Abstract
Although dapsone is a commonly used alternative agent for prophylaxis against Pneumocystis carinii pneumonia in children intolerant to trimethoprim-sulfamethoxazole, there are few data that describe dapsone pharmacokinetics in children. We studied dapsone pharmacokinetics in 30 children (median age, 2.8 years; age range, 0.3 to 12 years) receiving a new proprietary liquid preparation by three dosing regimens (1 mg/kg of body weight daily, 2 mg/kg daily, or 4 mg/kg weekly). Dosing of children with 2 mg/kg daily or 4 mg/kg weekly resulted in peak concentrations equivalent to those reached in adults receiving 100-mg tablets daily. For the entire population, the median half-life was 22.2 h (range, 7.1 to 40.3 h), the median oral clearance was 0.0365 liter/kg/h (range, 0.0104 to 0.1021 liter/kg/h), and the median oral apparent volume of distribution was 1.13 liters/kg (range, 0.50 to 2.32 liters/kg). The median dapsone oral clearance was significantly increased in those infants less than 2 years of age compared to the oral clearance in those over 2 years of age (0.0484 versus 0.0278 liter/kg/h; P = 0.011). These data suggest that absorption of this liquid preparation is adequate and that the concentrations in the sera of children receiving 2 mg/kg daily or 4 mg/kg weekly are equivalent to those seen in adults receiving standard dapsone dosing. Dapsone oral clearance appears to be increased in children under 2 years of age.
Prophylaxis against Pneumocystis carinii pneumonia (PCP) is recommended for high-risk immunocompromised children, including human immunodeficiency virus (HIV)-infected children with low CD4 lymphocyte counts (3). Dapsone is now commonly used as an alternative prophylactic agent for children intolerant to trimethoprim-sulfamethoxazole (TMP-SMX), the first-choice drug. Until recently, the use of dapsone had been limited to the treatment of leprosy and dermatitis herpetiformis. Little was known about the pharmacokinetics of dapsone in children, and the initial pediatric dosing suggestion of 1 mg/kg of body weight daily was made in the absence of pediatric pharmacokinetic data (2).
Two previous studies have investigated dapsone pharmacokinetics in children. A small preliminary study suggested that chronic dosing with 1-mg/kg daily doses of dapsone in children results in concentrations in serum roughly half those achieved in adults receiving 100-mg tablets daily (3 to 4 μg/ml) and that an extemporaneous ethanol-based liquid preparation had extremely poor bioavailability (14). Gatti and colleagues (5) reported on single-dose pharmacokinetics in 12 children receiving a 2-mg/kg preparation of pulverized tablets suspended in water. Absorption of this preparation was adequate, with the maximum concentration in serum (Cmax) averaging 1.12 μg/ml. Among these children, oral clearance (CL/F) and volume of distribution (V/F) decreased with advancing age.
A proprietary liquid preparation of dapsone is now available. The bioavailability of that preparation in adults is equivalent to that of tablets (13). We now report on the pharmacokinetics of dapsone in a large group of HIV-infected children during chronic dosing with this proprietary liquid formulation. While the most common current dapsone regimen for adults is 100 mg daily, less frequent dosing regimens, including weekly, biweekly, and monthly dosing, have been proposed (8, 9). We therefore studied children receiving dapsone daily and weekly in order to describe dapsone pharmacokinetic parameters in children and, since the concentration of dapsone in serum required for successful PCP prophylaxis is not known, to develop daily and weekly dosing regimens that would achieve serum dapsone concentrations in children equivalent to those observed in adults receiving standard doses of dapsone.
MATERIALS AND METHODS
Patient eligibility.
HIV-infected children who were between the ages of 1 month and 12 years, who were begun on PCP prophylaxis by their clinical care givers, and who demonstrated intolerance to TMP-SMX were eligible for enrollment in Pediatric AIDS Clinical Trials Group (PACTG) protocol no. 179. This protocol was an open-label, two-arm, randomized phase I-II study designed to compare daily and weekly dapsone dosing regimens. Children with glucose-6-phosphate dehydrogenase deficiency, previous dapsone treatment, known dapsone allergy, or serious or life-threatening reactions to TMP-SMX were excluded from the study. Study patients were randomized to receive dapsone either daily or weekly and were stratified by age (above or below 2 years of age). This age cutoff was chosen in order to include a significant number of children under age 2 years, the age group in which we presumed developmental changes would likely be most important. The first 20 patients (5 per age group and dosing regimen) were enrolled in a pharmacokinetic substudy. Those initial patients randomized to the daily dapsone arm received dosages of 1 mg/kg daily. On the basis of their pharmacokinetic data, the daily dose was increased to 2 mg/kg and 10 additional patients who received this higher dosage were studied. All patients enrolled in the weekly therapy arm received 4-mg/kg doses. The current report describes the results of this pharmacokinetic substudy. The results of the parent study that describe the safety, tolerance, and efficacy of dapsone in the entire study population will be published separately. The study was conducted with the approval of the institutional review boards at all participating institutions. Informed consent was obtained from the parents or guardians of study patients prior to entry.
