Bezzi previews work from Cvetkovic et al. that presents a neural organoid system to model human reactive astrocytes.
Abstract
The study of human reactive astrocytes has been limited by resource availability. In this issue, Cvetkovic et al. (2022. J. Cell Biol. https://doi.org/10.1083/jcb.202107135) develop multicellular organoid systems containing mature astrocytes to study the dynamics of human astrocytes reactivity and its downstream effects on neuronal activity.
Over the last 10 yr, it has become increasingly apparent that astrocytes, the most abundant glial cells in the central nervous system (CNS), play major roles in the physiology of the brain and spinal cord, including synaptogenesis, neurovascular coupling, neuronal bio-energetic support, clearance of proteins and waste, maintenance of redox and pH levels, and the regulation of neuronal activity by means of the buffering of ions and neurotransmitters and release of various neuroactive substances. Astrocytes are also involved in all forms of CNS disease and lesions, to which they respond by undergoing a series of cellular, molecular and functional changes known as “reactive astrogliosis” (1). These alterations may be transient or long-lasting, and interdisciplinary approaches combining-omics with physiology and genetic manipulations have shown that can have both harmful and beneficial effects, thus indicating that astrocytes have multiple states of reactivity and function depending on the myriad of intrinsic and extrinsic cues governing their post-injury gene expression and function (1). However, what remains unclear is whether the gene expression and functional characteristics of any particular reactive astrocyte subtype originally identified in rodents can be directly translated to human pathologies (Fig. 1).
Figure 1.
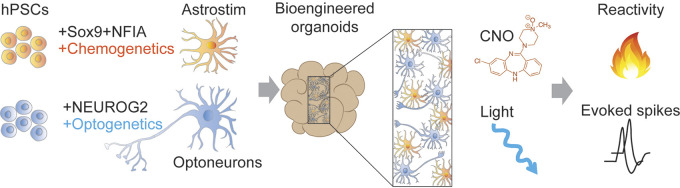
Human brain organoid system developed by Cvetkovic et al. Human induced pluripotent stem cells (hiPSCs) are differentiated into chemogenetic astrocytes (i.e., Astrostim) expressing modified human M3 muscarinic Gq protein-coupled (hM3Dq) receptors and into optogenetic neurons (i.e., Optoneurons) harboring Channelrhodopsin 2 (ChR2). The generation of multi-cellular sphere co-cultures of Optoneurons and Astrostims allows for dual specific modulation of calcium responses in astrocytes upon application of clozapine-N-oxidase (CNO) and for an increase in neuronal activity upon light stimulation.
The study of human reactive astrocytes has been limited by the availability of resources and, more importantly, because there are important transcriptional and functional differences between rodent and human astrocytes (2–5) that are even more pronounced during inflammatory responses (6). In the context of translational research toward reactive astrocyte-mediated neuroprotection as a strategy for neurorepair, these differences can be addressed by leveraging human induced pluripotent stem cell (hiPSC) technology which have allowed astrocytes with pathological phenotypes to be generated from tissues obtained from patients with CNS diseases. However, current techniques for studying human reactive astrocytes consist of anatomical studies of human post-mortem tissues and pro-inflammatory protein treatments to hiPSC-derived astrocytes that are co-cultured with neurons in two-dimensional models which mainly reflect features of immature astrocytes. Thus, to date, we lack an approach for studying the dynamics of mature reactive astrocytes in the neuronal environment. In this issue, Cvetkovic et al. address this technical shortcoming by developing bio-engineered neural organoid cultures containing mature astrocytes which allow for the investigation of the dynamics of astrocyte reactivity and its downstream effects on neuronal activity (7).
The approach of the authors mainly entails two modules: the differentiation of neurons and astrocytes from hiPSCs by inducing the expression of cell-type specific transcription factors and the separate activation of astrocytes and neurons by means of chemogenetics or optogenetics, respectively. They first forced hiPSCs to differentiate into astrocytes (iAstro) by combining the insertion of doxycycline (dox)-inducible gliogenic transcription factors Sox9 and NFIA and the addition of special-supportive media in the presence of dox. Interestingly, on post-induction day 12, RNA sequencing, proteomics, immunohistochemistry and calcium (Ca2+) imaging analyses showed that the iAstros had many of the recognized features of mature astrocytes. In particular, cross-referencing the iAstro transcriptome dataset and that of astrocytes in intact brain revealed that iAstros highly expressed 41 out of 50 human astrocyte signature genes, including those with known functions such as channels/transporters (GJA1, ATP1A2, AQP4, SLC1A3). Furthermore, immunohistochemistry analyses of astrocyte and synapse maturation over 3 wk revealed a progressively branching morphology and physical association with the synapses.
