Abstract
Streptococcus pneumoniae topoisomerase IV and DNA gyrase have been purified from a fluoroquinolone-susceptible Streptococcus pneumoniae strain, from first-step mutants showing low-level resistance to ciprofloxacin, sparfloxacin, levofloxacin, and ofloxacin, and from two clinical isolates showing intermediate- and high-level fluoroquinolone resistance by a gene cloning method that produces recombinant proteins from Escherichia coli. The concentrations of ciprofloxacin, sparfloxacin, levofloxacin, or ofloxacin required to inhibit wild-type topoisomerase IV were 8 to 16 times lower than those required to inhibit wild-type DNA gyrase. Furthermore, low-level resistance to these fluoroquinolones was entirely due to the reduced inhibitory activity of fluoroquinolones against topoisomerase IV. For all the laboratory strains, the 50% inhibitory concentration for topoisomerase IV directly correlated with the MIC. We therefore propose that with S. pneumoniae, ciprofloxacin, sparfloxacin, levofloxacin, and ofloxacin target topoisomerase IV in preference to DNA gyrase. Sitafloxacin, on the other hand, was found to be equipotent against either enzyme. This characteristic is unique for a fluoroquinolone. A reduction in the sensitivities of both topoisomerase IV and DNA gyrase are required, however, to achieve intermediate- or high-level fluoroquinolone resistance in S. pneumoniae.
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia. In the past, this bacterium has been readily treatable with penicillin or other related antibiotics. However, it has become increasingly apparent in recent years that S. pneumoniae has developed significant levels of resistance to penicillin in certain “hot spots” throughout the world (14). As a consequence, alternative therapies for S. pneumoniae infections have been sought. Options include the use of newer fluoroquinolones which have enhanced activity against gram-positive bacteria compared to that of ciprofloxacin, the main fluoroquinolone in clinical use at present.
The fluoroquinolones act by inhibiting the essential type II topoisomerases, DNA gyrase and topoisomerase IV, which alter DNA topology after insertion of a double-stranded DNA break (for a review, see reference 6). DNA gyrase exists as an A2B2 tetramer, encoded by the gyrA and gyrB genes, and catalyzes negative DNA supercoiling (9). This enzyme is thought to allow DNA replication to occur by removing positive supercoils ahead of the replication fork (39). Topoisomerase IV exists as a C2E2 tetramer encoded by the parC and parE genes and is involved in chromosome partitioning (20).
Our knowledge of the target specificity of fluoroquinolones against bacterial type II topoisomerases is based on two types of studies: first, those that investigate the mutations involved in bacterial resistance to fluoroquinolones (genetic studies) and, second, those that investigate the activities of fluoroquinolones against purified topoisomerases in vitro (enzymatic studies).
Genetic studies with Escherichia coli show that resistance to fluoroquinolones can occur due to single mutations in gyrA or gyrB (25). Mutations in parC or parE of topoisomerase IV alone do not confer fluoroquinolone resistance in E. coli (5). However, higher levels of fluoroquinolone resistance can occur in E. coli due to topoisomerase IV mutations if they are present within a mutated gyrA background (4, 15, 21, 22, 37). These data suggest that DNA gyrase is the primary target for fluoroquinolones against E. coli and that topoisomerase IV is the secondary target. Enzymatic studies confirm this hypothesis by demonstrating that a higher fluoroquinolone concentration is required to inhibit E. coli topoisomerase IV decatenation compared with the concentration required to inhibit E. coli DNA gyrase supercoiling (16).
In stark contrast, genetic studies with Staphylococcus aureus show that single mutations in grlA (equivalent to parC in E. coli) are able to cause fluoroquinolone resistance, but single mutations in gyrA are not (7, 8, 26). Therefore, in S. aureus, the target specificity for fluoroquinolones is the reverse of that seen in E. coli; i.e., the primary target is topoisomerase IV rather than DNA gyrase. As with E. coli, enzymatic studies with the type II topoisomerases purified from S. aureus confirm the results of genetic analyses; i.e., the drug concentrations required to inhibit DNA gyrase from S. aureus are higher than those required to inhibit topoisomerase IV from S. aureus (2). Unlike with E. coli, however, ofloxacin was found to be the exception to this rule, in that this fluoroquinolone was found to be equipotent against either staphylococcal type II topoisomerase (2).
With S. pneumoniae, only results for the genetic analysis of quinolone-resistant mutants have been published fully. These investigations show that the primary target of the majority of fluoroquinolones (ciprofloxacin, trovafloxacin, pefloxacin, PD-131628, temafloxacin, and Bay y3118) in S. pneumoniae is topoisomerase IV (3, 13, 18, 23, 28, 29, 32, 36), in accordance with that observed in S. aureus. Intriguingly, genetic investigations of stepwise sparfloxacin-resistant mutants indicate that the primary target for sparfloxacin in S. pneumoniae is DNA gyrase (30). Careful analysis of other studies investigating laboratory-generated sparfloxacin-resistant mutants and clinical isolates resistant to sparfloxacin also support this novel target specificity for sparfloxacin against S. pneumoniae (18, 32). The finding that target specificities vary between individual fluoroquinolones has important clinical implications (30).
To provide further data regarding the target specificities of fluoroquinolones against S. pneumoniae, we report here on the inhibition of recombinant DNA gyrase and topoisomerase IV enzymes purified from E. coli by using DNA from a wild-type pneumococcus, DNA from laboratory-generated fluoroquinolone-resistant mutants, and DNA from clinical isolates of S. pneumoniae resistant to fluoroquinolones. Some preliminary findings have been presented previously (10–12).
MATERIALS AND METHODS
Fluoroquinolones.
