Abstract
Background
Remnant cholesterol/high-density lipoprotein cholesterol (RC/HDL-C) ratio has been shown to be a good predictor of metabolic disease risk, but no studies have further investigated the role of RC/HDL-C ratio in non-alcoholic fatty liver disease (NAFLD) patients.
Methods
The participants were 14,251 adults who underwent a physical examination, all of whom underwent abdominal ultrasonography to determine whether they had NAFLD. Receiver operating characteristic curve analysis and multivariate logistic regression models were used to assess the association between the RC/HDL-C ratio and the risk of NAFLD.
Results
Multivariate logistic regression analysis showed that after fully adjusting the confounding factors, the higher RC/HDL-C ratio was independently positively correlated with the risk of NAFLD. Interaction tests suggested that the effect of RC/HDL-C ratio on NAFLD was significantly affected by sex. Additionally, receiver operating characteristic curve analysis showed that the area under the curve of RC/HDL-C ratio for identifying NAFLD was 0.82, which was significantly higher than that of other conventional lipid parameters.
Conclusions
This study indicates for the first time that the higher RC/HDL-C ratio in the general population may be closely related to the increased risk of NAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-022-02216-x.
Keywords: Remnant cholesterol/high-density lipoprotein cholesterol ratio, Remnant cholesterol, High-density lipoprotein cholesterol, Non-alcoholic fatty liver disease, RC/HDL-C ratio
Background
Non-alcoholic fatty liver disease (NAFLD) seems to have become the most common chronic disease in the world. According to a recent epidemiological survey of more than 8 million people in 22 countries by Younossi et al., the current prevalence of NAFLD is 25.24% in the world and 27.37% in Asia [1]. With the increasing prevalence of obesity and diabetes worldwide, the prevalence of NAFLD continues to go up and has become a global public health problem [1, 2]. NAFLD contains a series of liver histological changes ranging from mild hepatic steatosis to severe necrotizing inflammation. Without any interventions, it will eventually develop into liver cirrhosis or even liver cancer, leading to serious adverse consequences [3–5]. Additionally, many clinical studies have found that NAFLD also causes many adverse effects on other organs and systems outside the liver [6–8]. The widespread adverse consequences caused by NAFLD in and out of the liver have further increased the global burden of chronic diseases. Therefore, both medical institutions and the general public should pay attention to the preventive screening and management of NAFLD.
Atherogenic dyslipidemia is closely related to NAFLD, in which characteristic changes of triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) are common in NAFLD patients [9, 10]. Therefore, monitoring atherosclerotic lipids is an important way to assess the risk of NAFLD [9, 11]. Remnant cholesterol (RC) is an unconventional lipid that has been widely studied in recent years. It is a kind of lipoproteins rich in TG, that is, intermediate-density lipoprotein and very-low-density lipoprotein in fasting state, and in the nonfasting state also chylomicron remnants [12]. It is a key lipoprotein of atherosclerosis [13, 14]. Some previous clinical studies have also confirmed that RC is a major factor mediating the residual risk of major cardiovascular events and is independently related to the progression of atherosclerosis [14–16]. In recent years, a growing number of studies have found that high levels of RC significantly increased the risk of diabetes complications, hypertension and NAFLD; additionally, high levels of RC can also be used to predict cardio-cerebrovascular events in patients with NAFLD [17–20]. These pieces of evidence suggest that RC may be a good parameter for assessing the risk of metabolism-related disease. Recently, some scholars have pointed out that the parameter remnant cholesterol/high-density lipoprotein cholesterol ratio (RC/HDL-C ratio) after the combination of RC and HDL-C is a valuable independent predictor of myocardial injury in diabetic patients after receiving PCI; in addition, the RC/HDL-C ratio can also be used to evaluate intracranial atherosclerotic [21, 22]. However, there are no epidemiological studies to investigate the relationship between RC/HDL-C ratio and NAFLD, and it remains unclear whether the RC/HDL-C ratio is a risk factor for NAFLD. Here, in order to solve these problems, this study retrospectively analyzed the population data of NAGALA (NAfld in Gifu Area, Longitudinal Analysis) cohort to examine the relationship between RC/HDL-C ratio and NAFLD.
Methods
Study data and participants
This study was a secondary analysis of the NAGALA cohort data set to examine the relationship between the RC/HDL-C ratio and NAFLD. The NAGALA study is a cohort study based on general adults that have been ongoing since 1994 to assess risk factors for common chronic diseases, including NAFLD, in the general population. Details of the study design have been published elsewhere [23]. Available research data were uploaded to the Dryad database by Hamaguchi et al. According to the Dryad data usage service, different researchers can use the data set for in-depth analysis according to different research hypotheses, and need to indicate the source of the data [24].
This study extracted the available data from the NAGALA cohort from 2004 to 2015, and established new exclusion criteria based on the research hypothesis, including the following: (1) During the baseline interview, men drank more than 210 g per week or women drank more than 140 g per week (1952) [25]; (2) Participants who were still taking oral medication at the time of the baseline visit (2321); (3) Diagnosis of impaired fasting glucose or diabetes or alcoholic hepatitis or viral hepatitis or autoimmune hepatitis or liver disease due to other causes were made at the baseline visit (1547); (4) Participants with missing baseline information (873). Informed consent of participants had been obtained in previous studies [23]. In addition, as the previous study had been authorized by the ethics committee of Murakami Memorial Hospital, the Institutional Review Board (IRB) of Jiangxi Provincial People’s Hospital exempted the application for duplicate ethical authorization (IRB Number: 2021-066).
