Abstract
We evaluated several 3-day antimicrobial regimens in the treatment of experimental endocarditis caused by an oxacillin-resistant Staphylococcus aureus strain exhibiting intermediate susceptibility in vitro to vancomycin (VISA). Neither vancomycin alone nor trovafloxacin exhibited in vivo efficacy; addition of amikacin to vancomycin yielded a modest in vivo effect. In contrast, the combination of ampicillin and sulbactam was highly effective in vivo, causing a mean decrease in VISA vegetation densities of >5 log10 CFU/g versus those of untreated controls.
Until recently, vancomycin was uniformly active in vitro against all oxacillin-resistant Staphylococcus aureus (ORSA) strains. However, within the last few years, investigators in Japan and the United States have documented the isolation of ORSA strains with intermediate susceptibility to vancomycin (VISA) (MICs of 4 to 8 μg/ml) from patients with recalcitrant clinical infections who were failing vancomycin therapy (7, 8, 12).
The present study was designed to examine the in vivo efficacies of several antibiotic regimens, including that of the potent new fluoroquinolone agent trovafloxacin, in the treatment of a severe experimental infection caused by VISA, aortic-valve endocarditis. The rabbit animal model provides a rigorous test of antimicrobial efficacy (1, 4, 5, 13, 19).
VISA MU-50 was kindly provided by K. Hiramatsu, Tokyo, Japan, and has been described in detail previously (11). This organism was isolated from a child with a relapsing median sternotomy wound infection who was failing vancomycin therapy. Briefly, this strain exhibits heterotypic resistance to both oxacillin (ORSA) and vancomycin (VISA) by population analyses; moreover, compared to vancomycin-susceptible S. aureus strains, MU-50 demonstrates the characteristic VISA phenotypes of excessive cell wall thickness on electron microscopy, increases in penicillin-binding protein production, and upregulation of cell wall murein precursor biosynthesis (12, 17, 20).
Vancomycin, amikacin, and ampicillin were purchased from commercial sources (Eli Lilly, Indianapolis, Ind.; Faulding Pharmaceuticals, Aguadilla, P.R.; and Bristol-Myers Squibb, Princeton, N.J., respectively). Sulbactam and trovafloxacin (as the prodrug [alatrofloxacin]) were supplied by Pfizer Central Research (Groton, Conn.). For alatrofloxacin, 1 mg is equivalent to ∼0.80 mg of trovafloxacin (21). Antibiotics were reconstituted according to the manufacturers’ recommendations.
MICs for the VISA strain were determined by a National Committee for Clinical Laboratory Standards-recommended broth microdilution method (with cation [Ca2+ and Mg2+]-supplemented Mueller-Hinton [CSMH] broth [Difco, Detroit, Mich.] plus 2% NaCl) as previously described (3). The final VISA inoculum was 106 CFU/ml (to mirror readily achievable staphylococcal vegetation densities in the endocarditis model [13]). The antibiotic concentration range tested was 0.125 to 128 μg/ml, encompassing the concentrations in serum achieved by these agents in experimental infective endocarditis (IE) when they were administered in the dose regimens used in this study (see below). The MICs were defined as the lowest antibiotic concentrations yielding no visible growth after 24 h of incubation at 32°C. The capacity of sulbactam to enhance the growth inhibitory effects of ampicillin against the VISA strain were evaluated with the broth microtiter dilution system. Antibiotic ranges tested were 0.125 to 128 μg/ml for ampicillin and 0.0625 to 64 μg/ml for sulbactam, to parallel that of the clinically available formulation of this agent (Unasyn), which provides a 2:1 drug ratio. An enhanced growth inhibitory effect was defined as a reduction in both ampicillin and sulbactam MICs by at least fourfold by the drug combination (13).
