Abstract
Although Mycobacterium tuberculosis is eradicated rapidly during therapy in some patients with pulmonary tuberculosis, it can persist for many months in others. This study examined the relationship between mycobacterial drug tolerance (delayed killing in vitro), persistence, and relapse. It was performed with 39 fully drug-susceptible isolates from a prospective trial of standard short-course antituberculous therapy with sputum smear-positive, human immunodeficiency virus-uninfected subjects with pulmonary tuberculosis in Brazil and Uganda. The rate of killing in vitro was determined by monitoring the growth index (GI) in BACTEC 12B medium after addition of drug to established cultures and was measured as the number of days required for 99% sterilization. Drugs differed significantly in bactericidal activity, in the following order from greatest to least, rifampin > isoniazid-ethambutol > ethambutol (P < 0.001). Isolates from subjects who had relapses (n = 2) or in whom persistence was prolonged (n = 1) were significantly more tolerant of isoniazid-ethambutol and rifampin than isolates from other subjects (P < 0.01). More generally, the duration of persistence during therapy was predicted by strain tolerance to isoniazid and rifampin (P = 0.012 and 0.026, respectively). Tolerance to isoniazid-ethambutol and tolerance to rifampin were highly correlated (P < 0.001). Tolerant isolates did not differ from others with respect to the MIC of isoniazid; the rate of killing of a tolerant isolate by isoniazid-ethambutol was not increased at higher drug concentrations. These observations suggest that tolerance may not be due to drug-specific mechanisms. Tolerance was of the phenotypic type, although increased tolerance appeared to emerge after prolonged drug exposure in vivo. This study suggests that drug tolerance may be an important determinant of the outcome of therapy for tuberculosis.
There is substantial variability in the response to therapy of pulmonary tuberculosis (TB), even in those patients infected with fully drug-sensitive isolates. In some patients, Mycobacterium tuberculosis is rapidly eradicated and patients are cured after as little as 2 to 3 months of ultra-short-term chemotherapy (2–4, 14). Yet, in others, viable organisms persist in sputum for many months, despite drug susceptibility in vitro. The risk of relapse is increased in individuals in whom sputum sterilization is delayed (1, 8, 17). The factors responsible for mycobacterial persistence (prolonged culture positivity during therapy) are not well understood but may include mycobacterial and host biological factors, as well as host behavioral factors.
Studies of tolerance, the ability to evade the bactericidal activity of antimicrobial drugs, may be relevant to these observations. The hallmark of tolerance is bacteriostasis with reduced or delayed bactericidal activity (for a review, see reference 19). Tolerance in streptococci can be induced by selective depletion of the medium of certain nutrients, exposure to certain drugs, or more generally, existence in the stationary growth phase. Tolerance may be an important factor that determines the risk of relapse in patients with streptococcal endocarditis (13).
Mycobacterial persistence and relapse may in part be determined by drug tolerance. Like the streptococcus, M. tuberculosis resists killing when it is in the stationary growth phase. The sterilizing activity of isoniazid (INH), for example, is reduced nearly 1,000-fold in stationary-phase cultures (12). Sterilizing activity may be essential for prevention of relapse of TB, yet the relationship of mycobacterial drug tolerance to treatment outcome has not been studied.
Many clinical laboratories use the BACTEC TB 460 system (Becton Dickinson, Sparks, Md.) for rapid detection and susceptibility testing of mycobacteria. It is based on detection of 14CO2, produced by oxidation of [14C]palmitic acid. In the study described in this report, a simple method that used the BACTEC system to measure the rate of killing of M. tuberculosis was developed. The method was used to examine the relationship between tolerance in vitro and persistence in vivo during standard therapy for TB.
MATERIALS AND METHODS
Mycobacterial isolates and clinical data were obtained from a prospective study conducted in Uganda and Brazil and reported previously (21). Briefly, patients with initial episodes of sputum smear-positive pulmonary TB who had not received prior therapy were prospectively recruited at TB control clinics in Kampala, Uganda, and Vitória, Brazil. All patients gave informed written consent for human immunodeficiency virus testing and study participation. The study protocol was approved by the institutional review boards of Case Western Reserve University (Cleveland, Ohio), Makerere University (Kampala, Uganda), Universidade Federal do Espírito Santo (Vitória, Brazil), Duke University (Durham, N.C.), and the University of Arkansas (Little Rock). Tuberculosis was presumptively diagnosed by a positive acid-fast smear of sputum and a compatible chest radiograph and was subsequently confirmed by culture. Serology for human immunodeficiency virus type 1 was performed for all subjects; seropositive individuals were excluded from the study. Subjects were also subsequently excluded from this analysis if their initial isolates were resistant to INH, rifampin (RIF), pyrazinamide, or ethambutol (EMB) or the duration of follow-up was less than 180 days.
