Abstract
Introduction
Some patients diagnosed with cancer use medical cannabis to self-manage undesirable symptoms, including nausea and pain. To improve patient safety and oncological care quality, the routes of administration for use of medical cannabis, patients’ reasons, and prescribed indications must be better understood.
Methods
Based on the Joanna Briggs Institute guidelines, a scoping review was conducted to map the current evidence regarding the use of medical cannabis in oncological settings based on the experiences of patients diagnosed with cancer and their healthcare providers. A search strategy was developed with a scientific librarian which included five databases (CINAHL, Web of Science, Medline, Embase, and PsycINFO) and two grey literature sources (Google Scholar and ProQuest). The inclusion criteria were: 1) population: adults aged 18 and over diagnosed with cancer; 2) phenomena of interest: reasons for cannabis use and/or the prescribed indications for medical cannabis; 3) context: oncological setting. French- or English-language primary empirical studies, knowledge syntheses, and grey literature published between 2000 and 2021 were included. Data were extracted by two independent reviewers and subjected to a thematic analysis. A narrative description approach was used to synthesize and present the findings.
Results
We identified 5,283 publications, of which 163 met the eligibility criteria. Two main reasons for medical cannabis use emerged from the thematic analysis: limiting the impacts of cancer and its side effects; and staying connected to others. Our results also indicated that medical cannabis is mostly used for three approved indications: to manage refractory nausea and vomiting, to complement pain management, and to improve appetite and food intake. We highlighted 11 routes of administration for medical cannabis, with oils and oral solutions the most frequently reported.
Conclusion
Future studies should consider the multiple routes of administration for medical cannabis, such as inhalation and edibles. Our review highlights that learning opportunities would support the development of healthcare providers’ knowledge and skills in assessing the needs and preferences of patients diagnosed with cancer who use medical cannabis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09378-7.
Keywords: Cancer, Cannabidiol, Cannabis, Medical marijuana, Nabilone, Oncology
Introduction
Cannabis is one of the most widely used recreational drugs in the world [1]. It has been documented that some people diagnosed with cancer use cannabis to alleviate some of their symptoms, including pain, nausea, vomiting, stress, and lack of appetite [1–3]. Cannabis use is becoming increasingly popular for the management of cancer-related symptoms, with some patients incorporating it as a regular self-management behaviour [4–6]. Several surveys report cannabis use as ranging from 13 to 24% in this population [4, 7, 8].
Cannabis use for the management of cancer-related symptoms may have numerous benefits, including improved quality of life and potentially better adherence to chemotherapy and radiotherapy treatments [6]. Cannabis has chemical properties that may help reduce or control various adverse symptoms, such as cancer-associated pain [9–11]. It may also mitigate chemotherapy-induced nausea and vomiting [12–14], as well as sleep disorders [1]. Cancer patients sometimes use medical cannabis as complementary pain relief [15].
Although cannabis is traditionally been associated with inhalation, routes of administration have diversified in recent years, in conjunction with the legalization of cannabis in various North American jurisdictions [16]. Thus, medical cannabis is no longer administered via a single route, but instead is found in many forms, including tablets (i.e. Nabilone), sprays (i.e. Nabiximol), creams, edible products, or oils [16–19].
However, cannabis can cause various side effects, including respiratory problems (e.g. coughing) [20]; for people with predispositions, its use can also be associated with certain mental health problems, such as depression, mania, and psychosis [21–24]. Some authors also point out that regular cannabis use may affect cognitive functions (e.g. decreased attention and reflexes) and induce structural, functional, and chemical changes in the brain in people with predispositions [25–28]. To ensure safe use of medical cannabis by people diagnosed with cancer, oncology care providers must have the knowledge, skills, and open-mindedness to discuss patients’ needs and preferred routes of administration [29, 30]. However, many healthcare providers report not feeling adequately equipped to discuss the various aspects of medical cannabis use, such as patients’ reasons for use, the approved indications, and the possible routes of administration [29, 31–33].
A preliminary search of the Cumulative Index to Nursing and Allied Health Literature (CINAHL) showed no review of the literature has yet mapped the reasons for the use of medical cannabis, the indications for the prescription of cannabis, and the routes of administration based on the experiences of patients diagnosed with cancer and of their healthcare providers. The knowledge syntheses found in our search often present the efficacy of cannabis in managing the various symptoms cancer patients experience, such as chemotherapy-induced nausea and vomiting [12, 34], cancer pain [35, 36], or cancer cachexia [37]. We retrieved only two knowledge syntheses on the use of cannabis and its administration in oncology [18, 19]; however, neither included qualitative evidence from primary empirical studies, surveys, or grey literature. By deepening our understanding of optimal approaches for supporting patients’ decision-making around medical cannabis use and for providing high-quality care to people diagnosed with cancer, a synthesis of qualitative evidence from patient and/or provider experiences is expected to add to the current state of knowledge. Furthermore, as some authors point out [19], it would be appropriate for oncology care providers to become more familiar with the routes of administration, dosage, and potential risks of medical cannabis, and to make recommendations in consequence.
In light of our findings, the reasons for medical cannabis use by people diagnosed with cancer should be highlighted, since they may differ from approved-medical indications. This scoping review aims to map the current literature on the use of medical cannabis in oncological settings based on the experiences of patients diagnosed with cancer and their healthcare providers.
Methods
This review was developed and conducted according to the Joanna Briggs Institute [38] framework for scoping reviews and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews checklist (PRISMA-ScR) [39]. The following five steps were conducted: 1) elaboration of the research question; 2) identification of relevant studies; 3) selection of appropriate studies; 4) data analysis; and 5) data presentation.
Step 1: Elaboration of the research question
The overarching aim of this scoping review was to answer the following question: What do we know about the use of medical cannabis in oncology? The following three sub-questions were also formulated:
Why do people diagnosed with cancer use medical cannabis?
What are the approved indications for the prescription of medical cannabis in oncology?
By what routes of administration do people diagnosed with cancer use medical cannabis?
Step 2: Identification of relevant studies
The literature search was conducted in collaboration with a librarian who is an expert in the health sciences. To meet the aim of this scoping review, the literature included had to: 1) target adults over 18 years of age diagnosed with cancer (participants); 2) discuss the reasons for using medical cannabis or the approved indications for cannabis (concept); 3) take place within an oncology care setting, such as an outpatient clinic, a care unit, or a radiation oncology unit (context). The types of evidence sources selected were primary studies (e.g. randomized controlled trial, qualitative design) and knowledge syntheses (e.g. systematic review, meta-analysis, literature review, clinical guidelines) as they provide evidence of cannabis use via empirical and experiential data.
The search strategy developed included five scientific databases, namely CINAHL (EBSCOhost), Web of Science (Clarivate), Medline (Ovid), Embase (Ovid), and PsycINFO (Ovid), and two grey literature sources (Google Scholar and ProQuest). These databases were selected because they include extensive scientific literature targeting health sciences and oncology. The search strategy was initially performed in CINAHL (see Additional File 1) and then adapted to the other databases. The search was conducted on May 13, 2020, and updated on July 7, 2021.
These concepts were operationalized into keywords and MeSH related to: 1) people diagnosed with cancer (e.g. oncology patients, cancer patients, patients with tumours); 2) various cannabis-related terms (e.g. hashish, marijuana, weed), and 3) routes of administration (e.g. routes of administration, method of use, pill).
Step 3: Selection of appropriate studies
All references were uploaded in Covidence (Veritas Health Innovation, Melbourne, Australia) to facilitate the identification of relevant studies. The screening of titles and abstracts and the full-text reviews were conducted by two independent reviewers (BV and AEA), respecting the inclusion criteria. The inclusion criteria specified that studies must: 1) have been published between 2000 and 2021; 2) be written in French or English (to increase review feasibility); 3) have focused on adults over 18 years of age diagnosed with cancer; 4) discuss the reasons for use of medical cannabis or approved indications for cannabis; 5) have taken place in an oncology setting, such as an outpatient clinic, care unit, or radiation oncology unit; and 6) be a primary research study or knowledge synthesis. Non-human (i.e., laboratory or animal) studies using cannabis to treat cancer were excluded, due to the complexity of the antineoplastic treatments and receptors involved. The reference lists of the selected articles were consulted. Finally, we did not contact the selected articles’ authors since all were readily accessible to the first author.
Data were extracted using a data extraction form inspired by the Joanna Briggs Institute data extraction template [38]. A preliminary version of the data extraction form was pilot tested by three independent reviewers (BV, AEA, HM) who extracted the data from five studies. The form was then modified according to the reviewers’ comments. Data were extracted and compared by two independent reviewers (BV with AEA or HM or AMF) using Microsoft Excel (Microsoft, Redmond, United States) to facilitate data management. Any disagreements between reviewers were resolved through discussion or by a third reviewer (KB) in the case of a persistent disagreement.
The following data were extracted:
Article characteristics (first author’s name, year of publication, country of origin)
Study methods (aim, study design, sample size, and setting)
Population (cancer type, sex, and age of participants)
Reasons for medical cannabis use by people diagnosed with cancer
Approved indications for the prescription of medical cannabis in oncology
Routes of administration (e.g. pill, inhalation)
Step 4: Data analysis
A thematic analysis [40] was undertaken to analyze and synthesize the data collected. This approach includes three main procedures: 1) data condensation; 2) data display; and 3) drawing and verifying conclusions. Text segments on the reasons for the use of medical cannabis and on approved medicinal indications were exported from primary studies and knowledge syntheses to Word (Microsoft, Redmond, United States) and a descriptive coding was then used to create themes and subthemes. The first coding cycle was inspired by the domains of the Comprehensive Cancer Experience Measurement Framework [41]. This framework provides a better understanding of the perspective of patients diagnosed with cancer throughout their survivorship (i.e., from diagnosis to death) [41]. Next, a qualitative analysis expert who did not participate in the analysis (KB) validated the themes and subthemes. The same process was performed for the routes of administration used for medical cannabis.
Step 5: Data presentation
The first author (BV) assigned subthemes to the data extracted from the selected articles and presented them in tabular form. Frequencies were calculated to highlight the most frequently mentioned subthemes. Finally, the characteristics of the studies were grouped into tables.
