Human autoantibodies neutralizing specific cytokines can underlie specific infectious diseases in otherwise healthy patients. The autoantibodies mimic inborn errors of the corresponding cytokines or their response pathways. Four autoimmune phenocopies of inborn errors of cytokine immunity have already been reported.
Abstract
The vast interindividual clinical variability observed in any microbial infection—ranging from silent infection to lethal disease—is increasingly being explained by human genetic and immunological determinants. Autoantibodies neutralizing specific cytokines underlie the same infectious diseases as inborn errors of the corresponding cytokine or response pathway. Autoantibodies against type I IFNs underlie COVID-19 pneumonia and adverse reactions to the live attenuated yellow fever virus vaccine. Autoantibodies against type II IFN underlie severe disease caused by environmental or tuberculous mycobacteria, and other intra-macrophagic microbes. Autoantibodies against IL-17A/F and IL-6 are less common and underlie mucocutaneous candidiasis and staphylococcal diseases, respectively. Inborn errors of and autoantibodies against GM-CSF underlie pulmonary alveolar proteinosis; associated infections are less well characterized. In individual patients, autoantibodies against cytokines preexist infection with the pathogen concerned and underlie the infectious disease. Human antibody-driven autoimmunity can interfere with cytokines that are essential for protective immunity to specific infectious agents but that are otherwise redundant, thereby underlying specific infectious diseases.
Introduction
The clinical consequences of any infection vary greatly between individuals, ranging from silent infection to lethal disease (Casanova and Abel, 2020). The study of single-gene inborn errors of immunity (IEI) has led to the discovery of human genetic and immunological determinants of infectious diseases (Casanova and Abel, 2020; Casanova and Abel, 2021a; Casanova and Abel, 2021b). Most of the more than 450 IEI genetically characterized since 1985 confer a predisposition to infectious diseases (Bousfiha et al., 2020; Tangye et al., 2020; Tangye et al., 2021). Following their discovery in the 1950s, each IEI was thought to underlie various infectious diseases in individual patients. However, from 1996 onward, some IEI were found to underlie a single, specific infectious disease (Notarangelo et al., 2020). These IEI include inborn errors of specific cytokines or their response pathways disrupting immunity to specific microorganisms (Casanova and Abel, 2021a; Casanova and Abel, 2021b; Notarangelo et al., 2020; Tangye et al., 2020; Tangye et al., 2021). Indeed, otherwise healthy patients vulnerable to weakly virulent mycobacteria (Mendelian susceptibility to mycobacterial disease [MSMD]) and/or to the more virulent Mycobaterium tuberculosis, carry inborn errors of IFN-γ, type II IFN immunity (Bustamante, 2020; Yang et al., 2020). Patients with inborn errors of type I IFN immunity each suffer from one or a few viral diseases, including herpes simplex virus 1 encephalitis (Bastard et al., 2020a; Zhang, 2020b), influenza A virus pneumonia (Casanova and Abel, 2021b; Lim et al., 2019; Zhang, 2020a), severe rhinovirus pulmonary diseases (Asgari et al., 2017; Lamborn et al., 2017; Lamborn and Su, 2020), hypoxemic COVID-19 pneumonia (Asano et al., 2021a; Casanova and Abel, 2021b; Zhang et al., 2022; Zhang et al., 2020), or adverse reactions to live-attenuated measles (Hambleton et al., 2013; Hernandez et al., 2019) or yellow fever virus vaccines (Bastard et al., 2021b; Hernandez et al., 2019). Patients with chronic mucocutaneous candidiasis (CMC), which is occasionally associated with staphylococcal disease, carry inborn errors of IL-17A/IL-17F (IL-17A/F; Puel, 2020; Puel et al., 2011). Others, with cutaneous staphylococcal diseases, suffer from inborn errors of IL-6 (Chen et al., 2021a; Puel and Casanova, 2019). Finally, rare inborn errors of GM-CSF, which underlie pulmonary alveolar proteinosis (PAP), have not been associated with infections; yet, nocardiosis and cryptococcosis have been diagnosed in other patients with PAP (Trapnell et al., 2019).
These IEI preceded (type II IFN, GM-CSF) or followed (type I IFNs, IL-17A/F, IL-6) the discovery of autoantibodies (auto-Abs) neutralizing the corresponding cytokines in patients with the same or a similar infectious phenotype (Ku et al., 2020). By blocking their target cytokines, these auto-Abs underlie infectious phenocopies of inborn errors of the corresponding cytokine or response pathway (Browne, 2014; Ku et al., 2020; Shih et al., 2021; Table 1). Four autoimmune phenocopies of IEI of cytokines have been reported to date (Ku et al., 2020; Tangye et al., 2020; Fig. 1). Auto-Abs against cytokines may underlie mycobacterial disease (type II IFN), one or a few viral diseases (type I IFNs), mucocutaneous candidiasis (IL-17A/F), or staphylococcal disease (IL-6). The infectious diseases of patients with PAP (with no documented inborn error of GM-CSF) include invasive nocardiosis and cryptococcosis, which have also been seen in patients with auto-Abs to GM-CSF (with or without PAP). The pathogenesis of these auto-Abs is largely unknown, but they can be detected in children or adults with IEI underlying broader autoimmunity, such as germline loss-of-function biallelic (or monoallelic) mutations of AIRE underlying autoimmune polyendocrine syndrome type 1 (APS-1). Most patients with these auto-Abs have no diagnosed IEI (Bloomfield et al., 2019; Puel et al., 2008). Auto-Abs against cytokines are widely thought to underlie late-onset immunodeficiency (Ku et al., 2020; Tangye et al., 2020), as they are more commonly found in adults, typically with no known underlying IEI (Browne, 2014; Browne et al., 2012a; Browne and Holland, 2010; Ku et al., 2020). Little is known about the causes of these auto-Abs, their prevalence in patients with a given infection and in the general population, the changes in their levels during the life of the individual, their biochemical nature and diversity, their corresponding T and B cell epitopes, and their deleterious or beneficial clinical consequences. We review here the emerging field of anti-cytokine auto-Abs underlying infectious diseases.
Table 1.
Inborn errors of cytokines or their receptors, their corresponding autoimmune phenocopies (anti-cytokine auto-Abs), and monoclonal antibodies used in therapeutics, together with the associated infectious phenotypes
| Cytokine | Receptor of cytokine | Inborn error of immunity | Main infectious disease | Phenocopies (auto-Abs) | Infectious disease | Therapeutic with monoclonal Abs | Infectious disease |
|---|---|---|---|---|---|---|---|
| Type II IFN (IFN-γ) | IFN-γR1 IFN-γR2 |
IFNG IFNG-R1 IFNGR2 |
- Disseminated M. bovis–BCG disease - Disseminated environmental mycobacteria disease |
Auto-Abs to IFN-γ | - Disseminated environmental mycobacteria disease - Disseminated tuberculosis - Salmonellosis |
- Emapalumab - Fontolizumab - AMG811 |
- Disseminated histoplasmosis - Disseminated salmonellosis |
| Type I IFNs (IFN-α/β) | IFNAR1 IFNAR2 |
IFNAR1
IFNAR2 |
- Herpes virus encephalitis - Severe influenza - Yellow fever - Life-threatening COVID-19 pneumonia |
Auto-Abs to IFN-α2, other IFN-α, IFN-β, IFN-ω | - Life-threatening COVID-19 pneumonia - Yellow fever vaccine disease |
- Sifalimumab/MEDI545 - Rontalizumab/RG-7415 - AGS-009 - S95021/19D11 -Anifrolimab/MEDI-546 |
- Respiratory tract infections - Herpes zoster |
| IL-17A IL-17F |
IL-17RA IL-17RC |
IL17F
IL17RA IL17RC |
Chronic mucocutaneous candidiasis | Auto-Abs to IL-17A, IL-17F | - Chronic mucocutaneous candidiasis | - Secukinumab/AIN457 - Ixekizumab/LY2439821 - Brodalumab/AMG 827 - Bimekizumab |
- Chronic mucocutaneous candidiasis |
| IL-6 | IL-6R GP130/IL6ST |
IL6R
IL6ST |
Staphylococcal cutaneous infections | Auto-Abs to IL-6 | - Staphylococcal cutaneous infections | - Tocilizumab - Sarilumab - Satralizumab - Sirukumab - Siltuximab |
- Staphylococcal cellulitis - Pneumonia by S. aureus |
| GM-CSFa | CSF2RA CSF2RB |
CSF2RA
CSF2RB |
- Nocardiosis? - Cryptococcosis? |
Auto-Abs to GM-CSF | - Pulmonary and extra-pulmonary cryptococcosis - Pulmonary and extra-pulmonary nocardiosis |
- Lenzilumab - Namilumab - Gimsilumab - Otilimab - Mavrilimumab |
- Nasopharyngitis without microbe isolation |
As explained in the text, inborn errors of and auto-Abs to GM-CSF underlie PAP. The infectious diseases seen in these patients are relatively diverse and may result from PAP (including its therapy) and/or from impaired GM-CSF–dependent immunity in alveolar macrophages.
Figure 1.
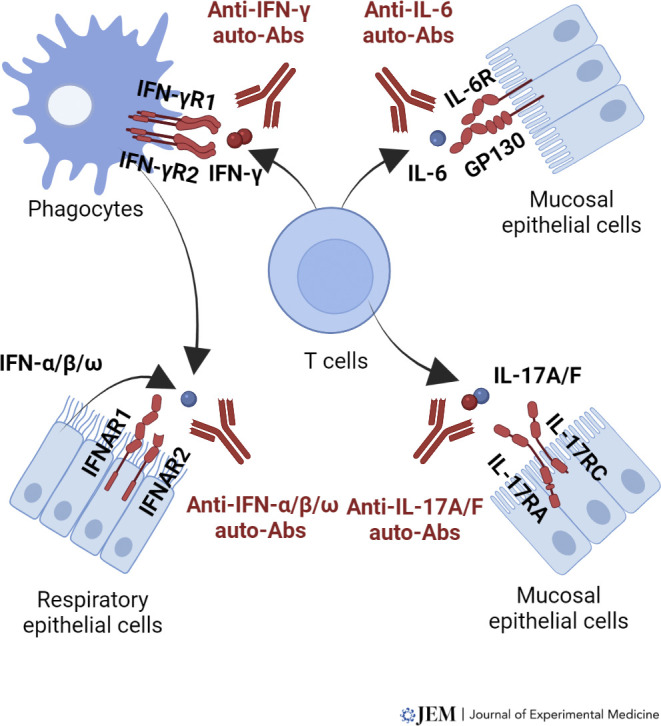
Human inborn errors of and auto-Abs to cytokines underlying infectious diseases. This figure illustrates the key actions of four of the cytokines reviewed in this article. Gene products mutated in patients with infectious diseases are shown in red. Auto-Abs neutralizing the cytokines are also shown. For the sake of simplicity, only the most important cell types involved in the biology of each of these cytokines are shown. Molecules, including cytokines and their receptors, are also shown only on key cell types. A more detailed description of the biology of each cytokine can be found in specific reviews.
Mycobacterial diseases in patients with anti–IFN-γ auto-Abs
In humans, IFN-γ is predominantly produced by activated natural killer and T cells (Schoenborn and Wilson, 2007; Yang et al., 2020). IFN-γ, which was first identified as a leukocyte antiviral IFN (Marcus and Salb, 1966), differs from the other IFNs in that it was later shown to be the macrophage-activating factor (Nathan et al., 1983). Human inborn errors of IFN-γ immunity underlie MSMD (Boisson-Dupuis and Bustamante, 2021; Bustamante, 2020). MSMD patients have a selective susceptibility to severe diseases caused by bacillus Calmette-Guérin (BCG) vaccines and environmental mycobacteria (EM). They are typically otherwise healthy and normally resistant to other microbes. The clinical severity of MSMD, ranging from localized to disseminated infections, varies considerably between, and even within affected kindreds, and increases with decreasing levels of IFN-γ activity (Bustamante, 2020; Kerner et al., 2020). Moreover, autosomal recessive (AR) complete IFN-γR1 (Jouanguy et al., 1996; Newport et al., 1996), IFN-γR2 (Dorman and Holland, 1998), and IFN-γ (Kerner et al., 2020) deficiencies are the only known etiologies of MSMD that clearly display complete penetrance in early childhood. Neutralizing anti–IFN-γ auto-Abs (nAIGA) cause an adult-onset and ethnicity-biased immunodeficiency characterized by susceptibility to mycobacterial disease, mostly due to EM (Ku et al., 2020; Shih et al., 2021). Since 2004, at least 500 patients with nAIGAs have been reported (Doffinger et al., 2004; Hoflich et al., 2004; Kampmann et al., 2005; Patel et al., 2005; Shih et al., 2021). All but two of these patients were adults (aged 40–70 yr; Liew et al., 2019), with a balanced sex ratio. These antibodies were initially reported only in patients from South Asia, East Asia, or Southeast Asia, but two patients of European descent were recently reported (Browne et al., 2012a; Hong et al., 2020; Ku et al., 2020; Shih et al., 2021). Over 85% of patients with nAIGAs suffered from disseminated mycobacterial diseases. At least 12 EM species have been isolated from these patients. The more virulent M. tuberculosis has also been found in 6% of all patients (Browne et al., 2012a; Kampitak et al., 2011; Shih et al., 2021). However, no infection with Mycobacterium bovis–BCG has been reported in these patients, in contrast to those with MSMD, suggesting that the development of auto-Abs occurs after neonatal vaccination. EM disease in patients with nAIGAs is an autoimmune phenocopy of adult MSMD.
