Abstract
The Tol/Pal system (also written as “The Tol-Pal system”) is a set of protein complexes produced by most Gram-negative bacteria. It comprises the inner membrane-associated and the outer membrane-anchored subunits composed of the TolA, TolQ, and TolR proteins and the TolB and Pal proteins, respectively. Although the Tol/Pal system was first defined as bacterial proteins involved in colicin uptake of Escherichia coli, its global roles have been characterized in several studies as mentioned in this article. Pathogenesis of many Gram-negative pathogens is sustained by the Tol/Pal system. It is also essential for cell growth and fitness in some pathogens. Therefore, the Tol/Pal system is proposed as a potential target for antimicrobial chemotherapy. Although the tol/pal mutants are low in virulence, they still have the ability to stimulate the immune system. The Pal protein is highly immunogenic and induces both adaptive and innate immune responses. Therefore, the tol/pal mutant strains and Pal proteins also have potential vaccine properties. For these reasons, the Tol/Pal system represents a promising research target in the development of antibacterial therapeutic strategies for refractory infections caused by multi-drug-resistant (MDR), Gram-negative pathogens. In this paper, we summarize studies on the Tol/Pal system associated with bacterial pathogenesis and vaccine development.
Keywords: Gram-negative bacteria, virulence, drug resistance, antimicrobial chemotherapy, vaccine, outer membrane protein, bacterial pathogenesis
1. Introduction
Antimicrobial agents are commonly used for the prevention and treatment of bacterial infections; however, an increasing number of bacteria have acquired resistance to conventional drugs. On account of this, there is an urgent need to develop alternative chemotherapy options, such as new drugs and vaccines.
Gram-negative bacteria ubiquitously have a lipid bilayer named the outer membrane, outside the cell wall and cytoplasmic inner membrane. The outer membrane is a permeability barrier that limits the entry of drugs into the cells [1]. Due to this, most Gram-negative bacteria are innately tolerant to some classes of antimicrobial agents [2,3]. They are hard to treat when additional resistance is acquired. Many classes of antimicrobial agents have been used in medical and industrial applications to prevent and treat infectious diseases caused by these pathogens, and their widespread application has resulted in the development of drug-resistant bacteria.
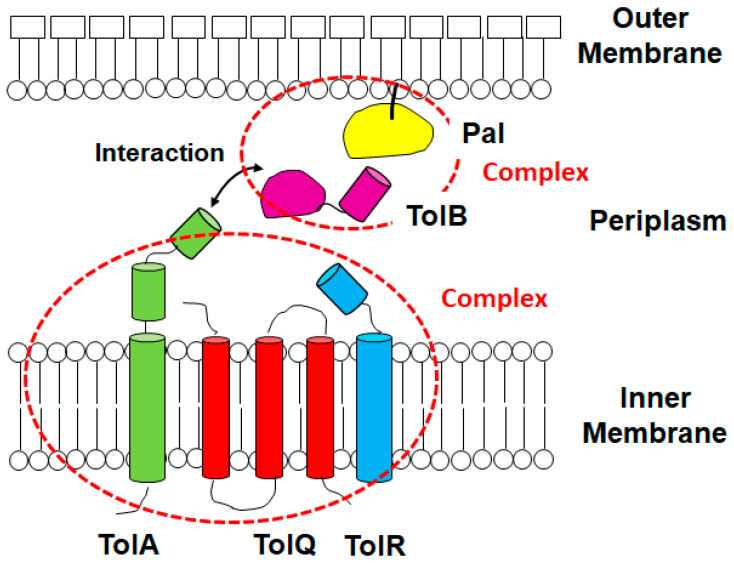
The Tol/Pal system is a set of protein complexes. It is composed of the TolA, TolB, TolQ, TolR, and Pal proteins, and they form two protein complexes that traverse the outer membrane, periplasmic space and inner membrane [4]. Although the tol/pal genes were originally identified in Escherichia coli, these orthologous genes are found in many Gram-negative bacteria [5]. The tol/pal genes are transcribed in two operons (ybgC-tolQ-tolR-tolA and tolB-pal-cpoB), while a single large (ybgC-tolQ-tolR-tolA-tolB-pal-cpoB) transcript is also observed [6,7]. Expression of tol/pal genes is regulated by RcsC, a sensor kinase of the two-component regulatory system, in E. coli, iron in Pseudomonas aeruginosa and CRP (cyclic AMP receptor protein) in Klebsiella pneumoniae [8,9,10]. The TolQ, TolR and TolA proteins form a complex in the inner membrane while the periplasmic TolB protein interacts with the Pal protein anchored in the outer membrane [11,12] (Figure 1). The TolQ-TolR-TolA inner membrane complex has structural similarities to the TonB-ExbB-ExbD iron/siderophore uptake system and the MotA-MotB flagellar motor complex [13,14]. Some in vitro studies show that TolB binds to the C-terminal domain of the TolA protein [15,16,17]. TolB can also interact with several proteins localized in the outer membrane, including OmpA, OmpF, and Lpp [18]. The function of the Tol/Pal system is disrupted when any of the tolA, tolB, tolQ, tolR and pal genes are mutated. The ybgC and cpoB (formally named ybgF) genes encode cytoplasmic lipid thioesterase and the periplasmic regulator of peptidoglycan peptide crosslinking, respectively [19,20]. Neither YbgC nor CpoB participates in the Tol/Pal system because these mutations do not yield the same phenotypes as tol/pal mutants. Mutations in the tol/pal genes have a pleiotropic effect [21]. The tol/pal genes were originally characterized as genes associated with tolerance to colicins that are bacteriocins produced by E. coli [22]. The tol/pal mutants were shown to be resistant to colicins with a low ability of colicin uptake, resulting in the cells escaping death. The following studies showed that mutations in tol/pal genes disturb the outer membrane integrity, induce the formation of mucoid colonies with increased production of colonic acid, and decrease the translocation of filamentous bacteriophages into the cytoplasm [4,8,23,24,25,26]. Remarkably, the disturbance of the outer membrane barrier leads to increased susceptibility to some antimicrobial agents, including colistin, β-lactams, vancomycin and novobiocin [27,28,29,30]. The tol/pal genes are also involved in the process of bacterial cell division and morphology, and inactivation of these genes induces filamentation of cells in several bacteria, such as E. coli, Pseudomonas putida, Vibrio cholerae [31,32,33].
Figure 1.
Structure representation of the Tol/Pal protein complexes. TolA, TolQ and TolR proteins form a complex in the inner membrane while the periplasmic TolB protein is associated with the outer membrane-associated Pal protein at the C-terminal site. The N-terminal domain in the TolB protein interacts with the C-terminal domain in the TolA protein.
