Abstract
Constitutive vesicle trafficking is the default pathway used by all cells for movement of intracellular cargoes between subcellular compartments and in and out of the cell. Classically, constitutive trafficking was thought to be continuous and unregulated, in contrast to regulated secretion, wherein vesicles are stored intracellularly until undergoing synchronous membrane fusion following a Ca2+ signal. However, as shown in the literature reviewed here, many continuous trafficking steps can be up- or down-regulated by Ca2+, including several steps associated with human pathologies. Notably, we describe a series of Ca2+ pumps, channels, Ca2+-binding effector proteins, and their trafficking machinery targets that together regulate the flux of cargo in response to genetic alterations as well as baseline and agonist-dependent Ca2+ signals. Here, we review the most recent advances, organized by organellar location, that establish the importance of these components in trafficking steps. Ultimately, we conclude that Ca2+ regulates an expanding series of distinct mechanistic steps. Furthermore, the involvement of Ca2+ in trafficking is complex. For example, in some cases, the same Ca2+ effectors regulate surprisingly distinct trafficking steps, or even the same trafficking step with opposing influences, through binding to different target proteins.
Keywords: vesicle trafficking, calcium, secretion, Golgi, endoplasmic reticulum, lysosomes, late endosomes, calcium channel, calcium signaling, vesicle coat, apoptosis-linked gene 2 (ALG-2)
Introduction
Eukaryotic cells are in a constant state of membrane maintenance, renewal, and expansion, accomplished by an interwoven web of constitutive vesicle trafficking pathways connecting the endoplasmic reticulum (ER), Golgi, plasma membrane (PM), and the endolysosomal system. Classically, constitutive secretion is thought to be continuous and unregulated. This contrasts with the highly controlled, regulated secretory pathway in which proteins stored in intracellular granules are released en masse in response to external factors or signals. However, constitutive secretion is also regulated. By way of example, during mitosis in mammalian cells, the Golgi is fragmented for partitioning into progeny cells. This transition from S-to-M phase necessitates that most, if not all, membrane trafficking pathways are temporarily shut down, only to be resumed later during cytokinesis1. Alternatively, the differentiation of B cells to plasma cells requires a marked increase in secretory capacity, driven in part by sequential waves of protein expression2. However, there is still much to learn about the regulation of constitutive secretion. The milieu of ions, lipids, and proteins that constitutive trafficking externalizes, degrades, or delivers to various organelles affects nearly every aspect of cell metabolism, making dynamic regulation of these pathways of great significance to biomedical research.
Intracellular Ca2+ is actively sequestered into several organelles for use in a myriad of signaling processes3. Many of these Ca2+ stores, such as the ER, Golgi, and lysosome, are prominent in constitutive vesicle trafficking pathways (Figure 1). Interestingly, the concentration of luminal Ca2+ decreases from the ER to the PM, the same direction as the flow of cargo, suggesting a role for Ca2+ in directing cargo progression or sorting. Ca2+ is well known for its role in activation of regulated exocytosis. However, Ca2+ has also been suggested to play a role as a regulator of constitutive secretion. An often-cited study showed that vesicular tubular cluster (VTC)-to-Golgi and Golgi-to-ER retrograde trafficking is inhibited by the fast Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) but not the slower chelator of comparable Ca2+ affinity, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA)4. In addition to illustrating a role for calcium in constitutive secretion, that study implied tight spatial coupling of the vesicular fusion machinery and Ca2+, wherein the putative fusion site is close to the Ca2+ release site. Since then, further evidence continued to mount and various groups have implicated Ca2+ in the fusion of yeast vacuoles5, the heterotypic fusion of late endosomes with lysosomes, or the reformation of lysosomes from hybrid compartments6.
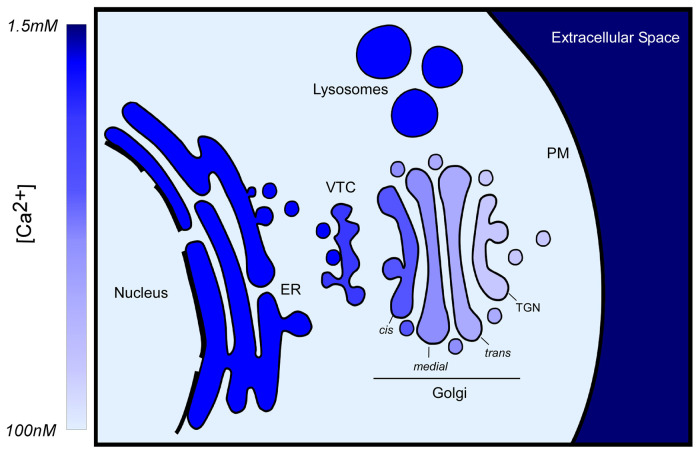
Figure 1. Ca2+ concentrations in constitutive trafficking.
