Abstract
Porous precision-templated scaffolds (PTS) with uniformly distributed 40 μm spherical pores have shown a remarkable ability in immunomodulating resident cells for tissue regeneration. While the pore size mediated pro-healing response observed only in 40 μm pore PTS has been attributed to selective macrophage polarization, monocyte recruitment and phenotype have largely been uncharacterized in regulating implant outcome. Here, we employ a double transgenic mouse model for myeloid characterization and a multifaceted phenotyping approach to quantify monocyte dynamics within subcutaneously implanted PTS. Within 40 μm PTS, myeloid cells were found to preferentially infiltrate into the scaffold. Additionally, macrophage receptor with collagenous structure (MARCO), an innate activation marker, was significantly upregulated within 40 μm PTS. When 40 μm PTS were implanted in monocyte-depleted mice, the transcription of MARCO was significantly decreased and an increase in pro-inflammatory inducible nitric oxide synthase (iNOS) and tumor necrosis factor alpha (TNFα) were observed. Typical of a foreign body response (FBR), 100 μm PTS significantly upregulated pro-inflammatory iNOS, secreted higher amounts of TNFα, and displayed a pore size dependent morphology compared to 40 μm PTS. Overall, these results identify a pore size dependent modulation of circulating monocytes and implicates MARCO expression as a defining subset of monocytes that appears to be responsible for regulating a pro-healing host response.
Keywords: biomaterial scaffolds, cellular morphology, immune polarization, macrophages, monocytes
1 |. INTRODUCTION
Porous precision-templated scaffolds (PTS) are three-dimensional biomaterial constructs in which pore size and pore interconnects are precisely tunable and uniform in size throughout the scaffold (Hady et al., 2020; Madden et al., 2010; Marshall & Ratner, 2005; Sussman et al., 2014). Regardless of the polymer used in construction, PTS with uniform pores between 30 and 40 μm have shown enhanced healing characteristics by decreasing fibrosis and increasing the vascular host response (Madden et al., 2010; Sussman et al., 2014). In contrast, PTS with smaller or larger pores undergo a prototypical foreign body response (FBR) and chronic inflammation (Sussman et al., 2014). Previous studies have demonstrated that macrophage phenotype is a key mediator of the PTS host immune response through a pore size dependent immunomodulation of macrophages (Madden et al., 2010; Sussman et al., 2014). Despite studies that have characterized the role of macrophages in the PTS host immune response, the mechanisms that gives rise and sustains the infiltration of macrophages to implanted PTS remains unclear.
Previously, monocytes were thought just to be circulating pre-cursors to tissue macrophages. However, in response to injury or biomaterial implantation, circulating monocytes quickly extravasate out of peripheral blood and into tissues where they are essential in the initiation, coordination, and resolution of inflammation (Crane et al., 2014; Rahman et al., 2017; Soehnlein & Lindbom, 2010). As part of the FBR, host proteins are spontaneously adsorbed onto the surface of biomaterials followed by integrin binding and cytoskeleton remodeling of monocytes to cover the foreign material (Anderson et al., 2008). Despite the growing literature investigating the monocyte response to biomaterial implants, monocytes have not been considered involved in the pro-healing response within 40 μm PTS. However, previous literature has found that biomaterial topography alters pro-inflammatory cytokine secretion and gene transcription in monocytes (Bota et al., 2010), which may contribute to the unique healing phenomenon observed in 40 μm PTS.
Of the monocytes involved in wound healing, two distinct monocyte subsets have been identified. Murine monocytes expressing high levels of lymphocyte antigen 6C (Ly6Chigh; characterized by the production of IL-1β, IL-6, tumor necrosis factor alpha [TNFα], and matrix metalloproteinases) are highly motile, phagocytotic, and promote a pro-inflammatory host response (Ginhoux & Jung, 2014; Italiani & Boraschi, 2014; Kimball et al., 2018). Conversely, Ly6Clow monocytes (characterized by the production of IL-10, TGF-β, and vascular endothelial growth factor) are implicated in a reparative role and readily patrol the endothelial lumen (Ginhoux & Jung, 2014; Italiani & Boraschi, 2014; Olingy et al., 2017). Within the monocyte subsets, monocytes with a high expression of scavenger receptors have been shown to contribute to overall wound healing and exert a regulatory role in toll-like receptor induced inflammatory responses (Kissick et al., 2014; Komai et al., 2017). Specifically, macrophage receptor with collagenous structure (MARCO), an innate activation marker of macrophages, has been implicated in mediating the phagocytosis of unopsonized particles, bacteria, and viruses (Palecanda et al., 1999). However, the role of MARCO as a positive or negative regulator of the inflammatory response remains uncharacterized within the context of PTS healing.
As evident from the current monocyte and macrophage classification, monocytes are highly versatile and heterogeneous effector cells (Das et al., 2015; Lauvau et al., 2014). They display a functional array of phenotypes from classically inflammatory to anti-inflammatory, as well as pro-fibrotic to pro-angiogenic. The plasticity of the monocyte-macrophage lineage allows these cells to modify both phenotype and functional response based on dynamic changes in microenvironment. However, these characterizations have relied heavily on the outdated “M1/M2” classification concept based on in vitro conditions. This makes the current phenotyping paradigm obsolete in unique microenvironments such as within the context of in vivo biomaterial-mediated tissue regeneration. A growing body of evidence has shown that in vivo biomaterial scaffold vascularization requires a mixed phenotype of both a pro-inflammatory and a pro-wound healing macrophage response (Agrawal et al., 2012; Spiller et al., 2014). Such findings show the ambiguity of using an “either/or M1/M2” polarization scheme and demonstrates the necessity for a holistic evaluation of monocyte and macrophage phenotype within PTS.
Here, we employ a multifaceted phenotyping approach that quantifies the role of monocytes in the enhanced healing observed only in 40 μm PTS implants. We hypothesize that a pore size dependent recruitment and infiltration of circulating monocytes influences the regenerative pro-healing effect observed only in 40 μm PTS. We use a double transgenic myeloid-labeled mouse model to describe the pore size dependent recruitment of circulating monocyte subsets to subcutaneously implanted PTS. Additionally, we identify MARCO expression within 40 μm PTS and characterize the PTS resident cells’ transcriptome, secreted cytokine profiles, and cellular morphology as a function of pore size. Overall, these findings quantify the effector function of circulating monocytes in porous PTS and provide insight into how these factors influence macrophage phenotype and morphology in regulating scaffold outcome.
2 |. MATERIALS AND METHODS
2.1 |. Poly (2-hydroxyethyl methacrylate) scaffold synthesis
Porous templated scaffolds were fabricated using a sphere-templating method to create uniform sized pores and interconnects (Hady et al., 2020; Madden et al., 2010; Marshall & Ratner, 2005; Ratner & Marshall, 2008; Sussman et al., 2014; Patent US2008/0075752A1, Figure S1). Briefly, poly (methyl methacrylate) (PMMA) spherical beads of 40 μm (Dynoseeds TA-40) and 100 μm (Dynoseeds TA-100) in diameter were purchased from Microbeads AS (Norway). A U-shaped mold was created by sandwiching 0.8 mm thick Teflon tape strips (5 mm wide) between two 75 × 25 × 1 mm glass slides. Beads of desired size, 40 or 100 μm, were poured into the mold and agitated for 2 h in a water bath sonicator to achieve close-packing. Molds filled with beads were then placed in an oven to sinter the beads at their contact points, forming a 3-D interconnected structure. Oven sintering temperatures and times were optimized to achieve an interconnect diameter to pore diameter ratio between 25% and 33% by heating at 177°C for 25.5 h (40 μm) or 169°C for 26 h (100 μm). Poly (2-hydroxyethyl methacrylate) precursor solution was formulated by mixing 5.1 ml 2-hydroxyethyl methacrylate (HEMA; Polysciences), 0.24 ml tetraethyleneglycol dimethacrylate (TEGDMA) (Polysciences), 1.5 ml ethylene glycol, 2.1 ml deionized water, and 20 mg 2,2-dimethoxy-1,2-diphenylethan-1-one (Irgacure 651; BASF, Freeport, LA). The precursor solution was infiltrated into the mold with the sphere template and degassed under vacuum. Broad-spectrum UV was used to cross-link the HEMA monomer solution into pHEMA via free-radical polymerization. The sphere template was removed from the glass mold and immersed in acetone to dissolve the PMMA beads. PTS were sterilized in 70% ethanol and rehydrated in ultrapure water. Scaffolds were cut into 6 mm round, 0.8 mm thick disks using a biopsy punch and lyophilized for storage. Before use, scaffolds were rehydrated in 1x phosphate buffered saline (PBS).
2.2 |. PTS characterization
Scanning electron microscopy (SEM) was performed on lyophilized samples to confirm that the scaffold interconnect-to-pore ratio was tightly controlled between 25% and 33%. Samples were sputter coated in three intervals of 60 s with gold/palladium (NanoImages MCM-200) and then imaged on a scanning electron microscope (NanoImages SNE-3200M) at 5 kV with a 1 mm working distance under high vacuum. A minimum of three top view and three cross-sectional images were taken of both the empty sphere template and the resultant pHEMA scaffolds from each batch. ImageJ software (National Institutes of Health) was used to measure pore size and the scaffold interconnects from the SEM images.
2.3 |. Double transgenic mouse model for myeloid characterization
All animal experiments were conducted according to protocols approved by the University of Washington Institutional Animal Care and Use Committee (Animal Welfare Assurance #A3464–01). Mice were housed in a pathogen-free facility at the University of Washington, Department of Comparative Medicine. C57BL/6J, G6.129P2-Lyz2tm1(cre)lfo/J (LysM-Cre), and B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,−EGFP)Luo/J (mTmG) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). LysM-Cre+/0:mT/mG+/0 double transgenic mice were generated by crossing LysM-Cre mice with mTmG mice, described previously (Hady et al., 2020). Briefly, mTmG mice possess a membrane-bound tdTomato region surrounded by LoxP sites with a Rosa26 constitutive promoter downstream followed by an enhanced Green Fluorescent Protein (EGFP) cassette. Upon LysM-Cre recombination, the floxed stuffer sequence is deleted allowing only cells that express LysM to express EGFP and all other cells types to express tdTomato. Genotyping was performed from ear clippings by polymerase chain reaction (PCR) using the appropriate primers, Table S1.
