Abstract
Glycopeptides (vancomycin and teicoplanin) and metronidazole are the drugs of choice for the treatment of Clostridium difficile infections, but trends in susceptibility patterns have not been assessed in the past few years. The objective was to study the MICs of glycopeptides and metronidazole for unrelated C. difficile strains isolated in 1991 (n = 100) and in 1997 (n = 98) by the agar macrodilution, the E-test, and the disk diffusion methods. Strain susceptibilities to erythromycin, clindamycin, tetracycline, rifampin, and chloramphenicol were also determined by the ATB ANA gallery (bioMérieux, La Balme-les-Grottes, France). The MICs at which 50% of isolates are inhibited (MIC50s) and MIC90s of glycopeptides and metronidazole remained stable between 1991 and 1997. All the strains were inhibited by concentrations that did not exceed 2 μg/ml for vancomycin and 1 μg/ml for teicoplanin. Comparison of MICs determined by the agar dilution method recommended by the National Committee for Clinical Laboratory Standards and the E test showed correlations (±2 dilutions) of 86.6, 95.9, and 99% for metronidazole, vancomycin, and teicoplanin, respectively. The E test always underestimated the MICs. Strains with decreased susceptibility to metronidazole (MICs, ≥8 μg/ml) were isolated from six patients (n = 4 in 1991 and n = 2 in 1997). These strains were also detected by the disk diffusion method (zone inhibition diameter, ≤21 mm); they belonged to nontoxigenic serogroup D (n = 5) and toxigenic serogroup H (n = 1). Decreased susceptibility to erythromycin (MICs, ≥1 μg/ml), clindamycin (MICs, ≥2 μg/ml), tetracycline (MICs, ≥8 μg/ml), rifampin (MICs, ≥4 μg/ml), and chloramphenicol (MICs, ≥16 μg/ml) was observed in 64.2, 80.3, 23.7, 22.7, and 14.6% of strains, respectively. Strains isolated in 1997 were more susceptible than those isolated in 1991, and this trend was correlated to a major change in serogroup distribution. Periodic studies are needed in order to detect changes in serogroups and the emergence of strains with decreased susceptibility to therapeutic drugs.
Clostridium difficile is an anaerobic, gram-positive rod. Toxigenic strains are responsible for 20 to 25% of cases of antibiotic-associated diarrhea and for virtually all cases of pseudomembranous colitis (4, 17, 20, 30). C. difficile is the most common agent of nosocomial diarrhea in adults from industrialized countries (22). Since 1980, outbreaks of C. difficile diarrhea have increasingly been reported among hospitalized patients (2, 9, 11, 15, 28).
Currently, the drugs most commonly used to treat diseases caused by C. difficile are metronidazole and vancomycin, both of which should be given orally for a full 10-day course. Clinical trials indicated that these two antibiotics are equivalent for the treatment of mild disease (29, 31, 32). Other glycopeptides such as teicoplanin have been shown to have efficacies equivalent to that of vancomycin (12).
In vitro determination of C. difficile susceptibility to these antibiotics is not routinely performed in France. The reasons are that the method is time-consuming and that the use of susceptibility breakpoints based on the levels of therapeutic drugs in serum are not relevant for itraluminal infections, in which higher drug concentrations can be achieved. Thus, resistance patterns of C. difficile still remain imprecise. Studies from the mid-1980s have shown that this bacterium is highly susceptible to metronidazole (MICs, 0.06 to 2 μg/ml), vancomycin (MICs, 0.125 to 4 μg/ml), and teicoplanin (MICs, 0.03 to 2 μg/ml); but antimicrobial susceptibility trends have not been assessed in the past few years (5, 16, 18, 25). One very recent report from Spain described a dramatic increase in the number of strains with decreased susceptibility to metronidazole in 1998 and the emergence of strains with decreased susceptibility to vancomycin (27). The aims of the present study were (i) to compare the MICs of metronidazole and glycopeptides for strains isolated in 1991 and 1997 by the agar macrodilution method and the E test; (ii) to determine trends in patterns of susceptibility to other drugs such as erythromycin, clindamycin, tetracycline, rifampin, and chloramphenicol; and (iii) to establish correlations between the resistance patterns of C. difficile strains and serogroups.