The comparative in vitro bactericidal effects of the various study drugs were delineated by the timed-kill curve method. A final inoculum of 106 CFU/ml of logarithmio-phase VISA cells was incorporated into CSMH broth plus 2% NaCl. The final antibiotic concentrations represented five times the in vitro MICs as determined above. For ampicillin-sulbactam combinations, the concentration of each individual drug used was based on the MIC results for the drugs in combination, as defined above. For the vancomycin-amikacin combination, the concentration of each individual drug was based on the results of the MIC studies described above. At 0, 4, 6, and 24 h of incubation at 32°C, 100 μl from each growth tube was quantitatively cultured in CSMH agar (plus 2% NaCl) for an additional 48 h and the numbers of surviving CFU were counted. A decline of ≥3 log10 CFU/ml after 24 h of incubation (versus the 0-h bacterial counts) was considered evidence of a bactericidal effect (13, 19).
The rabbit model of catheter-induced IE was used to evaluate therapeutic efficacy in this study as previously described (13, 19). Twenty-four hours after aortic-valve catheterization, animals were challenged intravenously (i.v.) with the 95%-infective-dose inoculum for the VISA strain as determined in pilot studies (∼2 × 106 CFU). Twenty-four hours postchallenge, blood samples were cultured to document induction of IE. Animals were then randomized to receive either no therapy or their first antibiotic treatment.
The pharmacokinetics of all the antibiotics in the dose regimens used in this study have previously been determined with the rabbit IE model and were not repeated (2, 3, 6, 9, 13, 19). Moreover, all antibiotic regimens were designed to attain peak levels in plasma above the MICs for all agents against the infecting VISA strain (2, 3, 6, 9).
Animals received either no therapy (controls) or one of the following antibiotic regimens for 3 days: trovafloxacin (25 mg/kg of body weight i.v., administered twice a day [b.i.d.] as the prodrug alatrofloxacin), vancomycin (15 mg/kg i.v., administered b.i.d.) alone or with amikacin (7.5 mg/kg intramuscularly [i.m.], administered b.i.d.), or ampicillin (200 mg/kg i.m., administered three times a day) plus sulbactam (20 mg/kg i.m., administered b.i.d.).
For assessment of treatment efficacy, all animals were sacrificed by i.v. sodium pentobarbital overdosage at least 24 h after the last drug dose, to minimize antibiotic carryover effects in vivo. At the time of sacrifice, proper catheter placement across the aortic valve was confirmed. Only animals with proper catheter placement and macroscopic vegetations on the aortic valve were further analyzed. All vegetations from a single animal were removed, weighed, homogenized, serially diluted, and quantitatively cultured. The serial-dilution strategy further minimized potential antibiotic carryover effects. For calculation of the median and mean bacterial densities per gram of vegetation, culture-negative vegetations were assigned a value based on vegetation weight and the lower limit of detection in CFU per gram (13, 19). For randomly selected vegetation homogenates from untreated controls, parallel plating for quantitative culture was performed with untreated or vancomycin (2 μg/ml)-containing CSMH agar plates to confirm retention of the VISA phenotype in vivo.
Fisher’s exact test was used for comparing proportional data, while Kruskal-Wallis analysis of variance with the Tukey post hoc correction for multiple comparisons was used for comparing differences between median vegetation staphylococcal densities. P values of ≤0.05 were considered statistically significant.
The MICs (in micrograms per milliliter) for the VISA strain were 8 for vancomycin, 64 for ampicillin, >128 for sulbactam, 0.5 for amikacin, and 2 for trovafloxacin (the trovafloxacin MIC was within the Food and Drug Administration-approved susceptible range [MIC breakpoint, ≤2 μg/ml] determined in 1998). Synergistic growth inhibition was exhibited against the VISA strain by the combination of ampicillin and sulbactam at plasma-achievable levels for both antibiotics in this experimental model (16 and 8 μg/ml, respectively).
