Abstract
Using an isolate of Aspergillus fumigatus that is less susceptible in vivo to amphotericin B than most other isolates, we compared different doses of liposomal nystatin (L-nystatin), liposomal amphotericin B (L-amphotericin), and amphotericin B lipid complex (ABLC) with amphotericin B deoxycholate. Four experiments with intravenously infected neutropenic mice were conducted. A dose of L-nystatin at 10 mg/kg of body weight was toxic (the mice had fits or respiratory arrest). The optimal dosage of L-nystatin was 5 mg/kg daily on days 1, 2, 4, and 7 (90% survival). This was superior to L-amphotericin (5 mg/kg [P = 0.24] and 1 mg/kg [P < 0.0001]), ABLC (5 mg/kg [P = 0.014] and 1 mg/kg [P < 0.0001]), and amphotericin B deoxycholate (5 mg/kg [P = 0.008]). In terms of liver and kidney cultures, L-nystatin (5 mg/kg) was superior to all other regimens (P = 0.0032 and <0.0001, respectively). Higher doses of L-amphotericin (25 and 50 mg/kg) in one earlier experiment were more effective (100% survival) than 1 mg of L-amphotericin per kg and amphotericin deoxycholate (5 mg/kg) in terms of mortality and both liver and kidney culture results and to L-amphotericin (5 mg/kg) in terms of liver and kidney culture results only. ABLC (25 mg/kg) given daily for 7 days was superior to ABLC (50 mg/kg [P = 0.03]) but not to ABLC at 5 mg/kg or amphotericin B deoxycholate in terms of mortality, although it was in terms of liver and kidney culture results. No dose-response for amphotericin B (5 and 1 mg/kg) was demonstrable. In conclusion, in this stringent model, high doses of L-amphotericin and ABLC could overcome reduced susceptibility to amphotericin B deoxycholate, but all were inferior to 5- to 10-fold lower doses of L-nystatin.
At best, amphotericin B is only moderately effective for the treatment of invasive aspergillosis (9). Recommended dosages (0.8 to 1.5 mg/kg of body weight daily) are at the limit of tolerability. There are conflicting data on the value of high amphotericin B doses for the treatment of invasive aspergillosis. High doses are recommended because the use of lower doses in neutropenic patients allowed breakthrough infection to occur (4), with subsequent improvement when the dose was increased. However, these were all leukemic patients, and resolution of the neutropenia probably contributed to their improvement. Breakthrough infections are not infrequent when higher doses (≈1 mg/kg daily) of amphotericin B are given empirically to profoundly neutropenic patients (5). A clear dose-response was not apparent in a retrospective analysis of patients who survived long enough to receive at least 2 weeks of therapy (7). Some animal model experiments are consistent with a dose-response with amphotericin B, but most are not (7).
Lipid-associated amphotericin B preparations are now widely used for the treatment of invasive aspergillosis (2, 10, 25, 26). Little dose range work has been done with animals or humans (11, 21). However, a comparison of 1 and 4 mg of liposomal amphotericin B (L-amphotericin; AmBisome) per kg in neutropenic patients with probable or definite aspergillosis did not reveal any differences in outcome between the doses (11). A dose escalation study with bone marrow transplant patients with amphotericin B colloidal dispersion (Amphotec) also did not favor any dose (2). However, the dose ranges used in the studies were relatively small, although they were commensurate with what is deliverable clinically.
In previous work we have shown substantial interisolate differences in the susceptibility of Aspergillus fumigatus to amphotericin B in vivo (17, 24). Unfortunately, these differences are not reflected in vitro, in our hands, with multiple in vitro test formats. Others have reported a correlation between therapeutic failure and death when amphotericin B was used for the treatment of infections caused by Aspergillus isolates for which MICs are ≥2 μg/ml (18), although most of these were Aspergillus flavus and Aspergillus terreus. Mutants resistant to amphotericin B in vitro (19) have been generated in the laboratory. Given the likelihood that some isolates of A. fumigatus are less susceptible to amphotericin B in vivo, we have selected one of these and evaluated different doses of liposomal nystatin (L-nystatin) and three formulations of amphotericin B in several experiments using our temporarily neutropenic murine model.
MATERIALS AND METHODS
A clinical isolate, A. fumigatus AF65, was used for the study. The strain has been deposited with the United Kingdom Collection of Pathogenic Fungi, held at the Mycology Reference Laboratory, Bristol, England, as NCPF 7097. The isolate was recovered from the lung of a leukemic patient who had an intermediate response to amphotericin B but whose aspergillosis later relapsed. The strain was maintained on slopes of Oxoid Sabouraud dextrose agar (Unipath Limited, Basingstoke, England) supplemented with 0.5% (wt/vol) chloramphenicol. In vitro tests of the susceptibility of AF65 to amphotericin B were performed previously (10, 17, 20), and the results are shown in Table 1.
TABLE 1.
In vitro susceptibility test results for AF65a
| Drug or formation | MIC (μg/ml) | MFCb (μg/ml) |
|---|---|---|
| Nystatin | 4 | >16 |
| L-nystatin | 1 | 8 |
| Amphotericin B deoxycholate | 0.5 | 4 |
| L-amphotericin | 1 | >16 |
| ABLC | 0.5 | >16 |
| Amphotericin B colloidal dispersion | 0.25 | >16 |
| Itraconazole | 0.25 | >16 |
Data are from reference 20.
MFC, minimum fungicidal concentration.
Animals.
