1001/jamainternmed.2021.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo S.W., Ahn J., Yoon U., Im K., Lee J.M., Tae Kim S., Ahn H.J., Chin J., Jeong Y., Na D.L. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J. Neuroimaging Off. J. Am. Soc. Neuroimaging. 2010;20:37–45. doi: 10.1111/j.1552-6569.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y., Wang Z.C., Guo Q.H., Cheng W., Chen Y.W. Is thyroid status associated with cognitive impairment in elderly patients in China? BMC Endocr. Disord. 2016;16:11. doi: 10.1186/s12902-016-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobsen J.P., Plenge P., Sachs B.D., Pehrson A.L., Cajina M., Du Y., Roberts W., Rudder M.L., Dalvi P., Robinson T.J., et al. The interaction of escitalopram and R-citalopram at the human serotonin transporter investigated in the mouse. Psychopharmacology. 2014;231:4527–4540. doi: 10.1007/s00213-014-3595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuels M.H. Thyroid disease and cognition. Endocrinol. Metab. Clin. N. Am. 2014;43:529–543. doi: 10.1016/j.ecl.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Taylor E., Heyland A. Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Mol. Cell. Endocrinol. 2017;459:14–20. doi: 10.1016/j.mce.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Koromilas C., Liapi C., Schulpis K.H., Kalafatakis K., Zarros A., Tsakiris S. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab. Brain Dis. 2010;25:339–354. doi: 10.1007/s11011-010-9208-8. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Lamo I., Montero-Pedrazuela A., Delgado-Garcia J.M., Guadano-Ferraz A., Gruart A. Effects of thyroid hormone replacement on associative learning and hippocampal synaptic plasticity in adult hypothyroid rats. Eur. J. Neurosci. 2009;30:679–692. doi: 10.1111/j.1460-9568.2009.06862.x. [DOI] [PubMed] [Google Scholar]
- 59.Lauffer P., Zwaveling-Soonawala N., Naafs J.C., Boelen A., van Trotsenburg A.S.P. Diagnosis and Management of Central Congenital Hypothyroidism. Front. Endocrinol. 2021;12:686317. doi: 10.3389/fendo.2021.686317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dratman M.B., Crutchfield F.L., Schoenhoff M.B. Transport of iodothyronines from bloodstream to brain: Contributions by blood:brain and choroid plexus:cerebrospinal fluid barriers. Brain Res. 1991;554:229–236. doi: 10.1016/0006-8993(91)90194-Z. [DOI] [PubMed] [Google Scholar]
- 61.Ceballos A., Belinchon M.M., Sanchez-Mendoza E., Grijota-Martinez C., Dumitrescu A.M., Refetoff S., Morte B., Bernal J. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3’-triiodo-L-thyronine. Endocrinology. 2009;150:2491–2496. doi: 10.1210/en.2008-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heuer H. The importance of thyroid hormone transporters for brain development and function. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:265–276. doi: 10.1016/j.beem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Zou Q.H., Zhu C.Z., Yang Y., Zuo X.N., Long X.Y., Cao Q.J., Wang Y.F., Zang Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bianco A.C., Kim B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 66.Sandler B., Webb P., Apriletti J.W., Huber B.R., Togashi M., Cunha Lima S.T., Juric S., Nilsson S., Wagner R., Fletterick R.J., et al. Thyroxine-thyroid hormone receptor interactions. J. Biol. Chem. 2004;279:55801–55808. doi: 10.1074/jbc.M410124200. [DOI] [PubMed] [Google Scholar]
- 67.Brent G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y.Y., Gusdon A.M., Qu S. Cross-talk between the thyroid and liver: A new target for nonalcoholic fatty liver disease treatment. World J. Gastroenterol. 2013;19:8238–8246. doi: 10.3748/wjg.v19.i45.8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz H.L., Strait K.A., Ling N.C., Oppenheimer J.H. Quantitation of rat tissue thyroid hormone binding receptor isoforms by immunoprecipitation of nuclear triiodothyronine binding capacity. J. Biol. Chem. 1992;267:11794–11799. doi: 10.1016/S0021-9258(19)49768-8. [DOI] [PubMed] [Google Scholar]
- 70.Heuer H., Mason C.A. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor α1. J. Neurosci. 2003;23:10604–10612. doi: 10.1523/JNEUROSCI.23-33-10604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopez-Juarez A., Remaud S., Hassani Z., Jolivet P., Pierre Simons J., Sontag T., Yoshikawa K., Price J., Morvan-Dubois G., Demeneix B.A. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10:531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Kapoor R., Desouza L.A., Nanavaty I.N., Kernie S.G., Vaidya V.A. Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J. Neuroendocrinol. 2012;24:1259–1271. doi: 10.1111/j.1365-2826.2012.02329.x. [DOI] [PubMed] [Google Scholar]
- 73.Cheng S.Y., Leonard J.L., Davis P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tousson E., Ibrahim W., Arafa N., Akela M.A. Monoamine concentrations changes in the PTU-induced hypothyroid rat brain and the ameliorating role of folic acid. Hum. Exp. Toxicol. 2012;31:282–289. doi: 10.1177/0960327111405863. [DOI] [PubMed] [Google Scholar]
- 75.Horikoshi S., Miura I., Kunii Y., Asano S., Kanno-Nozaki K., Mashiko H., Yabe H. Hashimoto encephalopathy with high plasma monoamine metabolite levels: A case report. Neuropsychiatr. Dis. Treat. 2017;13:1043–1045. doi: 10.2147/NDT.S131356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carageorgiou H., Pantos C., Zarros A., Stolakis V., Mourouzis I., Cokkinos D., Tsakiris S. Changes in acetylcholinesterase, Na+,K+-ATPase, and Mg2+-ATPase activities in the frontal cortex and the hippocampus of hyper- and hypothyroid adult rats. Metabolism. 2007;56:1104–1110. doi: 10.1016/j.metabol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Bell R.J., Rivera-Woll L., Davison S.L., Topliss D.J., Donath S., Davis S.R. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease—A community-based study. Clin. Endocrinol. 2007;66:548–556. doi: 10.1111/j.1365-2265.2007.02771.x. [DOI] [PubMed] [Google Scholar]
- 78.DeLong G.R., Stanbury J.B., Fierro-Benitez R. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism) Dev. Med. Child Neurol. 1985;27:317–324. doi: 10.1111/j.1469-8749.1985.tb04542.x. [DOI] [PubMed] [Google Scholar]
