This article has supplementary material on the web site: www.jdbp.org.
Index terms: children and adolescents, HIV disclosure, HIV care, LMIC, Africa, mental health
ABSTRACT:
Objective:
The aim of this study was to describe the disclosure process in children with perinatally acquired HIV infection (PHIV+) and its impact on their emotional well-being and adherence to antiretroviral therapy (ART) in South Africa.
Methods:
This prospective cohort study followed PHIV+ children aged 7 to 13 years attending counseling over 18 months. Standardized disclosure tools were used by a counselor with both child and caregiver present. Assessments included the Child Behavior Checklist (CBCL), Vineland Adaptive Behavior Scale (VABS), Child Depression Inventory (CDI), and Revised Children's Manifest Anxiety Scale (RCMAS). Adherence to ART was recorded through pharmacy pill returns. Changes over time and their differences from baseline were assessed by linear mixed models.
Results:
Thirty children with median age 10 years (interquartile range [IQR]: 9.0–11.0) were enrolled. The median time to disclosure was 48 weeks (IQR: 48.0–54.6). There was a significant decrease from baseline (p < 0.0001) and over time (p = 0.0037) in the total CDI score. A positive trend in the changes from baseline and over time was observed for internalizing (p values < 0.0001) and externalizing (p values < 0.0001) CBCL scales and Total Anxiety score of the RCMAS (p < 0.0001 and p < 0.0002, respectively). Only the Defensiveness median T-score increased during the follow-up (p = 0.004) and in the change from baseline (p = 0.0005). The adaptive (p = 0.0092) and maladaptive (p < 0.0001) scores of the VABS showed a decrease from baseline. ART adherence remained high throughout this study.
Conclusion:
Disclosure does not worsen the child's emotional well-being and adherence to ART over time. This study adds to research from low- and middle-income countries to alleviate fears that disclosure may have an adverse outcome on children with PHIV+.
Disclosure of HIV status is central in pediatric HIV care but requires long-term effort and an understanding of the child's cognitive and psychosocial stage of development. The World Health Organization (WHO) encourages a comprehensive approach that ensures the child's physical, emotional, cognitive, and social well-being is taken into account. The guidelines provided by the WHO and the South African National Department of Health suggest that school-age children, between the ages of 6 and 12 years, should be disclosed to in an ongoing, culturally, and age-appropriate incremental manner.1–4 Full disclosure is recommended before adolescence to enable the child to transition to becoming responsible for their own health care and before sexual debut.1,5,6 Furthermore, it is suggested that the child is provided with continued support and that the health care professionals should monitor the child's emotional state during the process.1,6
Research has shown that many children are unaware of their HIV status and that their parents and/or caregivers are hesitant to disclose their status to them.2 The cited reasons for delayed disclosure, and even nondisclosure, include the following: the social stigma surrounding the diagnosis, discrimination, the impact on the child's emotional/psychological health, the child's resentment, the inappropriate disclosure of HIV status to others, and the parent/caregiver's lack of knowledge on how and when to approach the disclosure questions.1–3,6,7 Contrary to these fears, studies indicate that disclosure leads to improved mental health, better adherence to antiretroviral therapy (ART), improved immunological status, less loss to follow-up, and more protective sexual behaviors in adolescence.1,8 Delayed disclosure, however, may promote self-stigmatization and affect the child's ability to adjust to the diagnosis.9
A systematic review highlighted that few studies assessed the impact of disclosure on the psychological and emotional effects of the child.10 The aim of this study was to describe the process of disclosure in children and to determine how disclosure affects the child's emotional well-being and their compliance to antiretroviral medication.
METHODS
Study Design
This was a prospective cohort study that followed children with perinatally acquired HIV infection (PHIV+) through an ongoing, gradual disclosure programme.
Participant Characteristics, Study Site, and Recruitment
This study was conducted at the Perinatal HIV Research Unit, South Africa, between October 2017 and January 2020. Children with PHIV+ were recruited from the wellness programme that provides HIV care and ART. Children between the ages of 7 and 13 years who were not yet aware of their HIV status were invited to participate in a sequential fashion until the accrual target was met. Their caregivers were part of this study and referred to the child's biological parent, legal guardian, foster parent, or another person who was responsible for the protection of their health, well-being, and development.11 Children were included if they were able to comprehend the assent process as well as communicate and follow instructions in a language of their choice. Informed consent and assent were obtained in English, Zulu, or Sotho.
Nondisclosure refers to the child being unaware of their illness and its effect on their body, partial disclosure refers to the child being made aware of their illness without actually naming HIV, and full disclosure is when the child is made aware of their illness, named HIV. Postdisclosure refers to counseling sessions received after the child is fully disclosed to.