Male CD1 mice (age, 4 to 5 weeks; weight, between 18 and 20 g) were purchased from Charles River UK Ltd. (Margate, United Kingdom). The mice were virus-free and were allowed free access to food and water. Mice were randomized into groups of 10 mice each. Each cage was inspected twice daily, and any infected animals unable to reach the drinker were culled.
Immunosuppression.
Cyclophosphamide (Sigma-Aldrich, Poole, United Kingdom) was administered intravenously via the lateral tail vein to all animals at a dose of 200 mg/kg. A state of profound neutropenia was achieved 3 days after administration and lasted for 4 days (8).
Inoculum.
The isolate was grown in a vented tissue culture flask containing Sabouraud dextrose agar (Oxoid) for 10 days. The Aspergillus conidia were harvested in 25 ml of sterile phosphate-buffered saline with 0.5% Tween 80 (Sigma, Poole, United Kingdom). The stock solution was adjusted to an inoculum that would give a 90% lethal dose (5 × 106 conidia/ml) on the basis of viability counts. Three days after immunosuppression all animals were infected with the 90% lethal dose via the lateral tail vein. The inoculum was rechecked from the remaining conidial suspension after the animals were infected.
Antifungal therapy.
Amphotericin B deoxycholate (Fungizone; E. R. Squibb, Hounslow, United Kingdom) was dissolved in 5% dextrose (Baxter Healthcare, Norfolk, United Kingdom) to a stock concentration of 5.0 mg/ml. Two doses of amphotericin B were used in the course of the experiment: 5.0 and 1.0 mg/kg. The stock solution of amphotericin B was diluted accordingly in 5% dextrose. All doses of amphotericin B (and a 5% dextrose control) were administered via intraperitoneal injection once daily at 24, 48, and 96 h and 7 days postinfection.
L-amphotericin (AmBisome; 50 mg; Nexstar Pharmaceuticals Ltd., Cambridge, United Kingdom) was reconstituted with 12 ml of sterile water to a stock concentration of 4 mg/ml. Several doses of L-amphotericin (from 1.0 to 50 mg/kg) were used in the course of the experiment, and the stock solution of L-amphotericin was diluted accordingly in 5% dextrose. Amphotericin B lipid complex (ABLC; Abelcet; 5 mg/ml; The Liposome Company, London, United Kingdom) was gently resuspended according to the manufacturer’s instructions. The same dose range used for L-amphotericin was used for ABLC in the course of the experiment. The stock solution of ABLC was diluted accordingly in 5% dextrose.
L-nystatin (Nyotran; 50 mg; Aronex Inc., Houston, Tex.) was reconstituted with the addition of 50 ml of 5% dextrose, the mixture was shaken vigorously for 1 min, and liposomes were then allowed to form at room temperature for 30 min. A pilot experiment with dosages of 2.5 mg/kg daily, 5 mg/kg daily and twice daily, and 10 mg/kg daily was performed with uninfected mice. In the treatment model, 5 different doses of L-nystatin were used in the course of the experiment. These varied from 2.5 mg/kg given twice daily to 0.5 mg/kg given twice daily for 7 days or on days 1, 2, 4, and 7. The stock solution of L-nystatin was diluted accordingly in 5% dextrose.
Control mice were treated with either 5% dextrose given intraperitoneally or empty liposomes given intravenously. The control treatments were given to groups of 10 each. Empty liposomes (Aronex Inc.) were reconstituted by the addition of 100 ml of 5% dextrose, the mixture was shaken vigorously for 1 min, and liposomes were then allowed to form at room temperature for 30 min.
All doses of L-amphotericin, amphotericin B deoxycholate, and empty liposomes were given via intravenous injection once daily at 24, 48 and 96 h and 7 days postinfection. ABLC was given every day for 7 days. All doses of L-nystatin were given once or twice daily via intravenous injection (at 12-h intervals) at 1, 2, 4, and 7 days postinfection; the first dose for all treatments was administered at 24 h postinfection.
On day 11 of the experiment all surviving mice were killed. The lungs, liver, and kidneys were removed and transferred to 2 ml of phosphate-buffered saline. The organs were homogenized in a tissue grinder (Polytron; Kinematica AG, Lucerne, Switzerland) for approximately 15 to 30 s and were then diluted 10−1 and 10−2. A total of 0.1 ml of the neat and diluted suspensions was then transferred to Sabouraud dextrose agar (Oxoid) and the liquid was spread over the surfaces of the plates. The plates were incubated at 37°C in a moist atmosphere and were examined daily for 5 days. Colony counts were recorded from all plates that showed growth. Single colonies were accorded a negative result, because of the possibility of airborne contamination. CFU data are reported for both lungs, both kidneys, and the whole liver.
Statistical analysis.
Mortality and culture data were analyzed by the Mann-Whitney U test or the Kruskall-Wallis test if it was not possible to perform the Mann-Whitney U test (i.e., if all values for one group were identical). Two-sided P values are given. Mice which died before day 10 were assumed to have organ counts at least as high as the highest counts in the surviving mice in the calculation of culture result statistics. All data analyses were performed with the computer package Arcus Quik Stat (Addison Wesley Longman Ltd.). Two-sided probability values are quoted in the text.
RESULTS
One pilot experiment was done with L-nystatin, followed by four experiments with multiple treatments and control treatments. These four experiments are described separately below, followed by a summary of all the data obtained in experiments with 1 and 5 mg of L-nystatin, L-amphotericin, ABLC, and amphotericin B per kg.
Dose range study with L-nystatin.