Study protocol.
A proprietary liquid preparation of dapsone (Jacobus Pharmaceutical Co., Princeton, N.J.) was administered to all except one of the children in the study. An older child received dapsone in tablet form because the volume of the liquid dose was unacceptable to the patient and family. Sampling for pharmacokinetic studies was performed after at least 4 weeks of continuous therapy without dose interruption. Blood samples were collected from all study patients before administration of the study dose and at 1, 2, 4, 6, 8, 24, and 48 h after administration of the dose. For patients receiving weekly doses, an additional sample was collected at 96 h after administration of the dose. Subsequent dapsone doses were held until the completion of pharmacokinetic sampling. Blood samples were allowed to clot and were then centrifuged and the serum was removed. Serum samples were frozen at −70°C until analysis.
Dapsone assay.
Serum dapsone and monoacetyldapsone (MADDS) concentrations were determined by high-performance liquid chromatography (HPLC) by a modification of the method of Zuidema et al. (19). HPLC was performed with a Bio-Rad 800 HRLC chromatograph with a Supelcosil LC-18 column (15 cm by 4.6 mm) maintained at 35°C. The mobile phase consisted of 16% acetonitrile and 84% acetic acid (1.5%) delivered at a flow rate of 2.2 ml/min. Absorbance was monitored at 305 nm. Serum samples were prepared for injection by mixing 125-μl aliquots of serum with 187.5 μl of acetonitrile, followed by the addition of 625 μl of acetic acid (1.5%) to approximate the composition of the mobile phase. Following centrifugation, 350-μl aliquots of the supernatant were injected onto the column. Calibration curves were constructed by preparing standards in blank serum and plotting peak height against the known concentrations. The standard curve was linear over the range of 0.13 to 8.0 μg/ml for dapsone and 0.25 to 8.0 μg/ml for MADDS, with correlation coefficients of >99.8%. Interday and intraday coefficients of variation were less than 11% for dapsone and 15% for monoacetyldapsone.
Pharmacokinetic calculations.
The Cmax, time to Cmax (Tmax), and whether the patient was at steady state at the time of dosing were determined by inspection of concentration-time plots. Pharmacokinetic calculations were performed by using two commercial personal computer programs (WinNonlin [Pharsight Corp., Palo Alto, Calif.] and Excel [Microsoft, Seattle, Wash.]). Terminal elimination half-lives (t1/2) for dapsone and MADDS were determined by weighted least-squares fitting. If the time zero (predosing) dapsone concentration was above the detectable limit of the assay, reverse superposition was used to calculate a derived curve for a single dose (1). The area under the serum concentration-time curve from time zero to infinity (AUC0–∞) was calculated by the trapezoidal rule with extrapolation to infinity. CL/F was determined from the formula CL/F = dose/AUC0–∞). V/F was determined from the formula Varea/F = dose/(λz · AUC0–∞), where λz is the terminal elimination rate constant. The acetylation ratio was determined by dividing the MADDS concentration by the dapsone concentration at 8 h postdosing. If at 8 h postdosing the dapsone and MADDS concentrations were not both available, then the concentrations at 6 or 4 h postdosing were used.
Statistical analysis.
Chi-square and Kruskal-Wallis tests were used to compare demographic characteristics and dapsone pharmacokinetic parameters among the three dosing groups. Dapsone pharmacokinetic parameters in children under 2 years of age were compared with those in children over 2 years of age by Student’s t test or, if the data were not normally distributed, the Mann-Whitney rank sum test. Spearman rank correlation analysis was used to compare dapsone pharmacokinetic parameters with the acetylation ratio and with MADDS pharmacokinetic parameters (17).