Disclosure Process
All study staff underwent training on the psychometric assessments and the disclosure counseling programmes. The “Right to Care Mini Flipster Disclosure”12 and the USAID disclosure tools13 were used to standardize the process for all children. Sessions 1 and 2 educate the child about their health, how germs and viruses affect their body, and the immune system response and the importance of taking medicine to remain healthy. The third session assesses the child's knowledge about HIV and provides education on what is unclear. Based on the child's comprehension of previous sessions, full disclosure is done by the caregiver with the support of the study counselor (session 4). Postcounseling sessions (5 and 6) focus on the child's feelings and making the child aware of their support structures. Their agency in their own care is emphasized, particularly their role in the quality of their health and therefore their lives. This process was a guideline and was individualized for each participant dependent on their developmental level and comprehension of information provided.
During the first visit, the caregivers attended a predisclosure discussion including counseling on the disclosure process. This equipped them with the necessary skills and knowledge for the joint disclosure to the child. Caregivers then completed the Child Behavior Checklist (CBCL), and the Vineland Adaptive Behavior Scale (VABS), with the assistance of study staff. The Child Depression Inventory (CDI) and Revised Children's Manifest Anxiety Scale (RCMAS) were administered to the child by the study counselor. These psychometric tests were used to determine the child's baseline psychological, cognitive, and behavioral functioning. Thereafter, the first disclosure counseling session was conducted.
The study duration was 18 months. This study involved a baseline visit with both a counseling session and an assessment at week 0, followed by an assessment visit every 12 weeks, starting at week 6 and then at weeks 18, 30, 42, 54, and 78. Counseling visits occurred every 12 weeks at weeks 12, 24, 36, 48, and 72. There was a scheduled break for 12 weeks between counseling sessions at week 48 because it was hoped that most children would be disclosed to, and therefore, a longer time was allocated to assess the child postdisclosure. Visits were expected to be long and therefore split between counseling and assessment, and this also gave opportunity for processing of information before testing was performed. Each session was led by the counselor with both the child and the caregiver present. Participants were encouraged to have ongoing discussions on topics covered at home. Although full disclosure was aimed for week 36 (session 4), the process was flexible based on each child's response to the disclosure programme, allowing for additional visits, revisiting of topics, and accelerating or decelerating the process.
Participants were followed up telephonically on a weekly basis and were provided with support where necessary. The child's viral load and CD4 counts were recorded at the beginning and at the end of this study. Adherence to ART was also recorded through pharmacy pill returns.
Assessment Tools
The Child Behavior Checklist
The CBCL is a validated instrument for the assessment of behavior, emotional, and social abilities within the past 6 months, as perceived by a child's primary caregiver even in children with HIV.14 The CBCL contains an Internalizing scale that corresponds to anxiety and mood disorders, as well as an Externalizing scale that corresponds to disruptive behavior disorders. It has been adapted cross-culturally more than any other psychological screening inventory for children with cross-cultural norms.15
The Vineland Adaptive Behavior Scale, Second Edition
The VABS measures children's adaptive functioning across 5 domains (communication, socialization, daily living skills, motor skills, and maladaptive behavior) and has been used in South Africa among people living with HIV.16 It is a standardized, norm-referenced assessment tool that can be used for measuring the child's daily functioning and adaptive behavior. Any deficits in adaptive behavior, as well as emotional and behavioral disturbances, are also assessed.
The Children's Depression Inventory
The CDI is a self-report scale designed to assess symptoms of depression in children and adolescents and has excellent validity.17 There are 5 subscales that measure different components of depression, namely, Anhedonia (the inability or decreased ability to experience joy), Negative Self-Esteem (the belief that you are not good at anything), Ineffectiveness (lack of motivation or inability to complete tasks), Interpersonal Problems (difficulty making and keeping close relationships), and Negative Mood (irritability or anger).
The Revised Children's Manifest Anxiety Scale
The RCMAS is a self-report measure used to assess symptoms of anxiety and negative effect and has previously been used in South Africa.18 The item responses yield 4 subscales, namely, Total Anxiety, Physiological Anxiety, Worry, and Social Anxiety. It establishes the source of anxiety and its severity. There are also 2 validity scales to note invalid or biased responses.
Statistical Analysis
Continuous data such as psychometric scales, CD4 count, and CD4% were assessed by medians and interquartile ranges. Frequencies and percentages were determined for categorical values. Scale measures were evaluated graphically at all the visits and presented with medians and 95% confidence intervals. The CDI was tested for reliability using the Cronbach alpha test.
Standardized scale measures were plotted during the follow-up to show their trajectory. In addition, change from baseline measures was also plotted. To assess whether there was a change in the measures during the follow-up, we tested the global null hypothesis of no change over time using linear mixed modeling. In the model, the standardized scale measures were fitted as dependent variables with follow-up weeks as the covariate and an unstructured covariance matrix. Similarly, we determined the median change from baseline and determined whether there were significant changes during the follow-up. Statistical analysis was conducted using SAS Enterprise Guide 7.15 using standard procedures.
Ethics/Protection of Human Subjects
Ethical clearance was obtained from the University of Witwatersrand Human Research Ethics Committee.