Clinical characteristics
As mentioned earlier, data on the demographic characteristics and lifestyle of study participants were recorded by trained medical staff using a standard questionnaire [23]. Anthropometric data collected included height, weight, waist circumference and blood pressure; lifestyle data included a habit of exercise, smoking and drinking status, history of chronic diseases and history of medication. Among them, the habit of exercise was evaluated according to whether they participated in exercise regularly or not, and the frequency was at least once a week. Smoking status and drinking status were classified according to past history asked during the baseline interview, in which smoking status was divided into three groups: non-smoking, past smoking and current smoking, and drinking status was divided into non-drinking or small, light and moderate groups.
Biochemical parameters
All venous blood samples were collected after fasting overnight, and the biochemical parameters such as aspartate aminotransferase (AST), HDL-C, total cholesterol (TC), hemoglobin A1c (HbA1c), gamma-glutamyl transferase (GGT), TG, fasting plasma glucose (FPG) and alanine aminotransferase (ALT) were determined by standard experimental method.
Definition and calculation
The study population underwent abdominal ultrasound to assess NAFLD. Liver sonograms were first acquired by an experienced technician using the AlokassD-650CL ultrasound system, and then evaluated by a gastroenterologist without knowledge of the participants’ other examination information. The main features of NAFLD under ultrasound were determined and scored according to the four criteria of deep elevation, hepatorenal echo contrast, liver brightness and vascular blurring. Participants with a final score greater than 2 were diagnosed with NAFLD [26].
RC was calculated as non-HDL-C – low-density lipoprotein cholesterol (LDL-C) [27], where non-HDL-C = TC – HDL-C and LDL-C (mg/ dL) = non-HDL-C x 90% – TG x 10% [28].
RC/HDL-C ratio was calculated as RC divided by HDL-C [21].
Statistical analysis
In this study, descriptive statistics were firstly carried out for the quartile grouping of the RC/HDL-C ratio. The descriptive data of the study population were expressed as the percentage of categorical variables and the mean (standard deviation) or median (interquartile range) of continuous variables. Comparisons between groups were performed using one-way analysis of variance or Kruskal-Wallis test or Chi-square test. Then, according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines, the OR (odds ratio) and 95% CI (confidence interval) of the ratio of RC/HDL-C to the risk of NAFLD in different multivariate logical regression models were reported [29]. Among which model I was regarded as a fine-tuning model, and sex and age were regarded as confounding factors. Model II takes non-collinear variables with an impact of > 10% on the risk of NAFLD associated with the RC/HDL-C ratio as confounders [30, 31]. Based on model II, model III further treated the significant covariables in the univariate analysis as confounding factors. Model IV was regarded as a fully adjusted model, in which all non-collinear covariables were taken as confounding factors (Additional file 1: Table S1) [30]. Sensitivity analysis was performed by limiting the RC/HDL-C ratio quartiles to continuous variables in four models. Additionally, we created stratified models to assess the NAFLD risk of RC/HDL-C ratios in people of different sexes, ages, BMI and habits of exercise. In order to evaluate the potential interaction, the likelihood ratio test was also used to compare the differences of subgroups in different models. Finally, to further evaluate the accuracy of the RC/HDL-C ratio in identifying NAFLD risk, we also used the receiver operating characteristic (ROC) curve to assess the area under the curve (AUC) of the RC/HDL-C ratio and other lipid parameters, and compared the AUC of RC/HDL-C ratio with that of other lipid parameters by DeLong test [32]. All statistical analyses were performed using R language version 3.4.3 and Empower (R) version 2.0. Double-tail P < 0.05 was considered to be statistically significant.
Results
Characteristics of study participants
The observational study involved 14,251 participants. The average age of the study population was 43 years old, and 6840 (48%) were women. According to the results of abdominal ultrasound, 2507 (17.59%) participants were diagnosed with NAFLD. Table 1 describes the clinical and biochemical characteristics of the study population according to the quartiles of RC/HDL-C ratios. We noted significant differences in all baseline information across the quartile groups of RC/HDL-C ratios. The main results were summarized below: (1) In the group with a higher RC/HDL-C ratio, the number of men was about 5 times higher than that of women (83.15% vs. 16.85%). This suggests that the RC/HDL-C ratio may be sex-dependent, and the NAFLD risk associated with the RC/HDL-C ratio may have a better distinction between the sexes. In addition, the group with a higher RC/HDL-C ratio was significantly older. (2) Anthropometric parameters were significantly higher in the higher RC/HDL-C ratio group. (3) Biochemical parameters except for HDL-C, other lipid parameters, glucose metabolism parameters and liver enzymology parameters were significantly higher in the high RC/HDL-C ratio groups. It should be noted that although liver enzymology indexes ALT, AST and GGT were gradually increased across the RC/HDL-C ratio quartiles elevated, they were all at normal levels in all groups, suggesting that most of the patients with NAFLD in this study may still be in the early stage. (4) In the group with a higher RC/HDL-C ratio, there were fewer people maintained exercise habits, and more people smoking and drinking alcohol. (5) The prevalence of NAFLD between the RC/HDL-C ratio quartile was gradually increased (1.94% vs. 6.29% vs. 18.48%, 43.58%).