Subjects were treated with INH, RIF, EMB, and pyrazinamide daily at standard doses for 2 months, followed by daily treatment with INH and RIF for 4 months. Patients were evaluated on days 0, 2, 4, 7, 14, and 30, monthly until therapy was completed, and then bimonthly. At each evaluation, a medical history was taken and a physical examination was performed. Multiple sputum specimens were obtained. These were processed and analyzed as described below. Patients were hospitalized for the initial 2 weeks of treatment, after which time therapy was self-administered. Compliance was assessed at each clinic visit by review of dispensing records, clinic attendance, and urinary isoniazid metabolite testing (MycoDyn Uritec; DynaGen, Inc., Cambridge, Mass.). Subjects were classified as treatment failures if M. tuberculosis was repeatedly isolated from sputum on days 120 to 180 in conjunction with symptoms and radiographic findings consistent with active tuberculosis.
Sputum processing and culture.
Specimens were digested with N-acetyl cysteine and were then decontaminated by addition of an equal volume of 4% NaOH–2.9% sodium citrate, as described previously (21). The specimen was then cultured on Middlebrook Cohn 7H10 and 7H10S (antibiotic containing) agar medium and in BACTEC 12B liquid medium. Decontamination of sputum with NaOH has been shown to reduce the numbers of CFU by 80% (23). However, this approach was adopted because use of NaOH resulted in reduced contamination rates compared to those from the use of antibiotic-containing medium and because the reduction in CFU was consistent among specimens in a pilot study. Sputum cultures were performed on site in Kampala and Vitória. Growth indices (GIs) were measured daily after inoculation into BACTEC 12B medium. The day of collection of the last sputum specimen to indicate growth in BACTEC 12B medium (GI ≥ 10) within 20 days of inoculation (rapid growth) was determined retrospectively for each subject. Specimens that were contaminated with organisms other than M. tuberculosis were excluded from analysis.
Measurement of drug tolerance.
Isolates were shipped to Case Western Reserve University for studies of tolerance. Isolates were placed into BACTEC 12B medium (Becton Dickinson) and were maintained in culture at 37°C without agitation. GIs were monitored daily until a value of 250 was reached, at which time 100-μl samples were removed for inoculation of four replicate bottles. These were cultured until the GI again reached 250, at which time EMB (final concentration, 2.5 μg/ml), is INH (0.1 μg/ml), or RIF (2.0 μg/ml) was added. Control cultures were maintained without drug. The GI was monitored daily. The mean coefficient of variation of GIs for replicate cultures on the day of drug addition (day 0) was 8.3%. The mean day 0 GI among all isolates was 277, with a coefficient of variation of 17.7%. To adjust for these minor differences in baseline values, GIs for each culture bottle in studies of clinical isolates were reported as GI × 250/day 0 GI.
Susceptibility and MIC testing.
Aliquots of 100 μl were removed from cultures in BACTEC 12B medium when the GI reached 250. These were placed into culture in fresh bottles to which drug had been added. Control cultures (without drug) were inoculated with an aliquot that had been diluted 1:100 in bovine serum albumin 2 mg/ml and Tween 80 (0.02%). The GI was monitored daily until the control cultures reached a GI of 30, at which time the increment in GI over the subsequent 24 h was recorded. The MIC was reported as the lowest concentration of drug resulting in an increment of GI less than that of the control culture.
Data analysis.
Statistical analysis was performed with SigmaStat (SPSS, Chicago, Ill.). Comparisons between groups were performed by two-tailed tests. Comparisons between multiple groups were performed by analysis of variance (ANOVA), followed by Tukey’s test, a conservative method for the identification of differences between pairs. Correlations were identified by the Pearson product method.
RESULTS
Measuring mycobacterial drug tolerance in BACTEC 12B medium.
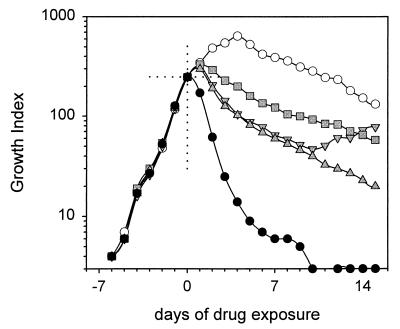
The essential features of the use of BACTEC 12B medium to study mycobacterial killing are illustrated in Fig. 1. In this experiment, M. tuberculosis H37Ra was allowed to grow in BACTEC 12B medium until the GI reached 250. At that time (day 0), EMB (2.5 μg/ml), INH (0.1 μg/ml), INH-EMB, or RIF (2.0 μg/ml) was added. These concentrations are routinely used for susceptibility testing in BACTEC 12B medium. GIs were then monitored daily.