Doses of L-nystatin of 10 mg/kg were toxic: mice had fits or suffered respiratory arrest immediately after dosing. This dose was not used further after administration of the second dose. The dosage of 5 mg/kg given twice daily was better tolerated, but two uninfected mice died shortly after dosing. The dosage of 5 mg/kg given once daily was better tolerated and was therefore the maximum dosage used in the subsequent treatment experiments, in which it was given as 2.5 mg/kg twice daily.
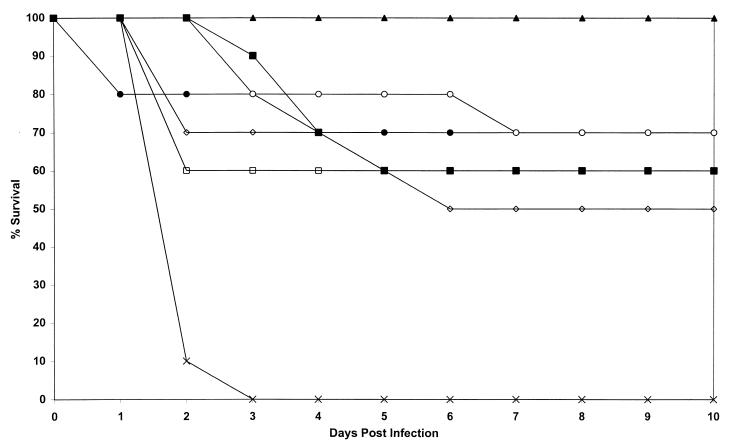
Treatment with all doses of L-nystatin and amphotericin B were superior to control treatment with liposomes in terms of survival (P = <0.0001 to 0.02) (Fig. 1). Administration of single daily doses of L-nystatin intravenously at 5 mg/kg was less effective than administration of half the dose twice daily, but not significantly so (P = 0.09). A lower dosage of L-nystatin (2.5 mg/kg/day) was less effective than one of 2.5 mg/kg twice daily (P = 0.03) when both were given on days 1, 2, 4, and 7. No L-nystatin treatment was statistically superior or inferior to amphotericin B treatment (P = 0.09 to 1.0).
FIG. 1.
Study of activity of L-nystatin at cumulative doses ranging from 10 to 35 mg/kg compared with those of amphotericin B and empty liposomes (control). Plots are of cumulative mortality against time after AF65 infection and treatment with L-nystatin. □, L-nystatin at 5 mg/kg on days 1 to 7; ●, L-nystatin at 5 mg/kg on days 1, 2, 4, and 7; ○, L-nystatin at 2.5 mg/kg on days 1 to 7; ▴, L-nystatin at 2.5 mg/kg twice daily on days 1, 2, 4, and 7; ◊, L-nystatin at 2.5 mg/kg on days 1, 2, 4, and 7; ■, amphotericin B at 5 mg/kg days on 1, 2, 4, and 7; ×, no active treatment.
No cultures of lung specimens were positive for any surviving animals.
Cultures of liver specimens were positive for 19 animals in all groups except the group treated with L-nystatin at 2.5 mg/kg over 4 days. These counts varied from 40 to 720 CFU. Two animals in the group treated with L-nystatin at 2.5 mg/kg twice daily for 4 days were positive, and both had counts of 140 CFU. With respect to culture results for the liver specimens, treatment with L-nystatin at 2.5 mg/kg twice daily for 4 days was statistically superior to treatment with L-nystatin at 2.5 mg/kg once daily for 4 days (P = 0.03) and 7 days (P = 0.002), L-nystatin at 5 mg/kg for 4 days (P = 0.047) or 7 days (P = 0.003), and amphotericin B (P = 0.006).
Only three survivors had positive cultures of kidney specimens: two in the amphotericin B group and one in the L-nystatin at 5 mg/kg for 4 days group. There were fewer culture differences for the kidney specimens, but L-nystatin at 2.5 mg/kg twice daily for 4 days was superior to L-nystatin at 2.5 mg/kg once daily for 4 days (P = 0.01) and amphotericin B (P = 0.002).
Dose range study with L-amphotericin.
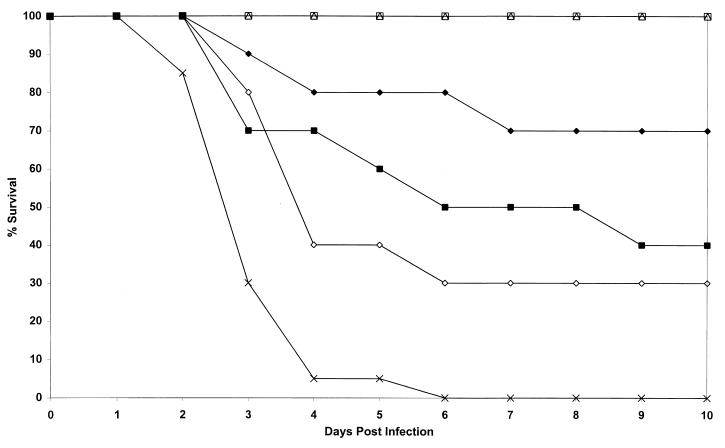
A wide spectrum of results was obtained for a 50-fold dose range of L-amphotericin (Fig. 2). All doses were given on days 1, 2, 4, and 7. All treatments were superior to the control treatments in terms of mortality (P = 0.001 to 0.03) with the exception of L-amphotericin at 1 mg/kg (P = 0.07). Both the 25- and 50-mg/kg treatments with L-amphotericin were 100% successful in terms of mortality and were apparently well tolerated by the mice. These two doses were statistically superior to amphotericin B at 5 mg/kg (P = 0.01) and L-amphotericin at 1 mg/kg (P = 0.003) and were statistically equivalent to L-amphotericin at 5 mg/kg (P = 0.21).