After extensively validating the morphology and gene expression of iAstro in vitro, Cvetkovic et al. investigated the effects of over-activating Ca2+ signaling in astrocytes on neuronal activity. They first produced chemogenetic iAstros (i.e., Astrostim) containing modified human M3 muscarinic Gq protein-coupled (hM3Dq) receptors, whose activation upon the application of clozapine-N-oxidase (CNO) elicited the robust up-regulation of Ca2+ responses. Then, they engineered a readout system to monitor neuronal activity in these bio-engineered organoids in the presence of Astrostim cells—they integrated inducible Channelrhodopsin2 (ChR2) in excitatory neurons derived from hiPSCs (i.e., OptoNeurons), which responded with increased neuronal activity upon being challenged with light. To ascertain whether the chronic chemogenetic activation of astrocytes alters neuronal function, the authors generated multi-cellular sphere co-cultures of OptoNeurons and Astrostims for dual cell-specific modulation, and reported a significant decrease in the light-induced firing rates in the CNO-treated groups compared with control co-cultures.
The observation of a reduced neuronal response in the presence of Astrostims led Cvetkovic et al. to speculate that the chronic activation of hM3Dq receptors mediate aberrant Ca2+ signaling in a similar manner to that widely described in reactive astrocytes in a wide range of pathologies (8). Taking advantage of the CNO chemogenic activation of astrocytes, they characterized the effects of aberrant Ca2+ on the acquisition of a reactive phenotype. CNO treatment rapidly induced changes in the gene expression pattern of Astrostim cells similar to those observed in reactive astrocytes, and this reactivity persisted for at least 11 d. Of note, the activation of the hM3Dq pathway in human astrocytes led to the expression of a plethora of factors whose levels may impact the physiological outcome by causing beneficial or detrimental effects. For example, the increased release of THBS1, which is a well-known promoter of synapse formation, is thought to benefit neuronal activity, but excessive THBS1 can elicit downstream neuropathic hyperactivity (9) which may further result in excitotoxicity. Surprisingly, they did not observe any of the neurotoxicity found in many other inflammatory reactivity studies. This could possibly be due to differences in their experimental paradigms (i.e., differences in the stage of maturation of neurons and astrocytes, the approach to induce astrocyte reactivity, endpoint measurements, etc.) or because an astrocyte-induced neurotoxic effect on neurons may require additional disease-associated stressors such as genetic mutations in neurons or micro-environmental factors.
Overall, the work of Cvetkovic et al. provides a new in vitro model for studying the dynamics of human reactive astrocytes and their impact on neuronal function. Future challenges will be the development of more complex models that include other cell types that interact with astrocytes in the human brain. Microglia, oligodendrocytes and vascular endothelial cells are also involved in the neuro-inflammatory processes associated with pathological conditions and it would be of interest to discover how each of these cell types alters the dynamics of reactive astrocytes. This technique will be extremely useful for modeling the human pathologies associated with neuro-inflammatory conditions to determine the extent to which the function of reactive astrocytes contributes to disease progression and to test potential therapies.
Acknowledgments
The author declares no competing financial interests.
References
- 1.Escartin, C., et al. 2021. Nat. Neurosci. 10.1038/s41593-020-00783-4 [DOI] [Google Scholar]
- 2.Zhang, Y., et al. 2016. Neuron. 10.1016/j.neuron.2015.11.013 [DOI] [Google Scholar]
- 3.Kelley, W.K., et al. 2018. Nat. Neurosci. 10.1038/s41593-018-0216-z [DOI] [Google Scholar]
- 4.Li, J., et al. 2021. Nat. Commun. 10.1038/s41467-021-24232-3 [DOI] [Google Scholar]
- 5.Krencik, R., et al. 2017. Stem Cell Reports. 10.1016/j.stemcr.2017.10.026 [DOI] [Google Scholar]
- 6.Tarassishin, L., et al. 2014. Glia. 10.1002/glia.22657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cvetkovic, C., et al. 2022. J. Cell Biol. 10.1083/jcb.202107135 [DOI] [Google Scholar]
- 8.Shigetomi, E., et al. 2019. Int. J. Mol. Sci. 10.3390/ijms20040996 [DOI] [Google Scholar]
- 9.Nagai, J., et al. 2019. Cell. 10.1016/j.cell.2019.03.019 [DOI] [Google Scholar]