Of the Ca2+ pools depicted, the low end is the cytosol, with a free [Ca2+] of about 100 nM. The concentration of Ca2+ decreases from the endoplasmic reticulum (ER) (~500 µM) to the cis-Golgi (~300 µM) to the trans-Golgi network (TGN) (50–100 µM)8,9, in the same direction as anterograde secretion. Lysosomes contain a similar [Ca2+] to the ER8,10. At the high end is the extracellular space with typical [Ca2+] of 1.2 to 1.5 mM8. PM, plasma membrane; VTC, vesicular tubular cluster.
In this review, we discuss multiple proteins that appear to regulate constitutive trafficking and have direct or indirect ties to Ca2+, focusing mainly on studies after 2007, when we published a review on the same topic7. We discuss a variety of secretory effectors, organized by the organelle wherein the primary secretory effects spatially occur, that modulate secretion via their capacity to bind Ca2+. In addition to these Ca2+ effectors, what has been most obvious in recent years is the rise in the number of calcium pumps or channels that have been found to affect constitutive trafficking. Channels mobilize Ca2+ and create a dynamic Ca2+ landscape in the cytoplasm8 but still must act upstream of Ca2+ sensors and trafficking machinery effectors to influence trafficking. Pumps, on the other hand, tend to affect trafficking on the luminal side by providing sufficiently high Ca2+ environments for Ca2+-dependent sorting or retention processes. Prominent example studies from the text are briefly summarized in Table 1 and Figure 2, Figure 3, and Figure 4. Some of the examples that we discuss, such as certain TRP channel family members of non-selective ion channels, have been implicated in secretion changes yet so far have not been matched up with Ca2+ sensors that could translate a Ca2+ flux into changes in secretion. This may mean that the critical Ca2+-binding effectors or channel-binding partners have not been defined yet.
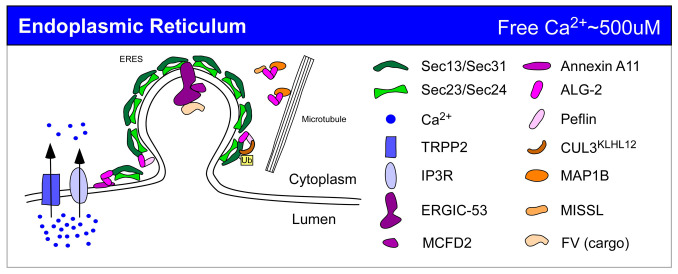
Figure 2. Ca2+ regulation of constitutive secretion at the endoplasmic reticulum.
Prominent examples from the text are depicted and color-coded at an example endoplasmic reticulum exit site (ERES). Greens: trafficking machinery; pinks/purples: Ca2+-binding proteins; blues: Ca2+ pumps or channels; oranges: accessory proteins or cargo. Ub, monoubiquitination.
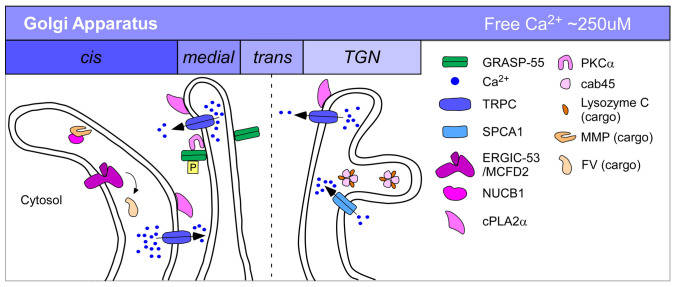
Figure 3. Ca2+ regulation of constitutive secretion through the Golgi and trans-Golgi network (TGN).
Prominent examples from the text are depicted and color-coded in various Golgi compartments. Greens: trafficking machinery; pinks/purples: Ca2+-binding proteins; blues: Ca2+ pumps or channels; oranges: accessory proteins or cargo. P, phosphorylation.
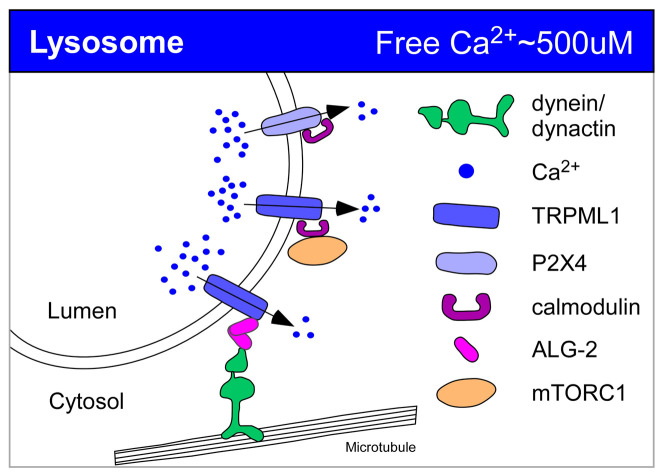
Figure 4. Ca2+ regulation of constitutive secretion at the lysosome.
Prominent examples from the text are depicted and color-coded. Greens: trafficking machinery; pinks/purples: Ca2+-binding proteins; blues: Ca2+ pumps or channels; oranges: accessory proteins or cargo.
