Abstract
Background:
Humans are exposed to complex mixtures of phthalate chemicals from a range of consumer products. Previous studies have reported significant associations between individual phthalate metabolites and pregnancy outcomes, but mixtures research is limited.
Objectives:
We used the Puerto Rico Testsite for Exploring Contamination Threats longitudinal pregnancy cohort to investigate associations between phthalate metabolite mixtures and pregnancy outcomes.
Methods:
Women ( carrying females, carrying males) provided up to three urine samples throughout gestation (median 18, 22, and 26 wk), which were analyzed for 13 phthalate metabolites. Pregnancy outcomes including preterm birth (PTB), spontaneous PTB, small and large for gestational age (SGA, LGA), birth weight z-score, and gestational age at delivery were abstracted from medical records. Environmental risk scores (ERS) were calculated as a weighted linear combination of the phthalates from ridge regression and adaptive elastic net, which are variable selection methods to handle correlated predictors. Birth outcomes were regressed on continuous ERS. We assessed gestational average and visit-specific ERS and stratified all analyses by fetal sex. Finally, we used Bayesian kernel machine regression (BKMR) to explore nonlinear associations and interactions between metabolites.
Results:
Differences in metabolite weights from ridge and elastic net were apparent between birth outcomes and between fetal sexes. An interquartile range increase in gestational average phthalate ERS was associated with increased odds of PTB [male ; 95% confidence interval (CI): 1.08, 2.27; female ; 95% CI: 1.23, 2.98], spontaneous PTB (male ; 95% CI: 1.46, 3.68; female ; 95% CI: 1.04, 3.82), and reduced gestational age at birth (male wk, 95% CI: , ; female wk, 95% CI: , ). Analyses by study visit suggested that exposure at wk (range 20–24 wk) was driving those associations. Bivariate plots from BKMR analysis revealed some nonlinear associations and metabolite interactions that were different between fetal sexes.
Discussion:
These results suggest that exposure to phthalate mixtures was associated with increased risk of early delivery and highlight the need to study mixtures by fetal sex. We also identified various metabolites displaying nonlinear relationships with measures of birth weight. https://doi.org/10.1289/EHP8990
Introduction
Preterm birth (PTB) is a major public health problem in the United States, where the rate of PTB is higher than that in most other developed nations.1 Of the children who died before the age of 5 y in 2010 worldwide, 14% died from complications of preterm birth.2 Being born preterm increases the risk of future morbidities including neurological impairments,3 vision and hearing loss,4 cerebral palsy,3 attention deficit disorder,5 and asthma.6 The etiology of preterm birth, as well as other adverse birth outcomes, is not well understood, but exposures to environmental contaminants may play an important role as demonstrated in a review by Ferguson et al.7 We believe that a better understanding of the environmental risk factors for adverse birth outcomes will help in the development of targeted intervention and prevention strategies, particularly among at-risk populations, such as pregnant women and those living in areas burdened by high environmental contamination.
Phthalates are a class of synthetic plasticizers commonly used in the manufacture of consumer products.8,9 High molecular weight phthalates are most commonly used in flexible plastic contained in flooring, food storage containers, and medical equipment.10 Conversely, low molecular weight phthalates are used in personal care products, including shampoos, lotions, and fragrances, as well as in lacquers and varnishes.10 Because of their different exposure sources, high molecular weight phthalate exposure typically occurs via ingestion, whereas low molecular weight phthalate exposure comes mostly from inhalation and dermal absorption.11 Phthalates are not covalently bound to the products they are used in and can easily leach into the environment; thus their widespread use results in ubiquitous human exposure to diverse mixtures of phthalate compounds.12 Case–control studies have shown phthalate metabolite concentrations measured throughout pregnancy to be significantly higher among women experiencing preterm birth13,14 and spontaneous abortion.15,16 Other studies have shown inconsistent relationships between gestational phthalate exposure and measures of fetal growth and development including birth weight,17,18 birth length,17 head circumference17,19,20 and femur length,19,20 as well as gestational age.17,18,20
The pathways by which phthalates may adversely affect pregnancy are not fully understood. Our previous work has shown significant associations between increased exposure to phthalates during pregnancy and various thyroid and reproductive hormones,21 as well as biomarkers of oxidative stress,22,23 inflammation,22,24,25 and lipid metabolism,25 which are all important for maintaining a healthy pregnancy. Phthalates may exert deleterious effects on pregnancy through disruption of these physiological processes.
The epidemiology literature implicating phthalate exposures in the occurrence of adverse birth outcomes has grown in recent years, but few studies have assessed mixtures of phthalate metabolites in relation to birth outcomes,26,27 and differences in statistical methods, exposure distributions, underlying cohort characteristics, and study exclusion criteria make it difficult to draw aggregate conclusions from these studies. Animal studies have shown that exposures to phthalate mixtures representative of human exposure resulted in transgenerational reproductive effects in female mice, including altered uterine morphology, cystic ovaries, and fertility complications.28,29 To our knowledge, regulatory bodies tend to rely on single pollutant associations with health outcomes for developing policy, which does not reflect a realistic body burden of phthalates among the general population. Therefore, it is critically important to assess the effects of complex phthalate mixtures on adverse birth outcomes to truly understand the health risks they pose.
The aim of this study was to evaluate the associations between mixtures of phthalate metabolites and adverse birth outcomes relating to gestational age at birth and birth weight among pregnant women in Puerto Rico. Associations between single phthalate metabolites and preterm birth, spontaneous preterm birth, and gestational age in a similar subset of the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort have been described previously.30 Various previous studies have observed greater risk of delivering preterm male babies than female babies,31,32 so we also aimed to explore differences in these associations based on fetal sex, which was not assessed in the previous analysis. We also assessed differences in associations by timing of exposure assessment. Finally, we sought to investigate potential nonlinear associations and interactions between phthalate metabolites within the mixture.
Methods
Study Population
We used data from the PROTECT cohort, a longitudinal birth cohort in the northern karst region of Puerto Rico, for the present analysis. PROTECT was originally conceptualized to investigate environmental contaminants in relation to adverse pregnancy outcomes. Details of the study design and recruitment protocols have been previously described.33 Briefly, women were recruited from 2011 to 2019 at wk gestation and were excluded from the study if they were younger than 18 y or older than 40 y old, participated in their first clinic visit after their 20th week of pregnancy,34 had taken oral contraceptives within 3 months of becoming pregnant, had used in vitro fertilization to become pregnant, or had preexisting medical or obstetric conditions. Demographics and other relevant health information was collected from questionnaires administered at the first study visit. Initially, there were 1,262 women with complete phthalate and specific gravity data across the study (1,082 complete at visit 1; 1,004 complete at visit 2; and 813 complete at visit 3). Of those, 1,011 women also provided data on at least one birth outcome, and nine of those women were missing data on fetal sex due to delays in data abstraction from medical records (schematic of sample sizes is shown in Figure S1).
This study was approved by the research and ethics committees of the University of Michigan School of Public Health, University of Puerto Rico, Northeastern University, and participating hospitals and clinics. All methods reported in this study were performed in accordance with relevant guidelines and regulations imposed by those institutions. All study participants provided full informed consent prior to participation.
Phthalate Exposure Assessment
All spot urine samples were frozen at and shipped over night on dry ice to the U.S. Centers for Disease Control and Prevention (U.S. CDC) for analysis. Samples were collected from participants at 16–20, 20–24, and 24–28 wk gestation. Urine was collected into polypropylene containers and aliquoted at the University of Puerto Rico before overnight shipment to the U.S. CDC at . Samples were analyzed using solid phase extraction high-performance liquid chromatography-isotope dilution tandem mass spectrometry, described in more detail elsewhere.35,36 All analytical runs included reagent blanks, calibration standards, and low- and high-concentration quality control materials. Nine different batches of samples were run on a rolling basis as they were collected. Samples in batches 1–6 were analyzed for 11 phthalate metabolites: mono(2-ethlhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), monoisobutyl phthalate (MiBP), mono(3-carboxypropyl) phthalate (MCPP), monocarboxynonyl phthalate (MCNP), and monocarboxyoctyl phthalate (MCOP). Monohydroxybutyl phthalate (MHBP) and monohydroxyisobutyl phthalate (MHiBP) were added to the analytical panel beginning in batch 7. Values detected below the limit of detection (LOD) were assigned a value of the LOD divided by the square root of 2,37 and LODs differed between batches.
Birth Outcome Assessment
Based on recommendations from the American College of Obstetricians and Gynecologists,38 self-reported date of the last menstrual period (LMP) and early ultrasound measurements were used to determine gestational age at birth. Briefly, the LMP was used as the gold standard and was compared to ultrasound measurements taken primarily before 14 wk gestation. Gestational age was changed from the LMP estimate to the ultrasound estimate if the difference between the two methods was d for ultrasounds conducted before 9 wk gestation, or d for ultrasounds conducted before 14 wk gestation. Estimates were changed from the LMP to the ultrasound method for 11% of the participants included in the present study (). PTB was defined as delivery before 37 wk gestation. We also assessed the spontaneous subtype of PTB, defined as PTB presenting with premature rupture of membranes, spontaneous preterm labor, or both.39 We calculated birth weight z-scores based on fetal sex and gestational age using widely accepted international standards.40 Those born with a birth weight percentile and percentile were considered small for gestational age (SGA) and large for gestational age (LGA), respectively.
Associations with individual phthalate metabolites.