RESULTS
Study population.
Thirty children were enrolled in the pharmacokinetic substudy of PACTG protocol no. 179. Thirteen children were females and 17 were males. Sixteen children were white, seven were black, and seven were Hispanic. Their ages at the time of sampling for the study ranged from 4 months to 12 years, with a median of 2.8 years. There were no differences in age, size, gender, or ethnicity among the dosing groups.
Dapsone pharmacokinetics.
Ten children were studied in each of the 1-mg/kg daily and 4-mg/kg weekly treatment arms. Eleven children were studied after receiving 2-mg/kg daily doses, including one child who was studied after receiving both a 1-mg/kg and a 2-mg/kg dose. Three of the children receiving 1-mg/kg daily doses were sampled for only 8 h after the dosing, allowing determination of Cmax and Tmax only. Two patients in both the 1- and 2-mg/kg daily dosing groups were not at steady state at the time of sampling, and Cmax data for these patients are not reported. Of the patients in the 4-mg/kg weekly dosing group, for all but one predose concentrations were below the detectable limit of the assay at the start of the pharmacokinetic study. The one exception was studied only 5 days after he had received his previous dose and had a predose concentration of 0.19 μg/ml. At 7 days after receiving his dose the concentration would have been below detectable limits by extrapolation of his concentration-time curve.
Pharmacokinetic parameters for the patients are listed in Table 1, and concentration-time curves for each of the dosing groups are plotted in Fig. 1 to 3. Tmax was similar in all three groups, with a median of 2.0 h postdosing. In the patients who were receiving daily doses and were at steady state at the time of sampling, the median Cmax equaled 1.39 μg/ml in the 1-mg/kg group and 3.09 μg/ml in the 2-mg/kg group. The median Cmax was 3.54 μg/ml in the children receiving 4-mg/kg weekly doses. There were no differences in t1/2, CL/F, or V/F among the dosing groups. For the entire population, the median t1/2 was 22.2 h (range, 7.1 h to 40.3 h), the median CL/F was 0.0365 liter/kg/h (range, 0.0104 to 0.1021 liter/kg/h), and the median V/F was 1.13 liters/kg (range, 0.50 to 2.32 liters/kg).
TABLE 1.
Dapsone pharmacokinetic parameters by dosing group
| Dosage | Age (yr) | Tmax (h) | Cmax (μg/ml) | AUC0–∞ (μg · h/ml) | CL/F (liter/kg/h) | V/F (liter/kg) | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| 1 mg/kg daily (n = 10) | 3.0 (0.7–11.1) | 2 (1–4) | 1.39 (0.50–2.33) | 19.6 (9.8–98.6) | 0.0541 (0.0104–0.1021) | 1.15 (0.50–2.32) | 15.8 (9.0–33.0) |
| 2 mg/kg daily (n = 11) | 2.0 (0.3–12.2) | 2 (1–8) | 3.09 (1.86–3.93) | 49.1 (30.3–121.7) | 0.0363 (0.0165–0.0650) | 1.20 (0.63–1.63) | 18.3 (14.3–34.5) |
| 4 mg/kg weekly (n = 10) | 3.2 (0.9–12.7) | 4 (1–6) | 3.54 (2.65–4.43) | 140.8 (42.8–267.9) | 0.0278 (0.0155–0.0906) | 0.94 (0.79–1.51) | 25.7 (7.1–40.3) |
| Total (n = 31) | 2.8 | 2.0 | 0.0365 | 1.13 | 22.2 |
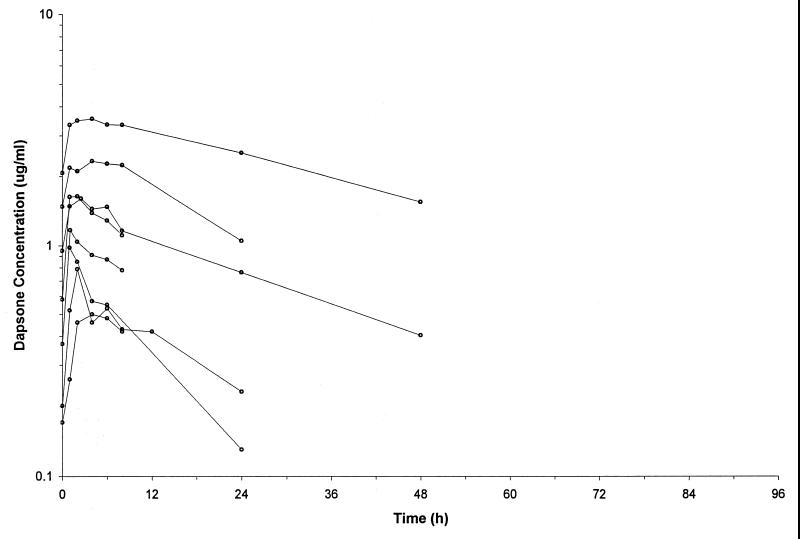
FIG. 1.