- 79.Gulseren S., Gulseren L., Hekimsoy Z., Cetinay P., Ozen C., Tokatlioglu B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch. Med. Res. 2006;37:133–139. doi: 10.1016/j.arcmed.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Demartini B., Ranieri R., Masu A., Selle V., Scarone S., Gambini O. Depressive symptoms and major depressive disorder in patients affected by subclinical hypothyroidism: A cross-sectional study. J. Nerv. Ment. Dis. 2014;202:603–607. doi: 10.1097/NMD.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 81.Beydoun M.A., Beydoun H.A., Rostant O.S., Dore G.A., Fanelli-Kuczmarski M.T., Evans M.K., Zonderman A.B. Thyroid hormones are associated with longitudinal cognitive change in an urban adult population. Neurobiol. Aging. 2015;36:3056–3066. doi: 10.1016/j.neurobiolaging.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beydoun M.A., Beydoun H.A., Kitner-Triolo M.H., Kaufman J.S., Evans M.K., Zonderman A.B. Thyroid hormones are associated with cognitive function: Moderation by sex, race, and depressive symptoms. J. Clin. Endocrinol. Metab. 2013;98:3470–3481. doi: 10.1210/jc.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kramer C.K., von Muhlen D., Kritz-Silverstein D., Barrett-Connor E. Treated hypothyroidism, cognitive function, and depressed mood in old age: The Rancho Bernardo Study. Eur. J. Endocrinol. 2009;161:917–921. doi: 10.1530/EJE-09-0606. [DOI] [PubMed] [Google Scholar]
- 84.Smith C.D., Grondin R., LeMaster W., Martin B., Gold B.T., Ain K.B. Reversible cognitive, motor, and driving impairments in severe hypothyroidism. Thyroid. 2015;25:28–36. doi: 10.1089/thy.2014.0371. [DOI] [PubMed] [Google Scholar]
- 85.SO E.S., Chan I.T., Lobo Santos M.A., Cohen M., de La Roque P.A.M., da Silva Almeida J., Simoes A., Givigi H.R., Vaisman M., Paixao C.M., Jr., et al. Impact of thyroid status and age on comprehensive geriatric assessment. Endocrine. 2014;47:255–265. doi: 10.1007/s12020-013-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brownlie B.E., Rae A.M., Walshe J.W., Wells J.E. Psychoses associated with thyrotoxicosis—‘thyrotoxic psychosis.’ A report of 18 cases, with statistical analysis of incidence. Eur. J. Endocrinol. 2000;142:438–444. doi: 10.1530/eje.0.1420438. [DOI] [PubMed] [Google Scholar]
- 87.Ceresini G., Lauretani F., Maggio M., Ceda G.P., Morganti S., Usberti E., Chezzi C., Valcavi R., Bandinelli S., Guralnik J.M., et al. Thyroid function abnormalities and cognitive impairment in elderly people: Results of the Invecchiare in Chianti study. J. Am. Geriatr. Soc. 2009;57:89–93. doi: 10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson P.T., Katsumata Y., Nho K., Artiushin S.C., Jicha G.A., Wang W.X., Abner E.L., Saykin A.J., Kukull W.A., Fardo D.W. Genomics and CSF analyses implicate thyroid hormone in hippocampal sclerosis of aging. Acta Neuropathol. 2016;132:841–858. doi: 10.1007/s00401-016-1641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Accorroni A., Giorgi F.S., Donzelli R., Lorenzini L., Prontera C., Saba A., Vergallo A., Tognoni G., Siciliano G., Baldacci F., et al. Thyroid hormone levels in the cerebrospinal fluid correlate with disease severity in euthyroid patients with Alzheimer’s disease. Endocrine. 2017;55:981–984. doi: 10.1007/s12020-016-0897-6. [DOI] [PubMed] [Google Scholar]
- 90.Rutigliano G., Accorroni A., Zucchi R. The Case for TAAR1 as a Modulator of Central Nervous System Function. Front. Pharmacol. 2017;8:987. doi: 10.3389/fphar.2017.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wortmann M. Dementia: A global health priority-highlights from an ADI and World Health Organization report. Alzheimer’s Res. Ther. 2012;4:40. doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu J.H., Han K., Park S., Cho H., Lee D.Y., Kim J.W., Seo J.A., Kim S.G., Baik S.H., Park Y.G., et al. Incidence and Risk Factors for Dementia in Type 2 Diabetes Mellitus: A Nationwide Population-Based Study in Korea. Diabetes Metab. J. 2020;44:113–124. doi: 10.4093/dmj.2018.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirza Z., Kamal M.A., Buzenadah A.M., Al-Qahtani M.H., Karim S. Establishing genomic/transcriptomic links between Alzheimer’s disease and type 2 diabetes mellitus by meta-analysis approach. Former. Curr. Drug Targets-CNS Neurol. Disord. 2014;13:501–516. doi: 10.2174/18715273113126660154. [DOI] [PubMed] [Google Scholar]
- 94.Kuo S.C., Lai S.W., Hung H.C., Muo C.H., Hung S.C., Liu L.L., Chang C.W., Hwu Y.J., Chen S.L., Sung F.C. Association between comorbidities and dementia in diabetes mellitus patients: Population-based retrospective cohort study. J. Diabetes Its Complicat. 2015;29:1071–1076. doi: 10.1016/j.jdiacomp.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 95.Kopf D., Frolich L. Risk of incident Alzheimer’s disease in diabetic patients: A systematic review of prospective trials. J. Alzheimer’s Dis. 2009;16:677–685. doi: 10.3233/JAD-2009-1011. [DOI] [PubMed] [Google Scholar]
- 96.Macauley S.L., Stanley M., Caesar E.E., Yamada S.A., Raichle M.E., Perez R., Mahan T.E., Sutphen C.L., Holtzman D.M. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J. Clin. Investig. 2015;125:2463–2467. doi: 10.1172/JCI79742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boles A., Kandimalla R., Reddy P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2017;1863:1026–1036. doi: 10.1016/j.bbadis.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bomfim T.R., Forny-Germano L., Sathler L.B., Brito-Moreira J., Houzel J.C., Decker H., Silverman M.A., Kazi H., Melo H.M., McClean P.L., et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Investig. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verdile G., Fuller S.J., Martins R.N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 2015;84:22–38. doi: 10.1016/j.nbd.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Rudolph J.D., de Graauw M., van de Water B., Geiger T., Sharan R. Elucidation of Signaling Pathways from Large-Scale Phosphoproteomic Data Using Protein Interaction Networks. Cell Syst. 2016;3:585–593 e583. doi: 10.1016/j.cels.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Kimura N. Diabetes Mellitus Induces Alzheimer’s Disease Pathology: Histopathological Evidence from Animal Models. Int. J. Mol. Sci. 2016;17:503. doi: 10.3390/ijms17040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takeda S., Sato N., Uchio-Yamada K., Sawada K., Kunieda T., Takeuchi D., Kurinami H., Shinohara M., Rakugi H., Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breteler M.M. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiol. Aging. 