Single pollutant associations with birth outcomes were assessed using linear and logistic regression models adjusted for maternal age and education, plus prepregnancy body mass index (BMI) for birth weight z-score models. Rather than stratifying the data, fetal sex-specific estimates were determined using phthalate interaction terms with sex indicator variables in statistical models. Models were run using gestational average phthalate concentrations and visit-specific phthalate concentrations.
Selection of Phthalate Predictors
Adaptive elastic net (adENET) was used to determine the subsets of phthalate metabolites within the mixture that were most predictive of individual birth outcomes. In addition, adENET functions as a variable selection tool with correlated predictors by employing two tuning parameters—lambda 1 and lambda 2 attached with two types of penalty. Although lambda 1 shrinks the coefficients of unimportant predictors exactly to zero, lambda 2 stabilizes selection in the presence of highly correlated predictors. The latter is particularly important in this application because many phthalate metabolites are highly correlated, especially those coming from the same parent compound. Five-fold cross-validation and optimization of cross-validated prediction errors were used to estimate lambda 1 and lambda 2 using the R package gcdnet. As a final output, adENET returns a vector of coefficients, which are weighted by their associated regression coefficients from a model with the outcome of interest, accompanied with large-sample confidence intervals.adENET variable selection is conditional, meaning that one predictor being selected into the model is wholly dependent on which other predictors have been selected into or dropped out of the model. Because of this conditional variable selection, predictor selections by adENET can be unstable. To remedy this, we supplemented our variable selection methods with ridge regression. Ridge is similar to adENET in that it stabilizes selection in the presence of highly correlated predictors, but it uses only one tuning parameter to shrink unimportant predictors toward zero (but never to exact zero) and thus provides more stable variable selection than adENET. Five-fold cross-validation and optimization of prediction errors were used to estimate the tuning parameter.adENET and ridge regression analyses were first conducted with geometric mean phthalate concentrations measured at up to three study visits. Before averaging, phthalate concentrations at each visit were first corrected for specific gravity at that visit using the formula:
where is the SG-adjusted average phthalate concentration, is the uncorrected average phthalate concentration, is the population median of the gestational average specific gravity concentrations (equal to 1.019 here), and is the gestational average specific gravity concentration.13 Specific gravity correction was done at each study visit to account for urinary dilution at the time of sample collection. Specific gravity was measured using a digital handheld refractometer (ATAGO Co., Ltd.) at the time of urine sample aliquoting. To take advantage of the repeated exposure measurements in our cohort, adENET and ridge regression analyses were also conducted separately for each study visit to assess potential windows of susceptibility, for which models were adjusted for specific gravity as an unpenalized covariate rather than using corrected phthalate concentrations. All phthalate metabolite concentrations were natural logarithm transformed. Analyses were conducted separately for each birth outcome and for women carrying male vs. female fetuses. We explored various possible confounders: maternal age, education level, marital status, employment status, annual household income, smoking status, exposure to environmental tobacco smoke, alcohol use, parity, and prepregnancy BMI. Covariates were added into the model in a forward stepwise manner; Akaike information criterion and change in the main effect estimate by more than 10% were used to arrive at final statistical models, which included maternal age, maternal education, and specific gravity as unpenalized covariates. Models assessing birth weight z-score also included maternal prepregnancy BMI, resulting in a lower sample size for these models because of incomplete data on prepregnancy BMI. adENET was conducted using the gcdnet package in R (version 3.5.1; R Development Core Team).
Prediction of Birth Outcomes
Coefficients derived from adENET and ridge regression were separately multiplied by each study participant’s matrix of observed phthalate metabolite concentrations to give weighted concentrations of each metabolite for every individual. Weighted concentrations were then summed across metabolites to arrive at the individual’s environmental risk score (ERS), which represents a weighted sum of their exposure to the mixture of phthalate metabolites. The ERS has previously been validated as a promising tool for assessing cumulative disease risk from exposure to chemical mixtures.41,42 Gestational average ERS were calculated using coefficients from the average adENET/ridge analysis, and visit-specific ERS were calculated using coefficients from adENET/ridge at each study visit. When the association between increased phthalate exposure and the outcome was expected to be inverse, based on single pollutant associations and assigned metabolite weights, ERS were multiplied by to reflect its interpretation as conferring risk of having the outcome. The ERS were then used as continuous exposure variables in multiple linear (for continuous outcomes) and logistic (for binary outcomes) regression, with effect estimates representing the unit change in, or odds of, the outcome with an interquartile range increase in ERS. All models were adjusted for maternal age and education level, and models for birth weight z-score also were adjusted for maternal prepregnancy BMI, based on a priori knowledge. Samples for models including prepregnancy BMI were smaller due to delays in medical record data abstraction. Models with visit-specific ERS further adjusted for specific gravity. Models with average ERS did not adjust for specific gravity because correction was implemented in the adENET analysis. Annotated R code for conduction adENET and ridge regression and subsequent creation and modeling of ERS can be found in the supplementary materials. The significance level was set to .
Bayesian Kernel Machine Regression
We used Bayesian kernel machine regression (BKMR) to explore the possibility of interactions between phthalate metabolites within the mixture. BKMR uses a kernel function to flexibly model relationships between a response variable and multiple predictors. It is a particularly useful tool for visualizing nonlinear interactions between predictors in the chemical mixture.43 We conducted all BKMR analyses using gestational average values of phthalate exposure. Metabolites included in models were chosen based on selections from adENET rather than including all 13 metabolites for model simplicity and ease of interpretability. Hierarchical models were used when highly correlated metabolites were included in the same model. Thus, adENET provides the first level of predictor selection, and then BKMR further interrogates selections made by adENET and explores possible nonlinear interactions between predictors. To ensure model convergence and adequate acceptance rates for predictor variables, we ran 10,000 iterations and set the tuning parameters r.jump1 and r.jump2 to 1 and 0.1, corresponding to the standard deviations of the proposal distributions in the switching and refinement steps of variable selection, respectively. Posterior inclusion probabilities (PIPs) for each predictor variable were calculated as a measure of variable importance. In cases where hierarchical models were implemented, the group PIP indicates the importance of that exposure group, and cond.PIP indicates the importance of each predictor within that group for determining the group-level importance.
Results
There were 1,325 mothers (620 female fetuses and 686 male fetuses) in the larger PROTECT cohort with full covariate information and data on at least one phthalate metabolite and one birth outcome. Because adENET analysis requires full exposure data, the present analysis was conducted on a subset of these PROTECT women () with full data on all 13 phthalate metabolites. Birth characteristics and demographics information are presented in Table 1. The median gestational age at birth was 39.3 wk [interquartile range (IQR): 1.79], and the median birth weight z-score was 0.09 (IQR: 1.31). Preterm birth was observed in 8.9% of women, spontaneous preterm birth in 7.1% of women, SGA in 9.2% of women, and LGA in 11.0% of women. Newborns were 46% female and 54% male. A similar imbalance in the fetal sex distribution has been observed in past PROTECT analyses,30,44,45 but more mothers carrying a male fetus in the present analysis had complete phthalate data, and thus more male fetuses were included in the final sample than female fetuses. The majority of women in the study were under the age of 30 y (65.9%), had attained at least some college education (80.7%), were employed (65.8%), lived in a home earning less than (61.1%), were either married or cohabitating (83.3%), had never smoked (86.6%) or been exposed to environmental tobacco smoke (91.5%), did not drink alcohol during pregnancy (94.2%), had fewer than two previous children (85.7%), and had a prepregnancy BMI of no more than (81.5%).
Table 1.
Maternal demographic and birth characteristics of 1,011 mothers in Puerto Rico from 2011 to 2019.
| Median (IQR) | |
|---|---|
| Gestationals age at delivery (wk) | 39.3 (1.79) |
| Birth weight z-score | 0.09 (1.31) |
| n (%) | |
|---|---|
| Preterm Birth | |
| Yes | 89 (8.9%) |
| No | 911 (90.1%) |
| Missing | 11 |
| Spontaneous preterm birth | |
| Yes | 52 (7.1%) |
| No | 921 (92.9%) |
| Missing | 38 |
| Small for gestational age | |
| Yes | 89 (9.2%) |
| No | 876 (90.8%) |
| Missing | 46 |
| Large for gestational age | |
| Yes | 106 (11%) |
| No | 859 (89%) |
| Missing | 46 |
| Maternal age (y) | |
| 18–24 | 357 (35.3%) |
| 25–29 | 309 (30.6%) |
| 30–34 | 214 (21.2%) |
| 35–41 | 131 (13.0%) |
| Missing | 0 |
| Maternal education | |
| GED or less | 195 (19.3%) |
| Some college | 337 (33.3%) |
| Bachelor’s or higher | 479 (47.4%) |
| Missing | 0 |
| Employment status | |
| No | 344 (34.2%) |
| Yes | 662 (65.8%) |
| Missing | 5 |
| Annual household income (USD$) | |
| 255 (28.4%) | |
| 293 (32.7%) | |
| 223 (24.9%) | |
| 126 (14%) | |
| Missing | 114 |
| Marital status | |
| Single | 168 (16.7%) |
| Married | 553 (54.9%) |
| Cohabitating | 286 (28.4%) |
| Missing | 4 |
| Smoking status | |
| Never | 873 (86.6%) |
| Ever | 118 (11.7%) |
| Current | 17 (1.7%) |
| Missing | 3 |
| Daily environmental tobacco smoke exposure | |
| Never | 848 (91.5%) |
| 1 h or less | 37 (4%) |
| 42 (4.5%) | |
| Missing | 84 |
| Alcohol use | |
| Never | 520 (51.6%) |
| Yes, before Pregnancy | 429 (42.6%) |
| Yes, currently | 58 (5.8%) |
| Missing | 4 |
| Number of previous children | |
| 0 | 327 (39.9%) |
| 1 | 375 (45.8%) |
| 2–5 | 117 (14.3%) |
| Missing | 192 |
| Prepregnancy BMI | |
| 515 (53.5%) | |
| 269 (28%) | |
| 178 (18.5%) | |
| Missing | 49 |
| Fetal sex | |
| Female | 462 (46%) |
| Male | 540 (54%) |
| Missing | 9 |
Note: BMI, body mass index; IQR, interquartile range.