Table 1. Summary of Ca2+ regulation of vesicle trafficking organized by organelle and order of appearance in the text.
| Endoplasmic reticulum | |||||
|---|---|---|---|---|---|
| Primary reference(s), by first author |
Ca2+ channel/ pump |
Ca2+-binding effector |
Links to Ca2+ flux/homeostasis | Links to trafficking machinery | Observed trafficking effects |
|
Le Corre (2014)16;
Sammels (2010)19 |
TRPP2 | - | TRPP2 KD increased releasable Ca2+ |
TRPP2 KD upregulated COPII expression |
TRPP2 KD increased collagen secretion |
| La Cour (2013)35 | - | ALG-2 | Ca2+-bound ALG-2 binds Sec31 | Ca2+-bound ALG-2 potentiated binding of Sec31 to Sec23 |
Ca2+-bound ALG-2 inhibited COPII vesicle budding |
| Shibata (2015)46 | - | ALG-2/AnxA11 | ALG-2 binds AnxA11 Ca2+- dependently |
ALG-2 couples AnxA11 to Sec31A | AnxA11 or ALG-2 KD increased ER-to- Golgi transport of VSV-G |
| Takahara (2017)48 | - | ALG-2/MISSL/ MAP1B |
MISSL colocalizes with ALG-2 in response Ca2+ |
MISSL-ALG-2-MAP1B may sequester ALG-2 |
KD of MISSL or ALG-2 decreased SEAP secretion |
| McGourty (2016)40 | - | ALG-2/peflin /CUL3KLHL12 |
Ca2+-dependent assoc. of KLHL12 with Sec31 |
CUL3KLHL12 monoubiquitinated sec31A |
Ubiq. complex required for collagen I secretion |
| Sargeant (2021)39 | IP3R | ALG-2/peflin | Pulse of Ca2+ signaling | Increased Sec31 targeting to ERES | Increased ER-to-Golgi transport |
| Sargeant (cont.)39 | IP3R | ALG-2/peflin | Continuous Ca2+ signaling | Decreased Sec31 targeting to ERES | Decreased ER-to Golgi transport |
| Held (2021)21 | IP3R | ALG-2/peflin | IP3R-3 KD increased Ca2+ signaling |
IP3R-3 KD increased ALG-2 and COPII coat at ERES |
IP3R-3 KD increased ER-to-Golgi transport |
|
Cho (2018)51;
(2020)50 |
- | - | 24-hour extremes of low or high Ca2+ |
Sec31 S694 O-GlcNACylated/de- acylated, respect. |
Golgi structure modulated by Sec31 acylation |
| Trychta (2018)55 | - | - | 24-hour TG, depleted luminal Ca2+ | Overwhelmed and upregulated KDEL receptors |
Secretion of ER-resident proteins |
| Zheng (2013)66 | - | ERGIC-53/ LMAN-3 |
Ca2+-dependent binding/release of luminal cargo |
ERGIC-53/LMAN-3 is a COPII client membrane protein |
Req. for trafficking of coag. factors, neurorec. and others |
| Golgi apparatus | |||||
| Primary reference(s), by first author |
Ca2+ channel/ pump |
Ca2+-binding effector |
Links to Ca2+ flux/homeostasis | Links to trafficking machinery | Observed trafficking effects |
| Lavender (2008)82 | TRPC3/TRPC7 | - | Presumed change of steady-state Ca2+ |
- | TRPC3,7 OE increased SEAP secretion 2x-4x |
| Ireland (2020)80 | - | PKCα | Ca2+ agonist or TG for up to 2 hours | PKCα-mediated phosphorylation of GRASP55 |
Golgi fragmentation and increased intra- Golgi transport |
|
San Pietro
(2009)88 |
- | cPLA2α | Presumed cargo-dependent Ca2+ release at Golgi |
cPLA2α KD or inhibition reduced inter- cisternal tubules |
cPLA2α KD inhibited intra-Golgi transport of VSV-G |
|
Regan-Klapisz
(2009)91 |
- | cPLA2α | Presumed Ca2+ release from Golgi | cPLA2α KD changed Golgi morphology |
KD accumulated junctional proteins in the Golgi |
|
Mukherjee
(2016)78 |
pmr1 (SPCA1 homolog) |
Possible direct effect of Ca2+ |
sly41 OE increased cytoplasmic Ca2+ |
Bypassed lack of p115 homolog Uso1 | Increased fusion of COPII vesicles with Golgi |
| Grice (2010)96 | SPCA1 | - | SPCA1 KD decreased Golgi luminal Ca2+ |
- | Blocked IGF1R trafficking/ maturation at the TGN |
|
Deng (2018)97;
von Blume (2012)98 |
SPCA1 | cab45 | SPCA1 KD decreased Golgi luminal Ca2+ |
cab45 oligomerized Ca2+-dependently with select cargoes in TGN |
SPCA1 or cab45 KD blocked sorting of lysozyme C in TGN |
|
Larkin (2016)103;
Brodeur (2009)100 |
- | NUCB1 | - | NUCB1 req. for rab7-dep. recruitment of retromer coat to LEs |
NUCB1 KD caused lysosomal accum. of Mann-6P receptors |
|
Pacheco-
Fernandez (2020)106 |
- | NUCB1 | NUCB1 KO reduces cis Golgi luminal Ca2+ |
NUCB1 directly bound cargo MMPs in cis-Golgi lumen |
NUCB1 KO delays intra-Golgi transport of MMPs |
| Endosome/Lysosome | |||||
| Primary reference(s), by first author |
Ca2+ channel/ pump |