There is increasing evidence that the Tol/Pal system is also required for virulence in many species of Gram-negative bacteria, and cell growth in some species. Therefore, the Tol/Pal system is a potential drug target while the tol/pal mutants may serve as potential live attenuated vaccines as they can significantly induce immune responses. Additionally, several studies showed that the Pal protein behaves as one of the bacterial surface antigens and induces an immune response although no clinical evidence has been provided, yet. In the following sections, we describe a series of studies focusing on the roles of the Tol/Pal system in bacterial pathogenesis. We also discuss the potential of Tol/Pal proteins in drug and vaccine development.
2. Roles of the Tol/Pal System in Pathogenesis
The involvement of the Tol/Pal system in bacterial pathogenesis has been described in studies that analyzed tol/pal mutants (summarized in Table 1). Alleviated bacterial burden in hosts infected with the mutants, reduced fitness and/or defective production of certain bacterial proteins required for their virulence, such as toxins and flagellar proteins, were observed. In some pathogens, the tol/pal genes are essential for growth and/or survival, i.e., tol/pal mutants cannot replicate and/or survive in the host.
Table 1.
Roles of the Tol/Pal system in Gram-negative pathogens.
| Bacterial Species | Pathogenesis | References |
|---|---|---|
| Enterohemorrhagic Escherichia coli (EHEC) | Secretion of the T3SS effector proteins, A/E lesion formation, Flagellar synthesis |
[34] |
| Citrobacter rodentium | Lethality and Enteritis in mouse | [34] |
| Uropathogenic Escherichia coli (UPEC) | Flagellar synthesis, Bacterial colonization within bladder epithelial cells and in the urinary tract of mice | [35] |
| Escherichia coli | Development of sepsis | [36] |
| Salmonella enterica (Typhimurium) | Bacterial survival in macrophage and mouse, Innate tolerance to bile acid and serum | [37,38,39,40] |
| Salmonella enterica (Choleraesuis) | Lethality in mice, innate tolerance to deoxycholate and vancomycin |
[41] |
| Shigella flexneri | Bacterial invasion and growth in epithelial cells, Innate tolerance to antibiotics, bile acid and human complement |
[42] |
| Pseudomonas aeruginosa | Bacterial growth | [43,44,45] |
| Burkholderia cenocepacia | Bacterial adhesion to lung cells, Lethality in G. mellonella |
[46] |
| Klebsiella pneumoniae | Innate tolerance to serum and phagocytosis, Lethality in mice |
[47,48] |
| Haemophilus ducreyi | Papules formation, Bacterial survival | [49,50] |
| Vibrio cholerae | Uptake of the CTXphi phase, Bacterial growth at high temperature | [33] |
| Edwardsiella ictaluri | Mortality in catfish | [51] |
|
Dickeya dadantii (Erwinia chrysanthemi) |
Activity of pectinolytic enzyme, Motility, Tissue injury on plant leaf | [52] |
2.1. Escherichia coli Pathogenic Subgroups
Although most E. coli strains settle in the intestine as members of normal intestinal flora, there are a few subtypes that cause severe infectious diseases. Enterohaemorrhagic E. coli (EHEC) is a well-known pathogenic subtype. EHEC produces effector proteins that are secreted via transporter machinery, termed “the type III secretion system (T3SS)” [53,54]. The effector proteins enable the bacteria to tightly attach to and injure the intestinal cells (This pathogenesis is commonly named “Attaching and effacing [A/E] lesion”) [55]. Therefore, the effector proteins and their transport machinery are required for EHEC pathogenesis. Deletion of tolB was shown to decrease the secretion of the effector proteins and the ability of EHEC to induce A/E lesions [34]. The tolB mutant of Citrobacter rodentium, the alternative model pathogen to evaluate the T3SS-associated virulence in mice, was avirulent because the mutant caused neither lethality nor enteritis symptoms in mice [34]. Thus, TolB is a potential target for the development of drugs to treat infection caused by EHEC.
Uropathogenic E. coli (UPEC) is another pathogenic subtype, and it causes urinary tract infections (UTIs). This bacterium is the most common causative agent for both uncomplicated and complicated UTIs [56]. When UPEC enters the urinary tract, the bacteria initially invade bladder epithelial cells, where they form multicellular microbial colonies [57]. The flagella of UPEC are required for bacterial colonization within bladder epithelial cells [58,59]. The tol/pal mutants produce defective flagella, resulting in the mutants forming colonies within the bladder epithelial cells, to a lesser extent [35]. Flagella also contribute to bacterial ascent from the bladder and entry into the kidneys [60,61]. Inoculation of tolB deletion mutants into the urinary tract of mice showed a relatively lower bacterial burden and colonization in the kidneys [35]. E. coli can cause bloodstream infections as a complication of UTIs and lead to sepsis. Pal was shown to be required for optimal virulence in septic mice because mutant E. coli strain with reduced levels of Pal or truncated Pal had lower abilities than the wild-type, to survive in blood and kill the mice [36]. Thus, the Tol/Pal system also contributes to the extraintestinal pathogenesis of E. coli.
2.2. Salmonella enterica
Salmonella enterica represents a major family of intestinal bacterial pathogens. S. enterica serovar Typhimurium (S. Typhimurium) is widely used as the alternative model pathogen for S. Typhi that causes human typhoid fever [62]. This bacterium causes similar typhoid disease in mice. It also causes diarrhea in humans. S. enterica can survive within macrophages by inhibiting the phagolysosome formation and thereby leads to systemic infection [63]. The tol/pal mutants were shown to have a low ability to survive in mouse macrophages and systemically infected mice [37,38]. The Tol/Pal system is also responsible for outer membrane homeostasis and resistance to bile acids and serum that aid bacteria to establish infections, mutations of the tol/pal genes alter the levels of glycerophospholipid (GPL) and lipopolysaccharide (LPS) within the outer membrane, and increase susceptibility to bile acids and serum, respectively [37,38,39,40]. S. enterica serovar Choleraesuis (S. Choleraesuis) is a non-typhoidal Salmonella and it causes diseases in pigs. The tolA, tolB and tolR mutants of this bacterium exhibited high sensitivities to deoxycholate and vancomycin with defective envelope integrity and motility [41]. These mutants also exhibited higher LD50 values than the wild-type strain in mice [41].
2.3. Shigella flexneri
Shigella flexneri is a Shigella species that causes human shigellosis [64]. This bacterium invades intestinal epithelial cells, causing severe inflammation and death of the cells. During infection, the bacteria can multiply intracellularly and spread to neighboring epithelial cells [65]. However, the tolR mutant exhibited low rates of invasiveness and growth within the cultured human epithelial cells and attenuated virulence, as defined by body weight loss, in mice [42]. This mutant was also shown to be highly sensitive to some chemical compounds, including antibiotics, bile salts, and human complement, affecting its fitness and survival in the host.