Distributions of phthalate metabolites, as well as the number of samples below the limit of detection, are shown in Table S1. Associations between single phthalate metabolites and birth outcomes specific to this subset of the PROTECT cohort are shown in Tables S2 (female fetuses) and S3 (male fetuses).
Ridge Regression and adENET Metabolite Selection
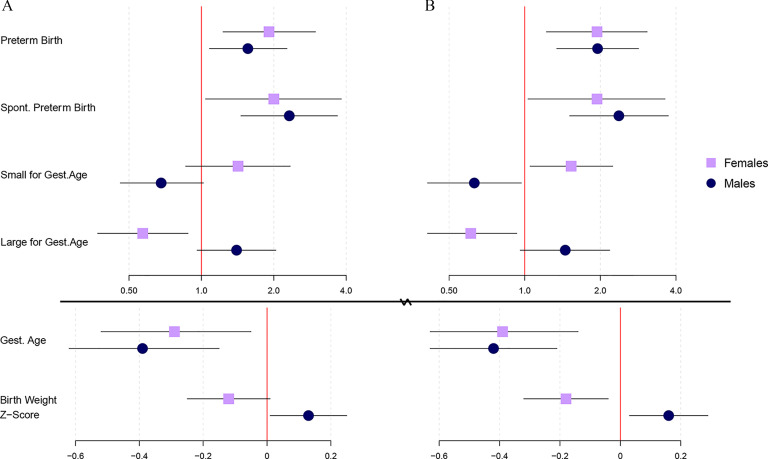
Gestational average metabolites that were selected as having a nonnull effect on birth outcomes from ridge regression and adENET, by fetal sex, are depicted in Figure 1. Corresponding weights can be found in Tables S4 (ridge, females), S5 (ridge, males), S6 (adENET, females), and S7 (adENET, males). Tables for adENET weights also contain large sample 95% confidence intervals (CI). Ridge regression gave weights low in magnitude among female and male fetuses for preterm birth, with MHBP being the strongest predictor among females () and MCNP () being the strongest predictor among males. adENET selected only MBP and MHBP for preterm birth among female fetuses [ (95% CI: , 0.60) and (95% CI: , 0.62), respectively], whereas MEHP, MiBP, and MCNP were selected among male fetuses [ (95% CI: , 0.28), (95% CI: , 0.24), and (95% CI: , 0.36), respectively].
Figure 1.
Heat maps depicting gestational average metabolite selections from (A) ridge regression, and (B) adaptive elastic net, between birth outcomes and by fetal sex in the PROTECT cohort. Box color corresponds to the weight assigned to each metabolite from ridge or adENET. Corresponding numeric data can be found in Tables S4–S7. Note: *The MCPP metabolite results from metabolism of multiple high molecular weight parent phthalates.
Different results were found when the spontaneous subtype of preterm birth was assessed. Ridge regression again gave weights low in magnitude among female fetuses, with MCPP being the strongest predictor (). In contrast, ridge regression gave various stronger weights among male fetuses, with MEHP () and MCNP () being the strongest predictors. Among female fetuses, adENET selected MHiBP (, 95% CI: , 0.59), MCPP (, 95% CI: , 0.69), and MCNP (, 95% CI: , 0.60). adENET selection of MCNP among males became much stronger for spontaneous preterm birth (, 95% CI: , 0.85) relative to all preterm births, and other positive selections for spontaneous PTB included MBP (, 95% CI: , 0.79), MiBP (, 95% CI: , 0.89), and MHiBP (, 95% CI: , 0.72). MEHP and MHBP were selected as having inverse relationships with odds of spontaneous preterm birth among male fetuses [ (95% CI: , 0.11) and (95% CI: , 0.56), respectively]. Ridge selections for gestational age among female fetuses were generally stronger than those for preterm and spontaneous preterm birth, with MBzP () and MHBP () contributing the strongest weights. In contrast, adENET selected only MBzP, and its weight was close to zero (, 95% CI: , 0.12). Ridge selections for gestational age among males were similar to those for spontaneous preterm birth, with MCNP and MBP () giving the strongest weights. All metabolites that were selected in adENET for gestational age among male fetuses (MEHP, MCNP, MBP) were also among the metabolites selected for spontaneous PTB, but weights were weaker for gestational age [ (95% CI: , 0.38), (95% CI: , 0.12), and (95% CI: , 0.07), respectively]. Weights for SGA and most weights for LGA from ridge regression and adENET were weak among both female and male fetuses ( for all selections). MBP among male fetuses was the only metabolite more strongly selected for LGA (, 95% CI: , 0.46) from adENET. Ridge regression weights for birth weight z-score among female fetuses were strongest and positive for MEHHP () and MHiBP (), and strongest and negative for MECPP () and MHBP (). In contrast, the only nonzero weight from adENET was for MCOP (, 95% CI: , 0.06). Birth weight z-score weights from ridge regression among males were weak (), whereas adENET selected MBzP (, 95% CI: , 0.05) and MBP (, 95% CI: , 0.20).
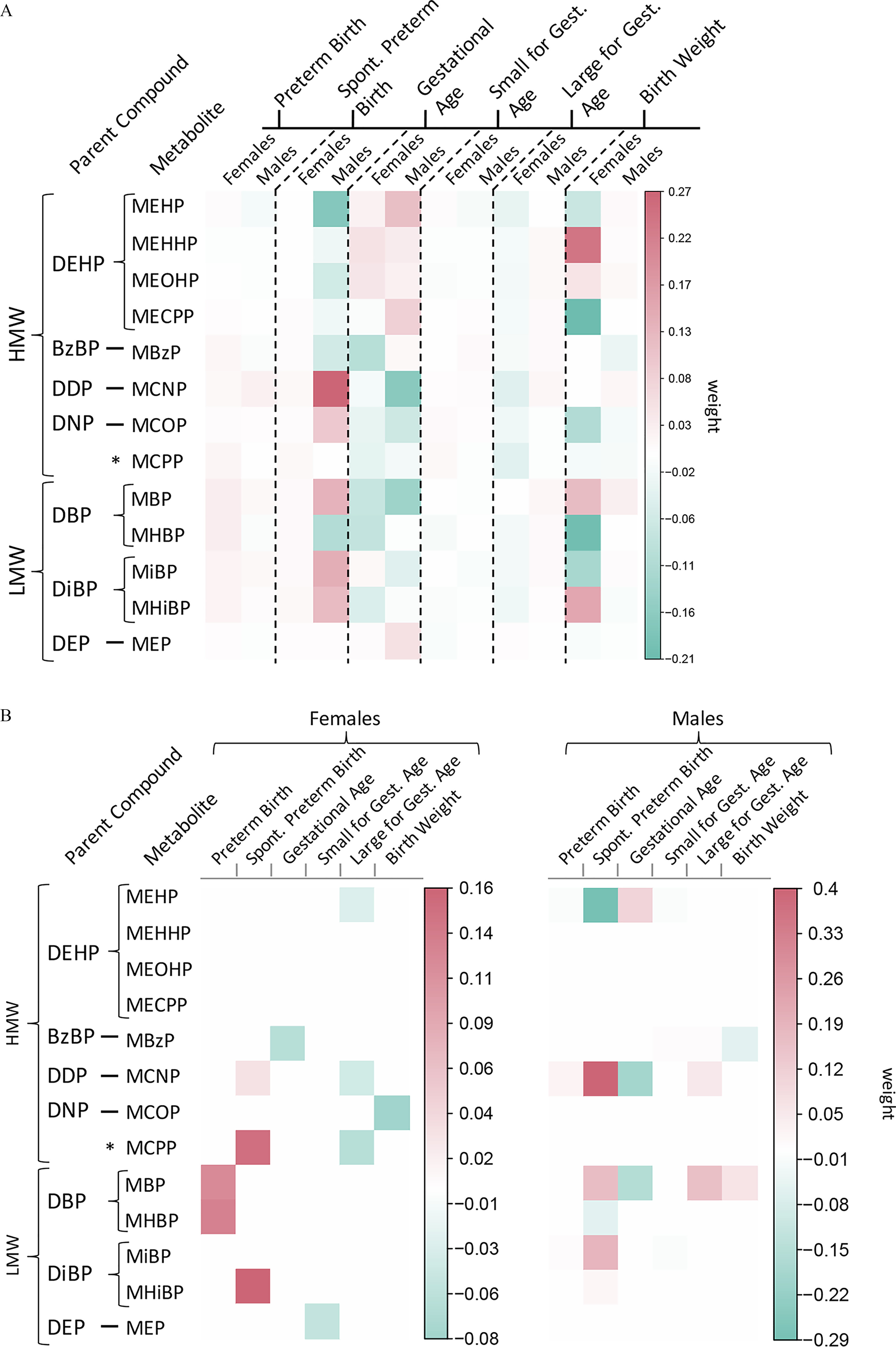
Metabolite selections from ridge regression and adENET at each study visit and by fetal sex are depicted in Figure 2. Corresponding weights (and 95% CI for adENET) can be found in Tables S3–S6. Weights from ridge regression for preterm birth among female fetuses were strongest at the second study visit and suggested that MHBP () and MHiBP () were the strongest predictors of preterm birth. adENET also selected MHBP (, 95% CI: , 0.75) and MHiBP (, 95% CI: , 0.52) at the second study visit as the strongest predictors of preterm birth. All weights from ridge regression for preterm birth among males were weak (). In contrast, adENET more strongly selected MCNP (, 95% CI: , 0.36), MBP (, 95% CI: , 0.33), and MiBP (, 95% CI: , 0.34) at visit 2, and MCNP (, 95% CI: , 0.45) at visit 3.
