Abstract
The emergence of antimicrobial-resistant Staphylococcus aureus has become a grave concern worldwide. In this study, 95 strains of S. aureus isolated from stool samples were collected from Busan, South Korea to characterize their antimicrobial susceptibility, enterotoxin genes, and molecular typing using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and random amplification of polymorphic DNA (RAPD) assay. Only two strains showed no drug resistance, whereas resistance to three or more antibiotics was observed in 87.4% of strains. Ampicillin resistance was the most common at 90% and all strains were susceptible to vancomycin. The distribution of enterotoxin genes encoded in isolates was sea (32.6%), sec (11.6%), seg (19%), sea & sec (2.1%), and sec & seg (34.7%). Molecular typing using both MALDI-TOF MS and RAPD indicated that S. aureus exhibited diverse clonal lineages and no correlations were observed among the profiling of enterotoxin, MALDI-TOF MS, and RAPD. This investigation provides useful information on foodborne pathogenic S. aureus that has a significant public health impact in South Korea.
Keywords: S. aureus, antimicrobial susceptibility, enterotoxin gene, MALDI-TOF MS, RAPD
1. Introduction
Staphylococcus aureus is a round, Gram-positive, facultative anaerobic bacterium that is part of the natural microflora of humans. However, this bacterium is also a pathogen associated with a number of diseases; in particular, it is the causative agent of staphylococcal enteritis (i.e., staphylococcal food poisoning), which is characterized by gastroenteritis, vomiting, diarrhea, abdominal pain, etc. [1]. Among many diseases by toxins including food poisoning and toxic shock syndrome, this toxin-mediated food poisoning is brought about by the enterotoxins produced by S. aureus. While proliferating in foods such as dairy, meat, eggs and vegetables, S. aureus releases more than 20 different staphylococcal enterotoxins (SE) toxins. Of them, SEA and SED are the most common toxins in staphylococcal food poisoning worldwide [2,3]. These toxins are thermostable and resistant to stomach proteases, and ingestion of S. aureus-contaminated food can be fatal. Moreover, spoiled or contaminated food may not show signs of spoilage (e.g., changes in odor, color and/or flavor), which generates public health threats [4].
The emergence of multidrug-resistant S. aureus is also a major concern in public health since S. aureus can acquire plasmids or transposons encoded with antimicrobial-resistant genes from other species and genera [5]. The spread of antimicrobial-resistant S. aureus such as methicillin-resistant S. aureus (MRSA) is one of the major problems in health care settings [6].
When a patient is suspected of suffering from foodborne illness, the conventional diagnosis usually consists of culturing a stool sample on selective agar or nutrient agar, followed by identification of the causative bacterium using biochemical tests, such as Gram staining and catalase, oxidase, and API tests [7,8]. These procedures are simple and inexpensive, but they can be laborious and time-consuming. Thus, a number of molecular biology techniques have been adapted for the rapid identification of some bacterial pathogens. For the detection of the SE toxins of S. aureus, for example, polymerase chain reaction (PCR) assays, enzyme-linked immunosorbent assays (ELISAs) and agglutination tests are recommended [2,4]. More recently, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), wherein the spectra of unknown microorganisms are compared with those of a reference microorganism, has shown promise for the rapid, accurate and inexpensive identification of bacteria [9].
Diverse methods have been used to assess the molecular typing and analysis of S. aureus isolates such as random amplified polymorphism DNA (RAPD) PCR [10], and MALDI-TOF MS [11]. These tools are considered useful for comparison of various types of bacteria in research laboratories [11].
Many studies have reported that S. aureus could transfer their antimicrobial resistances as well as virulent genes to adjacent bacteria through their mobile genetic elements [12]. Therefore, S. aureus in contaminated foods and feces could contribute to the spread of antimicrobial resistances and virulence to humans [1]. The aim of the present study is to demonstrate the distribution of antimicrobial resistance and virulence factors in S. aureus strains recovered from human stool samples in Korea. Further correlation of S. aureus strains was analyzed by two molecular typing methods, RAPD and MALDI-TOF.
2. Materials and Methods
2.1. S. aureus Isolation and Growth Conditions
Stool specimens were collected from 152 patients suffering from foodborne diarrheal diseases from 2014 to 2016 in Busan, South Korea. Each stool specimen was grown on Baird–Parker (BP) agar (Oxoid, Hampshire, UK) with 5% egg-yolk tellurite emulsion (Oxoid) at 35 °C overnight. Small black colonies with transparent zones on BP agar were selected and sub-cultured on blood agar plates (BAP) at 35 °C overnight. Gram staining, the catalase test, and the coagulase test were carried out, and biochemical tests were performed with an API 20 Staph kit (BioMerieux, Durham, NC, USA) to confirm that the isolates were S. aureus.