Ca2+-binding effector |
Links to Ca2+ flux/homeostasis | Links to trafficking machinery | Observed trafficking effects |
|
Yang (2019)115;
Cao (2017)113; Dong(2010)114 |
TRPML1 | CaM | TRPML1 OE enhanced LEL Ca2+ release |
CaM recruited mTORC1 to LEL | TRMPL1 KD produced enlarged LEL |
| Li (2019)112 | TRPML1 | - | TRPML1 Ca2+ release regulated by ceramidase |
TRPML1 inhibition blocked lysosome- MVB interactions |
TRPML1 inhibition stimulated exosome release |
| Li (2016)10 | TRPML1 | ALG-2/dynein- dynactin |
TRPML1 Ca2+ release regulated by PI(3,5)P2 |
Ca2+ recruited ALG-2 and dynein/ dynactin to TRPML1 |
Permits perinuclear positioning of LEL |
| Cao (2015)117 | P2X4 | CaM | P2X4 released Ca2+ from the LEL pH-dependently |
CaM presumed to activate fusion machinery |
P2X4 OE promotes endolysosome fusion |
CaM, calmodulin; ER, endoplasmic reticulum; ERES, endoplasmic reticulum exit site; KD, knockdown; KO, knockout; LE, late endosome; LEL, late endosome/lysosome; MMP, matrix metalloproteinase; MVB, multivesicular body; OE, over-expression; SEAP, secretory alkaline phosphatase; TG, trans-Golgi; TGN, trans-Golgi network.
Calcium and secretion
Endoplasmic reticulum
The ER folds and assembles newly synthesized proteins destined for trafficking via the secretory pathway, dysregulation of which leads to the unfolded protein response (UPR), reviewed elsewhere11,12 but not covered in this review except regarding Ca2+-specific observations. In addition, the ER acts as a principal Ca2+ store within cells facilitating Ca2+ signals in the cytosol or multiple organelles via ER-organelle contact sites8,13–15. In this section, we describe effects on secretion via ER-localized Ca2+ channels or their cytosolic effectors; finally, we note ways in which luminal calcium has been described to affect secretion.
Originally studied for its role in polycystic kidney disease, TRPP2 was suggested to have a role in altered extracellular matrix (ECM) integrity. TRPP2 localizes to the ER membrane, although it is also present at the PM and primary cilia16. TRPP2, like all TRP family members, functions as a cation channel, principally directing the flow of Ca2+ into the cytosol. TRPP2 knockdown (KD) caused enhanced production of type II collagen in the zebrafish notochord sheath16, a trait reversed by expression of an ER-retained mutant of TRPP217. This implied that TRPP2 activity in the ER could directly or indirectly affect collagen translation or vesicle trafficking. Indeed, TRPP2 depletion led to an increase in the mRNAs of inner COPII coat components Sec23B, Sec24C, and Sec24D16, factors predicted to increase rates of ER-to-Golgi trafficking. Given that the increased coat expression could also be a compensation mechanism for loss of TRPP2, it is unclear whether TRPP2 depletion itself increased secretion. However, COPII coat protein KD or chemical inhibition of ER-to-Golgi transport via low-level brefeldin A (BFA) treatment was sufficient to reverse the enhanced collagen and aberrant morphology in the notochord sheath. This implies that the secretory pathway was an important target for manifesting the ECM abnormalities. Ultimately, in that study, a specific effector mechanism was not implicated nor were Ca2+ dynamics monitored, but previous work had shown that TRPP2 significantly decreased ER Ca2+18 and increased ER agonist-stimulated release of Ca2+19 or reduced it in the case of mutant TRPP220. These findings are reminiscent of a study21 focusing on a separate Ca2+ channel: inositol trisphosphate receptor-3 (IP3R-3). IP3Rs are broadly localized; they are present at the Golgi22), nucleus23–25, and the PM26,27 but are by far most abundant at the cells’ largest Ca2+ store: the ER28. Held et al. showed that IP3R-3 depletion increased spontaneous Ca2+ oscillations and produced an increase in ER-to-Golgi trafficking of a VSV-G transmembrane cargo21. It is possible that the two channels—IP3R and TRPP2—regulate trafficking via the same mechanism, possibly mediated in part by the demonstrated ability of TRPP2 to bind to ER IP3R and potentiate its activity19.