Results
Characteristics of included studies
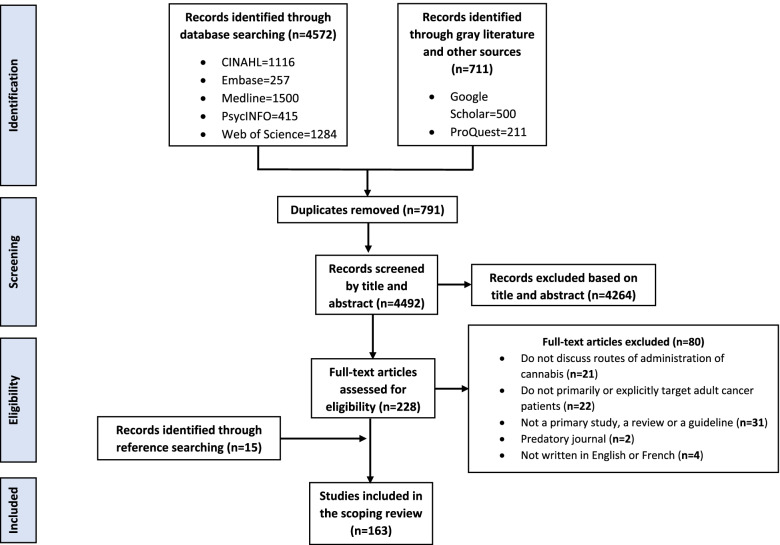
A total of 5,283 articles were imported into Covidence (Veritas Health Innovation, Melbourne, Australia) and 791 duplicates were removed. The titles and abstracts of 4,492 articles were evaluated for eligibility and then the full text of 228 articles was read, leading to the inclusion of 148 articles. Subsequently, the references of all selected articles were searched to obtain 15 additional references, resulting in a total of 163 papers (62 qualitative and quantitative studies, and 101 knowledge syntheses). All of the selected articles were written in English, except one study [42]. A PRISMA flow chart is shown in Fig. 1. A list of selected articles shows this in detail (see Additional File 2).
Fig. 1.
Prisma flowchart
Knowledge syntheses (n = 101) were varied and included literature reviews (n = 61), systematic reviews (n = 13), systematic reviews and meta-analysis (n = 6), guidelines (n = 3), meta-analysis (n = 3), scoping reviews (n = 3), comprehensive reviews (n = 2), overviews of systematic reviews (n = 2), systematic reviews of systematic reviews (n = 2), critical reviews (n = 1), integrated reviews (n = 1), a meta-analysis and meta-regression (n = 1), a protocol for a systematic review and meta-analysis (n = 1), a rapid review (n = 1) and a selective review (n = 1). Only three guidelines were identified, and these dealt with the management of chemotherapy-induced nausea and vomiting [43–45].
The characteristics of the selected primary studies (n = 62) are presented in Table 1. No studies have been identified regarding the experiences of healthcare providers. Surveys were the most frequent type of study (37.1%, n = 23/62) followed by randomized controlled trials (21%, n = 13/62). A large proportion of the primary studies identified were conducted in the United States (43.5%, n = 27/62); this was followed by Canada (14.8%, n = 9/62) and Australia (14.8%, n = 9/62). A total of 18,684 different participants were identified in the selected primary studies. The most common cancer diagnoses were gastrointestinal (n = 2,288), breast (n = 2,236), genitourinary (n = 1,835), and hematologic (n = 1,655). Most primary studies (n = 48) included a wide variety of cancer types (range 2 − 25). Only three studies [46–48] examined a single type of cancer. A few studies (n = 11) did not specify participants’ type of cancer [49–59]. Almost half of the cancer diagnoses (42.5%, n = 7,949/18,684) were not reported in the primary studies. The sex of participants was balanced (female 49.1% and male 48.0%) and sex was not stated in only 2.9% of data.
Table 1.
Characteristics of included primary studies
| Design (n = 62) | N (%) |
| Survey | 23 (37.1) |
| Randomized controlled trial | 13 (21.0) |
| Observational study | 9 (14.5) |
| Pilot study | 5 (8.1) |
| Qualitative study | 3 (4.8) |
| Phenomenology | 2 (3.2) |
| Case report | 2 (3.2) |
| Protocol for a randomized controlled trial | 2 (3.2) |
| Pre experimental study | 1 (1.6) |
| Quality improvement study | 1 (1.6) |
| Descriptive study | 1 (1.6) |
| Countries (n = 62) | N (%) |
| United States | 27 (43.5) |
| Canada | 9 (14.5) |
| Australia | 9 (14.5) |
| Israel | 8 (12.9) |
| United Kingdom | 3 (4.8) |
| Denmark | 1 (1.6) |
| France | 1 (1.6) |
| Germany | 1 (1.6) |
| Italy | 1 (1.6) |
| Mexico | 1 (1.6) |
| Spain | 1 (1.6) |
| Type of cancer (n = 18,684) | N (%) |
| Gastrointestinal (including colorectal, intestinal, liver, oesophageal, oral, pancreas, rectal, stomach) | 2288 (12.2) |
| Breast | 2236 (12.0) |
| Genitourinary (including bladder, cervical, ovarian, peritoneal, prostate, renal, testicular, vaginal) | 1835 (9.8) |
| Hematologic (including leukemia, lymphoma, multiple myeloma, myelodysplastic syndrome) | 1655 (8.9) |
| Lung | 1615 (8.6) |
| Skin (including melanoma) | 292 (1.6) |
| Neurological (including brain, central nervous system, neuroendocrine) | 291 (1.6) |
| Head and neck | 287 (1.5) |
| Sarcoma | 160 (0.9) |
| Hepatobiliary | 36 (0.2) |
| Kidney | 16 (0.1) |
| Musculoskeletal | 13 (0.1) |
| Thyroid | 11 (0.1) |
| Not reported | 7,949 (42.5) |
| Sex of participants (n = 20,069) *include protocols | N (%) |
| Female | 9857 (49.1) |
| Male | 9627 (48.0) |
| Not reported | 585 (2.9) |
Results for review question #1
Analysis of the results highlighted that the use of medical cannabis by people diagnosed with cancer can be influenced by beliefs, be it their own, their loved ones’ or those of the healthcare providers with whom they are in contact. Indeed, some use medical cannabis because they consider there to be enough evidence of the effectiveness of such substances [60], because they have heard others report benefits [61], or feel cannabis can mitigate certain symptoms [62].
Two themes—limiting the impacts of cancer and its side effects, and staying connected to others—were identified and separated into 11 reasons for use of medical cannabis by people diagnosed with cancer (see Table 2 and Additional File 3). The different reasons identified are presented according to the frequency they are mentioned in the selected literature (n = 163).
Table 2.
Reasons of use
| Themes | Reasons for use by people with cancer | Frequency n (%) | Approved indications | Examples |
|---|---|---|---|---|
| Limiting the impacts of cancer and its side effects | Managing refractory nausea and vomiting | 130/163 (79.8%) | √ |
•Reduce the frequency and severity of nausea •Treat anticipatory nausea and vomiting •Use with highly or moderately emetogenic chemotherapy •Manage nausea associated with radiotherapy •Limit delayed emesis |
| Complementary use to assist in pain management | 120/163 (73.6%) | √ |
•Relieve cancer-associated pain •Treat neuropathic pain •Adjuvant for cancer pain not completely relieved by opioid therapy •Use when refractory to opioids and conventional pain management techniques •Enhance the anti-nociceptive effect of morphine |
|
| Improving appetite and food intake | 88/163 (54%) | √ |
•Increase food enjoyment •Weight gain/stabilization •Limit anorexia and cachexia syndrome •Improve taste and smell |
|
| Helping to manage emotions | 59/163 (36.2%) |
•Reduce stress •Improve mood •Treat anxiety •Use to cope emotionally •Allow relief of psychological symptoms |
||
| Promoting sleep and reducing insomnia | 56/163 (34.4%) |
•Improve sleep quality •Facilitate sleep •Reinforce sleep habit •Reduce sleep disruptions |
||
| Easily perform activities of daily living and domestic activities | 23/163 (14.1%) |
•Boost energy and reduce fatigue •Facilitate daytime activities •Improve concentration and memory •Increase activity tolerance |
||
| Alleviating musculoskeletal symptoms | 10/163 (6.1%) |
•Combat muscle tension •Reduce spasticity •Treat arthritis •Decrease spasm and tremors •Control trismus |
||
| Managing respiratory symptoms | 3/163 (1.8%) | •Reduce dyspnea, shortness of breath and coughs | ||
| Staying connected to others | Recreational use | 11/163 (6.7%) | •Enjoyment | |
| Improving sexual function and libido | 5/163 (3.1%) | •Increase frequency of sexual intercourses | ||
| Stimulating social interactions | 3/163 (1.8%) |
•Enhance social interactions •Feel part of a group |
Almost all the selected studies and reviews (n = 160/163; 98.2%) associated cancer patients’ use of medical cannabis with physical health (i.e., managing refractory nausea and vomiting, complementing pain management, promoting sleep, and reducing insomnia, improving appetite and food intake, alleviating musculoskeletal symptoms, managing respiratory symptoms, and improving sexual function and libido). Indeed, only three studies were not associated with this physical health domain [51, 63, 64]. More than one third of the studies and reviews (n = 59/163; 36.2%) were related to emotional health (managing emotions) [2, 4, 8, 17–19, 30, 42, 46–49, 52–54, 57–62, 65–102]. In addition, 22/62 studies [7, 8, 48–50, 52, 58–60, 62, 65, 66, 69, 73, 77, 86, 88, 102–106] and 10/101 knowledge syntheses [17, 30, 79, 80, 85, 96, 100, 107–109] stated reasons for the use of medical cannabis related to overall quality of life (facilitating daily living and domestic activities, recreational use). Lastly, only three studies [30, 48, 87] stated social health reasons (stimulating social interactions).
Results for review question #2
Our findings highlighted three approved indications for the prescription of medical cannabis in oncology (see Table 2): 1) managing refractory nausea and vomiting, 2) complementary use to assist in pain management; and 3) improving appetite and food intake. However, the data analysis did not identify specific healthcare provider experiences of the reasons for their patients’ use of medical cannabis, as none of the reviewed articles addressed this element.
