Abstract
Background:
Nicotine and illicit stimulants are very addictive substances. Although associations between grey matter and dependence on stimulants have been frequently reported, white matter correlates have received less attention.
Methods:
Eleven international sites ascribed to the ENIGMA-Addiction consortium contributed data from individuals with dependence on cocaine (n = 147), methamphetamine (n = 132) and nicotine (n = 189), as well as non-dependent controls (n = 333). We compared the fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) of 20 bilateral tracts. Also, we compared the performance of various machine learning algorithms in deriving brain-based classifications on stimulant dependence.
Results:
The cocaine and methamphetamine groups had lower regional FA and higher RD in several association, commissural, and projection white matter tracts. The methamphetamine dependent group additionally showed lower regional AD. The nicotine group had lower FA and higher RD limited to the anterior limb of the internal capsule. The best performing machine learning algorithm was the support vector machine (SVM). The SVM successfully classified individuals with dependence on cocaine (AUC = 0.70, p < 0.001) and methamphetamine (AUC = 0.71, p < 0.001) relative to non-dependent controls. Classifications related to nicotine dependence proved modest (AUC = 0.62, p = 0.014).
Conclusions:
Stimulant dependence was related to FA disturbances within tracts consistent with a role in addiction. The multivariate pattern of white matter differences proved sufficient to identify individuals with stimulant dependence, particularly for cocaine and methamphetamine.
Keywords: Addiction, DTI, FA, Myelin, Machine learning
1. Introduction
Substance abuse has negative consequences for health and entails a heavy economic and societal burden (Degenhardt et al., 2018). Despite worldwide efforts in reducing its consumption, nicotine remains one of the most used legal drugs and is the leading cause of preventable and premature death due to smoking-related conditions such as cancer, respiratory, and cardiovascular illnesses (West, 2017). While less common, the use of illicit stimulants is on the rise (Center for Behavioral Health Statistics and Quality, 2018). Cocaine and methamphetamine are both very addictive and neurotoxic substances (Gonçalves et al., 2014). Whilst grey matter alterations relative to nicotine (Fritz et al., 2014; Hanlon et al., 2016; Kaag et al., 2018; Kuhn et al., 2010; Mackey et al., 2019; Wetherill et al., 2013) and illicit stimulants have been widely studied (Hall et al., 2015; Mackey et al., 2019; Mackey and Paulus, 2013; Yang et al., 2020), differences in white matter have received less attention.
Diffusion-tensor imaging (DTI) has been broadly used to assess white matter composition (Basser et al., 1994), and assumes that water diffusion within tissue is anisotropic, or highly coherent, due to the physical boundaries myelin sheaths impose (Basser et al., 1994). Fractional anisotropy (FA) considers the ratio of parallel to perpendicular diffusion. Values closer to 1 suggest greater anisotropy such that water is diffused in parallel to the tract’s predominant direction. Tracts can be ordered based on such direction as commissural (left-right, and vice versa), projection (top-down), or associative tracts (anterior-posterior) (Mori et al., 2008). While FA is very sensitive to microstructural differences it does not illuminate the exact source. Hence, incorporating other metrics may help in understanding the nature of white matter disruptions. For example, parallel or axial diffusivity (AD) is sensitive to axonal injury, perpendicular or radial diffusivity (RD) reflects myelin density. The average of AD and RD, mean diffusivity (MD), reveals the amount of diffused water irrespective of direction and may hint at edema or swelling (Alexander et al., 2007).
Findings on DTI for nicotine dependence are controversial as studies have shown both higher and lower FA among tobacco smokers (Huang et al., 2020; Van Ewijk et al., 2015; Wang et al., 2017; Yu et al., 2016; Zhang et al., 2010, 2013). It has been proposed that higher FA might be related to the age of onset of smoking and be transient (Gogliettino et al., 2016; Kochunov et al., 2013). Findings in cocaine and methamphetamine are less ambiguous since both have been related to lower FA (Huang et al., 2020; Lederer et al., 2016; Suchting et al., 2020). Still, most of the studies on illicit stimulants are likely underpowered and focus on a priori selected tracts to avoid multiple comparisons issues. In the current work, authors joined the ENIGMA-Addiction consortium’s data pooling initiative (https://www.ENIGMAaddiction.com) that provides larger sample sizes to both replicate and extend findings from the literature. Additionally, we tested a variety of machine learning algorithms to assess whether DTI-derived metrics can discriminate substance dependent individuals from controls. Machine learning implementation in substance dependence research has received increased attention in recent years (Barenholtz et al., 2020; Mak et al., 2019). However, the few studies that have tested brain-based classifications for stimulant dependence focused on other MRI modalities (Li et al., 2019; Mackey et al., 2019; Mete et al., 2016; Wetherill et al., 2019). Unlike conventional mass-univariate approaches, multivariate-based classification methods may detect interactions and non-linear relations that would otherwise pass unnoticed.
2. Materials and methods
2.1. Participants
Eleven sites from the ENIGMA-Addiction consortium contributed 14 studies with 808 participants for this project, including non-dependent controls (n = 333) and individuals with dependence on cocaine (n = 154), methamphetamine (n = 132), and nicotine (n = 189). A variety of tools served to diagnose substance dependence (see Table ST1 in the Supplementary materials). Participants were grouped according to their primary substance of choice and required to not meet any other axis-I psychiatric diagnoses, neurological diseases, or additional dependencies besides nicotine (see Table 1). Long-term abstinent individuals (> 365 days) were excluded (n = 7, all from the cocaine group; n = 147) to avoid confounding effects of recovery. This work was carried out under the code of ethics of the World Medical Association (Declaration of Helsinki). All sites obtained local ethical review and informed consent from all participants.
Table 1.
Age and sex distribution of each group (mean, SD).
| N | Age | Range | Females | |
|---|---|---|---|---|
| Control group | 333 | 30.7 (9.76) | 18 – 55 | 141 |
| Cocaine dependent group | 147 | 39.8 (9.14) | 19 – 58 | 22 |
| Subset co-dependent on nicotine | 109 | 39.0 (9.22) | 19 – 58 | 16 |
| Methamphetamine dependent group | 132 | 30.9 (7.67) | 18 – 54 | 46 |
| Subset co-dependent on nicotine | 115 | 31.2 (7.77) | 18 – 54 | 35 |
| Nicotine dependent group | 189 | 29.0 (9.94) | 18 – 54 | 81 |
2.2. Diffusion MRI acquisition and processing
Scanner and protocol details can be found in ST1. Eddy currents and b0 distortions were removed at each site accordingly with the ENIGMA DTI protocols (http://enigma.ini.usc.edu/protocols/dti-protocols/). After tensor fitting, scalar maps were eroded, registered, and projected to the ENIGMA’s template and skeleton (Jahanshad et al., 2013) as part of the Tract-based Spatial Statistics (TBSS) pipeline. Registration, vectors’ orientation, and projection distances to the skeleton were locally inspected. Following ENIGMA-DTI protocols, the average diffusivity metrics (i.e., FA, AD, RD, and MD) were derived from 43 tracts (i.e., 5 bilateral and 38 lateralized) in accordance with the ICBM-DTI-81 atlas (Mori et al., 2008). The corpus callosum, the internal capsule, and the corona radiata were removed in favor of their divisions (e.g., body, genu, and splenium of the corpus callosum). The inferior fronto-occipital fasciculus was excluded because of well-known issues in the ENIGMA DTI protocol with this tract (see ENIGMA-DTI protocol link for more details), i.e., one bilateral and three lateralized tracts were excluded (n = 7). Lateralized tracts (n = 32) were bilaterally averaged (n = 16) generating a total of 20 tracts to examine. Scanner influence was adjusted with ComBat while preserving group, age, age2, and sex effects. ComBat uses an empirical Bayes framework to improve the variance of the parameter estimates and has proven robust in settings where the biological covariate of interest (e.g., group) is not well-balanced across sites (Fortin et al., 2017). This approach has been recently used in various work from the ENIGMA consortium (Favre et al., 2019; Hatton et al., 2020; Villalón-Reina et al., 2020).
2.3. Statistical analyses
2.3.1. Univariate group comparisons
Linear regression models included the mean FA of each tract as the dependent variable. The main predictor, group, was a 4-level factor variable where non-dependent controls were the reference group. Age, age2, and sex were entered as covariates to account for linear and non-linear effects (Kochunov et al., 2012). All statistical analyses were performed using R version 4.1.0 (R Core Team, 2018). Assumptions of normality and homoscedasticity of residuals were examined for each model with Shapiro’s and Levene’s tests. Robust models were conducted if any of these assumptions were violated with the robustbase package (Maechler et al., 2021). The residuals of the FA of the posterior corona radiata and the splenium of the corpus callosum, and the residuals of the MD of the uncinate fasciculus were further tested with robust regressions as they violated the homoscedasticity assumption. Effect sizes were calculated using the effectsize package (Ben-Shachar et al., 2020). Results were plotted with the ggseg3d package (Mowinckel and Vidal--Piñeiro, 2019). All the resulting p-values from each test (i.e., three predefined contrasts [cocaine, methamphetamine, and nicotine vs non-dependent controls] for 20 tracts totaling 60 tests) were further corrected with a False Discovery Rate (FDR) adjustment (Benjamini and Hochberg, 1995). Significance level was set as p-corrected (q) < 0.05. Separate post-hoc tests were conducted on AD, RD, and MD on tracts where groups differed for FA. These p-values did not include further adjustments as they were deemed in-depth analyses to explore the underlying sources of differences in FA.