Changes in local Ca2+ concentrations, mediated by changes in channel expression (as described above) or signaling events, likely affect secretion via direct binding of Ca2+ to effectors. At the ER, no effectors are more prominent than the cytosolic penta-EF-hand (PEF) proteins. Common features of this family include (1) five serial Ca2+-binding EF-hand motifs, (2) capacity to dimerize via EF-hand domains, and (3) Ca2+-dependent effector binding, often at membrane sites. Of this family, the most studied is apoptosis-linked gene-2 (ALG-2). ALG-2 has more than 20 Ca2+-dependent-binding partners and possesses several binding pockets for effectors, implying that one ALG-2 homodimer can bind two or more target proteins simultaneously in response to Ca2+-induced conformational changes29. Furthermore, in mammalian cells, ALG-2 is present in two splice forms that have a different spectrum of interaction partners30. Early research on ALG-2 in secretion indicated that it localizes principally with Sec31 at ER exit sites (ERES) in a manner concomitant with intracellular calcium oscillations31–33, suggesting a role for ALG-2 in affecting ERES function in response to a Ca2+ signal. Other early work reported that recombinant ALG-2 inhibited COPII vesicle budding and/or fusion in vitro34,35, implying an inhibitory role for ALG-2. Other cellular studies showed that recombinant ALG-2 bound to Sec31 was able to stabilize ERES31,36, and dominant-negative ALG-237 and depletion38 studies also indicated a positive regulatory role. Recently, our lab helped reconcile some of these apparent contradictions by demonstrating that ALG-2 can either stimulate or inhibit ER-to-Golgi transport in the same system39. This duality in the regulatory role of ALG-2 may come down to multiple partners it can bind under different circumstances. In this review, we will discuss multiple ALG-2 protein complexes that can or do affect secretion.
In the basal state, in the absence of significant cytosolic Ca2+ oscillations, ALG-2 binds to the outer COPII coat subunit Sec3131,32, where it colocalizes with another PEF protein: peflin39,40. Likely, the colocalized peflin and ALG-2 at ERES are comprised of the earlier reported 1:1 heterodimer, observed in vitro to dissociate in response to Ca2+41. Interestingly, depletion of peflin in NRK cells produced increased ALG-2 at ERES38 and an increase in ER-to-Golgi trafficking of multiple COPII cargoes39, a situation reminiscent of ALG-2-binding to ERES in response to intracellular Ca2+ oscillations33, indicating a straightforward positive and negative role for ALG-2 and peflin at ERES, respectively. Furthermore, double KD of ALG-2 and peflin did not decrease transport below control levels, indicating that neither is actually required for secretion and both are purely regulatory in this context. Importantly, this result is in partial contrast to that of another study. McGourty et al. showed that the ubiquitin ligase CUL3 and its adaptor KLHL12 required both peflin and ALG-2 together for recognition and ubiquitination of Sec31 and further that both PEF proteins were positively required for collagen I secretion40. In that study, treatment of cells with histamine or ionomycin caused enlargement of COPII membrane sites or vesicles in an ALG-2- and peflin-dependent manner, suggesting activation of collagen export in response to surges in cytoplasmic Ca2+. The reason for the opposite effect of peflin depletion in Sargeant et al.39 and McGourty et al.40 is not known.
Other binding partners for ALG-2—for example, annexin A11 (Anxa11)—continue to support a regulatory role for ALG-2. The role of the annexin family of Ca2+- and lipid-binding proteins in trafficking is long and complex; a 2017 review discussed the potential involvement of annexins in a variety of plant membrane trafficking steps42. Briefly, there is very strong evidence that annexin A2 is involved in regulated exocytosis of chromaffin granules43 as well as several annexins being involved in Ca2+-dependent PM repair44,45, but the role of Anxa11 in ER-to-Golgi transport seems to be a unique example of an annexin playing a regulatory role at a constitutive trafficking step. At ERES, Anxa11, like ALG-2, has a role in stabilizing Sec31A46. There, Anxa11 likely anchors the ALG-2-Sec31A complex directly to the phospholipid bilayer, stabilizing the Sec31A interaction with ALG-2 rather than competing with it. Additionally, studies testing specific mutants that disrupt ALG-2-Anxa11-binding but not Sec31A-binding suggest that one ALG-2 molecule can bind Sec31A and Anxa11 at the same time, potentially through different binding sites. If this is true, then Anxa11 would not exactly be an additional ALG-2 target but rather an ALG-2 “co-effector” of Ca2+ since Anxa11 is itself a Ca2+-binding protein whose interactions with phospholipids could be independently modulated by Ca2+, separately from the Ca2+ modulation of ALG-2-binding to Anxa11 and Sec31A. This dual Ca2+-sensing unit could create complex functional responses, as has been observed in the Ca2+-dependent changes of ALG-2 in ER-to-Golgi transport. Also in support of more complex functions of ALG-2, ALG-2 has been reported to bind trk-fused gene (TFG) Ca2+-dependently at ERES in a Sec31A-independent manner47. GFP-MAPK1-interacting and spindle-stabilizing like (GFP-MISSL) also colocalized with ALG-2 in response to increased Ca2+48. The effector complex responsible for this targeting phenomenon appeared to be distinct from the Sec31A-ALG-2-Anxa11 or ALG-2-TFG complexes and was comprised of ALG-2 bound to both MISSL and MAP1B (microtubule-associated protein 1B) but not Sec31A. MISSL itself appeared to have a positive role in secretion as its KD attenuated secretion of SEAP (secretory alkaline phosphatase), possibly via sequestration of MAP1B, KD of which reversed the secretion defect. Furthermore, since the MISSL-ALG-2-MAP1B effector complex does not contain a member of the transport machinery, its effects on transport could be indirect (for example, by buffering ALG-2 availability for the Anxa11-ALG-2-Sec31A complex). Collectively, these studies indicate that ALG-2 has the capacity to serve multiple roles in secretion through binding to an array of target proteins.