2.4 |. Monocyte depletion
For the depletion of circulating monocytes, mice were intravenously injected through the retro-orbital venous sinus with 0.1 ml clodronate liposomes (Liposoma BV, Netherlands) per 10 g of body weight, 24 h prior to surgery. Mice were monitored and weighed daily to ensure no adverse effects and no more than 10% of body weight was lost during treatment. Monocyte depletion was confirmed by cardiac puncture blood draw 1, 2, 4, 6, and 8 days post-injection followed by flow cytometry analysis.
2.5 |. Scaffold surgery/harvest
Precision-templated scaffolds implantations were performed in 6–8 week old male or female LysM-Cre+/0:mT/mG+/0 mice. Briefly, mice were anaesthetized with 2% isoflurane by nose cone, and the dorso-lumbar skin was shaved and sterilized with three alternating betadine and 70% ethanol washes. A small 1 cm incision was made along the dorsal midline and two subcutaneous pockets were created, one on each side of the incision. One pHEMA scaffold (6 mm diameter, 0.8 mm thick) was implanted subcutaneously into each pocket, and the incision was closed with wound clips. At 3, 5, and 7 days after implantation surgery, scaffolds were excised, rinsed, then cultured for 48 h in complete scaffold medium (Roswell Park Memorial Institute (RPMI) 1640 supplemented with 2 mM L-glutamine, 10% fetal bovine serum (FBS), and 1x penicillin-streptomycin) at 37°C and 5% CO2. Next, cells were isolated by centrifugation, and both the cells and conditioned medium were collected for further analysis.
2.6 |. Flow cytometry
Peripheral blood was collected via cardiac puncture using heparinized syringes and diluted 1:1 with PBS. Diluted blood was layered over an equal volume of Ficoll-Plaque Plus (GE Healthcare, Sweden) and the peripheral blood mononuclear cells (PBMCs) were collected after centrifugation without brake. PBMCs were hemolyzed and resuspended in staining buffer (1x PBS/1% horse serum). Additionally, scaffold resident cells were washed and resuspended in staining buffer prior to antibody staining. Nonspecific binding to Fc receptors was blocked by incubating with an anti-mouse CD16/32 antibody (BD Biosciences, 1:500) at 4°C for 5 min followed by staining with fluorochrome-conjugated antibodies (see Table S2 for antibodies used in monocyte and macrophage identification). Cells were surface stained for 30 min on ice, washed with staining buffer, and resuspended in 200 μL staining buffer containing 1 μM propidium iodide (Invitrogen). Samples were analyzed on a BD FACSCanto™ flow cytometer (BD Biosciences; see Table S3 for instrument configuration) and the data analyzed using FlowJo software (TreeStar). Cells were gated using unstained negative controls.
2.7 |. Gene expression analysis
RNA was extracted from isolated scaffold resident cells using a RNeasy (Qiagen) column-based isolation as per the manufacturer’s instructions. cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. Real-time quantitative PCR was performed in a 384-well plate on an Applied Biosystems 7900HT Real-Time PCR system using TaqMan Universal Master Mix II (Applied Bio-systems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control and all plates contained a “no template” control. Relative expression of traditional M1-like genes, CD86, interleukin 1 beta (IL-1β), inducible nitric oxide synthase (iNOS), and TNFα; and traditional M2-like genes arginase 1 (ARG1), CD163, mannose receptor (CD206), found in inflammatory zone (FIZZ1), interleukin 10 (IL-10), transforming growth factor beta (TGF-β1), and chitinase-like 3 (YM1), were determined by RT-qPCR. TaqMan Assay IDs for individual genes are shown in Table S4. Relative fold change analysis was carried out by normalizing against the endogenous control using the 2−ΔCt method where −ΔCt is Cttarget − Ctendogenous control.
Semi-quantitative PCR was performed using DreamTaq Green PCR Master Mix (Thermo Scientific) as per the manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase was used as the endogenous control. Polymerase chain reaction products were run on a 1.5% agarose gel and Image Lab Software (Bio-Rad) was used for densitometry analysis. Fold change analysis was carried out by normalizing against the endogenous control (masstarget/massendogenous control) and gene expression was compared relative to 40 μm PTS. Semi-quantitative PCR primers are shown in Table S5. Enrichr (http://amp.pharm.msm.edu/Enrichr/) was used on differentially expressed genes for gene ontology (GO) analysis.
2.8 |. Cytokine quantification
Precision-templated scaffolds explanted from mice were cultured in complete scaffold medium for 48 h, then the cell-free conditioned medium was collected for cytokine analysis. Scaffold conditioned medium was tested by Sandwich ELISA for pro-inflammatory IL-1β (DuoSet, R&D Systems) and TNFα (DuoSet, R&D Systems); anti-inflammatory IL-10 (DuoSet, R&D Systems); and pleiotropic TGF-β (Invitrogen). ELISAs were performed according to manufacturer’s protocol. Absorbance readings were measured using a Multiskan FC plate reader (Thermo Fisher) at a wavelength of 450 nm. Cytokine concentration was determined based on an 8-point standard curve.
2.9 |. Tissue histology
Explanted scaffolds were cut in half, placed in a 0.7 × 0.7 cm disposable base mold, and immersed in optimum cutting temperature compound (Tissue-Tek Sakura). Slides were prepared from 5 μm thick tissue sections obtained by cryotome cutting. Trichrome staining (Abcam) was performed on slides according to manufacturer’s protocol using multi-tissue control slides as a positive control. Collagen was stained blue, cytoplasm was stained red, and nuclei was stained black. Stained slides were cleared using Histo-Clear II (National Diagnostics) and mounted using Omnimount (National Diagnostics) with a #1.5 coverslip. A minimum of five field of views were captured for each scaffold.
2.10 |. Explanted scaffold SEM imaging
Explanted scaffolds were fixed in a solution of 4% paraformaldehyde and 1% glutaraldehyde (Electron Microscopy Sciences) in PBS. Next, scaffolds were washed three times each in PBS followed by ultrapure water prior to staining in a solution of 0.2% osmium tetroxide (Electron Microscopy Sciences). Following staining, samples were washed three times in ultrapure water and incrementally dehydrated in ethanol (50%, 70%, 80%, 90%, and absolute ethanol). Scaffolds were cryofractured and critical point dried (Tousimis autosamdri 8780) in carbon dioxide. Samples were sputter coated in three intervals of 60 s with gold/palladium (NanoImages MCM-200) and then imaged on a scanning electron microscope (NanoImages SNE-3200M) at 5 kV with a 1 mm working distance under high vacuum. Scaffolds with 40 μm pores were imaged at 3000× magnification and 100 μm pore scaffolds were imaged at 2000× magnification.
2.11 |. Statistical analysis
Data are presented as mean standard error of mean ± Statistical analysis was performed in R (version 4.0.2) using the fisheries stock assessment, ggplot2, multtest, PMCMRplus, and qqplotr packages. All sample groups were tested for normality using the Shapiro-Wilks test to determine whether data was non-parametric or parametric with α = 0.05. Quantile-quantile (q–q) plots were used to confirm and visualize normal distribution of data. For studies with parametric data and more than two sample groups, a one-way analysis of variance with Tukey’s multiple comparison test was used to determine statistical significance. Kruskal-Wallis followed by Dunn’s test of multiple comparisons with a two-stage Benjamini and Hochberg correction was performed to determine statistical significance for studies with non-parametric data and more than two sample groups. Unless otherwise noted, statistical significance was defined as a p-value less than or equal to 0.05. Statistics on relative fold change gene expression was performed on log normalized data. In boxplots, the box encompasses the first and third quartiles with a vertical line through the median. Whiskers denote the minimum and maximum data point excluding values 1.5 greater than the interquartile range (IQR). Values greater than 1.5 times the IQR were represented as points and were included in all statistical analysis.
3 |. RESULTS
3.1 |. PTS generate distinct in vivo circulating blood monocyte profiles dependent on PTS pore size
We have generated a double transgenic mouse line (LysM-Cre+/0: mT/mG+/0) where the myeloid lineage express membrane-bound EGFP (green MyeloidEGFP+) while all other non-myeloid cells and tissues express membrane sequestered tdTomato (red mT). This allows for the selective tracking of monocytes, mature macrophages, dendritic cells, and granulocytes but not lymphocytes or other cell lineages. Circulating monocytes, collected via cardiac puncture, were identified as MyeloidEGFP+SSClowCD14+ (controls: 1.3 ± 0.2% of leukocytes) and myeloid subsets characterized by levels of Ly6C expression (controls: 54.0 ± 3.3% Ly6Chigh, 11.3 ± 0.9% Ly6Cmid, and 34.1 ± 4.7% Ly6Clow). Controls are defined as blood collected from LysM-Cre+/0:mT/mG+/0 mice not implanted with PTS. The dynamics of circulating monocytes and subsequent subsets were tracked at 3, 5, and 7 days after subcutaneous PTS implantation (Figure 1a,b). Relative to controls, both the 40 and 100 μm pore PTS doubled the total percentage of circulating blood monocytes at Day 3 (3.5 ± 0.4% [p = 0.039] and 3.4 ± 0.4% [p = 0.036], respectively, Figure 1c). Interestingly, implantation of the 100 μm PTS showed a significant decrease in inflammatory Ly6Chigh monocytes (35.6 ± 2.8%) compared to 40 μm PTS at Day 3 (61.2 ± 8.3%, p = 0.011, Figure 1d). At Day 5, a significantly higher amount of circulating CD14+ monocytes were found in the blood of mice receiving 100 μm PTS (4.8 ± 1.0%) compared to the blood of mice receiving 40 μm PTS (2.4 ± 0.1%, p = 0.031, Figure 1e). At Day 7, the trend is reversed (2.4 ± 0.3% and 3.6 ± 0.4%, respectively, Figure 1f). No differences were observed in the blood Ly6C subset expression at Day 5 or 7 in the 40 and 100 μm PTS. Together, these findings demonstrate a differential response in the expansion and phenotype of circulating monocytes as a function of PTS pore size.