2.2. PCR-Based Detection of Enterotoxin Genes
Genomic DNA was extracted from the S. aureus isolates by the boiling method [13]. Briefly, 1 mL of S. aureus cultured overnight in brain heart infusion broth (BHI, Oxoid) was centrifuged at 9000× g for 3 min. The pellet was suspended with 1 mL of PBS and centrifuged at 9000× g for 3 min. The washed pellet was suspended in 100 μL of sterile distilled water, boiled for 20 min, and centrifuged at 14,000× g for 10 min. The supernatant was used as the template for PCR. Two multiplex PCR methods designated sets A and B were used to detect the sea, seb, sec, sed, see and seg genes (Table S1). The Set A reaction contained 10 pmol each of the sea, seb and sec primer pairs, whereas the set B reaction contained 10 pmol each of the sed, see and seg primer pairs. The sequences of the primers are given in Table S1. Each multiplex PCR mixture included 2.5 units of i-StarMAXTM DNA polymerase (INTRON, Daejeon, Korea), 10 mM dNTP (2.5 mM each), 1 × PCR buffer, 2 μL of each primer set (10 pmol), and 2 μL of bacterial DNA. Distilled water was added to a final volume of 20 μL. PCR was performed using a T-100™ programmable thermal controller (Bio-Rad, Irvine, CA, USA) and the following conditions: one cycle of 94 °C for 5 min, 35 cycles of 95 °C for 1 min, 53 °C for 1 min and 72 °C for 1 min, and a final extension at 72 °C for 5 min. The amplified PCR products were visualized on 1.5% TAE agarose gels containing 0.5 μg/mL ethidium bromide.
2.3. Antimicrobial-Agent Susceptibility Test
The antibiotic resistance of S. aureus isolates was determined using the standard disk diffusion method described by the Clinical and Laboratory Standards Institute (CLSI) [14], with application of ampicillin (10 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), cefepime (30 μg), cefotetan (30 μg), clindamycin (2 μg), erythromycin (15 μg), gentamicin (10 μg), imipenem (10 μg), oxacillin (1 μg), penicillin (10 U), rifampin (5 μg), trimethoprim/sulfamethoxazole (1.25 μg/23.75 μg), tetracycline (30 μg) and vancomycin (30 μg). The isolates were spread on tryptone soya agar (TSA, Oxoid) and cultured at 35 °C for 24 h. Colonies were suspended in 3 mL of Mueller–Hinton broth (Oxoid), and the suspension turbidity was adjusted to 0.5 McFarland standard (BioMerieux, Durham, NC, USA). The bacterial solution was then evenly spread on Mueller–Hinton agar (Oxoid, UK), and antimicrobial susceptibility test disks (BD, Franklin Lakes, NJ, USA) were placed on the plates. After the plates were incubated for 24 h at 35 °C, clear zones were examined, and an electronic digital caliper (Fisher Scientific, Hampton, NH, USA) was used to measure the growth inhibition of the isolates in response to each antimicrobial agent. The susceptibilities of the isolates were determined based on the standard suggested by CLSI [14]. E. coli ATCC 25922 and S. aureus ATCC 29213 were used as control strains.
2.4. MALDI-TOF Mass Spectrometry
2.4.1. Sample Preparation
The direct colony and standard extraction methods were used to prepare the isolates for MALDI biotyping analysis as the manufacturer recommended. In brief, in the direct colony method, fresh bacterial colonies were applied directly onto an MSP 96 target polished steel plate (Bruker Daltonik, Bremen, Germany), air dried, mixed with 1 μL of HCCA matrix solution (a saturated solution of α-cyano-4-hydroxy-cinnamic acid in 50% acetonitrile and 2.5% trifluoroacetic acid) to crystallize the sample, and air dried.
2.4.2. MALDI-TOF MS
Mass spectra were obtained using a Microflex LT mass spectrometer (Bruker Daltonik) controlled by the Flexcontrol software (Version 3.0; Bruker Daltonik). Positive ions were extruded with an accelerating voltage of 20 kV and the spectra were analyzed within a mass/charge (m/z) ratio of 2000 to 20,000 in the positive linear mode. Each spectrum was calibrated with a bacterial test standard (BTS 255343, Bruker Daltonik). The generated spectra were automatically matched with the reference library and scored using integrated pattern-matching algorithm software (MALDI Biotyper RTC, Bruker Daltonik). Logarithmic scores of 0 to 3 were assigned according to the matching patterns of the spectral peaks. Scores of 0 to 1.699 indicated no reliable identification; 1.700 to 1.999 indicated a probable genus-level identification; 2.000 to 2.299 indicated a secure genus-level identification, probable species-level identification; and scores of 2.300 to 3.000 indicated a highly probable species-level identification. A main spectra library (MSP) dendrogram was generated using the MALDI Biotyper 3.0, with the distance level in the dendrogram set to a maximal value of 1000, as recommended by the manufacturer.