Additional comparisons were performed on tracts where the illicit stimulant dependent groups differed from non-dependent controls. Concretely, a subsample of individuals with dependence on cocaine (n = 108) and methamphetamine (n = 115) and co-dependence on nicotine was compared with the nicotine group to test if differences with controls were influenced by comorbid nicotine dependence. Furthermore, we repeated the main analysis with the lateralized version of all tracts to test for laterality effects (n = 36; all FDR-corrected).
2.3.2. Multivariate machine learning classifiers
Base out-of-sample performance was calculated for each classification task according to a 5-fold cross-validation (CV) scheme, where the ratio of case to control was matched between all training and validation sets. The main classifier examined was a support vector machine (SVM) with radial basis kernel function-based pipeline and a front-end robust scaler, where each feature, i.e., the site-adjusted FA, RD, AD and MD values of 20 tracts, was standardized by first removing the median and next scaled according to the 5th and 95th percentiles of its distribution (Amari and Wu, 1999). Age, age2, and sex were residualized to prevent estimations based on non-brain data (Schwarz et al., 2019). Hyper-parameters for the SVM, the strength of regularization as well as the kernel coefficient, were selected by a random hyper-parameter search. Sixty combinations were tested with a nested 3-fold CV. The best performing combination was used to train the final evaluated SVM following the 5-fold CV. Moreover, other machine learning pipelines (i.e., regularized logistic regressions, light gradient boosting, random forest, and AutoGluon AutoML) were compared to the SVM to validate its choice. More details are available in the supplementary materials (SM7). The average area under the receiving operating curve (AUC) of each machine learning algorithm, representing its performance, is reported. Feature importance of each SVM estimation was computed based on the greatest change in cost function after removing individual features (Guyon et al., 2002). Machine learning algorithms were implemented and evaluated with the python-based brain predictability toolbox (Hahn et al., 2021).
Permutation tests (Golland and Fischl, 2003; Noirhomme et al., 2014) were done to establish the statistical significance of each cross-validated classification (i.e., a certain classification was re-evaluated after labels were randomly permuted). The significance was calculated by comparing the average k-fold performance from the real dataset relative to the average k-fold performance from the randomly permuted dataset (i.e., the rank of the real average score within the sorted null distribution scores is calculated and converted to a p-value). Due to the unbalanced structure unique to this multi-site dataset, permutations were constrained to participants from the same imaging site because of concerns about case or control-only sites (Dinga et al., 2020).
3. Results
Relative to non-dependent controls, the cocaine dependent group had lower FA in the posterior thalamic radiation (t794 = −2.91, q = 0.025, d = −0.21 [−0.35, −0.07]), the retrolenticular part of the internal capsule (t794 = −3.59, q = 0.006, d = −0.26 [−0.40, −0.12]), and the sagittal stratum (t794 = −3.12, q = 0.019, d = −0.22 [−0.36, −0.08]) (see Fig. 1). Post-hoc contrasts revealed that individuals with cocaine dependence had higher RD in the retrolenticular part of the internal capsule (t475 = 3.16, p = 0.002, d = 0.29 [0.11, 0.47]) and the sagittal stratum (t475 = 2.12, p = 0.035, d = 0.20 [0.01, 0.38]), and higher MD in the retrolenticular part of the internal capsule (t475 = 2.83, p = 0.005, d = 0.26 [0.08, 0.44]), and the sagittal stratum (t475 = 3.13, p = 0.002, d = 0.29 [0.11, 0.47]). A summary of the results is available in the Supplementary materials (see ST2).
Fig. 1.
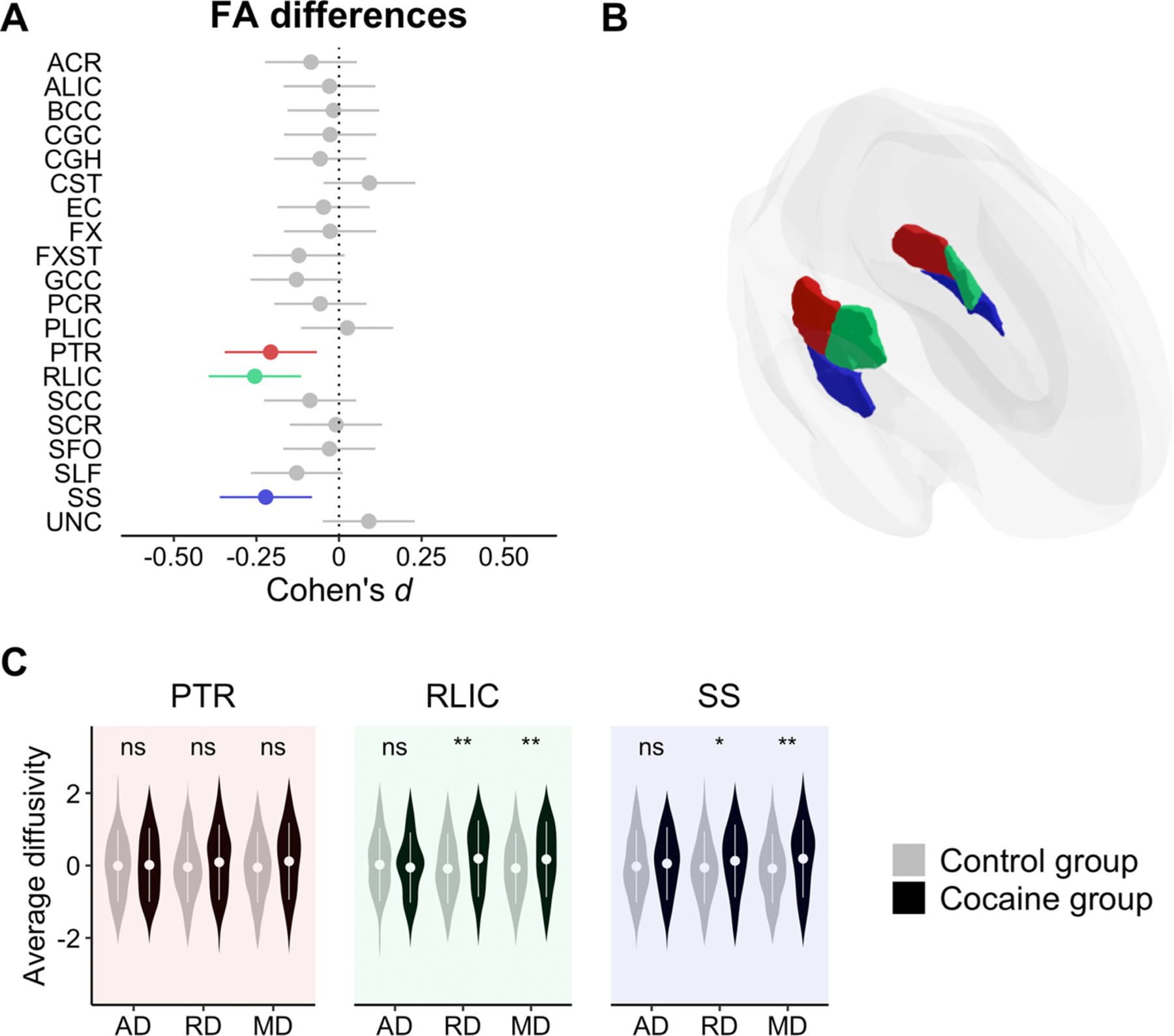
Panel A: Effect sizes and confidence intervals from group comparison. Highlighted bars indicate a significant effect (q < 0.05) of the cocaine dependent group as compared to the control group. Bars falling to the left indicate lower FA in the cocaine dependent group. Panel B: Location of the effects. Panel C: Post-hoc analyses on the rest of the DTI metrics (i.e., AD, RD, and MD along the X-axis) for those tracts showing a significant FA difference (ns, non-significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001). The Y-axis reflects site-adjusted AD, RD, and MD values after being residualizing for sex, age and age2. ACR: Anterior corona radiata, ALIC: Anterior limb of the internal capsule, BCC: Body of corpus callosum, CGC: Cingulum, CGH: Cingulum hippocampal part, CST: Corticospinal tract, EC: External capsule, FX: Fornix, FXST: Fornix stria terminalis, GCC: Genu of corpus callosum, PCR: Posterior corona radiata, PLIC: Posterior limb of the internal capsule, PTR: Posterior thalamic radiation, RLIC: Retrolenticular part of the internal capsule, SCC: Splenium of corpus callosum, SCR: Superior corona radiata, SFO: Superior fronto-occipital fasciculus, SLF: Superior longitudinal fasciculus, SS: Sagittal stratum, UNC: Uncinate fasciculus.