2000;21:153–160. doi: 10.1016/S0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 104.Marseglia A., Fratiglioni L., Laukka E.J., Santoni G., Pedersen N.L., Backman L., Xu W. Early Cognitive Deficits in Type 2 Diabetes: A Population-Based Study. J. Alzheimer’s Dis. 2016;53:1069–1078. doi: 10.3233/JAD-160266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alvarez E.O., Beauquis J., Revsin Y., Banzan A.M., Roig P., De Nicola A.F., Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009;198:224–230. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Taurino F., Stanca E., Siculella L., Trentadue R., Papa S., Zanotti F., Gnoni A. Mitochondrial proteome analysis reveals depression of the Ndufs3 subunit and activity of complex I in diabetic rat brain. J. Proteom. 2012;75:2331–2341. doi: 10.1016/j.jprot.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 107.Duarte J.M., Oses J.P., Rodrigues R.J., Cunha R.A. Modification of purinergic signaling in the hippocampus of streptozotocin-induced diabetic rats. Neuroscience. 2007;149:382–391. doi: 10.1016/j.neuroscience.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 108.Neumann K.F., Rojo L., Navarrete L.P., Farias G., Reyes P., Maccioni R.B. Insulin resistance and Alzheimer’s disease: Molecular links & clinical implications. Curr. Alzheimer Res. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- 109.Perez-Taboada I., Alberquilla S., Martin E.D., Anand R., Vietti-Michelina S., Tebeka N.N., Cantley J., Cragg S.J., Moratalla R., Vallejo M. Diabetes Causes Dysfunctional Dopamine Neurotransmission Favoring Nigrostriatal Degeneration in Mice. Mov. Disord. 2020;35:1636–1648. doi: 10.1002/mds.28124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grunberger G. Novel therapies for the management of type 2 diabetes mellitus: Part 1. pramlintide and bromocriptine-QR. J. Diabetes. 2013;5:110–117. doi: 10.1111/1753-0407.12034. [DOI] [PubMed] [Google Scholar]
- 111.Andersen J.V., Nissen J.D., Christensen S.K., Markussen K.H., Waagepetersen H.S. Impaired Hippocampal Glutamate and Glutamine Metabolism in the db/db Mouse Model of Type 2 Diabetes Mellitus. Neural Plast. 2017;2017:2107084. doi: 10.1155/2017/2107084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gingrich J.A., Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology. 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 113.Herrera-Marquez R., Hernandez-Rodriguez J., Medina-Serrano J., Boyzo-Montes de Oca A., Manjarrez-Gutierrez G. Association of metabolic syndrome with reduced central serotonergic activity. Metab. Brain Dis. 2011;26:29–35. doi: 10.1007/s11011-010-9229-3. [DOI] [PubMed] [Google Scholar]
- 114.Maratou E., Hadjidakis D.J., Peppa M., Alevizaki M., Tsegka K., Lambadiari V., Mitrou P., Boutati E., Kollias A., Economopoulos T., et al. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur. J. Endocrinol. 2010;163:625–630. doi: 10.1530/EJE-10-0246. [DOI] [PubMed] [Google Scholar]
- 115.Dessein P.H., Joffe B.I., Stanwix A.E. Subclinical hypothyroidism is associated with insulin resistance in rheumatoid arthritis. Thyroid. 2004;14:443–446. doi: 10.1089/105072504323150750. [DOI] [PubMed] [Google Scholar]
- 116.Khassawneh A.H., Al-Mistarehi A.H., Zein Alaabdin A.M., Khasawneh L., AlQuran T.M., Kheirallah K.A., Saadeh N.A., Beni Yonis O., Shawkat M., Obeidat N. Prevalence and Predictors of Thyroid Dysfunction Among Type 2 Diabetic Patients: A Case-Control Study. Int. J. Gen. Med. 2020;13:803–816. doi: 10.2147/IJGM.S273900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jun J.E., Jee J.H., Bae J.C., Jin S.M., Hur K.Y., Lee M.K., Kim T.H., Kim S.W., Kim J.H. Association Between Changes in Thyroid Hormones and Incident Type 2 Diabetes: A Seven-Year Longitudinal Study. Thyroid. 2017;27:29–38. doi: 10.1089/thy.2016.0171. [DOI] [PubMed] [Google Scholar]
- 118.Zhao W., Zeng H., Zhang X., Liu F., Pan J., Zhao J., Zhao J., Li L., Bao Y., Liu F., et al. A high thyroid stimulating hormone level is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2016;115:122–129. doi: 10.1016/j.diabres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 119.Fan J., Pan Q., Gao Q., Li W., Xiao F., Guo L. TSH Combined with TSHR Aggravates Diabetic Peripheral Neuropathy by Promoting Oxidative Stress and Apoptosis in Schwann Cells. Oxidative Med. Cell. Longev. 2021;2021:2482453. doi: 10.1155/2021/2482453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Volke L., Krause K. Effect of Thyroid Hormones on Adipose Tissue Flexibility. Eur. Thyroid J. 2021;10:1–9. doi: 10.1159/000508483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sinha R.A., You S.H., Zhou J., Siddique M.M., Bay B.H., Zhu X., Privalsky M.L., Cheng S.Y., Stevens R.D., Summers S.A., et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012;122:2428–2438. doi: 10.1172/JCI60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yin L., Zhang Y., Hillgartner F.B. Sterol regulatory element-binding protein-1 interacts with the nuclear thyroid hormone receptor to enhance acetyl-CoA carboxylase-α transcription in hepatocytes. J. Biol. Chem. 2002;277:19554–19565. doi: 10.1074/jbc.M111771200. [DOI] [PubMed] [Google Scholar]
- 123.Perra A., Simbula G., Simbula M., Pibiri M., Kowalik M.A., Sulas P., Cocco M.T., Ledda-Columbano G.M., Columbano A. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008;22:2981–2989. doi: 10.1096/fj.08-108464. [DOI] [PubMed] [Google Scholar]
- 124.Cable E.E., Finn P.D., Stebbins J.W., Hou J., Ito B.R., van Poelje P.D., Linemeyer D.L., Erion M.D. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49:407–417. doi: 10.1002/hep.22572. [DOI] [PubMed] [Google Scholar]
- 125.Dentin R., Girard J., Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): Two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 126.Ritter M.J., Amano I., Hollenberg A.N. Thyroid Hormone Signaling and the Liver. Hepatology. 2020;72:742–752. doi: 10.1002/hep.31296. [DOI] [PubMed] [Google Scholar]
- 127.Li Y., Wang L., Zhou L., Song Y., Ma S., Yu C., Zhao J., Xu C., Gao L. Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol. Cell. Endocrinol. 2017;446:70–80. doi: 10.1016/j.mce.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 128.Teng X., Liu Y.Y., Teng W., Brent G.A. COUP-TF1 Modulates Thyroid Hormone Action in an Embryonic Stem-Cell Model of Cortical Pyramidal Neuronal Differentiation. Thyroid. 2018;28:667–678. doi: 10.1089/thy.2017.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y.Y., Brent G.A. Thyroid hormone-dependent gene expression in differentiated embryonic stem cells and embryonal carcinoma cells: Identification of novel thyroid hormone target genes by deoxyribonucleic acid microarray analysis. Endocrinology. 2005;146:776–783. doi: 10.1210/en.2004-1177. [DOI] [PubMed] [Google Scholar]