2.4. Pseudomonas aeruginosa
Pseudomonas aeruginosa is an opportunistic pathogen and is often isolated as the causative agent of nosocomial infections. Since this bacterium is innately resistant to some classes of antimicrobial agents, chemotherapy options are limited [66]. In addition, most P. aeruginosa strains have a high ability to form a biofilm [67,68]. The important properties of this biofilm are extreme resistance to many conventional antimicrobial agents and host immune systems [69,70]. Therefore, biofilm formation enables bacteria to survive within the host, progressing the infection to an untreatable chronic state. One study showed that TolB is one of the most abundant periplasmic proteins in P. aeruginosa [43]. In a transcriptome analysis, it was shown that expression of the P. aeruginosa tol/pal genes was increased in the biofilm [44]. On the other hand, no P. aeruginosa strains with either of tol/pal genes inactivated, was obtained in conventional site-directed and random transposon mutagenesis methods [71,72,73,74]. Lo Sciuto et al. alternatively constructed a conditional tolB mutant by replacing the chromosomal tolB with an exogenous copy of the tolB gene under the control of an arabinose-dependent promoter [45]. The mutant was unable to grow when arabinose was absent in a medium, which indicated that TolB was indeed essential for the growth of P. aeruginosa and might be a good target for the development of drugs to combat P. aeruginosa infection.
2.5. Burkholderia cenocepacia
Burkholderia cenocepacia is a member of the Burkholderia cepacia complex of Gram-negative bacteria. This bacterium is a causative agent of chronic respiratory infections and is often isolated from people with cystic fibrosis (CF) [75]. Deletion of the pal gene was shown to reduce the ability of B. cenocepacia to attach to lung epithelial cells derived from a CF patient [46]. The pal mutant also exhibited a lower lethality in Galleria mellonella than the wild-type parent.
2.6. Klebsiella pneumoniae
Although Klebsiella pneumoniae is a normal bacterial member that settles in the mouth, skin, and intestines, it can cause infections in the lungs and the urinary tract, resulting in pneumonia and bacteremia, respectively [76]. The pal mutant of K. pneumoniae was shown to be more sensitive to serum and macrophages and lesser virulent in mice with septicemia, compared to the wild-type parent [47]. One study demonstrated that the Pal protein is released during bloodstream infections [48]. Thus, Pal of K. pneumoniae is closely associated with the development of bloodstream infections caused by the bacteria.
2.7. Haemophilus ducreyi
Haemophilus ducreyi is the etiologic agent of genital ulcer disease known as chancroid. The virulence of this bacterium can be experimentally estimated by measuring the area of papules, formed on the arms of healthy humans inoculated with the bacterial strain [77]. Pal of H. ducreyi was found as an outer membrane protein, with a significant homology to the Pal protein of E. coli [78]. This protein was shown to be abundantly expressed in the human model of experimental infection. The pal mutant H. ducreyi formed papule lesions to a lesser extent than the wild-type [49]. Thus, the expression of Pal contributes to the ability of H. ducreyi to develop the pustular disease. Moreover, Pal of H. ducreyi was shown to be important for its survival because treatment with anti-Pal sera significantly decreased the bacterial colony-forming units [50].
2.8. Vibrio cholerae
Cholera is one of the most common infectious diseases in the world, and it is caused by Vibrio cholerae [79,80]. Cholera toxin is the most important causative factor of the disease. CTXphi is a temperate bacteriophage carrying DNA code for cholera toxin, and it infects V. cholerae [81]. When its phage DNA is inserted into the bacterial chromosome, the lysogenized strains produce a toxin that causes severe diarrheal disease. The pathogenesis of V. cholera is closely associated with the ability to acquire the CTXphi phage. Heilpern et al. found one set of tolQRAB orthologues in a V. cholerae strain with significant similarity to a corresponding sequence in E. coli [33]. The tolQ, tolR, tolA and tolB mutants of V. cholerae had growth defect phenotypes when cultured at 42 °C. The tolA, tolQ and tolRgenes of V. cholera were shown to be necessary for the uptake of CTXphi. In contrast, tolB was unlikely to play an essential role in this process since the tolB mutant could be infected with CTXphi in a similar efficiency as that of the parent strain. This implies a biological function of TolB that does not depend on other Tol/Pal members although its molecular mechanism remains unknown.
2.9. Edwardsiella ictaluri
Edwardsiella ictaluri is a pathogen for fish, including many catfish species. It causes acute septicemia or chronic meningoencephalitis, called the enteric septicaemia of catfish (ESC) disease [82]. A set of homologous ORFs to tol/pal genes of E. coli was found in the chromosomal DNA of E. ictaluri. Abdelhamed et al., demonstrated that the tolQ and tolR genes contribute to the virulence of this bacterium [51]. Mortalities in catfish experimentally infected with the tolQ and tolR mutants were significantly lower than in those infected with the parent strain.
2.10. Dickeya dadantii (Formerly Named Erwinia chrysanthemi)
Dickeya dadantii is a phytopathogenic bacterium. It causes diseases, including necrosis, blight, and soft rot, in many plant species. The bacteria degrade the plant cell wall with the use of pectinolytic enzymes and lead to disruption of the parenchymatous tissues [83]. In addition, oligosaccharides, the degradation products from pectin, are used as growth substrates by the bacteria [84]. The tol/pal genes were shown to sustain the activity of pectinolytic enzymes and virulence in D. dadantii [52]. However, the activity of pectate lyase produced by the tolA, tolB, tolQ and pal mutants was not as virulent as that of the parent D. dadantii. The mutants induced tissue injury on plant leaves to a lower degree, compared to the parent strain. Motility also contributes to optimal virulence of D. dadantii [85]; these mutants were shown to be less motile than the parent strain [52].
3. Immunogenicity of the tol/pal Mutants and Outer Membrane Vesicles (OMVs)
Interestingly, though tol/pal mutants of some pathogens are avirulent or exhibit low virulence, they can still induce immune responses. Thus, the tol/pal mutant strains are potential attenuated live vaccines. The tolB mutant of C. rodentium, alternatively used to evaluate EHEC virulence in vivo, has an ability to induce the production of immunoglobulin G (IgG) and several cytokines, including IFN-γ and IL-17 and confer protection against the lethal parental wild-type strain [34]. Inoculation with the less-virulent tolA mutant strain protects mice against subsequent S. Typhimurium infection [86]. In comparison with naïve control mice, the mice immunized with the tolA mutant exhibited significantly lower bacterial burden when infected with the wild-type subsequently. All the mice immunized with the K. pneumoniae pal mutant could thus survive after infection with the highly virulent wild-type strain [47]. The tolQ and tolR mutants of E. ictaluri were also shown to provide resistance to infection with the highly virulent wild-type E. ictaluri [51].