FIG. 1.
Killing of M. tuberculosis H37Ra
by EMB, INH, and RIF in BACTEC 12B medium. INH resistance emerged in
cultures exposed to INH alone. This was prevented by addition of EMB
(upright triangles) without otherwise affecting the rate of killing.
○, no drug; ░⃞, EMB; 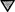 , INH;
, INH-EMB; ●, RIF.
, INH;
, INH-EMB; ●, RIF.
GIs in cultures to which drug was not added rose to a maximum of over 600 and gradually declined thereafter. Among cultures to which drug was added, the GIs declined the most rapidly in those with RIF and declined the least rapidly in those with EMB. Cultures with INH or INH-EMB showed intermediate responses that initially were identical, but those responses subsequently diverged. This was not due to the loss of potency of INH (data not shown) but, instead, was due to the emergence of INH resistance, confirmed by susceptibility testing on agar. This emergence of resistance was prevented by the addition of EMB-INH. The addition of EMB to INH did not otherwise affect the rate of sterilization. A two-drug combination was not required to study killing by rifampin, as resistance emerged only in a small number of isolates, and, even then, resistance emerged only after 3 weeks of culture. This may be due to the low frequency of RIF-resistant colonies in most susceptible populations.
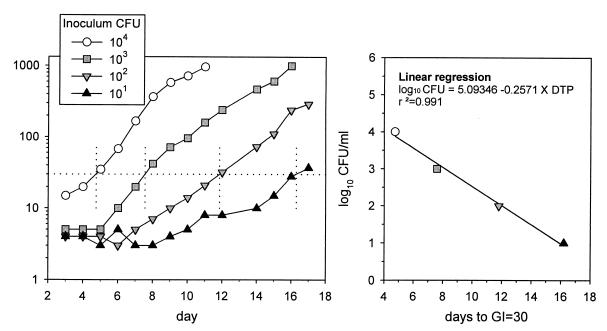
14CO2 production in BACTEC 12B medium reflects the product of the number of viable organisms and their average state of metabolic activity. Declines in GI thus do not a priori indicate killing. We therefore experimentally determined the number of viable bacilli each day by measuring the time required for subcultures to become positive in bottles without drug. As shown in Fig. 2, the number of days to reach a GI of 30 (days to positivity [DTP]), is inversely proportional to the log10 CFU of the inoculum (log10 CFU = 5.09 − 0.257 × DTP; r2 = 0.99), indicating that DTP in subcultures can serve as a surrogate for conventional CFU determination. The method is more sensitive than direct CFU determination and is unaffected by clumping.
FIG. 2.
Relationship of CFU of M. tuberculosis H37Ra to DTP in BACTEC 12B medium.
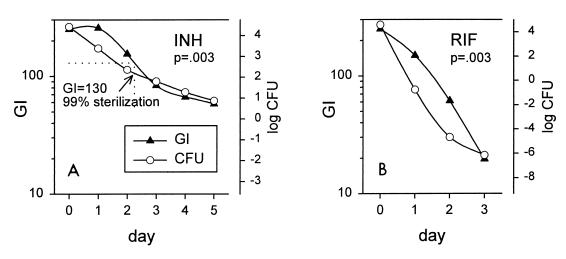
Therefore, aliquots of 100 μl were removed daily from cultures to which drug had been added. Bacilli were sedimented to minimize drug carryover; the medium in the original bottle was replenished with drug to maintain a constant concentration. The results for INH and RIF are indicated in Fig. 3. CFU values were calculated from DTP values for subcultures. The correlation between the GI and the calculated CFU was statistically significant at P equal to 0.003 for each drug. These experiments indicate that the rate of killing of M. tuberculosis in the presence of drugs can be determined by monitoring the rate of decline of GI. No such relationship was observed for cultures to which no drug was added (P = 0.27; data not shown). This occurred because subcultures were positive very quickly (<1 day) due to the large number of organisms and also because, late in the stationary phase, the GI declined somewhat without a concomitant decline in viability.
FIG. 3.
Relationship of GI to CFU in cultures with INH (A) or RIF (B). CFU values were calculated from DTP values for daily subcultures. The number of viable bacilli in cultures exposed to INH was reduced by 99% (2 log10) after slightly more than 2 days (A). The corresponding GI at that time was 130.