FIGURE 1.
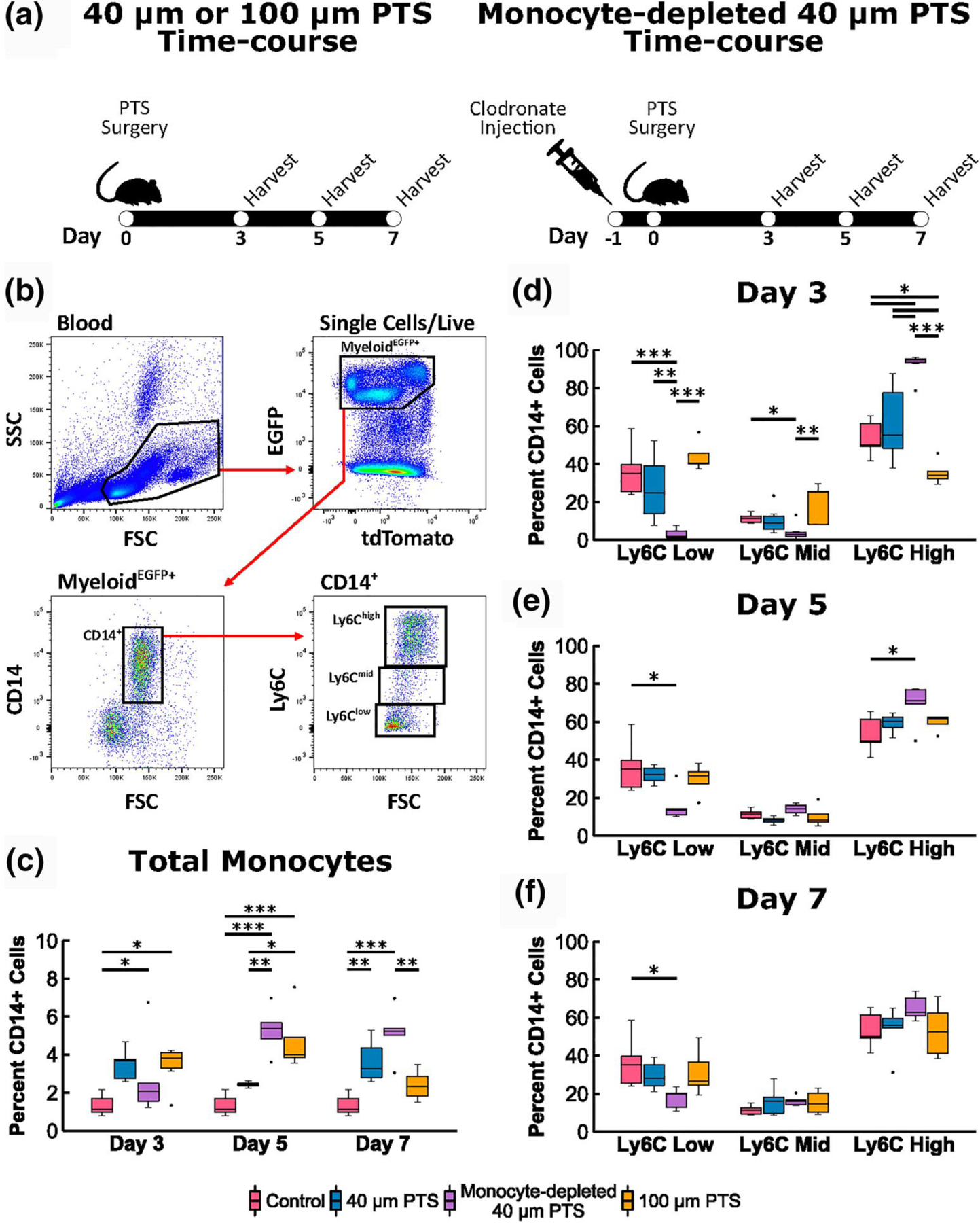
Flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) recovered from LysM-Cre+/0:mT/mG+/0 mice implanted subcutaneously with 40 μm precision-templated scaffolds (PTS) or 100 μm PTS, and from 40 μm PTS implanted subcutaneously in monocyte-depleted LysM-Cre+/0:mT/mG+/0 mice. (a) Schematic of scaffold surgery and harvest time-course. Clodronate liposomes were intravenously injected 24 h prior to scaffold surgery to deplete circulating monocytes. (b) PBMC were gated on SSC lowMyeloideGFP+CD14+ expression, and each monocyte subset was characterized as Ly6Chigh, Ly6Cmid, and Ly6Clow. Cell aggregates, doublets, and dead cells were excluded from analysis. (c) Total percentage of CD14+ monocytes from PBMCs. (d–f) Ly6C expression of CD14+ monocytes at (d) Day 3, (e) Day 5, and (f) Day 7 post-scaffold implantation. * denotes p < 0.05, ** denotes p < 0.01, and *** denotes p < 0.001; n = 4–7 animals per group
Due to the distinct circulating monocyte profiles generated from the 40 μm pore and 100 μm pore PTS, a transient depletion of monocytes was performed to examine the impact an impaired monocyte recruitment would have on the pro-healing response observed only in the 40 μm PTS. Previous studies have demonstrated that monocyte depletion reduces tissue vascularization, increases foreign body capsule size, and impairs wound healing (Bank et al., 2017; Goren et al., 2009; Mirza et al., 2009), which are outcomes seen in all wild-type mice implanted with PTS of pores larger than 40 μm as part of the FBR (Sussman et al., 2014). Further impairment by implanting a 100 μm PTS in monocyte-depleted mice would not prove fruitful; therefore, only monocyte depletion studies involving pro-healing 40 μm PTS were investigated. Clodronate liposomes were intravenously administered 24 h prior to surgical implantation of 40 μm PTS resulting in the depletion of 87.9 ± 5.8% circulating blood monocyte (Figure S2a). Ly6Chigh monocytes were shown to be the first monocyte subset to repopulate two days after depletion (Figure S2b), and with the implantation of the 40 μm PTS in monocyte-depleted mice, corresponding trends were observed with a predominantly higher circulating Ly6Chigh monocyte population (92.4 ± 2.8%, p = 0.034) at Day 3 (Figure 1d). Despite the monocyte depletion, the 40 μm PTS-associated inflammation led to the quick and significantly higher monocyte expansion at Day 5 and 7 compared to controls (5.3 ± 0.5% [p < 0.001] and 5.1 ± 0.6% [p < 0.001], respectively; Figure 1c).
3.2 |. Depleting circulating monocytes alters PTS resident myeloid cell population in a PTS pore size dependent manner
Following the characterization of 40 and 100 μm pore PTS effects on circulating monocytes, the subsequent trafficking and recruitment of monocytes colonizing the PTS was investigated. Tissue monocytes and macrophages were identified based on the expression of MyeloidEGFP+ and the monocyte lineage was further discriminated by the expression of CD68+ and CD14+ (Figure 2a). Since cells obtained from tissues contain more heterogeneous cell populations than in blood, the pan monocyte/macrophage marker CD68 was added to the scaffold analysis. At Day 3, analysis of scaffold-resident cells in 40 μm PTS implanted in monocyte-depleted mice showed a significant decrease in both the total myeloid (p = 0.023, Figure 2b) and CD68+CD14+ cells (p = 0.006, Figure 2c) compared to the 40 μm PTS implanted in non-treated mice. This indicates that scaffold resident cells appear to be derived from circulating monocytes. Furthermore, Day 7 40 μm PTS contained a higher proportion of resident myeloid cells (71.6 ± 6.9%) relative to the 100 μm PTS (52.2 ± 3.9%, p = 0.019). This trend paralleled the proportion of CD68+CD14+ cells resident within the 40 μm PTS at Day 7 compared to the 100 μm PTS, though not statistically significant (p = 0.063). Further analysis of monocyte subsets revealed a primarily unimodal distribution of Ly6C expression that differed from the distinct circulating monocyte populations found in the bloodstream. Based on overall Ly6C expression, analysis revealed scaffold resident cells expressed minimal amounts of Ly6C, primarily within the Ly6Clow and Ly6Cmid range. Comparison of the Ly6C median fluorescent intensity (MFI) showed no significant difference between scaffold groups at Day 3, 5, or 7 (Figure 2d). Collectively, these results further support the importance of the myeloid lineage in generating a pro-healing response in the 40 μm PTS.
FIGURE 2.