RESULTS
Participant Characteristics
A total of 30 children living with HIV with a median age of 10 years (interquartile range [IQR]: 9.0–11.0) were enrolled into this study. Of the 32 approached, 1 participant failed screening because of comprehension and communication problems and another knew their HIV status and was not eligible. All children had at least 1 parent alive; however, it was predominantly mothers who attended the sessions with the child (26 mothers vs 4 fathers). Of those, 28 caregivers were HIV infected and 27 on antiretroviral therapy (ART). Most of the adults were unemployed (67%), and 83% had received secondary level education. Ten caregivers reported using alcohol (33%), whereas none reported illicit substance use. Most participants lived in formal housing (73%), and the median household crowding index was 1.67 (IQR: 1.0–2.5) (Table 1).
Table 1.
Child and Caregiver Demographic and Clinical Characteristics
| Variable | Overall | Male | Female |
| Participant characteristics | |||
| No. of participants (%) | 30 (100) | 13 (43.33) | 17 (56.67) |
| Race: Black/African (%) | 29 (96.67) | 13 (100.00) | 16 (94.12) |
| Age (yr) (%) | |||
| 7–10 | 16 (53.33) | 5 (38.46) | 11 (64.71) |
| 11–12 | 14 (46.67) | 8 (61.54) | 6 (35.29) |
| Median age (IQR) | 10.00 (9.00–11.00) | 11.00 (10.00–12.00) | 10.00 (9.00–11.00) |
| Education level (%) | |||
| Grade 2 | 2 (6.67) | 1 (8.33) | 1 (5.56) |
| Grade 3 | 7 (23.33) | 3 (25.00) | 4 (22.22) |
| Grade 4 | 7 (23.33) | 3 (25.00) | 4 (22.22) |
| Grade 5 | 8 (26.67) | 3 (25.00) | 5 (27.78) |
| Grade 6 | 4 (13.33) | 1 (8.33) | 3 (16.67) |
| Grade 7 | 2 (6.67) | 1 (8.33) | 1 (5.56) |
| Clinical characteristics at enrollment | |||
| Virally suppressed at enrollment (VL < 400 cp/mL) | 25 (83.33) | 10 (76.92) | 15 (88.24) |
| Median (IQR) CD4 count (cells/mm3) | 812.00 (705.00–1155.0) | 997.00 (752.00–1180.0) | 722.50 (653.00–1092.5) |
| Median (IQR) CD4% | 35.65 (31.90–38.90) | 35.21 (30.40–38.58) | 35.97 (32.10–39.97) |
| Clinical characteristics at the final visit | |||
| Virally suppressed (%) | 27 (96.4) | 12 (100) | 15 (93.75) |
| Median (IQR) CD4 count (cells/mm3) | 807.50 (667.50–1172.5) | 787.50 (667.50–1055.0) | 807.50 (671.00–1269.5) |
| Median (IQR) CD4% | 37.45 (31.54–41.73) | 33.72 (29.99–38.89) | 40.86 (34.92–42.70) |
| Caregiver characteristics | |||
| Relationship to child (%) | |||
| Mother | 26 (86.67) | 11 (84.62) | 15 (88.24) |
| Father | 4 (13.33) | 2 (15.38) | 2 (11.76) |
| HIV positive (%) | 28 (93.33) | 11 (84.62) | 17 (100.00) |
| HIV-positive caregivers on ART (%) | 27 (96.43) | 10 (90.91) | 17 (100.00) |
| Education (%) | |||
| Secondary | 25 (83.33) | 11 (84.62) | 14 (82.35) |
| Postmatric | 3 (10.00) | 1 (7.69) | 2 (11.76) |
| Other | 2 (6.66) | 1 (7.69) | 1 (5.88) |
| Unemployed (%) | 20 (66.67) | 8 (61.54) | 12 (70.59) |
| Used alcohol (%) | 10 (33.33) | 5 (38.46) | 5 (29.41) |
| Never used substances before | 30 (100.00) | 13 (100.00) | 17 (100.00) |
| Live in brick house (%) | 22 (73.33) | 9 (69.23) | 13 (76.47) |
| Median (IQR) household crowding index | 1.67 (1.00–2.50) | 1.75 (1.00–3.00) | 1.25 (1.00–2.33) |
| Median (IQR) number of children < 18 yr in the household | 2.00 (2.00–3.00) | 3.00 (2.00–4.00) | 2.00 (1.00–3.00) |
ART, antiretroviral therapy; IQR, interquartile range; VL, viral load.
Timing of Disclosure
Most of the children (19 participants) were disclosed to at week 48/11 months (counseling session 5). Some (3/30) were able to receive full disclosure earlier than week 48, whereas nearly a quarter (7/30) were only ready to be disclosed to at the final visit. The median time to disclosure was 48 weeks (IQR: 48.0–54.6). Two participants were not disclosed to because they were lost to follow-up, and another participant was not cognitively mature enough for disclosure to occur during the study.