MATERIALS AND METHODS
Patients and strains.
One hundred ninety-eight C. difficile strains isolated in 1991 (n = 100) and 1997 (n = 98) from hospitalized adults suspected of having C. difficile diarrhea or colitis were studied. Strains were epidemiologically unrelated and nonrepetitive. They were isolated on TCCA medium (brain heart agar supplemented with 5% defibrinated horse blood, 0.1% taurocholate, 250 μg of cycloserine per ml, 10 μg of cefoxitin per ml). The plates were incubated for 48 h in an anaerobic atmosphere. Suspicious colonies (on the basis of morphology, Gram smear results, and odor) were identified by using RapID 32A galleries (bioMérieux, La Balme-les-Grottes, France).
The quality control strains used in susceptibility testing included Bacteroides fragilis ATCC 25285, Bacteroides thetaiotaomicron ATCC 29741, Clostridium perfringens ATCC 13124, and Clostridium difficile ATCC 9689.
MIC determination by agar dilution method.
MICs were determined by the agar dilution method described by the National Committee for Clinical Laboratory Standards (23) with a Steers replicator. Serial twofold dilutions of teicoplanin (Merrell Dow, Neuilly-sur-Seine, France), vancomycin (Lilly, Saint-Cloud, France), and metronidazole (Specia, Rhône-Poulenc Rorer, Paris, France) were incorporated into Wilkins-Chalgren agar (Oxoid, Dardilly, France), with antibiotic concentrations ranging from 0.016 to 32 μg/ml. Inocula were prepared from brain heart infusion broth (Diagnostics Pasteur, Marnes-la-Coquette, France) in which the organisms were grown at 37°C for 24 h. Cultures were adjusted to an optical density on the McFarland scale of 0.5, and 10 μl (105 CFU/spot) was applied with a Steers replicator to prereduced Wilkins-Chalgren agar. Assays were performed at least in duplicate for each strain. The plates were observed after 48 h of incubation in anaerobic jars (HP11; Oxoid, Dardilly, France) at 37°C. The MIC was defined as the lowest concentration of each antibiotic that inhibited visible growth.
MIC determination with E-test strips.
A C. difficile suspension (no. 1 McFarland standard) was swabbed in three directions on prereduced Wilkins-Chalgren agar and was then dried for 15 min on the bench. Strips of vancomycin, teicoplanin, and metronidazole (BMD, Marne-la-Vallée, France) were applied onto the agar surface, and the plates were incubated in an anaerobic atmosphere for 24 h. MICs were read at the point at which the zone of complete inhibition intersects the MIC scale.
Disk diffusion method.
Colonies of C. difficile were suspended in sterile saline buffer (no. 1 McFarland standard) and were swabbed on prereduced Wilkins-Chalgren agar. Standard disks of vancomycin (30 μg), teicoplanin (30 μg), and metronidazole (4 μg [Sanofi Diagnostics Pasteur, Marnes la Coquette, France] and 16 μg [Rosco, Taastrup, Denmark]) were used. The plates were incubated for 24 h in anaerobic jars.
ATB ANA susceptibility test.
The ATB ANA strips (bioMérieux, Marcy l’Etoile, France) permit determination of the susceptibility of anaerobic bacteria to antibiotics in a semisolid medium under conditions similar to those used for the agar dilution method. A suspension of no. 3 McFarland standard was prepared by homogenizing without shaking C. difficile colonies in 0.85% saline buffer, and 200 μl was transferred into an ampoule of ATB-S medium; 135 μl was then distributed into each cupule of the strip containing dehydrated antimicrobial agents. The strips were incubated for 24 h at 37°C in an anaerobic atmosphere. The turbidimetries of the cupules were observed by visual reading, and interpretation was performed according to the manufacturer’s recommendations. The breakpoints to be used for interpretation of the results were as follows: 1 to 4 μg/ml for erythromycin, 2 μg/ml for clindamycin, 8 μg/ml for tetracycline, 4 to 16 μg/ml for rifampin, and 16 μg/ml for chloramphenicol.