Other infectious diseases in patients with anti–IFN-γ auto-Abs
Other intra-macrophagic infections have also been reported in patients with nAIGAs, with or without mycobacterial diseases. About 18% of all patients with nAIGAs described to date have suffered from infections caused by Salmonella spp., Salmonella enteritidis B, Salmonella enteritidis D, and Salmonella typhi (Browne et al., 2012a; Shih et al., 2021). Such infections have also been observed in MSMD patients, particularly those with impaired IFN-γ production (Boisson-Dupuis and Bustamante, 2021). Various fungal infectious diseases have also been documented. About 6% of all patients with nAIGAs had cryptococcosis (principally caused by Cryptococcus neorformans; Chi et al., 2013; Pithukpakorn et al., 2015; Shih et al., 2021; Wongkulab et al., 2013), 3% had histoplasmosis (Histoplasma capsulatum; Pithukpakorn et al., 2015; Shih et al., 2021; Wongkulab et al., 2013), and 22% had talaromycosis (Talaromyces [Penicillium] marneffei; Kampitak et al., 2011; Shih et al., 2021; Tang et al., 2010; Wongkulab et al., 2013). In a recent study of 58 HIV-negative adults from South China with severe T. marneffei disease, almost 95% had nAIGAs, suggesting a critical role of IFN-γ in immunity to this fungus (Guo et al., 2020; Shih et al., 2021). Shingles was associated with intramacrophagic infections in about 22% of all patients with nAIGAs, suggesting a reactivation of varicella zoster virus. These patients each presented at least one episode of cutaneous shingles, but some displayed reactivation of latent varicella zoster virus infection (Browne et al., 2012a; Chi et al., 2013; Chi et al., 2016; Patel et al., 2005; Shih et al., 2021; Wongkulab et al., 2013). These viral infections have occasionally been seen in patients with AR complete IFN-γR1 or IFN-γR2 deficiency, or autosomal dominant (AD) IFN-γR1 deficiency (Boisson-Dupuis and Bustamante, 2021; Roesler et al., 2011). Thus, not only patients with EM disease but also those with severe infections caused by other intramacrophagic pathogens, such as T. marneffei, should be tested for nAIGAs.
Molecular characterization of anti–IFN-γ auto-Abs
AIGA levels are generally high in the blood of patients producing these antibodies. nAIGAs block cellular responses to up to 200 ng/ml IFN-γ, as shown by the analyses of the phosphorylation of STAT1 (with normal IFN-α–induced STAT1 phosphorylation), the expression of HLA-DR (human leukocyte antigen–DR isotype), the secretion of IL-12 and TNF, and the expression of IFN-γ response genes (Browne et al., 2012a; Krisnawati et al., 2019a; Krisnawati et al., 2019b; Nithichanon et al., 2020; Patel et al., 2005; Shima et al., 2014). Most nAIGAs are of the IgG type, usually of the IgG1, IgG3, and IgG4 subclasses; IgM antibodies, when identified, were not neutralizing, and no IgA antibodies have been detected (Browne et al., 2012a; Wipasa et al., 2018). Other auto-Abs, such as anti–GM-CSF auto-Abs, may also be found in rare patients (Browne et al., 2012a; Kim et al., 2014). Peptide scanning with overlapping peptides covering the human IFN-γ protein has led to the identification of a single major epitope targeted by the nAIGAs in the C-terminal part of IFN-γ (amino acids 121–131, P121–131). Amino acids 128–131 (KRKR) are crucial for IFN-γ bioactivity and are conserved across several species. In contrast, amino acids 121–127 are less conserved (SPAAKTG in humans and LPESSLR in mice), and plasma from patients with nAIGAs do not bind mouse IFN-γ, suggesting that their epitope involves amino acids 121–127 (Lin et al., 2016). By binding to the P121–131 epitope, nAIGAs neutralize IFN-γ–mediated signaling, a prerequisite for their pathogenicity (Lin et al., 2016). Indeed, nonneutralizing AIGAs in healthy individuals do not target P121–131 and do not affect IFN-γ bioactivity (Browne et al., 2012a; Lin et al., 2016). Finally, the P121–135 epitope has been shown to have a sequence highly similar to that of the noc2 ribosome assembly protein of Aspergillus spp. (Lin et al., 2016; Shih et al., 2021; Wipasa et al., 2018). The identification of a single major B cell epitope displaying similarity to a fungal protein in patients with nAIGAs suggests that molecular mimicry may underlie the development of these auto-Abs. The development of an engineered biologically active variant of IFN-γ, in which the epitope (amino acids 121–127) is modified, enabling it to escape neutralization by nAIGAs, is a possible therapeutic approach that could be considered in patients with nAIGAs (Lin et al., 2016). Various IgG mAbs have been used in clinics (Hommes et al., 2006; Locatelli et al., 2020; Reinisch et al., 2006; Welcher et al., 2015). These antibodies include emapalumab, a human IgG1 mAb, which has been used to treat children and adults with primary hemophagocytic lymphohistiocytosis. Unsurprisingly, albeit rarely, intra-macrophagic infections (e.g., disseminated histoplasmosis, disseminated Salmonella group D infection, each found in one patient) have been reported among the most serious adverse reactions (Boedigheimer et al., 2017; Locatelli et al., 2020; Table 1).
Human genetic underpinnings of anti–IFN-γ auto-Abs
Major human genetic risk factors for nAIGAs have been identified, in the form of specific HLA class II DRB1 and DQB1 alleles found in patients from Southeast Asia, mostly Taiwan and Thailand (Chi et al., 2013; Ku et al., 2016; Pithukpakorn et al., 2015). In a study of 44 Taiwanese patients and 102 controls, a strong enrichment in HLA-DRB1*16:02 (odds ratio [OR] = 8.36, P = 4.5 × 10−9) and HLA-DQB1*05:02 (OR = 6.54, P = 1.05 × 10−8) was observed in patients (frequencies of 43 and 48%, respectively) relative to controls (frequencies of 8 and 12%, respectively; Ku et al., 2016). A weaker association of nAIGAs was also observed with HLA-DRB1*15:02 and DRQB1*05:01. The same study also investigated a sample of 78 Thai patients and 101 controls and found a strong enrichment in HLA-DRB1*15:02 (OR = 5.33, P = 1.02 × 10−8) and HLA-DQB1*05:01 (OR = 4.19, P = 9.61 × 10−6) in patients (frequencies of 43 and 33%, respectively) relative to controls (frequencies of 12 and 10%, respectively; Ku et al., 2016). The presence of nAIGAs was also significantly associated with HLA-DRB1*16:02 (OR = 3.80, P = 3.8 × 10−6) and DRQB1*05:02 (OR = 2.56, P = 10−4). In addition, 100% (n = 78) of the Thai patients carried at least DRB1*15:02 or DRB1*16:02. In total, there were 17 homozygotes for DRB1*15:02, 6 homozygotes for DRB1*16:02, and 12 compound heterozygotes for DRB1*15:02/16:02, with ORs for nAIGA carriage ranging from 6.7 to 18.2, whereas the OR in heterozygotes was about 3.6. Similar patterns, but with lower OR, were observed with the two HLA-DQB1 alleles (Ku et al., 2016). Indeed, very strong linkage disequilibrium was observed between the associated DRB1 and DQB1 alleles, underlying two haplotypes, with HLA-DRB1*16:02/DQB1*05:02 common in Asian populations (2.6–18.8%), especially in Taiwan and South China, and HLA-DRB1*15:02/DQB1*05:01 common in Southeast Asia, especially in Thailand (Chi et al., 2013; Ku et al., 2016). HLA-DRB1*15:02 and HLA-DRB1*16:02 are common not only in Southeast Asians but also in Pacific Islanders and Amerindians (for HLA-DRB1*16:02). By contrast, they are rare in Europeans and Africans, in whom HLA-DQB1*05:01 and DQB1*05:02 are more widespread. All Asian patients with nAIGAs identified to date carry at least HLA-DRB1*15:02 or HLA-DRB1*16:02. Interestingly, the only non-Asian HLA-typed patient identified carried neither of these alleles, nor either of the linked HLA-DQB1*05:01 and DQB1*05:02 alleles (Ku et al., 2016). The mechanisms driving the occurrence of auto-Abs in individuals carrying these HLA haplotypes remain unclear, and the corresponding T cell epitopes are unknown.
Auto-Abs against type I IFNs
Type I IFNs are potent antiviral cytokines that operate as a first line of defense against many viruses (Hertzog and Williams, 2013; Isaacs and Lindenmann, 1957; Isaacs et al., 1957; Lazear et al., 2019; Meyts and Casanova, 2021; Munoz-Moreno et al., 2021). There are 17 type I IFNs (13 IFN-α subtypes, IFN-β, -κ, -ε, and -ω), all closely related phylogenetically, and all binding to the same receptor composed of the IFNAR1 and IFNAR2 chains (Lazear et al., 2019; Manry et al., 2011). Inborn errors of type I IFN immunity underlying severe viral diseases, including influenza and COVID-19 pneumonia, have been described since 2003 (Bastard et al., 2020a; Beck and Aksentijevich, 2020; Bousfiha et al., 2020; Duncan et al., 2015; Duncan et al., 2021; Hambleton et al., 2013; Hernandez et al., 2019; Hernandez et al., 2018; Kreins et al., 2015; Meyts and Casanova, 2021; Zhang et al., 2020). Neutralizing auto-Abs against type I IFNs were first detected in the 1980s, in patients treated with IFN-α or IFN-β for various indications (Rudick et al., 1998; Vallbracht et al., 1981) and in patients with systemic lupus erythematosus (SLE), a condition associated with high levels of type I IFNs in the blood (Gupta et al., 2016; Panem et al., 1982). Auto-Abs against type I IFNs are also observed in patients with thymic abnormalities, such as thymoma (Shiono et al., 2003), or myasthenia gravis (Bello-Rivero et al., 2004; Meager et al., 2003). The production of high levels of anti–type I IFN auto-Abs may be genetically driven and may occur in early childhood, as in patients with APS-1 carrying germline biallelic or monoallelic rare deleterious variants of AIRE (Levin, 2006; Meager et al., 2006; Meyer et al., 2016; Oftedal et al., 2015). They are also found in patients with biallelic hypomorphic mutations of RAG1 or RAG2 and combined immunodeficiency (Walter et al., 2015), in men with hemizygous mutations of FOXP3 and IPEX (Rosenberg et al., 2018), and in women with heterozygous null mutations of X-linked NEMO and incontinentia pigmenti (Bastard et al., 2020b). In 1984, Ion Gresser described a 77-yr-old woman with disseminated zoster and no history of severe viral disease in whom auto-Abs neutralizing type I IFNs were detected (Pozzetto et al., 1984). Nevertheless, over the last 40 yr, these auto-Abs have generally been considered to be clinically silent.
Neutralizing auto-Abs against type I IFNs underlie severe or critical COVID-19
In 2020, a large international cohort of patients infected with SARS-CoV-2 was tested for the presence of auto-Abs neutralizing 10 ng/ml IFN-α2 and/or -ω. At least 10% of patients with life-threatening COVID-19 pneumonia carried these neutralizing auto-Abs, which were not found in any of the individuals with asymptomatic or paucisymptomatic infection tested (Bastard et al., 2020b). These auto-Abs were mostly found in men (95%), and half the patients carrying them were over the age of 65 yr (Bastard et al., 2020b). These findings were subsequently widely replicated in independent cohorts (Abers et al., 2021; Acosta-Ampudia et al., 2021; Carapito et al., 2022; Chang et al., 2021; Chauvineau-Grenier et al., 2022; Goncalves et al., 2021; Koning et al., 2021; Raadsen et al., 2022; Solanich et al., 2021; Troya et al., 2021; van der Wijst et al., 2021; Vazquez et al., 2021; Wang et al., 2021; Zhang et al., 2022; Ziegler et al., 2021). Consistently, patients with APS-1 and preexisting anti–type I IFN auto-Abs were found to be at very high risk of severe disease upon SARS-CoV-2 infection (Bastard et al., 2020b; Beccuti et al., 2020; Carpino et al., 2021). In a large study of 22 APS-1 patients, most (n = 19, 86%) suffered from severe or critical COVID-19 and four patients died; the others had mild or asymptomatic infections, possibly due to prior or early medical interventions (Bastard et al., 2021c). Another group recently described four younger APS-1 patients with neutralizing auto-Abs, who developed only mild or moderate COVID-19 (Meisel et al., 2021). Overall, patients with APS-1, particularly those over the age of 25 yr, are at very high risk of developing severe or critical COVID-19 pneumonia. They should benefit from early vaccination and prompt treatment in cases of infection before vaccination (Bastard et al., 2021c). There are probably also other conditions, monogenic or otherwise, underlying the production of these auto-Abs and conferring a predisposition to life-threatening COVID-19 pneumonia.