Table 1.
Baseline characteristics of four groups
| RC/HDL-C ratio quartile | |||||
|---|---|---|---|---|---|
| Q1 (0.07–0.26) | Q2 (0.26–0.37) | Q3 (0.37–0.55) | Q4 (0.55–4.39) | P-value | |
| Sex | < 0.001 | ||||
| Women | 2785 (78.21%) | 2111 (59.30%) | 1344 (37.75%) | 600 (16.85%) | |
| Men | 776 (21.79%) | 1449 (40.70%) | 2216 (62.25%) | 2961 (83.15%) | |
| Age, years | 40.00 (35.00–46.00) | 42.00 (36.00–49.00) | 44.00 (38.00–51.00) | 45.00 (39.00–52.00) | < 0.001 |
| Weight, kg | 52.70 (8.11) | 57.08 (9.47) | 62.16 (10.55) | 69.10 (11.13) | < 0.001 |
| Height, cm | 161.70 (7.53) | 163.50 (8.46) | 165.72 (8.56) | 168.25 (7.88) | < 0.001 |
| BMI, kg/m2 | 20.09 (2.21) | 21.27 (2.56) | 22.55 (2.91) | 24.34 (3.08) | < 0.001 |
| WC, cm | 69.99 (6.69) | 73.53 (7.58) | 77.85 (8.15) | 83.36 (7.93) | < 0.001 |
| ALT, U/L | 14.00 (11.00–17.00) | 15.00 (12.00–19.00) | 17.00 (13.00–23.00) | 23.00 (17.00–32.00) | < 0.001 |
| AST, U/L | 16.00 (13.00–19.00) | 16.00 (13.75-20.00) | 17.00 (14.00–21.00) | 19.00 (15.00–23.00) | < 0.001 |
| GGT,U/L | 12.00 (10.00–15.00) | 13.00 (10.00–17.00) | 15.00 (12.00–22.00) | 21.00 (15.00–30.00) | < 0.001 |
| HDL-C, mmol/L | 1.90 (0.35) | 1.56 (0.25) | 1.33 (0.21) | 1.06 (0.19) | < 0.001 |
| Non-HDL-C, mmol/L | 2.80 (2.45–3.19) | 3.39 (3.00-3.80) | 3.86 (3.43–4.31) | 4.46 (3.96–4.99) | < 0.001 |
| TC, mmol/L | 4.73 (0.76) | 4.97 (0.78) | 5.22 (0.81) | 5.57 (0.88) | < 0.001 |
| LDL-C, mmol/L | 2.42 (2.10–2.77) | 2.90 (2.56–3.27) | 3.27 (2.88–3.68) | 3.65 (3.19–4.14) | < 0.001 |
| TG, mmol/L | 0.42 (0.32–0.53) | 0.60 (0.47–0.75) | 0.85 (0.69–1.05) | 1.45 (1.13–1.90) | < 0.001 |
| RC, mmol/l | 0.38 (0.33–0.43) | 0.48 (0.43–0.54) | 0.58 (0.53–0.65) | 0.78 (0.69–0.92) | < 0.001 |
| FPG, mmol/L | 4.97 (0.39) | 5.08 (0.40) | 5.21 (0.39) | 5.34 (0.37) | < 0.001 |
| HbA1c, % | 5.14 (0.30) | 5.15 (0.31) | 5.20 (0.33) | 5.23 (0.34) | < 0.001 |
| SBP, mmHg | 108.06 (13.08) | 111.38 (13.88) | 115.86 (14.43) | 120.42 (14.83) | < 0.001 |
| DBP, mmHg | 66.62 (9.28) | 69.27 (9.62) | 72.55 (9.93) | 76.04 (10.20) | < 0.001 |
| Habit of exercise | 671 (18.84%) | 639 (17.95%) | 634 (17.81%) | 524 (14.71%) | < 0.001 |
| Drinking status | < 0.001 | ||||
| Non or small | 3087 (86.69%) | 3024 (84.94%) | 2851 (80.08%) | 2840 (79.75%) | |
| Light | 370 (10.39%) | 400 (11.24%) | 505 (14.19%) | 479 (13.45%) | |
| Moderate | 104 (2.92%) | 136 (3.82%) | 204 (5.73%) | 242 (6.80%) | |
| Smoking status | < 0.001 | ||||
| Non | 2845 (79.89%) | 2478 (69.61%) | 1950 (54.78%) | 1469 (41.25%) | |
| Past | 421 (11.82%) | 543 (15.25%) | 761 (21.38%) | 831 (23.34%) | |
| Current | 295 (8.28%) | 539 (15.14%) | 849 (23.85%) | 1261 (35.41%) | |
| NAFLD | 69 (1.94%) | 224 (6.29%) | 658 (18.48%) | 1552 (43.58%) | < 0.001 |
Values were expressed as mean (standard deviation) or medians (quartile interval) or n (%)
RC/HDL-C ratio remnant cholesterol/high-density lipoprotein cholesterol ratio, BMI body mass index, WC waist circumference, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyl transferase, TC total cholesterol, LDL-C low density lipoprotein cholesterol, TG triglyceride, FPG fasting plasma glucose, HbA1c hemoglobin A1c, SBP systolic blood pressure, DBP diastolic blood pressure, NAFLD non-alcoholic fatty liver disease