Many Gram-negative bacteria release outer membrane vesicles (OMVs), which contain various immunogenic bacterial surface components, such as outer membrane proteins, phospholipids, and LPS [87,88]. The tol/pal mutants in some pathogens, including Salmonella, Shigella, Helicobacter pylori, and Buttiauxella agrestis, were shown to release a large amount of OMVs with an ability to induce immune responses [41,42,89,90,91,92]. Administration of OMVs from S. Choleraesuis wild-type, and the tolA, tolB, and tolR mutants conferred immunity in mice [41]. Compared to OMVs from the wild-type, mice immunized with OMVs from the tolA and tolR mutants exhibited a more prolonged survival after a lethal challenge. Interestingly, immunization with OMVs from the tolB mutant conferred 40% protection to mice. Thus, OMVs from the tolB mutant conferred stronger immunity in mice compared to OMVs from the tolA and tolR mutants. In another Salmonella study, OMVs from the nontyphoidal Salmonella tolR mutant were used to induce the production of IgG antibodies in mice, which reduced bacterial colonization when they were subsequently infected with the wild-type Salmonella strain [89]. Similarly, OMVs from the S. flexneri tolR mutant increased CD40 and MHC II molecules and induced the production of the IgG antibodies, and OMVs from the Shigella boydii tolA mutant elevated levels of mucosal IgG and IgA antibodies and some pro-inflammatory cytokines, including TNF-α, IL-6 and IFN-γ in mice [42,90]. OMVs from the H. pylori tolB and pal mutants were shown to induce IL-8 production in human gastric adenocarcinoma cells at a significantly higher level than the wild-type strain [91].
4. Pal Proteins for Vaccine Strategies
The bacterial surface molecules, such as lipoproteins, capsular, LPS, pilin, flagellin, serve as antigens to induce protective immunity. In the Tol/Pal system, Pal is a member of the bacterial lipoproteins [23]. Additionally, lipoprotein Pal induces the production of proinflammatory cytokines through the activation of Toll-like receptor 2 (TLR2) [93]. For these reasons, Pal acts as a potential immunogenic antigen to induce both adaptive and innate immune responses.
Some vaccine strategies using the whole recombinant Pal proteins and a synthetic partial peptide have been proposed. Immunization of mice with the recombinant Pal protein from Legionella pneumoniae induced the Pal-specific IgG antibodies and a couple of proinflammatory cytokines (IL-6 and TNF-α) and conferred protection against the subsequent L. pneumoniae infection [94,95]. Kim et al. determined an epitope of the L. pneumoniae Pal protein, responsible for the induction of the immune system, which contains sequences corresponding to amino acid residues 92–100 (Glu-Tyr-Leu-Lys-Thr-His-Pro-Gly-Ala) in the Pal protein [96]. Inoculation of a synthetic peptide containing this epitope sequence together with the CpG-oligodeoxynucleotides (ODN) that is the agonist of TLR9, as an adjuvant, significantly induced cytotoxic T cell responses (represented by elevated IFN-γ and TNF-α levels) and resulted in reduced bacterial burden and protection of mice against L. pneumoniae infection. McMahon et al. designed a peptide that contains an epitope sequence, within the Haemophilus influenzae Pal protein, to elicit T cell responses [97]. Immunization with this peptide resulted in promoted clearance of the bacteria in mice infected with H. influenzae. Spinola et al. established a monoclonal antibody recognizing the Pal protein of H. ducrevi from infected patients [78]. Interestingly, this antibody cross-reacted with the Pal proteins from some other Haemophilus species, such as H. influenzae [98].
DNA-encoded Pal protein has also been used as the other type of Pal vaccine candidate. A pioneering study demonstrated that the pal DNA from L. pneumoniae had the ability to induce Pal-specific IgG antibodies and cytotoxic T-lymphocyte responses [99]. In that study, the ability of the plasmid DNA carrying the pal gene to induce Pal-specific immune responses was compared with that of the inoculation of recombinant Pal protein into mice for vaccination. Remarkably, mice immunized with the pal DNA exhibited stronger cytotoxic T-lymphocyte responses than those immunized with the Pal protein. IgG1 and IgG2a production was also induced after vaccination with the pal DNA, though to a moderate extent when compared to that in mice immunized with the Pal protein. One study demonstrated that introduction of the pal DNA partially protected mice from L. pneumoniae when CD8+ T cells are depleted [96]. FlaA and PilE encode flagellin and type IV pilin in L. pneumoniae, respectively, and these proteins can act as bacterial surface antigens. Chen et al. used a hybrid DNA construct carrying the pal gene together with the pilE and flaA genes in a single plasmid for vaccination [100]. Vaccination with the plasmid DNA induced some Th1 and Th2 cytokines, including IFN-γ, TNF-α, IL-12, IL-4, and IL-10, together with the IgG antibodies, and protected the mice against the lethal L. pneumoniae. The authors also showed that the pal DNA vaccine has a higher ability to induce IgGs production compared to the flaA and pilE DNA vaccines and the vaccine effect of the pal DNA can be enhanced by additional administration of flaA and pilE DNA [100]. In another study, inoculation of DNA encoding the pal gene of Acinetobacter baumannii induced Th1/Th2/Th17 responses and IgG production [101]. The DNA vaccine conferred protection against acute A. baumannii infection in a mouse model of pneumonia, as shown by the alleviated bacterial burden and reduced inflammation in the lungs.
5. Conclusions
The Tol/Pal system brings about optimal virulence in many Gram-negative pathogens, while it is also required for bacterial growth and survival in some species. The Tol/Pal system is an effective novel antibacterial target, that is distinguished from conventional targets, such as cell wall synthases, ribosomes, RNA polymerase and primary metabolic enzymes. Therefore, disrupting the Tol/Pal system is a promising strategy in antibacterial therapy. Remarkably, any inhibitors of the Tol/Pal system may be potentially active against refractory multi-drug-resistant (MDR) pathogens that are resistant to several conventional drugs. As one idea, a series of molecules that interfere with a protein–protein interaction or bind any of the TolA, TolB, TolQ, TolR and Pal proteins may be promising compounds to disrupt the Tol/Pal system.