The following fluoroquinolones were used in this study: levofloxacin and ofloxacin (Hoechst Marion Roussel, Romainville, France), sparfloxacin (Rhône-Poulenc Rorer, Vitry Sur Seine, France), ciprofloxacin (Bayer UK, Newbury, United Kingdom), and sitafloxacin (DU-6859a; Daiichi Pharmaceutical Co. Ltd., Tokyo, Japan). The drugs were first diluted in 0.1 M NaOH and were then further diluted in sterile distilled water before use.
Determination of MICs.
S. pneumoniae was plated at an inoculum of about 105 CFU per spot onto plates of blood agar comprising nutrient broth no. 2 (Unipath, Basingstoke, United Kingdom) 1.5% (wt/vol) bacteriological agar (Unipath), and 7% (vol/vol) laked horse blood (Unipath), and various concentrations of fluoroquinolones. The plates were then incubated for 48 h at 37°C. The MIC was taken as the lowest concentration of fluoroquinolone required to prevent visible bacterial growth compared to the growth achieved with a drug-free control.
Selection of fluoroquinolone-resistant mutants.
Approximately 5 × 109 CFU of S. pneumoniae C3LN4 (a wild-type fluoroquinolone-susceptible strain) was spread onto standard 20-ml blood agar plates containing a fluoroquinolone at 2× the MIC, or approximately 5 × 1010 CFU was spread onto larger 80-ml plates, and the plates were incubated for 48 h at 37°C. Any colonies that were able to grow were then restreaked onto blood agar plates containing a fluoroquinolone at 2× the MIC. The MICs of the fluoroquinolones for those mutants present after subculturing were then evaluated. In addition, MICs were evaluated in the presence of 7.5 μg of reserpine per ml as an attempt to discount any mutants that were resistant due to fluoroquinolone efflux.
PCR cloning of topoisomerase genes for protein purification.
Chromosomal DNA was obtained from each pneumococcus by established methods, and this was used as a template for the PCR. Oligonucleotide primers for parC, parE, and gyrB were designed according to published sequences (29). The gyrA oligonucleotide sequence was kindly provided by Daiichi Pharmaceutical Co. Ltd. (Tokyo, Japan) and was found to be in accordance with that found by Balas et al. (1). The primer sequences used for protein purification are described in Table 1. The PCR amplification mixture consisted of 10 ng of template DNA, 35 pmol of each primer, 12.5 nmol of each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 2 U of Taq polymerase (Boehringer, Lewes, United Kingdom) in a final volume of 50 μl. The PCR conditions were, first, denaturation for 4 min at 95°C, followed by 25 cycles of 95°C for 30 s, 58°C (for parE only, 54°C was used) for 30 s, and 72°C for 2.5 min, and a final elongation at 72°C for 4 min. The PCR products were then treated with T4 DNA polymerase (New England Biolabs Ltd., Hitchin, United Kingdom), followed by T4 polynucleotide kinase (New England Biolabs Ltd.), according to the manufacturer’s instructions.
TABLE 1.
Oligonucleotide primers used to amplify the type II topoisomerase genes from S. pneumoniae for protein purification and DNA sequencing
| Application | Gene | Forward primer (nucleotide positions) | Reverse primer (nucleotide positions) |
|---|---|---|---|
| Protein purification | gyrA | 5′-ATGCAGGATAAAAATTTACTG (bp 1–21) | 5′-CTGATTTGTATTAATAAAATTGTTTAT (158–185 bp downstream of stop codon) |
| gyrB | 5′-ATGACAGAAGAAATCAAAAATCTG (bp 1–24) | 5′-CATATTTTCTAGACCAAGGGAAC (17–39 bp downstream of stop codon) | |
| parC | 5′-ATGTCTAACATTCAAAACATGTC (bp 1–23) | 5′-TTATTTATCTTCAGTAACTACTTC (7–30 bp downstream of stop codon) | |
| parE | 5′-ATGTCAAAAAAAGGAAATCAAT (bp 1–23) | 5′-TTAAAACACTGTCGCTTCTTC (1–22 bp downstream of stop codon) | |
| Target gene amplification | gyrA | 5′-TAAAAAACTTTGTCACGAATATGCC (130–105 bp upstream of start) | 5′-AACGATACGCTCACGACCAGT (bp 750–771) |
| gyrB | 5′-TGAAGGACAAACCAAGACCAAA (bp 1032–1053) | 5′-GTCCATTTCACCTAGCCCCTTATA (bp 1759–1782) | |
| parC | 5′-AAACCTACTCTACATTCTTTGAAAGGAG (134–106 bp upstream of start) | 5′-CAGTTGGGTGGTCAATCATGTAAA (bp 571–594) | |
| parE | 5′-ACCAAGGATAAACATGGAAGCC (bp 1026–1047) | 5′-CATTCATCTCACCAAGTCCTTTGTA (bp 1737–1762) | |
| QRDR amplification | gyrA | 5′-CGTTTTAGTGGTTTAGAGGC (85–66 bp upstream of start) | 5′-GACCAACTTCACTGCATCA (bp 567–585) |
| gyrB | 5′-TTCTCCGATTTCCTCATG (bp 1105–1122) | 5′-CCCGGCTGGATATATTCT (bp 1665–1682) | |
| parC | 5′-CGCCCTAGATACTGTGTGA (98–80 bp upstream of start) | 5′-AAATCCCAGTCGAACCAT (bp 493–510) | |
| parE | 5′-TGTGGATGGAATAGTGGC (bp 1054–1071) | 5′-ACCGAACTGTTTACGGAGT (bp 1697–1715) |
Sequencing of topoisomerase gene QRDRs.
