Abstract
A mutant, named 11B, hypersusceptible to aminoglycosides, tetracycline, and erythromycin was isolated after Tn501 insertion mutagenesis of Pseudomonas aeruginosa PAO1. Cloning and sequencing experiments showed that 11B was deficient in an, at that time, unknown active efflux system that contains homologs of MexAB. This locus also contained a putative regulatory gene, mexZ, transcribed divergently from the efflux operon. Introduction of a recombinant plasmid that carries the genes of the efflux system restored the resistance of 11B to parental levels, whereas overexpression of these genes strongly increased the MICs of substrate antibiotics for the PAO1 host. Antibiotic accumulation studies confirmed that this new system is an energy-dependent active efflux system that pumps out aminoglycosides. Furthermore, this system appeared to function with an outer membrane protein, OprM. While the present paper was being written and reviewed, genes with a sequence identical to our pump genes, mexXY of P. aeruginosa, have been reported to increase resistance to erythromycin, fluoroquinolones, and organic cations in Escherichia coli hosts, although efflux of aminoglycosides was not examined (Mine et al., Antimicrob. Agents Chemother. 43:415–417, 1999). Our study thus shows that the MexXY system plays an important role in the intrinsic resistance of P. aeruginosa to aminoglycosides. Although overexpression of MexXY increased the level of resistance to fluoroquinolones, disruption of the mexXY operon in P. aeruginosa had no detectable effect on susceptibility to these agents.
Multidrug active efflux systems have recently been recognized as efficient mechanisms of resistance in P. aeruginosa. For example, the MexAB-OprM system, which is expressed constitutively in wild-type cells, contributes greatly to the natural resistance of the pathogen to a wide range of antibacterial agents including β-lactams, β-lactamase inhibitors, quinolones, chloramphenicol, tetracycline, trimethoprim, sulfamethoxazole, and novobiocin (13, 17, 18, 21, 22, 28). Furthermore, MexAB-OprM may confer high levels of resistance to clinical strains when it is overexpressed as a result of mutations that occur mainly in the mexR regulatory gene (34).
Two other systems, namely, MexCD-OprJ and MexEF-OprN, which are homologous to MexAB-OprM but which are not constitutively produced in wild-type cells of P. aeruginosa, have also been reported to be involved in the multidrug resistance of laboratory mutants (6, 10, 14, 27) and clinical strains (7, 11). The latter efflux pumps, which display more restricted antibiotic substrate spectrums than MexAB-OprM, are still able to accommodate compounds as structurally different as quinolones, chloramphenicol, and trimethoprim (6, 10, 14, 20, 22, 26).
Up to the present, RND-type efflux systems of P. aeruginosa were believed to transport only lipophilic or amphiphilic compounds since very hydrophilic antibiotics such as aminoglycosides were not substrates of the already known pumps. Yet, P. aeruginosa strains usually show significant intrinsic levels of resistance to aminoglycosides. We therefore looked for a possible aminoglycoside efflux pump in P. aeruginosa by first isolating an aminoglycoside-hypersusceptible mutant and by then looking for genomic clones that could restore resistance in this mutant. We identified a new homolog of MexAB-MexCD-MexEF systems and then showed directly that this new system pumps out aminoglycosides. While the present work was being reviewed, a paper by Mine et al. (23) appeared. That paper described P. aeruginosa genomic clone mexXY, which was shown to increase levels of resistance to fluoroquinolones, erythromycin, and ethidium bromide in Escherichia coli host cells. This system was found to function cooperatively with OprM, the outer membrane component of MexAB-OprM, for the export of substrate antibiotics in E. coli. Mine et al. (23), however, did not examine the effect of MexXY on aminoglycoside resistance, nor did they study the function of this system in P. aeruginosa. In this paper we provide evidence that MexXY plays a crucial role in the intrinsic resistance of P. aeruginosa to aminoglycosides.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Wild-type strain P. aeruginosa PAO1 was from B. W. Holloway’s culture collection. PAO1T is an oprM::ΩHg derivative of PAO1 obtained by transduction (22); PAM1275 is a mexB::ΩHg mutant of PAO1 (18a). P. aeruginosa MT350 (met-9020 catA1 nar-9011 cnu-9001 puuE8 tyu-9025 rif-8003) was used as a donor for the conjugational transfer of plasmid pMT1000 to PAO1 (32). DNA manipulations were performed with E. coli DH5α [supE44 endA1 hsdR17 (rk−mk+) thi-1 recA1 Δ(argF-lac zya) U169 φ80 lacZ ΔM15], which was obtained from Gibco BRL. Strains of E. coli and P. aeruginosa were grown in Luria-Bertani broth (LB) or on Mueller-Hinton (MH) agar plates (Sanofi Pasteur Diagnostics). When necessary, ampicillin (Ap; 100 μg ml−1), kanamycin (25 μg ml−1), and tetracyline (30 μg ml−1) for E. coli or ticarcillin (Tic; 250 μg ml−1) and mercuric chloride (15 μg ml−1) for P. aeruginosa were added to the growth media. All bacterial cultures in LB were incubated at 37°C with shaking (200 rpm). As specified below, some mating experiments were done with the minimal agar medium of Davis and Mingioli (4) supplemented with 0.5% (wt/vol) glucose for selection of PAO1 transconjugants.