Figure 2.
Heat maps depicting repeated phthalate metabolite selections from (A) ridge regression, and (B) adaptive elastic net, between birth outcomes and by fetal sex in the PROTECT cohort. Box color corresponds to the weight assigned to each metabolite from ridge or adENET. Corresponding numeric data can be found in Tables S4–S7. Note: *The MCPP metabolite results from metabolism of multiple high molecular weight parent phthalates.
The strongest weights from ridge regression for spontaneous preterm birth among females were MCNP (), MiBP (), and MHiBP () at the third study visit. adENET also selected MiBP (, 95% CI: , 0.26) and MHiBP (, 95% CI: , 0.27) at the third study visit, but selection weights were stronger at visit 2 [MiBP (95% CI: , 0.89), MHiBP (95% CI: , 1.00), MCNP (95% CI: , 0.53), MEP (95% CI: , 0.37), and MHBP (95% CI: , 0.63)]. Ridge regression gave the strongest weights at the second study visit for a similar set of metabolites (MCNP , MiBP , MHiBP ) for spontaneous preterm birth among males, and adENET also selected similar set of metabolites at visit 2 [MCNP (95% CI: , 0.45), MCOP (95% CI: , 0.35), MBP (95% CI: , 0.35), MiBP (95% CI: , 0.50), MHiBP (95% CI: , 0.51)]. adENET also showed strong selection of MCNP at visit 3 (, 95% CI: , 0.74).
Visit-specific weights from ridge regression for gestational age among female fetuses were strongest for MEHP () and MHBP () at visit 2. adENET selected those same metabolites at visit 2 [MEHP (95% CI: , 0.16) and MHBP (95% CI: , 0.13)], and also selected MCPP at visit 1 (, 95% CI: , 0.12) and MBzP at visit 3 (, 95% CI: , 0.06). Ridge regression weights among male fetuses suggested that MCNP at visit 3 () was the strongest predictor of gestational age at birth. adENET also selected MCNP at visit 3 (, 95% CI: , 0.07) as the strongest predictor, and additionally selected MEHP at visit 1 (, 95% CI: , 0.33) and MCNP (, 95% CI: , 0.09) and MBP (, 95% CI: , 0.07) at visit 2. Ridge regression weights for SGA among female fetuses were strongest for MCPP (), MCOP (), and MHBP () at visit 2, and weight for metabolites selected in adENET were all relatively weak (). Ridge regression weights for SGA among males were all weak (), and adENET selected MEHP (, 95% CI: , 0.19) and MBzP (, 95% CI: , 0.33) at visit 3 as the most important predictors. LGA weights from ridge regression among females were weak (), and adENET selections were also mostly weak (), with MCPP at visit 1 contributing the strongest weight (, 95% CI: , 0.13). LGA weights from ridge regression among males were also mostly weak (), and adENET selected MBzP at visit 2 as the strongest predictor (, 95% CI: , 0.35). Weights for birth weight z-score across all three visits were weak among both male and female fetuses from ridge regression () and adENET ().
Prediction of Birth Outcomes with ERS
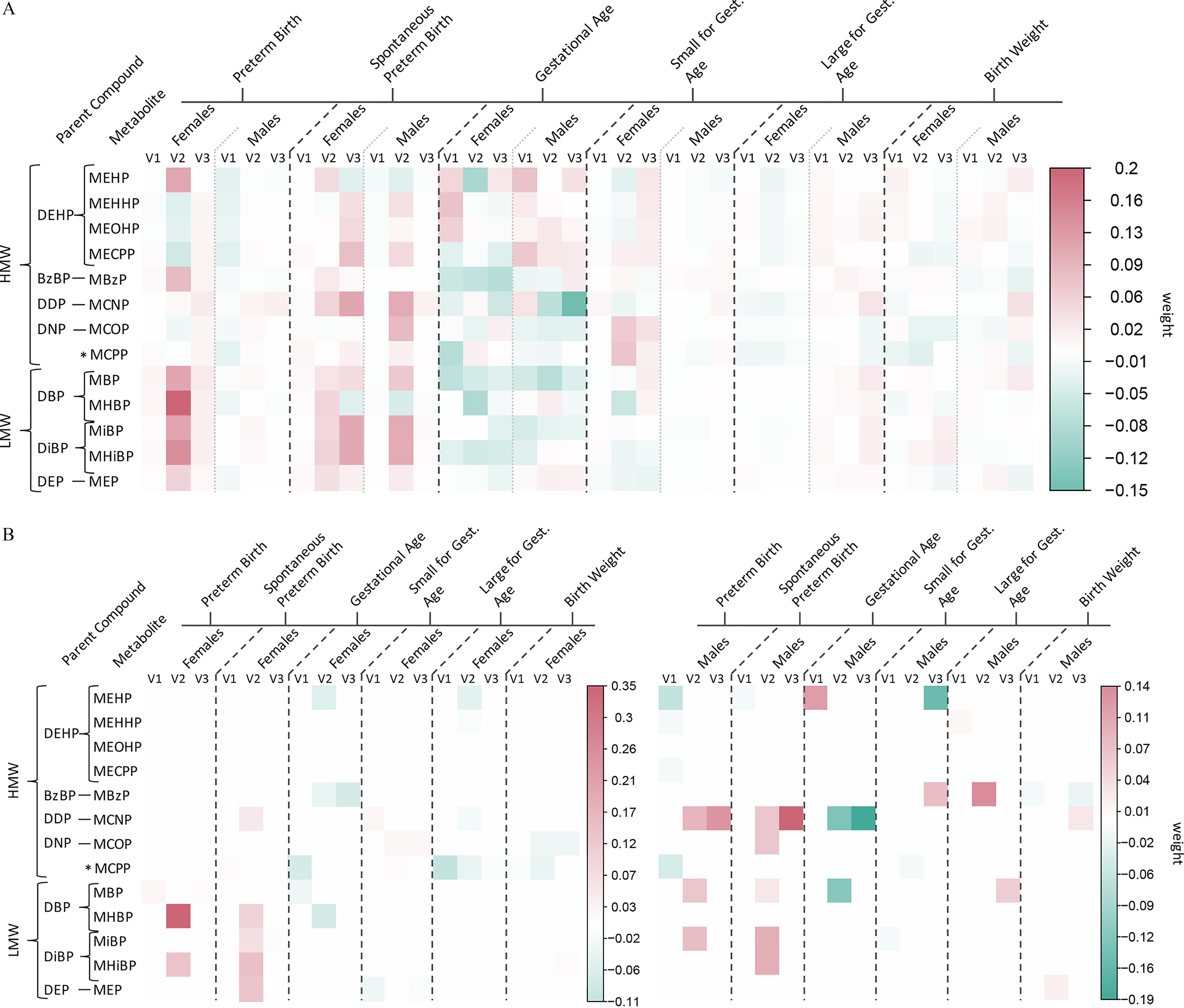
Phthalate ERS were used as exposure variables in linear and logistic regression models to assess associations between exposure to phthalate mixtures and adverse birth outcomes. Figure 3A depicts these associations with ERS calculated from adENET, and Figure 3B with ERS calculated from ridge regression, between fetal sexes with an IQR increase in gestational average phthalate ERS (corresponding effect estimates and 95% CI can be found in Table S8). Significant positive associations with phthalate ERS derived from adENET among both male and female fetuses were similar for preterm [male odds ratio (OR) ; 95% CI: 1.08, 2.27; female ; 95% CI: 1.23, 2.98] and spontaneous preterm birth [male ; 95% CI: 1.46, 3.68; female ; 95% CI: 1.04, 3.82]. These effect estimates were larger in magnitude than single pollutant associations (Tables S1–S2), though confidence intervals often overlapped. For example, no individual averaged phthalates were significantly associated with preterm birth among male fetuses, and an IQR increase in average MHBP was associated with 1.80 times greater odds of preterm birth among female fetuses (95% CI: 1.16, 2.80).
Figure 3.
Associations (points) and 95% CI (bars) from linear/logistic regression between an interquartile range increase in gestational average phthalate ERS derived from (A) adaptive elastic net, or (B) ridge regression, and birth outcomes between male and female fetuses in the PROTECT cohort. Change in gestational age at birth is presented in weeks. Solid vertical lines indicate the null values. All models adjust for maternal age and education, and birth weight models additionally adjust for maternal prepregnancy BMI. Corresponding numeric data can be found in Table S8. Note: BMI, body mass index; CI, confidence interval; ERS, environmental risk scores.