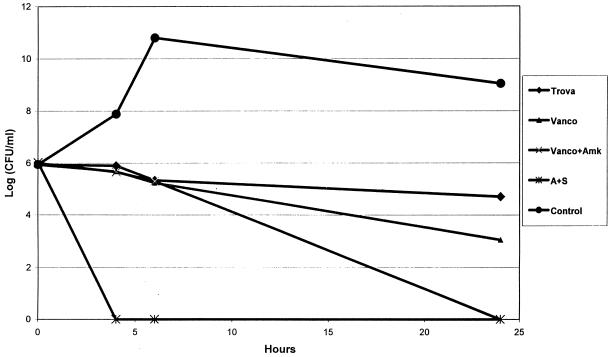
In timed-kill curves, ampicillin plus sulbactam exerted rapid and substantial bactericidal effects in vitro over the 24-h incubation period at five times the MIC (a mean decrease of 6 log10 CFU/ml by 4 h of incubation) (Fig. 1). In contrast, both trovafloxacin and vancomycin exerted slow and incomplete bactericidal effects (mean decreases of 1.3 and 2.6 log10 CFU/ml, respectively, by 24 h of incubation). This same slow in vitro bactericidal effect with trovafloxacin was observed at eight times the MICs (data not shown). Amikacin alone yielded a rapid bactericidal effect at 6 h of incubation; however, rapid regrowth was observed by 24 h of incubation (data not shown), and phenotypically small-colony variants were commonly observed at this time point. The addition of amikacin to vancomycin yielded a synergistic reduction in VISA density compared to that produced by vancomycin alone by 24 of incubation. In the untreated control animals, the VISA phenotype was retained during in vivo passage, with vegetation densities on antibiotic-free and vancomycin (2 μg/ml)-containing media generally being within ∼1 log10 CFU/g of each other. For the VISA strain, only the ampicillin-sulbactam regimen was active in terms of significant reductions in intravegetation densities compared to those of untreated controls (Table 1). Moreover, the proportion of vegetations from animals treated with this regimen that were rendered culture negative (85%) was significantly higher than those of vegetations from animals treated with the other antibiotic regimens (0%; P < 0.005). Although not reaching statistical significance, the addition of amikacin to vancomycin yielded a modest reduction in VISA vegetation densities, compared to those of animals treated with vancomycin alone (∼3-log10-CFU/g decrease).
FIG. 1.
Timed-kill curve of VISA MU-50 (inoculum, 106) versus the studied antibiotics, each at five times the MIC. Trova, trovafloxacin; Vanco, vancomycin; Vanco+Amk, vancomycin plus amikacin; A+S, ampicillin plus sulbactam; Control, medium alone. Data points represent the means of results from two independent assays performed on different days.
TABLE 1.
Efficacies of vancomycin, trovafloxacin, and ampicillin-sulbactam in experimental IE due to VISA MU-50 and vegetation densities
| Antibiotic | No. of animals | Mean log10 CFU/g ± SD | Median log10 CFU/g | No. of sterile vegetations/total no. of vegetations (%) |
|---|---|---|---|---|
| Control (no drug) | 12 | 9.2 ± 1.2 | 9.4 | 0/12 (0) |
| Vancomycin | 9 | 9.4 ± 1.4 | 9.6 | 0/9 (0) |
| Vancomycin + amikacin | 11 | 6.2 ± 2.4 | 7.1 | 0/11 (0) |
| Trovafloxacin | 11 | 8.4 ± 1.5 | 8.9 | 0/11 (0) |
| Ampicillin + sulbactam | 14 | 2.2 ± 0.4a | 2.0 | 12/14 (85)b |
P =0.05 versus vancomycin plus amikacin. P < 0.0001 versus vancomycin and trovafloxacin.
P < 0.005 versus all other regimens.
VISA strains have been shown to be relatively common in selected geographic locales. For example, Hiramatsu et al. (11) demonstrated, in a survey of more than 1,000 ORSA isolates from more than 200 Japanese hospitals and clinics, that ∼10% of such strains exhibited the VISA phenotype. The mechanisms underlying the VISA phenotype are incompletely delineated to date. However, VISA strains share the following phenotypic characteristics: (i) decreased cell wall autolytic activity in the face of normal or supranormal cell wall synthetic rates (20) (this dysregulation of cell wall turnover results in excessively thick and irregular cell walls in VISA strains morphologically [20]); (ii) increased expression of penicillin-binding proteins (17); (iii) a nonhydrolytic trapping capacity for glycopeptide antibiotics, in which situation the glycopeptide agent retains bioactivity and yet is prevented from reaching its synthetic target (i.e., cell wall synthetic sites close to the cytoplasmic membrane [20]); and (iv) lack of the vanA, vanB, or vanC genotype that is the sine quo non of vancomycin-resistant enterococci (18).