Recently, our lab showed that distinct calcium signals and patterns can cause opposite effects on ER export in an ALG-2-dependent manner39. In NRK cells, exposure to histamine or ATP for up to 2.5 hours caused an ALG-2-dependent depression of ER-to-Golgi trafficking, but in neuroendocrine PC12 cells, the same treatment caused ALG-2-dependent enhancement of transport. Another recent report found a stimulatory effect of ER Ca2+ release in the ER-to-Golgi transport of a bulk flow cargo in HeLa cells49. We noted that the important distinction between a stimulatory or inhibitory effect of Ca2+ is not the cell type per se but the pattern and longevity of the Ca2+ signal produced, since by adjusting the pattern of signaling using different protocols, it was found that NRK cells could either down-regulate or up-regulate ER export in response to Ca2+ signaling in an ALG-2-dependent manner39. Mechanistically, the Ca2+-dependent decrease of ER export co-occurred with less COPII outer shell and more peflin at ERES but the opposite was true for the Ca2+-dependent enhancement of ER export. We proposed that different Ca2+ signals were able to elicit different effects via ALG-2, likely via other ALG-2- binding partners that may bind in response to different intensities and durations of Ca2+ signals. The ability of ALG-2 to influence Sec31A targeting may relate to the earlier observation that ALG-2 binding to the Sec31 subunit of the outer coat heterodimer Sec13/31 seems to dramatically potentiate its interaction with the inner coat heterodimer sec23/2435; the inner-outer coat interaction is thought to be critical for cargo sorting and vesicle formation.
A completely different line of research has also focused on modification of the COPII subunit Sec31A in response to Ca2+ —and also relates to ALG-2. This work examined the effect of Ca2+ dysregulation on the O-GlcNAcylation and membrane targeting of Sec31A50. O-GlcNAc transferase (OGT) is an enzyme responsible for this newly appreciated cytosolic post-translational modification. OGT was found in a previous article to interact directly with Sec31A and mediate O-GlcNAcylation at position S96451. In the more recent article, extremely high Ca2+ for 24 hours (brought about with A23187 or A-beta peptide) caused disruption of Sec31A targeting to membranes whereas 24-hour exposure to EGTA caused enhanced Sec31A targeting. The low-Ca2+, Sec31A targeting-promoting condition was accompanied by increased O-GlcNACylation of Sec31A and less ALG-2 association whereas the high-Ca2+, Sec31A targeting-inhibiting condition was accompanied by decreased O-GlcNACylation and increased ALG-2 association. An S694A mutation of Sec31A that cannot be O-GlcNACylated was not subject to the targeting regulation by Ca2+ flux, suggesting that O-GlcNACylation is a powerful determinant of Sec31A targeting. One interesting speculation is that since the ALG-2 binding site on Sec31A (residues 839–85136) is relatively close to S694, ALG-2-binding and OGT-binding could be mutually exclusive, in which case Ca2+ could regulate Sec31A O-GlcNACylation through Ca2+-dependent ALG-2-binding.
The locus of action of Ca2+ in the preceding examples is presumed to be the cytoplasm, where released Ca2+ regulates the trafficking machinery, such as vesicle coats. However, Ca2+ inside the lumen of organelles can also affect trafficking by altering the function of receptors or other vesicle components that influence whether luminal cargo is recruited to a budding vesicle. Substantial depletion of ER Ca2+ has been associated with the abnormal secretion of ER-resident proteins normally retained in the ER52–54. This abnormal ER exit has been demonstrated to affect proteins containing a C-terminal KDEL retrieval sequence, and their exit can be suppressed by over-expression (OE) of KDEL receptors55. Although the mechanism of retrieval via the KDEL receptor is well established, it is not known how luminal Ca2+ contributes to the retention of ER proteins, a phenomenon first noted for the ER chaperone calreticulin in 199456. Several putative mechanisms have been proposed to explain this departure, including (i) proteins with KDEL sequences such as ER chaperones BiP or GRP94 bind Ca2+ with low affinity and interact with both each other and misfolded proteins, possibly forming a kind of retention matrix. A decrease in Ca2+ could reduce their interactions and cause them to be secreted. Or (ii) the ER environment may exist as a dynamic hydrogel because of a high concentration of Ca2+ and Ca2+-interacting proteins, and the hydrogel properties may limit access of resident proteins to ERES. A decrease in the Ca2+ concentration could thereby decrease the hydrogel viscosity, allowing ER resident proteins to escape and overwhelm the KDEL retrieval pathway. The precise mechanism for Ca2+-dependent ER protein retention remains to be explored and will have medical significance to the many diseases whose pathologies involve severe disruption of homeostasis (for example, ischemia)57.