2.5. Random Amplified Polymorphic DNA (RAPD) Analysis
Chromosomal DNA was extracted with an Accuprep genomic DNA extraction kit (Bioneer, Daejeon, Korea) following the manufacturer’s protocol. Random amplified polymorphic DNA (RAPD) analysis was performed using Ready-To-Go RAPD Analysis Beads (GE Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. Briefly, each 25 μL reaction volume contained 10 ng of template DNA and 25 pmol of RAPD analysis primer 5 (5′-d(AACGCGCAAC)-3′), which was found to generate unique banding patterns in our preliminary experiments (data not shown). Amplification was performed using a PT-100TM thermocycler (MJ Research, Watertown, MA, USA) and the following protocol: one cycle at 95 °C for 5 min, 45 cycles of 95 °C for 1 min, 36 °C for 1 min, and a final incubation at 72 °C for 2 min. The resulting PCR products were visualized as described above, and the results were analyzed using the Bionumerics software (Applied Maths, Austin, TX, USA) to generate the phylogenetic tree.
3. Results
3.1. Detection of Enterotoxins
A total of 95 S. aureus isolates were obtained from stool samples of diarrheal patients, and their identifications as S. aureus were confirmed through biochemical tests. Only one isolate per person was included for further analysis. Multiplex PCR assays of enterotoxin genes revealed that all of these foodborne S. aureus isolates harbored one or more of the genes—sea, sec and seg—but none of the isolates harbored the seb, sed or see genes (Table 1).
Table 1.
Distribution of enterotoxin genes in the 95 S. aureus strains.
| Enterotoxin Gene(s) | No. of Isolates Positive for Gene(s) (%) |
|---|---|
| sea | 31 (32.6) |
| sec | 11 (11.6) |
| seg | 18 (18.9) |
| sea & sec | 2 (2.1) |
| sec & seg | 33 (34.7) |
3.2. Antimicrobial-Agent Susceptibility Test
The S. aureus isolates were highly resistant to ampicillin (94.7%) and penicillin (95.8%); moderately resistant to cefepime, cefotetan, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, imipenem, oxacillin, tetracycline, and trimethoprim/sulfamethoxazole; and minimally resistant to rifampin (4.2%). All of the isolates were susceptible to vancomycin (Table 2). Ninety-two strains (96.8%) were multidrug-resistant. Two of the strains were resistant to 13 of the tested antibiotics. Among the multidrug-resistant isolates, many were resistant to 11 antibiotics (44.2%). Only one strain was resistant to just a single antibiotic (Table 3).
Table 2.
Antimicrobial-agent resistance profiles of the 95 S. aureus strains.
| Antimicrobial Agent | No. of Resistant Isolates (%) | No. of Intermediate Isolates (%) | No. of Susceptible Isolates (%) |
|---|---|---|---|
| Ampicillin | 90 (94.7) | 0 (0) | 5 (5.3) |
| Cefepime | 73 (76.8) | 1 (1.1) | 21 (22.1) |
| Cefotetan | 66 (69.5) | 3 (3.2) | 26 (27.3) |
| Chloramphenicol | 29 (30.5) | 1 (1.1) | 65 (68.4) |
| Ciprofloxacin | 41 (43.2) | 0 (0) | 54 (56.8) |
| Clindamycin | 56 (58.9) | 2 (2.1) | 37 (39.0) |
| Erythromycin | 71 (74.7) | 4 (4.2) | 20 (21.1) |
| Gentamicin | 72 (75.8) | 1 (1.1) | 22 (23.1) |
| Imipenem | 68 (71.6) | 0 (0) | 27 (28.4) |
| Oxacillin | 74 (77.9) | 0 (0) | 21 (22.1) |
| Penicillin | 91 (95.8) | 0 (0) | 4 (4.2) |
| Rifampin | 4 (4.2) | 2 (2.1) | 89 (93.7) |
| Tetracycline | 68 (71.6) | 1 (1.1) | 26 (27.3) |
| Trimethoprim/sulfamethoxazole | 39 (41.1) | 3 (3.2) | 53 (55.0) |
| Vancomycin | 0 (0) | 0 (0) | 95 (100.0) |
Table 3.