FIG. 2.
Study of activity of L-amphotericin at doses ranging from 1 to 50 mg/kg given four times to mice infected with strain AF65 compared with those of amphotericin B and dextrose (control). Plots are of cumulative mortality against time after AF65 infection and treatment with L-amphotericin. □, L-amphotericin at 50 mg/kg on days 1, 2, 4, and 7; ▵, L-amphotericin at 25 mg/kg on days 1, 2, 4, and 7; ⧫, L-amphotericin at 5 mg/kg on days 1, 2, 4, and 7; ◊, L-amphotericin at 1 mg/kg on days 1, 2, 4, and 7; ■, amphotericin B at 5 mg/kg on days 1, 2, 4, and 7; ×, no active treatment.
Aspergillus was eradicated from the lungs of all mice in all treatment groups.
Among the livers from mice treated with L-amphotericin at 25 and 50 mg/kg, only one liver from a mouse in each group was positive (200 and 460 CFU, respectively). Four of seven, one of three, and one of four livers among mice in the groups treated with L-amphotericin at 5 and 1 mg/kg and amphotericin B were culture positive, respectively (40 to 120 CFU). In terms of growth in cultures of liver specimens, L-amphotericin at 50 mg/kg was superior to L-amphotericin at 5 mg/kg (P = 0.03) and 1 mg/kg (P = 0.002) and amphotericin B (P = 0.02). Likewise, L-amphotericin at 25 mg/kg was superior to L-amphotericin at 5 mg/kg (P = 0.03) and 1 mg/kg (P = 0.01) and amphotericin B (P = 0.012) in terms of liver culture results.
All kidneys from mice in the group that received L-amphotericin at 25 mg/kg were sterile, and one kidney from a mouse that received L-amphotericin at 50 mg/kg was positive (300 CFU). Three of seven kidneys from mice in the L-amphotericin (5 mg/kg) group were positive (600 to 13,100 CFU). All three kidneys from mice in the L-amphotericin (1 mg/kg) group were positive (1,500 to 24,000 CFU). Two of four kidneys from mice in the amphotericin B group were positive (300 and 1,800 CFU). An identical pattern of superiority of 50 and 25 mg of L-amphotericin per kg compared to all other treatments was seen in terms of kidney results culture (P = <0.0001 to 0.003), as was the case for liver culture results. In addition, L-amphotericin at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.01).
Dose range study with ABLC.
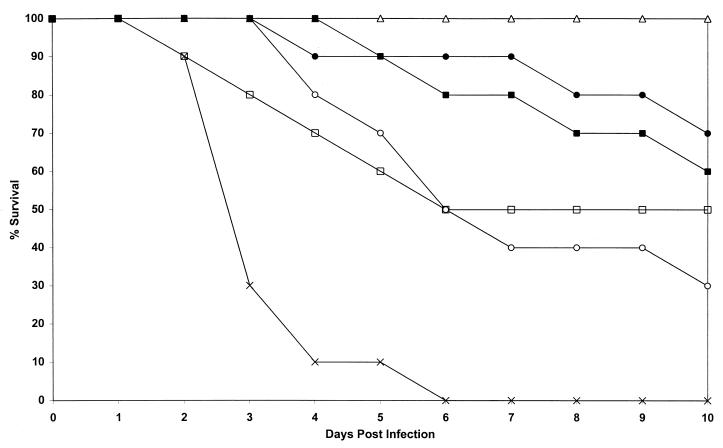
Again, a wide spectrum of survival results was seen with ABLC, depending on the dose (Fig. 3). ABLC was given daily for 7 days. Only ABLC at 25 mg/kg was 100% successful in terms of survival and was superior to treatment with 50 mg/kg (P = 0.03) and 1 mg/kg (P = 0.003) and the control treatments. ABLC at 25 mg/kg was not superior to ABLC at 5 mg/kg (P = 0.21) or amphotericin B (P = 0.09). The higher dose of 50 mg/kg was apparently toxic to the animals but was still superior to the control treatment (P = 0.01). Lower doses of 5 mg of ABLC per kg were equivalent to amphotericin B (P = 0.72) but not quite superior to ABLC at 1 mg/kg (P = 0.06). Amphotericin B was superior to control treatments (P = 0.001), as were all doses of ABLC (P = 0.01 to <0.0001).
FIG. 3.
Study of activity of ABLC at doses ranging from 1 to 50 mg/kg given seven times to mice infected with AF65 compared with those of amphotericin B and dextrose (control). Plots are of cumulative mortality against time after AF65 infection and treatment with ABLC. □, ABLC at 50 mg/kg on days 1 to 7; ▵, ABLC at 25 mg/kg on days 1 to 7; ●, ABLC at 5 mg/kg on days 1 to 7; ○, ABLC at 1 mg/kg on days 1 to 7; ■, amphotericin B at 5 mg/kg on days 1, 2, 4, and 7; ×, no active treatment.
No cultures of lung tissue from survivors were positive.
The mortality results are partly reflected by the culture results. Livers were sterilized in all the survivors that received ABLC at 50 and 25 mg/kg, but the livers of four of seven mice and all three mice in the groups that received ABLC at 5 and 1 mg/kg, respectively, were positive (40 to 120 CFU). The livers of two of the six amphotericin B-treated mice were positive by culture. Treatment with ABLC at 25 mg/kg was superior to treatment with ABLC at 5 mg/kg (P = 0.0001), ABLC at 1 mg/kg (P ≤ 0.0001), and amphotericin B at 5 mg/kg (P = 0.0001) in terms of liver culture results. Also, ABLC at 5 mg/kg was superior to ABLC at 1 mg/kg (P = 0.04) in terms of liver culture results.