DNA methodology.
Chromosomal DNA was prepared by the procedure of Chen and Kuo (3). Genomic libraries were constructed with restricted DNA fragments that were selected for the appropriate size (10 to 20 kbp) on 10 to 40% (wt/vol) linear sucrose gradients and that were subsequently ligated with a plasmid vector. Electrotransformation of E. coli DH5α (5) and P. aeruginosa (31) with plasmid DNA has been described in detail elsewhere. Plasmid DNA was routinely prepared by the alkaline lysis procedure (29) or by using Plasmid Midi Preps kit from Qiagen S.A. Selected restriction fragments were purified from agarose gels with the Jet-Sorb kit (Genome Inc.). Probes used in Southern and colony blotting experiments were labeled by random priming (Amersham) with [α-32P]dCTP (Dupont NEN) by following the manufacturer’s instructions. DNA hybridization and autoradiography were performed under standard conditions (29). Other reagents for molecular biology procedures were purchased from Gibco BRL, Stratagene Inc., or Sigma Chemical Co.
Transposon mutagenesis with pMT1000.
Tn501 tagging of the P. aeruginosa PAO1 chromosome was carried out with pMT1000, a plasmid that was derived from R68::Tn501 and that is temperature sensitive for replication and maintenance (32). Insertional mutants of PAO1 were selected on minimal glucose agar medium containing 15 μg of HgCl2 ml−1 at 42°C after conjugational transfer of pMT1000 from donor strain MT350 at 30°C. A total of 6,200 Tn501 mutants were isolated and were subsequently tested for resistance or hypersusceptibility to aminoglycosides by individual replication on MH plates containing 0.5 or 16 μg of amikacin ml−1.
Plasmid constructions.
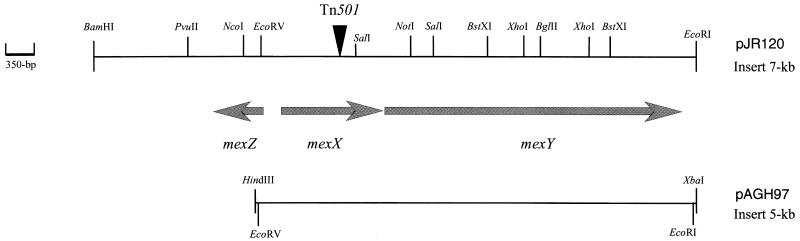
The recombinant plasmids used in this study were obtained as follows. Insertion of transposon Tn501 in the chromosome of mutant 11B was confirmed by Southern hybridization of a 1-kbp radiolabeled PCR product that encompasses the mercuric reductase gene (merA) of Tn501 with BamHI- and HindIII-restricted fragments of the mutant 11B genome. A BamHI-HindIII genomic DNA library from mutant 11B was then constructed in E. coli DH5α with the versatile plasmid vector pUC18 (Apr) (33) and was screened with the merA probe. Restriction mapping of positive clones revealed the presence of a 12.8-kbp insert consisting of a 4.6-kbp fragment from the mutant 11B chromosome adjacent to the 8.2-kbp transposon Tn501. The resultant recombinant vector was named pJR162. A 4.3-kbp EcoRI fragment from pJR162 was subcloned into phagemid pBlueScript KSII+ (Apr; Stratagene Inc.) to give pJR49. A 1.2-kbp internal SalI-NotI fragment from pJR49, selected from a set of 150- to 250-bp nested deletions produced with exonuclease III for sequence determination (see below), was used as a new probe to screen a BamHI-NotI genomic DNA library from PAO1 cloned into pBlueScript KSII+. One E. coli DH5α-positive transformant was selected for further studies and was shown to contain a 4.1-kbp fragment from the PAO1 genome in recombinant plasmid pJR41. A 3-kbp NotI fragment from pJR49 (the second NotI cleavage site is located in the polylinker of pBlueScript KSII+) was isolated by agarose gel electrophoresis and was subcloned into pJR41 to obtain pJR100, a plasmid that carries the mexZ, mexX, and mexY genes. Then, pJR120 (see Fig. 1) was constructed by excision of the whole 7-kbp insert of pJR100 and recloning into the broad-host-range vector pAK1900 (Apr Ticr) (28). Finally, a 5-kbp EcoRV-EcoRI fragment from pJR100 that contained mexXY but not mexZ was subcloned into pBlueScript SKII+ to obtain pBGH80; a 5-kbp HindIII-XbaI fragment of this construct was recloned into pAK1900 to yield pAGH97 (see Fig. 1).