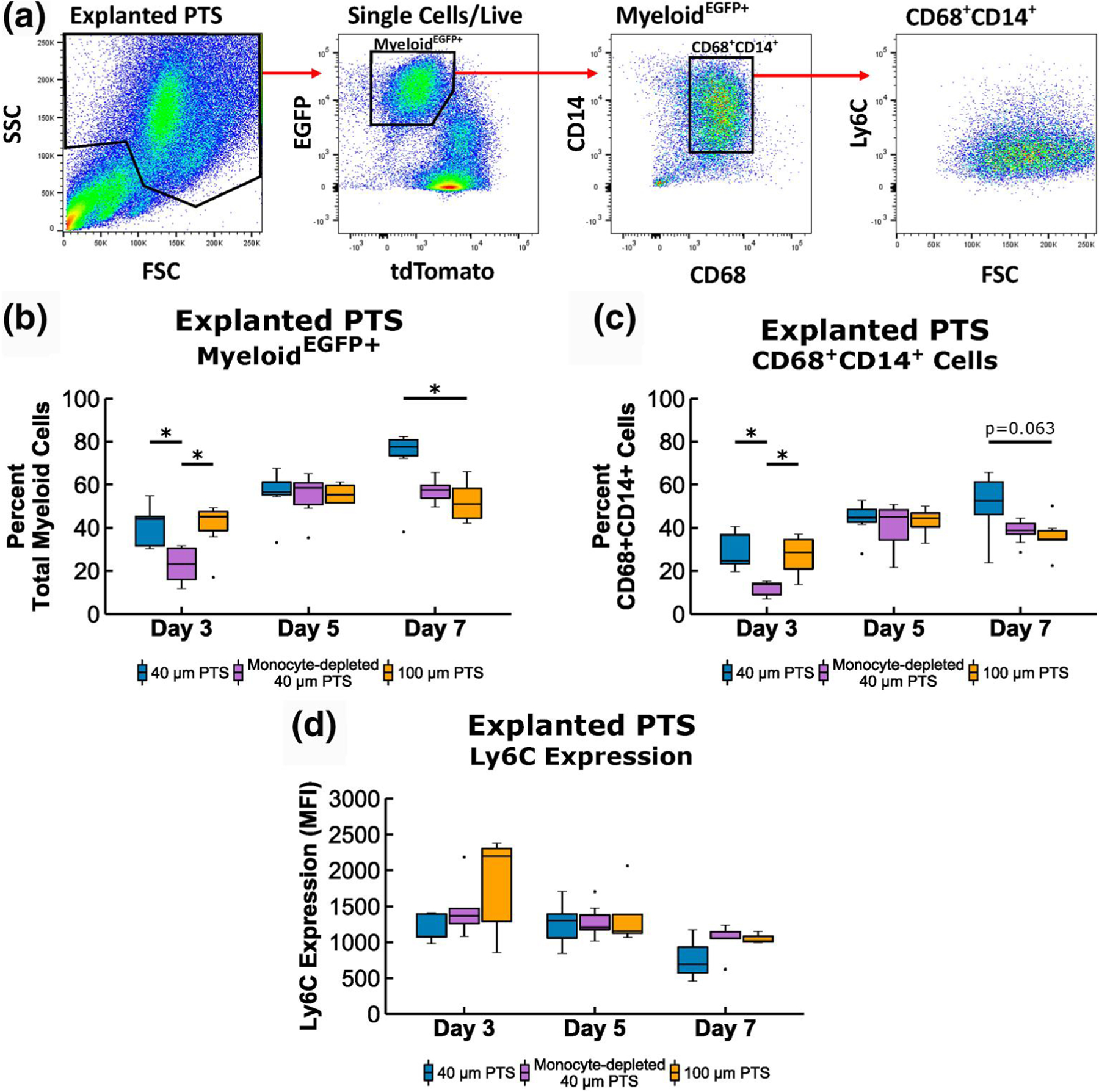
Flow cytometric analysis of scaffold resident cells recovered from 40 μm precision-templated scaffolds (PTS) or 100 μm PTS implanted subcutaneously in LysM-Cre+/0:mT/mG+/0 mice, and from 40 μm PTS implanted subcutaneously in monocyte-depleted LysM-Cre+/0:mT/mG+/0 mice. (a) Explanted scaffold-resident cells were gated on MyeloideGFP+ expression, and the monocyte linage was further discriminated by CD68+CD14+ expression. Cell aggregates, doublets, and dead cells were excluded from analysis. (b) Percentage of total myeloid cells from explanted PTS. (c) Percentage of MyeloideGFP+ CD68+CD14+ cells from explanted PTS. (d) median fluorescent intensity (MFI) of Ly6C expression from explanted PTS. * denotes p < 0.05 and ** denotes p < 0.01; n = 4–8 animals per group
3.3 |. Gene and cytokine expression analysis of scaffold-resident monocytes/macrophages
Scaffold resident cells were assessed for gene expression of MARCO and other markers to identify unique cellular subsets within 40 μm PTS at Day 3, 5, and 7 (Figures 3 and 4). Based on differentially regulated genes, gene ontology (GO) analysis demonstrated processes related to toll-like receptor signaling pathway, phagocytosis, and scavenger receptor activity (Figure 3c). In conjunction with transcriptomics, conditioned medium analysis was also performed to determine cytokine secretion profiles. Pro-inflammatory IL-1β and TNFα expression (generally associated with M1-like macrophages and Ly6Chigh monocytes; Figure 5), as well as anti-inflammatory IL-10 and pleiotropic TGF-β expression (generally associated with M2-like macrophages and Ly6Clow monocytes), were measured. However, no discernable levels of IL-10 were observed, and no statistical difference was observed in TGF-β expression at any of the time points.
FIGURE 3.
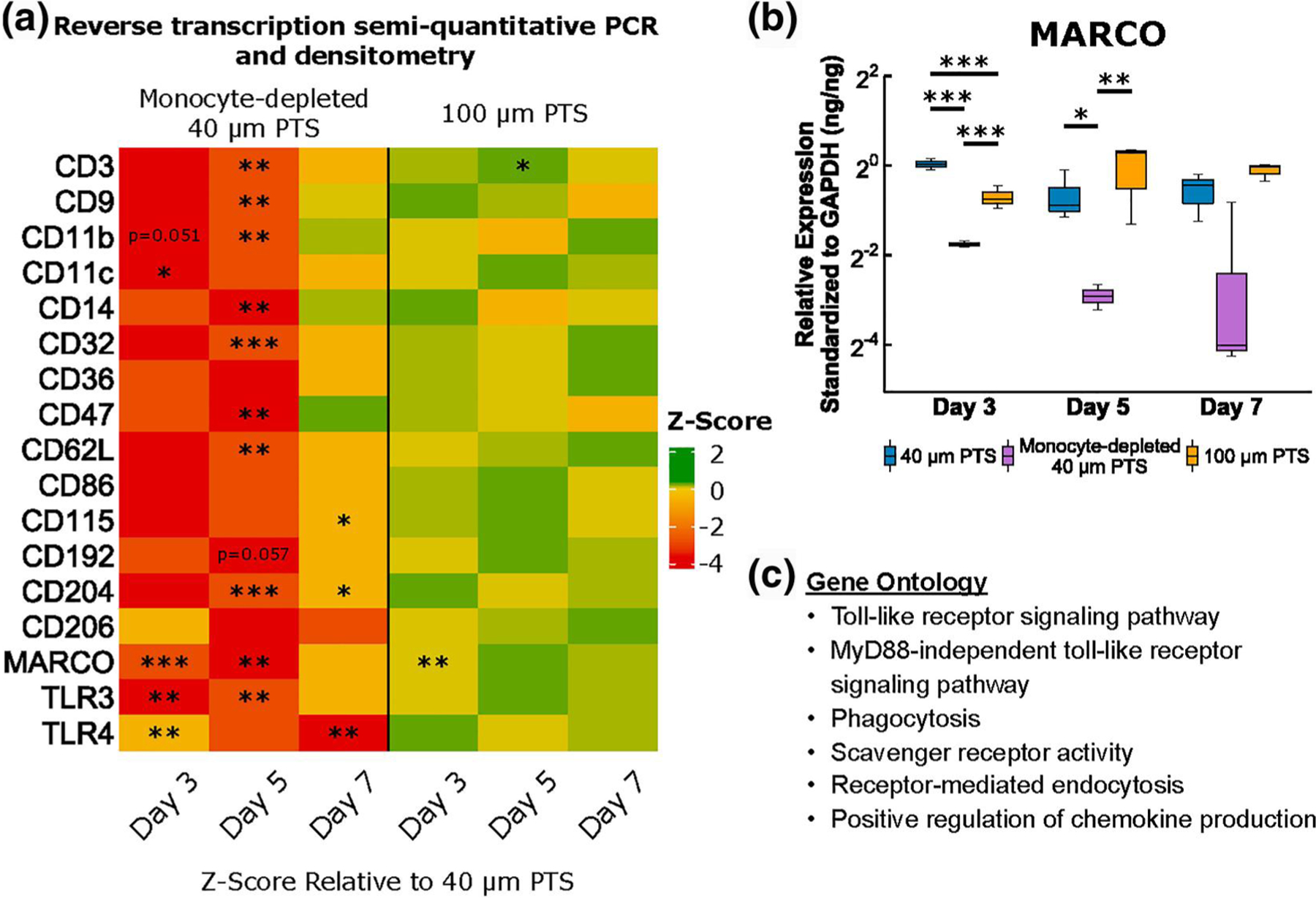
Surface marker transcriptome gene profiling on cells resident in 40 μm precision-templated scaffolds (PTS) or 100 μm PTS explanted from untreated LysM-Cre+/0:mT/mG+/0 mice, and cells resident in 40 μm PTS explanted from monocyte-depleted LysM-Cre+/0: mT/mG+/0 mice. (a) Average fold change of surface marker transcriptome relative to 40 μm PTS and standardized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Green indicates gene upregulation and red indicates gene downregulation relative to 40 μm PTS. (b) Relative expression of macrophage receptor with collagenous structure (MARCO) transcription standardized to GAPDH. (c) gene ontology (GO) analysis based on differentially regulated genes. * denotes p < 0.05, ** denotes p < 0.01, and *** denotes p < 0.001, n = 3 animals per group
FIGURE 4.