Neutralizing auto-Abs against type I IFNs in ∼20% of patients with severe or critical COVID-19
The use of more sensitive assays to detect auto-Abs neutralizing more physiological concentrations of type I IFNs (100 pg/ml in plasma diluted 1/10) revealed the presence of such Abs in up to 13.6% of patients of all ages with critical COVID-19. The prevalence of these auto-Abs increased with age, and they were found in more than 20% of patients with critical COVID-19 over the age of 80 yr, and accounted for almost 20% of all COVID-19 deaths (Bastard et al., 2021a). In addition, 6.8% of patients with severe, but not critical COVID-19 also carried such auto-Abs. These data strongly suggested that individuals with these auto-Abs were at higher risk of developing life-threatening disease. Indeed, the highest ORs were obtained for the patients having auto-Abs neutralizing both IFN-α2 and IFN-ω at 10 ng/ml or 100 pg/ml (OR = 67, P < 7.8 × 10−13 or OD = 54, P < 10−13), whereas ORs were lower, although still highly significant, for individuals carrying auto-Abs against IFN-α2 or IFN-ω alone. Auto-Abs against IFN-β were also found in about 1% of patients with critical COVID-19, with an OR of 5 (P = 0.043). Testing was not performed for auto-Abs against IFN-ε or IFN-κ. In the patients tested, the auto-Abs against type I IFNs were present before SARS-CoV-2 infection, as in patients with APS-1 (Bastard et al., 2021c). Their presence resulted in lower levels of IFN-stimulated gene expression in the blood (van der Wijst et al., 2021), and they were also found in the upper respiratory tract (de Prost et al., 2021; Lopez et al., 2021), along with diminished type I IFN activity in the nasal mucosae (Lopez et al., 2021). It is unknown whether these antibodies are present and functional in the lower respiratory tract. Overall, these data demonstrate that the neutralization of only one subtype or group of type I IFNs (the 13 IFN-α, or IFN-ω, or IFN-β) is sufficient to underlie life-threatening COVID-19 pneumonia (Casanova and Abel, 2021b; Zhang et al., 2022).
Neutralizing auto-Abs against type I IFNs in the general population
Given the greater risk of severe or critical COVID-19 in individuals with neutralizing auto-Abs against type I IFNs, especially in the elderly (Bastard et al., 2020b; van der Wijst et al., 2021), samples collected from a large general population cohort of over 34,000 individuals aged 20–100 yr before the COVID-19 pandemic were tested. Strikingly, the prevalence of auto-Abs neutralizing 10 ng/ml (and 100 pg/ml) IFN-α and/or IFN-ω increased significantly with age, with 0.17% of individuals under the age of 70 yr tested positive for these antibodies (1.1% for 100 pg/ml), and more than 1.4% of those over the age of 70 yr (4.4%). The prevalence of these antibodies even reached 4.2% (7.1%) for individuals between the ages of 80 and 85 yr. However, it decreased slightly after the age of 85 yr, perhaps because the individuals with these auto-Abs died before the COVID-19 pandemic from other illnesses related to the presence of the auto-Abs. No specific HLA class II alleles have been associated with the production of auto-Abs against type I IFNs. The B and T cell epitopes are unknown. These auto-Abs nevertheless contribute to the increase in the risk of critical COVID-19 in the elderly population. This increase in anti–type I IFN auto-Ab production in the elderly resembles that already described for various other auto-Abs since the 1960s (Hooper et al., 1972; Myasoedova et al., 2020; Parks et al., 2014; Potocka-Plazak et al., 1995; Shu et al., 1975). These auto-Abs appear to have remained clinically silent in these individuals until SARS-CoV-2 infection, although a more complete analysis of the medical history of these individuals is required. Indeed, auto-Abs against type I IFNs were recently shown to underlie severe adverse events following vaccination with the live attenuated yellow fever virus vaccine (Bastard et al., 2021b). It is therefore clearly possible that they may also underlie other severe viral or malignant diseases, especially in the elderly. Various anti-IFN mAbs have been used in clinical practice (e.g., sifalimumab, an anti–IFN-α IgG1 mAb, or anifrolumab, an anti-IFNAR1 mAb, used for the treatment of SLE, in particular), and have occasionally been linked to an increase in the incidence of shingles or respiratory tract infections (Furie et al., 2017; Khamashta et al., 2016; Petri et al., 2013; Tummala et al., 2021; Table 1).
IL-17A/F and mucocutaneous candidiasis
In humans, the essential and redundant roles of IL-17A/F have emerged through the molecular identification and cellular characterization of inborn errors of IL-17 immunity (Puel, 2020; Puel et al., 2012). Following the identification of STAT3 deficiency as the main genetic cause of AD “classical” hyper IgE syndrome (HIES; Holland et al., 2007; Minegishi et al., 2007; Renner et al., 2007), several teams reported low proportions of Th17 cells in HIES patients (de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008; Minegishi et al., 2009). Impaired IL-17 production, possibly due to impaired STAT3-dependent cellular responses to IL-6, IL-21, and/or IL-23 (Zhou and Littman, 2009), has been proposed as an explanation for the recurrent bacterial infections and CMC seen in these patients. Similarly, patients with AR IL-12p40 or IL-12Rβ1 deficiency, and a lack of IL-12 and IL-23 production or of response to these cytokines, respectively, also have abnormally small proportions of Th17 cells, possibly due to the absence of IL-23 signaling (de Beaucoudrey et al., 2008; Ma et al., 2016). These patients typically suffer from MSMD, but about 25% also display CMC (Bustamante, 2020). Finally, about two thirds of patients with AR deficiencies of caspase recruitment domain-containing protein 9—an adaptor acting downstream from the C-type lectin receptors that recognize fungal motifs and lead to the production of pro-inflammatory cytokines, including pro-Th17 cytokines (i.e., IL-6, IL-23)—have low proportions of Th17 cells (Corvilain et al., 2018). Most of these patients suffer from extensive/invasive fungal diseases mostly caused by ascomycete fungi, and about 40% have CMC (Corvilain et al., 2018; Li et al., 2017). These findings suggested a role for IL-17A/F in mucocutaneous protection against Candida albicans and, possibly, Staphylococcus aureus (Puel et al., 2010b). This role was definitively demonstrated by the identification of 13 inborn errors of IL-17 immunity, including AD IL-17F and JNK1 deficiencies and AR IL-17RA, IL-17RC, and ACT1 deficiencies, in particular, all of which impair or abolish IL-17A/F signaling and are associated with CMC (Puel, 2020). Some defects, such as AR IL-17RA, AR ACT1, and AD JNK1 deficiencies, are also associated with staphylococcal skin diseases (Li et al., 2019).
Neutralizing auto-Abs against IL-17A/F
CMC is one of the three most common clinical manifestations of APS-1 patients, often the earliest to appear (Cheng and Anderson, 2012). In 2010, two independent studies reported that almost all APS-1 patients, of all ages, tested, had high serum titers of IgG auto-Abs against at least one of the Th17 cytokines (IL-17A, IL-17F, and/or IL-22; Liang et al., 2006; Zheng et al., 2007), neutralizing up to 50 ng/ml IL-17A, 10 ng/ml IL-17F, and/or 0.5 ng/ml IL-22 (in plasma diluted 1/10; Kisand et al., 2010; Puel et al., 2010a). None of the healthy controls, healthy heterozygous relatives, or patients with various endocrine or autoimmune disorders tested in parallel had such auto-Abs (Kisand et al., 2010; Puel et al., 2010a), except two patients with thymoma, who were the only two patients with documented CMC out of the 35 patients with thymoma tested (Kisand et al., 2010). Apart from high levels of auto-Abs against IFN-α and IFN-ω, none of the patients had neutralizing auto-Abs against any of the 13 other cytokines tested (including nAIGAs and known antibodies against cytokines involved in Th17 cell differentiation or maintenance, such as IL-23). A few patients had high levels of auto-Abs against at least one of the three cytokines in the apparent absence of CMC (Kisand et al., 2010; Puel et al., 2010a), but the prevalence and titers of neutralizing auto-Abs were higher in patients with CMC than in those without CMC (Kisand et al., 2010). Auto-Abs against IL-17 cytokines were detectable before the onset of CMC in the informative serum samples of four patients with APS-1 and one with thymoma, with no clear increase in titer after CMC onset, suggesting that they were not triggered by candidiasis (Kisand et al., 2010). No specific HLA class II alleles or haplotypes have been associated with the production of anti–IL-17A/F auto-Abs. The T and B cell epitopes are unknown. Auto-Abs against IL-17A, IL-17F, and/or IL-22 are detected in >90% of patients with APS-1, in whom CMC is a hallmark of the disease (Kisand et al., 2021; Philippot et al., 2021; Puel and Casanova, 2021; Wolff et al., 2013). The lack of staphylococcal skin disease in most APS-I patients may result from residual IL-17A/F immunity, or the compensatory effect of other IL-17 cytokines. The identification of auto-Abs against IL-17A, IL-17F, and/or IL-22 in APS-1 solved the long-standing enigma of CMC in this disorder. The description of mild or moderate oral candidiasis in up to 21% of patients treated with therapeutic Abs blocking IL-17A/F signaling (Reich et al., 2021) was predicted by studies of APS-1 patients (Kisand et al., 2021; Philippot et al., 2021; Puel and Casanova, 2021; Table 1).
IL-6 and staphylococcal disease
The role of human IL-6, like that of IL-17A/F, has been progressively clarified by the study of IEI impairing its signaling (Chen et al., 2021a; Puel and Casanova, 2019). The description of dominant-negative mutations of STAT3 as the main cause of AD HIES (Asano et al., 2021b; Minegishi et al., 2007) revealed that impaired STAT3-dependent signaling downstream from several cytokines, including IL-6 (Kane et al., 2014), caused the complex clinical and cellular phenotype observed in these patients characterized by severe early-onset atopic dermatitis, recurrent skin and sino-pulmonary bacterial infections, CMC, poor or delayed clinical and biological signs of inflammation, eosinophilia, high serum IgE levels, low levels of memory B and Th17 cells, and various nonhematopoietic features (Tsilifis et al., 2021). Following this discovery, additional IEI associated with most, if not all of the clinical features observed in classical HIES, were reported, including AR deficiency of ZNF341, a transcription factor governing STAT3 expression and activity (Beziat et al., 2018; Frey-Jakobs et al., 2018), and partial AR (Chen et al., 2021b; Schwerd et al., 2017; Shahin et al., 2019) or AD (Beziat et al., 2020) deficiencies of GP130, the signaling receptor subunit common to all IL-6 family cytokines (Rose-John, 2018), suggesting that impaired IL-6 immunity underlies many of the key immunological and clinical features of HIES. Patients with AR IL-6R deficiency were first reported in 2019 (Nahum et al., 2020; Spencer et al., 2019). These patients displayed most of the clinical features of HIES, including severe atopic dermatitis, recurrent bacterial sinopulmonary infections, recurrent staphylococcal skin abscesses, poor inflammatory responses with undetectable C-reactive protein, high IgE levels, with or without eosinophilia, low-to-normal levels of memory B cells, and low but detectable levels of Th17 cells. These findings suggest that impaired IL-6 signaling drives most of the clinical presentations of HIES, and that this cytokine plays a crucial role in protection against bacterial mucocutaneous diseases, particularly those caused by staphylococci (Puel and Casanova, 2019).
Neutralizing auto-Abs against IL-6
Four patients with high levels of IgG (IgG1 or IgG4) auto-Abs neutralizing up to 50 ng/ml IL-6 (plasma diluted 1/10) have been reported since 2008 (Bloomfield et al., 2019; Nanki et al., 2013; Puel et al., 2008). These patients (aged from 11 mo to 67 yr) suffered from recurrent staphylococcal subcutaneous abscesses (n = 2), Escherichia coli and Streptococcus intermedius thoracic empyema (n = 1), or severe septic shock probably due to S. aureus (n = 1). None of the patients displayed any detectable increase in serum C-reactive protein concentration during infectious episodes. In the only patient tested, memory B cell counts were low, and serum IgE levels were high (Bloomfield et al., 2019). IL-6 was barely detectable in whole blood from the patients after stimulation, despite normal production by monocytes (as assessed by intracellular staining) or peripheral blood mononuclear cells tested in the absence of patients' plasma (Bloomfield et al., 2019; Puel et al., 2008). B cell epitope mapping was performed with 15-mer peptides overlapping by 10 amino acid residues, generated from the human IL-6 protein sequence. A peptide (LTKLQAQNQWLQDMT) was strongly bound by the serum samples of both patients tested (Nanki et al., 2013). However, no specific HLA class II allele or any other genetic variant, whether germline or somatic, has been associated with the occurrence of neutralizing anti–IL-6 auto-Abs. The T-cell epitope remains unknown. Collectively, inborn errors of the IL-6 pathway and their autoimmune phenocopies suggest that IL-6 is crucial for immunity to bacterial diseases, including staphylococcal skin diseases in particular, and for acute-phase inflammatory responses. Consistent with this conclusion, occasional bacterial skin and sinopulmonary infections, such as cellulitis and pneumonia, possibly caused by S. aureus, have been reported in patients following treatment with tocilizumab (a humanized monoclonal Ab against the IL-6 receptor), sirukumab, or siltuximab (anti–IL-6 mAbs; Aletaha et al., 2021; Pawar et al., 2019; Rose-John et al., 2017; Table 1).