FIG. 1.
Physical map of the mexZXY operon in P. aeruginosa. Inserts of recombinant plasmids pJR120 and pAGH97 are indicated. The site of insertion of transposon Tn501 in mutant 11B is indicated with an arrowhead.
DNA sequencing and protein analysis.
Unidirectional deletions of ca. 150 to 250 bp were produced from the BamHI site located in the polylinker of pJR49 by using exonuclease III and S1 nuclease (Nested Deletion Kit from Pharmacia Biotech). Sequencing was performed from the universal primer sequences of the plasmid vector. The 4.1-kbp BamHI-NotI insert of pJR41 was sequenced by using custom primers (Eurogentec S.A.). Nucleotide sequences of the overlapping fragments were determined on both strands by the dideoxy-chain determination method (30) with a 373A DNA Sequencer (Perking-Elmer Division, Applied Biosystems) at the Institut d’Etude et de Transfert de Gènes in Besançon, France. DNA sequences were edited with Navigator software (Perkin-Elmer). Searches for protein sequence homologies were carried out by using the BLAST program (1) provided by the National Center for Biotechnology Information. Open reading frames (ORFs) and predicted protein domains were established with the DNAStrider 1.0.1. program.
Assays for accumulation in intact cells.
The accumulation assays were performed as described by Li et al. (16) with exponentially growing bacteria. [3H]dihydrostreptomycin (specific activity, 20 Ci mmol−1) and [3H]tetracycline (0.55 Ci mmol−1) were from American Radiolabeled Chemicals, Inc. (St. Louis, Mo.). Radioactivity was quantitated with an Intertechnique SL30 liquid scintillation counter.
Antimicrobial susceptibility testing.
Bacterial susceptibilities to antimicrobial agents were determined by the standard broth microdilution method (2) in MH broth adjusted to 20 mg of Ca2+ liter−1 and 10 mg of Mg2+ liter−1 with inocula of 5 × 105 bacteria ml−1. Chemicals and antibiotics, if not specified, were from Sigma Chemical Co. The following antibiotics were kindly provided by the indicated manufacturers: isepamicin, netilmicin, and gentamicin, Schering-Plough; amikacin, Bristol-Myers Squibb; tobramycin, Lilly Laboratories; erythromycin, Abbott Laboratories; ciprofloxacin, Bayer Pharma; norfloxacin, Merck Sharpe & Dohme.
Nucleotide sequence accession numbers.
The DNA sequences of the genes described in this study were deposited in the GenBank database under the name mexZGH (accession no. AF073776; 22 June 1998), prior to the submission of the mexXY genes (accession no. AB 015853; 25 June 1998). Despite the earlier submission of mexZGH, we have adopted here the designation mexXY proposed by Mine et al. (23) to avoid further confusion.
RESULTS AND DISCUSSION
Isolation of a mutant hypersusceptible to aminoglycosides.