The cargo receptor ERGIC-53 (also called LMAN1) is another protein modulated by luminal Ca2+. ERGIC-53 Ca2+ dependently binds soluble glycoprotein cargoes in the ER lumen58,59. Example cargoes include cathepsins C and Z58,60, α1-antitrypsin61, and serotonin neuroreceptors62. In addition, ERGIC-53 forms a 1:1 Ca2+-dependent complex with a small Ca2+-binding protein MCFD2, both of which are necessary for the trafficking of coagulation factor V (FV) and factor VIII (FVIII)63. Mutations in ERGIC-53 or MCFD2 induce the autosomal recessive disorder combined deficiency of factor V and factor VIII (F5F8D)64. Previously, ERGIC-53 bound to cargo was thought to assemble in the ER lumen before dissociating in the ERGIC/Golgi in a manner dependent upon Ca2+ release facilitated by decreasing pH between organelle compartments65. However, a more recent study found that the carbohydrate-binding domain (CRD) of ERGIC-53 bound to Ca2+ was insensitive to the changes in pH predicted to occur between the ER and Golgi66. Importantly, that study also showed that CRD-ERGIC-53 had very little binding to a glycan-like moiety below 0.4 mM CaCl2. It remains unresolved whether differences in pH between the ER and Golgi, or differences in Ca2+ concentration, play a more significant role in regulating ERGIC-53 cargo loading and release.
Other ER luminal proteins also act as cargo receptors. Calumenin (CALU) is a Ca2+-binding protein from the CREC (Ca2+-binding protein of 45 kDa [Cab45], reticulocalbin, ER Ca2+-binding protein of 55 kDa [ERC-55], and calumenin) protein family. In striated muscle, CALU expression changes are coupled to Ca2+ cycling likely via Ca2+-dependent interaction with the SERCA pump or ryanodine receptors67, whereas CALU in thrombocytes may act as a cargo receptor for thrombospondin-1 (TSP1)—an ECM adhesive glycoprotein. In this system, CALU and TSP1 form a Ca2+-dependent complex and are released together in response to thrombin68. Whether CALU regulates cargo secretion in the same cells in which it regulates ER Ca2+ entry/exit remains to be seen. If such a role were observed, this could be an interesting example of a protein that regulates both the Ca2+ environment necessary for secretion and the actual secretion event itself.
Highlights of this section are summarized in Figure 2.
Golgi apparatus
The Golgi apparatus plays a central role in the trafficking and sorting of secretory cargo. Like the ER, the Golgi holds a vast reservoir of Ca2+ and is similarly endowed with Ca2+ pumps, Ca2+ release channels, and Ca2+-binding proteins69. The Ca2+ concentration at the Golgi diminishes from cis to trans (300 –> 150 μM), reaching the lowest concentration at the trans-Golgi network (TGN) (50–100 μM)9 (as depicted in Figure 1). Maintenance of this Ca2+ gradient appears to require a gradient of protein expression. Notably, the cis-Golgi maintains SERCA pumps for Ca2+ influx and IP3R channels for Ca2+ release, whereas, in the trans-Golgi compartment, Ca2+ transport is regulated mainly by the secretory pathway Ca2+ ATPase type 1 (SPCA1) and ryanodine receptors9,69–71. Similarly, three Golgi proteins considered Ca2+ regulators, two of which have direct roles in secretion (discussed below), are localized to distinct Golgi compartments: nucleobindin-1 in the cis-Golgi72–74, DNA-binding EF-hand acidic amino acid-rich non-glycosylated Ca2+-binding protein (NEFA/p54) in the medial-Golgi75, and Cab45 in the trans-Golgi and TGN76,77. It is possible that just as ERGIC-53 may use Ca2+ to regulate cargo loading and release, distinctive Ca2+ levels in the Golgi could aid cargo progression (although this is not directly explored here). In this section, we define specific instances of Ca2+ regulating secretion via its flux near the cytosolic surface or within luminal Golgi compartments.
Many studies have implicated Golgi Ca2+ channels and Ca2+ effectors in regulating trafficking. To begin, we discuss Sly41, an SLC-family solute transporter that constitutively cycles between the ER and Golgi in Saccharomyces cerevisiae. Although the substrate for the transporter remains unknown, genetic interactions with the PMR1 Golgi Ca2+ pump led to the discovery that Sly41 OE generated elevated cytosolic Ca2+78. This was an important trafficking discovery since Sly41 was originally identified in a screen for genes whose OE suppressed COPII-dependent membrane tethering deficiencies (SLY = suppressor of lethality of ypt1). Mechanistically, this meant that raising the cytosolic Ca2+ concentration through Sly41 OE compensated for the loss of an ER-to-Golgi vesicle tethering factor (Uso1; p115 in mammals) that is essential for intracellular transport pathways and efficient SNARE assembly78,79. These findings, on their own, suggest that Ca2+ promotes membrane fusion between COPII vesicles and the Golgi. Curiously, although the addition of Ca2+ in the absence of maximal vesicle tethering factors did indeed stimulate membrane fusion, it did nothing when saturating levels of vesicle tethering factors were present. A Ca2+ effector protein such as ALG-2 or calmodulin (CaM) that, for example, interacts with SNAREs or tethers and mediates the stimulation of transport was not identified. However, some Ca2+ effects on membrane fusion could instead be due to the fusion-promoting properties of Ca2+ itself without the requirement for a Ca2+ effector (to be discussed below).