Results for review question #3
Our findings suggest that people diagnosed with cancer opt for various routes of administration when using medical cannabis (see Table 3).
Table 3.
Routes of administration
| Authors (year) / Routes of administration | Oils and oral solutions | Edible | Capsule | Tablet | Oromucosal spray | Smoked | Vaporised | Suppository | Topical | Intramuscular | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrams (2018) | X | X | X | X | X | X | |||||
| Allan et al. (2018) | X | X | X | ||||||||
| Amato et al. (2016) | X | X | X | X | X | ||||||
| Anderson et al. (2019) | X | X | X | X | X | X | X | ||||
| Badowski (2017) | X | X | X | X | X | X | X | ||||
| Badowski & Yanful (2018) | X | X | |||||||||
| Bar-Lev Schleider et al. (2018) | X | X | X | ||||||||
| Bar-Sela et al. (2013) | X | X | X | X | X | ||||||
| Bar-Sela, Tauber, et al. (2019) | X | X | X | X | X | ||||||
| Bar-Sela, Zalman, et al. (2019) | X | X | X | X | |||||||
| Barakji et al. (2019) | X | X | X | X | X | X | X | ||||
| Bertrand et al. (2016) | X | ||||||||||
| Birdsall et al. (2016) | X | X | X | X | |||||||
| Blake et al. (2017) | X | X | X | ||||||||
| Blanton et al. (2019) | X | X | X | X | |||||||
| Braun et al. (2020) | X | X | X | X | X | X | |||||
| Brisbois et al. (2011) | X | X | |||||||||
| Brown et al. (2019) | X | X | X | X | X | ||||||
| Buchwald et al. (2020) | Unclear | ||||||||||
| Buhmeyer (2017) | X | X | X | X | X | X | X | ||||
| Byars et al. (2019) | X | X | X | X | X | X | X | ||||
| Campbell et al. (2001) | X | X | X | ||||||||
| Carr et al. (2019) | X | X | |||||||||
| Chapman et al. (2020) | X | ||||||||||
| Cheng et al. (2012) | X | X | X | ||||||||
| Chow et al. (2020) | X | X | |||||||||
| Clark (2018) | X | X | X | X | X | X | X | ||||
| Côté et al. (2016) | X | ||||||||||
| Cotter (2009) | X | X | X | ||||||||
| Darkovska-Serafimovska et al. (2018) | X | X | X | X | |||||||
| Davis (2008) | X | X | X | ||||||||
| Davis (2016) | X | X | X | X | X | ||||||
| De las Peñas et al. (2016) | X | X | |||||||||
| DiVall & Cersosimo (2007) | X | X | X | ||||||||
| Donovan et al. (2019) | X | X | X | X | |||||||
| Donovan et al. (2020) | Unclear | ||||||||||
| Donovan et al. (2021) | X | X | |||||||||
| Drosdowsky et al. (2020) | X | X | X | X | |||||||
| Duran et al. (2010) | X | X | X | X | X | X | |||||
| Dzierzanowski (2019) | X | X | X | X | X | X | X | X | |||
| Elliott et al. (2016) | X | X | X | X | |||||||
| Fallon et al. (2017) | X | ||||||||||
| Fraguas-Sánchez & Torres-Suárez (2018) | X | X | X | X | X | ||||||
| Garcia & Shamliyan (2018) | X | X | |||||||||
| Good et al. (2019) | X | ||||||||||
| Good et al. (2020) | X | ||||||||||
| Gouveia et al. (2019) | X | ||||||||||
| Green & De-Vries (2010) | X | X | X | X | X | ||||||
| Grimison et al. (2021) | X | X | X | ||||||||
| Hall et al. (2005) | X | X | X | X | X | X | |||||
| Häuser et al. (2018) | X | X | X | X | X | X | |||||
| Häuser et al. (2017) | X | X | X | ||||||||
| Hauser et al. (2019) | X | X | X | ||||||||
| Hawley & Gobbo (2019) | X | X | X | X | X | X | X | X | X | ||
| Hesketh et al. (2017) | X | X | |||||||||
| Highet et al. (2020) | X | X | X | X | X | X | X | ||||
| Hollister (2001) | X | X | X | ||||||||
| Huskey (2006) | X | X | X | X | X | ||||||
| Jatoi et al. (2002) | X | X | |||||||||
| Jensen et al. (2015) | X | X | X | X | |||||||
| Johannigman & Eschiti (2013) | X | X | X | ||||||||
| Johnson et al. (2010) | X | ||||||||||
| Johnson et al. (2013) | X | ||||||||||
| Karim et al. (2020) | Unclear | ||||||||||
| Keller (2020) | X | X | X | X | X | X | X | X | X | ||
| Kim et al. (2019) | X | X | X | X | |||||||
| Kleckner et al. (2019) | X | X | X | X | X | X | |||||
| Kramer (2015) | X | X | X | X | X | X | |||||
| Landa et al. (2018) | X | X | X | X | X | X | X | X | |||
| LeClair et al. (2020) | X | X | X | X | X | X | X | X | |||
| Lichtman et al. (2018) | X | ||||||||||
| Likar & Nahler (2017) | X | X | X | X | X | X | |||||
| Lintzeris et al. (2020) | X | X | X | X | X | X | X | ||||
| Lossignol (2019) | X | X | X | ||||||||
| Luckett et al. (2016) | X | X | X | X | X | X | X | X | X | X | |
| Lynch et al. (2014) | X | ||||||||||
| MacCallum & Russo (2018) | X | X | X | X | X | X | X | X | |||
| Machado Rocha et al. (2008) | X | X | X | X | |||||||
| Maida (2008) | X | ||||||||||
| Maida & Daeninck (2016) | X | X | X | X | X | X | X | X | |||
| Maida et al. (2008) | X | ||||||||||
| Makary et al. (2019) | X | X | X | X | X | X | |||||
| Martell et al. (2018) | X | X | X | X | |||||||
| May & Glode (2016) | X | X | X | X | X | X | X | ||||
| Meiri et al. (2007) | X | X | |||||||||
| Meng et al. (2020) | X | X | X | X | X | X | |||||
| Mersiades et al. (2020) | X | X | X | X | |||||||
| Morales et al. (2017) | X | X | X | ||||||||
| Mortimer et al. (2019) | X | X | X | X | X | X | |||||
| Mucke et al. (2018) | X | X | X | ||||||||
| Musty & Rossi (2001) | X | X | X | ||||||||
| National Academies of Sciences (2017) | X | X | X | X | X | X | X | X | |||
| National Comprehensive Cancer Network (2020) | X | X | |||||||||
| Navari (2009) | X | X | |||||||||
| Navari (2012) | X | X | |||||||||
| Panozzo et al. (2020) | X | X | |||||||||
| Parmar et al. (2016) | X | X | X | X | X | X | |||||
| Pawasarat et al. (2020) | X | X | X | ||||||||
| Peat (2010) | X | X | X | X | |||||||
| Peng et al. (2016) | X | X | X | X | |||||||
| Perez (2006) | X | X | X | ||||||||
| Pergolizzi Jr. et al. (2017) | X | X | X | X | X | ||||||
| Pergam et al. (2017) | X | X | X | X | X | ||||||
| Podda et al. (2020) | Unclear | ||||||||||
| Portenoy et al. (2012) | X | ||||||||||
| Potts et al. (2020) | Unclear | ||||||||||
| Rabgay et al. (2020) | X | X | X | X | X | X | |||||
| Reblin et al. (2019) | X | X | |||||||||
| Robson (2001) | X | X | X | X | X | X | |||||
| Robson (2013) | X | ||||||||||
| Romero-Sandoval et al. (2017) | X | X | X | X | X | ||||||
| Rosewall et al. (2020) | X | X | X | X | X | X | |||||
| Russo et al. (2007) | X | ||||||||||
| Russo (2008) | X | X | X | X | X | ||||||
| Saadeh & Rustem (2018) | X | X | X | X | X | X | X | ||||
| Santana et al. (2015) | X | X | |||||||||
| Sawtelle & Holle (2021) | X | X | X | X | X | X | X | X | X | X | |
| Schussel et al. (2018) | X | X | X | ||||||||
| Sharkey et al. (2014) | X | X | X | X | |||||||
| Shin et al. (2019) | X | ||||||||||
| Singh et al. (2019) | X | X | X | X | |||||||
| Smith et al. (2015) | X | X | X | ||||||||
| Steele et al. (2019) | X | X | X | X | |||||||
| Strasser et al. (2006) | X | X | X | ||||||||
| Sutton & Daeninck (2006) | X | X | X | X | |||||||
| Tafelski et al. (2016) | X | X | X | ||||||||
| Taha et al. (2019) | X | X | |||||||||
| Tallant (2020) | X | X | X | ||||||||
| Tanco et al. (2019) | X | X | |||||||||
| Tateo (2017) | X | X | X | X | |||||||
| Tečić Vuger et al. (2016) | X | X | X | X | |||||||
| Thielmann & Daeninck (2013) | X | X | X | X | X | X | |||||
| Todaro (2012) | X | X | X | X | X | X | |||||
| Tramér et al. (2001) | X | X | X | X | |||||||
| Trentham (2017) | X | X | X | X | X | X | X | X | X | X | |
| Turcott et al. (2018) | X | X | |||||||||
| Turgeman & Bar-Seta (2017) | X | X | X | X | |||||||
| Turgeman & Bar-Sela (2019) | X | X | X | X | X | ||||||
| Uberall (2020) | X | X | X | X | X | ||||||
| van den Beuken-van Everdingen et al. (2016) | X | ||||||||||
| Victorson et al. (2019) | X | X | X | ||||||||
| Villanueva (2019) | X | X | X | ||||||||
| Waissengrin et al. (2015) | X | X | X | ||||||||
| Walsh et al. (2003) | X | X | X | X | X | ||||||
| Wang et al. (2019a) | X | X | |||||||||
| Wang et al. (2019b) | X | X | |||||||||
| Ware et al. (2008) | X | X | X | ||||||||
| Welliver (2016) | X | X | X | ||||||||
| Whitcomb et al. (2020) | X | X | |||||||||
| Whiting et al. (2015) | X | X | X | X | X | X | X | ||||
| Wilkie et al. (2016) | X | X | X | X | X | ||||||
| Wilner & Arnold (2011) | X | X | X | X | X | ||||||
| Wilson et al. (2019) | Unclear | ||||||||||
| Wilson et al. (2021) | X | X | X | X | |||||||
| Yanes et al. (2019) | X | X | X | ||||||||
| Yeshurun et al. (2015) | X | ||||||||||
| Zaki et al. (2017) | X | X | X | ||||||||
| Zalman & Bar-Sela (2017) | X | X | X | X | X | ||||||
| Zarrabi et al. (2020) | X | X | X | X | X | X | |||||
| Zimmerman & Yarnell (2019) | X | X | X | X | |||||||
| Zolotov et al. (2021) | X | X | X | ||||||||
| Zhou et al. (2021) | X | X | |||||||||
| Zylla et al. (2021) | X | X | X | X | X | X | |||||
We identified 11 routes of administration, presented according to the frequency reported in the selected literature (n = 163), namely: 1) oils and oral solutions (n = 133/163, 81.6%); 2) capsules (n = 128/163, 78.5%); 3) smoked (n = 97/163, 59.5%); 4) oromucosal spray (n = 85/163, 52.1%); 5) edible (n = 45/163, 27.6%); 6) vaporized (vaping) (n = 50/163, 30.7%); 7) topical application (n = 29/132, 17.8%); 8) intramuscular (n = 28/163, 17.2%); 9) tablets (n = 18/163, 11%); 10) suppository (n = 17/163, 10.4%); and 11) other (n = 4/163, 2.5%). Six studies did not specify the routes of administration used [58, 70, 87, 88, 102, 109], while two studies [61, 77] reported the use a percutaneous endoscopic gastrotomy (other).