Reference strain PAO1 was mutagenized by random insertion of Tn501. Replication of 6,200 HgCl2r clones on selective agar plates containing different concentrations of amikacin led to the identification of three mutants unable to grow on 0.5 μg of amikacin ml−1 (the MIC of amikacin for PAO1 was 2 μg ml−1). One of them, named mutant 11B, was found to be hypersusceptible to erythromycin, tetracycline, and aminoglycosides (MICs were lowered four- to eightfold) and was selected for further investigation (Table 1). On the otherhand, mutant 11B demonstrated wild-type levels of resistance to ciprofloxacin, norfloxacin, chloramphenicol, trimethoprim, acriflavine, ethidium bromide, and 25 other antimicrobial agents (data not shown).
TABLE 1.
Antibiotic susceptibilities of various P. aeruginosa strains that are deficient in or that overexpress the mexZ and mexXY genes
| Strain (plasmid) | MIC (μg ml−1)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Akn | Gm | Tm | Isp | Net | Tet | Ery | Cip | Nor | |
| PAO1 | 2 | 1 | 0.5 | 2 | 1 | 16 | 256 | 0.03 | 0.125 |
| PAO1(pJR120) | 1 | 0.5 | 0.5 | 4 | 0.5 | 32 | 256 | 0.06 | 0.125 |
| PAO1(pAGH97) | 16 | 8 | 2 | 8 | 16 | 64 | 512 | 0.5 | 2 |
| 11Bb | 0.5 | 0.125 | 0.125 | 0.5 | 0.125 | 4 | 32 | 0.03 | 0.125 |
| 11B(pJR120) | 1 | 0.5 | 0.5 | 1 | 0.5 | 16 | 128 | 0.06 | 0.125 |
| 11B(pAGH97) | 16 | 8 | 2 | 16 | 16 | 64 | 512 | 0.5 | 2 |
| PAO1Tc | 0.5 | 0.125 | 0.125 | 0.25 | 0.06 | 2 | 64 | NDd | ND |
| PAO1T(pAGH97) | 0.5 | 0.25 | 0.25 | 0.5 | 0.125 | 2 | 64 | ND | ND |
| PAO1T(pAGH97; pOMI5) | 8 | 8 | 1 | 8 | 8 | 32 | 512 | ND | ND |
To maintain plasmids pJR120, pAGH97, or plasmid pOMI5 in transformed cells of P. aeruginosa, 50 μg of ticarcillin ml−1 or 50 μg of chloramphenicol ml−1 was added to the growth medium. No modification of MICs was observed for strain PAO1, 11B, or PAO1T when the strain was transformed with a control plasmid. Akn, amikacin; Gm, gentamicin; Tm, tobramycin; Isp, isepamicin; Net, netilmicin; Tet, tetracycline; Ery, erythromycin; Cip, ciprofloxacin; Nor, norfloxacin.
Mutant 11B was also more susceptible than wild-type strain PAO1 to fortimicin and apramycin by the disk method with the Aminoglycoside Resistance Test Kit from Schering Plough Research Institute.
No modification of MICs was observed for strain PAO1T transformed with pJR120.
ND, not determined.
Mutant 11B is deficient in MexXY.
A 7-kbp genomic fragment from strain PAO1 that encompassed the Tn501 insertion site of mutant 11B was cloned into E. coli as described in Materials and Methods. Sequencing of this 7-kbp DNA fragment revealed the presence of three complete ORFs (Fig. 1). ORF1 (1,191 bp) and ORF2 (3,135 bp) showed strong sequence similarity to multidrug efflux systems based on the RND family of transporters, such as AmrAB, which is responsible for aminoglycoside resistance in Burkholderia pseudomallei (51 and 65% identities, respectively) (24), and MexAB of P. aeruginosa (33 and 46% identities, respectively). The sequence was deposited in the data bank as mexGH (see Materials and Methods). We have most recently learned, however, that an identical sequence, mexXY, has subsequently been deposited in the data bank. Because the paper that describes mexXY has been published earlier (23), we will adopt here the nomenclature of Mine et al. and call ORF1 and ORF2, mexX and mexY, respectively. Our sequencing data clearly showed that mutant 11B owes its antibiotic hypersusceptibility phenotype to the disruption of mexXY (mexX::Tn501; Fig. 1). These results provide good evidence that MexXY is produced constitutively in wild-type cells of P. aeruginosa, where it contributes to intrinsic resistance to aminoglycosides.
Identification of mexZ.