- 130.Morte B., Diez D., Auso E., Belinchon M.M., Gil-Ibanez P., Grijota-Martinez C., Navarro D., de Escobar G.M., Berbel P., Bernal J. Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: Prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology. 2010;151:810–820. doi: 10.1210/en.2009-0958. [DOI] [PubMed] [Google Scholar]
- 131.Navarro D., Alvarado M., Morte B., Berbel D., Sesma J., Pacheco P., Morreale de Escobar G., Bernal J., Berbel P. Late maternal hypothyroidism alters the expression of Camk4 in neocortical subplate neurons: A comparison with Nurr1 labeling. Cereb. Cortex. 2014;24:2694–2706. doi: 10.1093/cercor/bht129. [DOI] [PubMed] [Google Scholar]
- 132.Redmond L., Kashani A.H., Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/S0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 133.Li J., Abe K., Milanesi A., Liu Y.Y., Brent G.A. Thyroid Hormone Protects Primary Cortical Neurons Exposed to Hypoxia by Reducing DNA Methylation and Apoptosis. Endocrinology. 2019;160:2243–2256. doi: 10.1210/en.2019-00125. [DOI] [PubMed] [Google Scholar]
- 134.Iniguez M.A., De Lecea L., Guadano-Ferraz A., Morte B., Gerendasy D., Sutcliffe J.G., Bernal J. Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology. 1996;137:1032–1041. doi: 10.1210/endo.137.3.8603571. [DOI] [PubMed] [Google Scholar]
- 135.Zhong L., Gerges N.Z. Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation. PLoS ONE. 2012;7:e41275. doi: 10.1371/journal.pone.0041275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miyakawa T., Yared E., Pak J.H., Huang F.L., Huang K.P., Crawley J.N. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 137.Alvarez-Dolado M., Ruiz M., Del Rio J.A., Alcantara S., Burgaya F., Sheldon M., Nakajima K., Bernal J., Howell B.W., Curran T., et al. Thyroid hormone regulates reelin and dab1 expression during brain development. J. Neurosci. 1999;19:6979–6993. doi: 10.1523/JNEUROSCI.19-16-06979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sui L., Ren W.W., Li B.M. Administration of thyroid hormone increases reelin and brain-derived neurotrophic factor expression in rat hippocampus in vivo. Brain Res. 2010;1313:9–24. doi: 10.1016/j.brainres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 139.Jossin Y., Cooper J.A. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci. 2011;14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herz J., Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 141.Thompson C.C., Potter G.B. Thyroid hormone action in neural development. Cereb. Cortex. 2000;10:939–945. doi: 10.1093/cercor/10.10.939. [DOI] [PubMed] [Google Scholar]
- 142.Gordon-Weeks P.R., Fournier A.E. Neuronal cytoskeleton in synaptic plasticity and regeneration. J. Neurochem. 2014;129:206–212. doi: 10.1111/jnc.12502. [DOI] [PubMed] [Google Scholar]
- 143.Silva J.E., Rudas P. Effects of congenital hypothyroidism on microtubule-associated protein-2 expression in the cerebellum of the rat. Endocrinology. 1990;126:1276–1282. doi: 10.1210/endo-126-2-1276. [DOI] [PubMed] [Google Scholar]
- 144.Aniello F., Couchie D., Bridoux A.M., Gripois D., Nunez J. Splicing of juvenile and adult tau mRNA variants is regulated by thyroid hormone. Proc. Natl. Acad. Sci. USA. 1991;88:4035–4039. doi: 10.1073/pnas.88.9.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koibuchi N., Chin W.W. Thyroid hormone action and brain development. Trends Endocrinol. Metab. 2000;11:123–128. doi: 10.1016/S1043-2760(00)00238-1. [DOI] [PubMed] [Google Scholar]
- 146.Kapoor R., Fanibunda S.E., Desouza L.A., Guha S.K., Vaidya V.A. Perspectives on thyroid hormone action in adult neurogenesis. J. Neurochem. 2015;133:599–616. doi: 10.1111/jnc.13093. [DOI] [PubMed] [Google Scholar]
- 147.Morte B., Bernal J. Thyroid hormone action: Astrocyte-neuron communication. Front. Endocrinol. 2014;5:82. doi: 10.3389/fendo.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kincaid A.E. Spontaneous circling behavior and dopamine neuron loss in a genetically hypothyroid mouse. Neuroscience. 2001;105:891–898. doi: 10.1016/S0306-4522(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 149.Lee E.H., Kim S.M., Kim C.H., Pagire S.H., Pagire H.S., Chung H.Y., Ahn J.H., Park C.H. Dopamine neuron induction and the neuroprotective effects of thyroid hormone derivatives. Sci. Rep. 2019;9:13659. doi: 10.1038/s41598-019-49876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mendes-de-Aguiar C.B., Alchini R., Decker H., Alvarez-Silva M., Tasca C.I., Trentin A.G. Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J. Neurosci. Res. 2008;86:3117–3125. doi: 10.1002/jnr.21755. [DOI] [PubMed] [Google Scholar]
- 151.Losi G., Garzon G., Puia G. Nongenomic regulation of glutamatergic neurotransmission in hippocampus by thyroid hormones. Neuroscience. 2008;151:155–163. doi: 10.1016/j.neuroscience.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 152.Richard S., Guyot R., Rey-Millet M., Prieux M., Markossian S., Aubert D., Flamant F. A Pivotal Genetic Program Controlled by Thyroid Hormone during the Maturation of GABAergic Neurons. iScience. 2020;23:100899. doi: 10.1016/j.isci.2020.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hashimoto H., Walker C.H., Prange A.J., Jr., Mason G.A. The effects of thyroid hormones on potassium-stimulated release of 3H-GABA by synaptosomes of rat cerebral cortex. Neuropsychopharmacology. 1991;5:49–54. [PubMed] [Google Scholar]
- 154.Da Settimo A., Primofiore G., Da Settimo F., Novellino E., Greco G., Martini C., Senatore G., Lucacehini A. Indole derivatives as probes to study the benzodiazepine binding site in GABA receptor complex. Adv. Exp. Med. Biol. 1996;398:677–683. doi: 10.1007/978-1-4613-0381-7_109. [DOI] [PubMed] [Google Scholar]
- 155.Manzano J., Cuadrado M., Morte B., Bernal J. Influence of thyroid hormone and thyroid hormone receptors in the generation of cerebellar gamma-aminobutyric acid-ergic interneurons from precursor cells. Endocrinology. 2007;148:5746–5751. doi: 10.1210/en.2007-0567. [DOI] [PubMed] [Google Scholar]