Statistical analysis of growth curves generally requires their reduction to a single number, such as DTP. Killing, like growth, occurs as a logarithmic process. In this study, the rate of killing was analyzed as the number of days required for 99% sterilization (days to sterilization [DTS]). This value rather than the value for complete sterilization (less than one viable organisms per sample) was selected as it has less inherent variability. Additionally, there is precedent for use of a 10−2 proportion in TB drug susceptibility testing. As indicated in Fig. 3, 99% sterilization was determined to occur when the GI had declined from 250 to 130. The DTS values of H37Ra with EMB, INH, INH-EMB, and RIF were 6.5, 3.3, 3.1, and 1.3 days, respectively.
Tolerance of clinical isolates.
Tolerance was then examined for 39 drug sensitive isolates, obtained from a prospective clinical trial in which two relapses occurred (as reported previously [21]). Both subjects with relapses had initially responded, with samples from the subjects becoming culture negative on agar after days 14 and 30, respectively, and in BACTEC 12B medium after days 30 and 90, respectively. Cultures on agar again became positive within several months after treatment had stopped, in association with clinical evidence of disease. In both cases, cultures in BACTEC 12B medium were rapidly positive during the last month of therapy; this reflects the greater sensitivity of BACTEC 12B medium for detection of small numbers of viable bacilli. Both relapse isolates remained fully drug sensitive. One subject (subject 29) was documented to have been noncompliant to the treatment regimen during the last month of therapy, by pill dispensing and clinic attendance records. The second subject with a relapse appeared to have been fully compliant with the treatment regimen. A third subject met the definition of treatment failure (repeated isolation of M. tuberculosis on or after day 120). This individual was ultimately cured by continued therapy and did not have a relapse. The rate of all adverse outcomes was 8%.
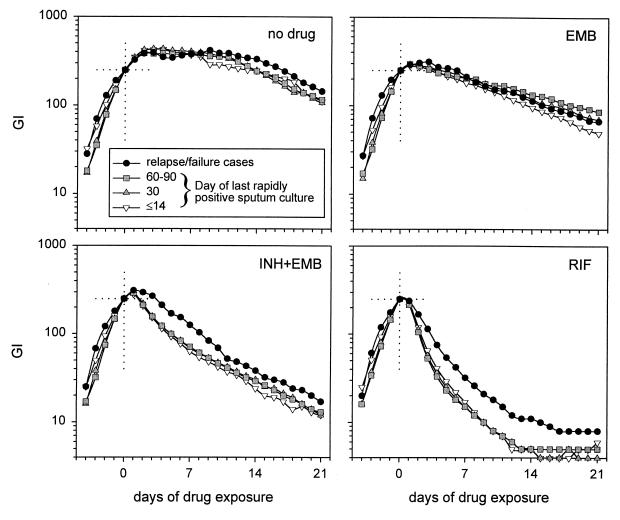
Pretreatment isolates from these 39 subjects were tested for tolerance to EMB, INH-EMB, and RIF. All isolates underwent one additional growth cycle in fresh BACTEC 12B medium bottles prior to measurement of tolerance, to ensure equal logarithmic-phase growth conditions among all isolates. As shown in Fig. 4 and Table 1, the rate of killing by INH and RIF of isolates from subjects who relapsed or failed treatment was markedly delayed compared to that for other isolates. This indicates that tolerance may predict failure and relapse in patients undergoing treatment for TB.
FIG. 4.
Killing of clinical M. tuberculosis isolates, grouped according to the duration of persistence in sputum during therapy. Isolates from subjects who had relapses or whose sputum cultures were persistently positive through day 120 of therapy (black circles) showed increased tolerance to INH-EMB and RIF compared to all other isolates.
TABLE 1.
DTS as a predictor of outcome in drug-sensitive pulmonary TBa
| Drug(s) | DTS
|
|||
|---|---|---|---|---|
| All isolates (n = 39) | Isolates from patients with relapses or treatment failures (n = 3) | All other isolates (n = 36) | P | |
| EMB | 15.4 ± 6.8 | 13.6 ± 6.8 | 15.5 ± 6.9 | 0.64 |
| INH-EMB | 4.22 ± 1.5 | 6.55 ± 2.3 | 4.03 ± 1.3 | 0.006 |
| RIF | 1.95 ± 0.7 | 3.41 ± 1.7 | 1.82 ± 0.33 | <0.001 |
Isolates from patients who subsequently had relapses or treatment showed significantly increased tolerance to INH and RIF compared to that of the isolates from other patients. In addition, the bactericidal activities of each drug or drug combination differed by ANOVA (P < 0.001).