The third ORF (ORF3), identified 263 bp upstream of but transcribed divergently from ORF1 (mexX), was found to contain 534 nucleotides and to encode a protein of 178 amino acid residues with a predicted molecular mass of 19,369 Da. The protein is a homolog of AmrR (41% identity and 50% similarity), the putative repressor of the amrAB-oprA operon from B. pseudomallei. Lower but still significant scores were also obtained with the MtrR protein which negatively controls the expression of the mtrCDE operon in Neisseria gonorrhoeae (8), and with the putative repressors of acrAB and acrEF operons in E. coli (18 and 21% identities and 35 and 37% homologies, respectively) (12, 19). The ORF3 product, designated MexZ, displays a helix-turn-helix motif characteristic of DNA binding domains at its N terminus. This domain was found to be highly conserved among MexZ, AcrR, AcrS, MtrR, and AmrR (data not shown). Several possible promoter sequences that showed some homology with the −35 and −10 consensus regions of E. coli promoters were pinpointed upstream of ORF3. Examination of the intergenic region that extends between ORF1 and ORF3 also allowed the detection of an inverted repeat sequence 20 bp ahead of ORF3, similar to that which precedes mtrR, the regulatory gene that encodes the MtrR protein in N. gonorrhoeae (identity of 10 of 13 nucleotides) (9). Altogether these results strongly support the notion that mexZ controls the expression of the mexXY operon.
MexXY exports aminoglycosides.
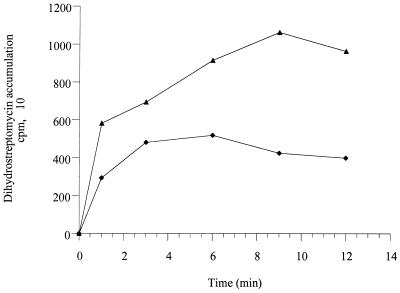
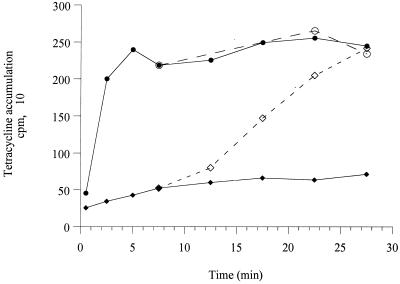
Mine et al. (23) have shown that MexXY actively pumps ethidium bromide out of E. coli cells transformed with the cloned mexXY operon. To unambiguously demonstrate that this efflux system is also involved in the transport of aminoglycosides, accumulation assays with [3H]dihydrostreptomycin were carried out with PAO1 and its hypersusceptible mutant, mutant 11B. Steady-state accumulation of the antibiotic in PAO1 was about 2.5-fold lower than that in mutant 11B (Fig. 2). Addition of the proton conductor carbonyl cyanide m-chlorophenylhydrazone (CCCP; 500 μM) to the cells prior to the uptake experiments did not, however, significantly increased the level of accumulation of [3H]dihydrostreptomycin (data not shown). A similar observation was reported by Moore et al. (24) with B. pseudomallei cells that express AmrAB-OprA. Such a phenomenon is likely to result from the complex inhibitory action of CCCP on both the active inward transport of the aminoglycoside across the cytoplasmic membrane and its efflux via MexXY (or AmrAB-OprA). We checked that MexXY is able to export tetracycline from P. aeruginosa by using a mexB null mutant (strain PAM1275) to circumvent efflux of the antibiotic by the MexAB-OprM system (Fig. 3). In this case, CCCP inhibited the energy-dependent efflux process mediated by MexXY and caused an increased level of accumulation of tetracycline.
FIG. 2.
Accumulation of [3H]dihydrostreptomycin by intact cells of P. aeruginosa PAO1 (⧫) and mutant 11B (▴). The MICs of dihydrostreptomycin for PAO1 and mutant 11B were 16 and 4 μg ml−1, respectively. The cells were grown in LB, harvested, washed (50 mM phosphate buffer, 100 mM LiCl), and resuspended in 50 mM of phosphate buffer (pH 7) containing 1 mM MgSO4 and 0.2% (wt/vol) glucose as a source of carbon. After 5 min at 37°C, a mixture of dihydrostreptomycin and [3H]dihydrostreptomycin (20 Ci mmol−1) was added to a final concentration of 8 μg ml−1; and the samples were taken and filtered, and the radioactivity was counted. The data are means for three independent experiments.