- 156.Mason G.A., Walker C.H., Prange A.J., Jr., Bondy S.C. GABA uptake is inhibited by thyroid hormones: Implications for depression. Psychoneuroendocrinology. 1987;12:53–59. doi: 10.1016/0306-4530(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 157.Cortes C., Eugenin E., Aliaga E., Carreno L.J., Bueno S.M., Gonzalez P.A., Gayol S., Naranjo D., Noches V., Marassi M.P., et al. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid. 2012;22:951–963. doi: 10.1089/thy.2010.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McNay E.C., Recknagel A.K. Brain insulin signaling: A key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol. Learn. Mem. 2011;96:432–442. doi: 10.1016/j.nlm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McNay E.C., Ong C.T., McCrimmon R.J., Cresswell J., Bogan J.S., Sherwin R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heni M., Kullmann S., Preissl H., Fritsche A., Haring H.U. Impaired insulin action in the human brain: Causes and metabolic consequences. Nat. Rev. Endocrinol. 2015;11:701–711. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- 161.Babri S., Badie H.G., Khamenei S., Seyedlar M.O. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn. 2007;64:86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 162.Ross A.P., Bartness T.J., Mielke J.G., Parent M.B. A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn Mem. 2009;92:410–416. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jolivalt C.G., Hurford R., Lee C.A., Dumaop W., Rockenstein E., Masliah E. Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp. Neurol. 2010;223:422–431. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gasparini L., Gouras G.K., Wang R., Gross R.S., Beal M.F., Greengard P., Xu H. Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhat R.V., Shanley J., Correll M.P., Fieles W.E., Keith R.A., Scott C.W., Lee C.M. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3β in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 167.Tsygankova O.M., Feshchenko E., Klein P.S., Meinkoth J.L. Thyroid-stimulating hormone/cAMP and glycogen synthase kinase 3β elicit opposing effects on Rap1GAP stability. J. Biol. Chem. 2004;279:5501–5507. doi: 10.1074/jbc.M305824200. [DOI] [PubMed] [Google Scholar]
- 168.Joffe B.I., Distiller L.A. Diabetes mellitus and hypothyroidism: Strange bedfellows or mutual companions? World J. Diabetes. 2014;5:901–904. doi: 10.4239/wjd.v5.i6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bathina S., Das U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Passaro A., Dalla Nora E., Morieri M.L., Soavi C., Sanz J.M., Zurlo A., Fellin R., Zuliani G. Brain-derived neurotrophic factor plasma levels: Relationship with dementia and diabetes in the elderly population. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015;70:294–302. doi: 10.1093/gerona/glu028. [DOI] [PubMed] [Google Scholar]
- 171.Gilbert M.E., Lasley S.M. Developmental thyroid hormone insufficiency and brain development: A role for brain-derived neurotrophic factor (BDNF)? Neuroscience. 2013;239:253–270. doi: 10.1016/j.neuroscience.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 172.Ohguni S., Notsu K., Kato Y. Correlation of plasma free thyroxine levels with insulin sensitivity and metabolic clearance rate of insulin in patients with hyperthyroid Graves’ disease. Intern. Med. 1995;34:339–341. doi: 10.2169/internalmedicine.34.339. [DOI] [PubMed] [Google Scholar]
- 173.Ruhla S., Arafat A.M., Weickert M.O., Osterhoff M., Isken F., Spranger J., Schofl C., Pfeiffer A.F., Mohlig M. T3/rT3-ratio is associated with insulin resistance independent of TSH. Horm. Metab. Res. 2011;43:130–134. doi: 10.1055/s-0030-1267997. [DOI] [PubMed] [Google Scholar]
- 174.Mills I., Barge R.M., Silva J.E., Larsen P.R. Insulin stimulation of iodothyronine 5’-deiodinase in rat brown adipocytes. Biochem. Biophys. Res. Commun. 1987;143:81–86. doi: 10.1016/0006-291X(87)90632-2. [DOI] [PubMed] [Google Scholar]
- 175.Lisboa P.C., Cabanelas A.P., Curty F.H., Oliveira K.J., Ortiga-Carvalho T.M., Moura E.G., Nascimento-Saba C.C., Rosenthal D., Pazos-Moura C.C. Modulation of type 2 iodothyronine deiodinase activity in rat thyroid gland. Horm. Metab. Res. 2007;39:538–541. doi: 10.1055/s-2007-984351. [DOI] [PubMed] [Google Scholar]
- 176.Ruhla S., Weickert M.O., Arafat A.M., Osterhoff M., Isken F., Spranger J., Schofl C., Pfeiffer A.F., Mohlig M. A high normal TSH is associated with the metabolic syndrome. Clin. Endocrinol. 2010;72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 177.Rong F., Dai H., Wu Y., Li J., Liu G., Chen H., Zhang X. Association between thyroid dysfunction and type 2 diabetes: A meta-analysis of prospective observational studies. BMC Med. 2021;19:257. doi: 10.1186/s12916-021-02121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buchberger B., Huppertz H., Krabbe L., Lux B., Mattivi J.T., Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84. doi: 10.1016/j.psyneuen.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 179.Wessels A.M., Scheltens P., Barkhof F., Heine R.J. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur. J. Pharmacol. 2008;585:88–96. doi: 10.1016/j.ejphar.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 180.Salkovic-Petrisic M., Tribl F., Schmidt M., Hoyer S., Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J. Neurochem. 2006;96:1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 181.Choi Y.M., Kim M.K., Kwak M.K., Kim D., Hong E.G. Association between thyroid hormones and insulin resistance indices based on the Korean National Health and Nutrition Examination Survey. Sci. Rep. 2021;11:21738. doi: 10.1038/s41598-021-01101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heemstra K.A., Smit J.W., Eustatia-Rutten C.F., Heijboer A.C., Frolich M., Romijn J.A., Corssmit E.P. Glucose tolerance and lipid profile in longterm exogenous subclinical hyperthyroidism and the effects of restoration of euthyroidism, a randomised controlled trial. Clin. Endocrinol. 2006;65:737–744. doi: 10.1111/j.1365-2265.2006.02660.x. [DOI] [PubMed] [Google Scholar]