In addition, the Tol/Pal system has considerable immunological properties and can be leveraged for the development of vaccines. The tol/pal mutants have abilities to elicit protective immunity against the parental wild-type strain and effectively induce an immune response, which may be partly attributed to OMV production in some species. Furthermore, the Pal proteins also stimulate both innate and adaptive immune systems. Pal acts as the ligand of TLR2 to induce proinflammatory cytokines, and also as the antigen recognized by antibodies from infected patients. Thus, the Tol/Pal system may be potentially targeted for vaccine development. Though the tol/pal mutants were shown to have low virulence, their safety needs to be nevertheless carefully assessed using animal models. The tol/pal mutants can be generated by inactivating any of the tolA, tolB, tolQ, tolR and pal genes and these mutants were previously believed to have identical characters. However, the tolB and pal mutants in D. dadantii were shown to be less virulent than the tolA and tolQ mutants [52]. OMVs from the tolB mutant induced IgG production and provided immunity at higher levels compared to the tolA and tolR mutants [41]. TolB directly interacts with Pal and several other outer membrane proteins, including OmpA required for virulence in some pathogens [18,102,103,104,105]. Therefore, inactivation of TolB or Pal, not TolA, TolQ, and TolR, may affect these outer membrane proteins. We suggest that a comprehensive study of the tol/pal mutants is essential to characterize which mutant is the lowest in virulence and causes optimal immune responses together with the highest safety.
With regard to vaccine development, a “bacteria-free non-toxin vaccine” based on the Pal protein may be relatively safe compared to a “live-bacterial vaccine” based on the tol/pal mutants. The pal DNA from L. pneumoniae induced specific antibodies in mice and its induction was enhanced by administrating together with the flaA and pilE DNA [100]. Thus, the Pal protein may be served to promote immune responses as another antigen. However, it is noteworthy that excessive induction of proinflammatory cytokines causes severe symptoms. The Pal protein induces proinflammatory cytokines via activation of TLR2; administration of the Pal protein develops bloodstream infection with E. coli and accelerates death in mice [36,93]. Therefore, care should be taken to address crucial concerns, such as the optimal dose of Pal-based vaccine to induce protective immunity significantly and to reduce pathological immunity minimally. We expect that Tol/Pal research in the near future would satisfactorily address all these issues.
Author Contributions
Writing—original draft preparation, H.H. and K.S.; writing—review and editing, H.T.; funding acquisition, H.H., K.S. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
H.H., K.S. and H.T., were financially supported by Japan Society for the Promotion of Science (JSPS) “Grant-in-Aid for Scientific Research (C)” (Grant No. 19K07533 to H.H. and 21K11669 to K.S.) and Japan Agency for Medical Research and Development (AMED) (Grant No. 19fk0108061h0502), respectively for the paper publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zgurskaya H.I., Lopez C.A., Gnanakaran S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches to Bypass It. ACS Infect. Dis. 2015;1:512–522. doi: 10.1021/acsinfecdis.5b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X.Z., Plesiat P., Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delcour A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazzaroni J.C., Germon P., Ray M.C., Vianney A. The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiol. Lett. 1999;177:191–197. doi: 10.1111/j.1574-6968.1999.tb13731.x. [DOI] [PubMed] [Google Scholar]
- 5.Krachler A.M., Sharma A., Cauldwell A., Papadakos G., Kleanthous C. TolA modulates the oligomeric status of YbgF in the bacterial periplasm. J. Mol. Biol. 2010;403:270–285. doi: 10.1016/j.jmb.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Muller M.M., Webster R.E. Characterization of the tol-pal and cyd region of Escherichia coli K-12: Transcript analysis and identification of two new proteins encoded by the cyd operon. J. Bacteriol. 1997;179:2077–2080. doi: 10.1128/jb.179.6.2077-2080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vianney A., Muller M.M., Clavel T., Lazzaroni J.C., Portalier R., Webster R.E. Characterization of the tol-pal region of Escherichia coli K-12: Translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J. Bacteriol. 1996;178:4031–4038. doi: 10.1128/jb.178.14.4031-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavel T., Lazzaroni J.C., Vianney A., Portalier R. Expression of the tolQRA genes of Escherichia coli K-12 is controlled by the RcsC sensor protein involved in capsule synthesis. Mol. Microbiol. 1996;19:19–25. doi: 10.1046/j.1365-2958.1996.343880.x. [DOI] [PubMed] [Google Scholar]
- 9.Duan K., Lafontaine E.R., Majumdar S., Sokol P.A. RegA, iron, and growth phase regulate expression of the Pseudomonas aeruginosa tol-oprL gene cluster. J. Bacteriol. 2000;182:2077–2087. doi: 10.1128/JB.182.8.2077-2087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Li F., Xu L., Wang J., Li M., Yuan J., Wang H., Yang R., Li B. Cyclic AMP-CRP Modulates the Cell Morphology of Klebsiella pneumoniae in High-Glucose Environment. Front. Microbiol. 2019;10:2984. doi: 10.3389/fmicb.2019.02984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouveret E., Derouiche R., Rigal A., Lloubes R., Lazdunski C., Benedetti H. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J. Biol. Chem. 1995;270:11071–11077. doi: 10.1074/jbc.270.19.11071. [DOI] [PubMed] [Google Scholar]
- 12.Derouiche R., Benedetti H., Lazzaroni J.C., Lazdunski C., Lloubes R. Protein complex within Escherichia coli inner membrane. TolA N-terminal domain interacts with TolQ and TolR proteins. J. Biol. Chem. 1995;270:11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- 13.Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: Nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J. Bacteriol. 1989;171:5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascales E., Lloubes R., Sturgis J.N. The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB. Mol. Microbiol. 2001;42:795–807. doi: 10.1046/j.1365-2958.2001.02673.x. [DOI] [PubMed] [Google Scholar]
- 15.Walburger A., Lazdunski C., Corda Y. The Tol/Pal system function requires an interaction between the C-terminal domain of TolA and the N-terminal domain of TolB. Mol. Microbiol. 2002;44:695–708. doi: 10.1046/j.1365-2958.2002.02895.x. [DOI] [PubMed] [Google Scholar]