The gyrA, gyrB, parC, and parE genes from S. pneumoniae C3LN4, CPFX1, SPFX1, and OFLX1 were amplified by PCR. The primers used for target gene amplification are described in Table 1. The PCR amplification mixture consisted of 200 ng of template DNA, 25 pmol of each primer, 0.2 mM of each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 2.5 U of Taq polymerase in a final volume of 100 μl. The PCR conditions were 35 cycles of 95°C for 45 s, 55°C for 30 s, and 72°C for 2.5 min and a final elongation at 72°C for 10 min. The PCR products were purified with Qiagen Q1A quick spin columns. Amplified templates for the quinolone resistance-determining region (QRDR) were sequenced by using an ABI PRISM 377 DNA sequencer (PE Applied Biosystems, Foster City, Calif.) with Big Dye terminator chemistry. The oligonucleotides used to prime the DNA sequencing reactions for sequencing of the QRDRs are described in Table 1. The sequences were assembled and edited with Sequencer, version 3.0 (Gene Codes Corp, Ann Arbor, Mich.).
Construction of protein-overexpressing E. coli.
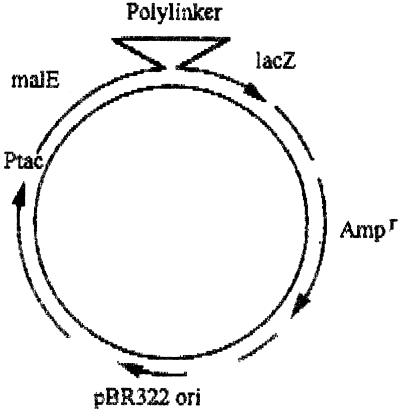
The pMAL-c2 protein fusion vector DNA (New England Biolabs UK Ltd.), as shown in Fig. 1, was cut within the polylinker sequence by using XmnI (New England Biolabs UK Ltd.) and was purified by gel electrophoresis.
FIG. 1.
pMAL-c2 vector.
Topoisomerase subunit PCR products were inserted separately into the cut polylinker sequence of the protein fusion vector by using T4 DNA ligase (New England Biolabs UK Ltd.). With this system each topoisomerase subunit gene was positioned adjacent to the malE gene (which encodes the maltose-binding protein [MBP]). Purified vector DNAs containing the separate topoisomerase genes were used to transform competent E. coli DH5α cells. Successful transformants were selected on agar plates containing 50 μg of ampicillin per ml. As was found with other gene products purified with this system (5), these clones overproduced the protein of interest (i.e., the topoisomerase subunit) fused to the MBP as deduced by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).
Purification of topoisomerase subunits.
Protein-overproducing clones were grown by shaking in Luria-Bertani broth containing 50 μg of ampicillin per ml at 37°C and were then induced with isopropyl-β-d-thiogalactopyranoside to overproduce fusion protein. The bacteria were harvested by centrifugation and were washed three times in buffer consisting of 20 mM Tris-HCl (pH 7.4), 200 mM NaCl, and 1 mM EDTA. The bacterial cells were then lysed by the addition of lysozyme (final concentration, 10 mg/ml [wt/vol]) and three repeated freezing-thawing steps. The lysed bacterial suspension was centrifuged, and the supernatant was stored at 4°C. The supernatant containing the fusion protein was loaded onto an amylose resin affinity chromatography column to which only the fusion protein can bind. Bound fusion protein was then eluted with 10 mM maltose. The GyrA, GyrB, ParC, or ParE protein was then cleaved from the fusion protein by factor Xa digestion at 23°C overnight. The separated MBP was removed by further amylose column chromatography.
Fluoroquinolone inhibition of topoisomerase IV.
The optimum reaction conditions for the decatenation activity of pneumococcal topoisomerase IV were deduced (data not shown). These optimum reaction mixtures (20 μl) contained 0.4 μg of kinetoplast DNA (kDNA; Topogen Inc., Columbus, Ohio), 40 mM Tris-HCl, 20 mM KCl, 5 mM MgCl2, 50 μg of bovine serum albumin per ml, 1 mM dithiothreitol, 0.5 mM ATP, and 1 U of topoisomerase IV. One unit of topoisomerase IV was defined as the amount of reconstituted ParC and ParE required to decatenate 0.4 μg of kDNA in 1 h at 37°C. After incubation with various fluoroquinolone concentrations at 37°C for 1 h, reactions were stopped by the addition of 5 μl of stopping solution (5% [vol/vol] Sarkosyl, 25% [vol/vol] bromophenol blue, 25% [vol/vol] glycerol). The samples were then subjected to 1% agarose gel electrophoresis, and the intensity of the decatenated DNA band was analyzed by using Grab-It and Gelworks 1D (UVP Life Sciences, Cambridge, United Kingdom). The inhibition of topoisomerase IV was expressed as a percentage of the intensity of the decatenated band compared to that for a drug-free control. The average of triplicate experiments was used to plot percent decatenation against the fluoroquinolone concentration. The fluoroquinolone concentration required to inhibit enzyme activity by 50% (IC50) was estimated from these plots.
Inhibition of DNA gyrase by fluoroquinolones.
The optimum reaction conditions for the supercoiling activity of pneumococcal DNA gyrase were deduced (data not shown). These optimum reaction mixtures (20 μl) contained 20 mM Tris HCl, 20 mM KCl, 8 mM MgCl2, 25 μg of bovine serum albumin per ml, 1 mM dithiothreitol, 5 mM ATP, 5 mM spermidine, 2.5 μg of tRNA, 0.2 μg of relaxed pBR322, and 1 U of DNA gyrase. One unit of DNA gyrase was defined as the amount of reconstituted enzyme required to supercoil 0.2 μg of relaxed DNA in 1 h at 37°C. After incubation with various fluoroquinolone concentrations (range, 1 to 200 μg/ml) at 37°C for 1 h, the reactions were stopped and analyzed as described above for topoisomerase IV. For DNA gyrase inhibition, IC50s were calculated by comparing the intensities of the supercoiled DNA bands.