FIG. 3.
Accumulation of [3H]tetracycline by intact cells of P. aeruginosa PAM1275 (●, ○) and PAM1275(pAGH97) (⧫, ◊). The MICs of tetracycline for PAM1275 and PAM1275(pAGH97) were 2 and 16 μg ml−1, respectively. The cells were prepared as described in the legend to Fig. 2. After 5 min at 37°C, a mixture of tetracycline and [3H]tetracycline (0.55 Ci mmol−1) was added to a final concentration of 2 μg ml−1; and the samples were taken and filtered, and the radioactivity was counted. After 7.5 min of incubation, a 500 μM final concentration of CCCP was added to one-half of the reaction mixture. Accumulation in CCCP-treated samples is represented by open symbols, and that in samples that did not receive CCCP is indicated by closed symbols. The data are means for three independent experiments. No modification of intracellular accumulation of antibiotic was observed when strains were transformed with pAK1900.
Overexpression of mexXY in P. aeruginosa.
In order to investigate the effects of overproduced MexXY on resistance levels, mutant 11B and its parent strain, PAO1, were transformed with pJR120, a recombinant plasmid carrying the mexZXY genes. The MICs of representative antibiotics for both strains, mutant 11B and strain PAO1, are presented in Table 1. While the resistance to aminoglycosides, tetracycline, and erythromycin was restored to parental levels in mutant 11B following the introduction of pJR120 (two to four times the MICs, that of PAO1 remained unchanged. The lack of influence of pJR120 on PAO1 resistance is consistent with the hypothesis that mexZ downregulates the expression of mexXY (see above). Transformation of 11B and PAO1 with plasmid pAGH97 (mexXY only; Fig. 1) produced substantial increases in the levels of resistance of both strains to substrate antibiotics (4 to 128 times the MICs), thus providing further evidence that overexpression of MexXY may determine multidrug resistance in P. aeruginosa. The MICs of fluoroquinolones, ciprofloxacin, and norfloxacin were also raised noticeably (16 times the MIC).
OprM is functional with MexXY for export of aminoglycosides.
It has recently been reported that OprM, the outer membrane component of the MexAB-OprM efflux system, may also be used by the MexXY proteins for the export of fluoroquinolones and various inhibitors (23). To gain some insight into the nature of components that may allow highly hydrophilic, polycationic antibiotics such as aminoglycosides to cross the outer membrane while they are exported by the MexXY system, we introduced plasmid pAGH97 into the oprM::Ω Hg PAO1 derivative PAO1T. In striking contrast to its OprM-proficient parent PAO1, no signifiant increase in resistance to aminoglycosides was noted for PAO1T when it was transformed with pAGH97 (Table 1). On the other hand, complementation of PAO1T(pAGH97) with the intact oprM gene carried on plasmid pOMI5 (34) resulted in multidrug resistance at levels similar to those exhibited by PAO1(pAGH97). Consistent with these data, deficiency in OprM (mutant PAO1T) rendered PAO1 cells more susceptible to aminoglycosides (Table 1). Thus, OprM appears to be used by both the MexAB and the MexXY machineries in wild-type cells for the export of a wide variety of compounds including aminoglycosides.
Role of MexXY in resistance of P. aeruginosa.
Although the physiological functions of bacterial efflux systems still remain unclear (25), it is interesting that the constitutive expression of two complementary multidrug transporters, MexAB-OprM and MexXY, in P. aeruginosa provides this organism with natural protection against an incredibly wide range of antibacterial molecules. MexAB-OprM contributes significantly to the intrinsic resistance to β-lactams (except imipenem [15]), fluoroquinolones, tetracyclines, and chloramphenicol (17, 21, 28), whereas MexXY plays an important role in the defense of the bacterium against aminoglycosides (and, to a lesser extent, tetracycline and erythromycin). By contrast, the involvement of MexXY in the natural resistance of P. aeruginosa to fluoroquinolones appears negligible (see mutant 11B, Table 1), even if this system can export these antibiotics (23).
Overexpression of MexAB-OprM has recently been shown to confer clinically relevant levels of resistance to β-lactams in strains isolated from hospitalized patients (34). Whether the overexpression of MexXY may be responsible for higher levels of resistance to aminoglycosides and fluoroquinolones in the clinical setting warrants further investigations.