The methamphetamine dependent group had lower FA in the cingulum (t794 = −3.95, q = 0.001, d = −0.28 [−0.42, −0.14]) and its hippocampal part (t794 = −3.92, q = 0.001, d = −0.28 [−0.42, −0.14]), the genu (t794 = −3.60, q = 0.002, d = −0.26 [−0.40, −0.12]) and splenium of the corpus callosum (t794 = −2.93, q = 0.014, d = −0.21 [−0.35, −0.07]; robust linear regression results: t794 = −3.16, p = 0.001, d = −0.22 [−0.36, −0.09]), the posterior thalamic radiation (t794 = −2.36, q = 0.046, d = −0.17 [−0.31, −0.03]), the superior fronto-occipital fasciculus (t794 = −2.39, q = 0.046, d = −0.17 [−0.31, −0.03]), the superior longitudinal fasciculus (t794 = −3.11, q = 0.010, d = −0.22 [−0.36, −0.08]), and the sagittal stratum (t794 = −2.48, q = 0.044, d = −0.18 [−0.32, −0.04]) when compared to non-dependent controls (see Fig. 2). Post-hoc tests shown that the methamphetamine dependent group had lower AD along the cingulum (t460 = −4.08, p < 0.001, d = −0.38 [−0.56, −0.20]), the genu of the corpus callosum (t460 = −2.33, p = 0.020, d = −0.22 [−0.40, −0.03]), and the superior longitudinal fasciculus (t460 = −3.37, p < 0.001, d = −0.31 [−0.50, −0.13]). Individuals from this group showed higher RD in the cingulum (t460 = 2.42, p = 0.016, d = 0.23 [0.04, 0.41]), its hippocampal part (t460 = 4.25, p < 0.001, d = 0.40 [0.21, 0.58]), and the genu (t460 = 2.35, p = 0.020, d = 0.22 [0.04, 0.40]) and splenium of the corpus callosum (t460 = 2.17, p = 0.027, d = 0.21 [0.02, 0.39]). Also, this group showed higher MD in the hippocampal part of the cingulum (t457 = 4.01, p < 0.001, d = 0.37 [0.19, 0.56]) (see ST3).
Fig. 2.
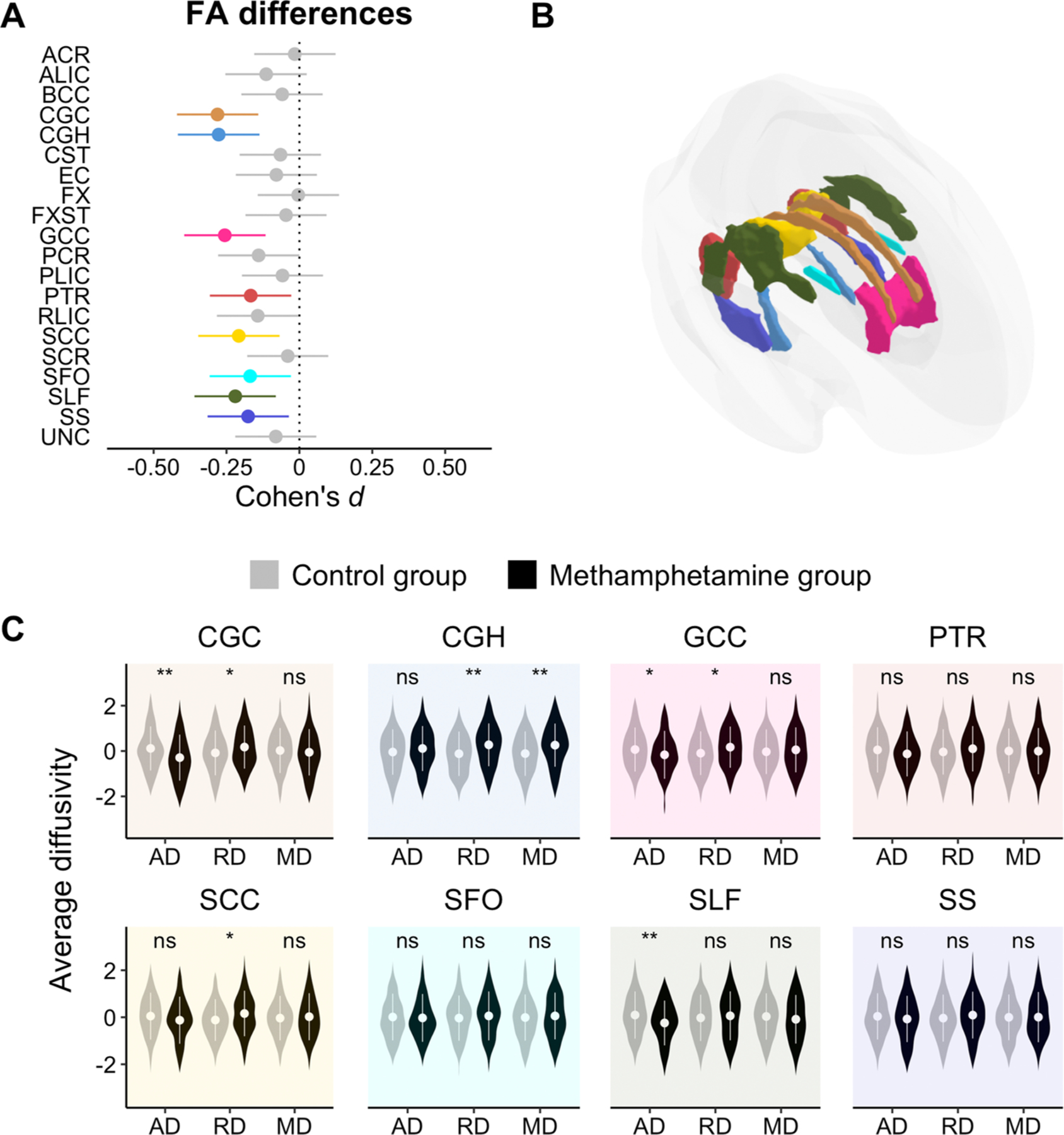
Panel A: Effect sizes and confidence intervals from group comparison. Highlighted bars indicate a significant effect (q < 0.05) of the methamphetamine dependent group as compared to the control group. Bars falling to the left suggest lower FA (negative deviation). Panel B: Location of the effects. Panel C: Post-hoc analyses on the rest of DTI metrics (i.e., AD, RD, and MD along the X-axis) only for those tracts previously showing a significant FA difference (ns, non-significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001). The Y-axis reflects site-adjusted AD, RD, and MD values after being residualizing for sex, age and age2. Tract abbreviations are the same as in Fig. 1.
The nicotine dependent group had lower FA in the anterior limb of the internal capsule (t794 = −3.17, q = 0.032, d = −0.23 [−0.37, −0.09]) (see Fig. 3) in comparison to non-dependent controls. Post-hoc contrasts revealed that individuals with nicotine dependence had higher RD (t517 = 3.57, p < 0.001, d = 0.31 [0.14, 0.49]) and higher MD (t517 = 2.18, p = 0.029, d = 0.19 [0.02, 0.36]) in this tract (see ST4).
Fig. 3.
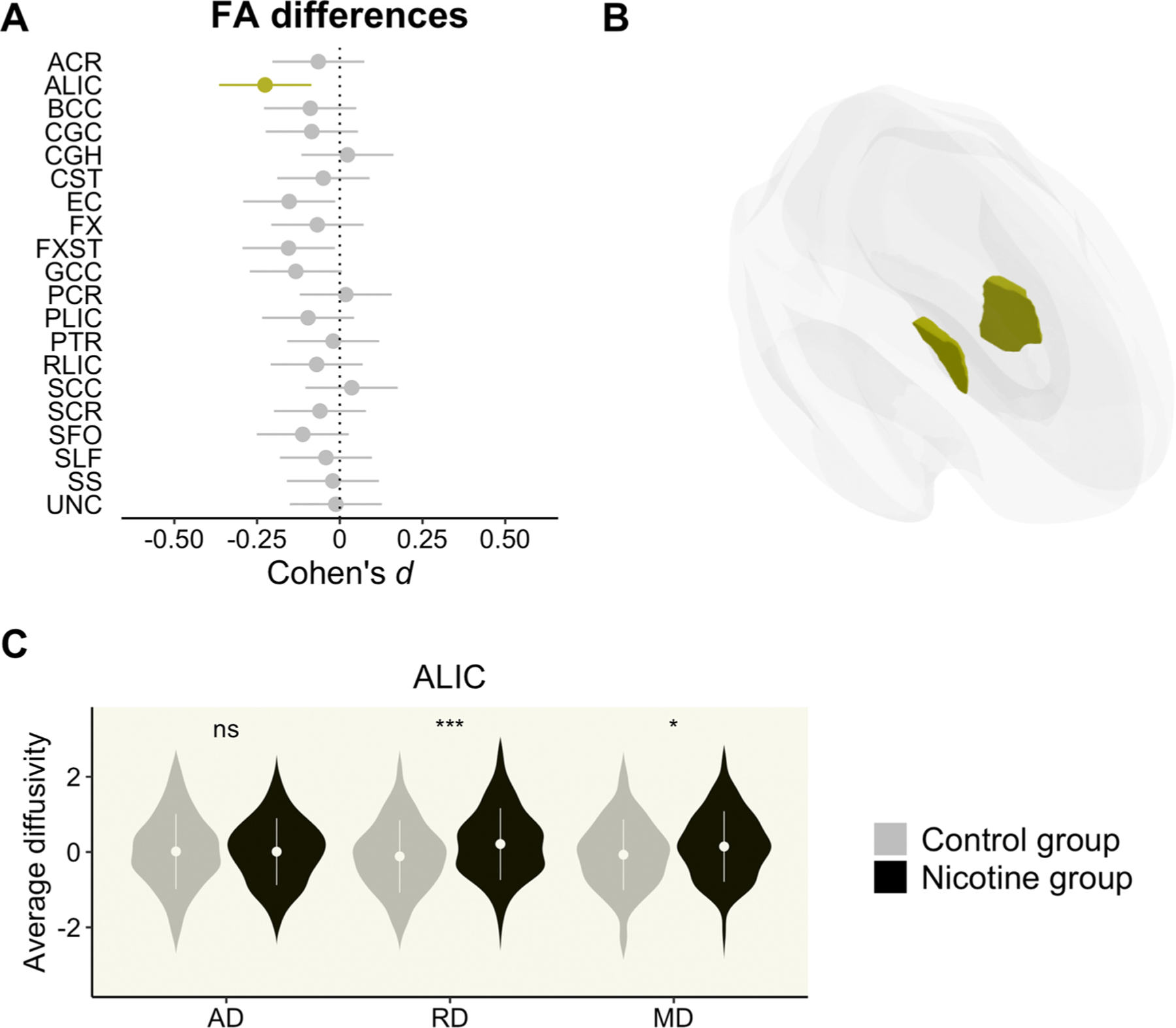
Panel A: Effect sizes and confidence intervals from group comparison. Highlighted bars indicate a significant effect (q < 0.05) of the nicotine dependent group as compared to the control group. Bars falling to the left suggest lower FA (negative deviation). Panel B: Location of the effects. Panel C: Post-hoc analyses on the rest of DTI metrics (i.e., AD, RD, and MD along the X-axis) only for those tracts previously showing a significant FA difference (ns, non-significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001). The Y-axis reflects site-adjusted AD, RD, and MD values after being residualizing for sex, age and age2. Tract abbreviations are the same as in Fig. 1.