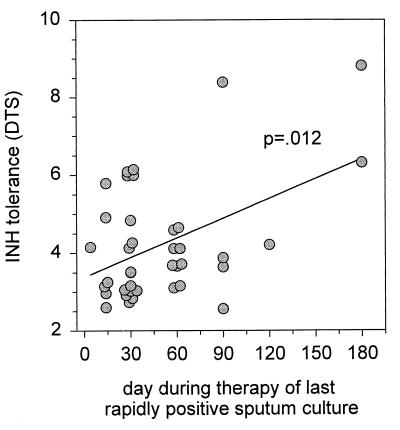
To determine whether a more general relationship existed between tolerance and persistence, subjects were classified according to the day of collection of the last sputum specimen to indicate growth within 20 days after inoculation into BACTEC 12B medium (last rapidly positive culture). This threshold was selected on the basis of the report of Epstein et al. (8) indicating a relationship of this parameter to the outcome of therapy. The median day of the last rapidly positive culture was 30 days (range, 4 to 180 days). Isolates from subjects with delayed clearance of viable bacilli from sputum (last rapidly positive culture on or after day 90; n = 7) were significantly more tolerant than all others (DTS values, INH-EMB, 5.4 ± 2 versus 4.0 ± 1 [P = 0.019]; RIF, 2.5 ± 1 versus 1.8 ± 0.3 [P = 0.017]). In addition, a linear relationship between day of last rapidly positive culture and days to 99% sterilization in vitro was identified for INH-EMB (P = 0.012) (Fig. 5) and RIF (P = 0.026) (data not shown) but not for EMB (P = 0.9) (data not shown). These findings suggest that the observations regarding the three failures or relapses were not likely due to chance.
FIG. 5.
Correlation between persistence (sputum culture positivity during therapy) and tolerance to INH (days required for 99% sterilization).
There was no apparent relationship between persistence and GI in the absence of drug, either in the logarithmic phase (before day 0) or in the stationary phase (P = 0.17 to 0.9 (Fig. 4). This indicates that the growth rate under ideal conditions or that under conditions of nutrient depletion does not appear to determine mycobacterial survival in the presence of drug. This negative finding must be taken in context, however, as our preliminary studies indicate that GI does not serve as a satisfactory surrogate for viability during late-stationary-phase growth.
As indicated in Table 1, the bactericidal activities of RIF, INH-EMB, and EMB differed by ANOVA (P < 0.001). All pairwise comparisons also showed significant differences (P < 0.05). Five isolates were studied with INH alone; these results did not differ from those for INH-EMB treatment (P = 0.22 by paired analysis), thus confirming the preliminary findings obtained with H37Ra.
Mechanisms of tolerance.
To determine whether the rate of killing was concentration dependent, one clinical isolate (isolate 80730, a tolerant pretreatment isolate from a patient who had a relapse, despite full compliance) was studied with a 40-fold range of concentrations of INH (0.025 to 1.0 μg/ml). All concentrations tested were greater than the MIC of INH for this strain. EMB was added to INH at a fixed concentration (2.5 μg/ml) to prevent the emergence of INH resistance. The rate of killing by INH did not vary over this range of INH concentrations, as indicated in Fig. 6.
FIG. 6.
Lack of effect of INH concentration on the rate of killing of a tolerant clinical M. tuberculosis isolate. All concentrations tested were greater than the MIC for this isolate.
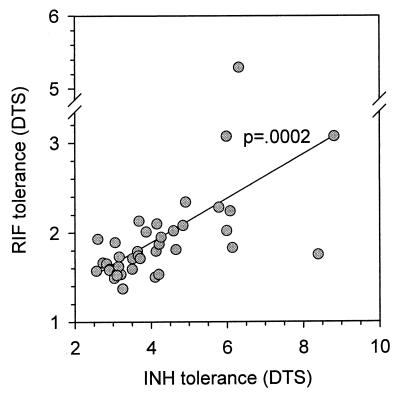
The majority of INH-sensitive M. tuberculosis isolates are highly susceptible (≤0.025 μg/ml), but for 40% of isolates MICs are 0.05 to 0.1 μg/ml (22). To determine whether differences in tolerance might be reflected in differences in MIC, the MICs for selected isolates were measured in BACTEC 12B medium. The findings for INH are shown in Table 2. No relationship between tolerance and MIC was identified. This indicates that tolerance is not likely due to expression of drug-specific factors. Such a hypothesis is further supported by the observation that tolerance to INH and RIF was highly intercorrelated among the clinical isolates (Fig. 7) (P = 0.0002), despite the marked difference in the mechanisms of action of these drugs.
TABLE 2.