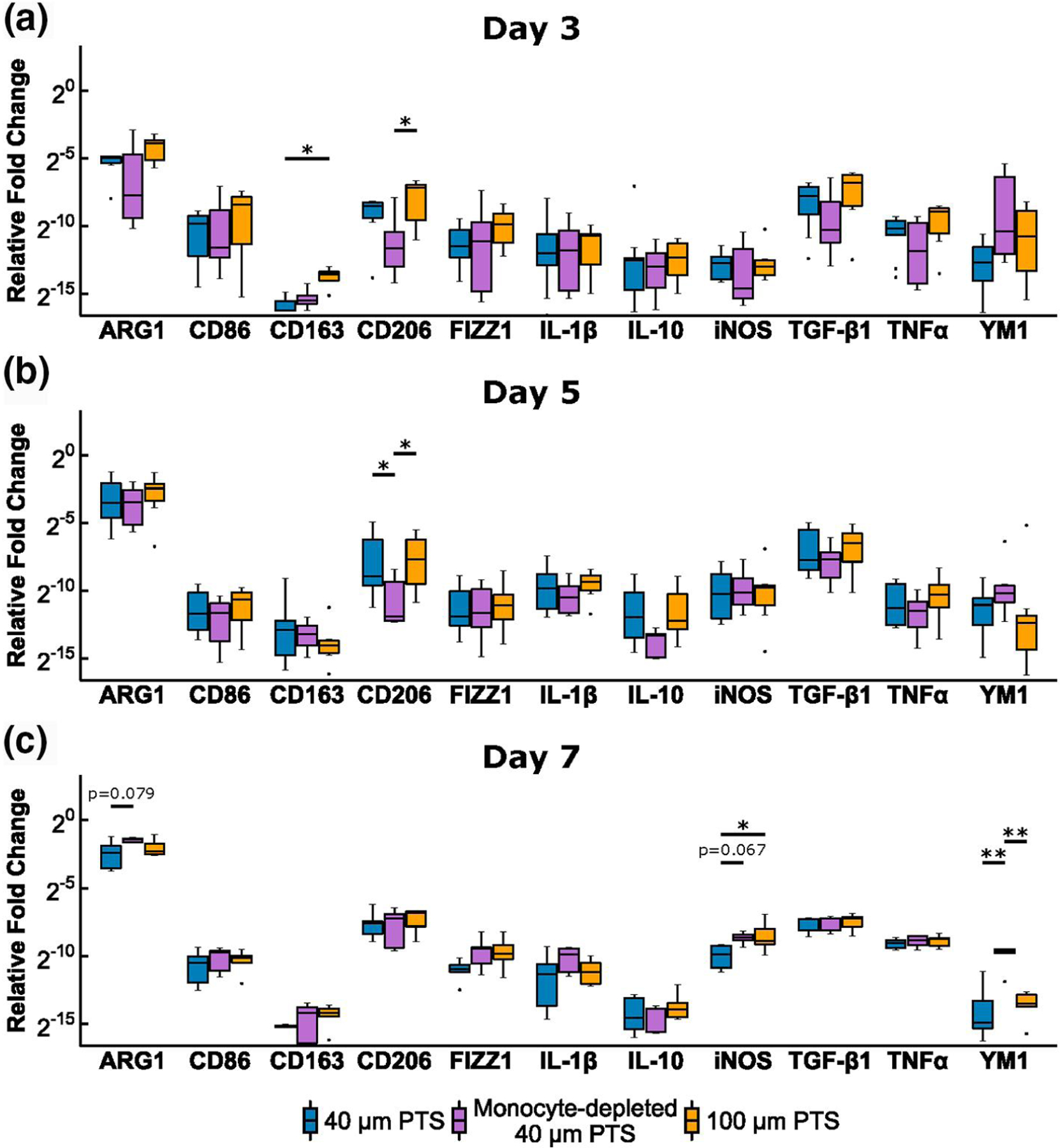
Polarization transcriptome gene profiling on cells resident in 40 μm precision-templated scaffolds (PTS) or 100 μm PTS explanted from untreated LysM-Cre+/0:mT/mG+/0 mice, and cells resident in 40 μm PTS explanted from monocyte-depleted LysM-Cre+/0: mT/mG+/0 mice. Scaffold explantation was performed at (a) Day 3, (b) Day 5, and (c) Day 7 post-implantation. * denotes p < 0.05 and **denotes p < 0.01, n = 5–10 animals per group
FIGURE 5.
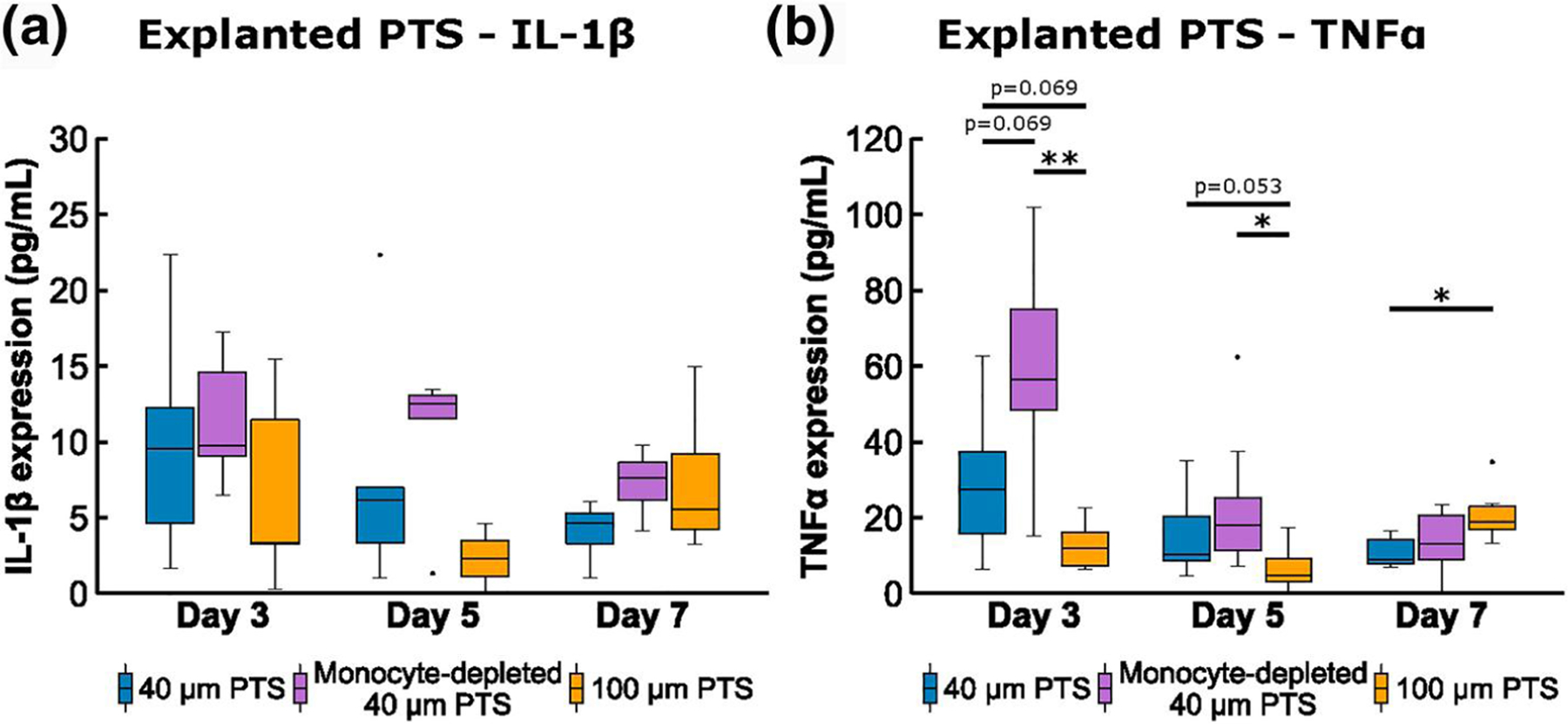
Conditioned medium analysis of pro-inflammatory (a) IL-1β, and (b) tumor necrosis factor alpha (TNFα), from cells resident in 40 μm precision-templated scaffolds (PTS) or 100 μm PTS explanted from untreated LysM-Cre+/0:mT/mG+/0 mice, and cells resident in 40 μm PTS explanted from monocyte-depleted LysM-Cre+/0:mT/mG+/0 mice. * denotes p < 0.05 and ** denotes p < 0.01; n = 8–11 animals per group
3.3.1 |. Day 3
Surface marker transcriptomics revealed that MARCO was significantly upregulated in 40 μm PTS in untreated mice relative to both 40 μm PTS in monocyte-depleted mice (p < 0.001) and 100 μm PTS in untreated mice (p = 0.002, Figure 3a and b). Additionally, transcription of Toll-like receptors, TLR3 and TLR4 were downregulated in 40 μm PTS in monocyte-depleted mice relative to 40 μm PTS in untreated mice (p = 0.007 and p = 0.005, respectively, Figure 3a). Given the higher expression of MARCO observed only within 40 μm PTS, these results suggest the presence of a unique subset of MARCO expressing monocytes involved in TLR activation within 40 μm PTS but not in 100 μm PTS. Additionally, early cellular infiltration to the PTS showed that 100 μm PTS upregulated CD163 relative to 40 μm PTS (p = 0.041, Figure 4a), indicative of a polarization toward a M2-like phenotype.
Conditioned medium analysis demonstrated an insignificant (p = 0.069) increase in TNFα expression from cells resident in 40 μm PTS (29.7 ± 6.9 pg/ml) versus cells resident in 100 μm PTS extracted from untreated mice (12.7 ± 2.1 pg/ml). Additionally, cells resident in 40 μm PTS implanted in monocyte-depleted mice induced a higher secretion of TNFα (61.1 ± 9.9 pg/ml, p = 0.069, Figure 5a). Similar trends were observed in IL-1β expression for 40 μm PTS in untreated mice (9.7 ± 2.4 pg/ml), 100 μm PTS in untreated mice (6.8 ± 2.9 pg/ml), and 40 μm PTS in monocyte-depleted mice (11.4 ± 1.2 pg/ml), suggesting a pro-inflammatory 40 μm PTS microenvironment.
3.3.2 |. Day 5
Similar to Day 3, MARCO transcription was significantly upregulated in 40 μm PTS in untreated mice compared to monocyte-depleted mice (p = 0.014; Figure 3a and b). However, the MARCO transcription between 40 μm PTS implanted in untreated mice and 100 μm PTS were similar. Surface marker transcription of CD3 was upregulated in 100 μm PTS (p = 0.048) yet downregulated in 40 μm PTS in monocyte-depleted mice (p = 0.001), relative to 40 μm PTS in untreated mice. Depletion of monocytes also resulted in the significant downregulation (p < 0.05) of CD9, CD11b, CD14, CD32, CD47, CD62L, CD204, and TLR3 in 40 μm PTS (Figure 3a). Cells resident in 40 μm PTS implanted in monocyte-depleted mice downregulated M2-like CD206 expression relative to 40 μm PTS implanted in untreated mice (p = 0.044, Figure 4b). Although insignificant (p = 0.053), TNFα expression in the conditioned medium at Day 5 remained elevated in 40 μm PTS (15.5 ± 4.0 pg/ml) versus TNFα expression from cells resident in 100 μm PTS (6.7 ± 2.3 pg/ml) extracted from untreated mice.