Concentration-time plots for the patients receiving dapsone at 1 mg/kg daily.
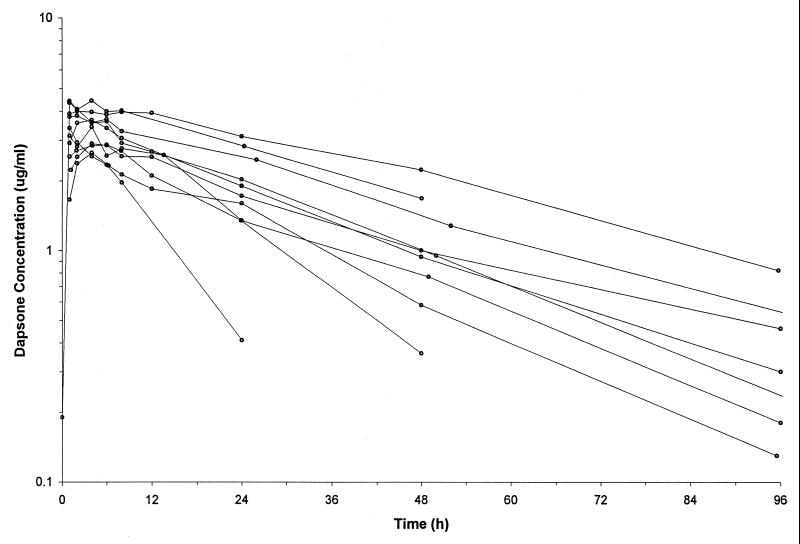
FIG. 3.
Concentration-time plots for the patients receiving dapsone at 4 mg/kg weekly.
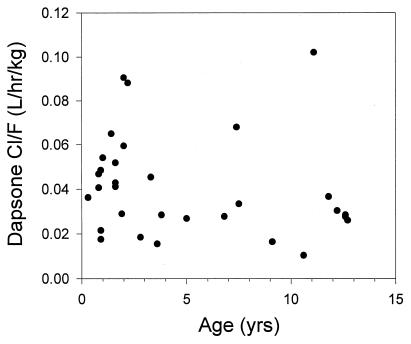
The median dapsone CL/F was significantly increased in those infants less than 2 years of age compared to that in those over 2 years of age (0.0484 versus 0.0278 liter/kg/h; P = 0.011) (Fig. 4). Median V/F and t1/2 were not significantly different between the two age groups. Comparison of Cmax and AUC0–∞ between the age groups for the 2- or 4-mg/kg dosing groups revealed that among all children, median AUC0–∞ and Cmax were lower in the younger children, although the differences did not achieve statistical significance. The median acetylation ratio (the ratio between the plasma MADDS concentration and the plasma dapsone concentration) was 0.51 (range, 0.21 to 2.52). While the acetylation ratio did not correlate with any of the dapsone pharmacokinetic parameters, there was a close correlation between the t1/2 of MADDS and that of dapsone (r = 0.79; P < 0.001).
FIG. 4.
Dapsone CL/F plotted against age for the entire population.
DISCUSSION
The use of dapsone in children was uncommon until the need for PCP prophylaxis in immunocompromised children became evident. When dapsone was first recommended as an alternative prophylactic regimen for PCP, the suggested dosage of 1 mg/kg daily was devised in the absence of pediatric pharmacokinetic data (2). A small preliminary study subsequently demonstrated that this dose results in concentrations in serum about one-half of those achieved in adults receiving 100-mg tablets and that absorption was poor after administration of an extemporaneous ethanol-based liquid preparation (14). In the only other study of dapsone pharmacokinetics in children, Gatti et al. (5) demonstrated adequate absorption after administration of single 2-mg/kg doses of a preparation of pulverized tablets suspended in water and described a correlation between increasing age and decreasing dapsone CL/F and V/F. The current study is the first to describe dapsone pharmacokinetics in children during chronic administration of daily and weekly doses of a proprietary liquid preparation.