Serotyping.
Serotyping of the C. difficile strains was performed by the method of Delmée et al. (14). Eleven antisera specific for serogroups A1, A5, A8, A9, A10, C, D, F, G, H, and K were used in an enzyme-linked immunosorbent assay format, as described previously (14).
Toxigenicity.
The presence of C. difficile toxin B was determined by demonstrating a specific cytopathic effect on MRC-5 cells, as described previously (1).
Statistical methods.
The significance of differences in susceptibility patterns or serogroup distribution was analyzed by the chi-square test or Fisher’s exact two-tailed test with EpiInfo 6.0 software (Centers for Disease Control and Prevention, Atlanta, Ga.). A P value of <0.05 was considered statistically significant.
RESULTS
Distribution of serogroups.
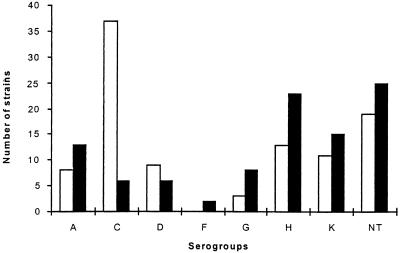
Among the 198 strains, only 21.7% were nontypeable with the 11 antisera that we used. Serogroups A, C, D, G, H, and K accounted for 9, 22, 8, 4, 19, and 14% of strains, respectively. The distribution of serogroups showed wide variations between 1991 and 1997 (Fig. 1). Indeed, strains from serogroup C were largely predominant in 1991 compared to their proportion in 1997 (36 versus 6%; P < 0.01).
FIG. 1.
Distribution of serogroups of C. difficile isolated in 1991 (□) and 1997 (■). NT, not typeable.
Susceptibility to glycopeptides and metronidazole.
All the strains were inhibited by concentrations that did not exceed 2 μg/ml for vancomycin and 1 μg/ml for teicoplanin, with MICs distributed over a narrow range (Table 1). There was no significant change in the MICs at which 50% of strains are inhibited (MIC50s) and the MIC90s of the glycopeptides between 1991 and 1997.
TABLE 1.
Susceptibility of C. difficile to vancomycin, teicoplanin, and metronidazole between 1991 and 1997 (agar dilution method)
| Agent and yr of isolation | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Range | Median | 50% | 90% | |
| Metronidazole | ||||
| 1991 | 0.12–>8 | 0.25 | 0.25 | 2 |
| 1997 | 0.06–>8 | 0.25 | 0.25 | 0.5 |
| Vancomycin | ||||
| 1991 | 0.125–2 | 1 | 1 | 2 |
| 1997 | 0.125–2 | 1 | 1 | 2 |
| Teicoplanin | ||||
| 1991 | 0.12–1 | 0.25 | 0.25 | 0.5 |
| 1997 | 0.12–1 | 0.25 | 0.25 | 0.5 |
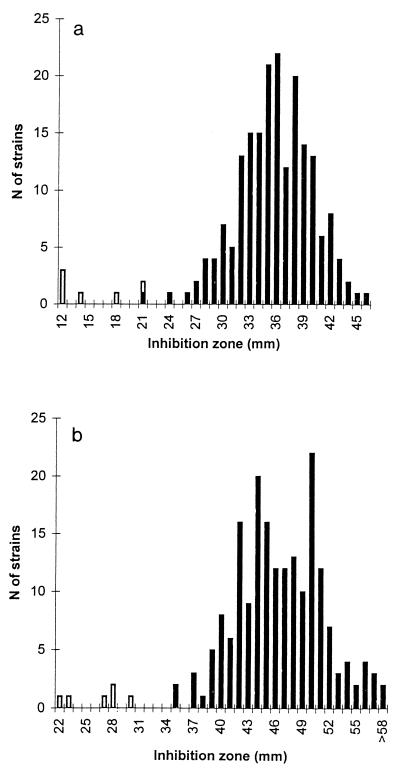
Decreased susceptibility to metronidazole was observed for six strains (one strain for which the MIC was 32 μg/ml, three strains for which the MICs were 16 μg/ml, and two strains for which the MICs were 8 μg/ml) (Table 2). Two strains were isolated in 1997 (2%), and four strains were isolated in 1991 (4%). All except one of these strains belonged to nontoxigenic serogroup D; the strain that was the exception