Association of RC/HDL-C ratio with risk of NAFLD
Four multivariate logistic regression models were developed to assess the association between RC/HDL-C ratio and NAFLD risk (Table 2). The unadjusted OR for the association between the RC/HDL-C ratio and NAFLD risk was 2.90 (95% CI 2.76–3.05). After adjusting for age and sex, the RC/HDL-C ratio was associated with an increased risk of NAFLD (Model I: OR 2.46, 95% CI 2.34–2.60). Model II adjusted the non-collinear variables that have an impact of more than 10% on the risk of NAFLD related to the ratio of RC/HDL-C, and the ratio of RC/HDL-C maintained a positive correlation with the risk of NAFLD disease (OR 1.58, 95% CI 1.49–1.67). Model III’s additional adjustment to significant variables in the univariate analysis only slightly weakened the results (OR 1.51, 95% CI 1.43–1.61). In a further fully adjusted model (Model IV), the association between the RC/HDL-C ratio and NAFLD was maintained to the same extent (OR 1.53, 95% CI 1.44–1.63). Additionally, sensitivity analysis showed similar results in the four models, and the risk of NAFLD increased with the increase of RC/HDL-C ratios (P-trend < 0.0001).
Table 2.
Logistic regression analyses for the association between RC/HDL-C ratio and NAFLD in different models
| RC/HDL-C ratio quartile (OR :95% CI) | ||||||
|---|---|---|---|---|---|---|
| Multivariable analysis (per SD increase) |
Q1 | Q2 | Q3 | Q4 | P-trend | |
| Unadjusted Model | 2.90 (2.76, 3.05) | Ref | 3.40 (2.58, 4.47) | 11.48(8.91,14.78) | 39.10 (30.53, 50.07) | < 0.0001 |
| Model I | 2.46 (2.34, 2.60) | Ref | 2.93 (2.22, 3.86) | 8.59 (6.63, 11.12) | 25.88 (20.03, 33.44) | < 0.0001 |
| Model II | 1.58 (1.49, 1.67) | Ref | 1.89 (1.41, 2.53) | 3.58 (2.72, 4.73) | 6.20 (4.71, 8.18) | < 0.0001 |
| Model III | 1.51 (1.43, 1.61) | Ref | 1.82 (1.35, 2.45) | 3.25 (2.45, 4.32) | 5.42 (4.06, 7.22) | < 0.0001 |
| Model IV | 1.53 (1.44, 1.63) | Ref | 1.80 (1.33, 2.42) | 3.25 (2.45, 4.32) | 5.49 (4.11, 7.33) | < 0.0001 |
RC/HDL-C ratio remnant cholesterol/high-density lipoprotein cholesterol ratio, OR odds ratios, CI confidence interval, SD standard deviation, NAFLD non-alcoholic fatty liver disease
Model I adjusted for sex and age
Model II adjusted for sex, BMI, TC, FPG and SBP
Model III adjusted for sex, age, BMI, ALT, AST, height, habit of exercise, GGT, TC, FPG, HbA1c and SBP
Model IV adjusted for sex, age, BMI, ALT, AST, height, habit of exercise, GGT, TC, FPG, HbA1c, SBP, drinking status and smoking status
Subgroup analysis of the relationship between RC/HDL-C ratio and the risk of NAFLD
This study investigated the relationship between the RC/HDL-C ratio and the risk of NAFLD in subgroups of different sexes, ages, BMI and habits of exercise. As shown in Table 3, although stratified analysis provided some reference information in different subgroups, we only observed an interaction between sex and RC/HDL-C ratio in relation to NAFLD risk. Among them, when the RC/HDL-C ratio was higher, the risk of NAFLD associated with women was significantly higher [OR: 2.07 (women) vs. 1.50 (men), P-interaction < 0.0001)].
Table 3.