The bioavailability of dapsone in adults is good, ranging from 86 to 104% (16). In adults receiving the standard dapsone dosage of a 100-mg tablet daily, Cmax averages 1.5 to 1.9 μg/ml after the administration of initial doses and doubles to about 3 to 4 μg/ml at steady state with chronic dosing (13, 18). In a bioequivalence study of dapsone liquid and tablets, median AUC0–∞ in uninfected adult volunteers receiving 100-mg doses was 56.4 μg · h/ml (range, 28.2 to 85.2 μg · h/ml) (13). Since the concentration of dapsone in serum required for successful prophylaxis against PCP is not known, a reasonable goal for pediatric dosing is to attain concentrations in serum comparable to those achieved with standard adult therapy. The pharmacokinetic data for the children enrolled in the daily dosing arms of this protocol confirm that the 1-mg/kg dose achieves levels much lower than those achieved with standard therapy in adults and that the 2-mg/kg dose achieves the desired target concentrations in serum. As a result of these data, the suggested daily dose for use in children has been revised to 2 mg/kg (3, 4). Children receiving a dosage of 4 mg/kg weekly achieved Cmax comparable to those for children receiving a dosage of 2 mg/kg daily but eliminated virtually all of the dose before administration of the next one. Intermittent dapsone dosing regimens in adults have generally been shown to be associated with less toxicity but also lower efficacy than daily dosing regimens (6).
In comparison to the values for adults, the values of the dapsone elimination parameters for our patients were more variable and were increased for the younger children. In uninfected adult volunteers receiving single 100-mg doses of a liquid and a tablet, median CL/F was 0.0288 liter/kg/h (range, 0.0166 to 0.0422 liter/kg/h) and median t1/2 was 20.8 h (range, 13.7 to 31.1 h) (13). The children in the current study demonstrated a nearly 10-fold variation in CL/F and a 5-fold variation in t1/2. Median CL/F was increased in children under age 2 years but was roughly equivalent to the median for adults in children over age 2 years. The predominant route of elimination of dapsone is metabolism, with only 5 to 15% of an administered dose found in the urine as unchanged drug in adults (18). Dapsone is extensively metabolized by both oxidative and conjugative processes, forming dapsone hydroxylamine and the N-acetylated metabolites MADDS and hydroxymonoacetyldapsone, respectively (11). Deacetylation of the acetylated metabolites also takes place, and an equilibrium between acetylation and deacetylation is reached within a few hours after dapsone administration, resulting in a relatively constant acetylation ratio. The absence of a relationship between the acetylation ratio and any pharmacokinetic parameter in our patients is consistent with findings from previous studies with adults and children and demonstrates that acetylation is not the major metabolic pathway responsible for dapsone elimination.
Data for adults suggest that N-hydroxylation, mediated by enzymes of the cytochrome P-450 3A4 subfamily, plays a central role in dapsone elimination (11). Rifampin, a known inducer of this enzyme system, has been associated with large increases in dapsone clearance in HIV-infected adults and healthy volunteers (6, 15). Previous observations of the developmental maturation of the activity of this enzyme system suggest that activity is low immediately after birth, increases during the first years of life, and then declines to the levels for adults during the rest of childhood and adolescence (10). The observation that dapsone CL/F was more rapid in the patients under age 2 years than in the patients over age 2 years or in adults is consistent with this pattern. However, this observation requires confirmation in a larger study, as our sample was too small to allow evaluation of potential confounding influences on dapsone CL/F, such as gender, ethnicity, and concomitant medications. Two of the older children in our study had CL/F values that greatly exceeded those for the other children over age 2 years. Concomitant medications do not appear to account for the increased dapsone CL/F in these children. Both of these patients were receiving dideoxyinosine, which has no known interaction with dapsone, and one was receiving fluconazole, which has been shown to be a weak inhibitor of cytochrome P-450 3A4 metabolism of other drugs (7, 12).