belonged to toxigenic serogroup H. The six strains were detected by the disk diffusion method (zone inhibition diameters, ≤21 and ≤30 mm with Diagnostic Pasteur tablets and Rosco tablets, respectively). The zone inhibition diameters for these strains were clearly different from those for susceptible strains (Fig. 2). Only one susceptible strain had a zone inhibition diameter of 21 mm (Pasteur tablets), but the MIC for this strain was 4 μg/ml.
TABLE 2.
Characterization of strains with decreased susceptibility to metronidazole
| Strain no. | Serogroup | Toxin B production | MTZa MIC (μg/ml)
|
MTZ inhibition zone diam (mm)
|
||
|---|---|---|---|---|---|---|
| Agar dilution method | E test | Pasteur tablets | Rosco tablets | |||
| 97-104 | D | − | 16 | 1.5 | 18 | 28 |
| 97-628 | H | + | 16 | 1.5 | 21 | 30 |
| 91-804 | D | − | 32 | 4 | 12 | 22 |
| 91-714 | D | − | 16 | 4 | 12 | 23 |
| 91-861 | D | − | 8 | 4 | 14 | 27 |
| 91-386 | D | − | 8 | 1.5 | 12 | 28 |
MTZ, metronidazole.
FIG. 2.
Distribution of inhibition zone for metronidazole (disk diffusion method). (a) Pasteur tablets. (b) Rosco tablets. C. difficile strains with decreased susceptibility to metronidazole are represented by white bars.
Comparison of MICs determined by the agar dilution method recommended by the National Committee for Clinical Laboratory Standards and the E test showed correlations (±2 dilutions) of 86.6, 95.9, and 99% for metronidazole, vancomycin, and teicoplanin, respectively. The E test always underestimated the MICs (Table 3). For the six strains with decreased susceptibility to metronidazole, the MIC was ≥1.5 μg/ml, as determined by the E test, and the strains were distinguishable from the fully susceptible strains (MICs, ≤0.5 μg/ml).
TABLE 3.
Analysis of agreement of susceptibility data obtained by the E test versus those obtained by the agar dilution method
| Antimicrobial agent | % of E-test MICs within the following concn (log2) of MICs obtained by agar dilution method
|
||||||
|---|---|---|---|---|---|---|---|
| <−2 | −2 | −1 | Same | +1 | +2 | >+2 | |
| Metronidazole | 12.9 | 23.2 | 34.5 | 25.8 | 3.1 | 0.5 | 0.5 |
| Vancomycin | 3.6 | 21.2 | 30.6 | 36.7 | 6.2 | 1 | 0.5 |
| Teicoplanin | 1 | 21.8 | 45.6 | 29.5 | 2.1 | 0 | 0 |
Correlation between serogroups and antimicrobial susceptibility.
Decreased susceptibilities to erythromycin (MICs, ≥1 μg/ml), clindamycin (MICs, ≥2 μg/ml), tetracycline (MICs, ≥8 μg/ml), rifampin (MICs, ≥4 μg/ml), and chloramphenicol (MICs, ≥16 μg/ml) were observed for 64.2, 80.3, 23.7, 22.7, and 14.6% of the strains respectively. Strains isolated in 1997 showed a pattern of greater susceptibility than those isolated in 1991 (Table 4).