Kidney tissues of 3 of 5 and 1 of 10 survivors in the groups that received 50 and 25 mg ABLC per kg, respectively, were positive by culture at autopsy. The kidneys of one of seven and two of three mice in the groups that received 5 and 1 mg of ABLC per kg, respectively, were positive culture at autopsy. Two of six amphotericin B-treated animals were positive. ABLC at 25 mg/kg was superior to ABLC at 5 mg/kg (P = 0.03), ABLC at 1 mg/kg (P = 0.0006), and amphotericin B (P = 0.04). ABLC at 5 mg/kg was superior to ABLC at 1 mg/kg (P = 0.03).
Comparison of 5 and 1 mg of L-nystatin per kg, L-amphotericin, ABLC, and amphotericin B.
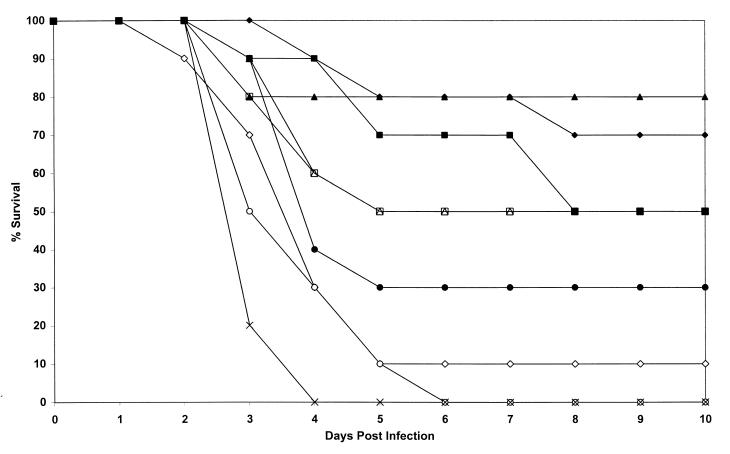
L-nystatin at 5 mg/kg was the most successful regimen in terms of survival and culture results. After L-nystatin (5 mg/kg), the rank order of the regimens in terms of survival were L-amphotericin (5 mg/kg) > ABLC (5 mg/kg) = amphotericin B (5 mg/kg and 1 mg/kg) = L-nystatin (1 mg/kg) > L-amphotericin (1 mg/kg) > ABLC (1 mg/kg). None of the regimens was 100% successful. L-nystatin at 5 mg/kg was statistically superior to L-amphotericin at 1 mg/kg (P = 0.01), ABLC at 1 mg/kg (P = 0.005), and the control treatments. L-nystatin at 1 mg/kg was superior to the control treatments (P = 0.0002). L-amphotericin at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.0025), ABLC at 5 mg/kg (P = 0.04), ABLC at 1 mg/kg (P = 0.002), and the control treatments (P < 0.0001). ABLC at 5 mg/kg was superior to the control treatments (P = 0.002). Amphotericin at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.009), ABLC at 1 mg/kg (P = 0.002), and the control treatments (P = 0.0002). All other comparisons of survival were insignificant.
All the regimens were effective in reducing organism counts in the lungs to very low levels; the only positive groups were those treated with L-nystatin at 1 mg/kg and L-amphotericin at 1 mg/kg (geometric mean, 2 CFU) (data not shown). Liver culture results mirrored survival results, and, to a lesser extent, so did kidney culture results. There was good concordance between culture results and mortality statistics. L-nystatin at 5 mg/kg was superior to all other treatment regimens except amphotericin B at 5 mg/kg in terms of both liver culture results (P = 0.005 to 0.02) and kidney culture results (P = 0.006 to 0.04). In addition L-amphotericin at 5 mg/kg was superior to L-amphotericin at 1 mg/kg and ABLC at 1 mg/kg. ABLC at 5 mg/kg was superior to ABLC at 1 mg/kg. All other comparisons were statistically insignificant.
Comparison of all data for 5- and 1-mg/kg daily doses for all drugs.
The mortality data are depicted in Fig. 4. These were obtained by using combined data for two experiments for all groups except the groups treated with L-nystatin at 1 mg/kg and amphotericin B at 1 mg/kg (for which data from only one experiment were used). The statistical comparisons of mortality are shown in Table 2.
FIG. 4.
Comparison of treatment with two doses (1 and 5 mg/kg) of L-nystatin, L-amphotericin, ABLC, and amphotericin B with control treatments in a model of invasive aspergillosis caused by AF65 in temporarily neutropenic mice. For all groups except L-nystatin at 1 mg/kg and amphotericin B at 1 mg/kg, the data are from two experiments. Plots are of cumulative mortality against time after AF65 infection and various treatments. ▴, L-nystatin at 2.5 mg/kg twice daily on days 1, 2, 4, and 7; ▵, L-nystatin at 0.5 mg/kg twice daily on days 1, 2, 4, and 7; ⧫, L-amphotericin at 5 mg/kg on days 1, 2, 4, and 7; ◊, L-amphotericin at 1 mg/kg on days 1, 2, 4, and 7; ●, ABLC at 5 mg/kg on days 1 to 7; ○, ABLC at 1 mg/kg on days 1 to 7; ■, amphotericin B at 5 mg/kg on days 1, 2, 4, and 7; □, amphotericin B at 1 mg/kg on days 1, 2, 4, and 7; ×, no active treatment.