In conclusion, our data demonstrate that absorption of the proprietary liquid formulation used in this study is adequate. Dosing of children with 2 mg/kg daily or 4 mg/kg weekly results in Cmax equivalent to those reached in adults receiving 100-mg tablets daily. Dapsone CL/F is increased in children less than 2 years of age, and younger children may tend to have lower serum dapsone concentrations than older children. Further studies are needed to investigate the relative efficacies and toxicities of these dosing regimens, to confirm the age-related changes in dapsone CL/F, and to evaluate the usefulness of monitoring of serum dapsone concentrations in young children.
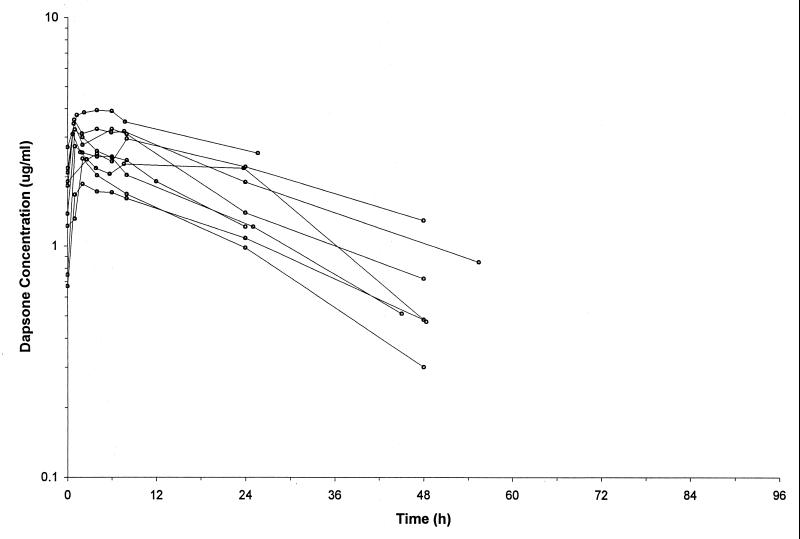
FIG. 2.
Concentration-time plots for the patients receiving dapsone at 2 mg/kg daily.
ACKNOWLEDGMENTS
This study was supported in part by the Pediatric AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases, by the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development, and by the General Clinical Research Center Units funded by the National Center for Research Resources.
We acknowledge the contributions of the many patients enrolled in the trial and their families and the following individuals of the clinical and administrative staffs at the various study sites: Ram Yogev, Children’s Memorial Hospital, Chicago, Ill.; Steve Spector, University of California San Diego; Andrea Rubin Hale, and Kelly Knox Burke, Children’s Hospital of Boston, Boston, Mass.; Anne Marie Regan, Boston Medical Center, Boston, Mass.; Barbara Stechenberg, Baystate Medical Center, Springfield, Mass.; Peter J. Krause, Connecticut Children’s Medical Center, Hartford; Michell Davi, Debra Hickey, and Daniel New, State University of New York at Stony Brook, Stony Brook; Vincent R. Bonagura, Susan J. Schuval, Steven J. Weiss, and Connie Colter, Schneider Children’s Hospital, New Hyde Park, N.Y.; Carol Vincent, Audrey Kamrin, Richard Rutstein, and Harold Lischner, Children’s Hospital of Philadelphia, Philadelphia, Pa.; Michael Lederman, Philip Toltzis, and Ina Adkins, Case Western Reserve University, Cleveland, Ohio; James M. Oleske, Margret Mukai, and George D. McSherry, University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Newark; Coleen K. Cunningham, Leonard B. Weiner, Kathie A. Contello, and Kim M. Kirkwood, State University of New York Health Sciences Center at Syracuse; Kathleen Mohan, Sandra Burchette, Morton Cohen, and Lisa M. Frenkel, Children’s Hospital and Medical Center, Seattle, Wash.; Gwendolyn B. Scott, Charles D. Mitchell, Brenda Haliburton-Jones, and Irma Infante, University of Miami, Miami, Fla.; L. D. Frenkel, Sunanda Guar, Robin Scudder, and Karen Stralkus, University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Foundation, Brunswick, N.J.; Elizabeth McFarland, Myron J. Levin, and Carol Salbenblatt, The Children’s Hospital, Denver, Colo.; Russell Van Dyke, Dawn Sokol, and Troy Lynn Maupin, Tulane University School of Medicine, New Orleans, La.; Karen Williams, Louisiana State University School of Medicine, Baton Rouge; Kenneth C. Rich, Karen Hayani, and Carolyn Everett, University of Illinois, Chicago; Mary Whitley, Statistical and Data Management Center, Harvard School of Public Health, Boston, Mass.; Beth Roy, PACTG Operations Center, Rockville, Md.; and Pamela Clax, Division of AIDS, Bethesda, Md.