Relationship between MIC of INH, tolerance in vitro, and persistence in vivo, for four clinical isolates of M. tuberculosis
| Subject | Day of last rapidly positive sputum culture | GI after 7 days of exposure to INH-EMB | INH MIC (μg/ml) |
|---|---|---|---|
| 80617 | 14 | 49 | ≤0.025 |
| 80831 | 14 | 43 | ≤0.025 |
| 80628 | 90 | 69 | ≤0.025 |
| 80730 | 180 | 217 | ≤0.025 |
FIG. 7.
Correlation between tolerance to RIF and INH in drug-sensitive clinical isolates of M. tuberculosis, measured as DTS.
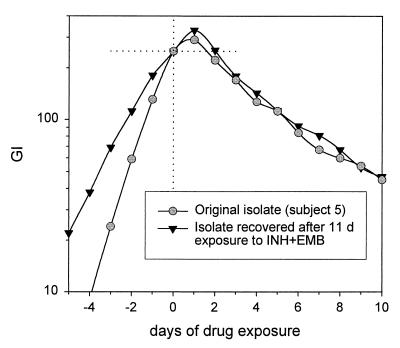
In gram-positive bacteria, two forms of tolerance have been described, and these are based on the characteristics of the fraction of organisms that survive drug exposure (19). In the phenotypic form of tolerance, surviving organisms are killed at the same rate as the parent strain when re-exposed to drugs. To characterize the type of tolerance in M. tuberculosis, two isolates were exposed to INH-EMB in vitro and the surviving bacilli were recovered. The isolate from subject 5, a subject with average characteristics, was exposed for 11 days, and that from subject 80730, a patient with relapse, was exposed for 26 days. The surviving bacilli showed tolerance equal to that of the parent strain (4.4 versus 4.2 days for surviving and parent strains of isolate 5, respectively, and 7.7 versus 8.8 days for surviving and parent strains of isolate 80730, respectively). The killing curves for the isolates from subject 5 are shown in Fig. 8. These data appear to indicate that tolerance is a stable phenomenon and that within each strain its expression is phenotypic.
FIG. 8.
Effect of prolonged drug exposure in vitro on expression of tolerance. The surviving bacilli of the isolate from subject 5 were recovered after 11 days of exposure to INH-EMB. The surviving bacilli exhibited tolerance identical to that of the original isolate.
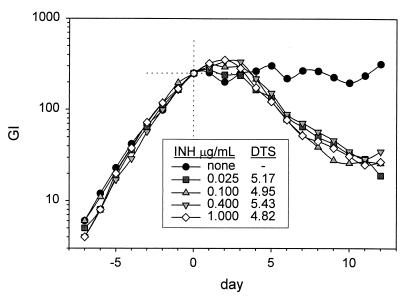
The effect of prolonged drug exposure in vivo during therapy was also studied with isolates from the two subjects with relapses. The isolates were obtained after therapy had been initiated. In the case of subject 80730, the relapse isolate was available for study. This isolate showed greater tolerance to INH-EMB than the original isolate, with the DTS increasing from 8.8 to 10.9 days. In the case of subject 29, isolates were available after 1 week and 1 month of standard therapy. DTS values for isolates treated with INH-EMB increased in a stepwise fashion during this brief interval, from 4.2 to 4.5 to 5.1 days. The isolate also became progressively more tolerant of EMB, with time to sterilization increasing from 8.9 to 15.6 days and, ultimately, to 18.5 days (Fig. 9). These experiments indicate that tolerance may arise in vivo during multidrug therapy.
FIG. 9.
Effect of prolonged drug exposure in vivo on expression of tolerance to INH. Isolates were collected from the sputum of subject 29 after 7 and 30 days of standard therapy. Tolerance increased in a stepwise manner. The subject ultimately had a relapse.
DISCUSSION
The eradication of TB has proven to be an elusive goal, often in individual patients as well as in large populations. It is believed that most actively replicating bacilli are killed rapidly during the first few weeks of therapy for TB. Prolonged treatment is required to eliminate persisting, nonreplicating organisms which exhibit reduced or otherwise altered metabolic activity and reduced susceptibility to the bactericidal activities of certain antimycobacterial drugs (11, 24).
This report may shed light on that phenomenon. In it, a method for the measurement of drug tolerance with the BACTEC TB 460 culture system was described. Its essential features are as follows: (i) the GI is high at the time when drug is added, and (ii) the GIs of tolerant isolates remain high, despite drug exposure. This differs fundamentally from susceptibility testing, in which the GI is low at the time of drug addition and in which the GIs of resistant isolates increase, despite drug exposure. The selection of 250 as the GI threshold at which time drug is added may have significantly influenced the results. At that GI, cultures are near the completion of the logarithmic growth phase and are approaching the stationary growth phase. This may have emphasized the effects of drug on non-replicating-stage bacilli. This emphasis may be clinically appropriate, given the significance of nonreplicating mycobacteria in clinical latency as well as in persistence during therapy.