RESULTS
Selection of fluoroquinolone-resistant mutants.
In an attempt to select for low-level fluoroquinolone resistance with fluoroquinolone concentrations at 2× the MIC, 12 mutants were selected with ciprofloxacin (mutation frequency, 1.4 × 10−9), 6 mutants were selected with sparfloxacin (mutation frequency, 2.4 × 10−9), and one mutant was selected with ofloxacin (mutation frequency, 1.4 × 10−10). Mutants resistant to levofloxacin or sitafloxacin were not selected, despite repeated attempts with higher inocula. It would appear, therefore, that the fluoroquinolones vary in their relative abilities to select for resistance. For all 12 mutant selected for resistance to ciprofloxacin fluoroquinolone MICs were identical and were resistant to ciprofloxacin but not to other quinolones (Table 2). All six mutants selected for resistance to sparfloxacin also shared an identical resistance pattern and again were resistant only to the selective agent (Table 2). The mutant selected for resistance to ofloxacin, on the other hand, was resistant to ciprofloxacin and sparfloxacin as well as to ofloxacin and levofloxacin (Table 2). None of the mutants was resistant to sitafloxacin (Table 2). The MICs for all the fluoroquinolone-resistant mutants selected were not affected by the presence of reserpine. From this it was concluded that the raised MICs were probably due to an alteration in the fluoroquinolone target or targets rather than an efflux-inducing mutation. Therefore, we were confident that we could continue with the purification of topoisomerases from these mutants.
TABLE 2.
Fluoroquinolone susceptibilities of S. pneumoniae C3LN4, first-step mutants, and clinical isolates
| Strain | Fluoroquinolone MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|
| LVFX | OFLX | CPFX | SPFX | STFX | |
| C3LN4 | 0.1 | 0.3 | 0.2 | 0.15 | 0.005 |
| SPFX1b | 0.1 | 0.3 | 0.2 | 0.4 | 0.005 |
| CPFX1c | 0.1 | 0.3 | 0.5 | 0.15 | 0.005 |
| OFLX1d | 0.3 | 0.75 | 0.6 | 0.8 | 0.005 |
| JP17e | 6.25 | 12.5 | 6.25 | 1.56 | 0.39 |
| JP27e | 25 | 50 | 50 | 25 | 0.39 |
LVFX, levofloxacin; OFLX, ofloxacin; CPFX, ciprofloxacin; SPFX, sparfloxacin; STFX, sitafloxacin. Those MICs greater than those for the wild type are shown in boldface type.
Selected with sparfloxacin (the MICs for the other five mutants were identical).
Selected with ciprofloxacin (MICs for the other 11 mutants were identical).
Selected with ofloxacin.
Clinical isolates.
Sequencing of mutant QRDRs.
When regions of all four topoisomerase genes were selected to include known QRDRs, each of the fluoroquinolone-resistant mutants was found to have gene sequences identical to those of the wild-type S. pneumoniae C3LN4 (data not shown). These results would suggest that mutations have occurred in genes other than those encoding topoisomerase IV or DNA gyrase. Alternatively, these results suggest that resistance may be due to mutations within a topoisomerase gene but beyond a known QRDR.
Purification of topoisomerases from S. pneumoniae.
Recombinant S. pneumoniae DNA gyrase and topoisomerase IV were purified from E. coli by using DNA from S. pneumoniae C3LN4, DNAs from two of the mutants selected for resistance to ciprofloxacin (mutants CPFX1 and CPFX2), two of the mutants selected for resistance to sparfloxacin (mutants SPFX1 and SPFX2), and the mutant selected for resistance to ofloxacin (mutant OFLX1). In addition, recombinant type II topoisomerases were also purified from two fluoroquinolone-resistant clinical isolates (isolates JP17 and JP27; kindly supplied by Daiichi Pharmaceutical Company Ltd.). The fluoroquinolone susceptibilities of these isolates are shown in Table 2. Pure topoisomerase subunit proteins were obtained after completion of all the stages of purification of topoisomerase IV and DNA gyrase from each strain. The SDS-PAGE results for C3LN4 are shown in Fig. 2. The subunits were assumed to be pure, i.e., without contamination from the MBP or other proteins, on account of the single bands produced after SDS-PAGE (Fig. 2, lanes 6 and 7). The estimated molecular masses for GyrA, GyrB, ParC, and ParE were 97, 74, 87, and 69 kDa, respectively. The specific activities of the reconstituted topoisomerase subunits ranged from 2.0 × 103 to 4.8 × 103 U per mg of protein. The fluoroquinolone-resistant enzymes were no less active than the fluoroquinolone-sensitive enzymes.
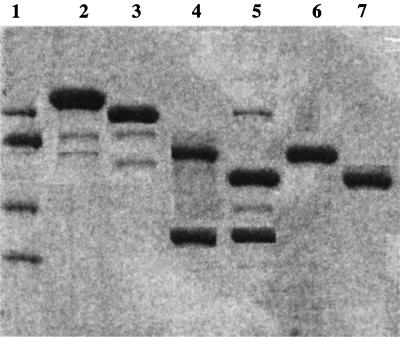
FIG. 2.
SDS-PAGE analysis of ParC and ParE subunits from S. pneumoniae C3LN4. Proteins at various steps were electrophoresed in an SDS–10% polyacrylamide gel and silver stained. Lane 1, molecular marker (116-, 97-, 66-, and 45-kDa proteins); lane 2, affinity-purified MBP-ParC fusion protein; lane 3, affinity-purified MBP-ParE fusion protein; lane 4, factor Xa digest of MBP-ParC; lane 5, factor Xa digest of MBP-ParE; lane 6, affinity-purified ParC; lane 7, affinity-purified ParE.
Fluoroquinolone inhibition of topoisomerases.