TABLE 2.
Two-sided probability values for all mortality data for 5- and 1-mg/kg daily doses of L-nystatin, L-amphotericin, ABLC, and amphotericin B for all experiments (except the pilot experiment) combined
| Inferior regimen |
P value for the following superior regimen:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| L-nystatina
|
L-amphotericin Ba
|
ABLCb
|
Amphotericin Ba
|
|||||
| 5 mg/kg | 1 mg/kgc | 5 mg/kg | 1 mg/kg | 5 mg/kg | 1 mg/kg | 5 mg/kg | 1 mg/kgc | |
| L-nystatin, 1 mg/kgac | 0.035 | 0.0004 | 0.024 | 0.56 | ||||
| L-amphotericin, 5 mg/kga | 0.24 | |||||||
| L-amphotericin, 1 mg/kga | <0.0001 | 0.032 | 0.005 | 0.017 | 0.49 | 0.002 | 0.072 | |
| ABLC, 5 mg/kgb | 0.014 | 0.2 | 0.72 | |||||
| ABLC, 1 mg/kgb | <0.0001 | 0.15 | 0.0006 | 0.044 | 0.005 | 0.24 | ||
| Amphotericin B, 5 mg/kga | 0.008 | 0.23 | ||||||
| Amphotericin B, 1 mg/kgac | 0.03 | 0.95 | 0.0003 | 0.15 | 0.51 | |||
| Liposomes and dextrose | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Given on days 1, 2, 4, and 7.
Seven doses given on days 1 to 7.
Data are for only a single experiment.
With respect to liver culture results (Table 3), treatment with L-nystatin at 5 mg/kg resulted in far fewer CFU than any other treatment (P = <0.0001 to 0.01) except that with L-amphotericin at 5 mg/kg (P = 0.052). L-amphotericin at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.007) and ABLC at 1 mg/kg (P = 0.001). ABLC at 5 mg/kg was superior to ABLC at 1 mg/kg (P = 0.03). Amphotericin B at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.035) and ABLC at 1 mg/kg (P = 0.008).
TABLE 3.
Survival and colony geometric mean counts for mice infected with A. fumigatus AF65 and treated with all drugs at 5 and 1 mg/kg dailya
| Group | No. of infected mice | No. (%) of mice that survived | Colony counts/organ
|
|
|---|---|---|---|---|
| Liver | Kidney | |||
| L-nystatin, 5 mg/kg (days 1, 2, 4, and 7) | 20 | 18 (90) | 1b | <1c |
| L-nystatin, 1 mg/kg (days 1, 2, 4, and 7) | 10 | 5 (50) | 35 | 63 |
| L-amphotericin, 5 mg/kg (days 1, 2, 4, and 7) | 20 | 14 (70) | 7 | 15 |
| L-amphotericin, 1 mg/kg (days 1, 2, 4, and 7) | 20 | 4 (20) | 72 | 211 |
| ABLC, 5 mg/kg (days 1 to 7) | 20 | 10 (50) | 19 | 22 |
| ABLC, 1 mg/kg (days 1 to 7) | 20 | 3 (15) | 114 | 245 |
| Amphotericin B, 5 mg/kg (days 1, 2, 4, and 7) | 20 | 11 (55) | 14 | 13 |
| Amphotericin B, 1 mg/kg (days 1, 2, 4, and 7) | 10 | 5 (50) | 54 | 68 |
| Controlsd | 70 | 0 (0) | ||
Results are for data pooled from all experiments (except the pilot experiment).
P = 0.0032 for L-nystatin at 5 mg/kg versus all doses of all drugs.
P = <0.0001 for L-nystatin at 5 mg/kg versus all doses of all drugs.
Five percent dextrose or empty liposomes.
A similar result was seen with renal culture results (Table 3), in which L-nystatin at 5 mg/kg was superior to all other regimens collectively and individually (P = <0.0001 to 0.001). L-amphotericin at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.005) and ABLC at 1 mg/kg (P = 0.002). ABLC at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.027) and ABLC at 1 mg/kg (P = 0.014). Amphotericin B at 5 mg/kg was superior to L-amphotericin at 1 mg/kg (P = 0.008) and ABLC at 1 mg/kg (P = 0.004).
DISCUSSION
The data presented here provide convincing evidence of the activity of L-nystatin given at a dosage of 5 mg/kg daily against experimental invasive aspergillosis using an isolate of A. fumigatus that has reduced susceptibility to amphotericin B. They also demonstrate the dose dependency of L-nystatin against this infection, just as Groll and colleagues did (13), with 1 mg/kg daily being substantially less effective, although it is as effective as amphotericin B at 5 mg/kg. Nystatin and amphotericin B are both polyene antifungal agents, and these drugs have been reported to have similar or identical modes of antifungal action against yeasts, which includes binding to ergosterol in the fungal membrane, resulting in altered membrane permeability, which allows the release of K+, sugars, and metabolites (23). The data generated in this study imply that the mechanism of resistance is not identical given the divergence of results between the compounds given at the same doses. Indeed, differences in the biochemical actions of nystatin and amphotericin B have been reported in Candida (6, 14). A lack of cross-resistance between amphotericin B and nystatin has been reported in Candida (3, 15). Our data obtained with an isolate of Aspergillus with a degree of in vivo resistance to amphotericin B suggest that very large doses of amphotericin B delivered in a lipid vehicle could overcome the reduced susceptibility nearly as well as lower doses of nystatin also delivered in a lipid vehicle. These data raise further uncertainty about the mode of action of the polyenes and imply that the lipid incorporation is not simply increasing local delivery of the drug (although this is possible) but, rather, is playing an intrinsically important part in augmenting the activity of amphotericin B (and, possibly, nystatin) against Aspergillus. The data that we previously generated in vitro, even taking into account all the uncertainties associated with in vitro testing, are consistent with this hypothesis (20). These data (20) showed that L-nystatin is active against a number of Aspergillus isolates for which the MICs of the lipid forms of amphotericin B are high (>16 μg/ml). We also showed that lipid incorporation of nystatin reduced MICs of nystatin and usually raised the MICs of amphotericin B. These data also further underline how little is known about the mechanism of action of amphotericin B. We could find only one paper that described an investigation of the mechanism of action of amphotericin B against Aspergillus spp. (22), despite 40 years of use. That paper showed that amphotericin B suppresses respiration and glycolysis and causes potassium and phosphorus leakage (22). Some dose dependency was demonstrated for all these effects over a 50-fold range upward from 1 U/ml in dimethyl sulfoxide.