Antimicrobial-agent resistance patterns of the 95 S. aureus stains.
| No. of Antimicrobials | Resistance Pattern | No. of Isolates |
|---|---|---|
| 0 | - | 2 |
| 1 | GM | 1 |
| 2 | GM, E | 1 |
| AM, P | 6 | |
| OX, P | 1 | |
| AM, P | 1 | |
| 3 | AM, FEP, P | 1 |
| AM, P, E | 3 | |
| AM, SXT, P | 2 | |
| AM, GM, P | 1 | |
| AM, OX, P | 1 | |
| 4 | AM, P, RA, E | 1 |
| AM, FEP, OX, P | 1 | |
| AM, GM, TE, P | 1 | |
| 5 | AM, OX, P, E, CC | 1 |
| AM, FEP, SXT, OX, P | 1 | |
| 7 | AM, FEP, TE, C, P, E, CC | 1 |
| 9 | AM, GM, FEP, CTT, CIP, IPM, OX, P, E | 1 |
| AM, GM, FEP, CIP, IPM, TE, OX, P, E | 1 | |
| 10 | AM, GM, FEP, CTT, CIP, IPM, OX, P, E, CC | 1 |
| AM, FEP, CTT, IPM, SXT, C, TE, OX, P, CC | 1 | |
| AM, GM, FEP, CTT, IPM, SXT, C, TE, OX, P | 2 | |
| AM, GM, FEP, CTT, CIP, IPM, TE, OX, P, CC | 1 | |
| AM, GM, FEP, CTT, CIP, IPM, TE, P, E, CC | 1 | |
| AM, GM, FEP, IPM, SXT, TE, OX, P, E, CC | 2 | |
| 11 | AM, GM, FEP, CTT, IPM, SXT, C, TE, OX, P, E | 1 |
| AM, GM, FEP, CTT, CIP, IPM, SXT, OX, P, E, CC | 1 | |
| AM, GM, FEP, CTT, IPM, SXT, TE, OX, P, E, CC | 1 | |
| AM, GM, FEP, CTT, SXT, C, TE, OX, P, E, CC | 1 | |
| AM, GM, FEP, CTT, IPM, SXT, C, TE, OX, P, E | 11 | |
| AM, GM, FEP, CTT, CIP, IPM, TE, OX, P, E, CC | 25 | |
| 12 | AM, GM, FEP, CTT, CIP, IPM, TE, OX, P, RA, E, CC | 2 |
| AM, GM, FEP, CTT, CIP, IPM, C, TE, OX, P, E, CC | 1 | |
| AM, GM, FEP, CTT, CIP, IPM, SXT, TE, OX, P, E, CC | 5 | |
| AM, GM, FEP, CTT, IPM, SXT, C, TE, OX, P, E, CC | 9 | |
| 13 | AM, GM, FEP, CTT, CIP, IPM, SXT, TE, OX, P, RA, E, CC | 1 |
| AM, GM, FEP, CTT, CIP, IPM, SXT, C, TE, OX, P, E, CC | 1 | |
| 37 patterns | 95 |
Abbreviations: GM, gentamicin; E, Erythromycin; AM, ampicillin; P, Penicillin; OX, Oxacillin; FEP, Cefepime; SXT, Trimethoprim/Sulfamethoxazole; RA, Rifampin; TE, Tetracycline, CC, Clindamycin; C, Chloramphenicol; CTT, Cefotetan; CIP, Ciprofloxacin; IPM, Imipenem.