Some of the identified routes of administration take the form of prescribed medical treatments, such as Nabilone (capsules), Dronabinol (capsules or oil), Namisol™ (tablets), Nabiximols (spray), and Levonantradol (intramuscular). Some cancer patients use cannabis leaves or buds to make other routes of administration (e.g. oils or oral solutions, edibles, suppositories, topical), or they purchase products using various routes of administration (e.g. oil or oral solution, capsule, vape oil or dry cannabis), whether legally, through licensed suppliers, or illegally.
Discussion
The purpose of this review was to map the current literature on the use of medical cannabis in oncological settings based on the experiences of patients diagnosed with cancer and their healthcare providers. To our knowledge, it is the first knowledge synthesis to focus on patients diagnosed with cancer experiences of using medical cannabis. Its findings bring further understanding of the reasons patients diagnosed with cancer use medical cannabis and the routes of administration they prefer.
Interestingly, primary studies found a similar proportion of male and female cannabis users: 48% and 49.1%, respectively. However, several studies point out that cannabis use is generally more widespread among male than female diagnosed with cancer [102, 111, 112]. Although this neither validates nor invalidates the presence of gender differences in the rate of cannabis use, it sheds a very useful light onto patients diagnosed with cancer participation in studies on cannabis use.
The next sections will discuss our results regarding the sub-questions of this knowledge synthesis.
Why do people diagnosed with cancer use medical cannabis?
Unsurprisingly, almost all the examined studies and reviews (98.2%) mention a reason related to physical health. The most frequently cited are associated with relieving refractory nausea and vomiting (n = 130; 79.8%), a finding that can be explained by the large number of knowledge syntheses and guidelines that support the use of medical cannabis in the management of chemotherapy-induced nausea and vomiting [1, 12, 34, 43–45, 113]. Pain relief was the second most commonly mentioned reason (n = 120; 73.6%), since many systematic reviews are on this topic [11, 36, 114–121].
Although people diagnosed with cancer may use medical cannabis primarily for therapeutic reasons, our results highlight that use can also be a way to stay connected with others. Indeed, it would seem that people diagnosed with cancer sometimes use cannabis to maintain or forge social relationships. These results echo those of various authors who point out that college students sometimes use cannabis to trigger social interactions with others [122]. Since, as Phillips points out (122, p.158), “marijuana use is a social activity,” it is not unreasonable to think that people diagnosed with cancer would also use it for a similar purpose.
In addition, 11 of the 62 selected primary studies [7, 8, 60, 62, 69, 73, 77, 85, 88, 108, 109] emphasize that people diagnosed with cancer may also use cannabis for recreational purposes. Such results are novel in that they provide insight into an area as of yet unexplored in the scientific literature; ours differ from the results of studies of other populations (i.e. people with HIV and their families [123, 124]) showing that recreation is frequently cited as a reason for cannabis use. These differences may be explained by the intensity of symptoms experienced or by the effectiveness of cannabis in relieving symptoms specific to cancer or its treatment (e.g. pain, chemotherapy-induced nausea and vomiting, cachexia).
Interestingly, 32 studies [7, 8, 17, 30, 48–50, 52, 58–60, 62, 65, 66, 69, 77, 79, 80, 85, 86, 88, 96, 100, 102–108] indicate that use of medical cannabis was linked to at least one overall quality-of-life reason. Some authors [6, 17, 51, 59, 125, 126] even suggest that cannabis use may influence quality of life of people diagnosed with cancer because of cannabis’ multidimensional effect. Furthermore, other studies [48, 127] have found medical cannabis to significantly improve the quality of life of people diagnosed with cancer. We did not explore this aspect, as the aim of our knowledge synthesis was to map the current literature regarding the use of medical cannabis based on patients’ and healthcare providers’ experiences.
What are the approved indications for the prescription of medical cannabis in oncology?
Surprisingly, the perspective of healthcare providers did not emerge in the data analysis although some keywords and MeSH were identified to highlight scientific literature targeting healthcare professionals (e.g., oncologic nursing, oncologic care). Most of the reasons associated with the use of medical cannabis (e.g. promoting sleep and reducing insomnia, alleviating musculoskeletal symptoms, and helping to manage emotions) were not related to an approved indication recognized by organizations like the National Health Service or Health Canada (e.g. for chemotherapy-induced nausea and vomiting, or cancer-induced pain). These findings are consistent with various studies pointing out that people diagnosed with cancer use medical cannabis to relieve a wide range of symptoms, not just chemotherapy-induced nausea and vomiting, or cancer-induce pain [1, 17, 58, 59, 77, 128]. Furthermore, several primary studies and knowledge syntheses show favourable results regarding the use of medical cannabis to increase appetite and aid weight gain in people diagnosed with cancer [37, 48, 67, 117]. Many surveys also suggest that people diagnosed with cancer perceive cannabis use as improving sleep or reducing insomnia [2, 59, 67, 87, 126, 129, 130]. Further scientific research is needed on certain therapeutic indications, such as for cancer cachexia, insomnia, emotion, and stress management, that are not currently recognized by various regulatory agencies (e.g. National Health Service).
By what routes of administration do people diagnosed with cancer use medical cannabis?
Our scoping review highlights that certain routes of administration for use of medical cannabis used in oncology are frequently mentioned in the selected articles. Oils and oral solutions (e.g. homemade oils or Dronabiol oral solution), capsules (e.g. homemade capsules, Dronabinol, and Nabilone), oromucosal sprays (e.g. Nabiximols), and smoked cannabis were cited in more than 50% of the studies and reviews. This may be explained by the broad range of products (such as oral solutions and oromucosal sprays) available in many countries, such as Australia, for purchase with a prescription [61, 64]; Canada also permits authorized retailers to sell cannabis [131]. Moreover, the results of a secondary data analysis [53] indicate that oral solutions (55.2%), oromucosal sprays (27.5%), and capsules (17.3%) are the routes of administration most-frequently purchased in a New York City dispensary. This difference could be explained by the fact that dispensaries offer only certain routes of administration, such as oral solutions, oromucosal sprays and capsules, i.e. those products authorized by the local legislation governing the purchase and sale of cannabis.
Many of the routes of administration identified in our knowledge synthesis are also found in a scoping review [16], although these authors do not exclusively focus on patients diagnosed with cancer who use cannabis (e.g. smoked, vaporized, edible). However, our results differ in that oils and oral solutions (e.g. Dronabinol oral solution) were mentioned in 133/163 of the studies reviewed. In addition, these authors group several routes of administration into the category “other” (e.g. suppositories, topical, tinctures, sprays). Our results indicate that suppositories were mentioned in 17 studies, topical administration came up in 29 studies, and percutaneous endoscopic gastrostomy was noted in two surveys [61, 77]. Moreover, as various surveys suggest, topical products may be used by 5 - 26% of people diagnosed with cancer, while suppository use may vary between 2 and 8% [49, 61, 77]. Our results show that further attention should be paid to certain routes of administration (notably suppositories and topical administration) since these seem to be used by people diagnosed with cancer.
Finally, no primary study or knowledge synthesis has explored the specific reasons for suppository or topical use of medical cannabis products in patients diagnosed with cancer. This demonstrates that these routes of administration are still poorly understood and little explored by researchers. Yet, people diagnosed with cancer may be tempted to use a cannabis suppository for its rapid onset of action (± 15 min) [19]. Indeed, many authors point out that the effects of medical cannabis vary by route of administration (e.g. onset of action, desired benefits, and potential side effects) [18, 19, 132]. Their results suggest that people diagnosed with cancer and the healthcare providers working with this clientele could be better informed on the different aspects of cannabis use.
Future research and practice recommendations
During data extraction, we found 11 primary studies that did not specify their participants’ type of cancer. In addition, the wide variety of cancers in the studies we selected for review (range 2 - 25) made it impossible to associate specific reasons with the prescription or use of medical cannabis.
As many reasons motivate the use of medical cannabis, studies examining different types of cancer (e.g. leukemia, breast, prostate), the treatments administered (e.g. highly emetogenic chemotherapy, pills, immunotherapy), and the disease trajectories (e.g. at diagnosis, during treatment, and post-treatment) would all seem to be worth more examination. Furthermore, such data should be systematically included in upcoming studies. Future primary studies should also explore the relationships between the wide range of routes of administration and the reasons for using medical cannabis. In doing so healthcare providers would be better informed about the routes of administration that are already is use by people diagnosed with cancer but that have not been well explored in the scientific literature. In addition, it would be interesting to conduct future studies to understand healthcare providers’ perspectives on their patients’ use of medical cannabis, as none of the selected studies and very few articles [133–135] examined this aspect.