GM-CSF and infections
PAP is a severe lung disease characterized by the accumulation of surfactant lipids and proteins in the alveolar space, resulting in progressive respiratory failure and an increase in the risk of infection (Trapnell et al., 2003). Rare patients with severe early-onset PAP due to inborn errors of the GM-CSF pathway have been reported since 1997, with AR deficiency of the βc receptor chain (Dirksen et al., 1997) common to the receptors for IL-3, IL-5, and GM-CSF, or AR deficiency of GM-CSFRα, encoded by the CSF2RA gene (Martinez-Moczygemba et al., 2008; Suzuki et al., 2008; Suzuki et al., 2010). These mutations may impair the terminal differentiation of alveolar macrophages, through impairment of the GM-CSF–dependent induction of expression of the transcription factor PU.1 in these cells, as shown in GM-CSF–deficient mice, resulting in a lower capacity to catabolize surfactant (Trapnell et al., 2019). Several cases of superinfections with unusual pathogens were reported from the early 1960s onward, before the identification of any genetic or immunological cause of PAP, some of which were caused by Nocardia spp. (Pascual et al., 1989). In a literature review aiming to identify all reported cases of PAP and unusual infections between 1950 and mid-2010, 75 cases were found, with nocardial infections being the most frequent, identified in n = 32 (43%) cases, with Nocardia asteroides as the causal agent in 19 cases (Punatar et al., 2012). Most patients suffered from pulmonary nocardiosis (n = 24), with or without infections at other sites, including the central nervous system (CNS, n = 6; Punatar et al., 2012). Other infections were also reported in these patients: mycobacterial (n = 28, 37%) in some, mostly due to M. tuberculosis (n = 21, 75%), with a few cases of Mycobacterium kansasii (n = 4, 14%) or Mycobacterium avium (n = 3, 11%) infections, or fungal in others (n = 15, 20%), mostly caused by Aspergillus spp. (n = 5, 33%), Cryptococcus spp. (n = 6, 40%), or Histoplasma capsulatum (n = 4, 27%; Punatar et al., 2012). Human GM-CSF is thus required for the efficient removal of surfactant by alveolar macrophages, and thereby and/or by hitherto unknown mechanisms for pulmonary defense against several pathogens, including Nocardia and Cryptococcus in particular. Yet, the rare patients with inborn errors of the GM-CSF receptor had PAP but no documented infection. There is therefore no causality between GM-CSF deficiency and nocardiosis or cryptococcosis.
Neutralizing auto-Abs against GM-CSF
High titers of neutralizing auto-Abs against GM-CSF have been reported in patients with idiopathic PAP worldwide since 1999 (Kitamura et al., 1999; Seymour and Presneill, 2002), in about 90% of the more than 400 PAP cases reported (Trapnell et al., 2019). These patients typically developed symptoms in adulthood (Seymour and Presneill, 2002). In addition to typical respiratory infections, these patients also displayed pulmonary and extrapulmonary (e.g., CNS) infections with various pathogens, including Nocardia spp., mycobacteria, Histoplasma spp., Cryptococcus spp., and Aspergillus spp. These infections may be secondary to PAP, the use of steroids, and/or impaired GM-CSF signaling directly compromising alveolar macrophage immunity to these pathogens (Punatar et al., 2012). Since 2013, high titers of IgG (mostly IgG1) auto-Abs neutralizing 10 ng/ml GM-CSF (plasma diluted 1/10) have been found in patients with adult-onset isolated idiopathic disseminated disease mostly due to Cryptococcus spp., almost exclusively Cryptococcus gattii (Applen Clancey et al., 2019; Browne et al., 2012a; Crum-Cianflone et al., 2017; Demir et al., 2018; Huynh et al., 2020; Kuo et al., 2017; Panackal et al., 2017; Perrineau et al., 2020; Rosen et al., 2013; Saijo et al., 2014; Stevenson et al., 2019; Viola et al., 2021), Nocardia spp. (Rosen et al., 2015), or more rarely, Aspergillus spp. (Arai et al., 2015). Some of these patients eventually developed PAP after their infectious disease (Rosen et al., 2013). The brain and lungs were the most frequently affected organs. Most of the patients were male (72%) and of various ancestries. A recent genome-wide association study of autoimmune PAP in patients and controls of Japanese ancestry found that the HLA class II allele HLA-DRB1*08:03, which is common in Asian populations (e.g., 8.3% in Japanese individuals) but very rare or absent in other populations, including Europeans (e.g., 0.3% in Germans, 0% in Italians), was associated (P = 0.035) with high levels of anti–GM-CSF auto-Abs in patients, suggesting an underlying genetic predisposition for the production of these auto-Abs, at least in individuals of Asian ancestry (Sakaue et al., 2021). In contrast, no HLA allele was associated with these auto-Abs in a study of 47 European patients with PAP (Anderson et al., 2019).
The T and B cell epitopes remain unknown. Thus, studies of inborn errors of GM-CSF and their autoimmune phenocopies suggest that GM-CSF is a crucial cytokine for immunity to Nocardia and Cryptococcus spp., particularly in the lungs and CNS, and that patients with idiopathic isolated cryptococcosis or nocardiosis may suffer from inborn errors of the GM-CSF pathway. Several anti–GM-CSF (KB003/lenzilumab, namilumab/AMG203, TJ003234, gimsilumab, otilimab/GSK3196165) or anti–GM-CSFRα (mavrilimumab) mAbs have been developed for clinical use in patients with various conditions (e.g., severe asthma, psoriasis, rheumatoid arthritis, COVID-19, chronic myelomonocytic leukemia). Rare infectious events have been reported (e.g., nasopharyngitis) with a slightly higher incidence than that for the placebo group. However, to our knowledge, no cases with features of PAP have been identified (Molfino et al., 2016; Patnaik et al., 2020; Table 1). Overall, while it is clear that inborn errors of and auto-Abs to GM-CSF underlie PAP, the infections seen in these patients are relatively diverse and may be a consequence of PAP itself (and its consequences, including steroid therapy) and/or of a dysfunction of GM-CSF–dependent immunity, especially in alveolar macrophages.
Concluding remarks
These studies provide a compelling evidence that autoimmunity may not only be triggered by infection (Bigley and Cooper, 2021; Knight et al., 2021), but that it can predate infection and be causal for infectious disease. The findings reviewed here have direct clinical implications for the diagnosis and management of patients with auto-Abs against any of these four or five cytokines. The depletion of auto-Abs or of the corresponding B cells may prevent relapses of infection, as shown for mycobacterial disease (Browne et al., 2012b; Czaja et al., 2014; Koizumi et al., 2017; Pruetpongpun et al., 2016). The detection of these auto-Abs before the occurrence of disease may lead to specific measures being taken, such as vaccination against the pathogen, or treatment with mAb against the pathogen, or early treatment with exogenous recombinant cytokines (e.g., IFN-β) following infection, as exemplified by COVID-19 (Vinh et al., 2021). The detection of these auto-Abs may also constitute a contraindication for some vaccinations, such as vaccination with live attenuated viruses in patients with auto-Abs against type I IFNs (Bastard et al., 2021b). These studies also raise the possibility that other infectious diseases may be caused by the same or other auto-Abs directed against cytokines. For example, other viral illnesses may develop due to the presence of auto-Abs against type I IFNs, and mycobacterial diseases or diseases caused by intramacrophagic microorganisms may develop due to the presence of auto-Abs against type II IFN (Bastard et al., 2021a; Shih et al., 2021). It will, therefore, be of interest to test for known auto-Abs in various cohorts of patients with idiopathic infectious diseases. Conversely, it may also be useful to test the hypothesis that these auto-Abs protect against some cytokine-dependent conditions (Uggenti et al., 2019). For example, it has been suggested that auto-Abs against type I IFNs are associated with a milder course of SLE (Gupta et al., 2016; Morimoto et al., 2011). The discovery of new auto-Abs directed against cytokines is another important challenge. Broad screening for auto-Abs in patients with infectious diseases would benefit from being performed at least 1 yr after infection, as infections can themselves trigger of the production of various auto-Abs. This search would also benefit from the discovery of new inborn errors of cytokines.
These studies also pose more fundamental biological questions. A first set of questions concerns their causes. The production of auto-Abs against type I IFN and IL-17 may result from IEI impairing T cell tolerance (Kisand et al., 2010; Meager et al., 2006; Puel et al., 2010a), but little is known about the genetic basis of other auto-Abs. All the various IEI underlying type I IFN auto-Abs impair T cell tolerance in the thymus or the periphery (Bastard et al., 2021c; Bastard et al., 2020b; Rosenberg et al., 2018; Walter et al., 2015). The sudden increase in the production of auto-Abs against type I IFNs in individuals over the age of 60 yr is another mystery (Bastard et al., 2021a). This increase may be due to genetic or epigenetic causes, which may be germline or somatic. The distribution of these auto-Abs by age, sex, and ancestry is known only for those directed against type I IFNs (age, sex) and type II IFN (ancestry; Bastard et al., 2021a; Bastard et al., 2020b; Browne et al., 2012a; Shih et al., 2021). It will also be important to determine the distribution of auto-Abs against other cytokines in the general population. The East Asian predominance of auto-Abs against type II IFN reflects the higher frequency of predisposing HLA-DRB1 alleles in these populations (Chi et al., 2013; Ku et al., 2016; Pithukpakorn et al., 2015), raising questions about possible HLA associations for other anti-cytokine auto-Abs, as suggested for anti–GM-CSF auto-Abs in PAP patients of Japanese ancestry (Sakaue et al., 2021). Another fundamental question is the nature of the cytokine-specific Igs and their B cell epitopes, which are known only for auto-Abs against type II IFN (Lin et al., 2016) and IL-6 (Nanki et al., 2013). The high prevalence of auto-Abs against type I IFN in the elderly European population, and in almost all patients with APS-1, together with the diversity of the type I IFNs recognized, suggests that there is unlikely to be an HLA association and implies the existence of multiple T cell epitopes, or a promiscuous T cell epitope. Auto-Abs against IL-17A/F are also found in most APS-1 patients. Investigations of the causes, nature, distribution, and consequences of known and newly discovered auto-Abs against cytokines promise to be an exciting area of study.
Acknowledgments
We warmly thank the members of the Human Genetics of Infectious Diseases laboratory for helpful discussions.
This work was supported by the Howard Hughes Medical Institute, The Rockefeller University, the St. Giles Foundation, the National Institutes of Health (R01AI088364, R01AI095983, R01AI127564, R01AI163029), the National Center for Advancing Translational Sciences, the National Institutes of Health Clinical and Translational Science Awards program (UL1 TR001866), the Fisher Center for Alzheimer’s Research Foundation, the Meyer Foundation, the JPB Foundation, the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (EQU201903007798), the Agence nationale de recherches sur le sida et les hépatites virales Nord-Sud (ANRS-COV05), ANR GENVIR (ANR-20-CE93-003), ANR AABIFNCOV (ANR-20-CO11-0001) and ANR GenMIS-C (ANR-21-COVR-0039) projects, ECOS-Nord (C19S0163407), the European Union’s Horizon 2020 research and innovation program under grant agreement No. 824110 (EASI-Genomics), the Square Foundation, Grandir–Fonds de solidarité pour l’Enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, the Institut National de la Santé et de la Recherche Médicale (INSERM), the French Ministry of Higher Education, Research, and Innovation (MESRI-COVID-19), REACTing-INSERM, and the University of Paris. P. Bastard was supported by the Fondation Recherche Médicale (EA20170638020) and the MD-PhD program of the Imagine Institute (with the support of the Fondation Bettencourt Schueller).
Author contributions: A. Puel: conceptualization, funding acquisition, resources, writing (original draft), writing (review & editing). P. Bastard: conceptualization, investigation, methodology, writing (original draft). J. Bustamante: conceptualization, funding acquisition, resources, writing (original draft). J.-L. Casanova: conceptualization, funding acquisition, project administration, resources, supervision, visualization, writing (original draft), writing (review & editing).