Previous work has demonstrated the pharmacokinetic equivalence of intraperitoneal and intravenous amphotericin B administration in mice (12). Uncertainty about the fate of lipid-complexed or liposome-encapsulated amphotericin B given intraperitoneally prevented us from attempting this with these compounds. However, such a mode of administration might have ameliorated some of the acute toxicity seen with 5 mg of L-nystatin per kg given as a single dose compared to that of L-nystatin given at 2.5 mg/kg twice daily and ABLC given at 50 mg/kg. Perhaps the use of split doses would enable larger doses to be given to patients.
The data are also consistent with the dose dependencies of L-amphotericin and ABLC over a 25-fold dose range. Lesser differences were seen between the 1- and 5-mg/kg and the 5- and 25-mg/kg doses. For L-amphotericin, no difference between doses of 25 and 50 mg/kg was seen. The relative difference between the two doses of L-nystatin studied over a fivefold range was smaller (but significant). Essentially no dose range effect could be shown for amphotericin B deoxycholate. However, the latter comparisons are limited because only one experiment included the lower doses of L-nystatin and amphotericin B. The relatively small increases in efficacy seen between 1 and 5 mg/kg and between 5 and 25 mg/kg is consistent with the in vivo response data for L-amphotericin and amphotericin B colloidal dispersion (2, 11). In the experiments described here, we used an isolate with reduced in vivo susceptibility to amphotericin B, and a dose-response could be demonstrated for the lipid-associated forms of amphotericin B but not conventional amphotericin B. In another study (17) we have generated data that showed a dose-response for one isolate (AF210) but not for AF65 (which was used in the model in the present study) or another fully susceptible isolate (AF294) against which all doses were effective. Thus, dose dependency of amphotericin B appears to be isolate dependent. Also, a large dose range (a dose range that is not generally used in patients) appears to be required to show dose dependency against some isolates. In any case, quite variable kinetics of L-amphotericin have been shown in ill patients (16). These two factors probably account, either partly or fully, for the lack of a convincing dose dependency of conventional amphotericin B against invasive aspergillosis (7). Furthermore, in patients other factors come into play, including the speed of diagnosis, the degree of immunocompromise, recovery of immune function and its timing, notably, recovery of neutropenia, and the possible impact of surgery.
The in vitro susceptibility methodology that we used to assess in vitro activity does not correlate with the in vivo outcome with the isolate that we used. This was noted previously (17, 24). Other in vitro test formats were also nonpredictive of outcome (17). Thus, we do not have a laboratory method that can be used to determine which patients might respond to “standard” doses of amphotericin B (in whatever formulation) and which patients might benefit from very large doses.
Therefore, this study does place into sharp relief the inadequacy of our present therapeutic decisions regarding the use of a lipid-based amphotericin B for the treatment of patients with life-threatening invasive aspergillosis. It is unlikely that another dose ranging clinical study of lipid-based amphotericin B for invasive aspergillosis will be undertaken, given the accrual difficulties and competition for patients for the study of new drugs. Should megadoses of L-amphotericin be used? Our data indicated that, for at least one isolate, the answer is yes and that L-amphotericin should be used. Perhaps L-nystatin would be better at moderate doses? Our data are consistent with this, but high doses of L-nystatin would not appear to be deliverable. The clinical data on response rates with L-nystatin will be critical to addressing these issues. The need for a reproducible means of predicting the clinical response to treatment of patients with invasive aspergillosis has never been greater.
ACKNOWLEDGMENTS
The study was funded by Aronex Pharmaceuticals and The Fungal Research Trust.
We are grateful to Hilary Gough for typing the manuscript.