Supplementary contrasts showed that the cocaine group had lower FA in the posterior thalamic radiation (t407 = −2.08, p = 0.038, d = −0.21 [−0.40, −0.01]) and the sagittal stratum (t407 = −2.02, p = 0.044, d = −0.20 [−0.40, −0.01]) relative to the nicotine group. Likewise, the methamphetamine group demonstrated lower FA in the cingulum (t407 = −2.12, p = 0.035, d = −0.21 [−0.41, −0.02]), its hippocampal part (t407 = −3.13, p = 0.002, d = −0.31 [−0.51, −0.12]), and the splenium of the corpus callosum (t407 = −2.56, p = 0.011, d = −0.25 [−0.45, −0.06]) compared to the nicotine group.
Results from the laterality analyses can be found in the Supplementary materials (see ST5-ST7). Differences in the cocaine group remained unchanged except for the posterior thalamic radiation whose effects were present in the left portion of the tract only. Results in the methamphetamine group were similar to the main analysis although three additional tracts, the left posterior limb (t794 = −3.11, q = 0.036, d = −0.18 [−0.32, −0. −04]) and the right anterior limb of the internal capsule (t794 = −2.40, q = 0.046, d = −0.17 [−0.31, −0. −03]), and the left posterior thalamic radiation (t794 = −3.11, q = 0.010, d = −0.22 [−0.36, −0. −08]), showed lower FA relative to controls. Nicotine’s group original results (i.e., anterior limb of the internal capsule) did not survive FDR correction (q = 0.06, both left and right portions).
The best performing machine learning algorithm was the SVM, with significant classifications for cocaine dependence (AUC = 0.70, p < 0.001), methamphetamine dependence (AUC = 0.71, p < 0.001), and nicotine dependence (AUC = 0.62, p = 0.014). SVM performance and the importance of each feature are available in Figs. 4 and 5, respectively. The performance of the remaining algorithms and the importance of each feature from the SVM classifications can be found in the Supplementary materials (SM8 and SM9).
Fig. 4.
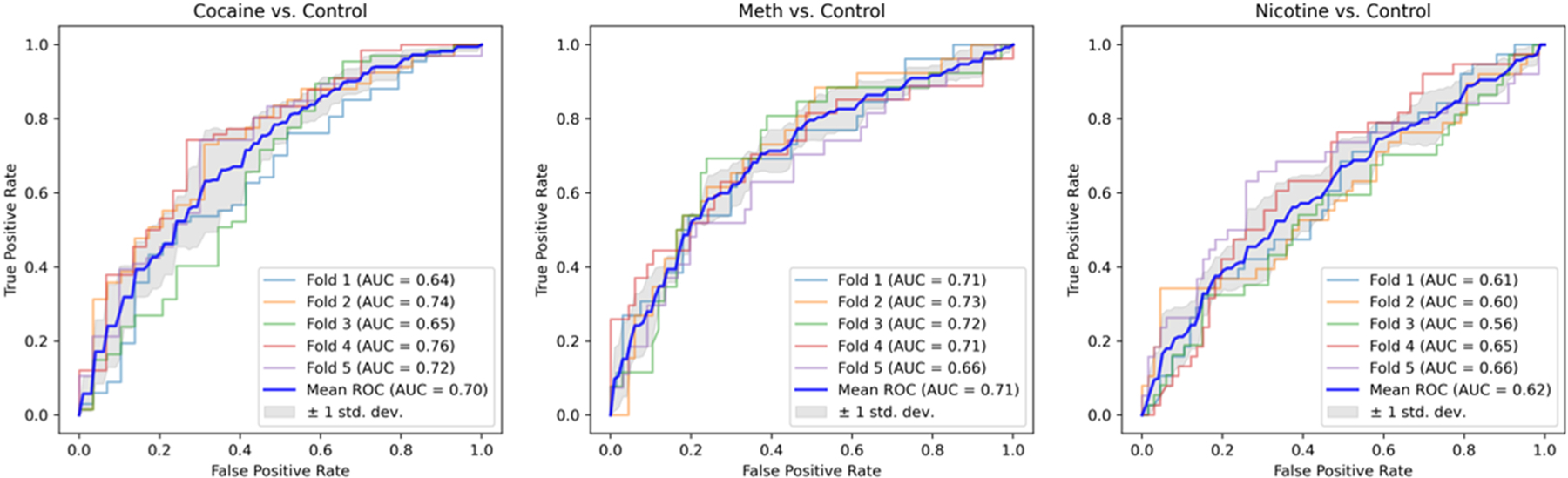
Area under the curve (AUC) of each of the 5-fold CV estimations on the SVM classifier for every group.
Fig. 5.
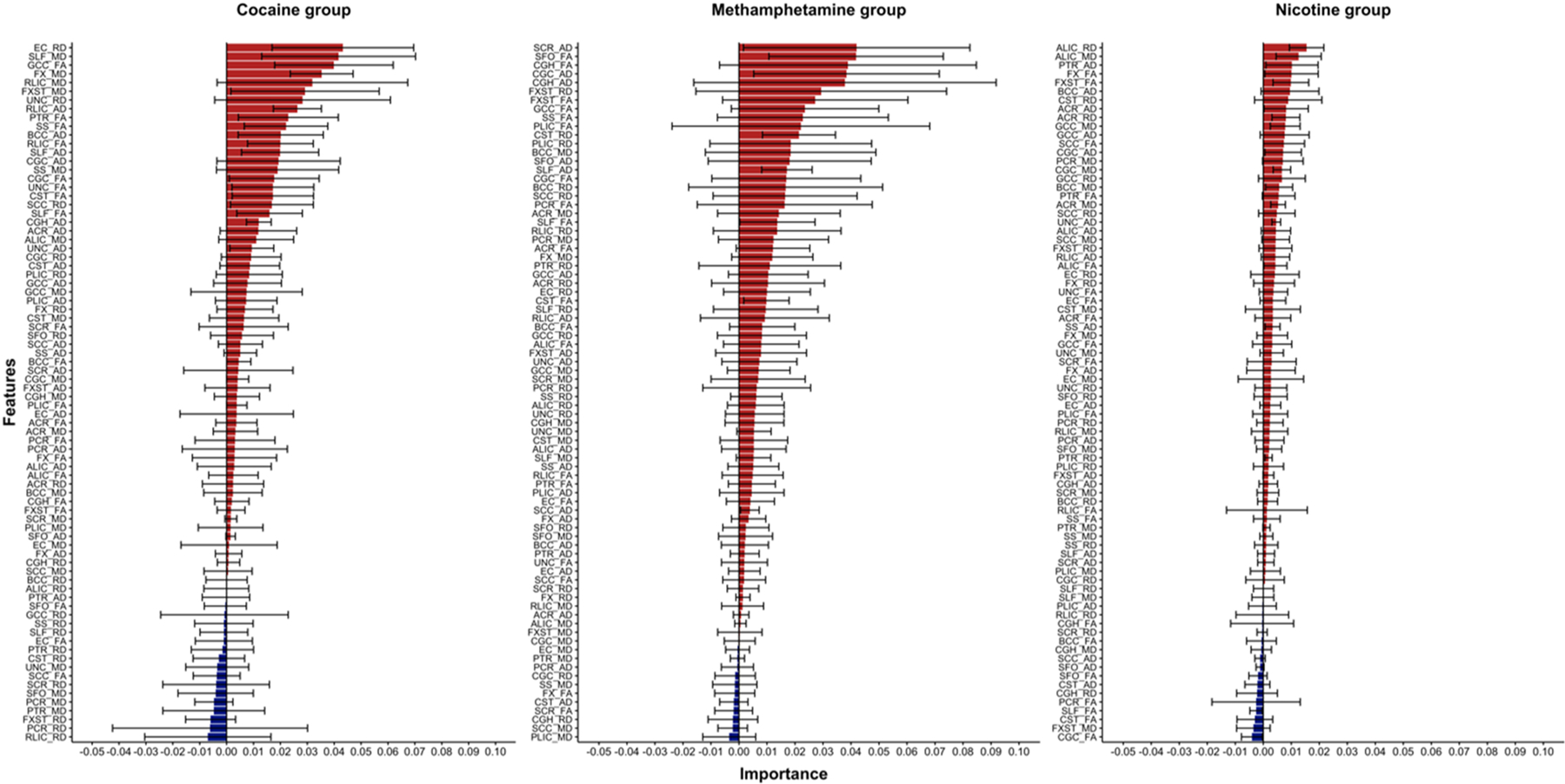
Mean and standard error of the importance of each feature in the SVM classifications. Note that the Y-axis is sorted by importance of each feature and thus it differs between groups. Red and blue colors suggest positive or negative contributions to the average AUC on the classification.