- 183.Jahagirdar V., McNay E.C. Thyroid hormone’s role in regulating brain glucose metabolism and potentially modulating hippocampal cognitive processes. Metab. Brain Dis. 2012;27:101–111. doi: 10.1007/s11011-012-9291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Miao Q., Zhang S., Guan Y.H., Ye H.Y., Zhang Z.Y., Zhang Q.Y., Xue R.D., Zeng M.F., Zuo C.T., Li Y.M. Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. Am. J. Neuroradiol. 2011;32:1034–1042. doi: 10.3174/ajnr.A2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brunetto E.L., Teixeira Sda S., Giannocco G., Machado U.F., Nunes M.T. T3 rapidly increases SLC2A4 gene expression and GLUT4 trafficking to the plasma membrane in skeletal muscle of rat and improves glucose homeostasis. Thyroid. 2012;22:70–79. doi: 10.1089/thy.2010.0409. [DOI] [PubMed] [Google Scholar]
- 186.Panveloski-Costa A.C., Silva Teixeira S., Ribeiro I.M., Serrano-Nascimento C., das Neves R.X., Favaro R.R., Seelaender M., Antunes V.R., Nunes M.T. Thyroid hormone reduces inflammatory cytokines improving glycaemia control in alloxan-induced diabetic wistar rats. Acta Physiol. 2016;217:130–140. doi: 10.1111/apha.12647. [DOI] [PubMed] [Google Scholar]
- 187.Bos M.M., Smit R.A.J., Trompet S., van Heemst D., Noordam R. Thyroid Signaling, Insulin Resistance, and 2 Diabetes Mellitus: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2017;102:1960–1970. doi: 10.1210/jc.2016-2816. [DOI] [PubMed] [Google Scholar]
- 188.Hage M., Zantout M.S., Azar S.T. Thyroid disorders and diabetes mellitus. J. Thyroid Res. 2011;2011:439463. doi: 10.4061/2011/439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Caria M.A., Dratman M.B., Kow L.M., Mameli O., Pavlides C. Thyroid hormone action: Nongenomic modulation of neuronal excitability in the hippocampus. J. Neuroendocrinol. 2009;21:98–107. doi: 10.1111/j.1365-2826.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 190.Cao X., Kambe F., Yamauchi M., Seo H. Thyroid-hormone-dependent activation of the phosphoinositide 3-kinase/Akt cascade requires Src and enhances neuronal survival. Biochem. J. 2009;424:201–209. doi: 10.1042/BJ20090643. [DOI] [PubMed] [Google Scholar]
- 191.Prieto-Almeida F., Panveloski-Costa A.C., Crunfli F., da Silva Teixeira S., Nunes M.T., Torrao A.D.S. Thyroid hormone improves insulin signaling and reduces the activation of neurodegenerative pathway in the hippocampus of diabetic adult male rats. Life Sci. 2018;192:253–258. doi: 10.1016/j.lfs.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 192.Bae S.S., Cho H., Mu J., Birnbaum M.J. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 193.Griffin R.J., Moloney A., Kelliher M., Johnston J.A., Ravid R., Dockery P., O’Connor R., O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J. Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 194.Mooradian A.D., Girgis W., Shah G.N. Thyroid hormone-induced GLUT-1 expression in rat cerebral tissue: Effect of age. Brain Res. 1997;747:144–146. doi: 10.1016/S0006-8993(96)01110-9. [DOI] [PubMed] [Google Scholar]
- 195.Kuruvilla A.K., Perez C., Ismail-Beigi F., Loeb J.N. Regulation of glucose transport in Clone 9 cells by thyroid hormone. Biochim. Biophys. Acta. 1991;1094:300–308. doi: 10.1016/0167-4889(91)90090-K. [DOI] [PubMed] [Google Scholar]
- 196.Grozovsky R., Ribich S., Rosene M.L., Mulcahey M.A., Huang S.A., Patti M.E., Bianco A.C., Kim B.W. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-gamma agonists in skeletal myocytes. Endocrinology. 2009;150:1976–1983. doi: 10.1210/en.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Estivalet A.A., Leiria L.B., Dora J.M., Rheinheimer J., Boucas A.P., Maia A.L., Crispim D. D2 Thr92Ala and PPARgamma2 Pro12Ala polymorphisms interact in the modulation of insulin resistance in type 2 diabetic patients. Obesity. 2011;19:825–832. doi: 10.1038/oby.2010.231. [DOI] [PubMed] [Google Scholar]
- 198.Sutherland M.K., Wong L., Somerville M.J., Handley P., Yoong L., Bergeron C., McLachlan D.R. Reduction of thyroid hormone receptor c-ERB A α mRNA levels in the hippocampus of Alzheimer as compared to Huntington brain. Neurobiol. Aging. 1992;13:301–312. doi: 10.1016/0197-4580(92)90043-W. [DOI] [PubMed] [Google Scholar]
- 199.Carlsson C.M. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease. J. Alzheimer’s Dis. 2010;20:711–722. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bowman G.L., Kaye J.A., Moore M., Waichunas D., Carlson N.E., Quinn J.F. Blood-brain barrier impairment in Alzheimer disease: Stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bowman G.L., Kaye J.A., Quinn J.F. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr. Gerontol. Geriatr. Res. 2012;2012:184042. doi: 10.1155/2012/184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Heverin M., Bogdanovic N., Lutjohann D., Bayer T., Pikuleva I., Bretillon L., Diczfalusy U., Winblad B., Bjorkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- 203.Papassotiropoulos A., Lutjohann D., Bagli M., Locatelli S., Jessen F., Buschfort R., Ptok U., Bjorkhem I., von Bergmann K., Heun R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J. Psychiatr. Res. 2002;36:27–32. doi: 10.1016/S0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- 204.Refolo L.M., Malester B., LaFrancois J., Bryant-Thomas T., Wang R., Tint G.S., Sambamurti K., Duff K., Pappolla M.A. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 205.Raffaitin C., Gin H., Empana J.P., Helmer C., Berr C., Tzourio C., Portet F., Dartigues J.F., Alperovitch A., Barberger-Gateau P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: The Three-City Study. Diabetes Care. 2009;32:169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mozaffarian D., Wu J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 207.Bogdanovic N., Bretillon L., Lund E.G., Diczfalusy U., Lannfelt L., Winblad B., Russell D.W., Bjorkhem I. On the turnover of brain cholesterol in patients with Alzheimer’s disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 2001;314:45–48. doi: 10.1016/S0304-3940(01)02277-7. [DOI] [PubMed] [Google Scholar]
- 208.Kojro E., Gimpl G., Lammich S., Marz W., Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc. Natl. Acad. Sci. USA. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Refolo L.M., Pappolla M.A., LaFrancois J., Malester B., Schmidt S.D., Thomas-Bryant T., Tint G.S., Wang R., Mercken M., Petanceska S.S., et al. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- 210.McLaurin J., Darabie A.A., Morrison M.R. Cholesterol, a modulator of membrane-associated Aβ-fibrillogenesis. Pharmacopsychiatry. 2003;36((Suppl. 2)):S130–S135. doi: 10.1111/j.1749-6632.2002.tb04840.x. [DOI] [PubMed] [Google Scholar]