Stratified associations between RC/HDL-C ratio and NAFLD by age, sex, BMI and habit of exercise
| Subgroup | unadjusted OR (95%CI) (Per SD increase) |
adjusted 0R (95%CI) (Per SD increase) |
P-interaction |
|---|---|---|---|
| Age (years) | 0.0345 | ||
| 18–29 | 10.71 (5.13, 22.32) | 0.73 (1.75, 12.75) | |
| 30–44 | 3.52 (3.26, 3.79) | 1.62 (1.49, 1.76) | |
| 44–59 | 2.41 (2.24, 2.59) | 1.50 (1.38, 1.63) | |
| ≥ 60 | 1.75 (1.45, 2.12) | 1.40 (1.14, 1.72) | |
| Sex | < 0.0001 | ||
| Women | 4.08 (3.60, 4.63) | 2.07 (1.80, 2.38) | |
| Men | 2.21 (2.09, 2.34) | 1.50 (1.41, 1.60) | |
| BMI (kg/m2) | 0.1560 | ||
| < 18.5 | 2.72 (0.55, 13.48) | 1.62 (0.23, 11.32 | |
| ≥ 18.5, < 25 | 2.38 (2.23, 2.53) | 1.77 (1.66, 1.90) | |
| ≥ 25, < 30 | 1.90 (1.72, 2.09) | 1.54 (1.39, 1.71) | |
| ≥ 30 | 2.13 (1.39, 3.26) | 1.52 (0.98, 2.37) | |
| Habit of exercise | 0.1817 | ||
| Yes | 2.51 (2.22, 2.83) | 1.46 (1.29, 1.66) | |
| No | 2.98 (2.82, 3.15) | 1.46 (1.29, 1.66) |
RC/HDL-C ratio remnant cholesterol/high-density lipoprotein cholesterol ratio, OR odds ratios, CI confidence interval, SD standard deviation, BMI body mass index, NAFLD non-alcoholic fatty liver disease; other abbreviations as in Table 1
Adjusted for sex, BMI, TC, FPG and SBP
Accuracy of RC/HDL-C ratio in identifying NAFLD
ROC curves were used to assess the ability of the RC/HDL-C ratio and other lipid parameters to identify NAFLD (Table 4). As shown in Fig. 1, most of the lipid parameters have moderate discrimination ability for NAFLD. Compared with HDL-C, TC, TG, non-HDL-C, LDL-C and RC, RC/HDL-C ratio has a significantly higher AUC value in the identification of NAFLD (AUC:0.82, all P < 0.0001).
Table 4.
Areas under the receiver operating characteristic curves for each lipid parameters in identifying non-alcoholic fatty liver disease
| AUC | 95%CI low | 95%CI upp | Best threshold | Specificity | Sensitivity | |
|---|---|---|---|---|---|---|
| HDL-C, mmol/L* | 0.76 | 0.75 | 0.77 | 1.34 | 0.65 | 0.75 |
| TC, mmol/L* | 0.63 | 0.62 | 0.64 | 5.21 | 0.60 | 0.61 |
| Non-HDL-C, mmol/L* | 0.73 | 0.72 | 0.74 | 3.77 | 0.64 | 0.72 |
| LDL-C, mmol/L* | 0.69 | 0.68 | 0.71 | 3.05 | 0.56 | 0.74 |
| RC, mmol/L* | 0.80 | 0.79 | 0.81 | 0.58 | 0.69 | 0.76 |
| TG, mmol/L* | 0.79 | 0.78 | 0.80 | 0.84 | 0.68 | 0.77 |
| RC/HDL-C ratio | 0.82 | 0.81 | 0.83 | 0.43 | 0.69 | 0.80 |
AUC area under the curve, CI confidence interval; other abbreviations as in Table 1
*P < 0.0001, compare with RC/HDL-C ratio by Delong test
Fig. 1.
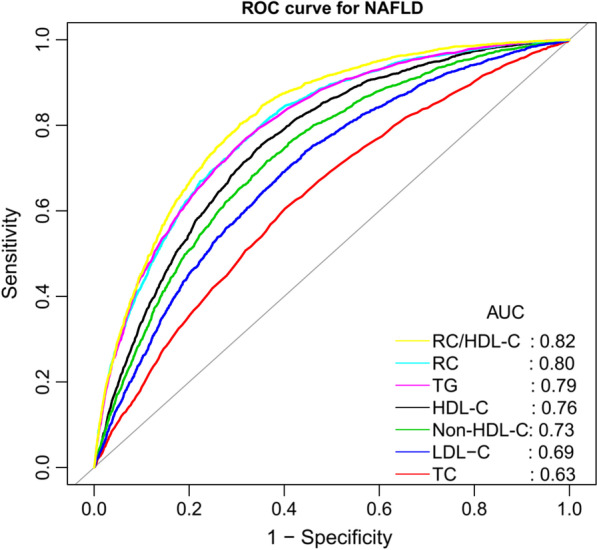
ROC curve analysis of NAFLD-related lipid parameters. ROC: receiver operating characteristic; AUC: area under the curve; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride; non-HDL-C: non-high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; RC: remnant cholesterol; RC/HDL-C ratio: remnant cholesterol/ high-density lipoprotein cholesterol ratio; NAFLD: non-alcoholic fatty liver disease
Discussion
In this secondary analysis of the NAGALA cohort, we found that the higher RC/HDL-C ratio in the general population may be closely related to the increased risk of NAFLD. Additionally, our results show that the combination of atherosclerotic lipid HDL-C and RC improves the recognition ability of NAFLD (AUC:0.82), and is significantly better than the traditional lipid parameters. As far as we know, this study proves for the first time that there is a correlation between RC/HDL-C ratio and NAFLD. More importantly, the calculation of the RC/HDL-C ratio is very simple, and this simple new parameter may provide an effective monitoring means of preventing and managing the NAFLD risk in the general population.