Associations between ERS derived from ridge regression and preterm and spontaneous preterm birth were similar in magnitude to those from adENET ERS among male and female fetuses, but lower bounds of 95% CI were further from the null for preterm birth among males. The association between phthalate ERS from adENET and SGA was not statistically significant for either fetal sex, but significant and inverse for ERS from ridge regression among male fetuses (; 95% CI: 0.41, 0.97) and significant and positive among female fetuses (; 95% CI: 1.05, 2.24). Phthalate ERS from adENET and ridge regression were inversely associated with odds of LGA among female fetuses (adENET , 95% CI: 0.37, 0.88; ridge , 95% CI: 0.41, 0.93), whereas only MCPP was significantly associated with LGA (; 95% CI: 0.38, 0.93) in single pollutant analyses. Neither phthalate ERS nor any individual metabolites were associated with LGA among male fetuses. Both fetal sexes displayed a reduction in gestational age (male wk, 95% CI: , ; female wk, 95% CI: , ) with increasing phthalate ERS from adENET, and results for ERS from ridge regression were similar in magnitude but further from the null in significance (male wk, 95% CI: , ; female wk, 95% CI: , ). In accordance with results for SGA and LGA, increases in phthalate ERS from ridge regression were associated with reduced birth weight z-score among female fetuses (; 95% CI: , ) and increased birth weight z-score among male fetuses ( 0.16; 95% CI: 0.03, 0.29). Results for ERS from adENET were similar but less significant (female , 95% CI: , 0.01; male , 95% CI: 0.01, 0.25). Effect sizes for ERS among males and females were greater in magnitude and/or significance than any individual averaged phthalate metabolite associations for both gestational age at birth and birth weight z-score.
Associations between birth outcomes and visit-specific phthalate ERS are shown in Figure 4A for female fetuses and Figure 4B for male fetuses (corresponding effect estimates and 95% CI can be found in Tables S9 and S10). Among female fetuses, associations between phthalate ERS from adENET and preterm birth and gestational age were strongest at visit 2 (PTB , 95% CI: 2.03, 10.3; gestational age , 95% CI: , ). Results were similar for ERS from ridge regression and strongest at the second study visit (PTB , 95% CI: 2.46, 14.3; gestational age , 95% CI: , ). The estimated effects with increasing ERS were stronger than those for individual phthalates; visit 2 MHBP was associated with 2.23 times greater odds of preterm birth (95% CI: 1.19, 4.19), and none of the visit 2 phthalates were associated with reductions in gestational age at birth. ERS from both adENET and ridge regression among male fetuses were all similarly associated with increased odds of preterm birth and reductions in gestational age at birth at each study visit. Phthalate ERS at visit 2 among male fetuses did, however, show a stronger association with spontaneous preterm birth (adENET , 95% CI: 1.68, 7.07; ridge , 95% CI: 1.83, 8.25) than the ERS at the other study visits. This effect was the stronger than any effects with individual metabolites at visit 2, the strongest of which was MHiBP (; 95% CI: 1.26, 4.11). Increased odds of having an SGA female and decreased odds of having an SGA male were marginally significant and did not differ appreciably between study visits. Accordingly, decreased odds of having an LGA female and increased odds of having an LGA male were similar across study visits and most significant and visit 2. Birth weight z-score was most significantly associated with ERS from ridge regression at visit 3 among both female (; 95% CI: , ) and male fetuses ( 0.30; 95% CI: 0.13, 0.47). Results from adENET were similar in magnitude but less significant than those from ridge.
Figure 4.
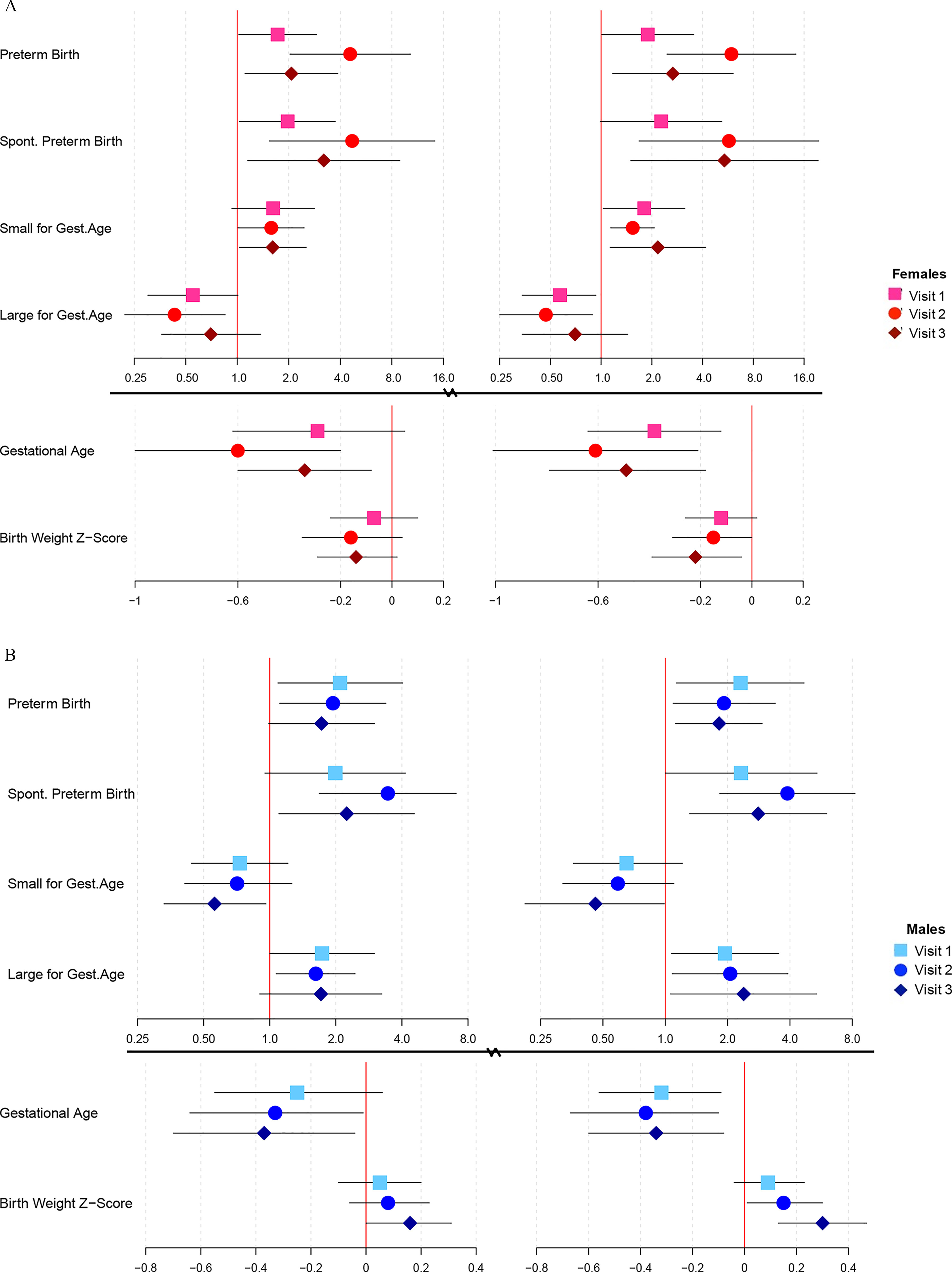
Associations (points) and 95% CI (bars) from linear/logistic regression between an interquartile range increase in visit-specific phthalate ERS, among (A) female, and (B) male fetuses, derived from adENET (left panels) and ridge regression (right panels) in the PROTECT cohort. Change in gestational age is presented in weeks. Solid vertical lines indicate the null values. All models adjust for maternal age and education, and birth weight models additionally adjust for maternal prepregnancy BMI. Corresponding numeric data can be found in Tables S9 (females) and S10 (males). Note: BMI, body mass index; CI, confidence interval; ERS, environmental risk scores.
BKMR
BKMR analysis was employed to investigate nonlinearity and interactions between gestational average phthalate metabolites selected in adENET as being predictive of adverse birth outcomes. The metabolites included in BKMR for each birth outcome by fetal sex, and their respective posterior inclusion probabilities, are shown in Table S11. Bivariate plots were used to assess linearity and interactions between phthalate metabolites (all bivariate plots can be found in Figure S2). From bivariate plots, the shape of the lines indicates the shape of the association between phthalate metabolites and outcomes, so a straight line would indicate a linear association. An interaction between metabolites is demonstrated when the shape of the association with a given outcome for one metabolite changes with varying quantiles of exposure to another phthalate. Among women carrying a female fetus, we did not observe interactions between metabolites for any birth outcomes assessed. Though most relationships were linear, we did observe some nonlinear associations. MCPP displayed a nonlinear relationship with odds of spontaneous PTB that was null at low concentrations and positive at high concentrations (Figure 5A). Additionally, MBP and MHBP displayed inverted U-shaped associations with gestational age at birth (Figure 5B). Among women carrying a male fetus, we also observed a small number of nonlinear relationships. The association between MBzP and SGA was U-shaped at low to moderate concentrations of MBzP but became positive at higher concentrations of MBzP (Figure 6A). A similarly shaped association was observed between MEHHP and LGA (Figure 6B). Birth weight z-score was the only birth outcome for which we observed any interactions between phthalate metabolites (Figure 6C). The association between MEP and birth weight z-score largely depended on concentrations of MBzP; inverse linear associations were observed with concentrations of MBzP at the 25th and 50th percentile, whereas positive linear associations were observed with concentrations of MBzP at the 75th and 90th percentile. MBzP displayed an inverted U-shaped association with birth weight z-score that did not depend heavily on concentrations of MEP.
Figure 5.