References
- Abers, M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. 2021. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 99:917–921. 10.1111/imcb.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Ampudia, Y., Monsalve D.M., Rojas M., Rodriguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. 2021. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 118:102598. 10.1016/j.jaut.2021.102598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aletaha, D., Bingham C.O., Karpouzas G.A., Takeuchi T., Thorne C., Bili A., Agarwal P., Hsu B., Rao R., Brown K., and Tanaka Y.. 2021. Long-term safety and efficacy of sirukumab for patients with rheumatoid arthritis who previously received sirukumab in randomised controlled trials (SIRROUND-LTE). RMD Open. 7:e001465. 10.1136/rmdopen-2020-001465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, K., Carey B., Martin A., Roark C., Chalk C., Nowell-Bostic M., Freed B., Aubrey M., Trapnell B., and Fontenot A.. 2019. Pulmonary alveolar proteinosis: An autoimmune disease lacking an HLA association. PLoS One. 14:e0213179. 10.1371/journal.pone.0213179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applen Clancey, S., Ciccone E.J., Coelho M.A., Davis J., Ding L., Betancourt R., Glaubiger S., Lee Y., Holland S.M., Gilligan P., et al. 2019. Cryptococcus deuterogattii VGIIa infection associated with travel to the pacific northwest outbreak region in an anti-granulocyte-macrophage colony-stimulating factor autoantibody-positive patient in the United States. mBio. 10. 10.1128/mbio.02733-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, T., Inoue Y., Akira M., Nakata K., and Kitaichi M.. 2015. Autoimmune pulmonary alveolar proteinosis following pulmonary aspergillosis. Intern. Med. 54:3177–3180. 10.2169/internalmedicine.54.5034 [DOI] [PubMed] [Google Scholar]
- Asano, T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. 2021a. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6:eabl4348. 10.1126/sciimmunol.abl4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, T., Khourieh J., Zhang P., Rapaport F., Spaan A.N., Li J., Lei W.T., Pelham S.J., Hum D., Chrabieh M., et al. 2021b. Human STAT3 variants underlie autosomal dominant hyper-IgE syndrome by negative dominance. J. Exp. Med. 218:e20202592. 10.1084/jem.20202592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari, S., Schlapbach L.J., Anchisi S., Hammer C., Bartha I., Junier T., Mottet-Osman G., Posfay-Barbe K.M., Longchamp D., Stocker M., et al. 2017. Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc. Natl. Acad. Sci. USA. 114:8342–8347. 10.1073/pnas.1704259114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. 2021a. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 6:eabl4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Manry J., Chen J., Rosain J., Seeleuthner Y., AbuZaitun O., Lorenzo L., Khan T., Hasek M., Hernandez N., et al. 2020a. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency. J. Clin. Invest. 131:e139980. 10.1172/jci139980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Michailidis E., Hoffmann H.H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., et al. 2021b. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 218:e20202486. 10.1084/jem.20202486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Orlova E., Sozaeva L., Levy R., James A., Schmitt M.M., Ochoa S., Kareva M., Rodina Y., Gervais A., et al. 2021c. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 218:e20210554. 10.1084/jem.20210554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Beziat V., et al. 2020b. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 370:eabd4585. 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccuti, G., Ghizzoni L., Cambria V., Codullo V., Sacchi P., Lovati E., Mongodi S., Iotti G.A., and Mojoli F.. 2020. A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in lombardy, Italy: Letter to the editor. J. Endocrinol. Invest. 43:1175–1177. 10.1007/s40618-020s4001323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, D.B., and Aksentijevich I.. 2020. Susceptibility to severe COVID-19. Science. 370:404–405. 10.1126/science.abe7591 [DOI] [PubMed] [Google Scholar]
- Bello-Rivero, I., Cervantes M., Torres Y., Ferrero J., Rodriguez E., Perez J., Garcia I., Diaz G., and Lopez-Saura P.. 2004. Characterization of the immunoreactivity of anti-interferon alpha antibodies in myasthenia gravis patients. Epitope mapping. J. Autoimmun. 23:63–73. 10.1016/j.jaut.2004.03.013 [DOI] [PubMed] [Google Scholar]
- Beziat, V., Li J., Lin J.X., Ma C.S., Li P., Bousfiha A., Pellier I., Zoghi S., Baris S., Keles S., et al. 2018. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci. Immunol. 3:eaat4956. 10.1126/sciimmunol.aat4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat, V., Tavernier S.J., Chen Y.H., Ma C.S., Materna M., Laurence A., Staal J., Aschenbrenner D., Roels L., Worley L., et al. 2020. Dominant-negative mutations in human IL6ST underlie hyper-IgE syndrome. J. Exp. Med. 217:e20191804. 10.1084/jem.20191804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley, T.M., and Cooper M.A.. 2021. Monogenic autoimmunity and infectious diseases: The double-edged sword of immune dysregulation. Curr. Opin. Immunol. 72:230–238. 10.1016/j.coi.2021.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield, M., Parackova Z., Cabelova T., Pospisilova I., Kabicek P., Houstkova H., and Sediva A.. 2019. Anti-IL6 autoantibodies in an infant with CRP-less septic shock. Front. Immunol. 10:2629. 10.3389/fimmu.2019.02629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedigheimer, M.J., Martin D.A., Amoura Z., Sanchez-Guerrero J., Romero-Diaz J., Kivitz A., Aranow C., Chan T.M., Chong Y.B., Chiu K., et al. 2017. Safety, pharmacokinetics and pharmacodynamics of AMG 811, an anti-interferon-gamma monoclonal antibody, in SLE subjects without or with lupus nephritis. Lupus Sci. Med. 4:e000226. 10.1136/lupus-2017-000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dupuis, S., and Bustamante J.. 2021. Mycobacterial diseases in patients with inborn errors of immunity. Curr. Opin. Immunol. 72:262–271. 10.1016/j.coi.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfiha, A., Jeddane L., Picard C., Al-Herz W., Ailal F., Chatila T., Cunningham-Rundles C., Etzioni A., Franco J.L., Holland S.M., et al. 2020. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J. Clin. Immunol. 40:66–81. 10.1007/s10875-020s1000758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, S.K. 2014. Anticytokine autoantibody-associated immunodeficiency. Annu. Rev. Immunol. 32:635–657. 10.1146/annurev-immunol-032713-120222 [DOI] [PubMed] [Google Scholar]
- Browne, S.K., Burbelo P.D., Chetchotisakd P., Suputtamongkol Y., Kiertiburanakul S., Shaw P.A., Kirk J.L., Jutivorakool K., Zaman R., Ding L., et al. 2012a. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 367:725–734. 10.1056/NEJMoa1111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, S.K., and Holland S.M.. 2010. Immunodeficiency secondary to anticytokine autoantibodies. Curr. Opin. Allergy Clin. Immunol. 10:534–541. 10.1097/ACI.0b013e3283402b41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, S.K., Zaman R., Sampaio E.P., Jutivorakool K., Rosen L.B., Ding L., Pancholi M.J., Yang L.M., Priel D.L., Uzel G., et al. 2012b. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood. 119:3933–3939. 10.1182/blood-2011b12-395707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante, J. 2020. Mendelian susceptibility to mycobacterial disease: Recent discoveries. Hum. Genet. 139:993–1000. 10.1007/s00439-020s0002120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapito, R., Li R., Helms J., Carapito C., Gujja S., Rolli V., Guimaraes R., Malagon-Lopez J., Spinnhirny P., Lederle A., et al. 2022. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci. Transl Med. 14:eabj7521. 10.1126/scitranslmed.abj7521 [DOI] [PubMed] [Google Scholar]
- Carpino, A., Buganza R., Matarazzo P., Tuli G., Pinon M., Calvo P.L., Montin D., Licciardi F., and De Sanctis L.. 2021. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in two siblings: Same mutations but very different phenotypes. Genes (Basel). 12:169. 10.3390/genes12020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.L., and Abel L.. 2020. The human genetic determinism of life-threatening infectious diseases: Genetic heterogeneity and physiological homogeneity? Hum. Genet. 139:681–694. 10.1007/s00439-020s0002184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.L., and Abel L.. 2021a. Lethal infectious diseases as inborn errors of immunity: Toward a synthesis of the germ and genetic theories. Annu. Rev. Pathol. 16:23–50. 10.1146/annurev-pathol-031920-101429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.L., and Abel L.. 2021b. Mechanisms of viral inflammation and disease in humans. Science. 374:1080–1086. 10.1126/science.abj7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., Barman L., Bennett K., Chakraborty S., Chang I., et al. 2021. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 12:5417. 10.1038/s41467-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvineau-Grenier, A., Bastard P., Servajean A., Gervais A., Rosain J., Jouanguy E., Cobat A., Casanova J.L., and Rossi B.. 2022. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J. Clin. Immunol. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.H., Spencer S., Laurence A., Thaventhiran J.E., and Uhlig H.H.. 2021a. Inborn errors of IL-6 family cytokine responses. Curr. Opin. Immunol. 72:135–145. 10.1016/j.coi.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.H., Zastrow D.B., Metcalfe R.D., Gartner L., Krause F., Morton C.J., Marwaha S., Fresard L., Huang Y., Zhao C., et al. 2021b. Functional and structural analysis of cytokine-selective IL6ST defects that cause recessive hyper-IgE syndrome. J. Allergy Clin. Immunol. 148:585–598. 10.1016/j.jaci.2021.02.044 [DOI] [PubMed] [Google Scholar]
- Cheng, M.H., and Anderson M.S.. 2012. Monogenic autoimmunity. Annu. Rev. Immunol. 30:393–427. 10.1146/annurev-immunol-020711-074953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, C.Y., Chu C.C., Liu J.P., Lin C.H., Ho M.W., Lo W.J., Lin P.C., Chen H.J., Chou C.H., Feng J.Y., et al. 2013. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 121:1357–1366. 10.1182/blood-2012b08-452482 [DOI] [PubMed] [Google Scholar]
- Chi, C.Y., Lin C.H., Ho M.W., Ding J.Y., Huang W.C., Shih H.P., Yeh C.F., Fung C.P., Sun H.Y., Huang C.T., et al. 2016. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore). 95:e3927. 10.1097/MD.0000000000003927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvilain, E., Casanova J.L., and Puel A.. 2018. Inherited CARD9 deficiency: Invasive disease caused by ascomycete fungi in previously healthy children and adults. J. Clin. Immunol. 38:656–693. 10.1007/s10875-018s1080539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone, N.F., Lam P.V., Ross-Walker S., Rosen L.B., and Holland S.M.. 2017. Autoantibodies to granulocyte-macrophage colony-stimulating factor Associated with severe and unusual manifestations of Cryptococcus gattii infections. Open Forum Infect. Dis. 4:ofx211. 10.1093/ofid/ofx211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja, C.A., Merkel P.A., Chan E.D., Lenz L.L., Wolf M.L., Alam R., Frankel S.K., Fischer A., Gogate S., Perez-Velez C.M., and Knight V.. 2014. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-gamma autoantibody. Clin. Infect. Dis. 58:e115–118. 10.1093/cid/cit809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey, L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Janniere L., et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205:1543–1550. 10.1084/jem.20080321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Prost, N., Bastard P., Arrestier R., Fourati S., Mahevas M., Burrel S., Dorgham K., Gorochov G., Tandjaoui-Lambiotte Y., Azzaoui I., et al. 2021. Plasma exchange to rescue patients with autoantibodies against type I interferons and life-threatening COVID-19 pneumonia. J. Clin. Immunol. 41:536–544. 10.1007/s10875-021-00994-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir, S., Chebib N., Thivolet-Bejui F., and Cottin V.. 2018. Pulmonary alveolar proteinosis following cryptococcal meningitis: A possible cause? BMJ Case Rep. 2018:bcr2017222940. 10.1136/bcr-2017-222940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen, U., Nishinakamura R., Groneck P., Hattenhorst U., Nogee L., Murray R., and Burdach S.. 1997. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. J. Clin. Invest. 100:2211–2217. 10.1172/jci119758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doffinger, R., Helbert M.R., Barcenas-Morales G., Yang K., Dupuis S., Ceron-Gutierrez L., Espitia-Pinzon C., Barnes N., Bothamley G., Casanova J.L., et al. 2004. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin. Infect. Dis. 38:e10–14. 10.1086/380453 [DOI] [PubMed] [Google Scholar]