3.3.3 |. Day 7
No differences in surface marker transcription were observed due to pore size. However, 40 μm PTS in monocyte-depleted mice significantly downregulated expression of CD115 (p = 0.012), CD204 (p = 0.013), and TLR4 (p = 0.001) compared to 40 μm PTS in untreated mice (Figure 3a). Analysis of polarization marker transcriptomics showed that the 100 μm PTS resident cells shifted to a pro-inflammatory phenotype, denoted by a higher expression of iNOS (p = 0.038) compared to cells resident in 40 μm PTS implanted in untreated mice (Figure 4c). Additionally, cells resident in 40 μm PTS implanted in monocyte-depleted mice transcribed significantly more YM1 (p = 0.002) along with a statistically insignificant increase in ARG1 (p = 0.079) and iNOS (p = 0.067) transcription over cells resident in 40 μm PTS implanted in untreated mice, representing a mixed phenotype of conventional M1-like and M2-like genes.
As a function of pore size, cells inhabiting 100 μm PTS secreted nearly double the amount of TNFα (21.1 ± 3.1 pg/ml) than cells resident to 40 μm PTS (10.6 ± 1.3 pg/ml, p = 0.030, Figure 5b). IL-1β expression profiles showed similar corresponding trends to TNFα with moderately higher IL-1β expression for cells inhabiting 100 μm PTS explanted from untreated mice (7.3 ± 1.8 pg/ml, Figure 5a). Cells inhabiting 40 μm PTS explanted from monocyte-depleted mice (7.3 ± 0.8 pg/ml) also showed increased IL-1β expression versus cells inhabiting 40 μm PTS (4.2 ± 0.6 pg/ml) explanted from untreated mice but were not statistically significant (p = 0.097). Taken together, these results demonstrate the progression and pore size immunomodulation of the host immune response in the context of PTS healing.
3.4 |. PTS demonstrate morphological changes as a function of monocyte depletion and pore size
The morphological and cellular structure of one-week implanted PTS were compared for 40 μm PTS in untreated mice, 40 μm PTS in monocyte-depleted mice, and 100 μm PTS in untreated mice by trichrome staining. Scaffolds with a uniform 40 μm pore size (Figure 6a) exhibited an atypical FBR with individual cells compartmentalized within pores and minimal evidence of fibrotic tissue formation. In comparison, the 40 μm PTS implanted in monocyte-depleted mice (Figure 6b) contained a distinctly higher degree of cellular fusion that spread across multiple adjacent pores and collagen staining at the implant surface. Similarly, the 100 μm PTS (Figure 6c) exhibited a thick collagen-rich foreign body capsule at the implant interface, extensive pore-spanning cellular networks, and evidence of collagen deposition on the pore surface. These results substantiate the role of recruited monocytes in influencing cellular fusion and collagen deposition within 40 μm PTS and regulating tissue resolution by modulating the FBR.
FIGURE 6.
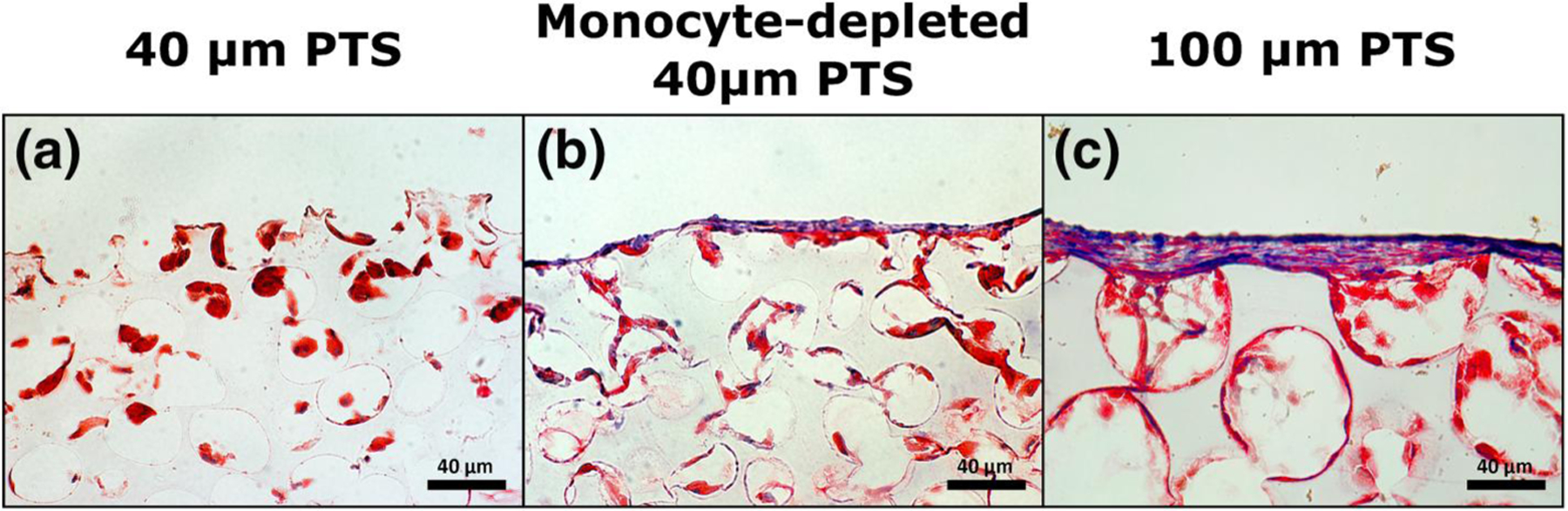
Representative trichrome stain of pHEMA scaffolds (40× magnification) 7 days post-implantation. Collagen is stained blue, cellular cytoplasm is stained red, and nuclei are stained black. (a) 40 μm precision-templated scaffolds (PTS) in untreated mice, (b) 40 μm PTS in monocyte-depleted mice, and (c) 100 μm PTS in untreated mice
Additionally, high magnification SEM images were taken of in vivo scaffold resident cells to further characterize cellular morphology as a function of pore size and monocyte depletion. Scaffold resident cells within 40 μm pores (Figure 7a–c) appeared distinctly smaller in size and tightly bundled within pores. There was evidence of membrane ruffling and relatively less extracellular matrix (ECM) protein adhesion. Cells resident within 40 μm PTS implanted within monocyte-depleted mice (Figure 7d–f) featured a flat, fibrous structure, with minimal cell attachment to scaffolds walls. At Day 5 and 7, the scaffold contained large amounts of cellular debris and ECM protein deposits adherent to the pore surface. Moreover, the un-controlled fibrous cellular structure at Day 7 of the 40 μm PTS in monocyte-depleted mice resembled characteristics more distinctly apparent in the 100 μm PTS rather than the 40 μm PTS in untreated mice. Cells within the 100 μm PTS (Figure 7g–i) were larger in size and exhibit an elongated, “star-shaped” structure that stretched across whole pores. These cells also displayed increasing fibrous and mesh-like characteristics over time, which allowed for thin cellular connections to adjacent pores. Taken together, these observations further demonstrate a morphological pore size dependence in PTS and that monocytes appear to be responsible for the pro-healing response in 40 μm PTS.
FIGURE 7.