4. Discussion
The study of white matter differences in relation to dependence on nicotine and illicit stimulants has received less attention than grey matter differences. While findings on nicotine dependence are often contradictory, cocaine and methamphetamine dependence work tend to be seriously confounded by comorbid dependence on other drugs and underpowered. Here, we found drug-specific white matter differences in a relatively large sample of individuals dependent on cocaine, methamphetamine, or nicotine. Dependent groups showed lower regional FA compared to non-dependent controls. The greatest differences were observed in methamphetamine dependence. Lower regional FA was found together with higher RD in all groups. Lower regional AD was related to methamphetamine dependence. Finally, we demonstrated that the SVM classifier successfully identified individuals dependent on cocaine and methamphetamine and to a lesser extent nicotine.
The cocaine group had lower FA in projection tracts such as the posterior thalamic radiation, the retrolenticular part of the internal capsule, and the sagittal stratum. While there is limited prior evidence implicating the posterior thalamic radiation in cocaine dependence (Yip et al., 2017), the thalamus has been proposed to play a role in substance use and dependence (Huang et al., 2018). FA differences in the internal capsule have been reported previously in cocaine addiction (He et al., 2020; van Son et al., 2016; Yip et al., 2017) and correlated to long-term abstinence, compulsive-like behaviors, and distress (Kopell and Greenberg, 2008). Lower FA along the sagittal stratum have been related to cocaine dependence (Ma et al., 2017; Yip et al., 2017).
The methamphetamine dependent group showed lower FA in the cingulum and its hippocampal part, the genu and splenium of the corpus callosum, the superior fronto-occipital fasciculus, and the superior longitudinal fasciculus. Similar to the observed cocaine effects, the methamphetamine group had lower FA in the posterior thalamic radiation and the sagittal stratum. While effects in the genu of the corpus callosum have been widely replicated (Huang et al., 2020; Kim et al., 2009; Lederer et al., 2016; Salo et al., 2009; Tobias et al., 2010), differences in the splenium have not. A recent TBSS work also found lower FA in the cingulum, superior longitudinal fasciculus, superior fronto-occipital fasciculus, sagittal stratum, and posterior thalamic radiation in participants with methamphetamine dependence (Huang et al., 2020). Most of these tracts have been implicated in cognitive control and emotion regulation (Bubb et al., 2018; Fitsiori et al., 2011; Kamali et al., 2014). Lower FA within these tracts has been associated with both impulsivity and aggression in methamphetamine dependent individuals (Huang et al., 2020; Lederer et al., 2016). Notably, this group showed the most substantial FA differences. Beyond statistical significance, all differences moved in the same direction suggesting a global pattern of lower FA. Methamphetamine remains longer in the brain than cocaine and blocks dopaminergic reuptake while increasing its release. Thus, it is more neurotoxic and entails a greater risk for serious psychiatric and cognitive sequelae (Yang et al., 2018a, 2018b). Moreover, individuals with methamphetamine dependence are more likely to have abused multiple drugs in their life which can have a severe impact on the brain (Kaag et al., 2017; van Son et al., 2016).
The cocaine and methamphetamine groups had higher RD in tracts showing lower FA, which is in line with prior reports (Huang et al., 2020; Kaag et al., 2017; Lane et al., 2010; Lederer et al., 2016; Salo et al., 2009). Higher RD suggests that lower FA may be linked to demyelination. There are several pathological processes concerning the abuse of such stimulants that are deleterious to myelin (Gonçalves et al., 2014; Pereira et al., 2015; Yang et al., 2018a, 2018b). Both substances reduce glial cell efficiency in regulating glutamate homeostasis (Bachtell et al., 2017; Matute et al., 2007), boost reactive oxygen species presence triggering oxidative stress responses (Lassmann and van Horssen, 2016), and down-regulate myelin expression (Smith et al., 2014). Stimulant-type drugs also cause vasoconstriction and increase the risk of hypoperfusion (Buttner, 2012; Polesskaya et al., 2011), expose the brain to toxins due to blood-brain barrier dysfunctions (Sajja et al., 2016), and prompt neuroinflammation by priming glial cells into relentlessly releasing pro-inflammatory cytokines (Bachtell et al., 2017). The methamphetamine group had lower AD within the cingulum, the genu of the corpus callosum, and the superior longitudinal fasciculus, which points instead to axonal damage. Our results contradict prior work reporting higher AD (Huang et al., 2020; Uhlmann et al., 2016) or null effects (Beard et al., 2019; Breen et al., 2017). Combined with lower FA, bidirectional AD changes might still suggest axonal damage. Concretely, higher AD may hint at neurofilament damage. This would provoke axons to be less tightly packed and lead to a widening of the interstitial space resulting in increases in the amount of water to diffuse (i.e., higher MD, lower FA) (Moore et al., 2018; Winklewski et al., 2018). This pattern of lower FA together with higher AD and MD was seen in Huang et al. (2020) and Uhlmann et al. (2016). Here, we exposed lower AD indicating axonal damage or fragmentation. Aggregation of cellular debris, disordered microtubules, and damaged neurofilaments following axonal injury barricade longitudinal water diffusion overall resulting in lower FA (Aung et al., 2013).
The nicotine group had lower FA in the anterior limb of the internal capsule, a projection tract that connects thalamic, basal ganglia, and prefrontal areas. Prior evidence is conflicting as various studies have reported higher (Jacobsen et al., 2007; Van Ewijk et al., 2015; Yu et al., 2016) and lower FA (Savjani et al., 2014; Wang et al., 2017; Yuan et al., 2018; Zhang et al., 2010) in this tract. The potential benefits of nicotine exposure during adolescence remain a matter of debate. Nicotine appears to promote glial maturation, boost nerve growth factor release, and prevent arachnoid acid-induced injury and apoptosis (Hudkins et al., 2012; Jacobsen et al., 2007; Liao et al., 2011; Van Ewijk et al., 2015; Yu et al., 2016). Other researchers have suggested the effects of nicotine exposure not only fade but reverse as lifetime use escalates and addiction develops (Gogliettino et al., 2016; Paul et al., 2008; Umene-Nakano et al., 2014; Yu et al., 2016). Similarly, lower FA was found together with greater RD (Savjani et al., 2014; Yuan et al., 2018). Studies in mice found that nicotine exposure was related to lower myelin expression (Cao et al., 2013) and other potential myelin insults such as vasoconstriction, oxidative stress, or inflammation (Liao et al., 2011; Sajja et al., 2016).
The best performing machine learning algorithm in the present dataset was the SVM that successfully detected individuals with cocaine and methamphetamine dependence relative to non-dependent controls. Although significant, neither the SVM nor the rest of the algorithms were as successful in classifying nicotine dependence (AUC = 0.62). Of note, the SVM algorithm favored features that were omitted in the mass-univariate tests suggesting that multivariate methods may be more useful in the development of neuroimaging markers of stimulant dependence than those exploring brain regions in isolation. Our results add to a growing body of work that leverages machine learning methods to identify patterns associated with stimulant dependence using MRI data (Li et al., 2019; Mackey et al., 2019; Mete et al., 2016; Wetherill et al., 2019). This is the first application of machine learning using DTI data in relation to stimulant dependence.
Comparisons of the nicotine group with the cocaine and methamphetamine groups revealed that the nicotine group showed higher FA. Differences in FA were localized on tracts where the control group also showed higher FA relative to the cocaine and methamphetamine groups. Nevertheless, the differences between the nicotine and the other stimulant groups were less widespread than those observed in comparison to the control group. Thus, it is possible that being dependent on both nicotine and cocaine or methamphetamine is associated with additional deleterious effects. In a recent report, FA negatively correlated with the number of additional drugs used by cocaine dependent individuals. In that study, however, most participants were co-dependent on nicotine making it difficult to divorce the results from this particular effect (Kaag et al., 2017).