Atherogenic dyslipidemia is an important feature of NAFLD and has been recognized as a risk factor for NAFLD [9, 11]. RC is an unconventional lipid, calculated as non-HDL-C – LDL-C [28]. In previous studies, RC has been shown to be a major lipid parameter mediating residual risk of cardiovascular disease, mainly due to its strong atherogenic effect [14–16]. It is reported that RC can promote the formation of foam cells and cause atherosclerosis in many ways. In addition, RC is also involved in the inflammation of the arterial wall, resulting in vascular injury [33–35]. Aside from being closely related to cardiovascular disease, several recent studies have found that higher levels of RC also significantly increase the risk of diabetic complications, hypertension and NAFLD [17–20]. These results suggested that RC has the potential to be used as a predictor of metabolic diseases. HDL-C is an anti-atherosclerotic lipid parameter, and many studies in the past have shown that HDL-C is closely related to a variety of metabolic diseases [9, 10, 36]. So can the combination of RC and HDL-C improve the discrimination ability of NAFLD? Can the combined parameters be used to assess NAFLD risk? In order to solve these problems, a series of analyses were carried out in this study. The study showed that the combination of RC and HDL-C significantly improved the ability of NAFLD identification, and was significantly better than other conventional lipid parameters. Additionally, this study revealed for the first time that RC/HDL-C ratio is an independent risk factor for NAFLD. At present, there are very few studies on RC/HDL-C ratio, and only a few studies have carried out some correlation analysis [21, 22, 37]. As early as 1998, Masuoka et al. described RC/HDL-C ratio for the first time. They evaluated 124 patients who had received coronary angiography, and found that RC/HDL-C ratio was valuable as a predictor of coronary artery disease [37]. Then, several recent studies specifically evaluated the value of the RC/HDL-C ratio in predicting intracranial atherosclerotic and diabetic complications [21, 22]. Combined with our current research, these results suggest that RC/HDL-C ratio is a metabolic-related marker with good potential, which should be paid attention to by more researchers.
In this study, there were some special findings in the subgroup analysis. Subgroup analysis showed that there was a significant interaction between sex and the risk of NAFLD related to the RC/HDL-C ratio, and when the RC/HDL-C ratio was higher, the risk of NAFLD in men was significantly lower than that in women. However, according to the comparison of baseline information in Table 1, it can be seen that the proportion of men was about 5 times higher than that of women in the higher RC/HDL-C ratio group. It may seem odd that there were fewer women in the group with the higher RC/HDL-C ratio but the risk of developing NAFLD is higher. To clarify this particular association, we summarized the baseline characteristics of NAFLD patients according to sex in the highest RC/HDL-C ratio (Q4). As shown in Table 5, women with NAFLD were significantly older than men in Q4, and dyslipidemia appeared more severe in women with NAFLD than in men. Older age in women means aging of the ovaries and decreased estrogen secretion, which leads to a higher risk of NAFLD [38, 39]. In addition, estrogen deficiency in women promotes atherosclerotic lipid abnormalities, visceral weight gain and insulin resistance (IR), which increases the risk of liver disease and heart metabolism [40, 41].
Table 5.
Baseline characteristics of NAFLD patients in Q4 group of RC/HDL-C ratio
| Women | Men | P-value | |
|---|---|---|---|
| No. of patients | 184 | 1368 | |
| Age, years | 50.00 (42.00–54.00) | 43.00 (38.00–50.00) | < 0.001 |
| Weight, kg | 64.74 (10.07) | 75.47 (10.46) | < 0.001 |
| Height, cm | 156.77 (5.03) | 170.64 (5.93) | < 0.001 |
| BMI, kg/m2 | 26.31 (3.60) | 25.87 (2.95) | 0.070 |
| WC, cm | 84.79 (9.21) | 87.54 (7.10) | < 0.001 |
| ALT, U/L | 21.50 (16.00–29.00) | 31.00 (23.00–44.00) | < 0.001 |
| AST, U/L | 19.00 (16.00–22.00) | 21.50 (17.00–27.00) | < 0.001 |
| GGT, U/L | 16.00 (13.00–21.00) | 26.00 (19.00–37.00) | < 0.001 |
| HDL-C, mmol/L | 1.13 (0.18) | 1.03 (0.18) | < 0.001 |
| Non-HDL-C, mmol/L | 4.73 (4.21–5.30) | 4.52 (4.04–5.03) | < 0.001 |
| TC, mmol/L | 5.91 (0.88) | 5.59 (0.83) | < 0.001 |
| LDL-C, mmol/L | 3.85 (3.47–4.35) | 3.67 (3.20–4.13) | < 0.001 |
| TG, mmol/L | 1.48 (1.21–1.92) | 1.60 (1.25–2.16) | 0.043 |
| RC, mmol/L | 0.81 (0.74–0.95) | 0.83 (0.71–0.98) | 0.725 |
| RC/HDL-C ratio | 0.70 (0.61–0.86) | 0.78 (0.65-1.00) | < 0.001 |
| FPG, mmol/L | 5.35 (0.37) | 5.43 (0.35) | 0.003 |
| HbA1c, % | 5.46 (0.32) | 5.28 (0.34) | < 0.001 |
| SBP, mmHg | 124.58 (18.16) | 124.67 (14.42) | 0.937 |
| DBP, mmHg | 77.37 (11.08) | 79.08 (10.01) | 0.032 |
| Habit of exercise | 24 (13.04%) | 185 (13.52%) | 0.858 |
| Drinking status | < 0.001 | ||
| Non or small | 179 (97.28%) | 1110 (81.14%) | |
| Light | 5 (2.72%) | 177 (12.94%) | |
| Moderate | 0 (0.00%) | 81 (5.92%) | |
| Smoking status | < 0.001 | ||
| Non | 157 (85.33%) | 485 (35.45%) | |
| Past | 9 (4.89%) | 391 (28.58%) | |
| Current | 18 (9.78%) | 492 (35.96%) |
Values were expressed as mean (standard deviation) or medians (quartile interval) or n (%)
Abbreviations as in Table 1
The underlying mechanism of the association between RC/HDL-C ratio and NAFLD is still uncertain, but IR may be involved in the association. Previous studies have found that TG to HDL-C ratio was an effective substitute marker for IR and had good IR prediction performance [42, 43], while RC is TG-rich cholesterol, and previous studies have also found that RC was closely related to IR [44]. Therefore, we speculated that RC/HDL-C ratio may be closely related to IR. However, IR information was not measured in this study and therefore could not be evaluated. Further studies are needed to clarify the correlation between RC/HDL-C ratio and IR.