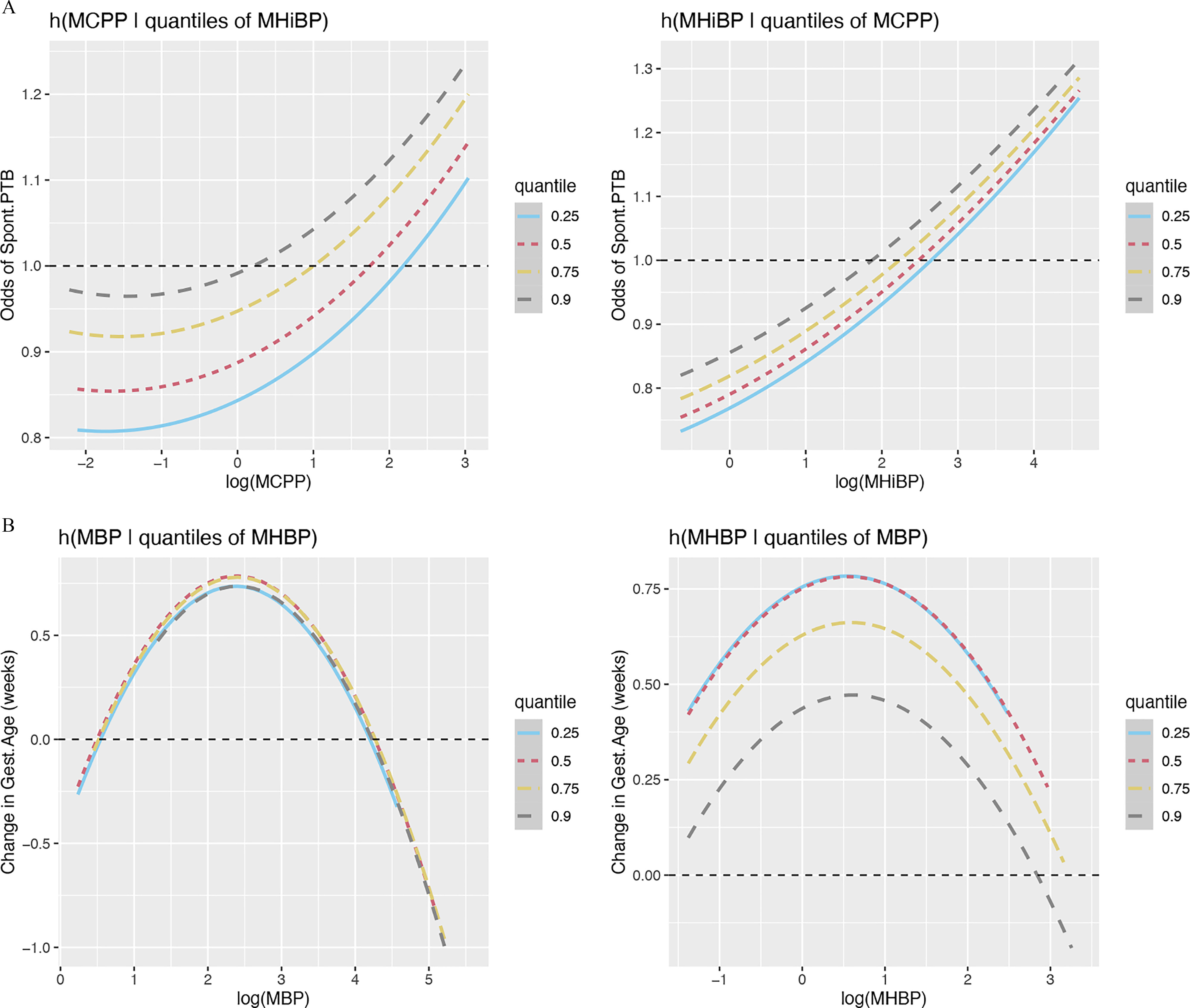
Exposure response function using Bayesian kernel machine regression among women carrying a female fetus in the PROTECT cohort. (A) Spontaneous preterm birth response as a function of MCPP conditional on differing quantiles of MHiBP (left) or MHiBP conditional on differing quantiles of MCPP (right), while fixing the rest of the mixture at its 50th percentile. (B) Gestational age at birth response as a function of MBP conditional on differing quantiles of MHBP (left) or MHBP conditional on differing quantiles of MBP (right), while fixing the rest of the mixture at its 50th percentile. Note: MBP, mono-n-butyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MHBP, monohydroxybutyl phthalate; MHiBP, monohydroxyisobutyl phthalate.
Figure 6.
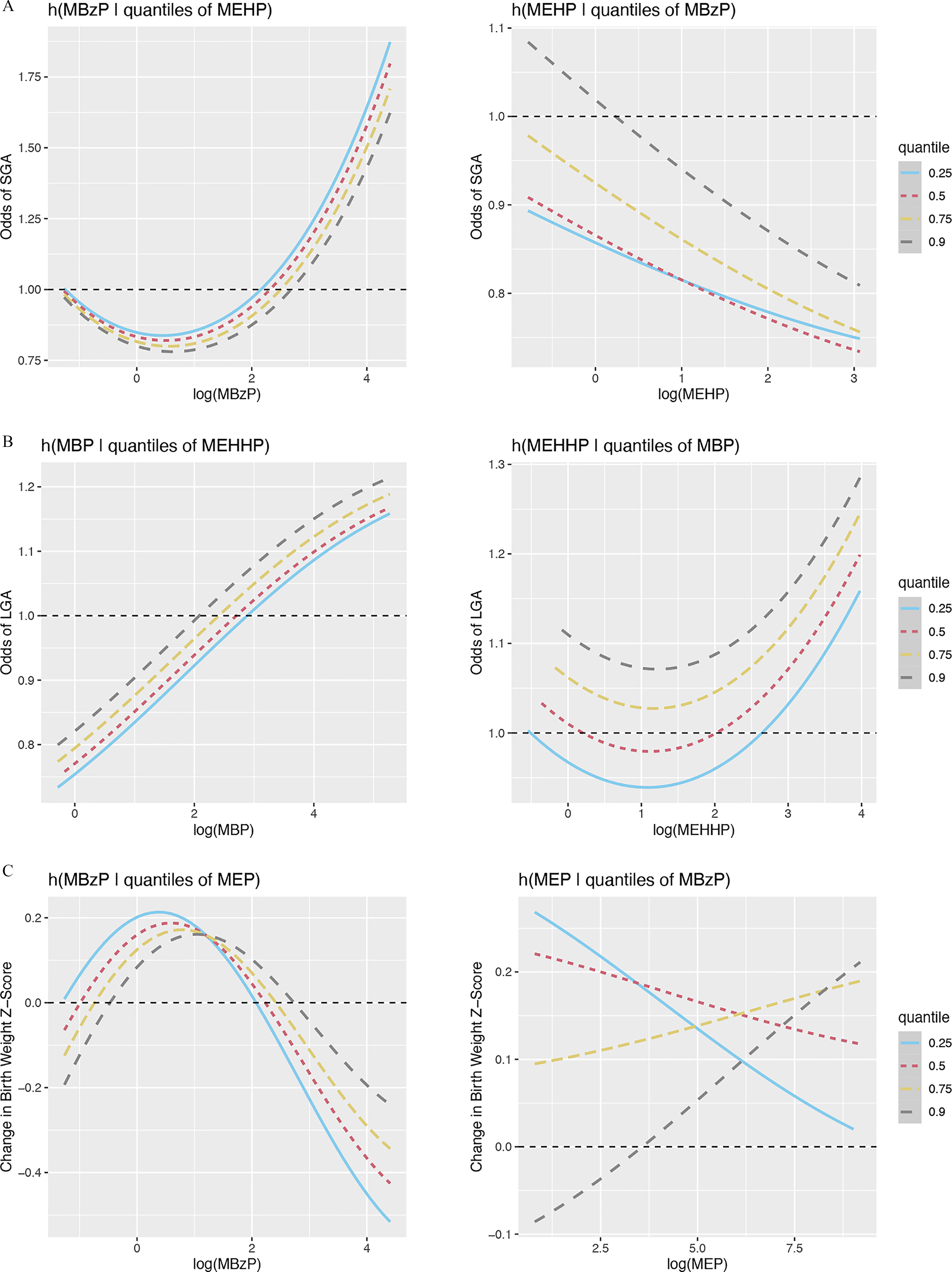
Exposure response function using Bayesian kernel machine regression among women carrying a male fetus in the PROTECT cohort. (A) SGA response as a function of MBzP conditional on differing quantiles of MEHP (left) or MEHP conditional on differing quantiles of MBzP (right), while fixing the rest of the mixture at its 50th percentile. (B) LGA response as a function of MBP conditional on differing quantiles of MEHHP (left) or MEHHP conditional on differing quantiles of MBP (right), while fixing the rest of the mixture at its 50th percentile. (C) Birth weight z-score response as a function of MBzP conditional on differing quantiles of MEP (left) or MEP conditional on differing quantiles of MBzP (right), while fixing the rest of the mixture at its 50th percentile. Note: LGA, large for gestational age; MBP, mono--butyl phthalate; MBzP, monobenzyl phthalate monobutyl phthalate (minor); MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; SGA, small for gestational age.
Discussion
In this study, we explored the associations between exposure to a mixture of phthalates and adverse birth outcomes and how those associations may differ between fetal sexes. Various metabolites stood out as being predictive of adverse birth outcomes, including those from DDP, DBP, and DiBP parent compounds, and differences by fetal sex were apparent. Increased exposure to the mixture of phthalate metabolites, given by ERS derived using metabolite weights from adaptive elastic net and ridge regression, was associated with increased risk of preterm and spontaneous preterm birth and reduced gestational age at birth among all pregnancies, increased odds of having small female babies, and increased odds of having large male babies. Visit-specific analyses suggested that exposure at the second study visit, occurring around 22 wk (range 20–24 wk), may be driving some of our observed associations. Last, BKMR analyses revealed that several associations were nonlinear, with low-vs. high-level exposure conferring differential risk for preterm birth, and that interactions between phthalate metabolites may have a significant effect on birth weight.