Our scoping review indicates that people diagnosed with cancer use many routes of administration for medical cannabis. Thus, it would seem important to develop training activities (i.e. modules, webinars) and educational materials (i.e. checklists, posters) to help oncology care providers become knowledgeable about the routes of administration and the reasons for use of medical cannabis in people diagnosed with cancer. Such training would promote safer and more adequate follow-up for people diagnosed with cancer who use medical cannabis to self-manage their symptoms. The summary of routes of administration and definitions provide below (see Table 4) could be used to support healthcare providers’ clinical practice.
Table 4.
Key findings to guide healthcare professionals
| Routes of administration | Definitions | Examples | Questions regarding cannabis use |
|---|---|---|---|
| Oils and oral solutions | Solution (e.g., oil) containing synthetic or homemadse component of cannabis, generally administered with a liquid dropper | •Dronabinol oral solution (Syndros™, Benuvia Therapeutics Inc., Chandler, United States) | •How would you describe the effects of this treatment on your symptoms? |
| Capsule | Soft gelatine capsules containing synthetic components of cannabis |
•Dronabinol capsule (Marinol™, Solvay Pharmaceuticals, Inc., Georgia, United States) •Nabilone (Cesamet™, Valeant Canada Ltd., Laval, Canada) •Cannabics™ (Cannabics Pharmaceuticals Inc., Maryland, United States) |
•What benefits do you experience when taking a capsule containing cannabis (eg, Dronabinol)? •What side effects do you think are associated with taking cannabis capsules (eg, Nabilone)? |
| Tablet | Solid dosage form containing synthetic components of cannabis | •Namisol™ (Echo Pharmaceuticals, The Netherlands) |
•Does the dose of Namisol™ provide adequate relief from your symptoms? •What could your interprofessional team do to help you manage your symptoms with Namisol™? |
| Edibles | Food products or liquid infused with cannabis extract | •Brownies, lozenges, cookies, candies, gummies, chocolate bars, cakes, tinctures (cannabis-infused alcohol) and beverages |
•Have you produced or used edibles containing cannabis to relieve your cancer symptoms? •What were your reasons for choosing to ingest cannabis edibles? |
| Oromucosal spray | Administration with a sprayer under the tongue, inside the cheek or within the mouth of a cannabis extract | •Nabiximols (Sativex™, GW Pharmaceuticals, Carlsbad, United States) |
•Does the frequency and dose of spray you receive each day seem adequate? •How does the oromucosal spray modify your symptoms? |
| Smoked | Inhalation of fresh or dried cannabis leaves, fresh or dried cannabis leaves with tobacco, resin, or hashish |
•Bong •Cigarette (‘joint’) •Pipe •Hookah |
•What are your reasons for using cannabis by inhalation? •What compromise would you be willing to make to avoid using cannabis by inhalation? |
| Vaporized (‘vaping’) | Use of distillate or oil with a vape pen or heating cannabis with a vaporizer | •Not applicable | •What are the reasons that would lead you to use another route of administration than the vaporizer? |
| Suppository | Rectal administration of a tablet or a capsule containing cannabis oil | •Not applicable |
•What are your reasons for using cannabis-containing suppositories? •How would you like to receive your cannabis to avoid using a suppository? |
| Topical | Application of a lotion, a cream, an ointment, a transdermal patch, or a gel on a body part | •Not applicable |
•What are your reasons for using creams/lotions/ointments containing cannabis? •What are the advantages and disadvantages of using creams/lotions/ointments containing cannabis? |
| Intramuscular | Administration of synthetic components of cannabis | •Levonantradol | •What route of administration would you like to use to receive your cannabis dose? |
| Percutaneous endoscopic gastrostomy | Administration of a concentrated hash oil, hemp oil or cannabis oil inserted in a percutaneous endoscopic gastrostomy | •Not applicable |
•What were the reasons for your administration of cannabis derivatives through your gastrostomy? •By which route of administration, other than your gastrostomy, would you like to receive your cannabis? |
Strengths and limitations
Our knowledge synthesis followed the recommendations of the Joanna Briggs Institute [38] for the development of a scoping review. To ensure the reproducibility of the study, its results were presented according to the PRISMA-ScR checklist [39]. We conducted an exhaustive literature search with a librarian, who is an expert in health-science databases. To ensure methodological rigour, many steps (e.g. screening and data extraction) were performed independently by two reviewers, and a third independent author adjudicated any disagreements. The addition of the Comprehensive Cancer Experience Measurement Framework [41] was useful to better understand the reasons associated with the use of medical cannabis by people diagnosed with cancer.
The quality of the selected literature was not assessed, as the purpose of this article was to map what is known about the use of medical cannabis in oncology, regardless of its quality. As pointed out by the Joanna Briggs Institute [38], some scoping reviews do not evaluate the quality of articles.
To increase our review’s feasibility, to reflect a more contemporary approach to cannabis use (i.e., harm reduction), and to highlight the shift in mindset that has come with new medical cannabis (e.g. Nabilone, Dronabinol, Nabiximols), the articles were limited to adult cancer patients and to studies published between 2000 and 2021. It is possible that including articles published before 2000 or targeting pediatrics could influence the results presented by this scoping review.
Conclusion
Our review mapped the current literature on the use of medical cannabis in oncology, mainly from the perspective of cancer patients. This scoping review is the first knowledge synthesis to explore the reasons for the use of medical cannabis, the approved indications for oncology patients, and the routes of administration that people diagnosed with cancer use for medical cannabis.
This review found that several routes of administration other than pills, smoked cannabis, and oral solutions, and that people with cancer use medical cannabis for many reasons. These include therapeutic purposes (to complement pain management, to promote sleep and reduce insomnia, to improve appetite and food intake, etc.) and three medically approved indications for the prescription of cannabis in oncology. The reasons patients use medical cannabis were not limited to therapeutic indications currently recognized by different regulatory agencies (such as Health Canada), underscoring the need for further scientific research into the effects of medical cannabis use. Lastly, the results of our scoping review provide food for thought on the routes of administration people diagnosed with cancer use but that have gone largely unexplored by scientific studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Anne Bourbonnais, RN, PhD, Professor at the University of Montreal, and Dr. Maria-Pilar Ramírez García, RN, PhD, Professor at the University Montreal for their contribution in commenting on this article. We would also like to thank Mr. Rafael Rangel Braga, M.S.I., librarian at the University of Montreal for providing us with his expertise in developing the search strategy as well as Mrs. Anna-Maréva Ferville, RN, BSN, for her dedicated time and support during data extraction.
Abbreviations
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- HIV
Human immunodeficiency virus
- MeSH
Medical Subject Headings
- PRISMA-ScR
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews checklist
Authors’ contributions
BV: Conceptualization, formal analysis, investigation, writing – original draft, visualization. JC: Conceptualization, formal analysis, writing – review, supervision. AEA: Investigation, writing – review and editing. HM: Investigation, writing – review and editing. GC: Conceptualization, writing – review and editing. KB: Conceptualization, formal analysis, writing – review and editing, supervision.
Funding
This scoping review was developed and conducted as part of the doctoral studies of the first author and for which he receives scholarships from the following: the Ministère de l’Éducation et de l’Enseignement supérieur-Université (QC, Canada), the Quebec Network on Nursing Intervention Research (QC, Canada) and the Research Chair in Innovative Nursing Practices (QC, Canada). The first author has also received financial support from the philanthropic donors of the University of Montreal Faculty of Nursing (QC, Canada) in developing and publishing this article.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 2.Zolotov Y, Eshet L, Morag O. Preliminary Assessment of Medical Cannabis Consumption by Cancer Survivors. Complement Ther Med. 2021;57:102592. doi: 10.1016/j.ctim.2020.102592. [DOI] [PubMed] [Google Scholar]
- 3.McLennan A, Kerba M, Subnis U, Campbell T, Carlson LE. Health care provider preferences for, and barriers to, cannabis use in cancer care. Curr Oncol. 2020;27(2):e199–205. doi: 10.3747/co.27.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saadeh CE, Rustem DR. Medical Marijuana Use in a Community Cancer Center. Journal of Oncology Practice. 2018;14(9):e566–e578. doi: 10.1200/JOP.18.00057. [DOI] [PubMed] [Google Scholar]
- 5.Brigden M, England D. Medical marijuana and community oncology practice: the good, the bad, and the potentially ugly. Oncology Exchange. 2018;17(3):10–16. [Google Scholar]
- 6.Daeninck PJ, Maida V. The clinical use of cannabinoid therapies in oncology patients. In: Wedman-St.Louis B, editor. Cannabis : a clinician's guide: Taylor & Francis Group; 2018.