- Dorman, S.E., and Holland S.M.. 1998. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Invest. 101:2364–2369. 10.1172/jci2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, C.J., Mohamad S.M., Young D.F., Skelton A.J., Leahy T.R., Munday D.C., Butler K.M., Morfopoulou S., Brown J.R., Hubank M., et al. 2015. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci. Transl Med. 7:307ra154. 10.1126/scitranslmed.aac4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, C.J.A., Randall R.E., and Hambleton S.. 2021. Genetic lesions of type I interferon signalling in human antiviral immunity. Trends Genet. 37:46–58. 10.1016/j.tig.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey-Jakobs, S., Hartberger J.M., Fliegauf M., Bossen C., Wehmeyer M.L., Neubauer J.C., Bulashevska A., Proietti M., Frobel P., Noltner C., et al. 2018. ZNF341 controls STAT3 expression and thereby immunocompetence. Sci. Immunol. 3:eaat4941. 10.1126/sciimmunol.aat4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie, R., Khamashta M., Merrill J.T., Werth V.P., Kalunian K., Brohawn P., Illei G.G., Drappa J., Wang L., Yoo S., and Investigators C.D.S.. 2017. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69:376–386. 10.1002/art.39962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, D., Mezidi M., Bastard P., Perret M., Saker K., Fabien N., Pescarmona R., Lombard C., Walzer T., Casanova J.L., et al. 2021. Antibodies against type I interferon: Detection and association with severe clinical outcome in COVID-19 patients. Clin. Transl Immunol. 10:e1327. 10.1002/cti2.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J., Ning X.Q., Ding J.Y., Zheng Y.Q., Shi N.N., Wu F.Y., Lin Y.K., Shih H.P., Ting H.T., Liang G., et al. 2020. Anti-IFN-gamma autoantibodies underlie disseminated Talaromyces marneffei infections. J. Exp. Med. 217:e20190502. 10.1084/jem.20190502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S., Tatouli I.P., Rosen L.B., Hasni S., Alevizos I., Manna Z.G., Rivera J., Jiang C., Siegel R.M., Holland S.M., et al. 2016. Distinct functions of autoantibodies against interferon in systemic lupus erythematosus: A comprehensive analysis of anticytokine autoantibodies in common rheumatic diseases. Arthritis Rheumatol. 68:1677–1687. 10.1002/art.39607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton, S., Goodbourn S., Young D.F., Dickinson P., Mohamad S.M., Valappil M., McGovern N., Cant A.J., Hackett S.J., Ghazal P., et al. 2013. STAT2 deficiency and susceptibility to viral illness in humans. Proc. Natl. Acad. Sci. USA. 110:3053–3058. 10.1073/pnas.1220098110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, N., Bucciol G., Moens L., Le Pen J., Shahrooei M., Goudouris E., Shirkani A., Changi-Ashtiani M., Rokni-Zadeh H., Sayar E.H., et al. 2019. Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J. Exp. Med. 216:2057–2070. 10.1084/jem.20182295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, N., Melki I., Jing H., Habib T., Huang S.S.Y., Danielson J., Kula T., Drutman S., Belkaya S., Rattina V., et al. 2018. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J. Exp. Med. 215:2567–2585. 10.1084/jem.20180628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog, P.J., and Williams B.R.. 2013. Fine tuning type I interferon responses. Cytokine Growth Factor. Rev. 24:217–225. 10.1016/j.cytogfr.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Hoflich, C., Sabat R., Rosseau S., Temmesfeld B., Slevogt H., Docke W.D., Grutz G., Meisel C., Halle E., Gobel U.B., et al. 2004. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 103:673–675. 10.1182/blood-2003blo04-1065 [DOI] [PubMed] [Google Scholar]
- Holland, S.M., Deleo F.R., Elloumi H.Z., Hsu A.P., Uzel G., Brodsky N., Freeman A.F., Demidowich A., Davis J., Turner M.L., et al. 2007. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 357:1608–1619. 10.1056/nejmoa073687 [DOI] [PubMed] [Google Scholar]
- Hommes, D.W., Mikhajlova T.L., Stoinov S., Stimac D., Vucelic B., Lonovics J., Zakuciova M., D’Haens G., Van Assche G., Ba S., et al. 2006. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut. 55:1131–1137. 10.1136/gut.2005.079392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, G.H., Ortega-Villa A.M., Hunsberger S., Chetchotisakd P., Anunnatsiri S., Mootsikapun P., Rosen L.B., Zerbe C.S., and Holland S.M.. 2020. Natural history and evolution of anti-interferon-gamma autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin. Infect. Dis. 71:53–62. 10.1093/cid/ciz786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, B., Whittingham S., Mathews J.D., Mackay I.R., and Curnow D.H.. 1972. Autoimmunity in a rural community. Clin. Exp. Immunol. 12:79–87. [PMC free article] [PubMed] [Google Scholar]
- Huynh, J., Saddi V., Cooper P., Cheng A.T., Meyer W., Chen S., and Isaacs D.. 2020. Unusual presentation of severe endobronchial obstruction caused by Cryptococcus gattii in a child. J. Pediatr. Infect. Dis. Soc. 9:67–70. 10.1093/jpids/piy100 [DOI] [PubMed] [Google Scholar]
- Isaacs, A., and Lindenmann J.. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258–267. 10.1089/jir.1987.7.429 [DOI] [PubMed] [Google Scholar]
- Isaacs, A., Lindenmann J., and Valentine R.C.. 1957. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:268–273. 10.1098/rspb.1957.0049 [DOI] [PubMed] [Google Scholar]
- Jouanguy, E., Altare F., Lamhamedi S., Revy P., Emile J.F., Newport M., Levin M., Blanche S., Seboun E., Fischer A., and Casanova J.L.. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956–1961. 10.1056/nejm199612263352604 [DOI] [PubMed] [Google Scholar]
- Kampitak, T., Suwanpimolkul G., Browne S., and Suankratay C.. 2011. Anti-interferon-gamma autoantibody and opportunistic infections: Case series and review of the literature. Infection. 39:65–71. 10.1007/s15010-010s1500067-3 [DOI] [PubMed] [Google Scholar]
- Kampmann, B., Hemingway C., Stephens A., Davidson R., Goodsall A., Anderson S., Nicol M., Scholvinck E., Relman D., Waddell S., et al. 2005. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J. Clin. Invest. 115:2480–2488. 10.1172/JCI19316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, A., Deenick E.K., Ma C.S., Cook M.C., Uzel G., and Tangye S.G.. 2014. STAT3 is a central regulator of lymphocyte differentiation and function. Curr. Opin. Immunol. 28:49–57. 10.1016/j.coi.2014.01.015 [DOI] [PubMed] [Google Scholar]
- Kerner, G., Rosain J., Guerin A., Al-Khabaz A., Oleaga-Quintas C., Rapaport F., Massaad M.J., Ding J.Y., Khan T., Ali F.A., et al. 2020. Inherited human IFN-gamma deficiency underlies mycobacterial disease. J. Clin. Invest. 130:3158–3171. 10.1172/JCI135460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamashta, M., Merrill J.T., Werth V.P., Furie R., Kalunian K., Illei G.G., Drappa J., Wang L., Greth W., and CD1067 study investigators . 2016. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75:1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K., Waterer G., Thomson R., Yang I.A., Nashi N., Tan D.B., and Price P.. 2014. Levels of anti-cytokine antibodies may be elevated in patients with pulmonary disease associated with non-tuberculous mycobacteria. Cytokine. 66:160–163. 10.1016/j.cyto.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Kisand, K., Boe Wolff A.S., Podkrajsek K.T., Tserel L., Link M., Kisand K.V., Ersvaer E., Perheentupa J., Erichsen M.M., Bratanic N., et al. 2010. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207:299–308. 10.1084/jem.20091669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand, K., Meager A., Hayday A., and Willcox N.. 2021. Comment on “Aberrant type 1 immunity drives susceptibility to mucosal fungal infections”. Science. 373:eabi6235. 10.1126/science.abi6235 [DOI] [PubMed] [Google Scholar]
- Kitamura, T., Tanaka N., Watanabe J., UchidaKanegasaki S., Yamada Y., and Nakata K.. 1999. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 190:875–880. 10.1084/jem.190.6.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, J.S., Caricchio R., Casanova J.L., Combes A.J., Diamond B., Fox S.E., Hanauer D.A., James J.A., Kanthi Y., Ladd V., et al. 2021. The intersection of COVID-19 and autoimmunity. J. Clin. Invest. 131:e154886. 10.1172/JCI154886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi, Y., Sakagami T., Nishiyama N., Hirai J., Hayashi Y., Asai N., Yamagishi Y., Kato H., Hagihara M., Sakanashi D., et al. 2017. Rituximab restores IFN-gamma-STAT1 function and ameliorates disseminated Mycobacterium avium infection in a patient with anti-interferon-gamma autoantibody. J. Clin. Immunol. 37:644–649. 10.1007/s10875-017-0425-3 [DOI] [PubMed] [Google Scholar]
- Koning, R., Bastard P., Casanova J.L., Brouwer M.C., van de Beek D., and the Amsterdam U.M.C. COVID-19 Biobank Investigators . 2021. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 47:704–706. 10.1007/s00134-021-06392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreins, A.Y., Ciancanelli M.J., Okada S., Kong X.F., Ramirez-Alejo N., Kilic S.S., El Baghdadi J., Nonoyama S., Mahdaviani S.A., Ailal F., et al. 2015. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J. Exp. Med. 212:1641–1662. 10.1084/jem.20140280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisnawati, D.I., Liu Y.C., Lee Y.J., Wang Y.T., Chen C.L., Tseng P.C., and Lin C.F.. 2019a. Functional neutralization of anti-IFN-gamma autoantibody in patients with nontuberculous mycobacteria infection. Sci. Rep. 9:5682. 10.1038/s41598-019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisnawati, D.I., Liu Y.C., Lee Y.J., Wang Y.T., Chen C.L., Tseng P.C., Shen T.J., and Lin C.F.. 2019b. Blockade effects of anti-interferon- (IFN-) gamma autoantibodies on IFN-gamma-regulated antimicrobial immunity. J. Immunol. Res. 2019:1629258. 10.1155/2019/1629258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, C.L., Chi C.Y., von Bernuth H., and Doffinger R.. 2020. Autoantibodies against cytokines: Phenocopies of primary immunodeficiencies? Hum. Genet. 139:783–794. 10.1007/s00439-020s0002180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, C.L., Lin C.H., Chang S.W., Chu C.C., Chan J.F., Kong X.F., Lee C.H., Rosen E.A., Ding J.Y., Lee W.I., et al. 2016. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J. Allergy Clin. Immunol. 137:945–948.e948. 10.1016/j.jaci.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Kuo, C.Y., Wang S.Y., Shih H.P., Tu K.H., Huang W.C., Ding J.Y., Lin C.H., Yeh C.F., Ho M.W., Chang S.C., et al. 2017. Disseminated cryptococcosis due to anti-granulocyte-macrophage colony-stimulating factor autoantibodies in the absence of pulmonary alveolar proteinosis. J. Clin. Immunol. 37:143–152. 10.1007/s10875-016s1080364-4 [DOI] [PubMed] [Google Scholar]
- Lamborn, I.T., Jing H., Zhang Y., Drutman S.B., Abbott J.K., Munir S., Bade S., Murdock H.M., Santos C.P., Brock L.G., et al. 2017. Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J. Exp. Med. 214:1949–1972. 10.1084/jem.20161759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamborn, I.T., and Su H.C.. 2020. Genetic determinants of host immunity against human rhinovirus infections. Hum. Genet. 139:949–959. 10.1007/s00439-020s0002137-3 [DOI] [PubMed] [Google Scholar]
- Lazear, H.M., Schoggins J.W., and Diamond M.S.. 2019. Shared and distinct functions of type I and type III interferons. Immunity. 50:907–923. 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. 2006. Anti-interferon auto-antibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 3:e292. 10.1371/journal.pmed.0030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Ritelli M., Ma C.S., Rao G., Habib T., Corvilain E., Bougarn S., Cypowyj S., Grodecka L., Levy R., et al. 2019. Chronic mucocutaneous candidiasis and connective tissue disorder in humans with impaired JNK1-dependent responses to IL-17A/F and TGF-beta. Sci. Immunol. 4:eaax7965. 10.1126/sciimmunol.aax7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Vinh D.C., Casanova J.L., and Puel A.. 2017. Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr. Opin. Microbiol. 40:46–57. 10.1016/j.mib.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., and Fouser L.A.. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279. 10.1084/jem.20061308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, W.K., Thoon K.C., Chong C.Y., Tan N.W.H., Cheng D.T., Chan B.S.W., Ng M.S.Y., Das L., Arkachaisri T., Huang C.H., et al. 2019. Juvenile-onset immunodeficiency secondary to anti-interferon-gamma autoantibodies. J. Clin. Immunol. 39:512–518. 10.1007/s10875-019s1000652-1 [DOI] [PubMed] [Google Scholar]
- Lim, H.K., Huang S.X.L., Chen J., Kerner G., Gilliaux O., Bastard P., Dobbs K., Hernandez N., Goudin N., Hasek M.L., et al. 2019. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J. Exp. Med. 216:2038–2056. 10.1084/jem.20181621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.H., Chi C.Y., Shih H.P., Ding J.Y., Lo C.C., Wang S.Y., Kuo C.Y., Yeh C.F., Tu K.H., Liu S.H., et al. 2016. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat. Med. 22:994–1001. 10.1038/nm.4158 [DOI] [PubMed] [Google Scholar]
- Locatelli, F., Jordan M.B., Allen C., Cesaro S., Rizzari C., Rao A., Degar B., Garrington T.P., Sevilla J., Putti M.C., et al. 2020. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N. Engl. J. Med. 382:1811–1822. 10.1056/NEJMoa1911326 [DOI] [PubMed] [Google Scholar]