ACKNOWLEDGMENTS
We thank Colette Godard and Karine Joseph for technical assistance and O. Lomovskaya for supplying strain PAM1275. We are also grateful to the Pseudomonas Genome Project for providing the PAO1 chromosome sequence on-line (28a).
This work was supported by the Association Française de Lutte contre la Mucoviscidose, research grant AI-09644 from the National Institutes of Health, and a grant from France-Berkeley fund.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Grapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3409. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: ASM Press; 1991. [Google Scholar]
- 3.Chen W P, Kuo T T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993;21:2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis B D, Mingioli E S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950;60:17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda H, Hosaka M, Hirai K, Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990;34:1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda H, Hosaka M, Iyobe S, Gotoh N, Nishino T, Hirai K. nfxC-type quinolone resistance in a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:790–792. doi: 10.1128/AAC.39.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagman K E, Pan W B, Spratt B G, Balthazar J T, Judd R C, Shafer W M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology UK. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 9.Hagman K E, Shafer W M. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol. 1995;177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai K, Suzue S, Irikura T, Iyobe S, Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987;31:582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakics E B, Iyobe S, Hirai K, Fukuda H, Hashimoto H. Occurrence of the nfxB type mutation in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:2562–2565. doi: 10.1128/aac.36.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein J R, Henrich B, Plapp R. Molecular analysis and nucleotide sequence of the envCD operon of Escherichia coli. Mol Gen Genet. 1991;230:230–240. doi: 10.1007/BF00290673. [DOI] [PubMed] [Google Scholar]
- 13.Köhler T, Kok M, Michéa Hamzehpour M, Plésiat P, Gotoh N, Nishino T, Curty L K, Pechère J C. Multidrug efflux in intrinsic resistance to trimethoprim and sulfamethoxazole in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:2288–2290. doi: 10.1128/aac.40.10.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köhler T, Michéa Hamzehpour M, Henze U, Gotoh N, Kocjancic Curty L, Pechère J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 15.Köhler T, Michéa Hamzehpour M, Epp S F, Pechère J C. Carbapenem activities against Pseudomonas aeruginosa: respective contributions of OprD and efflux systems. Antimicrob Agents Chemother. 1999;43:424–427. doi: 10.1128/aac.43.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X Z, Ma D, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to beta-lactam resistance. Antimicrob Agents Chemother. 1994;38:1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X Z, Srikumar R, Poole K. β-Lactamase inhibitors are substrates for the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:399–403. doi: 10.1128/aac.42.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Lomoskaya, O. Personal communication.
- 19.Ma D, Alberti M, Lynch C, Nikaido H, Hearst J E. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 20.Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:909–913. doi: 10.1128/aac.40.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michéa Hamzehpour M, Pechère J C, Plésiat P, Köhler T. OprK and OprM define two genetically distinct multidrug efflux systems in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:2392–2396. doi: 10.1128/aac.39.11.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore R A, DeShazer D, Reckseidler S, Weissman A, Woods D E. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother. 1999;43:465–470. doi: 10.1128/aac.43.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyfakh A A. Natural functions of bacterial multidrug transporters. Trends Microbiol. 1997;5:309–313. doi: 10.1016/S0966-842X(97)01064-0. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki T, Hirai K. Cloning and nucleotide sequence of the Pseudomonas aeruginosa nfxB gene, conferring resistance to new quinolones. FEMS Microbiol Lett. 1992;97:197–202. doi: 10.1016/0378-1097(92)90386-3. [DOI] [PubMed] [Google Scholar]
- 27.Poole K, Gotoh N, Tsujimoto H, Zhao Q X, Wada A, Yamasaki T, Neshat S, Yamagishi J I, Li X Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 28.Poole K, Krebes K, Mcnally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Pseudomonas Genome Project. 15 March 1999, posting date. [16 March 1999, last date accessed.]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith A W, Iglewski B H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989;17:10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda M, Harayama S, Lino T. Tn501 insertion mutagenesis in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1984;196:494–500. doi: 10.1007/BF00436198. [DOI] [PubMed] [Google Scholar]
- 33.Yanisch-Perron C, Vieria J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13 mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 34.Ziha-Zarifi I, Llanes C, Köhler T, Pechère J C, Plésiat P. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob Agents Chemother. 1999;43:287–291. doi: 10.1128/aac.43.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]