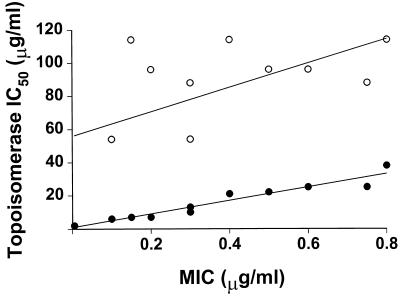
The experiments described here were designed to ascertain which topoisomerase is the most susceptible to fluoroquinolone inhibition and thereby indicate which topoisomerase is the primary target in S. pneumoniae. Figure 3 shows the effects of a range of ciprofloxacin concentrations on the decatenating activity of topoisomerase IV from S. pneumoniae C3LN4. Inhibition of topoisomerase IV is indicated by a reduction in the intensity of the decatenated monomer DNA band. Figure 4 shows the effects of a range of ciprofloxacin concentrations on the supercoiling activity of DNA gyrase from S. pneumoniae C3LN4. Inhibition of DNA gyrase is indicated by a reduction in the intensity of the supercoiled DNA band. It can be seen that the concentration of ciprofloxacin required to inhibit DNA gyrase is considerably higher than that required to inhibit topoisomerase IV. The IC50 of each fluoroquinolone for each topoisomerase is shown in Table 3. It can be seen that the IC50s of levofloxacin, ciprofloxacin, sparfloxacin, or ofloxacin for topoisomerase IV from S. pneumoniae C3LN4 were 8 to 16 times lower than those for DNA gyrase. This indicates that topoisomerase IV is the primary target of all these fluoroquinolones in S. pneumoniae, even though no mutations were found in the topoisomerase IV QRDRs. Interestingly, sparfloxacin was the least active fluoroquinolone against DNA gyrase, despite genetic studies that suggest that DNA gyrase is the primary target of sparfloxacin in S. pneumoniae (30). In stark contrast, the IC50 of sitafloxacin for topoisomerase IV was identical to that obtained for DNA gyrase. For the first-step fluoroquinolone-resistant mutants, the IC50s for topoisomerase IV were raised, but the IC50s for DNA gyrase remained the same. For each mutant the IC50s of fluoroquinolones for topoisomerase IV were increased only for those fluoroquinolones to which they were resistant. This adds further evidence to the hypothesis that the primary quinolone target in S. pneumoniae is topoisomerase IV and that first-step resistance occurs due to changes in the sensitivity of this enzyme (with the exception of sitafloxacin). Furthermore, when the MIC data from Table 2 (excluding the data for the clinical isolates) were plotted against IC50 for topoisomerase IV (Table 3), an almost perfect correlation was observed (Fig. 5). However, when the same process was repeated with the IC50s for DNA gyrase, little or no correlation occurred (Fig. 5). This strongly suggests once more that topoisomerase IV inhibition is the major factor in fluoroquinolone inhibition of S. pneumoniae and hence that topoisomerase IV is the primary target. The levels of fluoroquinolone inhibition of topoisomerases from laboratory-generated mutants with intermediate- or high-level fluoroquinolone resistance were not evaluated in this study. However, the fluoroquinolone sensitivities of topoisomerases from one intermediate-level fluoroquinolone-resistant clinical isolate and one high-level fluoroquinolone-resistant clinical isolate were investigated (Table 3). It can be seen that the IC50s of all the fluoroquinolones with the exception of sitafloxacin were greater than 200 μg/ml. This indicates that intermediate- and high-level fluoroquinolone resistance in S. pneumoniae requires dramatic changes in the fluoroquinolone sensitivities of both topoisomerase IV and DNA gyrase. With sitafloxacin, IC50s for both topoisomerase IV and DNA gyrase from these clinical isolates were also found to be raised, but only to those levels of the other fluoroquinolones that are required to inhibit wild-type pneumococcal topoisomerases. This is in keeping with the fact that these clinical isolates were susceptible to sitafloxacin, even though they were resistant to the other fluoroquinolones (Table 2).
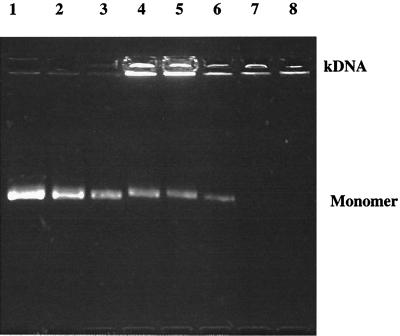
FIG. 3.
Inhibitory activity of ciprofloxacin on the decatenation reaction of topoisomerase IV from S. pneumoniae C3LN4. Lane 1, drug-free control; lanes 2 to 8, ciprofloxacin at 2, 4, 6, 8, 10, 12, and 14 μg per ml, respectively; kDNA, catenated kinetoplast DNA; monomer, decatenated monomer DNA.
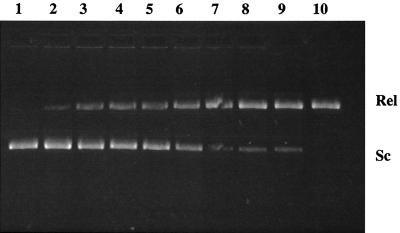
FIG. 4.
Inhibitory activity of ciprofloxacin on the supercoiling reaction of DNA gyrase from S. pneumoniae C3LN4. Lane 1, drug-free control; lanes 2 to 10, ciprofloxacin at 8, 16, 24, 32, 40, 48, 56, 64, and 72 μg per ml, respectively; Sc, supercoiled pBR322 DNA; Rel, relaxed pBR322 DNA.
TABLE 3.