3.3. RAPD Analysis
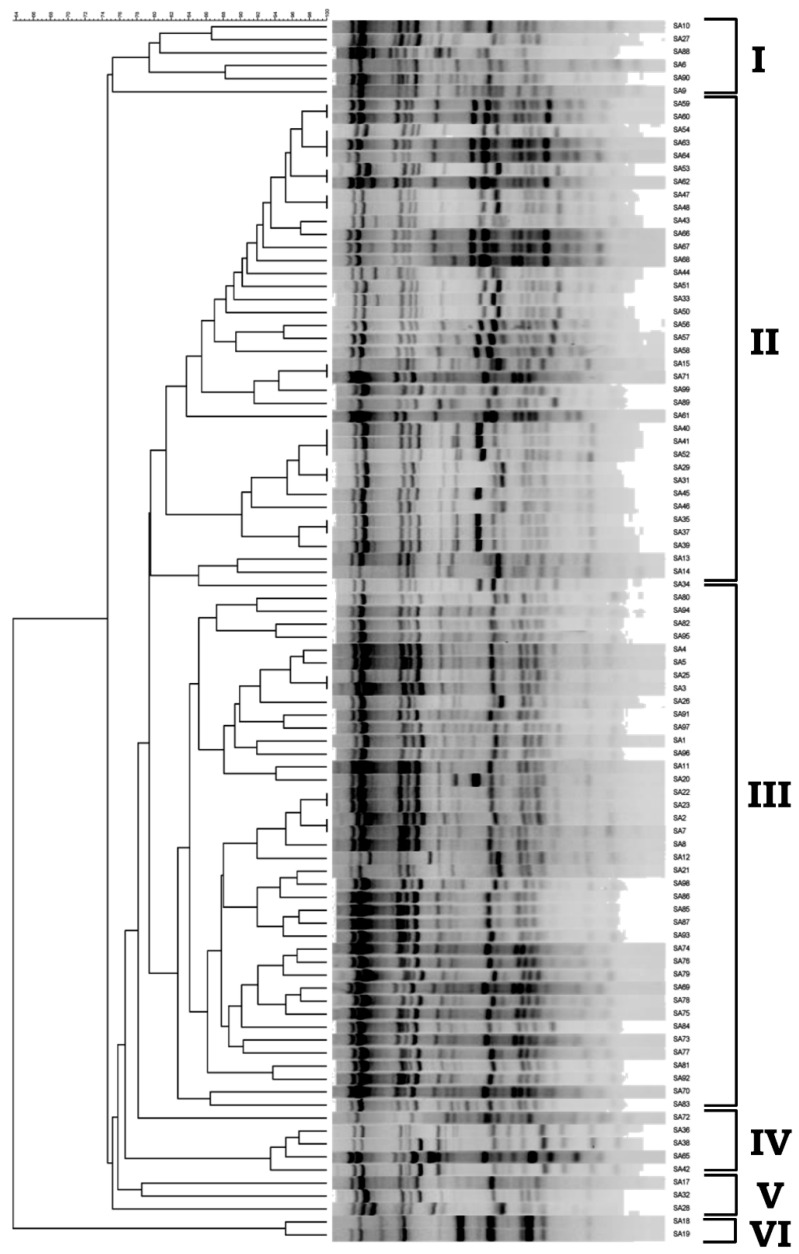
RAPD analysis was performed using RAPD primer 5 of the Ready-To-Go-RAPD Analysis kit. This primer was chosen from the six provided primers because 20 preliminary tests showed that it could generate clear and diverse amplification patterns (data not shown). The same amplification patterns were observed in three independent experiments. Unweighted pair group method with arithmetic mean (UPGMA) clustering was performed using the Bionumerics software, and identified six distinct groups/clusters of S. aureus isolates at a genetic distance of 78 (Figure 1).
Figure 1.
UPGMA clustering of the random amplified polymorphic DNA (RAPD) profiles of S. aureus isolates. Profiles were generated by RAPD primer 5 (Ready-To-Go-RAPD Analysis kit) and the UPGMA clustering (I to VI) was generated using Bionumerics.