- 7.Martell K, Fairchild A, LeGerrier B, Sinha R, Baker S, Liu H, et al. Rates of cannabis use in patients with cancer. Curr Oncol. 2018;25(3):219–225. doi: 10.3747/co.25.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pergam SA, Woodfield MC, Lee CM, Cheng G-S, Baker KK, Marquis SR, et al. Cannabis Use Among Patients at a Comprehensive Cancer Center in a State With Legalized Medicinal and Recreational Use. Cancer. 2017;123(22):4488–4497. doi: 10.1002/cncr.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin S, Mitchell C, Mannion K, Smolyn J, Meghani SH. An Integrated Review of Cannabis and Cannabinoids in Adult Oncologic Pain Management. Pain Management Nursing. 2018. [DOI] [PubMed]
- 10.Aviram J, Samuelly-Leichtag G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta- Analysis of Randomized Controlled Trials. Pain Physician. 2017;20(6):E755–E796. [PubMed] [Google Scholar]
- 11.Rabgay K, Waranuch N, Chaiyakunapruk N, Swawangjt R, Ingkaninan K, Dilokthornsakul P. The effects of cannabis, cannabinoids, and their administration routes on pain control efficacy and safety: A systematic review and network meta-analysis. J Am Pharm Assoc. 2020;60(1):225–234. doi: 10.1016/j.japh.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Allan G, Finley C, Ton J, Perry D, Ramji J, Crawford K, et al. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64(2):e78–e94. [PMC free article] [PubMed] [Google Scholar]
- 13.Schussel V, Kenzo L, Santos A, Bueno J, Yoshimura E, Latorraca Oliveira Cruz C, et al. Cannabinoids for nausea and vomiting related to chemotherapy Overview of systematic reviews. Phytotherapy Research. 2018;32:567–76. doi: 10.1002/ptr.5975. [DOI] [PubMed] [Google Scholar]
- 14.Tafelski S, Haüser W, Schäfer M. Efficacy, tolerability, and safety of cannabinoids for chemotherapy-induced nausea and vomiting—a systematic review of systematic reviews. Der Schmerz. 2016;30(1):14–24. doi: 10.1007/s00482-015-0092-3. [DOI] [PubMed] [Google Scholar]
- 15.Vyas MB, Lebaron VT, Gilson AM. The use of cannabis in response to the opioid crisis: A review of the literature. Nurs Outlook. 2018;66(1):56–65. doi: 10.1016/j.outlook.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use – basic prevalence and related health outcomes: A scoping review and synthesis. International Journal of Drug Policy. 2018;52:87–96. doi: 10.1016/j.drugpo.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Steele G, Arneson T, Zylla D. A Comprehensive Review of Cannabis in Patients with Cancer: Availability in the USA, General Efficacy, and Safety. Curr Oncol Rep. 2019;21(10):1–12. doi: 10.1007/s11912-019-0757-7. [DOI] [PubMed] [Google Scholar]
- 18.Brown D, Watson M, Schloss J. Pharmacological evidence of medicinal cannabis in oncology: a systematic review. Support Care Cancer. 2019;27(9):3195–3207. doi: 10.1007/s00520-019-04774-5. [DOI] [PubMed] [Google Scholar]
- 19.Sawtelle L, Holle LM. Use of Cannabis and Cannabinoids in Patients With Cancer. Ann Pharmacother. 2021;57(7):870–890. doi: 10.1177/1060028020965224. [DOI] [PubMed] [Google Scholar]
- 20.Martinasek MP, McGrogan JB, Maysonet A. A Systematic Review of the Respiratory Effects of Inhalational Marijuana. Respir Care. 2016;61(11):1543–1551. doi: 10.4187/respcare.04846. [DOI] [PubMed] [Google Scholar]
- 21.Memedovich KA, Dowsett LE, Spackman E, Noseworthy T, Clement F. The adverse health effects and harms related to marijuana use: an overview review. CMAJ Open. 2018;6(3):E339–E346. doi: 10.9778/cmajo.20180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: A systematic review and meta-analysis. J Affect Disord. 2015;171:39–47. doi: 10.1016/j.jad.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44(4):797–810. doi: 10.1017/S0033291713001438. [DOI] [PubMed] [Google Scholar]
- 24.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull. 2016;42(5):1262–1269. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition—A Systematic Review. Biol Psychiat. 2016;79(7):557–567. doi: 10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Cohen K, Weinstein A. The Effects of Cannabinoids on Executive Functions: Evidence from Cannabis and Synthetic Cannabinoids—A Systematic Review. Brain Sci. 2018;8(40):1–19. doi: 10.3390/brainsci8030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nader DA. Sanchez ZM. Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. 2017;44(1):1–15. doi: 10.1080/00952990.2017.1306746. [DOI] [PubMed] [Google Scholar]
- 28.Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, Gur RC. Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults A Systematic Review and Meta-analysis. JAMA Psychiat. 2018;75(6):585–595. doi: 10.1001/jamapsychiatry.2018.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balneaves LG, Alraja A, Ziemianski D, McCuaig F, Ware M. A National Needs Assessment of Canadian Nurse Practitioners Regarding Cannabis for Therapeutic Purposes. Cannabis and Cannabinoid Research. 2018;3(1):66–73. doi: 10.1089/can.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark CS. Medical Cannabis: The oncology nurse’s role in patient education about the effects of marijuana on cancer palliation. Clin J Oncol Nurs. 2018;22(1):E1–E6. doi: 10.1188/18.CJON.E1-E6. [DOI] [PubMed] [Google Scholar]
- 31.Gardiner KM, Singleton JA, Sheridan J, Kyle GJ, Nissen LM. Health professional beliefs, knowledge, and concerns surrounding medicinal cannabis - A systematic review. PLoS One. 2019;14(5):e0216556. doi: 10.1371/journal.pone.0216556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corroon J, Sexton M, Bradley R. Indications and administration practices amongst medical cannabis healthcare providers: a cross-sectional survey. BMC Family Practice. 2019;20:1–12. doi: 10.1186/s12875-019-1059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan L, Klein T, Wilson MG, Graves J. Knowledge, Practices, and Attitudes of Washington State Health Care Professionals Regarding Medical Cannabis. Cannabis and Cannabinoid Research. 2020;5(2):172–182. doi: 10.1089/can.2019.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LA, Azariah F, Lavender VT, Stoner NS, Beltiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database of Systematic Reviews. 2015(11). [DOI] [PMC free article] [PubMed]
- 35.Chapman EJ, Edwards Z, Boland JW, Maddocks M, Fettes L, Malia C, et al. Practice review: Evidence-based and effective management of pain in patients with advanced cancer. Palliat Med. 2020;34(4):444–453. doi: 10.1177/0269216319896955. [DOI] [PubMed] [Google Scholar]
- 36.Darkovska-Serafimovska M, Serafimovska T, Arsova-Sarafinovska Z, Stefanoski S, Keskovski Z, Balkanov T. Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. J Pain Res. 2018;11:837–842. doi: 10.2147/JPR.S160556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Wang Y, Tong M, Pan H, Li D. Medical Cannabinoids for Cancer Cachexia: A Systematic Review and Meta-Analysis. BioMed Research International. 2019.
- 38.Joanna Briggs Institute. Chapter 11: Scoping reviews 2020 [Available from: https://wiki.jbi.global/display/MANUAL/Chapter+11%3A+Scoping+reviews.
- 39.Tricco AC, Lillie E, Zrin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 40.Miles MB, Huberman AM, Saldaña J. Qualitative data analysis: A methods sourcebook. 3. Thousand Oaks, Californie: SAGE Publications Inc.; 2014. [Google Scholar]
- 41.Loiselle CG, Howell D, Nicoll I, Fitch M. Toward the development of a comprehensive cancer experience measurement framework. Support Care Cancer. 2019;27:2579–2589. doi: 10.1007/s00520-018-4529-y. [DOI] [PubMed] [Google Scholar]
- 42.Bertrand A, Boyle H, Moreaux J, Guillot L, Chvetzoff G, Charbonnel J-F, et al. Does consumption of tobacco, alcohol, and cannabis in adolescents and young adults with cancer affect the use of analgesics during hospitalizations? Arch Pediatr. 2016;23(4):353–359. doi: 10.1016/j.arcped.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network. Antiemesis - Version 1.2021 2021 [Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
- 44.Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240–3261. doi: 10.1200/JCO.2017.74.4789. [DOI] [PubMed] [Google Scholar]
- 45.Peñas De las R, Blasco A, De Castro J, Escobar Y, García-Campelo R, Gúrpide A, et al. SEOM Clinical Guideline update for the prevention of chemotherapy-induced nausea and vomiting (2016) Clinical and Translational Oncology. 2016;18:1237–42. doi: 10.1007/s12094-016-1583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott DA, Nabavizadeh N, Romer JL, Chen Y, Holland JM. Medical marijuana use in head and neck squamous cell carcinoma patients treated with radiotherapy. Support Care Cancer. 2016;24(8):3517–3524. doi: 10.1007/s00520-016-3180-8. [DOI] [PubMed] [Google Scholar]
- 47.Reblin M, Sahebjam S, Peeri NC, Martinez YC, Thompson Z, Egan KM. Medical Cannabis Use in Glioma Patients Treated at a Comprehensive Cancer Center in Florida. Journal of Palliative Medicine. 2019;22(10):1202–1207. doi: 10.1089/jpm.2018.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turcott JG, del Rocío Guillen Núñez M, Flores-Estrada D, Oñate-Ocaña LF, Zatarain-Barrón ZL, Barrón F, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients a randomized, double-blind clinical trial. Supportive Care in Cancer. 2018;26(9):3029–38. doi: 10.1007/s00520-018-4154-9. [DOI] [PubMed] [Google Scholar]
- 49.Anderson SP, Zylla DM, McGriff DM, Arneson TJ. Impact of Medical Cannabis on Patient-Reported Symptoms for Patients With Cancer Enrolled in Minnesota's Medical Cannabis Program. Journal of Oncology Practice. 2019;15(4):e338–e345. doi: 10.1200/JOP.18.00562. [DOI] [PubMed] [Google Scholar]
- 50.Fallon MT, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. 2017;11(3):119–133. doi: 10.1177/2049463717710042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Good P, Haywood A, Gogna G, Martin J, Yates P, Greer R, et al. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a doubleblind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD) BMC Palliative Care. 2019;187(1):1–7. doi: 10.1186/s12904-019-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Highet BH, Lesser ER, Johnson PW, Kaur JS. Tetrahydrocannabinol and Cannabidiol Use in an Outpatient Palliative Medicine Population. Am J Hosp Palliat Med. 2020;37(8):589–593. doi: 10.1177/1049909119900378. [DOI] [PubMed] [Google Scholar]
- 53.Kim A, Kaufmann CN, Ko R, Li Z, Han BH. Patterns of Medical Cannabis Use among Cancer Patients from a Medical Cannabis Dispensary in New York State. J Palliat Care. 2019;22(10):1196–1201. doi: 10.1089/jpm.2018.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maida V, Ennis M, Irani S, Corbo M, Dolzhykov M. Adjunctive nabilone in cancer pain and symptom management: a prospective observational study using propensity scoring. Journal of Supportive Oncology. 2008;6(3):119–124. [PubMed] [Google Scholar]