In the present work, we have demonstrated that dependence on stimulants is related to lower regional FA, and that such differences are more prominent in the cocaine and methamphetamine dependent groups. We based our analyses on tracts extracted according to the ICBM-DTI-81 atlas (Mori et al., 2008) to facilitate comparisons of results to other ENIGMA disease working groups (Favre et al., 2019; Hatton et al., 2020; Villalón-Reina et al., 2020). Most ROI-based studies on substance dependence have adopted manual segmentation approaches on a priori selected tracts, such as the corpus callosum in methamphetamine dependence research, that limit sample size and delays replication. We offer new evidence on white matter differences related to methamphetamine dependence beyond the corpus callosum. Also, we assessed other DTI-derived metrics to clarify the underlying sources of lower FA, which is often overlooked or explored in ways precluding interpretation (i.e., when lacking FA differences). Additionally, we confirmed the potential of multivariate-based machine learning methods using DTI-derived data to classify dependence on stimulants. The current work also has several limitations. Besides demyelination and axonal injury, differences in FA may also emerge from non-pathological sources such as fiber alignment differences or the presence of crossing fibers (Jones et al., 2013). Therefore, we cannot confirm that differences in FA were solely related to demyelination or axonal damage. With cross-sectional data, it is also possible that the observed effects existed before any drug exposure. To control the number of tests, we used bilaterally averaged tracts that may have masked lateralized effects. However, we showed that the pattern of effects remained similar using lateralized tracts. The cocaine group was significantly older and thus age was controlled in all the analyses. We additionally tested whether results changed with an age-matched subsample of controls (n = 147, 38.64 ± 8.48 age) and effects remained unaltered. Another limitation was the number of males within the cocaine group (n = 125, 85%). Although sex was controlled in all the analyses, we did not have sufficient statistical power to explore sex-specific effects. The comorbid dependence on nicotine in the cocaine (74%) and methamphetamine (87%) groups made it difficult to isolate the effects of being dependent on illicit stimulants from those of being dependent on nicotine too. However, similar effects were observed when comparing a subset of individuals with co-dependence on illicit stimulants and nicotine with the nicotine dependent only group and the non-dependent control group. Finally, and despite excluding individuals with dependence on other substances, information about recreational or sub-clinical use of other drugs was unavailable at most sites.
In summary, in a relatively large and well-defined multi-site sample, we found lower regional FA in the cocaine and methamphetamine groups in various white matter tracts. Lower FA was also observed in the nicotine group but limited to the anterior limb of the internal capsule. Lower regional FA was found together with higher regional RD, suggesting demyelination in all groups. The methamphetamine group also exhibited lower regional AD consistent with axonal damage. Significant brain-based classifications identified through the SVM algorithm indicate that there is sufficient signal within DTI-derived patterns of effects to identify individuals with dependence on stimulants. The best classifications were achieved for cocaine and methamphetamine dependent individuals relative to non-dependent controls.
Supplementary Material
Author disclosures
Dr Garavan was funded (1R01DA047119-01) by the National Institutes on Drug Abuse (NIDA). Dr Goldstein received financial support from NIDA (R21DA034954, 1R01DA041528, 1R01DA047851, and 1R01DA048301). Dr Li was supported with funds from NIDA (R01AA021449, R01DA023248, and K25DA040032). NIDA (R01DA020726), the Thomas P. and Katherine K. Pike Chair in Addiction Studies, the Endowment from the Marjorie Greene Family Trust, and UCLA contract 20063287 with Philip Morris USA funded Dr London. Dr Momenan and the Clinical NeuroImaging Research Core was supported (ZIA-AA000123) by the National Institutes on Alcohol Abuse and Alcoholism (NIAAA), Division of Intramural Clinical and Biological Research. Dr Luijten and Dr Veltman received funds from VIDI grant 016.08.322 from Netherlands Organization for Scientific Research (NWO), awarded to Ingmar H.A. Franken. Dr Verdejo-García was supported by the Career Development Fellowship from the Australian Medical Research Future fund (MRF1141214). Dr Zhao was funded by the National Key Research and Development Program of China (2017YFC1310400), the National Nature Science Foundation of China (81771436), and the Shanghai Municipal Health and Family Planning Commission (2018YQ045). Authors have no more financial aspects to disclose.
Footnotes
CRediT authorship contribution statement
JOG contributed to the work’s conception and design and was responsible for doing the analyses, preparing the manuscript. AU contributed to the work’s design and conception, data acquisition, and drafting and approving its final version. ZC, RC, and NS revised and approved the final version of the work. NA and SH contributed to the analysis and reviewed and approved its final version. NAK, HE, JPF, RZG, CSRL, CL, EDL, ML, SM, RM, MAO, AR, DJS, EAS, DJV, AVG, SZ, MZ, NZ, NJ, PMT, and PC made contributions to the acquisition of the data, critically revised the manuscript, and gave final approval for publication. HG and SM contributed to the conception and design, data acquisition, interpretation of the results, revising and approving the final version of the work.
Conflict of interests
None of the authors have conflict of interests to declare.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2021.109185.
References
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329. 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amari S, Wu S, 1999. Improving support vector machine classifiers by modifying kernel functions. Neural Netw. 12 10.1016/S0893-6080(99)00032-5. [DOI] [PubMed] [Google Scholar]
- Aung WY, Mar S, Benzinger TL, 2013. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 5, 427–440. 10.2217/iim.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, Comer SD, 2017. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend. 180, 156–170. 10.1016/j.drugalcdep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholtz E, Fitzgerald ND, Hahn WE, 2020. Machine-learning approaches to substance-abuse research: emerging trends and their implications. Curr. Opin. Psychiatry 33, 334–342. 10.1097/YCO.0000000000000611. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D, 1994. MR diffusion tensor spectroscopy and imaging. Biophys. J 66, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CL, Schmitz JM, Soder HE, Suchting R, Yoon JH, Hasan KM, Narayana PA, Moeller FG, Lane SD, 2019. Regional differences in white matter integrity in stimulant use disorders: a meta-analysis of diffusion tensor imaging studies. Drug Alcohol Depend. 201, 29–37. 10.1016/j.drugalcdep.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 57, 289–300. [Google Scholar]
- Ben-Shachar M, Makowski D, Lüdecke D, 2020. Compute and interpret indices of effect size. CRAN. R Package [Google Scholar]
- Breen MS, Uhlmann A, Ozcan S, Chan M, Pinto D, Bahn S, Stein DJ, 2017. Parallel changes in serum proteins and diffusion tensor imaging in methamphetamine-associated psychosis. Sci. Rep 7, 43777. 10.1038/srep43777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Metzler-Baddeley C, Aggleton JP, 2018. The cingulum bundle: anatomy, function, and dysfunction. Neurosci. Biobehav. Rev 92, 104–127. 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner A, 2012. Neuropathological alterations in cocaine abuse. Curr. Med. Chem 19, 5597–5600. 10.2174/092986712803988947. [DOI] [PubMed] [Google Scholar]
- Cao J, Wang J, Dwyer JB, Gautier NM, Wang S, Leslie FM, Li MD, 2013. Gestational nicotine exposure modifies myelin gene expression in the brains of adolescent rats with sex differences. e247–e247 Transl. Psychiatry 3. 10.1038/tp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, 2018. Results from the 2017 National Survey on Drug Use and Health: Detailed tables. Prevalence Estimates, Etandard Errors, P Values, and Sample sizes 2871. [Google Scholar]
- Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, Whiteford H, Leung J, Naghavi M, Griswold M, Rehm J, Hall W, Sartorius B, Scott J, Vollset SE, Knudsen AK, Haro JM, Patton G, Kopec J, Carvalho Malta D, Topor-Madry R, McGrath J, Haagsma J, Allebeck P, Phillips M, Salomon J, Hay S, Foreman K, Lim S, Mokdad A, Smith M, Gakidou E, Murray C, Vos T, 2018. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 5, 987–1012. 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinga R, Schmaal L, Penninx BWJH, Veltman DJ, Marquand AF, 2020. Controlling for effects of confounding variables on machine learning predictions. bioRxiv. 〈 10.1101/2020.08.17.255034〉. [DOI] [Google Scholar]
- Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abé C, Alda M, Alloza C, Alonso-Lana S, Andreassen OA, Baune BT, Benedetti F, Busatto GF, Canales-Rodríguez EJ, Caseras X, Chaim-Avancini TM, Ching CRK, Dannlowski U, Deppe M, Eyler LT, Fatjo-Vilas M, Foley SF, Grotegerd D, Hajek T, Haukvik UK, Howells FM, Jahanshad N, Kugel H, Lagerberg TV, Lawrie SM, Linke JO, McIntosh A, Melloni EMT, Mitchell PB, Polosan M, Pomarol-Clotet E, Repple J, Roberts G, Roos A, Rosa PGP, Salvador R, Sarró S, Schofield PR, Serpa MH, Sim K, Stein DJ, Sussmann JE, Temmingh HS, Thompson PM, Verdolini N, Vieta E, Wessa M, Whalley HC, Zanetti MV, Leboyer M, Mangin J-F, Henry C, Duchesnay E, Houenou J, 2019. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology 44, 2285–2293. 10.1038/s41386-019-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsiori A, Nguyen D, Karentzos A, Delavelle J, Vargas MI, 2011. The corpus callosum: white matter or terra incognita. Br. J. Radiol 84, 5–18. 10.1259/bjr/21946513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE, Schultz RT, Verma R, Shinohara RT, 2017. Harmonization of multi-site diffusion tensor imaging data. NeuroImage 161, 149–170. 10.1016/j.neuroimage.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H-C, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N, Lotze M, 2014. Current smoking and reduced gray matter volume—a voxel-based morphometry study. Neuropsychopharmacology 39, 2594–2600. 10.1038/npp.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliettino AR, Potenza MN, Yip SW, 2016. White matter development and tobacco smoking in young adults: a systematic review with recommendations for future research. Drug Alcohol Depend. 162, 26–33. 10.1016/j.drugalcdep.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golland P, Fischl B, 2003. Permutation Tests for Classification: Towards Statistical Significance in Image-Based Studies. 10.1007/978-3-540-45087-0_28. [DOI] [PubMed] [Google Scholar]
- Gonçalves J, Baptista S, Silva AP, 2014. Psychostimulants and brain dysfunction: a review of the relevant neurotoxic effects. Neuropharmacology 87, 135–149. 10.1016/j.neuropharm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Guyon I, Weston J, Barnhill J, 2002. Gene selection for cancer classification using Support Vector Machine. Mach. Learn 46, 389–422. 10.1023/A:1012487302797. [DOI] [Google Scholar]
- Hahn S, Yuan DK, Thompson WK, Owens M, Allgaier N, Garavan H, 2021. Brain Predictability toolbox: a Python library for neuroimaging-based machine learning. Bioinformatics 37. 10.1093/bioinformatics/btaa974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MG, Alhassoon OM, Stern MJ, Wollman SC, Kimmel CL, Perez-Figueroa A, Radua J, 2015. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am. J. Drug Alcohol Abus 41, 290–299. 10.3109/00952990.2015.1044607. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, Hartwell KJ, 2016. Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict. Biol 21, 185–195. 10.1111/adb.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton SN, Huynh KH, Bonilha L, Abela E, Alhusaini S, Altmann A, Alvim MKM, Balachandra AR, Bartolini E, Bender B, Bernasconi N, Bernasconi A, Bernhardt B, Bargallo N, Caldairou B, Caligiuri ME, Carr SJA, Cavalleri GL, Cendes F, Concha L, Davoodi-bojd E, Desmond PM, Devinsky O, Doherty CP, Domin M, Duncan JS, Focke NK, Foley SF, Gambardella A, Gleichgerrcht E, Guerrini R, Hamandi K, Ishikawa A, Keller SS, Kochunov PV, Kotikalapudi R, Kreilkamp BAK, Kwan P, Labate A, Langner S, Lenge M, Liu M, Lui E, Martin P, Mascalchi M, Moreira JCV, Morita-Sherman ME, O’Brien TJ, Pardoe HR, Pariente JC, Ribeiro LF, Richardson MP, Rocha CS, Rodríguez-Cruces R, Rosenow F, Severino M, Sinclair B, Soltanian-Zadeh H, Striano P, Taylor PN, Thomas RH, Tortora D, Velakoulis D, Vezzani A, Vivash L, von Podewils F, Vos SB, Weber B, Winston GP, Yasuda CL, Zhu AH, Thompson PM, Whelan CD, Jahanshad N, Sisodiya SM, McDonald CR, 2020. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA-Epilepsy study. Brain 143, 2454–2473. 10.1093/brain/awaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Li D, Turel O, Bechara A, Hser YI, 2020. White matter integrity alternations associated with cocaine dependence and long-term abstinence: Preliminary findings. Behav. Brain Res 379 (August 2019) 10.1016/j.bbr.2019.112388. In press. [DOI] [PubMed] [Google Scholar]
- Huang AS, Mitchell JA, Haber SN, Alia-Klein N, Goldstein RZ, 2018. The thalamus in drug addiction: from rodents to humans. Philos. Trans. R. Soc. B: Biol. Sci 373, 20170028. 10.1098/rstb.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Yang W, Luo J, Yan C, Liu J, 2020. White matter abnormalities based on TBSS and its correlation with impulsivity behavior of methamphetamine addicts. Front. Psychiatry 11, 1–9. 10.3389/fpsyt.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudkins M, O’Neill J, Tobias MC, Bartzokis G, London ED, 2012. Cigarette smoking and white matter microstructure. Psychopharmacology 221, 285–295. 10.1007/s00213-011-2621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE, 2007. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J. Neurosci 27, 13491–13498. 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC, 2013. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage 81, 455–469. 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R, 2013. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage 73, 239–254. 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kaag AM, van Wingen GA, Caan MWA, Homberg JR, van den Brink W, Reneman L, 2017. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict. Biol 22, 1048–1056. 10.1111/adb.12375. [DOI] [PubMed] [Google Scholar]
- Kaag AM, Schulte MHJ, Jansen JM, van Wingen G, Homberg J, van den Brink W, Wiers RW, Schmaal L, Goudriaan AE, Reneman L, 2018. The relation between gray matter volume and the use of alcohol, tobacco, cocaine and cannabis in male polysubstance users. Drug Alcohol Depend. 187, 186–194. 10.1016/j.drugalcdep.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM, 2014. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct. Funct 219, 269–281. 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I-S, Kim Y-T, Song H-J, Lee J-J, Kwon D-H, Lee HJ, Kim M-N, Yoo D-S, Chang Y, 2009. Reduced corpus callosum white matter microstructural integrity revealed by diffusion tensor eigenvalues in abstinent methamphetamine addicts. NeuroToxicology 30, 209–213. 10.1016/j.neuro.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC, 2012. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol. Aging 33, 9–20. 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Du X, Moran LV, Sampath H, Wijtenburg SA, Yang Y, Rowland LM, Stein EA, Hong LE, 2013. Acute nicotine administration effects on fractional anisotropy of cerebral white matter and associated attention performance. Front. Pharmacol 4 10.3389/fphar.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell BH, Greenberg BD, 2008. Anatomy and physiology of the basal ganglia: Implications for DBS in psychiatry. Neurosci. Biobehav. Rev 32, 408–422. 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Schubert F, Gallinat J, 2010. Reduced thickness of medial orbitofrontal cortex in smokers. Biol. Psychiatry 68, 1061–1065. 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG, 2010. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One 5. 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, van Horssen J, 2016. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta BBA Mol. Basis Dis 1862, 506–510. 10.1016/j.bbadis.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Lederer K, Fouche J-P, Wilson D, Stein DJ, Uhlmann A, 2016. Frontal white matter changes and aggression in methamphetamine dependence. Metab. Brain Dis 31, 53–62. 10.1007/s11011-015-9775-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Cui Z, Liao Q, Dong H, Zhang J, Shen W, Zhou W, 2019. Support vector machine-based multivariate pattern classification of methamphetamine dependence using arterial spin labeling. Addict. Biol 24, 1254–1262. 10.1111/adb.12705. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Deng Q, Deng Y, Luo T, Wang X, Chen H, Liu T, Chen X, Brody AL, Hao W, 2011. Bilateral fronto-parietal integrity in young chronic cigarette smokers: a diffusion tensor imaging study. PLoS ONE 6. 10.1371/journal.pone.0026460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Wang Q, Schmitz JM, Boone EL, Narayana PA, Moeller FG, 2017. A preliminary longitudinal study of white matter alteration in cocaine use disorder subjects. Drug Alcohol Depend. 173, 39–46. 10.1016/j.drugalcdep.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Paulus M, 2013. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci. Biobehav. Rev 37, 300–316. 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, Brooks S, Caparelli E, Chye YY, Cousijn J, Dagher A, Desrivieres S, Feldstein-Ewing S, Foxe JJ, Goldstein RZ, Goudriaan AE, Heitzeg MM, Hester R, Hutchison K, Korucuoglu O, Li CSR, London E, Lorenzetti V, Luijten M, Martin-Santos R, May A, Momenan R, Morales A, Paulus MP, Pearlson G, Rousseau ME, Salmeron BJ, Schluter R, Schmaal L, Schumann G, Sjoerds Z, Stein DJ, Stein EA, Sinha R, Solowij N, Tapert S, Uhlmann A, Veltman D, Van Holst R, Whittle S, Wright MJ, Yücel M, Zhang S, Yurgelun-Todd D, Hibar DP, Jahanshad N, Evans A, Thompson PM, Glahn DC, Conrod P, Garavan H, 2019. Mega-analysis of gray matter volume in substance dependence: General and substance-specific regional effects. Am. J. Psychiatry 176, 119–128. 10.1176/appi.ajp.2018.17040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak KK, Lee K, Park C, 2019. Applications of machine learning in addiction studies: A systematic review. Psychiatry Res. 275, 53–60. 10.1016/j.psychres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Sánchez-Gómez M-V, Pérez-Samartín A, Rodríguez-Antigüedad A, Pérez-Cerdá F, 2007. Excitotoxic damage to white matter. J. Anat 210, 693–702. 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mete M, Sakoglu U, Spence JS, Devous MD, Harris TS, Adinoff B, 2016. Successful classification of cocaine dependence using brain imaging: A generalizable machine learning approach. BMC Bioinforma. 17 10.1186/s12859-016-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EE, Hohman TJ, Badami FS, Pechman KR, Osborn KE, Acosta LMY, Bell SP, Babicz MA, Gifford KA, Anderson AW, Goldstein LE, Blennow K, Zetterberg H, Jefferson AL, 2018. Neurofilament relates to white matter microstructure in older adults. Neurobiol. Aging 70, 233–241. 10.1016/j.neurobiolaging.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J, 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40, 570–582. 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler M, Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian-Barrera M, Verbeke T, Koller M, Conceicao EL, Anna di Palma M, 2021. robustbase: Basic Robust Statistics. R package version 0.93–8. [Google Scholar]
- Mowinckel AM, Vidal-Piñeiro D, 2019. Visualisation of Brain Statistics with R-packages ggseg and ggseg3d.