- 16.Bonsor D.A., Hecht O., Vankemmelbeke M., Sharma A., Krachler A.M., Housden N.G., Lilly K.J., James R., Moore G.R., Kleanthous C. Allosteric beta-propeller signalling in TolB and its manipulation by translocating colicins. EMBO J. 2009;28:2846–2857. doi: 10.1038/emboj.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szczepaniak J., Holmes P., Rajasekar K., Kaminska R., Samsudin F., Inns P.G., Rassam P., Khalid S., Murray S.M., Redfield C., et al. The lipoprotein Pal stabilises the bacterial outer membrane during constriction by a mobilisation-and-capture mechanism. Nat. Commun. 2020;11:1305. doi: 10.1038/s41467-020-15083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clavel T., Germon P., Vianney A., Portalier R., Lazzaroni J.C. TolB protein of Escherichia coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol. Microbiol. 1998;29:359–367. doi: 10.1046/j.1365-2958.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Z., Song F., Martin B.M., Dunaway-Mariano D. The YbgC protein encoded by the ybgC gene of the tol-pal gene cluster of Haemophilus influenzae catalyzes acyl-coenzyme A thioester hydrolysis. FEBS Lett. 2002;516:161–163. doi: 10.1016/S0014-5793(02)02533-4. [DOI] [PubMed] [Google Scholar]
- 20.Gray A.N., Egan A.J., Van’t Veer I.L., Verheul J., Colavin A., Koumoutsi A., Biboy J., Altelaar A.F., Damen M.J., Huang K.C., et al. Coordination of peptidoglycan synthesis and outer membrane constriction during Escherichia coli cell division. eLife. 2015;4:e07118. doi: 10.7554/eLife.07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szczepaniak J., Press C., Kleanthous C. The multifarious roles of Tol-Pal in Gram-negative bacteria. FEMS Microbiol. Rev. 2020;44:490–506. doi: 10.1093/femsre/fuaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel de Zwaig R., Luria S.E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J. Bacteriol. 1967;94:1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascales E., Bernadac A., Gavioli M., Lazzaroni J.C., Lloubes R. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J. Bacteriol. 2002;184:754–759. doi: 10.1128/JB.184.3.754-759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riechmann L., Holliger P. The C-terminal domain of TolA is the coreceptor for filamentous phage infection of E. coli. Cell. 1997;90:351–360. doi: 10.1016/S0092-8674(00)80342-6. [DOI] [PubMed] [Google Scholar]
- 25.Mouslim C., Latifi T., Groisman E.A. Signal-dependent requirement for the co-activator protein RcsA in transcription of the RcsB-regulated ugd gene. J. Biol. Chem. 2003;278:50588–50595. doi: 10.1074/jbc.M309433200. [DOI] [PubMed] [Google Scholar]
- 26.Click E.M., Webster R.E. The TolQRA proteins are required for membrane insertion of the major capsid protein of the filamentous phage f1 during infection. J. Bacteriol. 1998;180:1723–1728. doi: 10.1128/JB.180.7.1723-1728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazdunski C., Shapiro B.M. Isolation and some properties of cell envelope altered mutants of Escherichia coli. J. Bacteriol. 1972;111:495–498. doi: 10.1128/jb.111.2.495-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies J.K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: Cross-resistance among colicins of group A. J. Bacteriol. 1975;123:102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onodera K., Rolfe B., Bernstein A. Demonstration of missing membrane proteins in deletion mutants of E. coli K12. Biochem. Biophys. Res. Commun. 1970;39:969–975. doi: 10.1016/0006-291X(70)90419-5. [DOI] [PubMed] [Google Scholar]
- 30.Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: Two new classes of tolerant mutants. J. Bacteriol. 1973;116:885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meury J., Devilliers G. Impairment of cell division in tolA mutants of Escherichia coli at low and high medium osmolarities. Biol. Cell. 1999;91:67–75. doi: 10.1111/j.1768-322X.1999.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 32.Llamas M.A., Ramos J.L., Rodriguez-Herva J.J. Mutations in each of the tol genes of Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J. Bacteriol. 2000;182:4764–4772. doi: 10.1128/JB.182.17.4764-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heilpern A.J., Waldor M.K. CTXphi infection of Vibrio cholerae requires the tolQRA gene products. J. Bacteriol. 2000;182:1739–1747. doi: 10.1128/JB.182.6.1739-1747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hueck C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 1998;62:379–433. doi: 10.1128/MMBR.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galan J.E., Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 36.Kenny B., DeVinney R., Stein M., Reinscheid D.J., Frey E.A., Finlay B.B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/S0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 37.Hirakawa H., Suzue K., Takita A., Awazu C., Kurushima J., Tomita H. Roles of the Tol-Pal system in the Type III secretion system and flagella-mediated virulence in enterohemorrhagic Escherichia coli. Sci. Rep. 2020;10:15173. doi: 10.1038/s41598-020-72412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson G.G., Palermo J.J., Schilling J.D., Roth R., Heuser J., Hultgren S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 40.Wright K.J., Seed P.C., Hultgren S.J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 2005;73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane M.C., Lockatell V., Monterosso G., Lamphier D., Weinert J., Hebel J.R., Johnson D.E., Mobley H.L. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 2005;73:7644–7656. doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirakawa H., Suzue K., Kurabayashi K., Tomita H. The Tol-Pal System of Uropathogenic Escherichia coli Is Responsible for Optimal Internalization into and Aggregation Within Bladder Epithelial Cells, Colonization of the Urinary Tract of Mice, and Bacterial Motility. Front. Microbiol. 2019;10:1827. doi: 10.3389/fmicb.2019.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichon C., Hechard C., du Merle L., Chaudray C., Bonne I., Guadagnini S., Vandewalle A., Le Bouguenec C. Uropathogenic Escherichia coli AL511 requires flagellum to enter renal collecting duct cells. Cell Microbiol. 2009;11:616–628. doi: 10.1111/j.1462-5822.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 44.Hirakawa H., Suzue K., Takita A., Kamitani W., Tomita H. Roles of OmpX, an Outer Membrane Protein, on Virulence and Flagellar Expression in Uropathogenic Escherichia coli. Infect. Immun. 2021;89:e00721-20. doi: 10.1128/IAI.00721-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellman J., Roberts J.D., Jr., Tehan M.M., Allaire J.E., Warren H.S. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J. Biol. Chem. 2002;277:14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 46.Sabbagh S.C., Forest C.G., Lepage C., Leclerc J.M., Daigle F. So similar, yet so different: Uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010;305:1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 47.Palmer A.D., Slauch J.M. Mechanisms of Salmonella pathogenesis in animal models. Hum. Ecol. Risk Assess. 