Fluoroquinolone susceptibilities of purified type II topoisomerases
| Strain | Fluoroquinolone | IC50 (μg/ml)
|
B/A ratio | |
|---|---|---|---|---|
| Topoisomerase IV (A) | DNA gyrase (B) | |||
| C3LN4 | Levofloxacin | 6 | 54 | 9.0 |
| Ciprofloxacin | 7 | 96 | 13.7 | |
| Sparfloxacin | 7 | 114 | 16.3 | |
| Ofloxacin | 10 | 88 | 8.8 | |
| Sitafloxacin | 2 | 2 | 1.0 | |
| SPFX1a | Levofloxacin | 6 | 54 | 9.0 |
| Ciprofloxacin | 7 | 96 | 13.7 | |
| Sparfloxacin | 21b | 114 | 5.4 | |
| Ofloxacin | 10 | 88 | 8.8 | |
| Sitafloxacin | 2 | 2 | 1.0 | |
| CPFX1c | Levofloxacin | 6 | 54 | 9.0 |
| Ciprofloxacin | 22 | 96 | 4.4 | |
| Sparfloxacin | 7 | 114 | 16.3 | |
| Ofloxacin | 10 | 88 | 8.8 | |
| Sitafloxacin | 2 | 2 | 1.0 | |
| OFLX1 | Levofloxacin | 13 | 54 | 4.2 |
| Ciprofloxacin | 25 | 96 | 3.8 | |
| Sparfloxacin | 38 | 114 | 3.0 | |
| Ofloxacin | 25 | 88 | 3.5 | |
| Sitafloxacin | 2 | 2 | 1.0 | |
| JP17 | Levofloxacin | >200 | >200 | |
| Ciprofloxacin | >200 | >200 | ||
| Sparfloxacin | >200 | >200 | ||
| Ofloxacin | >200 | >200 | ||
| Sitafloxacin | 8 | 4 | 0.5 | |
| JP27 | Levofloxacin | >200 | >200 | |
| Ciprofloxacin | >200 | >200 | ||
| Sparfloxacin | >200 | >200 | ||
| Ofloxacin | >200 | >200 | ||
| Sitafloxacin | 10 | 6 | 0.6 | |
Identical results were obtained with CPFX2.
Values in boldface type indicate an increase in IC50 over that found for the wild type.
Identical results were obtained with SPFX2.
FIG. 5.
Correlation between topoisomerase IC50 and MIC determined by using S. pneumoniae C3LN4 and its fluoroquinolone-resistant mutants. ●, topoisomerase IV (correlation = 0.97); ○, DNA gyrase (correlation = 0.57).
DISCUSSION
This study was designed to evaluate the sensitivities of recombinant type II topoisomerases derived from a wild-type S. pneumoniae and laboratory-generated first-step mutants to ascertain the primary fluoroquinolone target in pneumococci. The recombinant proteins that were produced had specific activities lower than those reported for other bacterial topoisomerases. This lower than expected enzyme activity is not due to any vector-associated modification of the recombinant protein because the pMAL-c2 vector system does not add any vector-specific residues to the N-terminal end of the protein (24). The C-terminal end of the protein is similarly unaffected by vector residues because transcription of the gene is stopped by the “natural” stop codon of each specific pneumococcal gene. We feel that a more likely cause of this low specific activity was the overnight incubation step at 23°C required to separate each topoisomerase subunit from the MBP. Because fluoroquinolone-sensitive topoisomerases had specific activities equal to those of fluoroquinolone-resistant topoisomerases, we conclude that the inherent low specific activities of the recombinant proteins have no effect on fluoroquinolone inhibition.
It is perhaps worth mentioning that we originally tried to purify these topoisomerases directly from pneumococcal cultures, but our attempts were rendered impossible due to the inherently high DNase activities within the bacterial extracts. As a consequence we used the recombinant protein method described here.
The data generated from this study show that topoisomerase IV purified from a wild-type S. pneumoniae is more sensitive to fluoroquinolones than DNA gyrase purified from this bacterium and that low-level fluoroquinolone resistance occurs due to changes in topoisomerase IV sensitivity alone. The exception to this rule is sitafloxacin, which was found to be equipotent against either topoisomerase from S. pneumoniae. This is consistent with the findings of other studies that have investigated the inhibition of these enzymes (27). Intermediate- or high-level fluoroquinolone resistance (as demonstrated by the two clinical isolates), on the other hand, is caused by a dramatic reduction in the activities of fluoroquinolones against both topoisomerase IV and DNA gyrase. This is in agreement with genetic studies with S. pneumoniae (24, 28, 35, 36).
For the majority of fluoroquinolones used in this study, our topoisomerase inhibition results are to be expected because genetic studies have shown that first-step fluoroquinolone-resistant mutants of S. pneumoniae contain mutations in the so-called QRDR of the parC or parE gene of topoisomerase IV (13, 18, 23, 28, 29, 32, 35). However, the results obtained with sparfloxacin in this study would appear to contradict the findings of Pan and Fisher (30), who suggested that a single mutation in the gyrA QRDR rendered pneumococci resistant to sparfloxacin but not to ciprofloxacin. The investigators in that study claimed, therefore, that sparfloxacin targets pneumococcal DNA gyrase and that ciprofloxacin targets pneumococcal topoisomerase IV. We have also shown a lack of cross-resistance between mutants selected for resistance to ciprofloxacin and mutants selected for resistance to sparfloxacin in the study presented here. However, our results indicate that this lack of cross-resistance is not due to a different target specificity because the raised MICs of both drugs were directly attributable to increased IC50s for topoisomerase IV. Intriguingly, the mutants generated in this study did not contain mutations in the traditional QRDRs of gyrA, gyrB, parC or parE. Because the mutants possessed fluoroquinolone-resistant topoisomerase IV, these results would suggest that mutations beyond the topoisomerase IV QRDRs are the cause of fluoroquinolone resistance. This offers an explanation for the high-level fluoroquinolone resistance seen with clinical isolates of S. pneumoniae that also do not possess mutations in any QRDR (19). We suggest that future studies on fluoroquinolone resistance in S. pneumoniae should include sequencing of whole topoisomerase genes so that precise S. pneumoniae QRDRs can be deduced and are not just based on the QRDRs predicted from data for E. coli and S. aureus.