- 211.Murakami K., Shimizu M., Yamada N., Ishibashi S., Shimano H., Yazaki Y., Akanuma Y. Apolipoprotein E polymorphism is associated with plasma cholesterol response in a 7-day hospitalization study for metabolic and dietary control in NIDDM. Diabetes Care. 1993;16:564–569. doi: 10.2337/diacare.16.4.564. [DOI] [PubMed] [Google Scholar]
- 212.Graff-Radford N.R., Green R.C., Go R.C., Hutton M.L., Edeki T., Bachman D., Adamson J.L., Griffith P., Willis F.B., Williams M., et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch. Neurol. 2002;59:594–600. doi: 10.1001/archneur.59.4.594. [DOI] [PubMed] [Google Scholar]
- 213.Wong W.B., Lin V.W., Boudreau D., Devine E.B. Statins in the prevention of dementia and Alzheimer’s disease: A meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol. Drug Saf. 2013;22:345–358. doi: 10.1002/pds.3381. [DOI] [PubMed] [Google Scholar]
- 214.Hyman B.T., Strickland D., Rebeck G.W. Role of the low-density lipoprotein receptor-related protein in β-amyloid metabolism and Alzheimer disease. Arch. Neurol. 2000;57:646–650. doi: 10.1001/archneur.57.5.646. [DOI] [PubMed] [Google Scholar]
- 215.Fan Y.C., Hsu J.L., Tung H.Y., Chou C.C., Bai C.H. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: A population-based cohort study. Alzheimer’s Res. Ther. 2017;9:7. doi: 10.1186/s13195-017-0236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Helzner E.P., Luchsinger J.A., Scarmeas N., Cosentino S., Brickman A.M., Glymour M.M., Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch. Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Watanabe T., Koba S., Kawamura M., Itokawa M., Idei T., Nakagawa Y., Iguchi T., Katagiri T. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia. Metabolism. 2004;53:476–482. doi: 10.1016/j.metabol.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 218.Evans R.M., Emsley C.L., Gao S., Sahota A., Hall K.S., Farlow M.R., Hendrie H. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: A population-based study of African Americans. Neurology. 2000;54:240–242. doi: 10.1212/WNL.54.1.240. [DOI] [PubMed] [Google Scholar]
- 219.Solomon A., Kareholt I., Ngandu T., Wolozin B., Macdonald S.W., Winblad B., Nissinen A., Tuomilehto J., Soininen H., Kivipelto M. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol. Aging. 2009;30:1006–1009. doi: 10.1016/j.neurobiolaging.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 220.Burgess B.L., McIsaac S.A., Naus K.E., Chan J.Y., Tansley G.H., Yang J., Miao F., Ross C.J., van Eck M., Hayden M.R., et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant Aβ in plasma. Neurobiol. Dis. 2006;24:114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 221.Reynolds C.A., Gatz M., Prince J.A., Berg S., Pedersen N.L. Serum lipid levels and cognitive change in late life. J. Am. Geriatr. Soc. 2010;58:501–509. doi: 10.1111/j.1532-5415.2010.02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Trkanjec Z., Bene R., Martinic-Popovic I., Jurasic J.M., Lisak M., Seric V., Demarin V. Serum HDL, LDL and total cholesterol in patients with late-life onset of Alzheimer’s disease versus vascular dementia. Acta Clin. Croat. 2009;48:259–263. [PubMed] [Google Scholar]
- 223.Reitz C., Tang M.X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010;67:1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang X., Song Y., Feng M., Zhou X., Lu Y., Gao L., Yu C., Jiang X., Zhao J. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J. Lipid Res. 2015;56:963–971. doi: 10.1194/jlr.M047654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kuusi T., Saarinen P., Nikkila E.A. Evidence for the role of hepatic endothelial lipase in the metabolism of plasma high density lipoprotein2 in man. Atherosclerosis. 1980;36:589–593. doi: 10.1016/0021-9150(80)90251-8. [DOI] [PubMed] [Google Scholar]
- 226.Gambo Y., Matsumura M., Fujimori K. Triiodothyronine enhances accumulation of intracellular lipids in adipocytes through thyroid hormone receptor α via direct and indirect mechanisms. Mol. Cell. Endocrinol. 2016;431:1–11. doi: 10.1016/j.mce.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 227.Liu S., Jing F., Yu C., Gao L., Qin Y., Zhao J. AICAR-Induced Activation of AMPK Inhibits TSH/SREBP-2/HMGCR Pathway in Liver. PLoS ONE. 2015;10:e0124951. doi: 10.1371/journal.pone.0124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gao C.X., Yang B., Guo Q., Wei L.H., Tian L.M. High thyroid-stimulating hormone level is associated with the risk of developing atherosclerosis in subclinical hypothyroidism. Horm. Metab. Res. 2015;47:220–224. doi: 10.1055/s-0034-1394370. [DOI] [PubMed] [Google Scholar]
- 229.Klieverik L.P., Coomans C.P., Endert E., Sauerwein H.P., Havekes L.M., Voshol P.J., Rensen P.C., Romijn J.A., Kalsbeek A., Fliers E. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009;150:5639–5648. doi: 10.1210/en.2009-0297. [DOI] [PubMed] [Google Scholar]
- 230.Nedvidkova J., Haluzik M., Bartak V., Dostalova I., Vlcek P., Racek P., Taus M., Behanova M., Svacina S., Alesci S., et al. Changes of noradrenergic activity and lipolysis in the subcutaneous abdominal adipose tissue of hypo- and hyperthyroid patients: An in vivo microdialysis study. Ann. N. Y. Acad. Sci. 2004;1018:541–549. doi: 10.1196/annals.1296.067. [DOI] [PubMed] [Google Scholar]
- 231.Lam K.S., Chan M.K., Yeung R.T. High-density lipoprotein cholesterol, hepatic lipase and lipoprotein lipase activities in thyroid dysfunction--effects of treatment. QJM Int. J. Med. 1986;59:513–521. [PubMed] [Google Scholar]
- 232.D’Ambrosio R., Campi I., Maggioni M., Perbellini R., Giammona E., Stucchi R., Borghi M., Degasperi E., De Silvestri A., Persani L., et al. The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liver disease (NAFLD) PLoS ONE. 2021;16:e0249614. doi: 10.1371/journal.pone.0249614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hazlehurst J.M., Tomlinson J.W. Non-alcoholic fatty liver disease in common endocrine disorders. Eur. J. Endocrinol. 2013;169:R27–R37. doi: 10.1530/EJE-13-0296. [DOI] [PubMed] [Google Scholar]
- 234.Tan K.C., Shiu S.W., Kung A.W. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J. Clin. Endocrinol. Metab. 1998;83:140–143. doi: 10.1210/jc.83.1.140. [DOI] [PubMed] [Google Scholar]
- 235.Zhao M., Liu L., Wang F., Yuan Z., Zhang X., Xu C., Song Y., Guan Q., Gao L., Shan Z., et al. A Worthy Finding: Decrease in Total Cholesterol and Low-Density Lipoprotein Cholesterol in Treated Mild Subclinical Hypothyroidism. Thyroid. 2016;26:1019–1029. doi: 10.1089/thy.2016.0010. [DOI] [PubMed] [Google Scholar]