This observational study has several strengths: (1) This study provides the first evidence that RC/HDL-C ratio is independently positively correlated with NAFLD. (2) In this study, a relatively strict statistical model adjustment strategy is implemented, and the sample size is large, so the conclusion of the study can be considered to be reliable. (3) The participants in this study are all people who have undergone physical examination, and the results of the study are very suitable for promotion in the general population.
Several limitations are also worth mentioning: (1) As mentioned above, IR was not measured in this study, so the association mechanism between RC/HDL-C ratio and NAFLD needs to be confirmed by further studies. (2) In this study, NAFLD was diagnosed by ultrasound, and some mild hepatic steatosis may be missed compared with liver biopsy. In addition, the current study excluded people who were taking oral drugs at baseline and people with abnormal blood glucose, which may also lead to the underestimation of the prevalence of NAFLD in the current sample and a certain selection bias. But from another point of view, this study found a correlation between RC/HDL-C ratio and NAFLD in the case of low prevalence, which further suggests that there is a strong correlation between them. (3) In this study, there is a lack of dietary information and some measurement parameters (such as neck and chest circumference), which may help to further understand the relationship between the two. (4) Since this study was designed as a cross-sectional investigation type, it could not prove whether there is a causal relationship between the two, and further prospective studies are needed.
Conclusion
The work of this study found an independent positive correlation between RC/HDL-C ratio and NAFLD for the first time. Compared with traditional lipid parameters, the RC/HDL-C ratio has a better ability to identify NAFLD in the general population. Based on these strong results, the RC/HDL-C ratio may be a simple, reliable and easy to popularize parameter, which can be used to evaluate the risk of NAFLD in the general population and provide new ideas for improving the risk stratification of NAFLD.
Supplementary Information
Additional file 1: Table S1. Collinearity diagnostics steps.
Acknowledgements
The authors thank all the staff and participants of this study for their important contributions.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- RC
Remnant cholesterol
- HDL-C
High-density lipoprotein cholesterol
- ROC
Receiver operating characteristic curve
- RC/HDL-C ratio
Remnant cholesterol/high-density lipoprotein cholesterol ratio
- TG
Triglycerides
- NAGALA
NAfld in Gifu Area, Longitudinal Analysis
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
Gamma-glutamyl transferase
- TC
Total cholesterol
- HbA1c
Hemoglobin A1c
- FPG
Fasting plasma glucose
- LDL-C
Low-density lipoprotein cholesterol
- OR
Odds ratio
- CI
Confidence interval
- AUC
Area under the curve
- IR
Insulin resistance
Authors’ contributions
YZ and YL-C conceived and designed the study. YZ, CH and YL-C classified data and conducted the statistical analysis. YZ, MB-K and YL-C wrote the first draft of the manuscript. All authors commented and contributed to the interpretation of results and the final manuscript. All authors read and approved the final manuscript.
Funding
No.
Availability of data and materials
The datasets that support the conclusions of this article can be found in the Dryad repository, and we confirm that this data set is publicly available in the Dryad database (https://datadryad.org/stash/dataset/doi:10.5061%2Fdryad.8q0p192).