An obvious question arises from this study. Why should the genetic studies with sparfloxacin (30) contradict the enzymatic studies with sparfloxacin presented here, and why should the genetic studies with ciprofloxacin (28) agree with the enzymatic studies presented here? One possibility is the selection pressure used to isolate first-step fluoroquinolone-resistant mutants. As mentioned earlier, in this study we chose 2× the MIC, as did Pan et al. (28) when they selected ciprofloxacin-resistant mutants. Pan et al. (28) found a mutation frequency of 5.8 × 10−8 for first-step ciprofloxacin resistance, and this mutation frequency is of an order of magnitude similar to the mutation frequency of 1.4 × 10−9 that we found to be associated with ciprofloxacin resistance. In addition, for the ciprofloxacin-resistant mutants selected in both studies, MICs were about three times greater than that for the original wild-type strain. However, when investigating the development of resistance to sparfloxacin, Pan and Fisher (30) used a selective pressure of 4× the MIC, producing mutation frequencies of between 5.0 × 10−10 and 8.0 × 10−10. For these sparfloxacin-resistant mutants, MICs were eight times greater than that for the parent strain. When we used a lower sparfloxacin selective pressure of 2× the MIC, we obtained a mutation frequency of 2.4 × 10−9, i.e., a mutation frequency similar to that observed with ciprofloxacin. Furthermore, for our sparfloxacin-resistant mutants MICs were three times greater than that for the parent. It would appear, therefore, that it is more difficult to select resistant mutants with sparfloxacin at 4× the MIC than at 2× the MIC and that for mutants selected at 4× the MIC the MICs are much higher. Taking these facts into account, it is quite possible that the mutants selected by Pan and Fisher (30) were not true first-step mutants at all but were really second-step mutants that harbored an undetected mutation, i.e., a mutation outside of the gyrA or parC QRDR. These mutants could have been of a genotype similar to that of the first-step mutants selected in this study. Because the QRDR is only a small portion of any one topoisomerase gene, it is possible that such mutations could readily be missed. Sequencing of complete topoisomerase genes and transformation “knockout” experiments would be required to test this hypothesis. Enzymatic studies, such as that presented here, would not be prone to the problems associated with basing resistance studies on putative QRDR sequences alone.
Another possibility that cannot be excluded is that the DNA gyrase gene could contain mutations that affect the fluoroquinolone MIC but that do not affect the fluoroquinolone IC50 for DNA gyrase and that are therefore not detectable by DNA gyrase inhibition studies. However, with the mutants selected in this study we suggest that this hypothesis is unlikely because of the strong correlation between the IC50 for topoisomerase IV and the MIC. Nevertheless, it would be interesting to evaluate this hypothesis.
It is indisputable from the studies by Pan and Fisher (30) and from the data presented here that there is no cross-resistance between ciprofloxacin-resistant first-step mutants and the equivalent mutants selected with sparfloxacin. As postulated above, it is possible that the mutations involved in fluoroquinolone resistance may not occur within recognized QRDRs. Such as lack of at least some form of cross-resistance is unusual with fluoroquinolone resistance, but one other example has been observed and well characterized. The nalC mutation within the gyrB gene of E. coli (17), later renamed nal-31 (38), confers resistance to nalidixic acid and all other quinolones that lack a C-7 piperazine (33). However, this mutation does not confer resistance to those quinolones that possess the piperazine, it actually confers hypersusceptibility (33). The nal-31 mutation replaces lysine (a neutral amino acid) with glutamic acid (a negatively charged amino acid) within GyrB. The explanation for the unusual resistance phenomenon associated with nal-31 is that the new increased negative charge repels the nonpiperazine derivatives but attracts the positive charge associated with C-7 piperazine derivatives (34). It may follow, therefore, that the resistance observed for sparfloxacin or ciprofloxacin in S. pneumoniae may be due to a similar type of mutation that selectively repels one compound and not the other. Unfortunately, the results observed with the mutant selected for resistance to ofloxacin further confuse the issue because this mutant shows complete cross-resistance (except to sitafloxacin). However, it is interesting that the frequency of the mutation for ofloxacin resistance found in this study was 1.4 × 10−10. Because this mutation rate is similar to that observed by Pan and Fisher (30) with sparfloxacin, as mentioned above, it may be that the ofloxacin-resistant mutant contains more than one mutation within topoisomerase IV. As mentioned above, genetic analysis of the entire sequences of the DNA gyrase and topoisomerase IV genes of fluoroquinolone-resistant S. pneumoniae strains would clarify a number of issues.
One fluoroquinolone that especially stands out in this study is sitafloxacin, which is uniquely equipotent against both type II topoisomerases from S. pneumoniae. It has been hypothesized that this equipotency may reduce the frequency at which resistance to sitafloxacin develops, because mutations in both topoisomerase IV and DNA gyrase would have to occur simultaneously (12). The lack of ability to select mutants with sitafloxacin in this study would appear to support this hypothesis. However, resistant mutants were also absent under levofloxacin selection. Further studies would be required to differentiate between the resistance-selecting capabilities of levofloxacin and sitafloxacin. The data obtained from examination of the two clinical isolates appear to support the fact that sitafloxacin is a novel and potentially very useful fluoroquinolone which, if it comes into clinical use, would offer considerable advantages over currently available fluoroquinolones. Interestingly, genetic studies suggest that clinafloxacin may also share the equipotent attributes of sitafloxacin (31). We await enzymatic studies to confirm this.