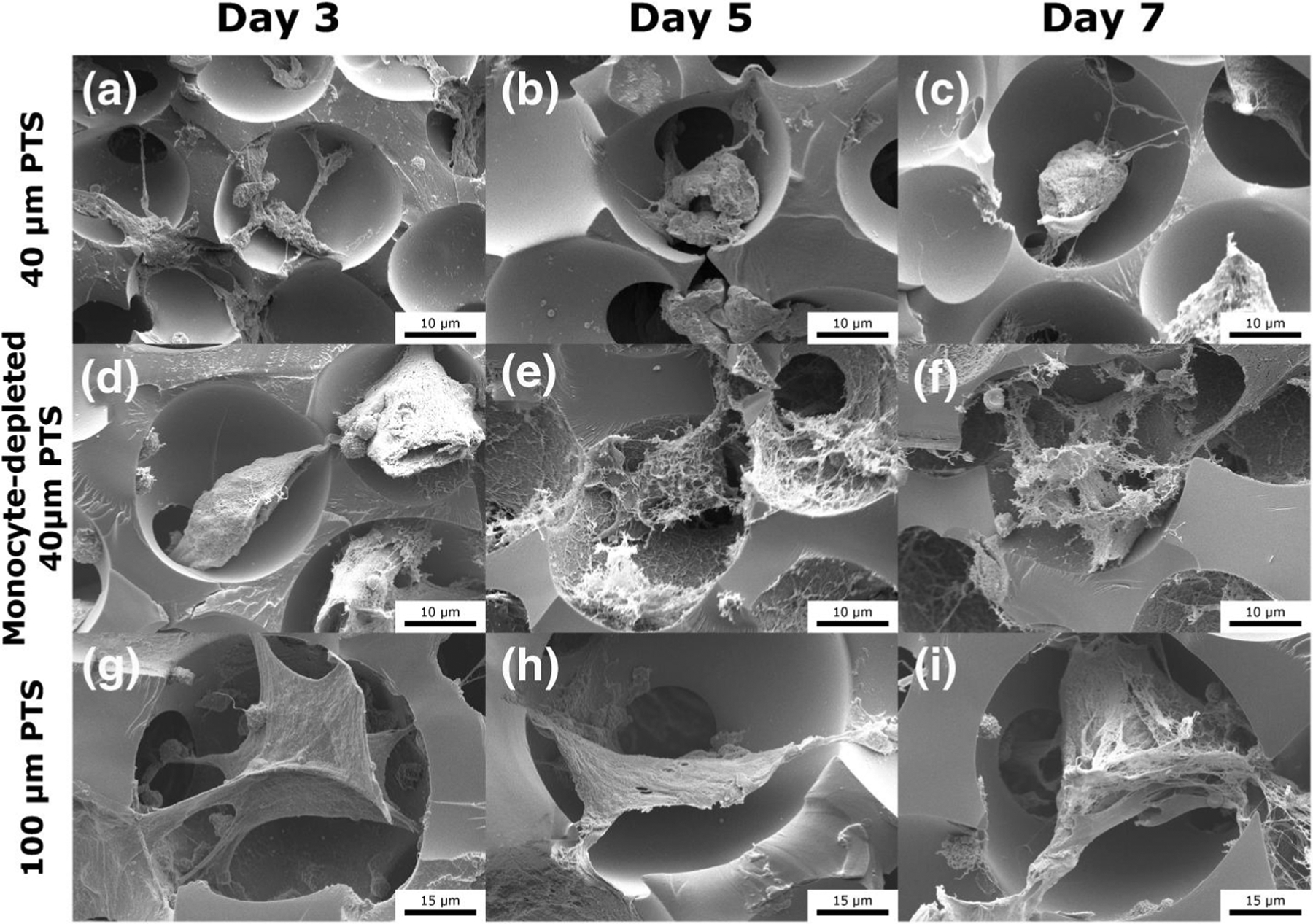
Scanning electron microscopy images of subcutaneously implanted scaffolds in LysM-Cre+/0:mT/mG+/0 mice at Day 3, Day 5, and Day 7 post-implantation. (a–c) 40 μm precision-templated scaffolds (PTS) in untreated mice, (d–f) 40 μm PTS in monocyte-depleted mice, and (g–i) 100 μm PTS in untreated mice
4 |. DISCUSSION
Monocytes are critical in initiating the early inflammatory response in wound healing by first promoting the initial clearance of invading pathogens, followed by the subsequent restoration and repair of tissue integrity. In situations where biomaterials are implanted, literature has reported that classical Ly6Chigh monocytes are the predominant infiltrating population followed by the later recruitment of Ly6Clow monocytes (Crane et al., 2014). Although both the 40 and 100 μm PTS increased the total number of circulating monocytes 3 days after surgery, there was a significant decrease in Ly6Chigh monocytes within 100 μm PTS. As Ly6Chigh monocytes are classified to be pro-inflammatory in nature (Kimball et al., 2018) and 100 μm PTS (non-healing) are associated with a canonical FBR, this suggests that Ly6Chigh monocytes are primarily infiltrating the 100 μm PTS due to the reduction of Ly6Chigh monocytes in circulation. Apart from the expansion of circulating monocytes in 40 μm PTS, the relative proportion of monocyte subtypes remained comparable to the non-scaffold control. Considering that PTS implantation involves a major surgery, the unaffected proportion of monocyte subtypes in 40 μm PTS suggests a quicker return to homeostasis and the return of damaged tissue to optimal function.
To investigate the role monocytes play in the pro-healing 40 μm PTS, clodronate liposomes were administered 24 h prior to 40 μm PTS implantation resulting in the highest monocyte depletion at the time of the scaffold surgery. Although this method provides a transient monocyte depletion, the typical monocyte immune response is severely impacted resulting in a significant delay to the typical monocyte kinetics infiltrating the PTS. By profiling the phenotype of 40 μm PTS implanted in monocyte-depleted mice versus 40 μm PTS implanted in non-treated mice, the role of in vivo monocytes in the host immune response to PTS can be determined. Despite the repopulation of immature monocytes in circulation with an amplified Ly6Chigh expression at Day 3, our results demonstrate that the initial infiltration of monocytes are essential in establishing the initial microenvironment and therefore, subsequent implant fate.
Analysis of scaffold resident cells at Day 3 from 40 μm PTS implanted in monocyte-depleted mice showed a decrease in both the total myeloid and CD68+CD14+ cells indicating that monocytes appear to be directly trafficked from circulation to subcutaneously implanted PTS. As intravenously injected clodronate liposomes cannot interact with cells outside of the vascular system (Gabizon & Papahadjopoulos, 1988), the majority of PTS recruited myeloid cells likely originate from circulating monocytes rather than through local tissue macrophages. Interestingly, 40 μm PTS contained more myeloid cells than 100 μm PTS at Day 7, demonstrating either a pore size dependent positive chemotaxis of myeloid cells or greater myeloid lineage proliferation. It is reasonable to suspect that CD14+ monocytes directly result in the greater myeloid infiltration within 40 μm PTS, as parallel blood analysis corroborates an expansion of circulating CD14+ monocytes at Day 7. Even though no other differences in myeloid infiltration or Ly6C subsets were observed between 40 and 100 μm PTS, it is important to note that both monocyte and macrophage phenotype are highly dependent on polarization stimuli within the microenvironment. Although increased myeloid infiltration in 40 μm PTS does not fully explain the pro-healing response, it further enforces the importance of the myeloid lineage in tissue healing and the necessity of gene and cytokine expression analysis to accurately phenotype in vivo scaffold resident macrophages.
Within the 40 μm PTS, a distinct population of MARCO expressing monocytes was identified that uniquely transcribed higher amounts of MARCO compared to 40 μm PTS in monocyte-depleted mice and 100 μm PTS in untreated mice. This suggests that a population of monocyte-derived MARCO effector cells are more prevalent in 40 μm PTS than 100 μm PTS. While MARCO is important for the innate sensing of bacterial, fungal, and viral pathogens, our study represents the first analysis demonstrating a unique sensing in biomaterial pore size by MARCO. As 40 μm PTS exhibit pro-healing properties, the findings of elevated MARCO transcription implicates a population of MARCO expressing monocytes as likely contributors in maintaining an optimal balance of tissue repair and regeneration within 40 μm PTS. Additionally, these findings identify MARCO as an important new target for further analysis in characterizing macrophage and biomaterial scaffold interactions.
A population of MARCO expressing monocytes in 40 μm PTS with tissue regenerative properties is further supported through gene expression of polarization markers. Studies have revealed MARCO expression as a negative regulator of inflammation and linked with an impaired transcription of iNOS (Xu et al., 2017). In 40 μm PTS, pro-inflammatory iNOS was downregulated at Day 7 compared to both 40 μm PTS implanted in monocyte-depleted mice and 100 μm PTS. Expression of iNOS is important in anti-microbial and anti-tumor activities and is associated with a classical pro-inflammatory macrophage activation. As 100 μm PTS tend toward chronic inflammation in the FBR, higher transcription of iNOS is expected and an indicating factor of unfavorable wound healing progression. Although 40 μm PTS in untreated mice displayed lower transcription of iNOS, the absence of monocytes resulted in higher iNOS transcription with levels comparable to 100 μm PTS. This demonstrates that MARCO monocytes are important in attenuating pro-inflammatory iNOS expression and further enforces MARCO expression as a defining subset of monocytes responsible for pro-healing in 40 μm PTS.
Within the scaffold microenvironment, cytokine analysis demonstrates that infiltrating monocytes appear to be important in regulating the secretion of pro-inflammatory cytokines. Although activated monocytes and macrophages are primarily responsible for the secretion of TNFα, the general trend shows an increase in both TNFα and IL-1β expression within the first week as a result of monocyte depletion in 40 μm PTS. Tumor necrosis factor alpha has been shown to suppress macrophage scavenger receptor function (van Lenten & Fogelman, 1992) and could contribute to the downregulation of MARCO in 40 μm PTS implanted in monocyte-depleted mice. However, in comparison to 100 μm PTS, data at Day 3 suggests that 40 μm PTS rather than 100 μm PTS may induce a stronger pro-inflammatory TNFα response. Although a strong initial inflammatory response could lead to chronic inflammation and a less favorable wound resolution, the initial inflammatory phase in wound healing is important for both tissue resolution and subsequent remodeling (Soehnlein & Lindbom, 2010). Nevertheless, 100 μm PTS significantly increased the secretion of TNFα at Day 7 and further supports previous long-term findings of 100 μm PTS tending toward chronic inflammation and a canonical FBR. Together, our data suggests the role of monocytes in regulating initial TNFα expression within 40 μm PTS, and the progression of 100 μm PTS toward a pro-inflammatory phenotype.
In response to scaffold implantation, monocytes are actively involved in returning tissue back to optimal conditions by repairing and regenerating damaged tissue. Although certain monocyte functions, such as MARCO expression, appear to contribute to the pro-healing effect observed in 40 μm PTS, other aspects appear to be essential to the overall wound healing process occurring within the PTS microenvironment. Both CD206 and YM1 were polarization markers that demonstrated significant changes in transcription from monocyte depletion yet remained similar regardless of pore size. At Day 3 and Day 5, 40 μm PTS in monocyte-depleted mice downregulated CD206 transcription compared to 40 and 100 μm PTS, and at Day 7, 40 μm PTS in monocyte-depleted mice significantly upregulated YM1 transcription. Traditionally, both CD206 and YM1 are associated with alternatively activated macrophages. As previous literature has shown that non-classical Ly6Clow monocytes preferentially give rise to CD206+ wound healing macrophages in soft tissue injuries (Olingy et al., 2017), this suggests that CD206+Ly6Clow monocytes mature to pro-healing macrophages in both PTS microenvironments. Additionally, Ikeda et al. have discovered a distinct subset of immunoregulatory YM1+Ly6Chigh monocytes that appear to mediate the recovery phase of tissue injury and could explain the upregulation of YM1 in monocyte-depleted mice due to the repopulation with immature Ly6Chigh monocytes (Ikeda et al., 2018). Although not responsible for the pore size mediated pro-healing phenomenon in PTS, these results further demonstrate the necessity of monocytes in the initial tissue repair within both scaffold microenvironments.