- 55.Mersiades AJ, Tognela A, Haber PS, Stockler M, Lintzeris N, Simes J, et al. Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: a study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV) BMJ Open. 2020;8(9):e020745. doi: 10.1136/bmjopen-2017-020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh V, Zarrabi AJ, Curseen KA, Sniecinski R, Welsh JW, McKenzie-Brown AM, et al. Concerns of Patients With Cancer on Accessing Cannabis Products in a State With Restrictive Medical Marijuana Laws: A Survey Study. Journal of Oncology Practice. 2019;15(10):531–538. doi: 10.1200/JOP.19.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis Impacts Tumor Response Rate to Nivolumab in Patients with Advanced Malignancies. Oncologist. 2019;24(4):549–554. doi: 10.1634/theoncologist.2018-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson MM, Masterson E, Broglio K. Cannabis Use among Patients in a Rural Academic Palliative Care Clinic. J Palliat Med. 2019;22(10):1224–1226. doi: 10.1089/jpm.2018.0534. [DOI] [PubMed] [Google Scholar]
- 59.Zarrabi AJ, Welsh JW, Sniecinski R, Curseen K, Gillespie T, Baer W, et al. Perception of Benefits and Harms of Medical Cannabis among Seriously Ill Patients in an Outpatient Palliative Care Practice. J Palliat Med. 2020;23(4):558–562. doi: 10.1089/jpm.2019.0211. [DOI] [PubMed] [Google Scholar]
- 60.Drosdowsky A, Blaschke S, Koproski T, Fullerton S, Thackerar A, Ellen S, et al. Cancer patients’ use of and attitudes towards medicinal cannabis. Aust Health Rev. 2020;44(4):650–655. doi: 10.1071/AH19066. [DOI] [PubMed] [Google Scholar]
- 61.Luckett T, Phillips J, Allsop D, Lee J, Solowij N, Martin J, et al. Clinical trials of medicinal cannabis for appetite-related symptoms from advanced cancer: a survey of preferences, attitudes and beliefs among patients willing to consider participation. Intern Med J. 2016;46(11):1269–1275. doi: 10.1111/imj.13224. [DOI] [PubMed] [Google Scholar]
- 62.Tanco K, Dumlao D, Kreis R, Nguyen K, Dibaj S, Liu D, et al. Attitudes and Beliefs About Medical Usefulness and Legalization of Marijuana among Cancer Patients in a Legalized and a Nonlegalized State. J Palliat Med. 2019;22(10):1213–1220. doi: 10.1089/jpm.2019.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeshurun M, Shpilberg O, Herscovici CLS, Dreyer J, Peck A, et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation Results of a Phase II Study. Biology of Blood and Marrow Transplantation. 2015;21(10):1770–5. doi: 10.1016/j.bbmt.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 64.Lintzeris N, Mills L, Suraev A, Bravo M, Arkell T, Arnold JC, et al. Medical cannabis use in the Australian community following introduction of legal access: the 2018–2019 Online Cross- Sectional Cannabis as Medicine Survey (CAMS-18) Harm Reduction Journal. 2020;17(37):1–12. doi: 10.1186/s12954-020-00377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bar-Sela G, Tauber D, Mitnik I, Sheinman-Yuffe H, Bishara-Frolova T, Aharon-Peretz J. Cannabis-related cognitive impairment: a prospective evaluation of possible influences on patients with cancer during chemotherapy treatment as a pilot study. Anticancer Drugs. 2019;30(1):91–97. doi: 10.1097/CAD.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 66.Bar-Sela G, Vorobeichik M, Drawsheh S, Omer A, Goldberg V, Muller E. The Medical Necessity for Medicinal Cannabis: Prospective, Observational Study Evaluating the Treatment in Cancer Patients on Supportive or Palliative Care. Evidence-based Complementary and Alternative Medicine. 2013;2013:1–8. doi: 10.1155/2013/510392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bar-Sela G, Zalman D, Semenysty V, Ballan E. The Effects of Dosage-Controlled Cannabis Capsules on Cancer-Related Cachexia and Anorexia Syndrome in Advanced Cancer Patients: Pilot Study. Integr Cancer Ther. 2019;18:1–8. doi: 10.1177/1534735419881498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birdsall SM, Birdsall TC, Tims LA. The Use of Medical Marijuana in Cancer. Curr Oncol Rep. 2016;18(40):1–9. doi: 10.1007/s11912-016-0530-0. [DOI] [PubMed] [Google Scholar]
- 69.Braun IM, Nayak MM, Revette A, Wright AA, Chai PR, Yusufov M, et al. Cancer Patients’ Experiences With Medicinal Cannabis-Related Care. Cancer. 2020;127(1):67–73. doi: 10.1002/cncr.33202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buchwald D, Brønnum D, Melgaard D, Leutscher PDC. Living with a Hope of Survival Is Challenged by a Lack of Clinical Evidence: An Interview Study among Cancer Patients Using Cannabis-Based Medicine. J Palliat Med. 2020;23(8):1090–1093. doi: 10.1089/jpm.2019.0298. [DOI] [PubMed] [Google Scholar]
- 71.Byars T, Theisen E, Bolton DL. Using Cannabis to Treat Cancer-Related Pain. Semin Oncol Nurs. 2019;35(3):300–309. doi: 10.1016/j.soncn.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Cheng K-C, Li Y-X, Cheng J-T. The Use of Herbal Medicine in Cancer-related Anorexia/Cachexia Treatment Around the World. Curr Pharm Des. 2012;18(31):4810–4826. doi: 10.2174/138161212803216979. [DOI] [PubMed] [Google Scholar]
- 73.Donovan KA, Portman DG. Effect of COVID-19 Pandemic on Cannabis Use in Cancer Patients. Journal of Hospice and Palliative Medicine. 2021;38(7):850–853. doi: 10.1177/1049909121999784. [DOI] [PubMed] [Google Scholar]
- 74.Good PD, Greer RM, Huggett GE, Hardy JR. An Open-Label Pilot Study Testing the Feasibility of Assessing Total Symptom Burden in Trials of Cannabinoid Medications in Palliative Care. J Palliat Med. 2020;23(5):650–655. doi: 10.1089/jpm.2019.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall W, Christie M, Currow D. Cannabinoids and cancer: causation, remediation, and palliation. Lancet Oncology. 2005;6(1):35–42. doi: 10.1016/S1470-2045(04)01711-5. [DOI] [PubMed] [Google Scholar]
- 76.Haüser W, Welsch P, Klose P, Radbruch L, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain A systematic review with meta-analysis of randomised controlled trials. Der Schmerz. 2019;33:424–436. doi: 10.1007/s00482-019-0373-3. [DOI] [PubMed] [Google Scholar]
- 77.Hawley P, Gobbo M. Cannabis use in cancer: a survey of the current state at BC Cancer before recreational legalization in Canada. Curr Oncol. 2019;26(4):e425–e432. doi: 10.3747/co.26.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huskey A. Cannabinoids in cancer pain management. J Pain Palliat Care Pharmacother. 2006;20(3):43–46. [PubMed] [Google Scholar]
- 79.Keller R. Medical Cannabis in Cancer Care. Radiat Ther. 2020;29(1):55–71. [Google Scholar]
- 80.Kleckner AS, Kleckner IR, Kamen CS, Tejani MA, Janelsins MC, Morrow GR, et al. Opportunities for cannabis in supportive care in cancer. Therapeutic Advances in Medical Oncology. 2019;11:1–29. doi: 10.1177/1758835919866362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LeClair JN, Chamberlin KW, Clement J, Holle LM. Documentation of medical marijuana use in cancer patients. J Oncol Pharm Pract. 2020;26(5):1117–1127. doi: 10.1177/1078155219883912. [DOI] [PubMed] [Google Scholar]
- 82.Likar R, Nahler G. The use of cannabis in supportive care and treatment of brain tumor. Neuro-Oncology Practice. 2017;4(3):151–160. doi: 10.1093/nop/npw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lossignol D. Cannabinoids: a new approach for pain control? Curr Opin Oncol. 2019;31(4):275–279. doi: 10.1097/CCO.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 84.Panozzo S, Le B, Collins A, Weil J, Whyte J, Barton M, et al. Who is asking about medicinal cannabis in palliative care? Intern Med J. 2020;50(2):243–246. doi: 10.1111/imj.14732. [DOI] [PubMed] [Google Scholar]
- 85.Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638–654. doi: 10.1016/j.sapharm.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Pawasarat IM, Schultz EM, Frisby JC, Mehta S, Angelo MA, Hardy SS, et al. The Efficacy of Medical Marijuana in the Treatment of Cancer-Related Pain. J Palliat Med. 2020;23(6):809–816. doi: 10.1089/jpm.2019.0374. [DOI] [PubMed] [Google Scholar]
- 87.Podda M, Pagani Bagliacca E, Sironi G, Veneroni L, Silva M, Angi M, et al. Cannabinoids use in adolescents and young adults with cancer: a single-center survey. Tumori Journal. 2020;106(4):281–285. doi: 10.1177/0300891620912022. [DOI] [PubMed] [Google Scholar]
- 88.Potts JM, Getachew B, Vu M, Nehl E, Yeager KA, Leach CR, et al. Use and Perceptions of Opioids Versus Marijuana among Cancer Survivors. Journal of Cancer Education. 2020. [DOI] [PMC free article] [PubMed]
- 89.Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178(2):107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- 90.Rosewall T, Feuz C, Bayley A. Cannabis and Radiation Therapy: A Scoping Review of Human Clinical Trials. Journal of Medical Imaging and Radiation Sciences. 2020;51(2):342–349. doi: 10.1016/j.jmir.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Trentham K. Medical Cannabis: Considerations for Dietitians Working in Oncology. Oncology Nutrition Connection. 2017;20(2):34–43. [Google Scholar]
- 92.Turgeman I, Bar-Sela G. Cannabis for cancer–illusion or the tip of an iceberg: a review of the evidence for the use of Cannabis and synthetic cannabinoids in oncology. Expert Opin Investig Drugs. 2019;28(3):285–296. doi: 10.1080/13543784.2019.1561859. [DOI] [PubMed] [Google Scholar]
- 93.Victorson D, McMahon M, Horowitz B, Glickson S, Parker B, Mendoza-Temple L. Exploring cancer survivors' attitudes, perceptions, and concerns about using medical cannabis for symptom and side effect management: A qualitative focus group study. Complement Ther Med. 2019;47:102204. doi: 10.1016/j.ctim.2019.102204. [DOI] [PubMed] [Google Scholar]
- 94.Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of Use of Medical Cannabis Among Israeli Cancer Patients: A Single Institution Experience. J Pain Symptom Manage. 2015;49(2):223–230. doi: 10.1016/j.jpainsymman.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 95.Walsh C, Currin-McCulloch J, Simon P, Zebrack B, Jones BA. Shifting Needs and Preferences: Supporting Young Adult Cancer Patients During the Transition from Active Treatment to Survivorship Care. J Adolesc Young Adult Oncol. 2019;8(2):114–121. doi: 10.1089/jayao.2018.0083. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Wang Y, Tong M, Pan H, Li D. New Prospect for Cancer Cachexia: Medical Cannabinoid. J Cancer. 2019;10(3):716–720. doi: 10.7150/jca.28246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ware MA, Daeninck P, Maida V. A review of nabilone in the treatment of chemotherapy-induced nausea and vomiting. Ther Clin Risk Manag. 2008;4(1):99–107. doi: 10.2147/tcrm.s1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Welliver M. Cannabinoid agonists for nausea and vomiting. Gastroenterol Nurs. 2016;39(2):137–138. doi: 10.1097/SGA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 99.Wilson A, Davis C. Attitudes of Cancer Patients to Medicinal Cannabis Use: A Qualitative Study. Australian Social Work. 2021.