ACKNOWLEDGMENTS
We are grateful to Hoechst Marion Roussel for financial support and to Daiichi Pharmaceutical Co. Ltd. for unpublished data.
We thank Richard Warren and Chris Traini (SmithKline Beecham Pharmaceuticals, Collegeville, Pa.) for sequencing the QRDRs.
REFERENCES
- 1.Balas D, Fernández-Moreira E, De La Campa A. Molecular characterisation of the gene encoding the DNA gyrase A subunit of Streptococcus pneumoniae. J Bacteriol. 1998;180:2854–2861. doi: 10.1128/jb.180.11.2854-2861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanche F, Cameron B, Bernard F-X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breines D M, Ouabdesselam S, Ng E Y, Tankovic J, Shah S, Soussy C J, Hooper D C. Quinolone resistance locus nfxD of Escherichia coli is a mutant allele of the parE gene encoding a subunit of topoisomerase IV. Antimicrob Agents Chemother. 1997;41:175–179. doi: 10.1128/aac.41.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C-R, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome; quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 5.Diguan C, Li P, Riggs P D, Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to the maltose-binding protein. Gene. 1988;67:21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 6.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gellert M, Mizuuchi K, O’Dea M H, Nash H A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George J T, Morrissey I. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1997. Purification of topoisomerase IV from staphylococcus aureus C3LN4 and inhibition by fluoroquinolones, abstr. C-90; pp. 61–62. [Google Scholar]
- 11.George J T, Morrissey I. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1998. Activity of levofloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against DNA gyrase from S. pneumoniae C3LN4, abstr. C-176; p. 120. [Google Scholar]
- 12.George J T, Morrissey I. Programme and abstracts of the 6th International Symposium on New Quinolones. 1998. The unique equipotency of sitafloxacin against topoisomerase IV and DNA gyrase from Streptococcus pneumoniae; p. 55. [Google Scholar]
- 13.Gootz T D, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer R J. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. doi: 10.1128/aac.40.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grüneberg R N, Felmingham D the Alexander Project Group. Results of the Alexander Project: a continuing, multicenter study of the antimicrobial susceptibility of community-acquired lower respiratory tract bacterial pathogens. Diagn Microbiol Infect Dis. 1996;25:169–181. doi: 10.1016/s0732-8893(96)00135-6. [DOI] [PubMed] [Google Scholar]
- 15.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino K, Kitamura A, Morrissey I, Sato K, Kato J-I, Ikeda H. Comparison of inhibition of Escherichia coli topoisomerase IV by quinolones with DNA gyrase inhibition. Antimicrob Agents Chemother. 1994;38:2623–2627. doi: 10.1128/aac.38.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue S, Ohue T, Yamagishi J, Nakamura S, Shimizu M. Mode of incomplete cross-resistance among pipemidic, piormidic, and nalidixic acids. Antimicrob Agents Chemother. 1978;14:240–245. doi: 10.1128/aac.14.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janoir C, Zeller V, Kitzis M-D, Moreau N J, Guttmann L. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob Agents Chemother. 1996;40:2760–2764. doi: 10.1128/aac.40.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson A P, Stewart B, Woodford N, Warner M. Programme and abstracts of the 6th International Symposium on New Quinolones. 1998. Activity of grepafloxacin against ciprofloxacin-resistant clinical isolates of Streptococcus pneumoniae; p. 50. [Google Scholar]
- 20.Kato J, Nishima Y, Imamura R, Niki H, Higari S, Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 21.Khodursky A B, Zechiedrich E L, Cozzarelli N R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai Y, Kato J-I, Hoshino K, Akasaka T, Sato K, Ikeda H. Quinolone-resistant mutants of Escherichia coli DNA topoisomerase IV parC gene. Antimicrob Agents Chemother. 1996;40:710–714. doi: 10.1128/aac.40.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muñoz R, De La Campa A G. ParC subunit of topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai K, Thogersen S C. Synthesis of sequence-specific proteolysis of hybrid protein produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura S, Nakamura M, Kojima T, Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989;33:254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onodera Y, Uchida Y, Tanaka M, Sato K. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1998. Dual inhibitory activity of DU-6859a against DNA gyrase and topoisomerase IV of Streptococcus pneumoniae, abstr. C-175; p. 119. [Google Scholar]
- 28.Pan X-S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan X-S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan X-S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan X-S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perichon B, Tankovic J, Courvalin P. Characterization of a mutation in the parE gene that confers fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1166–1167. doi: 10.1128/aac.41.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J T. Awakening the slumbering potential of the 4-quinolone antibacterials. Pharm J. 1984;233:299–305. [Google Scholar]
- 34.Smith J T, Lewin C S. Chemistry and mechanism of action of the quinolone antibacterials. In: Andriole V T, editor. The quinolones. London, United Kingdom: Academic Press; 1988. pp. 23–82. [Google Scholar]
- 35.Taba H, Kusano N. Sparfloxacin resistance in clinical isolates of Streptococcus pneumoniae: involvement of multiple mutations in gyrA and parC genes. Antimicrob Agents Chemother. 1998;42:2193–2196. doi: 10.1128/aac.42.9.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tankovic J, Perichon B, Duval J, Courvalin P. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob Agents Chemother. 1996;40:2505–2510. doi: 10.1128/aac.40.11.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vila J, Ruiz J, Goñi P, De Anta M T J. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:491–493. doi: 10.1128/aac.40.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagishi J-I, Yoshida H, Yamayoshi M, Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986;204:367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- 39.Zechiedrich E L, Cozzarelli N R. Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev. 1995;9:2859–2869. doi: 10.1101/gad.9.22.2859. [DOI] [PubMed] [Google Scholar]