3.4. MALDI-TOF MS
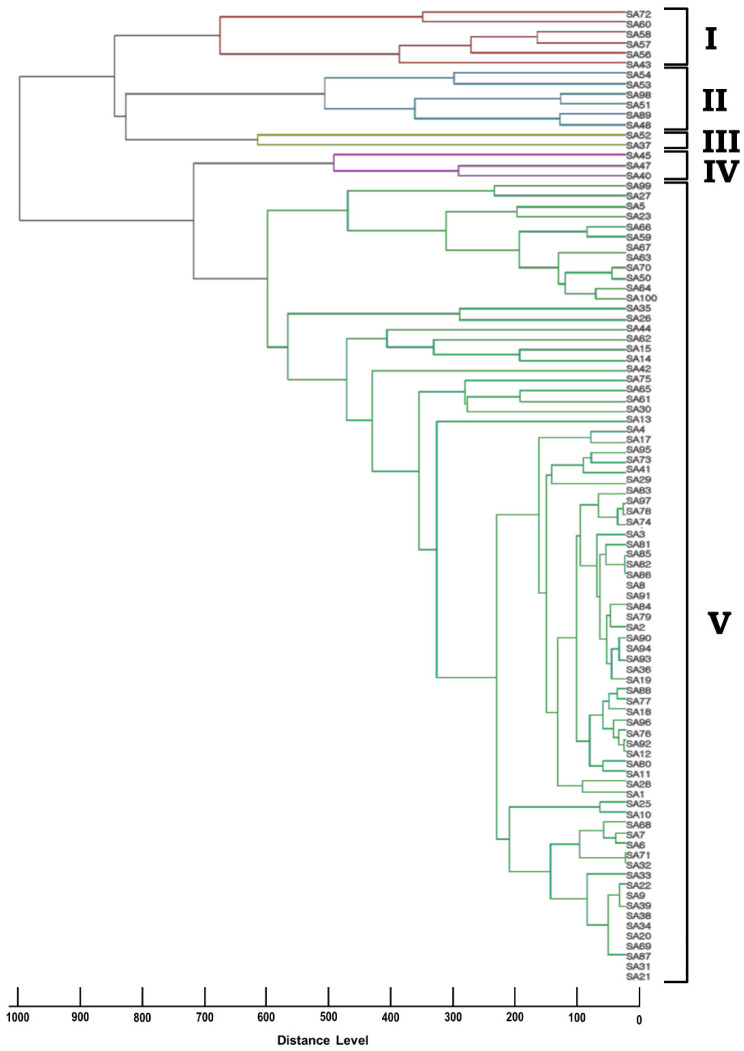
All of the S. aureus isolates were identified to the species level (log scores ≥ 2.0) using both the direct colony and standard extraction methods. The generated MSP dendrogram yielded five groups/clusters (Figure 2). We failed to observe any correlation in the clusters of our RAPD analysis and MSP dendrogram.
Figure 2.
Cluster analysis of the MSP dendrograms generated by the MALDI Biotyper 3.0 for S. aureus isolates. The MSP dendrogram results are clustered as groups (I to V) based on their distance levels. The distance level in the dendrogram was set at a maximal value of 1000.
4. Discussion
Staphylococci are ubiquitous, can be isolated from food products, and are responsible for a number of animal and human diseases [15]. The enterotoxins released by S. aureus are responsible for the bacterial food poisoning caused by this pathogen, with the SEA and SED pathotypes (i.e., strains expressing the sea and/or sed genes) highly associated with disease [16,17]. To detect the pathogen and identify its expressed toxin(s), a multiplex PCR assay is usually recommended [4,16,17]. A previous study on 430 S. aureus isolates obtained from dairy products found eight SEA strains, three SEB strains, and two SED strains [4]. In several studies on isolates obtained from food, SEA, SEB, and SED were found to be the major enterotoxins [4,18,19]. In the present study, in contrast, multiplex PCR revealed that the most dominant toxins expressed in the 95 isolated strains were SEC and SEG (33 strains), followed by SEA (31 strains), SEG (18 strains), SEC (11 strains) and SEA plus SEC (2 strains). This apparent discrepancy may reflect differences in number and origin of the samples/isolates.
MALDI-TOF MS using both the direct colony and standard extraction methods confirmed the identification of our S. aureus isolates (100%). An earlier study demonstrated that 94% of S. aureus isolates can be identified to the genus level using the standard extraction method [9]. Another report showed that MALDI-TOF MS could perform species-level detection for eight of 20 S. aureus isolates [20]. The 100% success rate obtained in the present study suggests the importance of using freshly cultured isolates, and the advantage of using the latest software for bacterial isolation. Additionally, the use of MALDI-TOF MS is accurate, inexpensive, faster and requires less expertise compared to conventional microbiological methods such as spa-typing or MLST-typing or time consuming, expensive methods such as whole genome sequencing [11].
Earlier studies showed that the results from MALDI-TOF MS and other clustering analyses (e.g., RAPD) may show correlations based on their hosts and geographic origins [21,22,23]. However, we failed to find any correlation between the MALDI-TOF MS dendrogram and RAPD fingerprints of our S. aureus isolates. Similarly, a previous study failed to find any correlation between the typing patterns obtained from RAPD and MALDI-TOF MS of various Enterococci [24]. In our case, the lack of correlation may reflect that all of the isolates originated from a single city (Busan, South Korea) and host (human).
Antibiotics are often used to treat foodborne diseases [25], but their indiscriminate and unmonitored use has led to drug resistance in certain bacterial species. Here, we show that all of the obtained foodborne S. aureus isolates were resistant to at least one antibiotic, and that 96.8% were resistant to multiple antibiotics. Most of the isolates showed resistance to ampicillin (94.7%) and penicillin (95.8%). A recent study also showed that 70% of the tested S. aureus strains were resistant to ampicillin and penicillin [26]. The resistance to oxacillin (77.9%) was noteworthy, which contains public health concerns such as the emergence of methicillin-resistant S. aureus (MRSA) [27]. Fortunately, all of the isolates were found to be susceptible to vancomycin.