Evaluation of Mental Health over Time
The Child Depression Inventory (CDI), measuring depression, showed a median score of 9.5 (IQR: 6–12) at the first visit, with only 1 participant having a score suggesting depressive symptoms. Over time, the CDI score remained low for all participants and was lower at the final assessment, with a median score of 5.0 (IQR: 2.5–7.0) (Fig. 1). There was a significant decrease in the overall CDI score during the follow-up (p = 0.0037) and change from baseline (p < 0.0001). Similarly, the subscales Negative Moods, Interpersonal Problems, and Anhedonia decreased significantly (Supplementary Fig. S1 and Table S1, Supplemental Digital Content 1, http://links.lww.com/JDBP/A326).
Figure 1.
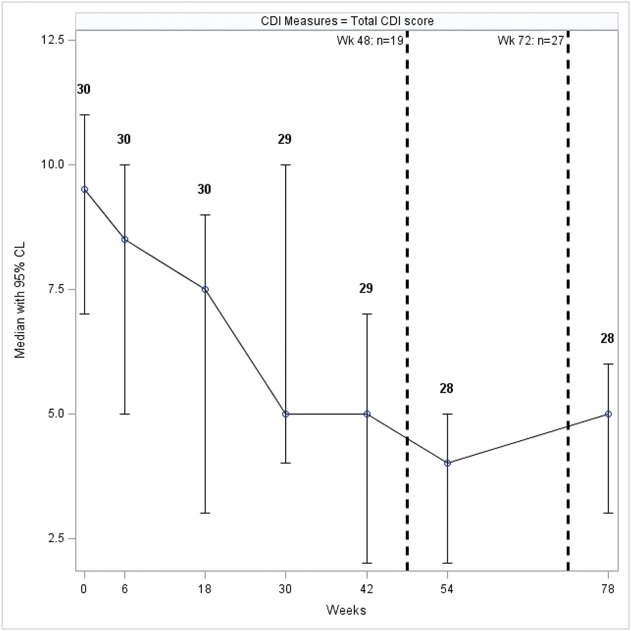
Median (95% confidence interval) of overall CDI scores during the follow-up. Dashed lines indicate cumulative number of participants disclosed to at 2 time points during the study. CDI, Child Depression Index; CL, confidence level.
Using the RCMAS the median T-score for Anxiety and Worry were 53 (IQR: 47–56) and 57 (IQR: 50–61), respectively. The Worry component scores suggested that 22 participants (73%) fell in the normal range, whereas 8 (27%) had a moderate problem with worry. Over the period of this study, the RCMAS's total T-score remained low with a median T-score of 42.50 (IQR: 34.0–50.5) at the final visit (Fig. 2). The Worry subscale decreased substantially over the course of the intervention, with a final median T-score of 45.00 (IQR: 38.0–52.0).
Figure 2.
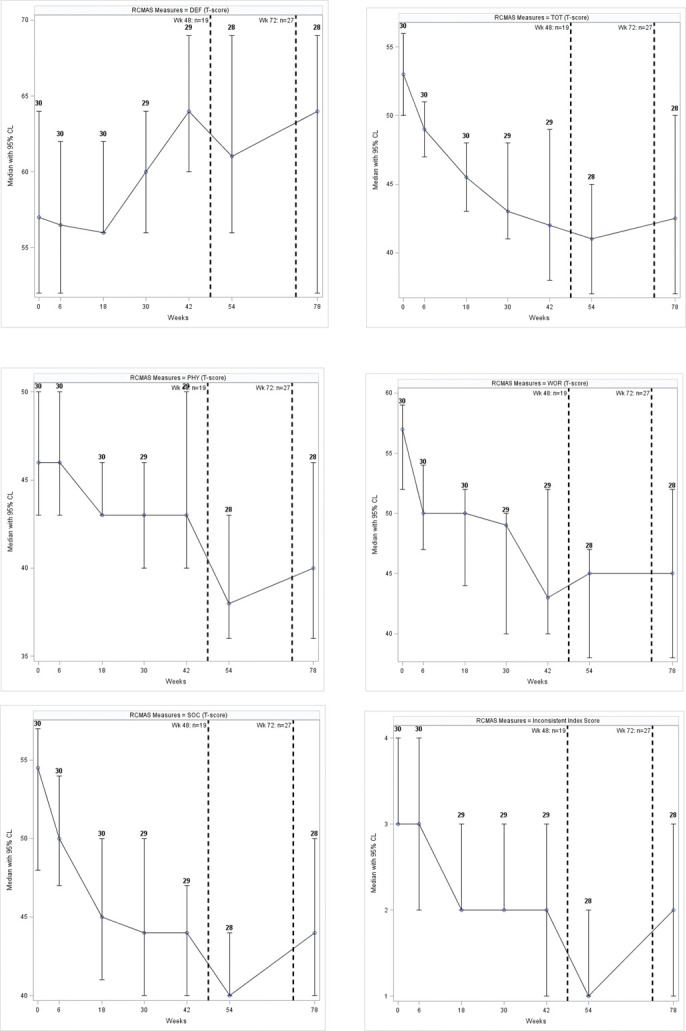
Median (95% confidence interval) RCMAS scores during the follow-up. Dashed lines indicate cumulative number of participants disclosed to at 2 time points during the study. CL, confidence level; DEF, Defensiveness; PHY, Physiological Anxiety; RCMAS, Revised Children's Manifest Anxiety Scale; SOC, Social Anxiety; TOT, Total Anxiety; WOR, Worry.