- Noirhomme Q, Lesenfants D, Gomez F, Soddu A, Schrouff J, Garraux G, Luxen A, Phillips C, Laureys S, 2014. Biased binomial assessment of cross-validated estimation of classification accuracies illustrated in diagnosis predictions. NeuroImage: Clin. 4 10.1016/j.nicl.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Grieve S, Niaura R, David S, Laidlaw D, Cohen R, Sweet L, Taylor G, Clark R, Pogun S, Gordon E, 2008. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob. Res 10, 137–147. 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RB, Andrade PB, Valeñtao P, 2015. A comprehensive view of the neurotoxicity mechanisms of cocaine and ethanol. Neurotox. Res 28, 253–267. 10.1007/s12640-015-9536-x. [DOI] [PubMed] [Google Scholar]
- Polesskaya O, Silva J, Sanfilippo C, Desrosiers T, Sun A, Shen J, Feng C, Polesskiy A, Deane R, Zlokovic B, Kasischke K, Dewhurst S, 2011. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 1373, 91–100. 10.1016/j.brainres.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A Language and Environment for Statistical Computing.
- Sajja RK, Rahman S, Cucullo L, 2016. Drugs of abuse and blood-brain barrier endothelial dysfunction: a focus on the role of oxidative stress. J. Cereb. Blood Flow. Metab 36, 539–554. 10.1177/0271678X15616978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, Galloway GP, Leamon MH, 2009. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol. Psychiatry 65, 122–128. 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani RR, Velasquez KM, Thompson-Lake DGY, Baldwin PR, Eagleman DM, De La Garza R, Salas R, 2014. Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug Alcohol Depend. 145, 134–142. 10.1016/j.drugalcdep.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Doan NT, Pergola G, Westlye LT, Kaufmann T, Wolfers T, Brecheisen R, Quarto T, Ing AJ, di Carlo P, Gurholt TP, Harms RL, Noirhomme Q, Moberget T, Agartz I, Andreassen OA, Bellani M, Bertolino A, Blasi G, Brambilla P, Buitelaar JK, Cervenka S, Flyckt L, Frangou S, Franke B, Hall J, Heslenfeld DJ, Kirsch P, McIntosh AM, Nöthen MM, Papassotiropoulos A, de Quervain DJ-F, Rietschel M, Schumann G, Tost H, Witt SH, Zink M, Meyer-Lindenberg A, 2019. Reproducible grey matter patterns index a multivariate, global alteration of brain structure in schizophrenia and bipolar disorder. Transl. Psychiatry 9. 10.1038/s41398-018-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJR, Nader MA, Porrino LJ, 2014. Regionally-specific alterations in myelin proteins in nonhuman primate white matter following prolonged cocaine self-administration. Drug Alcohol Depend. 137, 143–147. 10.1016/j.drugalcdep.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting R, Beard CL, Schmitz JM, Soder HE, Yoon JH, Hasan KM, Narayana PA, Lane SD, 2020. A meta-analysis of tract-based spatial statistics studies examining white matter integrity in cocaine use disorder. Addict. Biol, e12902 10.1111/adb.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED, 2010. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology 209, 13–24. 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann A, Fouche JP, Lederer K, Meintjes EM, Wilson D, Stein DJ, 2016. White matter microstructure and impulsivity in methamphetamine dependence with and without a history of psychosis. Hum. Brain Mapp 37, 2055–2067. 10.1002/hbm.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene-Nakano W, Yoshimura R, Kakeda S, Watanabe K, Hayashi K, Nishimura J, Takahashi H, Moriya J, Ide S, Ueda I, Hori H, Ikenouchi-Sugita A, Katsuki A, Atake K, Abe O, Korogi Y, Nakamura J, 2014. Abnormal white matter integrity in the corpus callosum among smokers: tract-based spatial statistics. PLoS One 9, 1–6. 10.1371/journal.pone.0087890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ewijk H, Groenman AP, Zwiers MP, Heslenfeld DJ, Faraone SV, Hartman CA, Luman M, Greven CU, Hoekstra PJ, Franke B, Buitelaar J, Oosterlaan J, 2015. Smoking and the developing brain: Altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum. Brain Mapp 36, 1180–1189. 10.1002/hbm.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Son D, Wiers RW, Catena A, Perez-Garcia M, Verdejo-García A, 2016. White matter disruptions in male cocaine polysubstance users: associations with severity of drug use and duration of abstinence. Drug Alcohol Depend. 168, 247–254. 10.1016/j.drugalcdep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Villalón-Reina JE, Martínez K, Qu X, Ching CRK, Nir TM, Kothapalli D, Corbin C, Sun D, Lin A, Forsyth JK, Kushan L, Vajdi A, Jalbrzikowski M, Hansen L, Jonas RK, van Amelsvoort T, Bakker G, Kates WR, Antshel KM, Fremont W, Campbell LE, McCabe KL, Daly E, Gudbrandsen M, Murphy CM, Murphy D, Craig M, Emanuel B, McDonald-McGinn DM, Vorstman JAS, Fiksinski AM, Koops S, Ruparel K, Roalf D, Gur RE, Eric Schmitt J, Simon TJ, Goodrich-Hunsaker NJ, Durdle CA, Doherty JL, Cunningham AC, van den Bree M, Linden DEJ, Owen M, Moss H, Kelly S, Donohoe G, Murphy KC, Arango C, Jahanshad N, Thompson PM, Bearden CE, 2020. Altered white matter microstructure in 22q11.2 deletion syndrome: a multisite diffusion tensor imaging study. Mol. Psychiatry 25, 2818–2831. 10.1038/s41380-019-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zuo L, Jiang T, Peng P, Chu S, Xiao D, 2017. Abnormal white matter microstructure among early adulthood smokers: a tract-based spatial statistics study. Neurol. Res 39, 1094–1102. 10.1080/01616412.2017.1379277. [DOI] [PubMed] [Google Scholar]
- West R, 2017. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32, 1018–1036. 10.1080/08870446.2017.1325890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF, 2013. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology 230, 663–671. 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Rao H, Hager N, Wang J, Franklin TR, Fan Y, 2019. Classifying and characterizing nicotine use disorder with high accuracy using machine learning and resting-state fMRI. Addict. Biol 24, 811–821. 10.1111/adb.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A, 2018. Understanding the physiopathology behind axial and radial diffusivity changes—what do we know? Front. Neurol 9 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang Y, Li Q, Zhong Y, Chen L, Du Y, He J, Liao L, Xiong K, Yi C, Yan J, 2018a. The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front. Mol. Neurosci 11 10.3389/fnmol.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang Y, Li Q, Zhong Y, Chen L, Du Y, He J, Liao L, Xiong K, Yi C, Yan J, 2018b. The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front. Mol. Neurosci 11 10.3389/fnmol.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang Y, Cheng J, Zheng R, 2020. Meta-analysis of brain gray matter changes in chronic smokers. Eur. J. Radiol, 109300 10.1016/j.ejrad.2020.109300. [DOI] [PubMed] [Google Scholar]
- Yip SW, Morie KP, Xu J, Constable RT, Malison RT, Carroll KM, Potenza MN, 2017. Shared microstructural features of behavioral and substance addictions revealed in areas of crossing fibers. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 2. 10.1016/j.bpsc.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C, Luo L, Zhai J, Zhao L, Zhao Y, Gu Y, Xue T, Liu X, Lu X, Qin W, Tian J, 2016. White matter integrity in young smokers: a tract-based spatial statistics study. Addict. Biol 21, 679–687. 10.1111/adb.12237. [DOI] [PubMed] [Google Scholar]
- Yuan K, Yu D, Zhao M, Li M, Wang R, Li Y, Manza P, Shokri-Kojori E, Wiers CE, Wang GJ, Tian J, 2018. Abnormal frontostriatal tracts in young male tobacco smokers. NeuroImage 183, 346–355. 10.1016/j.neuroimage.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stein EA, Hong LE, 2010. Smoking and schizophrenia independently and additively reduce white matter integrity between striatum and frontal cortex. Biol. Psychiatry 68, 674–677. 10.1016/j.biopsych.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lee MR, Salmeron BJ, Stein DJ, Hong LE, Geng X, Ross TJ, Li N, Hodgkinson C, Shen P-H, Yang Y, Goldman D, Stein EA, 2013. Prefrontal white matter impairment in substance users depends upon the catechol-o-methyl transferase (COMT) val158met polymorphism. NeuroImage 69, 62–69. 10.1016/j.neuroimage.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