- 236.Pearce E.N. Update in lipid alterations in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012;97:326–333. doi: 10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- 237.Willard D.L., Leung A.M., Pearce E.N. Thyroid function testing in patients with newly diagnosed hyperlipidemia. JAMA Intern. Med. 2014;174:287–289. doi: 10.1001/jamainternmed.2013.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Arikan S., Bahceci M., Tuzcu A., Celik F., Gokalp D. Postprandial hyperlipidemia in overt and subclinical hypothyroidism. Eur. J. Intern. Med. 2012;23:e141–e145. doi: 10.1016/j.ejim.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 239.Martin S.S., Daya N., Lutsey P.L., Matsushita K., Fretz A., McEvoy J.W., Blumenthal R.S., Coresh J., Greenland P., Kottgen A., et al. Thyroid Function, Cardiovascular Risk Factors, and Incident Atherosclerotic Cardiovascular Disease: The Atherosclerosis Risk in Communities (ARIC) Study. J. Clin. Endocrinol. Metab. 2017;102:3306–3315. doi: 10.1210/jc.2017-00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dong X., Yao Z., Hu Y., Yang N., Gao X., Xu Y., Wang G. Potential harmful correlation between homocysteine and low-density lipoprotein cholesterol in patients with hypothyroidism. Medicine. 2016;95:e4291. doi: 10.1097/MD.0000000000004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Stein S., Stepan H., Kratzsch J., Verlohren M., Verlohren H.J., Drynda K., Lossner U., Bluher M., Stumvoll M., Fasshauer M. Serum fibroblast growth factor 21 levels in gestational diabetes mellitus in relation to insulin resistance and dyslipidemia. Metabolism. 2010;59:33–37. doi: 10.1016/j.metabol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 242.Xiao F., Zeng J., Huang P., Yan B., Zeng X., Liu C., Shi X., Wang L., Song H., Lin M., et al. Independent Association of Serum Fibroblast Growth Factor 21 Levels With Impaired Liver Enzymes in Hyperthyroid Patients. Front. Endocrinol. 2018;9:800. doi: 10.3389/fendo.2018.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Adams A.C., Astapova I., Fisher F.M., Badman M.K., Kurgansky K.E., Flier J.S., Hollenberg A.N., Maratos-Flier E. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARα-dependent manner. J. Biol. Chem. 2010;285:14078–14082. doi: 10.1074/jbc.C110.107375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.van der Boom T., Jia C., Lefrandt J.D., Connelly M.A., Links T.P., Tietge U.J.F., Dullaart R.P.F. HDL Cholesterol Efflux Capacity is Impaired in Severe Short-Term Hypothyroidism Despite Increased HDL Cholesterol. J. Clin. Endocrinol. Metab. 2020;105:E3355–E3362. doi: 10.1210/clinem/dgaa411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gharib H., Tuttle R.M., Baskin H.J., Fish L.H., Singer P.A., McDermott M.T., American Association of Clinical E., American Thyroid A., Endocrine S. Consensus Statement #1: Subclinical thyroid dysfunction: A joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. Thyroid. 2005;15:24–28. doi: 10.1089/thy.2005.15.24. response 32–23. [DOI] [PubMed] [Google Scholar]
- 246.Hugo J., Ganguli M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vanderpump M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 248.Modesto T., Tiemeier H., Peeters R.P., Jaddoe V.W., Hofman A., Verhulst F.C., Ghassabian A. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr. 2015;169:838–845. doi: 10.1001/jamapediatrics.2015.0498. [DOI] [PubMed] [Google Scholar]
- 249.Sweatt J.D. Hippocampal function in cognition. Psychopharmacology. 2004;174:99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- 250.Salazar P., Cisternas P., Codocedo J.F., Inestrosa N.C. Induction of hypothyroidism during early postnatal stages triggers a decrease in cognitive performance by decreasing hippocampal synaptic plasticity. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2017;1863:870–883. doi: 10.1016/j.bbadis.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 251.Chaalal A., Poirier R., Blum D., Laroche S., Enderlin V. Thyroid Hormone Supplementation Restores Spatial Memory, Hippocampal Markers of Neuroinflammation, Plasticity-Related Signaling Molecules, and β-Amyloid Peptide Load in Hypothyroid Rats. Mol. Neurobiol. 2019;56:722–735. doi: 10.1007/s12035-018-1111-z. [DOI] [PubMed] [Google Scholar]
- 252.Nam S.M., Kim J.W., Yoo D.Y., Jung H.Y., Chung J.Y., Kim D.W., Hwang I.K., Yoon Y.S. Hypothyroidism increases cyclooxygenase-2 levels and pro-inflammatory response and decreases cell proliferation and neuroblast differentiation in the hippocampus. Mol. Med. Rep. 2018;17:5782–5788. doi: 10.3892/mmr.2018.8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang F., Lin X., Liu A., Chen J., Shan Z., Teng W., Yu X. Maternal Subclinical Hypothyroidism in Rats Impairs Spatial Learning and Memory in Offspring by Disrupting Balance of the TrkA/p75(NTR) Signal Pathway. Mol. Neurobiol. 2021;58:4237–4250. doi: 10.1007/s12035-021-02403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sui L., Anderson W.L., Gilbert M.E. Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol. Sci. 2005;85:647–656. doi: 10.1093/toxsci/kfi095. [DOI] [PubMed] [Google Scholar]
- 255.Parsaik A.K., Singh B., Roberts R.O., Pankratz S., Edwards K.K., Geda Y.E., Gharib H., Boeve B.F., Knopman D.S., Petersen R.C. Hypothyroidism and risk of mild cognitive impairment in elderly persons: A population-based study. JAMA Neurol. 2014;71:201–207. doi: 10.1001/jamaneurol.2013.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dong J., Yin H., Liu W., Wang P., Jiang Y., Chen J. Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology. 2005;26:417–426. doi: 10.1016/j.neuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 257.Sui L., Gilbert M.E. Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology. 2003;144:4195–4203. doi: 10.1210/en.2003-0395. [DOI] [PubMed] [Google Scholar]
- 258.Gerges N.Z., Alkadhi K.A. Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus. 2004;14:40–45. doi: 10.1002/hipo.10165. [DOI] [PubMed] [Google Scholar]
- 259.Gilbert M.E. Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain Res. Dev. Brain Res. 2004;148:11–18. doi: 10.1016/j.devbrainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 260.Harper P.C., Roe C.M. Thyroid medication use and subsequent development of dementia of the Alzheimer type. J. Geriatr. Psychiatry Neurol. 2010;23:63–69. doi: 10.1177/0891988709342723. [DOI] [PMC free article] [PubMed] [Google Scholar]