The Defensiveness median T-score was 57 (IQR: 52–64); 17 participants (57%) had a normal level of defensiveness, 11 (37%) had a moderate problem, and 2 (6%) had extremely problematic defensiveness. The Defensiveness median T-score increased during the follow-up (p = 0.004) and in the change from baseline (p = 0.0005). The Total, Physiological, Worry, Social Anxiety, and inconsistent index scores decreased significantly during the follow-up and in their changes from baseline (Supplementary Fig. S2 and Table S1, Supplemental Digital Content 1, http://links.lww.com/JDBP/A326).
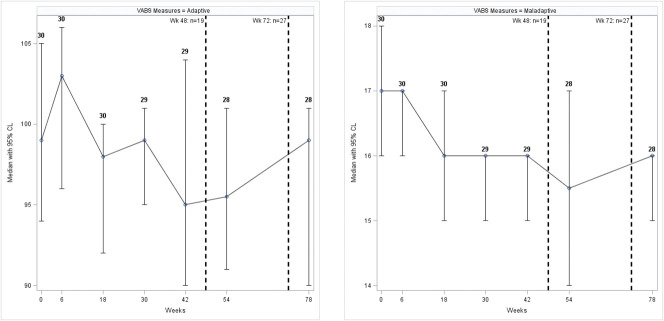
The median Vineland questionnaire score for the adaptive measure was 99.0 (IQR: 93.0–109.0), with 26 participants (86%) having adequate adaption, 3 (10%) having moderately high levels, and 1 (3%) having moderately low levels of adaption. Conversely, the median score for maladaptation was 17.0 (IQR: 16–18). Twenty (67%) had an average maladaptation, and 10 (33%) had elevated levels of maladaptation (Fig. 3). There was no significant difference in the Vineland questionnaire during the follow-up. However, there was a significant decrease in the change from baseline measures in both the Adaptive (p = 0.0092) and Maladaptive (p < 0.0001) scores (Supplementary Fig. S3, Supplemental Digital Content 1, http://links.lww.com/JDBP/A326).
Figure 3.
Median (95% confidence interval) VABS scores during the follow-up. Dashed lines indicate cumulative number of participants disclosed to at 2 time points during the study. CL, confidence level; VABS, Vineland Adaptive Behavioral Scale.
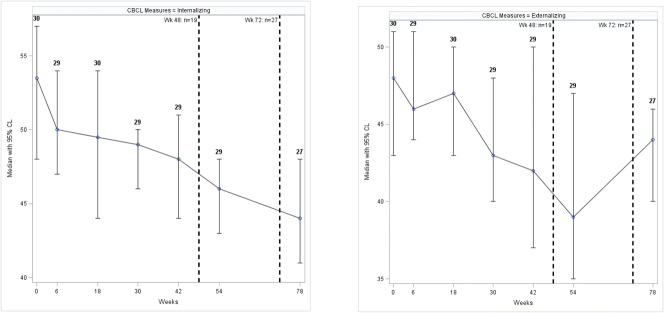
The T-score for the Child Behavior Checklist (CBCL) internalized problems showed 25 (93%) participants within the normal range, whereas 2 (7%) were borderline for possible clinical problems. The externalized problems scale showed similar results. The median T-score for internalizing decreased over time (Fig. 4). A significant decrease was observed during the follow-up and in the change from baseline for both the internalizing (both p values < 0.0001) and externalizing CBCL (both p values < 0.0001) scales (Supplementary Fig. S3, Supplemental Digital Content 1, http://links.lww.com/JDBP/A326).
Figure 4.
Median (95% confidence interval) CBCL scores during the follow-up for internalizing and externalizing behaviors, assessed by caregivers. Dashed lines indicate cumulative number of participants disclosed to at 2 time points during the study. CBCL, Child Behavioral Checklist; CL, confidence level.
Evaluation of Adherence
At the start of this study, the median viral load was 39 copies/mL (IQR: 20.0–40.0), and the CD4 count was 803 (IQR: 701.0–1030.0) cells/mm3. At the last visit, 27 of the 28 participants had viral load < 400 copies/mL with a median CD4 count of 807.5 (IQR: 667.5–1172.5) cells/mm3 (Table 1). Participants were on a range of different ARTs, with the most common combination being Zidovudine or Abacavir with Lamivudine and Kaletra. There was no significant change in adherence over the course of this study. The percentage of participants who had adherence > 95% for Protease Inhibitor and Nuclease Reverse Transcriptase Inhibitor at the start and end of this study was 60% and 63%, respectively.