TABLE 4.
Patterns of C. difficile susceptibility to erythromycin, tetracycline, rifampin, clindamycin, and chloramphenicol between 1991 and 1997
| Antimicrobial agent (MIC [μg/ml]) | % of strains
|
||
|---|---|---|---|
| 1991 (n = 100) | 1997 (n = 98) | Total (n = 198) | |
| Erythromycin (≥1) | 76 | 52 | 64.2 |
| Clindamycin (≥2) | 86 | 74.5 | 80.3 |
| Tetracycline (≥8) | 37 | 10.2 | 23.7 |
| Rifampin (≥4) | 38 | 7.1 | 22.7 |
| Chloramphenicol (≥16) | 28 | 1 | 14.6 |
Strains for which chloramphenicol MICs were ≥16 μg/ml belonged almost exclusively to serogroup C. Strains of serogroup G exhibited the pattern of the greatest susceptibility. Decreased susceptibility to tetracycline (MICs, ≥8 μg/ml) was common in serogroups C and K (Table 5). Fifty percent of serogroup C strains were characterized by a multiple-drug resistance pattern, with resistance to erythromycin, rifampin, tetracycline, and chloramphenicol, and this pattern was observed only among strains of this serogroup.
TABLE 5.
Correlation between serogroups of C. difficile and antimicrobial susceptibility patterns
| Antimicrobial agent (MIC [μg/ml]) | % of strains of the following serogroup
|
||||||
|---|---|---|---|---|---|---|---|
| A (n = 21) | C (n = 43) | D (n = 15) | G (n = 11) | H (n = 36) | K (n = 26) | NTa(n = 44) | |
| Erythromycin (≥1) | 47.6 | 90.5 | 86.7 | 22.2 | 66.7 | 53.8 | 48.8 |
| Clindamycin (≥2) | 52.4 | 97.6 | 93.3 | 66.7 | 80.6 | 73.1 | 76.7 |
| Tetracycline (≥8) | 9.5 | 73.8 | 0 | 0 | 0 | 34.6 | 9.3 |
| Rifampin (≥4) | 14.3 | 78.6 | 20 | 0 | 5.6 | 7.7 | 0 |
| Chloramphenicol (≥16) | 0 | 61.9 | 0 | 0 | 2.8 | 0 | 4.7 |
NT, not typeable.
DISCUSSION
Among the 198 strains studied, strains of serogroups C, H, K, and A were predominant. These data are consistent with those from a previous report (1). Nevertheless, the percentage of serogroup C strains was higher in 1991 than in 1997. This observation cannot be explained by outbreaks of C. difficile-associated diarrhea since patients have been hospitalized in different wards and different hospitals and strains were selected to be epidemiologically unrelated. We can hypothesize that the high rate of C. difficile from serogroup C observed in 1991 is biased by the high proportion of strains isolated from human immunodeficiency virus-positive patients treated with clindamycin for cerebral toxoplasmosis. This antimicrobial agent has previously been shown to select for strains of serogroup C which are usually resistant to clindamycin (3, 13). In 1997, since the introduction of protease inhibitors, the incidence of opportunistic infections such as cerebral toxoplasmosis among human immunodeficiency virus-positive patients declined, as has the level of use of clindamycin (26).
Our results show that there was no significant change in the MIC50s and MIC90s of glycopeptides between 1991 and 1997. All the strains were inhibited by concentrations that did not exceed 2 μg/ml for vancomycin and 1 μg/ml for teicoplanin, with the MICs distributed over a narrow range. In fact, in agreement with previous findings (5, 6, 18, 25), the MICs of teicoplanin ranged from 0.12 to 1 μg/ml, generally being two or four times lower than those of vancomycin, which ranged from 0.12 to 2 μg/ml. We did not confirm the recent data from a Spanish survey that found that 10% of clinical strains of C. difficile had decreased susceptibility to vancomycin (27).