This work builds on a previous analysis in which associations between single phthalate metabolites and preterm birth, spontaneous preterm birth, and gestational age at birth were assessed among a similar cohort of PROTECT women.30 Though results in that study combined all pregnancies and thus cannot be directly compared to the present sex-stratified results, most findings were consistent. In that study, the greatest risk of delivering preterm was observed with increasing average concentrations of MBP, MiBP, and MHBP. We also observed selection of MBP and MHBP for preterm birth, but only among mothers carrying a female fetus. The aforementioned study also found MiBP and MHiBP to be associated with spontaneous preterm birth and that those effect estimates were larger in magnitude than those observed for all preterm births combined. An interesting finding was that we also observed stronger selection for MiBP and MHiBP with spontaneous preterm birth, but only among women carrying a male. We additionally observed strong selection of MCNP for spontaneous preterm birth among only mothers carrying a male; however, MCNP was not significantly associated with any birth outcomes in the previous analysis. Using a similar source population and distinct statistical methods, our results substantiate previous findings that phthalate exposures at the second study visit were most important for driving associations and that metabolites of DDP, DBP, and DiBP may be particularly important for predicting risk of adverse birth outcomes. In addition, the present study adds new evidence that effects of these phthalate metabolites on birth outcomes differ between fetal sexes.
Another previous study used various statistical techniques, distinct from the adaptive elastic net and ridge regression methods used in the present study, to assess phthalate mixture associations with preterm birth and gestational age at delivery in the LIFECODES pregnancy cohort in Boston, Massachusetts.26 Although each method resulted in different selections of predictive metabolites, average MECPP and MBP were generally found to be positively predictive of delivering preterm, and average MiBP was generally found as inversely predictive of delivering preterm. We also observed positive selection of MBP for preterm birth among women carrying a female, but we did not find MECPP to be predictive of any birth outcomes, nor did we observe inverse associations between preterm birth and MiBP. Lack of fetal sex stratification, plus differing methods for assessing phthalate mixtures and for dealing with issues of multicollinearity, likely led to differing results between studies. Additionally, phthalate exposure profiles have been shown to be associated with socioeconomic status (SES),46 for which covariate adjustment differs substantially between the LIFECODES and PROTECT cohorts. Thus, differences in SES and how it is assessed in the LIFECODES cohort relative to PROTECT could also be contributing to differing results.
One previous study used elastic net to assess phthalates, along with other environmental pollutants, in relation to birth weight.27 Those researchers observed that MEHHP was inversely predictive of birth weight, which did not agree with results from the present study. We did observe weak selection of MEHHP as having a positive association with risk of LGA when measured at the first study visit among female fetuses, which contradicts the results of the previous study. However, Lenters et al. did not stratify their analyses by fetal sex, and thus their results cannot be directly compared with ours. Another possible source of differing results is that the former study measured phthalate metabolites in serum instead of urine, which presents a major limitation of that study because urine has been established as the best matrix for phthalate measurement due to very short half-lives in blood.47 Additionally, the panel of phthalate metabolites measured in the previous study does not overlap well with our study and importantly did not include metabolites of DBP or DiBP, which were found to be important in the present study. Because elastic net selections are conditional on other predictors put into the model, selection of differing final sets of predictors is not surprising. Finally, the former study was conducted in various European countries that presented a much lower preterm birth rate (3.84%) than that of Puerto Rico and that warranted differential covariate adjustment than that in the present study.
Most of the current toxicology literature focuses on DEHP exposures and effects on male reproductive outcomes, so there is little knowledge of the physiological pathways on which DBP and DiBP may act to adversely affect pregnancy outcomes. However, various epidemiology studies have shown significant associations between metabolites of DBP and DiBP and endogenous biomarkers that may serve as mediators on the causal pathway between phthalate exposure and preterm birth. MBP and MHBP have both shown positive associations with thyroid hormones [free thyroxine (fT4) and total triiodothyronine (T3)],21 possibly via alteration of thyroid hormone transcription48,49 or receptor antagonistic activity.50,51 These thyroid hormones have also shown positive associations with odds of preterm birth,52,53 which may occur through direct action on the placenta for transfer of maternal thyroid hormones54 or promotion of fetal growth and development.55
Phthalates may also exert their effects on timing of delivery via oxidative stress or inflammatory pathways. Previous research has shown positive associations between phthalate metabolites, including MBP and MHBP, and biomarkers of oxidative stress and inflammation.22,56,57 These pathways are particularly important when considering the spontaneous subtype of preterm birth, which can be characterized as having an inflammatory uterine environment.39 Oxidative stress and inflammation are tightly linked, and therefore elevated levels of phthalate exposure could lead to an increase in inflammation in the maternal–fetal environment and subsequent early initiation of labor.58
The present study was subject to various limitations. Despite having phthalate measurements repeated at up to three time points during pregnancy, metabolite measurements have been shown to have significant within-individual variability over time,34 and so three measurements may not be sufficient to ascertain true exposure levels during pregnancy. Further, we did not assess exposure at critical developmental windows early or late in pregnancy, at which times phthalate exposure may exert differential effects on pregnancy outcomes. Replacing phthalate concentrations measured below the LOD by the LOD divided by the square root of 2 reduces the variance of exposure data and leads to lower -values. We observed the greatest proportion of concentrations below the LOD for MEHP, MCPP, and MHBP, but these were all measured above the LOD in at least 80% of samples, and so the reduction in variance was minimal. Our study methodology involved excluding women with preexisting conditions, which may reduce the generalizability of our results. However, exclusion of those with preexisting conditions does allow us to home in on the associations between health effects and our exposures of interest more precisely. Relative to studies assessing phthalate exposures and birth outcomes in other cohorts, we adjusted final statistical models for a small number of potential confounders. However, all available markers of SES (education level, employment status, and annual household income) were assessed for associations with exposures and outcomes, and our final set of covariates (maternal age, education, and prepregnancy BMI) are consistent with previous research conducted in the PROTECT cohort. Finally, because the data set used to create the ERS was same the same as that used to obtain final effect estimates for associations between ERS and birth outcomes, it is possible that our models are overfit and thus produced effect estimates that were biased away from the null. However, adENET was still used as our primary method because of its variable selection utility and ability to handle large covariate spaces, whereas BKMR, in contrast, tends to have difficulty modeling a large number of predictors.42
Despite these limitations, this study was also strong in various ways. First, this is one of few studies, and the largest, to assess phthalate mixtures in relation to birth outcomes. We are also the first to investigate differences in phthalate mixture associations between male and female pregnancies. To our knowledge, there is currently no epidemiological evidence to support a mechanism for these fetal sex differences, but the toxicology literature shows in vitro evidence of greater pro-inflammatory responses by male fetal cells vs. greater anti-inflammatory responses from female fetal cells.31 Additionally, using a phthalate ERS as the exposure measure instead of individual phthalate metabolites reduces the potential for bias due to correlation between metabolites and also reduces the potential for confounding by one metabolite on observed associations for another. Further, this method of exposure assessment also allows for risk assessment and ascertainment of the biological pathways implicated with exposure to a whole class of environmental contaminants. Finally, we present associations for the spontaneous subtype of preterm birth, which has not been deeply studied in the past and is likely to represent a more etiologically homogenous group that also reduces outcome misclassification.
In conclusion, this study provided evidence that exposure to a mixture of phthalates may increase the risk of early delivery. This work adds to existing data showing that metabolites of DBP and DiBP, which are low molecular weight phthalates found in personal care products and many other sources, are of particular importance for predicting adverse birth outcomes in Puerto Rico and underscores the importance of policy regulations that aim to provide safer personal care products to consumers. Further, we have contributed evidence that phthalate exposure affects male and female pregnancies differently, particularly in relation to birth size, which had not been sufficiently explored in previous epidemiology research. Finally, we showed that some phthalate metabolites were differentially associated with birth outcomes when exposures were high vs. low, which is important for future studies hoping to use environmental exposure data for predicting pregnancies that are at risk for adverse birth outcomes. Future work will aim to explore the biological pathways, including endocrine disruption and oxidative stress, which could mediate our observed associations.
Supplementary Material
Acknowledgments
The authors would like to extend our gratitude to all PROTECT study participants and their families. The authors also thank the nurses and research staff who participated in cohort recruitment and follow-up, as well as the Federally Qualified Health Centers in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc., and the Delta OBGyn Group in Manati, Puerto Rico, as well as the Manati Medical Center and the Metro Pavia Hospital in Arecibo, Puerto Rico.
A.L.C. analyzed and interpreted data and drafted the original article. J.D.M., J.F.C., A.N.A., and C.M.V. made substantial contributions to the conception and design of the study. D.J.W. and Z.Y.R. contributed to acquisition of data. B.M. assisted with interpretation of data. All authors provided substantial revisions and gave approval for the final version to be published.
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198). Additional support was provided from NIEHS grant number P30ES017885 and the Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health.