In this study, we evaluated the efficacies of trovafloxacin, ampicillin-sulbactam, and vancomycin (alone or in combination with amikacin) in the therapy of experimental VISA IE due to a well-characterized, heterotypic strain (MU-50). The choice of these antibiotic regimens was based on recent experiences with both human and experimental invasive staphylococcal infections: (i) several recent studies, including one from our own laboratory, have confirmed the in vivo efficacy of trovafloxacin in experimental endocarditis caused by oxacillin-susceptible S. aureus and ORSA strains, as well as oxacillin-resistant Staphylococcus epidermidis (ORSE) strains (3, 14, 15); (ii) studies from our laboratory have demonstrated the efficacy of high-dose ampicillin-sulbactam regimens in the therapy and prophylaxis of experimental ORSA and ORSE IE (13, 19); and (iii) in several published cases of VISA infection (7, 8, 12), patients were cured with appropriate debridement and/or catheter removals plus antibiotic therapy with ampicillin-sulbactam regimens combined with the aminoglycoside arbekacin. Since arbekacin is unavailable in the United States but is closely related to amikacin, the latter agent was used in our investigation.
Several notable findings emerged from this study. As expected, vancomycin monotherapy was ineffective at reducing VISA vegetation densities. In contrast, the combination of amikacin and vancomycin resulted in a modest reduction in VISA vegetation densities, paralleling the reduction in VISA densities achieved in vitro by the addition of amikacin to vancomycin in timed-kill curves. Although the aberrantly thick cell walls of VISA strains appear to nonhydrolytically trap vancomycin, it is conceivable that sufficient levels of the agent reach the target sites to induce facilitated aminoglycoside uptake (16). Moreover, it should be emphasized that the treatment course in the present study was only 3 days; it is plausible that a longer treatment regimen with the drug combination may well have yielded an even more salutary outcome against VISA IE. Despite the efficacy of trovafloxacin in several recent investigations of oxacillin-susceptible S. aureus, ORSA, and ORSE IE (3, 14, 15), this agent had little demonstrable in vivo efficacy against VISA IE caused by the MU-50 strain. It should be emphasized that in those studies, the infecting staphylococcal strains were more susceptible in vitro to trovafloxacin than the MU-50 VISA strain (MICs ranging from 0.06 to 1 μg/ml versus 2 μg/ml, respectively). It is not known whether the abnormally thick cell wall is capable of trapping trovafloxacin as it does vancomycin. The combination of ampicillin and sulbactam was highly active both in vitro and in vivo against the VISA strain in the present investigation, paralleling its excellent efficacy in previous models of IE due to other staphylococcal strains (3, 13, 19).
Because of the abnormally thick cell walls of VISA strains, which apparently act as a sink for vancomycin, novel treatment strategies directed against the cell wall may be considered in the future. In this regard, Climo et al. (10) and Patron et al. (18a) have recently shown that lysostaphin, a 27-kDa peptidase that specifically cleaves the pentaglycine cross-links unique to the cell wall of S. aureus, was significantly more active in vivo at reducing vegetation densities of an ORSA strain and a VISA strain, respectively, than vancomycin alone in the rabbit IE model. If the efficacy of lysostaphin is confirmed for VISA strains in this model, this agent may prove useful, either alone or in combination with conventional antibiotic agents, in the therapy of invasive VISA infections.
Acknowledgments
This study was supported in part by a research grant from Pfizer Inc., New York, N.Y.