DISCUSSION
This study has demonstrated that a systematic process of disclosure to young children at a HIV clinic can be achieved and that it does not worsen the child's emotional well-being over time. Importantly, the caregiver was involved in the process of disclosure. This study adds to limited research from low- and middle-income countries (LMICs) to alleviate fears of both health care workers (HCWs) and caregivers who think that disclosure may negatively affect young children in their care. We documented the process longitudinally and attempted to disclose to younger children, which has been a concern because the literature suggests that disclosure occurs at a statistically older age in LMICs.19
Few studies from sub-Saharan Africa (SSA) focus on HIV disclosure, and most have looked at the likelihood of disclosure occurring, as well as barriers to disclosure.10,20 HCWs feel ill-equipped to start and engage in this process. Anxiety over the child's reaction to the news of their HIV status is given as a barrier by both caregivers and HCWs. However, only 1 study in SSA has looked at the mental health outcomes associated with the disclosure process. This Kenyan study showed that rates of depression increased in children who were in the disclosure arm versus the control arm at 6 months into the disclosure intervention but then improved during the next 18 months of follow-up.21 Another study from Zambia retrospectively compared adolescents who had been disclosed to versus those who had not, and the nondisclosure group were more than twice as likely to have emotional difficulties.22
An in-depth look at the mental well-being of children during the disclosure process was undertaken in this study, as well as assessing the caregiver's perception of the child's emotional functioning. The results are encouraging because children who participated did not have worsening of their mental health outcomes throughout this study. It is worth noting that scores at baseline and throughout this study were within the normal range. The baseline assessment is important to understand the child's psychological functioning before starting the disclosure process. At baseline, the median scores for depression and anxiety were low and continued to decrease, regardless of whether disclosure had occurred. This is in contrast to the study from Kenya, where there was initially an increase in depression scores before decreasing.21 The assessments performed at the time points during the process of disclosure allowed monitoring of the children's emotional well-being during what could have been a stressful 18-month period. Although actual HIV disclosure was not out in the open yet, it is likely that they were aware that some important event was unfolding. This could have led to externalizing or internalizing behavior if they were struggling. However, the preservation of mental health functioning was echoed by the caregivers, providing an external measure of the child's well-being and corresponding with the experience of the child. Both scores for internal and external symptoms of emotional distress on the Child Behavior Checklist (CBCL) decreased according to the caregivers. This suggests that the caregivers were able to acknowledge that there was no severe distress for the child throughout the disclosure process. Although longer follow-up of the children after disclosure would have been ideal, there was close monitoring during a time of transition, and there seemed to be adaption during this period.
The current findings are similar to an international study from Thailand documenting a disclosure process for children aged 7 to 18 years.23 The mean scores for depression, quality of life, and the CBCL were either unchanged or decreased. Another longitudinal study from the United States found no difference in the quality of life before and after disclosure with lower values after disclosure.24 Although there are few prospective studies assessing the child's well-being, these are reassuring findings.
This study took into account that disclosure should be a process that allows for the child's understanding and developmental level. Most disclosure occurred after at least 4 visits over a year. Previous reviews from LMICs showed that many children infected with HIV find out their status as a one-time event.10 A study from Tanzania assessing mental health retrospectively demonstrated that adolescents who were purposefully told their status in a structured manner had lower scores on mental health screening and better adherence.25 Our findings show that developing a process that is flexible to the participants and their caregivers is important.
The Revised Children's Manifest Anxiety Scale questionnaire showed an improving trend in most of the Anxiety subscales, except for the Defensiveness scale. Although within the normal range, the Defensiveness scores showed to be worsening over the course of the program. This scale measures the tendency to represent oneself in a socially desirable manner.26 This pattern has been seen in other studies, particularly among younger children (age 6–10 years), in which children have higher social desirability.26 This may be an indicator of cognitive immaturity and development. The increase in the Defensiveness score as the child was disclosed to indicate that the participants may have had social anxiety. This anxiety, particularly around the topic of HIV, resulted in feelings of isolation or fear of rejection and the need to be accepted, hence the increase in social desirability. This repressive coping response to HIV disclosure is consistent with studies that reported this style of coping among children who recently learned about their cancer diagnosis and was also characteristic of children living with chronic illness.27 Longitudinal research needs to be performed to assess whether the repressive style of coping is transient or becomes part of the child's permanent coping mechanisms.