- Lopez, J., Mommert M., Mouton W., Pizzorno A., Brengel-Pesce K., Mezidi M., Villard M., Lina B., Richard J.C., Fassier J.B., et al. 2021. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 218:e20211211. 10.1084/jem.20211211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., and Cook M.C.. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551–1557. 10.1084/jem.20080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C.S., Wong N., Rao G., Nguyen A., Avery D.T., Payne K., Torpy J., O’Young P., Deenick E., Bustamante J., et al. 2016. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J. Exp. Med. 213:1589–1608. 10.1084/jem.20151467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manry, J., Laval G., Patin E., Fornarino S., Itan Y., Fumagalli M., Sironi M., Tichit M., Bouchier C., Casanova J.L., et al. 2011. Evolutionary genetic dissection of human interferons. J. Exp. Med. 208:2747–2759. 10.1084/jem.20111680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, P.I., and Salb J.M.. 1966. Molecular basis of interferon action: Inhibition of viral RNA translation. Virology. 30:502–516. 10.1016/0042-6822(66)90126-7 [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba, M., Doan M.L., Elidemir O., Fan L.L., Cheung S.W., Lei J.T., Moore J.P., Tavana G., Lewis L.R., Zhu Y., et al. 2008. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J. Exp. Med. 205:2711–2716. 10.1084/jem.20080759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager, A., Visvalingam K., Peterson P., Moll K., Murumagi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., and Willcox N.. 2006. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 3:e289. 10.1371/journal.pmed.0030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager, A., Wadhwa M., Dilger P., Bird C., Thorpe R., Newsom-Davis J., and Willcox N.. 2003. Anti-cytokine autoantibodies in autoimmunity: Preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin. Exp. Immunol. 132:128–136. 10.1046/j.1365-2249.2003.02113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel, C., Akbil B., Meyer T., Lankes E., Corman V.M., Staudacher O., Unterwalder N., Kolsch U., Drosten C., Mall M.A., et al. 2021. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Invest. 131:e150867. 10.1172/JCI150867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, S., Woodward M., Hertel C., Vlaicu P., Haque Y., Karner J., Macagno A., Onuoha S.C., Fishman D., Peterson H., et al. 2016. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 166:582–595. 10.1016/j.cell.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts, I., and Casanova J.L.. 2021. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. Eur. J. Immunol. 51:1039–1061. 10.1002/eji.202048793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 452:773–776. 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi, Y., Saito M., Nagasawa M., Takada H., Hara T., Tsuchiya S., Agematsu K., Yamada M., Kawamura N., Ariga T., et al. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 206:1291–1301. 10.1084/jem.20082767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi, Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., et al. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 448:1058–1062. 10.1038/nature06096 [DOI] [PubMed] [Google Scholar]
- Molfino, N.A., Kuna P., Leff J.A., Oh C.K., Singh D., Chernow M., Sutton B., and Yarranton G.. 2016. Phase 2, randomised placebo-controlled trial to evaluate the efficacy and safety of an anti-GM-CSF antibody (KB003) in patients with inadequately controlled asthma. BMJ Open. 6:e007709. 10.1136/bmjopen-2015-007709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, A.M., Flesher D.T., Yang J., Wolslegel K., Wang X., Brady A., Abbas A.R., Quarmby V., Wakshull E., Richardson B., et al. 2011. Association of endogenous anti-interferon-alpha autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 63:2407–2415. 10.1002/art.30399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Moreno, R., Martinez-Romero C., and Garcia-Sastre A.. 2021. Induction and evasion of type-I interferon responses during influenza A virus infection. Cold Spring Harb Perspect. Med. 11:a038414. 10.1101/cshperspect.a038414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasoedova, E., Davis J., Matteson E.L., and Crowson C.S.. 2020. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014. Ann. Rheum. Dis. 79:440–444. 10.1136/annrheumdis-2019-216694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum, A., Sharfe N., Broides A., Dadi H., Naghdi Z., Mandola A.B., Vong L., Arbiv A., Dalal I., Brami I., et al. 2020. Defining the biological responses of IL-6 by the study of a novel IL-6 receptor chain immunodeficiency. J. Allergy Clin. Immunol. 145:1011–1015.e1016. 10.1016/j.jaci.2019.11.015 [DOI] [PubMed] [Google Scholar]
- Nanki, T., Onoue I., Nagasaka K., Takayasu A., Ebisawa M., Hosoya T., Shirai T., Sugihara T., Hirata S., Kubota T., et al. 2013. Suppression of elevations in serum C reactive protein levels by anti-IL-6 autoantibodies in two patients with severe bacterial infections. Ann. Rheum. Dis. 72:1100–1102. 10.1136/annrheumdis-2012-202768 [DOI] [PubMed] [Google Scholar]
- Nathan, C.F., Murray H.W., Wiebe M.E., and Rubin B.Y.. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670–689. 10.1084/jem.158.3.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport, M.J., Huxley C.M., Huston S., Hawrylowicz C.M., Oostra B.A., Williamson R., and Levin M.. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941–1949. 10.1056/nejm199612263352602 [DOI] [PubMed] [Google Scholar]
- Nithichanon, A., Chetchotisakd P., Matsumura T., Takahashi Y., Ato M., Sakagami T., and Lertmemongkolchai G.. 2020. Diagnosis of NTM active infection in lymphadenopathy patients with anti-interferon-gamma auto-antibody using inhibitory ELISA vs. indirect ELISA. Sci. Rep. 10:8968. 10.1038/s41598-020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo, L.D., Bacchetta R., Casanova J.L., and Su H.C.. 2020. Human inborn errors of immunity: An expanding universe. Sci. Immunol. 5:eabb1662. 10.1126/sciimmunol.abb1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftedal, B.E., Hellesen A., Erichsen M.M., Bratland E., Vardi A., Perheentupa J., Kemp E.H., Fiskerstrand T., Viken M.K., Weetman A.P., et al. 2015. Dominant mutations in the autoimmune regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity. 42:1185–1196. 10.1016/j.immuni.2015.04.021 [DOI] [PubMed] [Google Scholar]
- Panackal, A.A., Rosen L.B., Uzel G., Davis M.J., Hu G., Adeyemo A., Tekola-Ayele F., Lisco A., Diachok C., Kim J.D., et al. 2017. Susceptibility to cryptococcal meningoencephalitis associated with idiopathic CD4+ lymphopenia and secondary germline or acquired defects. Open Forum Infect. Dis. 4:ofx082. 10.1093/ofid/ofx082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panem, S., Check I.J., Henriksen D., and Vilcek J.. 1982. Antibodies to alpha-interferon in a patient with systemic lupus erythematosus. J. Immunol. 129:1–3. [PubMed] [Google Scholar]
- Parks, C.G., Miller F.W., Satoh M., Chan E.K., Andrushchenko Z., Birnbaum L.S., Jusko T.A., Kissling G.E., Patel M.D., Rose K.M., et al. 2014. Reproductive and hormonal risk factors for antinuclear antibodies (ANA) in a representative sample of U.S. women. Cancer Epidemiol. Biomarkers Prev. 23:2492–2502. 10.1158/1055-9965.epi-14-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual, J., Gomez Aguinaga M.A., Vidal R., Maudes A., Sureda A., Gomez Mampaso E., and Fogue L.. 1989. Alveolar proteinosis and nocardiosis: A patient treated by bronchopulmonary lavage. Postgrad. Med. J. 65:674–677. 10.1136/pgmj.65.767.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S.Y., Ding L., Brown M.R., Lantz L., Gay T., Cohen S., Martyak L.A., Kubak B., and Holland S.M.. 2005. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J. Immunol. 175:4769–4776. 10.4049/jimmunol.175.7.4769 [DOI] [PubMed] [Google Scholar]
- Patnaik, M.M., Sallman D.A., Mangaonkar A.A., Heuer R., Hirvela J., Zblewski D., Al-Kali A., Binder M., Balasis M.E., Newman H., et al. 2020. Phase 1 study of lenzilumab, a recombinant anti-human GM-CSF antibody, for chronic myelomonocytic leukemia. Blood. 136:909–913. 10.1182/blood.2019004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar, A., Desai R.J., Solomon D.H., Santiago Ortiz A.J., Gale S., Bao M., Sarsour K., Schneeweiss S., and Kim S.C.. 2019. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study. Ann. Rheum. Dis. 78:456–464. 10.1136/annrheumdis-2018-214367 [DOI] [PubMed] [Google Scholar]
- Perrineau, S., Guery R., Monnier D., Puel A., and Lanternier F.. 2020. Anti-GM-CSF autoantibodies and Cryptococcus neoformans var. grubii CNS vasculitis. J. Clin. Immunol. 40:767–769. 10.1007/s10875-020s1000796-5 [DOI] [PubMed] [Google Scholar]
- Petri, M., Wallace D.J., Spindler A., Chindalore V., Kalunian K., Mysler E., Neuwelt C.M., Robbie G., White W.I., Higgs B.W., et al. 2013. Sifalimumab, a human anti-interferon-alpha monoclonal antibody, in systemic lupus erythematosus: A phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 65:1011–1021. 10.1002/art.37824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot, Q., Casanova J.L., and Puel A.. 2021. Candidiasis in patients with APS-1: Low IL-17, high IFN-gamma, or both? Curr. Opin. Immunol. 72:318–323. 10.1016/j.coi.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithukpakorn, M., Roothumnong E., Angkasekwinai N., Suktitipat B., Assawamakin A., Luangwedchakarn V., Umrod P., Thongnoppakhun W., Foongladda S., and Suputtamongkol Y.. 2015. HLA-DRB1 and HLA-DQB1 are associated with adult-onset immunodeficiency with acquired anti-interferon-gamma autoantibodies. PLoS One. 10:e0128481. 10.1371/journal.pone.0128481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocka-Plazak, K., Pituch-Noworolska A., and Kocemba J.. 1995. [Prevalence of autoantibodies in serum of healthy persons over 85 years of age]. Przegl Lek. 52:544–546. [PubMed] [Google Scholar]
- Pozzetto, B., Mogensen K.E., Tovey M.G., and Gresser I.. 1984. Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J. Infect. Dis. 150:707–713. 10.1093/infdis/150.5.707 [DOI] [PubMed] [Google Scholar]
- Pruetpongpun, N., Khawcharoenporn T., Damronglerd P., Suthiwartnarueput W., Apisarnthanarak A., Rujanavej S., and Suwantarat N.. 2016. Disseminated Talaromyces marneffei and Mycobacterium abscessus in a patient with anti-interferon-gamma autoantibodies. Open Forum Infect. Dis. 3:ofw093. 10.1093/ofid/ofw093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A. 2020. Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum. Genet. 139:1011–1022. 10.1007/s00439-020-02141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., and Casanova J.L.. 2019. The nature of human IL-6. J. Exp. Med. 216:1969–1971. 10.1084/jem.20191002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., and Casanova J.L.. 2021. Comment on “Aberrant type 1 immunity drives susceptibility to mucosal fungal infections”. Science. 373:eabi5459. 10.1126/science.abi5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., Cypowyj S., Bustamante J., Wright J.F., Liu L., Lim H.K., Migaud M., Israel L., Chrabieh M., Audry M., et al. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 332:65–68. 10.1126/science.1200439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., Cypowyj S., Marodi L., Abel L., Picard C., and Casanova J.L.. 2012. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr. Opin. Allergy Clin. Immunol. 12:616–622. 10.1097/aci.0b013e328358cc0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., Doffinger R., Natividad A., Chrabieh M., Barcenas-Morales G., Picard C., Cobat A., Ouachee-Chardin M., Toulon A., Bustamante J., et al. 2010a. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207:291–297. 10.1084/jem.20091983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., Picard C., Cypowyj S., Lilic D., Abel L., and Casanova J.L.. 2010b. Inborn errors of mucocutaneous immunity to Candida albicans in humans: A role for IL-17 cytokines? Curr. Opin. Immunol. 22:467–474. 10.1016/j.coi.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., Picard C., Lorrot M., Pons C., Chrabieh M., Lorenzo L., Mamani-Matsuda M., Jouanguy E., Gendrel D., and Casanova J.L.. 2008. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J. Immunol. 180:647–654. 10.4049/jimmunol.180.1.647 [DOI] [PubMed] [Google Scholar]
- Punatar, A.D., Kusne S., Blair J.E., Seville M.T., and Vikram H.R.. 2012. Opportunistic infections in patients with pulmonary alveolar proteinosis. J. Infect. 65:173–179. 10.1016/j.jinf.2012.03.020 [DOI] [PubMed] [Google Scholar]
- Raadsen, M.P., Gharbharan A., Jordans C.C.E., Mykytyn A.Z., Lamers M.M., van den Doel P.B., Endeman H., van den Akker J.P.C., GeurtsvanKessel C.H., Koopmans M.P.G., et al. 2022. Interferon-alpha2 auto-antibodies in convalescent plasma therapy for COVID-19. J. Clin. Immunol. 42:232–239. 10.1007/s10875-021s1001168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich, K., Warren R.B., Lebwohl M., Gooderham M., Strober B., Langley R.G., Paul C., De Cuyper D., Vanvoorden V., Madden C., et al. 2021. Bimekizumab versus secukinumab in plaque psoriasis. N. Engl. J. Med. 385:142–152. 10.1056/NEJMoa2102383 [DOI] [PubMed] [Google Scholar]
- Reinisch, W., Hommes D.W., Van Assche G., Colombel J.F., Gendre J.P., Oldenburg B., Teml A., Geboes K., Ding H., Zhang L., et al. 2006. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut. 55:1138–1144. 10.1136/gut.2005.079434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner, E.D., Torgerson T.R., Rylaarsdam S., Anover-Sombke S., Golob K., LaFlam T., Zhu Q., and Ochs H.D.. 2007. STAT3 mutation in the original patient with Job’s syndrome. N. Engl. J. Med. 357:1667–1668. 10.1056/NEJMc076367 [DOI] [PubMed] [Google Scholar]