REFERENCES
- 1.Bauer L A, Gibaldi M. Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci. 1983;72:978–979. doi: 10.1002/jps.2600720843. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Guidelines for prophylaxis against Pneumocystis carinii pneumonia for children infected with human immunodeficiency virus. Morbid Mortal Weekly Rep. 1991;40(No. RR-2):1–13. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Revised guidelines for prophylaxis against Pneumocystis carinii pneumonia in children infected with or perinatally exposed to human immunodeficiency virus. Morbid Mortal Weekly Rep. 1995;44(No. RR-4):1–11. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Morbid Mortal Weekly Rep. 1997;46(No. RR-12):4–6. [PubMed] [Google Scholar]
- 5.Gatti G, Loy A, Casazza R A, Miletich F, Cruciani M, Bassetti D. Pharmacokinetics of dapsone in human immunodeficiency virus-infected children. Antimicrob Agents Chemother. 1995;39:1101–1106. doi: 10.1128/aac.39.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatti G, Merrigh M, Hossein J, Travaini S, Casazza R, Karlsson M, Cruciani M, Bassetti D. Population pharmacokinetics of dapsone administered biweekly to human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1996;40:2743–2748. doi: 10.1128/aac.40.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honig P K, Worham D C, Zamani X, Mullin J C, Conner D P, Cantilena L R. The effect of fluconazole on the steady-state pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine in humans. Clin Pharmacol Ther. 1993;53:630–636. doi: 10.1038/clpt.1993.83. [DOI] [PubMed] [Google Scholar]
- 8.Hughes W T. Comparison of dosages, intervals, and drugs in the prevention of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1988;32:623–625. doi: 10.1128/aac.32.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes W T, Kennedy W, Dugdale M, et al. Prevention of Pneumocystis carinii pneumonitis in AIDS patients with weekly dapsone. Lancet. 1990;ii:1066. doi: 10.1016/0140-6736(90)92533-n. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 10.Leeder J S, Kearns G L. Pharmacogenetics in pediatrics: implications for practice. Pediatr Clin N Am. 1997;44:55–77. doi: 10.1016/s0031-3955(05)70463-6. [DOI] [PubMed] [Google Scholar]
- 11.May D G, Proter J A, Uetrectt J P, Wilkinson G R, Branch R A. The contribution of N-hydroxylation and acetylation to dapsone pharmacokinetics in normal subjects. Clin Pharmacol Ther. 1990;48:619–627. doi: 10.1038/clpt.1990.204. [DOI] [PubMed] [Google Scholar]
- 12.Michalets E L. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18:84–112. [PubMed] [Google Scholar]
- 13.Mirochnick, M., and D. Jacobus. Unpublished data.
- 14.Mirochnick M, Michaels M, Clarke D, Breña A, Regan A M R, Pelton S. Pharmacokinetics of dapsone in children. J Pediatr. 1993;122:806–809. doi: 10.1016/s0022-3476(06)80033-8. [DOI] [PubMed] [Google Scholar]
- 15.Occhipinti, D. J., A. Choi, K. Deyo, L. H. Danziger, and J. H. Fisher. Influence of rifampin and clarithromycin on dapsone disposition and methemoglobin concentrations, abstr. OI-B-2. In Abstracts of papers of the 96th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics. American Society for Clinical Pharmacology and Therapeutics, Norristown, Pa.
- 16.Pieters F A J M, Zuidema J. The absolute oral bioavailability of dapsone in dogs and humans. Int J Clin Pharmacol Ther Toxicol. 1987;25:396–400. [PubMed] [Google Scholar]
- 17.Rosner B. Fundamentals of biostatistics. Boston, Mass: Duxbury Press; 1986. [Google Scholar]
- 18.Zuidema J, Hilbers-Modderman E S M, Merkus F W H M. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986;11:299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]
- 19.Zuidema J, Modderman E S M, Hilbers H W, Merkus F W H M. Rapid high-performance liquid chromatographic method for the determination of dapsone and monoacetyladapsone in biological fluids. J Chromatogr. 1980;182:130–135. doi: 10.1016/s0378-4347(00)81662-x. [DOI] [PubMed] [Google Scholar]