The role of disclosure in antiretroviral therapy (ART) adherence has been a driver for disclosure to occur. Reviews from LMICs have garnered mixed results, with a range of no impact, a negative impact, and a positive impact on ART adherence.28 The authors noted that most studies were cross-sectional, used self-report as the adherence measure, and were retrospective. A study from South Africa assessed all adolescents on ART from a rural district and showed that knowledge of their HIV status was associated with higher adherence to ART among adolescents with perinatally acquired HIV infection and that if disclosure had occurred before 12 years, it was associated with better adherence.29 Our study did not show any difference in ART adherence over time, regardless of whether disclosure had occurred. However, this was in the setting of high adherence at baseline and little variability in adherence results over time and a younger age group, which may be why no difference was seen. This finding is similar to a Thai study that also found little variance in adherence throughout the disclosure process because the participants had high adherence at baseline and throughout this study.30
Limitations
This study had a small sample size that may have limited variability. Those who participated in this study were long-standing patients within the Perinatal HIV Research Unit Wellness clinic. The caregivers seem to have been motivated and knowledgeable about the research process, resulting in their commitment. Both the caregivers and the participants had developed relationships with the staff, and this would have possibly led them to feel secure, understood, contained and emotionally supported, and crucial during the disclosure process. This may not be the same in a clinic where the staff members are unknown to the participants. Moreover, this process was undertaken over an 18-month period, and each participant was assessed emotionally 6 weeks after each disclosure session. The psychological impact of the disclosure process may have been dampened by this extended period. Added to this, disclosure occurred later than anticipated in 7 of the 28 participants who completed the study, and 1 participant did not have full disclosure. The short-term impact of the full disclosure was therefore not evaluated in these participants and could have resulted in psychological disturbances being missed in some participants. However, the participants were closely evaluated over a period of transition, and thus, one could expect signs of psychological distress to be detected at a number of time points if they were to occur.
CONCLUSION
The disclosure process is an important step in setting a foundation for children transitioning into adolescence to take responsibility for their health. This is, to the best of our knowledge, the first study from sub-Saharan Africa to include both the child and the caregiver's perception of the child's mental well-being and is an important finding that it did not worsen over time, as many caregivers have reservations about the child managing the disclosure of their HIV status. Longer-term follow-up of patients would be helpful to assess emotional functioning and adherence to antiretroviral therapy. Further analysis has been performed on qualitative data from caregivers and health care workers involved in this study and published to add to this literature. Future research should replicate this disclosure process in other low- and middle-income countries to look at variations in processes and outcomes and possibly include psychological assessments of the caregivers during this time. It is hoped that this study helps to promote the early disclosure to children with perinatally acquired HIV infection in a resource-limited setting.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Nonhlanhla Mazaleni for the support she provided to study participants and families; Dr. Stacy-Lee Sigamoney for writing the first draft of the protocol; Right to Care Foundation, Dr. Leon Levin, and Dr. Julia Turner for the informative workshop on the “Right to Care Mini Flipster Disclosure Tool for Adolescents Ages 12 and up”; and the PHRU clinic staff, Faith Madiehe, Mirriam Kunene, and Nkata Kekana, for their care and guidance provided to our patients and caregivers during this study.
Footnotes
The current work was funded through South African Medical Research Council's extramural funding to the Perinatal HIV Research Unit.
Disclosure: The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jdbp.org).
A. Liberty and G. Leshabane performed the research. C. Joyce and A. Violari designed the research study. K. Otwombe, M. E. Lebotsa, J. Buckley, C. Joyce, G. Leshabane, C. Ramsammy, and L. Galvin analyzed the data. J. Buckley, C. Joyce, C. Ramsammy, and K. Otwombe wrote the article.
Contributor Information
Kennedy Otwombe, Email: otwombek@phru.co.za.
Celeste Joyce, Email: joycec@phru.co.za.
Given Leshabane, Email: leshabaneg@phru.co.za.
Lisa Galvin, Email: galvinl@phru.co.za.
Candice Ramsammy, Email: ramsammyc@phru.co.za.
Moshoko Emily Lebotsa, Email: moshoko.e@gmail.com.
Afaaf Liberty, Email: libertya@phru.co.za.
Avy Violari, Email: violari@mweb.co.za.
REFERENCES
- 1.WHO. Guideline on HIV Disclosure Counselling for Children up to 12 Years of Age. World Health Organisation; 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241502863_eng.pdf. Accessed Accessed September 2, 2020. [PubMed] [Google Scholar]
- 2.Dahourou D, Raynaud J, Leroy V. The challenges of timely and safe HIV disclosure among perinatally HIV-infected adolescents in sub-Saharan Africa. Curr Opin HIV AIDS. 2018;13:220–229. [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Siberry G, Alarcon J, et al. Prevalence and associated characteristics of HIV-infected children in Latin America who know their HIV status. J Pediatr Infect Dis Soc. 2018;7:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Department of Health. Disclosure Guidelines for Children and Adolescents in the Context of HIV, TB and Non-communicable Diseases. Pretoria, South Africa: South African National Department of Health; 2016. [Google Scholar]