REFERENCES
- 1.Bowden R, Chandrasekar P, White M, van Burik J A, Wingard J, et al. Abstracts of the International Immunocompromised Host Society Meeting. 1998. A double-blind randomised controlled trial of Amphocil (ABCD) versus amphotericin B (AmB) for treatment of invasive aspergillosis in immunocompromised patients, abstr. 091. [Google Scholar]
- 2.Bowden R A, Cays M, Gooley T, Mamelok R D, van Burik J-A. Phase I study of amphotericin B colloidal dispersion for the treatment of invasive fungal infections after marrow transplant. J Infect Dis. 1996;173:1208–1215. doi: 10.1093/infdis/173.5.1208. [DOI] [PubMed] [Google Scholar]
- 3.Broughton M C, Bard M, Lees N D. Polyene resistance in ergosterol producing strains of Candida albicans. Mycoses. 1991;34:75–83. doi: 10.1111/j.1439-0507.1991.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 4.Burch P A, Karp J E, Merz W G, Kuhlman J E, Fishman E K. Favourable outcome of invasive aspergillosis in patients with acute leukemia. J Clin Oncol. 1987;5:1985–1993. doi: 10.1200/JCO.1987.5.12.1985. [DOI] [PubMed] [Google Scholar]
- 5.Caillot D, Casasnovas O, Bernard A, Couailler J F, Durand D, Cuisenier B, Solary E, Piard F, Petrella T, Bonnin A, Couillault G, Dumas M, Guy H. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–147. doi: 10.1200/JCO.1997.15.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Chen W C, Chou D-L, Feingold D S. Dissociation between ion permeability and the lethal action of polyene antibiotics on Candida albicans. Antimicrob Agents Chemother. 1978;13:914–917. doi: 10.1128/aac.13.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W, Hall L, Jackson M, Hollis S. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob Agents Chemother. 1995;39:1809–1814. doi: 10.1128/aac.39.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning D W. Therapeutic outcome of invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 10.Denning D W, Radford S A, Oakley K, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome for Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 11.Ellis M, Spence D, de Pauw B, Meunier F, Marinus A, Colette L, Sylvester R, Meis J, Boogaerts M, Selleslag D, Krcmery V, von-Sinner W. An EORTC International multicenter randomised trial (EORTC Number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin Infect Dis. 1998;27:1406–1412. doi: 10.1086/515033. [DOI] [PubMed] [Google Scholar]
- 12.Graybill J R, Kaster S R. Experimental murine aspergillosis: comparison of amphotericin B and a new polyene antifungal drug, SCH 28191. Am Rev Respir Dis. 1984;129:292–295. [PubMed] [Google Scholar]
- 13.Groll A H, Gonzalez C E, Giri N, Kligys K, Love W, Peter J, Feuerstein E, Bacher J, Piscitelli S P, Walsh T J. Liposomal nystatin against experimental pulmonary aspergillosis in persistently neutropenic rabbits: efficacy, safety and non-compartmental pharmacokinetics. J Antimicrob Chemother. 1999;43:95–103. doi: 10.1093/jac/43.1.95. [DOI] [PubMed] [Google Scholar]
- 14.Hammond S M. Biological activity of polyene antibiotics. W Prog Med Chem. 1977;14:106–179. doi: 10.1016/s0079-6468(08)70148-6. [DOI] [PubMed] [Google Scholar]
- 15.Hebeka E, Solotorovsky M. Development of resistance to polyene antibiotics in Candida albicans. J Bacteriol. 1965;89:1533–1539. doi: 10.1128/jb.89.6.1533-1539.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinemann V, Bosse D, Jehn U, Kahny B, Wachhol K, Debus A, Scholz P, Kolb H J, Wilmanns W. Pharmacokinetics of liposomal amphotericin B (AmBisome) in critically ill patients. J Antimicrob Chemother. 1997;41:1275–1280. doi: 10.1128/aac.41.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, E., K. L. Oakley, S. Radford, C. B. Moore, P. Warn, D. W. Warnock, and D. W. Denning. Lack of correlation of in vitro susceptibility testing methods with in vivo outcome for Aspergillus fumigatus for amphotericin B. J. Antimicrob. Chemother. [DOI] [PubMed]
- 18.Lass-Florl C, Kofler G, Kropshofer G, Hermans J, Kreczy A, Dierich M P, Niederwieser D. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J Antimicrob Chemother. 1998;42:497–502. doi: 10.1093/jac/42.4.497. [DOI] [PubMed] [Google Scholar]
- 19.Manavathu E K, Alangaden G J, Chandrasekar P H. In vitro isolation and antifungal susceptibility of amphotericin B-resistant isolates of Aspergillus fumigatus. J Antimicrob Chemother. 1998;41:615–619. doi: 10.1093/jac/41.6.615. [DOI] [PubMed] [Google Scholar]
- 20.Oakley K L, Moore C B, Denning D W. Comparison of in vitro activity of liposomal nystatin against Aspergillus species, with those of nystatin; amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazole. Antimicrob Agents Chemother. 1999;5:1264–1266. doi: 10.1128/aac.43.5.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentice H G, Hann I M, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, Pinkerton C R, Schey S A, Jacobs F, Oakhill A, Stevens R F, Darbyshire P J, Givson B E. A randomized comparison of liposomal versus conventional amphotericin B for treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol. 1997;98:711–718. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandhu D K. Effect of amphotericin B on the metabolism of Aspergillus fumigatus. Mycopathlogia. 1979;1:23–29. doi: 10.1007/BF00490387. [DOI] [PubMed] [Google Scholar]
- 23.Thomas A H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J Antimicrob Chemother. 1986;17:269–279. doi: 10.1093/jac/17.3.269. [DOI] [PubMed] [Google Scholar]
- 24.Verweij P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Efficacy of LY303366 against amphotericin B “susceptible” and “resistant” A. fumigatus infection in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh T J, Hiemenz J W, Seibel N L, Perfect J R, Horwith G, Lee L, Silber J L, DiNubile M J, Reboli A, Bow E, Lister J, Anaissie E J. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–1396. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 26.White M H, Anaissie E J, Kusne S, Wingard J R, Helmenz J W, Cantor A, Gurwith M, Du-Mond C, Mamelok R D, Bowden R A. Amphotericin B colloidal dispersion vs amphotericin B as therapy for invasive aspergillosis. Clin Infect Dis. 1997;24:635–642. [PubMed] [Google Scholar]