- 100.Zalman D, Bar-Sela G. Cannabis and synthetic cannabinoids for cancer patients: Multiple palliative indications together with promising laboratory antineoplastic effects. In: Preedy VR, editor. Handbook of cannabis and related pathologies: Biology, pharmacology, diagnosis, and treatment. San Diego, CA, US: Elsevier Academic Press; 2017. pp. 859–868. [Google Scholar]
- 101.Zhou G, Stoltzfus JC, Houldin AD, Parks SM, Swan BA. Knowledge, Attitudes, and Practice Behaviors of Oncology Advanced Practice Nurses Regarding Advanced Care Planning for Patients With Cancer. Oncol Nurs Forum. 2010;37(6):E400–E410. doi: 10.1188/10.ONF.E400-E410. [DOI] [PubMed] [Google Scholar]
- 102.Donovan KA, Oberoi-Jassal R, Chang YD, Rajasekhara S, Haas MF, Randich AL, et al. Cannabis Use in Young Adult Cancer Patients. J Adolesc Young Adult Oncol. 2020;9(1):30–35. doi: 10.1089/jayao.2019.0039. [DOI] [PubMed] [Google Scholar]
- 103.Bar-Lev Schleider L, Mechoulam R, Lederman V, Hilou M, Lencovsky O, Betzalel O, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37–43. doi: 10.1016/j.ejim.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 104.Brisbois TD, de Hock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M, et al. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol. 2011;22(9):2086–2093. doi: 10.1093/annonc/mdq727. [DOI] [PubMed] [Google Scholar]
- 105.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39(2):167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 106.Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24(21):3394–3400. doi: 10.1200/JCO.2005.05.1847. [DOI] [PubMed] [Google Scholar]
- 107.JL Kramer 2015 Medical marijuana for cancer. CA: a cancer journal for clinicians. 65 2 109 22 [DOI] [PubMed]
- 108.Maida V, Daeninck PJ. A user's guide to cannabinoid therapies in oncology. Curr Oncol. 2016;23(6):398–406. doi: 10.3747/co.23.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meng H, Dai T, Hanlon JG, Downar J, Alibhai SM, Clarke H. Cannabis and cannabinoids in cancer pain management. Current Opinion in Supportive Palliative Care. 2020;14(2):87–93. doi: 10.1097/SPC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 110.Karim S, Cheung WY, Bu J, Jess E, Kerba M. Medical Cannabis Authorization in Patients With Cancer in the Prelegalization Era: A Population-Based Study. J Pain Symptom Manage. 2020;59(6):1223–1231. doi: 10.1016/j.jpainsymman.2019.12.367. [DOI] [PubMed] [Google Scholar]
- 111.Milam J, Slaughter R, Meeske K, Ritt-Olson A, Sherman-Bien S, Freyer DR, et al. Substance use among adolescent and young adult cancer survivors. Psychooncology. 2017;25(11):1357–1362. doi: 10.1002/pon.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poghosyan H, Poghosyan A. Marijuana use among cancer survivors: Quantifying prevalence and identifying predictors. Addictive Behaviors. 2021;112:106634. doi: 10.1016/j.addbeh.2020.106634. [DOI] [PubMed] [Google Scholar]
- 113.Machado Rocha FC, Stéfano SC, De Cassia HR, Rosa Oliveira LMQ, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care. 2008;17(5):431–443. doi: 10.1111/j.1365-2354.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 114.Amato L, Davoli M, Minozzi S, Mitrova Z, Parmelli E, Saulle R, et al. Systematic review of safeness and therapeutic efficacy of cannabis in patients with multiple sclerosis, neuropathic pain, and in oncological patients treated with chemotherapy 2016 [Available from: https://www.who.int/medicines/access/controlled-substances/Systematic_reviews_on_therapeutic_efficacy_and_safety.pdf?ua=1. [DOI] [PubMed]
- 115.Campbell FA, Tramer MR, Carroll D, Reynolds DJM, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323:1–6. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gouveia DN, Guimarães AG, Santos WBDR, Quintans-Júnior LJ. Natural products as a perspective for cancer pain management: A systematic review. Phytomedicine. 2019;58:152766. doi: 10.1016/j.phymed.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 117.Mucke M, Weier M, Carter C, Copeland J, Degenhardt L, Cuhls H, et al. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle. 2018;9:220–234. doi: 10.1002/jcsm.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tallant J. Cannabinoids for the treatment of cancer-related pain: a systematic review. Cancer Nursing Practice. 2020;19(2):37–42. [Google Scholar]
- 119.van den Beuken-van Everdingen MHJ, de Graeff A, Jongen JLM, Dijkstra D, Mostovaya I, Vissers KC. Pharmacological Treatment of Pain in Cancer Patients: The Role of Adjuvant Analgesics, a Systematic Review. Pain Pract. 2017;17(3):409–419. doi: 10.1111/papr.12459. [DOI] [PubMed] [Google Scholar]
- 120.Tateo S. State of the evidence: Cannabinoids and cancer pain-A systematic review. J Am Assoc Nurse Pract. 2017;29(2):94–103. doi: 10.1002/2327-6924.12422. [DOI] [PubMed] [Google Scholar]
- 121.Häuser W, Welsch P, Klose P, Radbruch L, Fitzcharles M-A. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: A systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424–436. doi: 10.1007/s00482-019-0373-3. [DOI] [PubMed] [Google Scholar]
- 122.Phillips KT, Phillips MM, Lalonde TL, Prince MA. Does social context matter? An ecological momentary assessment study of marijuana use among college students. Addict Behav. 2018;83:154–159. doi: 10.1016/j.addbeh.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 123.Costiniuk CT, Saneei Z, Salahuddin S, Cox J, Routy J-P, Rueda S, et al. Cannabis Consumption in People Living with HIV: Reasons for Use, Secondary Effects, and Opportunities for Health Education. Cannabis and Cannabinoid Research. 2019;4(3):204–213. doi: 10.1089/can.2018.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruce D, Bouris AM, Bowers S, Blocker O, Young Lee S, Glidden MF, et al. Medical, therapeutic, and recreational use of cannabis among young men who have sex with men living with HIV. Addiction Research & Theory. 2020;28(3):250–259. doi: 10.1080/16066359.2019.1629427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davis MP. Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opin Investig Drugs. 2008;17(1):85–95. doi: 10.1517/13543784.17.1.85. [DOI] [PubMed] [Google Scholar]
- 126.Zaki P, Blake A, Wolt A, Chan S, Liying Z, Wan A, et al. The use of medical cannabis in cancer patients. Journal of Pain Management. 2017;10(4):353–362. [Google Scholar]
- 127.Zhang H, Xie M, Archibald SD, Jackson BS, Gupta MK. Association of marijuana use with psychosocial and quality of life outcomes among patients with head and neck cancer. JAMA Otolaryngology-head & Neck Surgery. 2018;144(11):1017–1022. doi: 10.1001/jamaoto.2018.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donovan KA, Chang YD, Oberoi-Jassal R, Rajasekhara S, Smith J, Haas M, et al. Relationship of Cannabis Use to Patient-Reported Symptoms in Cancer Patients Seeking Supportive/Palliative Care. J Palliat Med. 2019;22(10):1191–1195. doi: 10.1089/jpm.2018.0533. [DOI] [PubMed] [Google Scholar]
- 129.Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An Open-Label Extension Study to Investigate the Long-Term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal THC Spray in Patients With Terminal Cancer-Related Pain Refractory to Strong Opioid Analgesics. J Pain Symptom Manage. 2013;46(2):207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 130.Webster EM, Yadav GS, Gysler S, McNamara B, Black J, Tymon-Rosario J, et al. Prescribed medical cannabis in women with gynecologic malignancies: A single-institution survey-based study. Gynecologic Oncology Reports. 2020;34:100667. doi: 10.1016/j.gore.2020.100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Government of Canada. Cannabis Act (S.C. 2018, c. 16) 2020 [Available from: https://laws-lois.justice.gc.ca/eng/acts/c-24.5/.
- 132.MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–19. doi: 10.1016/j.ejim.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Braun IM, Wright A, Peteet J, Meyer FL, Yuppa DP, Bolcic-Jankovic D, et al. Medical Oncologists’ Beliefs, Practices, and Knowledge Regarding Marijuana Used Therapeutically: A Nationally Representative Survey Study. J Clin Oncol. 2018;36(19):1957–1962. doi: 10.1200/JCO.2017.76.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sannes TS, Nayak MM, Tung S, et al. United States oncologists' clinical preferences regarding modes of medicinal cannabis use. Cancer Commun. 2021;41(6):528–531. doi: 10.1002/cac2.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corroon J, Sexton M, Bradley R. Indications and administration practices amongst medical cannabis healthcare providers: a cross-sectional survey. BMC Family Practice. 2019;20(174):1–12. doi: 10.1186/s12875-019-1059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].