Pretreatment isolates from patients who ultimately failed treatment or who had relapses after standard therapy showed greater tolerance than those from patients whose treatment was successful. More generally, a linear relationship between strain tolerance and the duration of persistence of viable mycobacteria in sputum during treatment was identified. This is the first study to indicate that a microbial factor, aside from RIF or multidrug resistance, may affect the outcome of therapy for TB. Further studies are warranted to verify this finding, given the small number of adverse outcomes described in this report. Additional studies are also warranted to determine whether measurement of strain tolerance can be used prospectively to improve outcomes and reduce treatment costs. Further studies are also needed to determine the variability and other potential limitations of the method.
The potential mechanisms for tolerance remain uncertain. The high correlation in tolerance to RIF and INH is in distinct contrast to mycobacterial resistance to these drugs. Multidrug resistance is most often the result of the accumulation of mutations in the genes of drug targets, sometimes in conjunction with decreased permeability of the mycobacterial cell wall. The data presented here appear to indicate that these factors are unlikely to be involved in tolerance. Rather, it may be more likely that differences between isolates in the time of transition to the stationary phase of growth may be more relevant. Further studies will be required to examine this question.
This report also indicates that significant differences exist in the bactericidal activities of standard anti-TB drugs, with RIF being the most active, EMB being the least active, and INH being intermediately active. This ranking is consistent with clinical observations regarding the sterilizing activities of these drugs (16). In this context, “sterilizing activity” refers to the ability to clear viable organisms from sputum rapidly during therapy (17). RIF and pyrazinamide have high levels of sterilizing activity in vivo. The incorporation of these drugs into standard anti-TB therapy has allowed treatment to be shortened from 12 to 6 months and preventive therapy to be shorted from 9 to 2 months (5, 7, 10, 18, 20). EMB, in contrast, has little sterilizing activity (6, 24). Relapse rates increase when EMB is substituted for RIF in the continuation phase of anti-TB therapy (9). Mitchison (12) has argued that the development of new drugs with high levels of sterilizing activity is critical if anti-TB therapy is to be shortened and its effectiveness increased. This is the first report to indicate that a correlate of sterilizing activity can be readily measured in an in vitro assay. It may indicate an important potential role for this assay in anti-TB drug development.
The factors that determine the bactericidal activity of a drug are not known, but one may speculate that its mechanism of action is critical. EMB acts through the inhibition of synthesis of the arabinan component of cell wall arabinogalactan (15). This biosynthetic pathway may not be a critical one in nonreplicating bacilli. In contrast, RIF, by inhibiting RNA polymerase, may be more likely to affect essential processes in organisms in all stages of growth, particularly those in nonreplicating states. This hypothesis is supported by studies that indicate that drugs that affect cell wall synthesis have markedly reduced bactericidal activities when they are tested against non-replicating-stage bacilli, whereas the activities of RIF and streptomycin are less profoundly affected by the replication stage of the bacilli (11).
In summary, this report describes a novel method for measurement of the rate of killing of isolates of M. tuberculosis. The method may be of significance in the treatment of patients with TB and in the evaluation of new anti-TB drugs.
ACKNOWLEDGMENTS
This study was supported by grants AI45244 and AI41911 from the National Institutes of Health.