5. Conclusions
Here, we analyzed the characteristics of S. aureus isolated from the stool samples of diarrheal patients. We found that all of the isolated S. aureus strains possessed at least one enterotoxin gene, and most were multidrug-resistant. We were also able to show that MALDI-TOF MS can be a practical and faster method for identifying bacterial isolates using either the direct colony or standard extraction methods. These results may assist in the establishment of treatment protocols for S. aureus-mediated illnesses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10030642/s1, Table S1: PCR primer pairs used to detect enterotoxin genes in S. aureus strains and their predicted amplification size. [28].
Author Contributions
Conceptualization, S.Y., Y.K.P. and S.B.P.; methodology, S.B.P. and Y.K.P.; software, S.B.P.; validation, T.S.J.; formal analysis, S.Y.; investigation, Y.K.P.; resources, Y.K.P.; data curation, Y.K.P.; writing—original draft preparation, S.Y.; writing—review and editing, T.S.J. and S.B.P.; visualization, S.B.P.; supervision, T.S.J. and S.B.P.; project administration, T.S.J.; funding acquisition, T.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Research Foundation, grant number NRF-2021R1A2B5B02002220.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le Loir Y., Baron F., Gautier M. Staphylococcus aureus and Food Poisoning. Genet. Mol. Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 2.Klotz M., Opper S., Heeg K., Zimmermann S. Detection of Staphylococcus aureus Enterotoxins A to D by Real-Time Fluorescence PCR Assay. J. Clin. Microbiol. 2003;41:4683–4687. doi: 10.1128/JCM.41.10.4683-4687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinchuk I.V., Beswick E.J., Reyes V.E. Staphylococcal Enterotoxins. Toxins. 2010;2:2177–2197. doi: 10.3390/toxins2082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ertas N., Gonulalan Z., Yildirim Y., Kum E. Detection of Staphylococcus aureus Enterotoxins in Sheep Cheese and Dairy Desserts by Multiplex PCR Technique. Int. J. Food Microbiol. 2010;142:74–77. doi: 10.1016/j.ijfoodmicro.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Malachowa N., Deleo F.R. Mobile Genetic Elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010;67:3057–3071. doi: 10.1007/s00018-010-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:e00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett W.E., Tarr P.I. Enteric Infections and Diagnostic Testing. Curr. Opin. Gastroenterol. 2009;25:1–7. doi: 10.1097/MOG.0b013e32831ba094. [DOI] [PubMed] [Google Scholar]
- 8.Pawlowski S.W., Warren C.A., Guerrant R. Diagnosis and Treatment of Acute or Persistent Diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neville S.A., LeCordier A., Ziochos H., Chater M.J., Gosbell I.B., Maley M.W., van Hal S.J. Utility of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Following Introduction for Routine Laboratory Bacterial Identification. J. Clin. Microbiol. 2011;49:2980–2984. doi: 10.1128/JCM.00431-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zare S., Derakhshandeh A., Haghkhah M., Naziri Z., Broujeni A.M. Molecular Typing of Staphylococcus aureus from Different Sources by RAPD-PCR Analysis. Heliyon. 2019;5:e02231. doi: 10.1016/j.heliyon.2019.e02231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbehiry A., Marzouk E., Hamada M., Al-Dubaib M., Alyamani E., Moussa I.M., AlRowaidhan A., Hemeg H.A. Application of MALDI-TOF MS Fingerprinting as a Quick Tool for Identification and Clustering of Foodborne Pathogens Isolated from Food Products. New Microbiol. 2017;40:269–278. [PubMed] [Google Scholar]