Declarations
Ethics approval and consent to participate
As the previous study had been authorized by the ethics committee of Murakami Memorial Hospital, the Institutional Review Board of this study exempted the application for duplicate ethical authorization (IRB Number: 2021-066). We confirm that all the methods used in this study were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM. Non-alcoholic fatty liver disease: a global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65(8):1017–25. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47-64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Ballestri S, Mantovani A, Nascimbeni F, Lugari S, Lonardo A. Extra-hepatic manifestations and complications of nonalcoholic fatty liver disease. Future Med Chem. 2019;11(16):2171–2192. doi: 10.4155/fmc-2019-0003. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 9.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–23. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Corey KE, Chalasani N. Management of dyslipidemia as a cardiovascular risk factor in individuals with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(7):1077–84. doi: 10.1016/j.cgh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou Y, Zhong L, Hu C, Zhong M, Peng N, Sheng G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis. 2021;20(1):28. doi: 10.1186/s12944-021-01457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varbo A, Nordestgaard BG. Remnant lipoproteins. Curr Opin Lipidol. 2017;28(4):300–307. doi: 10.1097/MOL.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 13.Salinas CAA, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol. 2020;31(3):132–139. doi: 10.1097/MOL.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 14.Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb. 2009;16(3):145–54. doi: 10.5551/jat.e598. [DOI] [PubMed] [Google Scholar]
- 15.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 16.Authors/Task Force Members. ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Cao YX, Zhang HW, Jin JL, et al. The longitudinal association of remnant cholesterol with cardiovascular outcomes in patients with diabetes and pre-diabetes. Cardiovasc Diabetol. 2020;19(1):104. doi: 10.1186/s12933-020-01076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Fan F, Zheng B, et al. Associations between remnant lipoprotein cholesterol and central systolic blood pressure in a Chinese community-based population: a cross-sectional study. Lipids Health Dis. 2021;20(1):60. doi: 10.1186/s12944-021-01490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin J, Mori TA, Adams LA, et al. Association between remnant lipoprotein cholesterol levels and non-alcoholic fatty liver disease in adolescents. JHEP Rep. 2020;2(6):100150. doi: 10.1016/j.jhepr.2020.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastori D, Baratta F, Novo M, Cocomello N, Violi F, Angelico F, Del Ben M. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non-alcoholic fatty liver disease. J Clin Med. 2018;7(11):378. doi: 10.3390/jcm7110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng RX, Li S, Zhang MZ, et al. Remnant cholesterol predicts periprocedural myocardial injury following percutaneous coronary intervention in poorly-controlled type 2 diabetes. J Cardiol. 2017;70(2):113–120. doi: 10.1016/j.jjcc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Yang WS, Li R, Shen YQ, et al. Importance of lipid ratios for predicting intracranial atherosclerotic stenosis. Lipids Health Dis. 2020;19(1):160. doi: 10.1186/s12944-020-01336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond) 2019;43(1):139–148. doi: 10.1038/s41366-018-0076-3. [DOI] [PubMed] [Google Scholar]
- 24.Okamura Takuro, et al. Data from: Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Dataset: Dryad; 2019. [DOI] [PubMed] [Google Scholar]
- 25.Choi JH, Sohn W, Cho YK. The effect of moderate alcohol drinking in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(4):662–669. doi: 10.3350/cmh.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol Am J Gastroenterol. 2007;102:2708–15. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 27.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Zhang X, Pan B, et al. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010;9:52. doi: 10.1186/1476-511X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitchett EJA, Seale AC, Vergnano S, et al. Strengthening the reporting of observational studies in epidemiology for newborn infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis. 2016;16:e202-e213. doi: 10.1016/S1473-3099(16)30082-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–569. doi: 10.4097/kja.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 32.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 33.Mearns BM, Dyslipidaemia Role of remnant cholesterol in IHD. Nat Rev Cardiol. 2013;10(10):553. doi: 10.1038/nrcardio.2013.132. [DOI] [PubMed] [Google Scholar]
- 34.Bernelot Moens SJ, Verweij SL, Schnitzler JG, et al. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humans. Arterioscler Thromb Vasc Biol. 2017;37(5):969–975. doi: 10.1161/ATVBAHA.116.308834. [DOI] [PubMed] [Google Scholar]
- 35.Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10(4):328–35. doi: 10.2174/138945009787846434. [DOI] [PubMed] [Google Scholar]
- 36.Kjeldsen EW, Nordestgaard LT, Frikke-Schmidt R. HDL cholesterol and non-cardiovascular disease: a narrative review. Int J Mol Sci. 2021;22(9):4547. doi: 10.3390/ijms22094547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuoka H, Ishikura K, Kamei S, et al. Predictive value of remnant-like particles cholesterol/high-density lipoprotein cholesterol ratio as a new indicator of coronary artery disease. Am Heart J. 1998;136(2):226–30. doi: 10.1053/hj.1998.v136.89586. [DOI] [PubMed] [Google Scholar]
- 38.Turola E, Petta S, Vanni E, et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis Model Mech. 2015;8(9):1037–46. doi: 10.1242/dmm.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 40.Robeva R, Mladenović D, Vesković M, et al. The interplay between metabolic dysregulations and non-alcoholic fatty liver disease in women after menopause. Maturitas. 2021;151:22–30. doi: 10.1016/j.maturitas.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17(1):47–66. doi: 10.1038/s41574-020-00431-8. [DOI] [PubMed] [Google Scholar]
- 42.Yeh WC, Tsao YC, Li WC, Tzeng IS, Chen LS, Chen JY. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: a cross-sectional study. Lipids Health Dis. 2019;18(1):176. doi: 10.1186/s12944-019-1123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannini C, Santoro N, Caprio S, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–74. doi: 10.2337/dc10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohnishi H, Saitoh S, Takagi S, et al. Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis. 2002;164(1):167–70. doi: 10.1016/s0021-9150(02)00057-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Collinearity diagnostics steps.
Data Availability Statement
The datasets that support the conclusions of this article can be found in the Dryad repository, and we confirm that this data set is publicly available in the Dryad database (https://datadryad.org/stash/dataset/doi:10.5061%2Fdryad.8q0p192).