Six C. difficile strains (3%) exhibited decreased susceptibility to metronidazole, with the MICs for the strains ranging from 8 to 32 μg/ml. The frequency of occurrence of these strains remained stable from 1991 (4%) to 1997 (2%). These results are concordant with the rate of 3% usually reported. Nevertheless, two recent studies suggested the increasing emergence of strains with decreased susceptibility to metronidazole; these strains represented 20% of equine isolates of C. difficile (19) and 14% of clinical strains isolated in Spain in 1998 (versus 6% in 1993) (27). The clinical impact of these strains needs to be further evaluated. Indeed, the MICs for these strains might be above the concentrations of 3.3 μg/g (wet weight of feces) usually found in feces of patients who ingested 500 mg of metronidazole twice a day (8). In the present study, the clinical impact of such strains seems to be limited since five of six strains belonged to serogroup D, which is never toxigenic and which is thus never implicated in diarrhea or colitis. Nevertheless, these findings highlight the need for periodic surveys of the antimicrobial susceptibilities of C. difficile strains. The disk diffusion method seems to be a reliable method for detection of strains with decreased susceptibility to metronidazole since all the strains exhibited zone inhibition diameters of ≤21 and ≤30 mm with Pasteur tablets and Rosco tablets, respectively.
We found a good correlation (±2 dilutions) between the E test and the agar dilution method, although the E test always underestimated the MICs. The E test was performed according to the recommendations of Bolmström (7), who showed that results obtained with a suspension of a no. 1 McFarland standard and a reading after 24 h of incubation best matched those obtained by the standard agar dilution procedure. The good correlation between the E test and the agar dilution method for anaerobic bacteria has previously been demonstrated by Citron et al. (10), Bolmström (7), and Wüst and Hardegger (33) for various combinations of antimicrobial agents and bacteria. This is the first large-scale report of a correlation for C. difficile with glycopeptides and metronidazole.
Decreased susceptibility to erythromycin, clindamycin, tetracycline, rifampin, and chloramphenicol was observed in 64.2, 80.3, 23.7, 22.7, and 14.6% of the strains, respectively. These findings suggest a pattern of greater resistance compared to those found in others studies (13, 21, 24) except for that for chloramphenicol. Indeed, antimicrobial susceptibility depends on the different methodologies and breakpoints used and may fluctuate from one medical center to another as well as from one geographic region to another.
We tried to correlate susceptibility to erythromycin, clindamycin, tetracycline, rifampin, and chloramphenicol to serogroups from an epidemiological point of view. In vitro resistance to chloramphenicol was almost exclusively observed among strains of serogroup C. Resistance to tetracycline was common in strains of serogroups C and K. Serogroup C was characterized by a typical multiple-drug resistance pattern and was the only serogroup resistant to both chloramphenicol and tetracycline or rifampin. All these data are consistent with those reported by Delmée and Avesani (13), who studied 308 strains of C. difficile from different origins in 1988. The high prevalence of serogroup C in 1991 can account for the antimicrobial resistance patterns that we observed.
In conclusion, the MIC50s and MIC90s of glycopeptides and metronidazole remained stable between 1991 and 1997, and these antimicrobial agents still remain the drugs of choice for the treatment of C. difficile infections. Results obtained by the agar dilution method and the E test showed a good correlation, although the E test always underestimated the MICs. Three percent of strains, most of which belonged to nontoxigenic serogroup D, exhibited decreased susceptibility to metronidazole, but the clinical impact of such strains seems to be limited. These strains were easily detected by the disk diffusion method. Strains isolated in 1997 were more susceptible to erythromycin, clindamycin, tetracycline, rifampin, and chloramphenicol than those isolated in 1991 because of a major change in serogroup distribution. Periodic studies are needed in order to detect changes in serogroups and the emergence of strains resistant to therapeutic drugs.