REFERENCES
- 1.Aber V R, Nunn A J. Short term chemotherapy of tuberculosis. Factors affecting relapse following short term chemotherapy. Bull Int Union Tuberc. 1978;53:276–280. [PubMed] [Google Scholar]
- 2.Anonymous. A controlled trial of 2-month, 3-month, and 12-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 60 months. Am Rev Respir Dis. 1984;130:23–28. doi: 10.1164/arrd.1984.130.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. A controlled trial of 3-month, 4-month, and 6-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 5 years. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1989;139:871–876. doi: 10.1164/ajrccm/139.4.871. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian R, Sivasubramanian S, Vijayan V K, Ramachandran R, Jawahar M S, Paramasivan C N, Selvakumar N, Somasundaram P R. Five year results of a 3-month and two 5-month regimens for the treatment of sputum-positive pulmonary tuberculosis in south India. Tubercle. 1990;71:253–258. doi: 10.1016/0041-3879(90)90037-9. [DOI] [PubMed] [Google Scholar]
- 5.Bishai W R, Chaisson R E. Short-course chemoprophylaxis for tuberculosis. Clin Chest Med. 1997;18:115–122. doi: 10.1016/s0272-5231(05)70360-9. [DOI] [PubMed] [Google Scholar]
- 6.Botha F J, Sirgel F A, Parkin D P, van-de W B, Donald P R, Mitchison D A. Early bactericidal activity of ethambutol, pyrazinamide and the fixed combination of isoniazid, rifampicin and pyrazinamide (Rifater) in patients with pulmonary tuberculosis. S Afr Med J. 1996;86:155–158. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Use of short-course tuberculosis preventive therapy regimens in HIV-seronegative persons. JAMA. 1998;280:1736. doi: 10.1001/jama.280.20.1736. [DOI] [PubMed] [Google Scholar]
- 8.Epstein M D, Schluger N W, Davidow A L, Bonk S B, Rom W N, Hanna B. Time to detection of M. tuberculosisin sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest. 1997;113:379–386. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Felten M K. Importance of rifampicin in combined daily/intermittent chemotherapy for tuberculosis. S Afr Med J. 1989;75:524–526. [PubMed] [Google Scholar]
- 10.Halsey N A, Coberly J S, Desormeaux J, Losikoff P, Atkinson J, Moulton L H, Contave M, Johnson M, Davis H, Geiter C, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351:786–792. doi: 10.1016/S0140-6736(97)06532-X. [DOI] [PubMed] [Google Scholar]
- 11.Heifets L, Lindholm-Levy P. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis. 1992;145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 12.Herbert D, Paramasivan C N, Venkatesan P, Kubendiran G, Prabhakar R, Mitchison D A. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:2296–2299. doi: 10.1128/aac.40.10.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K S, Bayer A S. Significance of in-vitro penicillin tolerance in experimental enterococcal endocarditis. J Antimicrob Chemother. 1987;19:475–485. doi: 10.1093/jac/19.4.475. [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra M L, Gautam K D, Chaube C K. Shortest possible acceptable effective chemotherapy in ambulatory patients with pulmonary tuberculosis. Part II. Results during the 24 months after the end of chemotherapy. Am Rev Respir Dis. 1984;129:1016–1017. doi: 10.1164/arrd.1984.129.6.1016. [DOI] [PubMed] [Google Scholar]
- 15.Mikusova K, Slayden R A, Besra G S, Brennan P J. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother. 1995;39:2484–2489. doi: 10.1128/aac.39.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchison D A. The action of antituberculosis drugs in short-course chemotherapy. Tubercle. 1985;66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 17.Mitchison D A. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis. 1993;147:1062–1063. doi: 10.1164/ajrccm/147.4.1062. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 18.Mitchison D A, Nunn A J. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 19.Tuomanen E. Phenotypic tolerance: the search for beta-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis. 1986;8(Suppl. 3):S279–S291. doi: 10.1093/clinids/8.supplement_3.s279. [DOI] [PubMed] [Google Scholar]
- 20.Villarino M E, Ridzon R, Weismuller P C, Elcock M, Maxwell R M, Meador J, Smith P J, Carson M L, Geiter L J. Rifampin preventive therapy for tuberculosis infection: experience with 157 adolescents. Am J Respir Crit Care Med. 1997;155:1735–1738. doi: 10.1164/ajrccm.155.5.9154885. [DOI] [PubMed] [Google Scholar]
- 21.Wallis R S, Perkins M, Phillips M, Joloba M, Demchuk B, Namale A, Johnson J C, Williams D, Wolski K, Teixeira L, et al. Induction of the antigen 85 complex of M. tuberculosisin sputum: a determinant of outcome in pulmonary tuberculosis. J Infect Dis. 1998;178:1115–1121. doi: 10.1086/515701. [DOI] [PubMed] [Google Scholar]
- 22.Yajko D M, Madej J J, Lancaster M V, Sanders C A, Cawthon V L, Gee B, Babst A, Hadley W K. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:2324–2327. doi: 10.1128/jcm.33.9.2324-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yajko D M, Wagner C, Tevere V J, Kocagoz T, Hadley W K, Chambers H F. Quantitative culture of Mycobacterium tuberculosisfrom clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J Clin Microbiol. 1995;33:1944–1947. doi: 10.1128/jcm.33.7.1944-1947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamori S, Ichiyama S, Shimokata K, Tsukamura M. Bacteriostatic and bactericidal activity of antituberculosis drugs against Mycobacterium tuberculosis, Mycobacterium avium-Mycobacterium intracellulare complex and Mycobacterium kansasii in different growth phases. Microbiol Immunol. 1992;36:361–368. doi: 10.1111/j.1348-0421.1992.tb02035.x. [DOI] [PubMed] [Google Scholar]