- 5.Murnane P, Sigamoney S, Pinillos F, et al. Extent of disclosure: what perinatally HIV-infected children have been told about their own HIV status. AIDS Care. 2017;29:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright S, Amzel A, Ikoro N, et al. Talking to children about their HIV status: a review of available resources, tools, and models for improving and promoting pediatric disclosure. AIDS Care. 2017;29:1019–1025. [DOI] [PubMed] [Google Scholar]
- 7.Woollett N, Cluver L, Brahmbhat H. Reticence in disclosure of HIV infection and reasons for bereavement: impact on perinatally infected adolescents' mental health and understanding of HIV treatment and prevention in Johannesburg, South Africa. Afr J AIDS Res. 2017;16:175–184. [DOI] [PubMed] [Google Scholar]
- 8.Toska E, Pantelic M, Hodes R. To Know or Not to Know? HIV-Status Disclosure and Protective Sexual Practices Among Adolescent Girls and Boys in South Africa. Cape Town, South Africa: University of Cape Town; 2017. [Google Scholar]
- 9.McHugh G, Chikwari C, Mujuru H, et al. Familial silence surrounding HIV and non-disclosure of HIV status to older children and adolescents. AIDS Care. 2018;30:830–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vreeman R, Gisore P, Scanion M, et al. Disclosure of HIV status to children in resource-limited settings: a systemic review. J Int AIDS Soc. 2014;16:18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Children's Act No. 38 (SA). 2005. [Google Scholar]
- 12.Vujovic M, Meyersfeld S. Mini Flipster disclosure tool for adolescents ages 12 and up. Johannesburg: Right to Care; 2016: 47. [Google Scholar]
- 13.Disclosure of Pediatric and Adolescent HIV Status Toolkit. Washington, DC: Elizabeth Glaser Pediatric AIDS Foundation; 2018. [Google Scholar]
- 14.Bachanas P, Kullgren K, Schwartz K. Predictors of psychological adjustment in school-age children infected with HIV. J Pediatr Psychol. 2001;26:343–352. [DOI] [PubMed] [Google Scholar]
- 15.Ivanova M, Dumenci L, Rescorla L, et al. Testing the 8-syndrome structure of the Child Behavior Checklist in 30 societies. J Clin Child Adolesc Psychol. 2007;36:405–417. [DOI] [PubMed] [Google Scholar]
- 16.Eloff I, Finestone M, Makin J, et al. A randomized clinical trial of an intervention to promote resilience in young children of HIV-positive mothers in South Africa. AIDS. 2014;28:S347–S357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockings E, Lee Y, Mihalopoulos C, et al. Symptom screening scales for detecting major depressive disorder in children and adolescents: a systemic review and meta-analysis of reliability, validity and diagnostic utility. J Affect Disord. 2015;15:447–463. [DOI] [PubMed] [Google Scholar]
- 18.West N, Mudavanhu M, Hanrahan C, et al. Mental health in South African adolescents living with HIV. AIDS Care. 2019;31:117–124. [DOI] [PubMed] [Google Scholar]
- 19.Pinzon-Iregui M, Beck-Sague C, Malow R. Disclosure of their HIV status to infected children: a review of the literature. J Trop Pediatr. 2013;59:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paintsil E, Kyriakides T, Antwi S, et al. Clinic-based pediatric disclosure intervention trial improves pediatric HIV status disclosure in Ghana. J Acquir Immune Defic Syndr. 2020;84:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vreeman R, Mwangi A, McAteer C, et al. Evaluating a patient-centred intervention to increase disclosure and promote resilience for children living with HIV in Kenya. AIDS. 2019;33(suppl 1):S93–S101. [DOI] [PubMed] [Google Scholar]
- 22.Menon A, Glazebrook C, Campain N, et al. Mental health and disclosure of HIV status in Zambian adolescents with HIV infection: implications for peer-support programs. J Acquir Immune Defic Syndr. 2007;46:349–354. [DOI] [PubMed] [Google Scholar]
- 23.Boon-Yasidhi V, Naiwatanakul T, Chokephaibulkit K, et al. Effect of HIV diagnosis disclosure on psychosocial outcomes in Thai children with perinatal HIV infection. Int J STD AIDS. 2016;27:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler A, Howland L, Storm D, et al. Impact of disclosure of HIV infection on health-related quality of life among children and adolescents with HIV infection. Pediatrics. 2009;123:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos J, Mmbaga B, Turner E, et al. Modality of primary HIV disclosure and association with mental health, stigma and antiretroviral therapy adherence in Tanzanian youth living with HIV. AIDS Patient Care STDS. 2018;32:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan DE, Claar RL, Scharff L. Social desirability response bias and self-report of psychological distress in pediatric chronic pain patients. Pain. 2008;136:366–372. [DOI] [PubMed] [Google Scholar]
- 27.Phipps S, Steele R. Repressive adaptive style in children with chronic illness. Psychosom Med. 2002;64:34–42. [DOI] [PubMed] [Google Scholar]
- 28.Nichols J, Steinmetz A, Paintsil E. Impact of HIV-status disclosure on adherence to antiretroviral therapy among HIV-infected children in resource-limited settings: a systematic review. AIDS Behav. 2017;21:59–69. [DOI] [PubMed] [Google Scholar]
- 29.Cluver L, Hodes R, Toska E, et al. “HIV is like a tsotsi. ARVs are your guns”: associations between HIV-disclosure and adherence to antiretroviral treatment among adolescents in South Africa. AIDS. 2015;29(suppl 1):S57–S65. [DOI] [PubMed] [Google Scholar]
- 30.Sirikum C, Sophonphan J, Chuanjaroen T, et al. HIV disclosure and its effect on treatment outcomes in perinatal HIV-infected Thai children. AIDS Care. 2014;26:1144–1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.