- Roesler, J., Hedrich C., Laass M.W., Heyne K., and Rosen-Wolff A.. 2011. Meningoencephalitis caused by varicella-zoster virus reactivation in a child with dominant partial interferon-gamma receptor-1 deficiency. Pediatr. Infect. Dis. J. 30:265–266. 10.1097/inf.0b013e3181f6f78a [DOI] [PubMed] [Google Scholar]
- Rose-John, S. 2018. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 10:a028415. 10.1101/cshperspect.a028415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John, S., Winthrop K., and Calabrese L.. 2017. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 13:399–409. 10.1038/nrrheum.2017.83 [DOI] [PubMed] [Google Scholar]
- Rosen, L.B., Freeman A.F., Yang L.M., Jutivorakool K., Olivier K.N., Angkasekwinai N., Suputtamongkol Y., Bennett J.E., Pyrgos V., Williamson P.R., et al. 2013. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J. Immunol. 190:3959–3966. 10.4049/jimmunol.1202526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, L.B., Rocha Pereira N., Figueiredo C., Fiske L.C., Ressner R.A., Hong J.C., Gregg K.S., Henry T.L., Pak K.J., Baumgarten K.L., et al. 2015. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin. Infect. Dis. 60:1017–1025. 10.1093/cid/ciu968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, J.M., Maccari M.E., Barzaghi F., Allenspach E.J., Pignata C., Weber G., Torgerson T.R., Utz P.J., and Bacchetta R.. 2018. Neutralizing anti-cytokine autoantibodies against interferon-alpha in immunodysregulation polyendocrinopathy enteropathy X-linked. Front. Immunol. 9:544. 10.3389/fimmu.2018.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick, R.A., Simonian N.A., Alam J.A., Campion M., Scaramucci J.O., Jones W., Coats M.E., Goodkin D.E., Weinstock-Guttman B., Herndon R.M., et al. 1998. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology. 50:1266–1272. 10.1212/wnl.50.5.1266 [DOI] [PubMed] [Google Scholar]
- Saijo, T., Chen J., Chen S.C., Rosen L.B., Yi J., Sorrell T.C., Bennett J.E., Holland S.M., Browne S.K., and Kwon-Chung K.J.. 2014. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 5:e00912-00914. 10.1128/mBio.00912-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue, S., Yamaguchi E., Inoue Y., Takahashi M., Hirata J., Suzuki K., Ito S., Arai T., Hirose M., Tanino Y., et al. 2021. Genetic determinants of risk in autoimmune pulmonary alveolar proteinosis. Nat. Commun. 12:1032. 10.1038/s41467-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn, J.R., and Wilson C.B.. 2007. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 96:41–101. 10.1016/s0065-2776(07)96002-2 [DOI] [PubMed] [Google Scholar]
- Schwerd, T., Twigg S.R.F., Aschenbrenner D., Manrique S., Miller K.A., Taylor I.B., Capitani M., McGowan S.J., Sweeney E., Weber A., et al. 2017. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J. Exp. Med. 214:2547–2562. 10.1084/jem.20161810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour, J.F., and Presneill J.J.. 2002. Pulmonary alveolar proteinosis: Progress in the first 44 years. Am. J. Respir. Crit. Care Med. 166:215–235. 10.1164/rccm.2109105 [DOI] [PubMed] [Google Scholar]
- Shahin, T., Aschenbrenner D., Cagdas D., Bal S.K., Conde C.D., Garncarz W., Medgyesi D., Schwerd T., Karaatmaca B., Cetinkaya P.G., et al. 2019. Selective loss of function variants in IL6ST cause Hyper-IgE syndrome with distinct impairments of T-cell phenotype and function. Haematologica. 104:609–621. 10.3324/haematol.2018.194233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, H.P., Ding J.Y., Yeh C.F., Chi C.Y., and Ku C.L.. 2021. Anti-interferon-gamma autoantibody-associated immunodeficiency. Curr. Opin. Immunol. 72:206–214. 10.1016/j.coi.2021.05.007 [DOI] [PubMed] [Google Scholar]
- Shima, K., Sakagami T., Tanabe Y., Aoki N., Moro H., Koya T., Kagamu H., Hasegawa T., Suzuki E., and Narita I.. 2014. Novel assay to detect increased level of neutralizing anti-interferon gamma autoantibodies in non-tuberculous mycobacterial patients. J. Infect. Chemother. 20:52–56. 10.1016/j.jiac.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Shiono, H., Wong Y.L., Matthews I., Liu J.L., Zhang W., Sims G., Meager A., Beeson D., Vincent A., and Willcox N.. 2003. Spontaneous production of anti-IFN-alpha and anti-IL-12 autoantibodies by thymoma cells from myasthenia gravis patients suggests autoimmunization in the tumor. Int. Immunol. 15:903–913. 10.1093/intimm/dxg088 [DOI] [PubMed] [Google Scholar]
- Shu, S., Nisengard R.J., Hale W.L., and Beutner E.H.. 1975. Incidence and titers of antinuclear, antismooth muscle, and other autoantibodies in blood donors. J. Lab Clin. Med. 86:259–265. [PubMed] [Google Scholar]
- Solanich, X., Rigo-Bonnin R., Gumucio V.D., Bastard P., Rosain J., Philippot Q., Perez-Fernandez X.L., Fuset-Cabanes M.P., Gordillo-Benitez M.A., Suarez-Cuartin G., et al. 2021. Pre-existing autoantibodies neutralizing high concentrations of type I interferons in almost 10% of COVID-19 patients admitted to intensive care in barcelona. J. Clin. Immunol. 41:1733–1744. 10.1007/s10875-021s1001136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, S., Kostel Bal S., Egner W., Lango Allen H., Raza S.I., Ma C.A., Gurel M., Zhang Y., Sun G., Sabroe R.A., et al. 2019. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J. Exp. Med. 216:1986–1998. 10.1084/jem.20190344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, B., Bundell C., Mulrennan S., McLean-Tooke A., Murray R., and Brusch A.. 2019. The significance of anti-granulocyte-macrophage colony-stimulating factor antibodies in cryptococcal infection: Case series and review of antibody testing. Intern. Med. J. 49:1446–1450. 10.1111/imj.14637 [DOI] [PubMed] [Google Scholar]
- Suzuki, T., Sakagami T., Rubin B.K., Nogee L.M., Wood R.E., Zimmerman S.L., Smolarek T., Dishop M.K., Wert S.E., Whitsett J.A., et al. 2008. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med. 205:2703–2710. 10.1084/jem.20080990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., Sakagami T., Young L.R., Carey B.C., Wood R.E., Luisetti M., Wert S.E., Rubin B.K., Kevill K., Chalk C., et al. 2010. Hereditary pulmonary alveolar proteinosis: Pathogenesis, presentation, diagnosis, and therapy. Am. J. Respir. Crit. Care Med. 182:1292–1304. 10.1164/rccm.201002-0271OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, B.S., Chan J.F., Chen M., Tsang O.T., Mok M.Y., Lai R.W., Lee R., Que T.L., Tse H., Li I.W., et al. 2010. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin. Vaccin. Immunol. 17:1132–1138. 10.1128/CVI.00053-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye, S.G., Al-Herz W., Bousfiha A., Chatila T., Cunningham-Rundles C., Etzioni A., Franco J.L., Holland S.M., Klein C., Morio T., et al. 2020. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J. Clin. Immunol. 40:24–64. 10.1007/s10875-019-00737-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye, S.G., Al-Herz W., Bousfiha A., Cunningham-Rundles C., Franco J.L., Holland S.M., Klein C., Morio T., Oksenhendler E., Picard C., et al. 2021. The ever-increasing array of novel inborn errors of immunity: An interim update by the IUIS committee. J. Clin. Immunol. 41:666–679. 10.1007/s10875-021s1000980-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, B.C., Nakata K., Bonella F., Campo I., Griese M., Hamilton J., Wang T., Morgan C., Cottin V., and McCarthy C.. 2019. Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers. 5:16. 10.1038/s41572-019 [DOI] [PubMed] [Google Scholar]
- Trapnell, B.C., Whitsett J.A., and Nakata K.. 2003. Pulmonary alveolar proteinosis. N. Engl. J. Med. 349:2527–2539. 10.1056/NEJMra023226 [DOI] [PubMed] [Google Scholar]
- Troya, J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., Torres J., Martinez A., Abel L., Casanova J.L., and Pujol A.. 2021. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in madrid, Spain. J. Clin. Immunol. 41:914–922. 10.1007/s10875-021s1001036-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilifis, C., Freeman A.F., and Gennery A.R.. 2021. STAT3 hyper-IgE syndrome-an update and unanswered questions. J. Clin. Immunol. 41:864–880. 10.1007/s10875-021s1001051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala, R., Abreu G., Pineda L., Michaels M.A., Kalyani R.N., Furie R.A., and Morand E.F.. 2021. Safety profile of anifrolumab in patients with active SLE: An integrated analysis of phase II and III trials. Lupus Sci. Med. 8:e000464. 10.1136/lupus-2020-000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggenti, C., Lepelley A., and Crow Y.J.. 2019. Self-Awareness: Nucleic acid-driven inflammation and the type I interferonopathies. Annu. Rev. Immunol. 37:247–267. 10.1146/annurev-immunolan042718-041257 [DOI] [PubMed] [Google Scholar]
- Vallbracht, A., Treuner J., Flehmig B., Joester K.E., and Niethammer D.. 1981. Interferon-neutralizing antibodies in a patient treated with human fibroblast interferon. Nature. 289:496–497. 10.1038/289496a0 [DOI] [PubMed] [Google Scholar]
- van der Wijst, M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. 2021. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 13:eabh2624. 10.1126/scitranslmed.abh2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez, S.E., Bastard P., Kelly K., Gervais A., Norris P.J., Dumont L.J., Casanova J.L., Anderson M.S., and DeRisi J.L.. 2021. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J. Clin. Immunol. 41:1169–1171. 10.1007/s10875-021s1001060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh, D.C., Abel L., Bastard P., Cheng M.P., Condino-Neto A., Gregersen P.K., Haerynck F., Cicalese M.P., Hagin D., Soler-Palacin P., et al. 2021. Harnessing type I IFN immunity against SARS-CoV-2 with early administration of IFN-beta. J. Clin. Immunol. 41:1425–1442. 10.1007/s10875-021-01068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola, G.M., Malek A.E., Rosen L.B., DiNardo A.R., Nishiguchi T., Okhuysen P.C., Holland S.M., and Kontoyiannis D.P.. 2021. Disseminated cryptococcosis and anti-granulocyte-macrophage colony-stimulating factor autoantibodies: An underappreciated association. Mycoses. 64:576–582. 10.1111/myc.13247 [DOI] [PubMed] [Google Scholar]
- Walter, J.E., Rosen L.B., Csomos K., Rosenberg J.M., Mathew D., Keszei M., Ujhazi B., Chen K., Lee Y.N., Tirosh I., et al. 2015. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 125:4135–4148. 10.1172/JCI80477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. 2021. Diverse functional autoantibodies in patients with COVID-19. Nature. 595:283–288. 10.1038/s41586-021s4103631-y [DOI] [PubMed] [Google Scholar]
- Welcher, A.A., Boedigheimer M., Kivitz A.J., Amoura Z., Buyon J., Rudinskaya A., Latinis K., Chiu K., Oliner K.S., Damore M.A., et al. 2015. Blockade of interferon-gamma normalizes interferon-regulated gene expression and serum CXCL10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 67:2713–2722. 10.1002/art.39248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipasa, J., Chaiwarith R., Chawansuntati K., Praparattanapan J., Rattanathammethee K., and Supparatpinyo K.. 2018. Characterization of anti-interferon-gamma antibodies in HIV-negative immunodeficient patients infected with unusual intracellular microorganisms. Exp. Biol. Med. (Maywood). 243:621–626. 10.1177/1535370218764086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, A.S., Sarkadi A.K., Marodi L., Karner J., Orlova E., Oftedal B.E., Kisand K., Olah E., Meloni A., Myhre A.G., et al. 2013. Anti-cytokine autoantibodies preceding onset of autoimmune polyendocrine syndrome type I features in early childhood. J. Clin. Immunol. 33:1341–1348. 10.1007/s10875-013s1089938-6 [DOI] [PubMed] [Google Scholar]
- Wongkulab, P., Wipasa J., Chaiwarith R., and Supparatpinyo K.. 2013. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS One. 8:e76371. 10.1371/journal.pone.0076371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R., Mele F., Worley L., Langlais D., Rosain J., Benhsaien I., Elarabi H., Croft C.A., Doisne J.M., Zhang P., et al. 2020. Human T-bet governs innate and innate-like adaptive IFN-gamma immunity against mycobacteria. Cell. 183:1826–1847.e1831. 10.1016/j.cell.2020.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. 2020a. Human genetics of life-threatening influenza pneumonitis. Hum. Genet. 139:941–948. 10.1007/s00439-019s0002108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Bastard P., Effort C.H.G., Cobat A., and Casanova J.L.. 2022. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature. 10.1038/s41586-022-04447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. 2020. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 370:eabd4570. 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S.Y. 2020b. Herpes simplex virus encephalitis of childhood: Inborn errors of central nervous system cell-intrinsic immunity. Hum. Genet. 139:911–918. 10.1007/s00439-020s0002127-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., and Ouyang W.. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 445:648–651. 10.1038/nature05505 [DOI] [PubMed] [Google Scholar]
- Zhou, L., and Littman D.R.. 2009. Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol. 21:146–152. 10.1016/j.coi.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, C.G.K., Miao V.N., Owings A.H., Navia A.W., Tang Y., Bromley J.D., Lotfy P., Sloan M., Laird H., Williams H.B., et al. 2021. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 184:4713–4733.e4722. 10.1016/j.cell.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]