2017;23:1877–1892. doi: 10.1080/10807039.2017.1353903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowe F., Lipps C.J., Tsolis R.M., Groisman E., Heffron F., Kusters J.G. At least four percent of the Salmonella typhimurium genome is required for fatal infection of mice. Infect. Immun. 1998;66:3372–3377. doi: 10.1128/IAI.66.7.3372-3377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masilamani R., Cian M.B., Dalebroux Z.D. Salmonella Tol-Pal Reduces Outer Membrane Glycerophospholipid Levels for Envelope Homeostasis and Survival during Bacteremia. Infect. Immun. 2018;86:e00173-18. doi: 10.1128/IAI.00173-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prouty A.M., Van Velkinburgh J.C., Gunn J.S. Salmonella enterica serovar typhimurium resistance to bile: Identification and characterization of the tolQRA cluster. J. Bacteriol. 2002;184:1270–1276. doi: 10.1128/JB.184.5.1270-1276.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mouslim C., Groisman E.A. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 2003;47:335–344. doi: 10.1046/j.1365-2958.2003.03318.x. [DOI] [PubMed] [Google Scholar]
- 52.Li Q., Li Z., Fei X., Tian Y., Zhou G., Hu Y., Wang S., Shi H. The role of TolA, TolB, and TolR in cell morphology, OMVs production, and virulence of Salmonella choleraesuis. AMB Express. 2022;12:5. doi: 10.1186/s13568-022-01347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jennison A.V., Verma N.K. Shigella flexneri infection: Pathogenesis and vaccine development. FEMS Microbiol. Rev. 2004;28:43–58. doi: 10.1016/j.femsre.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki T., Sasakawa C. Molecular basis of the intracellular spreading of Shigella. Infect. Immun. 2001;69:5959–5966. doi: 10.1128/IAI.69.10.5959-5966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pastor Y., Camacho A.I., Zuniga-Ripa A., Merchan A., Rosas P., Irache J.M., Gamazo C. Towards a subunit vaccine from a Shigella flexneri DeltatolR mutant. Vaccine. 2018;36:7509–7519. doi: 10.1016/j.vaccine.2018.10.066. [DOI] [PubMed] [Google Scholar]
- 56.Poole K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harmsen M., Yang L., Pamp S.J., Tolker-Nielsen T. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunol. Med. Microbiol. 2010;59:253–268. doi: 10.1111/j.1574-695X.2010.00690.x. [DOI] [PubMed] [Google Scholar]
- 58.Hoiby N., Ciofu O., Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 59.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 60.Ciofu O., Tolker-Nielsen T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019;10:913. doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imperi F., Ciccosanti F., Perdomo A.B., Tiburzi F., Mancone C., Alonzi T., Ascenzi P., Piacentini M., Visca P., Fimia G.M. Analysis of the periplasmic proteome of Pseudomonas aeruginosa, a metabolically versatile opportunistic pathogen. Proteomics. 2009;9:1901–1915. doi: 10.1002/pmic.200800618. [DOI] [PubMed] [Google Scholar]
- 62.Whiteley M., Bangera M.G., Bumgarner R.E., Parsek M.R., Teitzel G.M., Lory S., Greenberg E.P. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–864. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 63.Dennis J.J., Lafontaine E.R., Sokol P.A. Identification and characterization of the tolQRA genes of Pseudomonas aeruginosa. J. Bacteriol. 1996;178:7059–7068. doi: 10.1128/jb.178.24.7059-7068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs M.A., Alwood A., Thaipisuttikul I., Spencer D., Haugen E., Ernst S., Will O., Kaul R., Raymond C., Levy R., et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liberati N.T., Urbach J.M., Miyata S., Lee D.G., Drenkard E., Wu G., Villanueva J., Wei T., Ausubel F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skurnik D., Roux D., Aschard H., Cattoir V., Yoder-Himes D., Lory S., Pier G.B. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 2013;9:e1003582. doi: 10.1371/journal.ppat.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lo Sciuto A., Fernandez-Pinar R., Bertuccini L., Iosi F., Superti F., Imperi F. The periplasmic protein TolB as a potential drug target in Pseudomonas aeruginosa. PLoS ONE. 2014;9:e103784. doi: 10.1371/journal.pone.0103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drevinek P., Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010;16:821–830. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- 69.Dennehy R., Romano M., Ruggiero A., Mohamed Y.F., Dignam S.L., Mujica Troncoso C., Callaghan M., Valvano M.A., Berisio R., McClean S. The Burkholderia cenocepacia peptidoglycan-associated lipoprotein is involved in epithelial cell attachment and elicitation of inflammation. Cell Microbiol. 2017;19:e12691. doi: 10.1111/cmi.12691. [DOI] [PubMed] [Google Scholar]
- 70.Podschun R., Ullmann U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998;11:589–603. doi: 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsieh P.F., Liu J.Y., Pan Y.J., Wu M.C., Lin T.L., Huang Y.T., Wang J.T. Klebsiella pneumoniae peptidoglycan-associated lipoprotein and murein lipoprotein contribute to serum resistance, antiphagocytosis, and proinflammatory cytokine stimulation. J. Infect. Dis. 2013;208:1580–1589. doi: 10.1093/infdis/jit384. [DOI] [PubMed] [Google Scholar]
- 72.Hellman J., Warren H.S. Outer membrane protein A (OmpA), peptidoglycan-associated lipoprotein (PAL), and murein lipoprotein (MLP) are released in experimental Gram-negative sepsis. J. Endotoxin Res. 2001;7:69–72. doi: 10.1177/09680519010070010101. [DOI] [PubMed] [Google Scholar]
- 73.Bauer M.E., Spinola S.M. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 2000;68:2309–2314. doi: 10.1128/IAI.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spinola S.M., Hiltke T.J., Fortney K., Shanks K.L. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect. Immun. 1996;64:1950–1955. doi: 10.1128/iai.64.6.1950-1955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fortney K.R., Young R.S., Bauer M.E., Katz B.P., Hood A.F., Munson R.S., Jr., Spinola S.M. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 2000;68:6441–6448. doi: 10.1128/IAI.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiltke T.J., Bauer M.E., Klesney-Tait J., Hansen E.J., Munson R.S., Jr., Spinola S.M. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 77.Deen J., Mengel M.A., Clemens J.D. Epidemiology of cholera. Vaccine. 2020;38((Suppl. S1)):A31–A40. doi: 10.1016/j.vaccine.2019.07.078. [DOI] [PubMed] [Google Scholar]