References
- 1.Frey HA, Klebanoff MA. 2016. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 21(2):68–73, PMID: , 10.1016/j.siny.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379(9832):2151–2161, PMID: , 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group. 2005. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 352(1):9–19, PMID: , 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 4.Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, et al. 2018. Visual and hearing impairments after preterm birth. Pediatrics 142(2):e20173888, PMID: , 10.1542/peds.2017-3888. [DOI] [PubMed] [Google Scholar]
- 5.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. 2009. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124(2):717–728, PMID: , 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 6.Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. 2013. The association of preterm birth with severe asthma and atopic dermatitis: a national cohort study. Pediatr Allergy Immunol 24(8):782–787, PMID: , 10.1111/pai.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson KK, O’Neill MS, Meeker JD. 2013. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev 16(2):69–113, PMID: , 10.1080/10937404.2013.775048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ATSDR (Agency for Toxic Substances and Disease Registry). 2001. Toxicological Profile for Di-n-butyl Phthalate. https://www.atsdr.cdc.gov/toxprofiles/tp135.pdf [accessed 15 October 2020]. [PubMed]
- 9.ATSDR. 2022. Toxicological Profile for Di(2-ethylhexyl) Phthalate (DEHP). https://www.atsdr.cdc.gov/ToxProfiles/tp9.pdf [accessed 15 October 2020]. [PubMed]
- 10.Schettler T. 2006. Human exposure to phthalates via consumer products. Int J Androl 29(1):134–135, PMID: , 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 11.Koch HM, Lorber M, Christensen KLY, Pälmke C, Koslitz S, Brüning T. 2013. Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 216(6):672–681, PMID: , 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Hauser R, Calafat AM. 2005. Phthalates and human health. Occup Environ Med 62(11):806–818, PMID: , 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. 2009. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environ Health Perspect 117(10):1587–1592, PMID: , 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson KK, McElrath TF, Meeker JD. 2014. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168(1):61–67, PMID: , 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng F, Ji W, Zhu F, Peng D, Yang M, Liu R, et al. 2016. A study on phthalate metabolites, bisphenol A and nonylphenol in the urine of chinese women with unexplained recurrent spontaneous abortion. Environ Res 150:622–628, PMID: , 10.1016/j.envres.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Mu D, Gao F, Fan Z, Shen H, Peng H, Hu J, et al. 2015. Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss. Environ Sci Technol 49(17):10651–10657, PMID: , 10.1021/acs.est.5b02617. [DOI] [PubMed] [Google Scholar]
- 17.Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GMB. 2015. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ Health 14:73, PMID: , 10.1186/s12940-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. 2016. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: a preliminary analysis. Reprod Toxicol 65:59–66, PMID: , 10.1016/j.reprotox.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernández MF, Garcia-Esteban R, et al. 2016. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell cohort. Environ Health Perspect 124(4):521–528, PMID: , 10.1289/ehp.1409190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson KK, Meeker JD, Cantonwine DE, Chen Y-H, Mukherjee B, McElrath TF, et al. 2016. Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environ Int 94:531–537, PMID: , 10.1016/j.envint.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cathey AL, Watkins D, Rosario ZY, Vélez C, Alshawabkeh AN, Cordero JF, et al. 2019. Associations of phthalates and phthalate replacements with CRH and other hormones among pregnant women in Puerto Rico. J Endocr Soc 3(6):1127–1149, PMID: , 10.1210/js.2019-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cathey AL, Eaton JL, Ashrap P, Watkins DJ, Rosario ZY, Vélez Vega C, et al. 2021. Individual and joint effects of phthalate metabolites on biomarkers of oxidative stress among pregnant women in Puerto Rico. Environ Int 154:106565, PMID: , 10.1016/j.envint.2021.106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD. 2015. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect 123(3):210–216, PMID: , 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol 48(12):7018–7025, PMID: , 10.1021/es502076j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, et al. 2021. Cross-Sectional estimation of endogenous biomarker associations with prenatal phenols, phthalates, metals, and polycyclic aromatic hydrocarbons in single-pollutant and mixtures analysis approaches. Environ Health Perspect 129(3):37007, PMID: , 10.1289/EHP7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boss J, et al. 2018. Associations between mixtures of urinary phthalate metabolites with gestational age at delivery: a time to event analysis using summative phthalate risk scores. Environ Health 17:56, PMID: , 10.1186/s12940-018-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenters V, Portengen L, Rignell-Hydbom A, Jönsson BAG, Lindh CH, Piersma AH, et al. 2016. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ Health Perspect 124(3):365–372, PMID: , 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C, Gao L, Flaws JA. 2017. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 158(6):1739–1754, PMID: , 10.1210/en.2017-00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K, Liszka M, Zhou C, Brehm E, Flaws JA, Nowak RA, et al. 2020. Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reprod Toxicol 93:178–190, PMID: , 10.1016/j.reprotox.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, et al. 2019. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int 132:105099, PMID: , 10.1016/j.envint.2019.105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. 2013. Fetal sex and preterm birth. Placenta 34(2):95–99, PMID: , 10.1016/j.placenta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 32.McGregor JA, Leff M, Orleans M, Baron A. 1992. Fetal gender differences in preterm birth: findings in a North American cohort. Am J Perinatol 9(1):43–48, PMID: , 10.1055/s-2007-994668. [DOI] [PubMed] [Google Scholar]
- 33.Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, et al. 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol 47(7):3439–3447, PMID: , 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantonwine DE, Cordero JF, Rivera-González LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int 62:1–11, PMID: , 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC (U.S. Centers for Disease Control and Prevention). 2013. Metabolites of Phthalates and Phthalate Alternatives: Urine. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/PHTHTE_G_met.pdf [accessed 15 October 2020].
- 36.CDC. 2017. Metabolites of Phthalates and Phthalate Alternatives: Urine. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/PHTHTE-J-MET-508.pdf [accessed 15 October 2020].
- 37.Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 38.Pettker C, Goldberg J, El-Sayed Y, Copel J. 2017. Committee Opinion No 700: methods for estimating the due date. Obstet Gynecol 129:e150–e154, PMID: , 10.1097/AOG.0000000000002046. [DOI] [PubMed] [Google Scholar]
- 39.McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. 2008. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 168(9):980–989, PMID: , 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384(9946):857–868, PMID: , 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 41.Park SK, Tao Y, Meeker JD, Harlow SD, Mukherjee B. 2014. Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PLoS One 9(6):e98632, PMID: , 10.1371/journal.pone.0098632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SK, Zhao Z, Mukherjee B. 2017. Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Health 16(1):102, PMID: , 10.1186/s12940-017-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: , 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashrap P, Watkins DJ, Mukherjee B, Rosario-Pabón Z, Vélez-Vega CM, Alshawabkeh A, et al. 2021. Performance of urine, blood, and integrated metal biomarkers in relation to birth outcomes in a mixture setting. Environ Res 200:111435, PMID: , 10.1016/j.envres.2021.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silver MK, Fernandez J, Tang J, McDade A, Sabino J, Rosario Z, et al. 2021. Prenatal exposure to glyphosate and its environmental degradate, aminomethylphosphonic acid (AMPA), and preterm birth: a nested case–control study in the PROTECT cohort (Puerto Rico). Environ Health Perspect 129(5):57011, PMID: , 10.1289/EHP7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Carmona Y, Ashrap P, Calafat AM, Ye X, Rosario Z, Bedrosian LD, et al. 2020. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J Expo Sci Environ Epidemiol 30(1):56–69, PMID: , 10.1038/s41370-019-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johns LE, Cooper GS, Galizia A, Meeker JD. 2015. Exposure assessment issues in epidemiology studies of phthalates. Environ Int 85:27–39, PMID: , 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibhazehiebo K, Koibuchi N. 2011. Thyroid hormone receptor-mediated transcription is suppressed by low dose phthalate. Niger J Physiol Sci 26(2):143–149, PMID: . [PubMed] [Google Scholar]
- 49.Breous E, Wenzel A, Loos U. 2005. The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol Cell Endocrinol 244(1–2):75–78, PMID: , 10.1016/j.mce.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Hu X, Shi W, Zhang F, Cao F, Hu G, Hao Y, et al. 2013. In vitro assessment of thyroid hormone disrupting activities in drinking water sources along the Yangtze River. Environ Pollut 173:210–215, PMID: , 10.1016/j.envpol.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Shen O, Wu W, Du G, Liu R, Yu L, Sun H, et al. 2011. Thyroid disruption by Di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) in Xenopus laevis. PLoS One 6(4):e19159. PMID: , 10.1371/journal.pone.0019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korevaar TIM, Chaker L, Medici M, de Rijke YB, Jaddoe VWV, Steegers EAP, et al. 2016. Maternal total T4 during the first half of pregnancy: physiologic aspects and the risk of adverse outcomes in comparison with free T4. Clin Endocrinol (Oxf) 85(5):757–763, PMID: , 10.1111/cen.13106. [DOI] [PubMed] [Google Scholar]
- 53.Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Seely EW, Meeker JD, et al. 2017. Longitudinal profiles of thyroid hormone parameters in pregnancy and associations with preterm birth. PLoS One 12(1):e0169542, PMID: , 10.1371/journal.pone.0169542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, et al. 2011. Thyroid hormone receptors and reproduction. J Reprod Immunol 90(1):58–66, PMID: , 10.1016/j.jri.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Glinoer D. 1997. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology. Endocr Rev 18(3):404–433, PMID: , 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson KK, Chen Y-H, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B, et al. 2017. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environ Health Perspect 125(3):488–494, PMID: , 10.1289/EHP282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holland N, Huen K, Tran V, Street K, Nguyen B, Bradman A, et al. 2016. Urinary phthalate metabolites and biomarkers of oxidative stress in a Mexican-American cohort: variability in early and late pregnancy. Toxics 4(1):7, PMID: , 10.3390/toxics4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F, et al. 2009. Inflammation and pregnancy. Reprod Sci 16(2):206–215, PMID: , 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.