REFERENCES
- 1.Bayer A S, Crowell D, Bradley D, Yih J, Norman D C. Differential antimicrobial pharmacokinetics and pharmacodynamics in right-sided versus left-sided vegetations in experimental Pseudomonas aeruginosa endocarditis. J Infect Dis. 1988;158:355–359. doi: 10.1093/infdis/158.2.355. [DOI] [PubMed] [Google Scholar]
- 2.Bayer A S, Lam K. Efficacy of vancomycin plus rifampin in experimental aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis. 1985;151:157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- 3.Bayer A S, Li C, Ing M. Efficacy of trovafloxacin, a new quinolone antibiotic, in experimental staphylococcal endocarditis due to oxacillin-resistant strains. Antimicrob Agents Chemother. 1998;42:1837–1841. doi: 10.1128/aac.42.7.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer A S, Norman D C. Valve-site specific pathogenetic differences between right-sided and left-sided bacterial endocarditis. Chest. 1990;98:200–205. doi: 10.1378/chest.98.1.200. [DOI] [PubMed] [Google Scholar]
- 5.Bayer A S, Norman D C, Chiu C Y, Nast C. Pathogenic effects of neutropenia, monocytopenia and steroid treatment in experimental Pseudomonas endocarditis. Chemotherapy. 1989;35:278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- 6.Bayer A S, Tu J. Chemoprophylactic efficacy against experimental endocarditis caused by a β-lactamase-producing, aminoglycoside-resistant enterococci is associated with prolonged serum inhibitory activity. Antimicrob Agents Chemother. 1990;34:1068–1074. doi: 10.1128/aac.34.6.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46(33):765–766. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46(35):813–815. [PubMed] [Google Scholar]
- 9.Choi C, Bayer A S, Fujita N K, Lam K, Guze L B, Yoshikawa T T. Therapy of experimental Pseudomonas endocarditis with high-dose amikacin and ticarcillin. Cheotherapy. 1983;29:303–312. doi: 10.1159/000238213. [DOI] [PubMed] [Google Scholar]
- 10.Climo M W, Patron R L, Goldstein B P, Archer G L. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob Agents Chemother. 1998;42:1355–1360. doi: 10.1128/aac.42.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hore S, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Hirano L, Bayer A S. β-Lactam–β-lactamase–inhibitor combinations are active in experimental endocarditis caused by β-lactamase-producing oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1991;35:685–690. doi: 10.1128/aac.35.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz G W, Seo S M, Aeschlimann J R, Houlihan H H, Mercier R-C, Rybak M J. Efficacy of trovafloxacin against experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1998;42:254–256. doi: 10.1128/aac.42.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y S, Liu Q, Chow L L, Chambers H F, Tauber M G. Comparative efficacy of trovafloxacin in experimental endocarditis caused by ciprofloxacin-sensitive, methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:3325–3327. doi: 10.1128/aac.42.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moellering R C, Jr, Wennersten C, Weinberg A N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971;77:790–800. [PubMed] [Google Scholar]
- 17.Moreira B, Boyle-Vavra S, deJonge B L M, Daum R S. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray B E. Vancomycin-resistant enterococci. Am J Med. 1997;101:284–293. doi: 10.1016/S0002-9343(99)80270-8. [DOI] [PubMed] [Google Scholar]
- 18a.Patron R L, Climo M W, Goldstein B P, Archer G L. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 1999;43:1754–1755. doi: 10.1128/aac.43.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos M C, Ing M, Kim E, Witt M D, Bayer A S. Ampicillin-sulbactam is effective in prevention and therapy of experimental endocarditis caused by β-lactam-producing coagulase-negative staphylococci. Antimicrob Agents Chemother. 1996;40:97–101. doi: 10.1128/aac.40.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieradski K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent J, Venitz J, Teng R, Baris B A, Willavize S A, Polzer R J, Friedman H L. Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J Antimicrob Chemother. 1997;39:75–80. doi: 10.1093/jac/39.suppl_2.75. [DOI] [PubMed] [Google Scholar]