ACKNOWLEDGMENTS
This work was supported by grants from INSERM (grant PARMIFR 9609) and from the UPRES Research Group on Clostridium difficile.
We thank M. Gomis and D. LeCunff for technical assistance.
REFERENCES
- 1.Barbut F, Corthier G, Charpack Y, Cerf M, Monteil H, Fosse T, Trévoux A, De Barbeyrac B, Boussougant Y, Tigaud S, Tytgat F, Sédallian A, Duborgel S, Collignon A, LeGuern M E, Bernasconi P, Petit J C. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Arch Intern Med. 1996;156:1449–1454. [PubMed] [Google Scholar]
- 2.Barbut F, Mario N, Meyohas M C, Binet D, Frottier J, Petit J C. Investigation of a nosocomial outbreak of Clostridium difficile-associated diarrhoea among AIDS patients with random amplified polymorphic DNA. J Hosp Infect. 1994;26:181–189. doi: 10.1016/0195-6701(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 3.Barbut F, Meynard J L, Guiguet M, Avesani V, Bochet M V, Meyohas M C, Delmée M, Tilleul P, Frottier J, Petit J C. Clostridium difficile-associated diarrhea in HIV-infected patients: epidemiology and risk factors. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:176–181. doi: 10.1097/00042560-199711010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett J G, Chang T W, Gurwith M, Gorbach S L, Onderdonk A B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 5.Bartolini A, Colao M G, Orsi A, Dei R, Giganti E, Parenti F. In vitro activity of vancomycin, teicoplanin, daptomycin, ramoplanin, MLD62873 and other agents against staphylococci, enterococci and Clostridium difficile. J Antimicrob Chemother. 1990;26:627–633. doi: 10.1093/jac/26.5.627. [DOI] [PubMed] [Google Scholar]
- 6.Biavasco F, Manso E, Varaldo P E. In vitro activities of ramoplanin and four glycopeptide antibiotics against clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 1991;35:195–197. doi: 10.1128/aac.35.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolmström A. Susceptibility testing of anaerobes with Etest. Clin Infect Dis. 1993;16(Suppl. 4):S367–S370. doi: 10.1093/clinids/16.supplement_4.s367. [DOI] [PubMed] [Google Scholar]
- 8.Bolton R P, Culshaw M A. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169–1172. doi: 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartmill T D I, Panigrahi H, Worsley M A, McCann D C, Nice C N, Keith E. Management and control of a large outbreak of diarrhoea due to Clostridium difficile. J Hosp Infect. 1994;27:1–15. doi: 10.1016/0195-6701(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 10.Citron D M, Ostovari M I, Karlsson A, Goldstein E J. Evaluation of the E test for susceptibility testing of anaerobic bacteria. J Clin Microbiol. 1991;29:2197–2203. doi: 10.1128/jcm.29.10.2197-2203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clabots C R, Johnson S, Olson M M, Peterson L R, Gerding D L. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166:561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 12.DeLalla F, Nicolin R, Rinaldi E, Scarpellini P, Rigoli R, Manfrin R, Tramarin A. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 1992;36:2192–2196. doi: 10.1128/aac.36.10.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmée M, Avesani V. Correlation between serogroup and susceptibility to chloramphenicol, clindamycin, erythromycin, rifampicin and tetracycline among 308 isolates of Clostridium difficile. J Antimicrob Agents. 1988;22:325–331. doi: 10.1093/jac/22.3.325. [DOI] [PubMed] [Google Scholar]
- 14.Delmée M, Depitre C, Corthier G, Ahoyo A, Avesani V. Use of enzyme-linked immunoassay for Clostridium difficile serogrouping. J Clin Microbiol. 1993;31:2526–2528. doi: 10.1128/jcm.31.9.2526-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmée M, Vandercam B, Avesani V, Michaux J L. Epidemiology and prevention of Clostridium difficile infections in a leukemia unit. Eur J Clin Microbiol. 1987;6:623–627. doi: 10.1007/BF02013056. [DOI] [PubMed] [Google Scholar]