- 78.Harris J.B., LaRocque R.C., Qadri F., Ryan E.T., Calderwood S.B. Cholera. Lancet. 2012;379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waldor M.K., Mekalanos J.J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 80.Klesius P.H., Shoemaker C.A. Development and use of modified live Edwardsiella ictaluri vaccine against enteric septicemia of catfish. Adv. Vet. Med. 1999;41:523–537. doi: 10.1016/s0065-3519(99)80039-1. [DOI] [PubMed] [Google Scholar]
- 81.Abdelhamed H., Lu J., Lawrence M.L., Karsi A. Involvement of tolQ and tolR genes in Edwardsiella ictaluri virulence. Microb. Pathog. 2016;100:90–94. doi: 10.1016/j.micpath.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 82.Hugouvieux-Cotte-Pattat N., Condemine G., Nasser W., Reverchon S. Regulation of pectinolysis in Erwinia chrysanthemi. Annu. Rev. Microbiol. 1996;50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 83.Hugouvieux-Cotte-Pattat N., Blot N., Reverchon S. Identification of TogMNAB, an ABC transporter which mediates the uptake of pectic oligomers in Erwinia chrysanthemi 3937. Mol. Microbiol. 2001;41:1113–1123. doi: 10.1046/j.1365-2958.2001.02564.x. [DOI] [PubMed] [Google Scholar]
- 84.Dubuisson J.F., Vianney A., Hugouvieux-Cotte-Pattat N., Lazzaroni J.C. Tol-Pal proteins are critical cell envelope components of Erwinia chrysanthemi affecting cell morphology and virulence. Microbiology. 2005;151:3337–3347. doi: 10.1099/mic.0.28237-0. [DOI] [PubMed] [Google Scholar]
- 85.Grenier A.M., Duport G., Pages S., Condemine G., Rahbe Y. The phytopathogen Dickeya dadantii (Erwinia chrysanthemi 3937) is a pathogen of the pea aphid. Appl. Environ. Microbiol. 2006;72:1956–1965. doi: 10.1128/AEM.72.3.1956-1965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paterson G.K., Northen H., Cone D.B., Willers C., Peters S.E., Maskell D.J. Deletion of tolA in Salmonella typhimurium generates an attenuated strain with vaccine potential. Microbiology. 2009;155:220–228. doi: 10.1099/mic.0.021576-0. [DOI] [PubMed] [Google Scholar]
- 87.Kulp A., Kuehn M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Camacho A.I., de Souza J., Sanchez-Gomez S., Pardo-Ros M., Irache J.M., Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29:8222–8229. doi: 10.1016/j.vaccine.2011.08.121. [DOI] [PubMed] [Google Scholar]
- 89.Micoli F., Rondini S., Alfini R., Lanzilao L., Necchi F., Negrea A., Rossi O., Brandt C., Clare S., Mastroeni P., et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA. 2018;115:10428–10433. doi: 10.1073/pnas.1807655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitra S., Sinha R., Mitobe J., Koley H. Development of a cost-effective vaccine candidate with outer membrane vesicles of a tolA-disrupted Shigella boydii strain. Vaccine. 2016;34:1839–1846. doi: 10.1016/j.vaccine.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 91.Turner L., Praszkier J., Hutton M.L., Steer D., Ramm G., Kaparakis-Liaskos M., Ferrero R.L. Increased Outer Membrane Vesicle Formation in a Helicobacter pylori tolB Mutant. Helicobacter. 2015;20:269–283. doi: 10.1111/hel.12196. [DOI] [PubMed] [Google Scholar]
- 92.Takaki K., Tahara Y.O., Nakamichi N., Hasegawa Y., Shintani M., Ohkuma M., Miyata M., Futamata H., Tashiro Y. Multilamellar and Multivesicular Outer Membrane Vesicles Produced by a Buttiauxella agrestis tolB Mutant. Appl. Environ. Microbiol. 2020;86:e01131-20. doi: 10.1128/AEM.01131-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang M.D., Bagchi A., Warren H.S., Tehan M.M., Trigilio J.A., Beasley-Topliffe L.K., Tesini B.L., Lazzaroni J.C., Fenton M.J., Hellman J. Bacterial peptidoglycan-associated lipoprotein: A naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J. Infect. Dis. 2005;191:939–948. doi: 10.1086/427815. [DOI] [PubMed] [Google Scholar]
- 94.Shim H.K., Kim J.Y., Kim M.J., Sim H.S., Park D.W., Sohn J.W., Kim M.J. Legionella lipoprotein activates toll-like receptor 2 and induces cytokine production and expression of costimulatory molecules in peritoneal macrophages. Exp. Mol. Med. 2009;41:687–694. doi: 10.3858/emm.2009.41.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mobarez A.M., Rajabi R.A., Salmanian A.H., Khoramabadi N., Hosseini Doust S.R. Induction of protective immunity by recombinant peptidoglycan associated lipoprotein (rPAL) protein of Legionella pneumophila in a BALB/c mouse model. Microb. Pathog. 2019;128:100–105. doi: 10.1016/j.micpath.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 96.Kim S.J., Sin J.I., Kim M.J. CD8(+) T Cells Directed Against a Peptide Epitope Derived from Peptidoglycan-Associated Lipoprotein of Legionella pneumophila Confer Disease Protection. Front. Immunol. 2020;11:604413. doi: 10.3389/fimmu.2020.604413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMahon M., Murphy T.F., Kyd J., Thanavala Y. Role of an immunodominant T cell epitope of the P6 protein of nontypeable Haemophilus influenzae in murine protective immunity. Vaccine. 2005;23:3590–3596. doi: 10.1016/j.vaccine.2005.01.151. [DOI] [PubMed] [Google Scholar]
- 98.Spinola S.M., Griffiths G.E., Bogdan J., Menegus M.A. Characterization of an 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi that contains a conserved surface-exposed epitope. Infect. Immun. 1992;60:385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon W.S., Park S.H., Park Y.K., Park S.C., Sin J.I., Kim M.J. Comparison of responses elicited by immunization with a Legionella species common lipoprotein delivered as naked DNA or recombinant protein. DNA Cell Biol. 2002;21:99–107. doi: 10.1089/104454902753604970. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y., Yang Z., Dong Y., Chen Y. Recombinant PAL/PilE/FlaA DNA vaccine provides protective immunity against Legionella pneumophila in BALB/c mice. BMC Biotechnol. 2020;20:28. doi: 10.1186/s12896-020-00620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lei L., Yang F., Zou J., Jing H., Zhang J., Xu W., Zou Q., Zhang J., Wang X. DNA vaccine encoding OmpA and Pal from Acinetobacter baumannii efficiently protects mice against pulmonary infection. Mol. Biol. Rep. 2019;46:5397–5408. doi: 10.1007/s11033-019-04994-2. [DOI] [PubMed] [Google Scholar]
- 102.Hirakawa H., Suzue K., Takita A., Tomita H. Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli. Pathogens. 2021;10:1496. doi: 10.3390/pathogens10111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prasadarao N.V., Wass C.A., Weiser J.N., Stins M.F., Huang S.H., Kim K.S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect. Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sukumaran S.K., Shimada H., Prasadarao N.V. Entry and intracellular replication of Escherichia coli K1 in macrophages require expression of outer membrane protein A. Infect. Immun. 2003;71:5951–5961. doi: 10.1128/IAI.71.10.5951-5961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nicholson T.F., Watts K.M., Hunstad D.A. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect. Immun. 2009;77:5245–5251. doi: 10.1128/IAI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.