- 12.Alibayov B., Baba-Moussa L., Sina H., Zdeňková K., Demnerová K. Staphylococcus aureus Mobile Genetic Elements. Mol. Biol. Rep. 2014;41:5005–5018. doi: 10.1007/s11033-014-3367-3. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.H., Jung B.Y., Rayamahji N., Lee H.S., Jeon W.J., Choi K.S., Kweon C.H., Yoo H.S. A multiplex real-time PCR for differential detection and quantification of Salmonella spp., Salmonella enterica serovar Typhimurium and Enteritidis in meats. J. Vet. Sci. 2009;10:43–51. doi: 10.4142/jvs.2009.10.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein M.P., Patel J.B., Bobenchik A.M., Campeau S., Cullen S.K., Galas M.F., Gold H., Humphries R.M., Kirn T.J. M100 ED31. Volume 31 Clinical and Laboratory Standards Institute; Malvern, PA, USA: 2021. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 15.Payne D.N., Wood J.M. The Incidence of Enterotoxin Production in Strains of Staphylococcus aureus Isolated from Foods. J. Appl. Bacteriol. 1974;37:319–325. doi: 10.1111/j.1365-2672.1974.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S.Y., Kim S.H., Jang E.J., Kwon N.H., Park Y.K., Koo H.C., Jung W.K., Kim J.M., Park Y.H. Novel Multiplex PCR for the Detection of the Staphylococcus aureus Superantigen and Its Application to Raw Meat Isolates in Korea. Int. J. Food Microbiol. 2007;117:99–105. doi: 10.1016/j.ijfoodmicro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Pelisser M.R., Klein C.S., Ascoli K.R., Zotti T.R., Arisi A.C.M. Ocurrence of Staphylococcus aureus and Multiplex Pcr Detection of Classic Enterotoxin Genes in Cheese and Meat Products. Braz. J. Microbiol. 2009;40:145–148. doi: 10.1590/S1517-83822009000100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Normanno G., Firinu A., Virgilio S., Mula G., Dambrosio A., Poggiu A., Decastelli L., Mioni R., Scuota S., Bolzoni G., et al. Coagulase-Positive Staphylococci and Staphylococcus aureus in Food Products Marketed in Italy. Int. J. Food Microbiol. 2005;98:73–79. doi: 10.1016/j.ijfoodmicro.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Tkáčiková L., Tesfaye I.M. Detection of the Genes for Staphylococcus aureus Enterotoxins by PCR. Acta Vet. BRNO. 2003;72:627–630. doi: 10.2754/avb200372040627. [DOI] [Google Scholar]
- 20.Alatoom A.A., Cunningham S.A., Ihde S.M., Mandrekar J., Patel R. Comparison of Direct Colony Method versus Extraction Method for Identification of Gram-Positive Cocci by Use of Bruker Biotyper Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2011;49:2868–2873. doi: 10.1128/JCM.00506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albesharat R., Ehrmann M.A., Korakli M., Yazaji S., Vogel R.F. Phenotypic and Genotypic Analyses of Lactic Acid Bacteria in Local Fermented Food, Breast Milk and Faeces of Mothers and Their Babies. Syst. Appl. Microbiol. 2011;34:148–155. doi: 10.1016/j.syapm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Firacative C., Trilles L., Meyer W. MALDI-TOF MS Enables the Rapid Identification of the Major Molecular Types within the Cryptococcus neoformans/C. gattii Species Complex. PLoS ONE. 2012;7:e37566. doi: 10.1371/journal.pone.0037566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmon K.E., Fang D.C., Tesic V., Khot P.D., Giangeruso E., Bolesta E.S., Wagner-Reiss K.M., Fisher M.A., Petti C.A., Han X.Y., et al. Isolation and Characterization of “Pseudomonas andersonii” from Four Cases of Pulmonary Granulomas and Emended Species Description. J. Clin. Microbiol. 2011;49:1518–1523. doi: 10.1128/JCM.02219-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenstrauß A.G., Pavlovic M., Bringmann A., Behr J., Ehrmann M.A., Vogel R.F. Comparison of Genotypic and Phenotypic Cluster Analyses of Virulence Determinants and Possible Role of CRISPR Elements towards Their Incidence in Enterococcus faecalis and Enterococcus faecium. Syst. Appl. Microbiol. 2011;34:553–560. doi: 10.1016/j.syapm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne Illness Acquired in the United States-Major Pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu C., Wei Y., Chuang S.T., Yu C., Changchien C.H., Su Y. Differences in Virulence Genes and Genome Patterns of Mastitis-Associated Staphylococcus aureus among Goat, Cow, and Human Isolates in Taiwan. Foodborne Pathog. Dis. 2013;10:256–262. doi: 10.1089/fpd.2012.1278. [DOI] [PubMed] [Google Scholar]
- 27.Shin E., Hong H., Park J., Oh Y., Jung J., Lee Y. Characterization of Staphylococcus aureus faecal isolates associated with food-borne disease in Korea. J. Appl. Microbiol. 2016;121:277–286. doi: 10.1111/jam.13133. [DOI] [PubMed] [Google Scholar]
- 28. Monday S.R., Bohach G.A. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in Staphylococcal isolates. J. Clin. Microbiol. 1999;37:3411–3414. doi: 10.1128/jcm.37.10.3411-3414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.