- 16.Dzink J, Bartlett J G. In vitro susceptibility of Clostridium difficile from patients with antibiotic-associated diarrhoea or colitis. Antimicrob Agents Chemother. 1980;17:695–698. doi: 10.1128/aac.17.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George W L, Rolfe R D, Finegold S M. Clostridium difficile and its cytotoxin in feces of patients with antimicrobial agent-associated pseudomembranous colitis and miscellaneous conditions. J Clin Microbiol. 1982;15:1049–1053. doi: 10.1128/jcm.15.6.1049-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruer L. Susceptibility of Clostridium difficile strains to new antibiotics: quinolones, efrotomycin, teicoplanin and imipenem. J Antimicrob Chemother. 1985;15:648–649. doi: 10.1093/jac/15.5.648-a. [DOI] [PubMed] [Google Scholar]
- 19.Jang S S, Hansen L M, Breher J E, Riley D A, Magdesian K G, Madigan J E, Tang Y J, Silva J, Jr, Hirsh D C. Antimicrobial susceptibilities of equine isolates of Clostridium difficile and molecular characterization of metronidazole-resistant strains. Clin Infect Dis. 1997;25(Suppl. 2):S266–S267. doi: 10.1086/516235. [DOI] [PubMed] [Google Scholar]
- 20.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 21.Levett P N. Antimicrobial susceptibility of Clostridium difficile determined by disc diffusion and breakpoint methods. J Antimicrob Chemother. 1988;22:167–173. doi: 10.1093/jac/22.2.167. [DOI] [PubMed] [Google Scholar]
- 22.McFarland L V, Mulligan M E, Kwok R Y Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 4th ed. Approved standard M11-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 24.Niyogi S K. Antimicrobial susceptibility of Clostridium difficile strains isolated from hospitalised patients with acute diarrhoea. J Diarrhoeal Dis Res. 1992;10:156–158. [PubMed] [Google Scholar]
- 25.Nord C E. In vitro activity of quinolones and other antimicrobial agents against anaerobic bacteria. Clin Infect Dis. 1996;23(Suppl. 1):S15–S18. doi: 10.1093/clinids/23.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 26.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 27.Pelaez T, Martinez-Sanchez L, Alcala L, Munoz P, Garcia-Lechuz J M, Rodriguez-Creixems M, Bouza E. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1998. Metronidazole resistance in Clostridium difficile: a new emerging problem?, abstr. E-173; p. 219. [Google Scholar]
- 28.Samore M H, DeGirolami P C, Tlucko A, Lichtenberg D A, Melvin Z A, Karchmer A W. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis. 1994;18:181–187. doi: 10.1093/clinids/18.2.181. [DOI] [PubMed] [Google Scholar]
- 29.Teasley D G, Olson M M, Gebhard R L, Gerding D N, Peterson L R, Schwartz M J, Lee J T., Jr Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhoea and colitis. Lancet. 1983;i:1043–1046. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 30.Viscidi R, Willey S, Bartlett J G. Isolation rates and toxigenic potential of Clostridium difficile isolated from various patient populations. Gastroenterology. 1981;81:5–9. [PubMed] [Google Scholar]
- 31.Wenisch C, Parschalk B, Hasenhündi M, Hirschl A M, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–818. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox M H, Howe R. Diarrhoea caused by Clostridium difficile: response time for treatment with metronidazole and vancomycin. J Clin Microbiol. 1995;36:673–679. doi: 10.1093/jac/36.4.673. [DOI] [PubMed] [Google Scholar]
- 33.Wüst J, Hardegger U. Comparison of the Etest and a reference agar dilution method for susceptibility testing of anaerobic bacteria. Eur J Clin Microbiol Infect Dis. 1992;11:1169–1173. doi: 10.1007/BF01961139. [DOI] [PubMed] [Google Scholar]