Morphologically, cellular structure is an important feature in immunological phenotyping as functional characteristics can be derived from these observations (McWhorter et al., 2015). In the context of biomaterial scaffolds, physical properties such as architecture (Madden et al., 2010), geometry (Almeida et al., 2014), and surface topography (Bota et al., 2010; Chen et al., 2010; Luu et al., 2015) can be specifically engineered to immunomodulate scaffold-resident cells. Therefore, these factors can elicit conformational changes and affect the behaviors in both the cellular and host response. Trichrome staining of porous scaffolds demonstrated higher amounts of cellular fusion and collagen deposition within the 100 μm PTS compared to 40 μm PTS, which corroborates previously reported findings (Sussman et al., 2014). Additionally, SEM imaging depicts 40 μm PTS resident cells as compact and tightly bundled within pores, demonstrating a spatial modulation of cell shape. Monocytes are known to exhibit high levels of scavenging activity, as such, 40 μm PTS in monocyte-depleted mice displayed a prominent increase in ECM protein deposits. Collectively, these results illustrate morphological changes as a function of PTS pore size and strongly implies that circulating monocytes are crucial in regulating host immune responses.
In this study, we take a multifaceted phenotyping approach by quantifying cellular recruitment, transcriptome, cytokine secretion, and the morphology of PTS resident cells as a function of pore size to elucidate the role of circulating monocytes on implant outcome. We demonstrate that implantation of 40 μm PTS results in the greater infiltration of myeloid cells and 100 μm PTS reduces the proportion of Ly6Chigh monocytes in circulation, suggesting an infiltration of pro-inflammatory Ly6Chigh monocytes to 100 μm PTS. Macrophage receptor with collagenous structure, an innate activation marker of macrophages, was found to be significantly upregulated in 40 μm PTS in untreated mice, demonstrating MARCO expression as a defining subset of monocytes responsible for pro-healing in 40 μm PTS and a potent negative regulator of inflammatory iNOS and TNFα. Independent of pore size, CD206+ monocytes were found to be essential in the overall wound healing of both 40 and 100 μm PTS. However, 40 μm PTS explanted from monocyte-depleted mice and 100 μm PTS both exhibit a thick collagen-rich foreign body capsule and high degrees of cellular fusion, indicative of an implant with unfavorable healing. Overall, these findings demonstrate the role of circulating monocytes in PTS healing and further illustrates the heterogeneity and plasticity of the monocyte and macrophage lineages within PTS as a function of pore size.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Dental and Craniofacial Research (NIDCR) and the National Institute of General Medical Sciences (NIGMS; Grant #: NIDCR 5R01DE018701-10 and NIGMS 1R01GM128991-01, respectively). Thank you to the Buddy Ratner lab (University of Washington Engineered Biomaterials, UWEB21) for the use of their scaffold synthesis equipment and SEM. Additional research support was provided by the Cell Analysis Facility Flow Cytometry Shared Resource Lab in the Department of Immunology at the University of Washington.
Funding information
National Institute of Dental and Craniofacial Research, Grant/Award Number: 5R01DE018701-10; National Institute of General Medical Sciences, Grant/Award Number: 1R01GM128991-01
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agrawal H, Tholpady SS, Capito AE, Drake DB, & Katz AJ (2012). Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices. Open Journal of Regenerative Medicine, 01(03), 51–59. 10.4236/ojrm.2012.13008 [DOI] [Google Scholar]
- Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, & Navarro M (2014). Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomaterialia, 10(2), 613–622. 10.1016/j.actbio.2013.10.035 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, & Chang DT (2008). Foreign body reaction to biomaterials. Seminars in Immunology, 20(2), 86–100. 10.1016/j.smim.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank RA, Zandstra J, Room H, Petersen AH, & van Putten SM (2017). Biomaterial encapsulation is enhanced in the early stages of the foreign body reaction during conditional macrophage depletion in transgenic macrophage Fas-induced apoptosis mice. Tissue Engineering—Part A, 23(19–20), 1078–1087. 10.1089/ten.tea.2016.0499 [DOI] [PubMed] [Google Scholar]
- Bota PCS, Collie AMB, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, & Stayton PS (2010). Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. Journal of Biomedical Materials Research—Part A, 95 A(2), 649–657. 10.1002/jbm.a.32893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Jones JA, Xu Y, Low HY, Anderson JM, & Leong KW (2010). Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials, 31(13), 3479–3491. 10.1016/j.biomaterials.2010.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL, & Albina JE (2014). The monocyte to macrophage transition in the murine sterile wound. PLoS One, 9(1), e86660. 10.1371/journal.pone.0086660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, & Roy S (2015). Monocyte and macrophage plasticity in tissue repair and regeneration. American Journal of Pathology, 185(10), 2596–2606. 10.1016/j.ajpath.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon A, & Papahadjopoulos D (1988). Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors (phospholipid vesicles/drug delivery systems/cancer therapy/glycolipids). Proceedings of the National Academy of Sciences of the United States of America, 85 (18), 6949–6953. https://pubmed.ncbi.nlm.nih.gov/3413128/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, & Jung S (2014). Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nature Reviews Immunology, 14(6), 392–404. 10.1038/nri3671 [DOI] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, Schürmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, & Frank S (2009). A transgenic mouse model of inducible macrophage depletion: Effects of diphtheria toxin-driven lysozyme m-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. American Journal of Pathology, 175(1), 132–147. 10.2353/ajpath.2009.081002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hady TF, Pusic AD, Hwang B, Waworuntu RL, Mulligan M, Ratner B, & Bryers JD (2020). Uniform 40-μm-pore diameter precision templated scaffolds promote a pro-healing host response by extracellular vesicle immune communication. Journal of Tissue Engineering and Regenerative Medicine, 15, 24–36. 10.1002/term.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda N, Asano K, Kikuchi K, Uchida Y, Ikegami H, Takagi R, Yotsumoto S, Shibuya T, Makino-Okamura C, Fukuyama H, Watanabe T, Ohmuraya M, Araki K, Nishitai G, & Tanaka M (2018). Emergence of immunoregulatory Ym1 + Ly6C hi monocytes during recovery phase of tissue injury. Science Immunology, 3. https://www.science.org [DOI] [PubMed] [Google Scholar]
- Italiani P, & Boraschi D (2014). From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Frontiers in Immunology, 5, 514. 10.3389/fimmu.2014.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball A, Schaller M, Joshi A, Davis FM, DenDekker A, Boniakowski A, Bermick J, Obi A, Moore B, Henke PK, Kunkel SL, & Gallagher KA (2018). Ly6CHiblood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(5), 1102–1114. 10.1161/ATVBAHA.118.310703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissick HT, Dunn LK, Ghosh S, Nechama M, Kobzik L, & Arredouani MS (2014). The scavenger receptor MARCO modulates TLR-induced responses in dendritic cells. PLoS One, 9(8), e104148. 10.1371/journal.pone.0104148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai K, Shichita T, Ito M, Kanamori M, Chikuma S, & Yoshimura A (2017). Role of scavenger receptors as damage-associated molecular pattern receptors in Toll-like receptor activation. International Immunology, 29(2), 59–70. 10.1093/intimm/dxx010 [DOI] [PubMed] [Google Scholar]
- Lauvau G, Chorro L, Spaulding E, & Soudja SMH (2014). Inflammatory monocyte effector mechanisms. Cellular Immunology, 291(1–2), 32–40. 10.1016/j.cellimm.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TU, Gott SC, Woo BWK, Rao MP, & Liu WF (2015). Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Applied Materials and Interfaces, 7(51), 28665–28672. 10.1021/acsami.5b10589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, & Ratner BD (2010). Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proceedings of the National Academy of Sciences of the United States of America, 107(34), 15211–15216. 10.1073/pnas.1006442107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AJ, & Ratner BD (2005). Quantitative characterization of sphere-templated porous biomaterials. AIChE Journal, 51(4), 1221–1232. 10.1002/aic.10390 [DOI] [Google Scholar]
- McWhorter FY, Davis CT, & Liu WF (2015). Physical and mechanical regulation of macrophage phenotype and function. Cellular and Molecular Life Sciences, 72(7), 1303–1316. 10.1007/s00018-014-1796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza R, DiPietro LA, & Koh TJ (2009). Selective and specific macrophage ablation is detrimental to wound healing in mice. American Journal Of Pathology, 175(6), 2454–2462. 10.2353/ajpath.2009.090248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, & Botchwey EA (2017). Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Scientific Reports, 7(1), 1–16. 10.1038/s41598-017-00477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, & Kobzik L (1999). Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. Journal of Experimental Medicine, 189(9), 1497–1506. http://www.jem.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, Loke P, & Fisher EA (2017). Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. Journal of Clinical Investigation, 127(8), 2904–2915. 10.1172/JCI75005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner BD, & Marshall A (2008). US20080075752A1—novel porous biomaterials. https://patents.google.com/patent/US20080075752A1/en
- Soehnlein O, & Lindbom L (2010). Phagocyte partnership during the onset and resolution of inflammation. Nature Reviews Immunology, 10(6), 427–439. 10.1038/nri2779 [DOI] [PubMed] [Google Scholar]
- Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, & Vunjak-Novakovic G (2014). The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials, 35(15), 4477–4488. 10.1016/j.biomaterials.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman EM, Halpin MC, Muster J, Moon RT, & Ratner BD (2014). Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Annals of Biomedical Engineering, 42(7), 1508–1516. 10.1007/s10439-013-0933-0 [DOI] [PubMed] [Google Scholar]
- van Lenten BJ, & Fogelman AM (1992). Lipopolysaccharide-induced inhibition of scavenger receptor expression in human monocyte-macrophages is mediated through tumor necrosis factor-alpha. The Journal of Immunology, 148(1), 112–116. http://www.jimmunol.org/ [PubMed] [Google Scholar]
- Xu J, Flaczyk A, Neal LM, Fa Z, Cheng D, Ivey M, Moore BB, Curtis JL, Osterholzer JJ, & Olszewski MA (2017). Exploitation of scavenger receptor, macrophage receptor with collagenous structure, by cryptococcus neoformans promotes alternative activation of pulmonary lymph node CD11b+ conventional dendritic cells and non-protective Th2 Bias. Frontiers in Immunology, 8, 1231. 10.3389/fimmu.2017.01231 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