A recent study investigated the effect of high cytosolic Ca2+ concentrations on Golgi structure80. The authors exposed HeLa cells to the Ca2+ agonist histamine or the SERCA inhibitor thapsigargin for 0.5 to 2 hours. They noted morphological fragmentation of Golgi stacks via a mechanism that involved PKCα-mediated phosphorylation of Golgi reassembly stacking protein 55 (GRASP-55). The fragmentation response appeared to be independent of ER stress and UPR activation. Satisfyingly, in this example, we have both an established Ca2+ effector (PKCα) and the trafficking machinery impacted (GRASP-55). However, the trafficking purpose for the effect is less clear. The authors suggested that it was part of a Golgi stress adaptive mechanism or signaling pathway. Cargo trafficking effects through the Golgi were regarded as mild; however, there was measurable acceleration that could have contributed to increased secretion. It would be interesting to know whether the PKCα-mediated response to Ca2+ signaling in the Golgi works in concert with changes in ER export mediated by ALG-2 that were discussed above.
As with the ER possessing TRPP2, the Golgi also possesses resident TRP channels that appear to affect secretion. The transient receptor potential canonical (TRPC) sub-family of TRP channels are non-selective cation channels that are known to open in response to phospholipase C activation, although their precise ligands (IP3, diacylglycerol) vary81. Human TRPC3 and 7 are localized on the PM but also are found in intracellular pools at the TGN and Golgi stack82. Furthermore, OE of either of these channels was found to increase constitutive secretion of SEAP by two- to four-fold82. The precise locus of the secretion effects observed in that study was not elucidated, and it cannot be eliminated that the over-expressed channels mobilized ER Ca2+ in addition to Golgi Ca2+ and that ER Ca2+ was the functional site, possibly via the effects of ALG-2 on COPII targeting, as discussed above. However, it is tempting to speculate that what occurred was local stimulation of intra-Golgi cargo transport, a process conventionally thought to be facilitated, through the process of cisternal maturation, by COPI-coated vesicles (although a competing hypothesis is intra-Golgi tubules, discussed below). Interestingly, two groups have presented evidence that COPI targeting to membranes, like COPII targeting, is Ca2+-dependent34,83 but that ALG-2 did not affect COPI targeting34. It would be very exciting if a separate Ca2+ effector regulated COPI; furthermore, this putative effector could be the key to understanding TRPC effects on secretion.
A prominent Golgi Ca2+ effector is the cytosolic phospholipase A2 alpha (cPLA2α). cPLA2α is a member of the superfamily of phospholipase A2 enzymes (PLA2) reviewed extensively elsewhere84. In general, PLA2 hydrolyze the fatty acid from the sn-2 position of membrane phospholipids creating a lysophospholipid and a free fatty acid. An inverted-cone-shaped lipid, the lysophospholipid favors positive curvature of lipid membranes and thereby promotes membrane tubule formation. Tubule-mediated (as opposed to vesicle-mediated) trafficking is gaining recognition for its contributions to intra-Golgi transport as well as Golgi-to-PM transport of certain cargoes85. Three specific PLA2 enzymes have been implicated in tubule regulation at the Golgi85–87, including cPLA2α88. Whereas cPLA2α is Ca2+ activated, the other two Golgi-associated PLAs are not but could instead be activated by G protein βγ complexes89. Notably, cPLA2α is specifically recruited to the Golgi complex, likely via its C2 domain90, in response to transient increases in Ca2+ apparently initiated by the arrival of secretory cargo88. There it was required for intra-Golgi transport of VSV-G through the Golgi, apparently via induction of small inter-stack tubules. In the study by San Pietro et al., depletion of cPLA2α specifically inhibited transport through the Golgi and did not impair ER-to-Golgi transport of VSV-G or delivery of VSV-G from the TGN to the PM or retrograde Golgi-to-ER transport of the KDEL receptor88. Another article showed that cPLA2α contributes to the delivery of transmembrane proteins to junction complexes in endothelial cells91. This point was demonstrated by noting that the inhibition or RNA interference-mediated KD of cPLA2α prevented delivery of VE-cadherin, occludin, and claudin-5 to cell-to-cell contacts. Instead, these proteins accumulated in the Golgi, indicating that either transport through the Golgi or transport from it was inhibited91. The evidence for cPLA2α in the regulation of intra-Golgi transport is thus quite strong, but the Ca2+ signaling that activates cPLA2α on the Golgi is less clear. One report suggested that Ca2+ is released from the Golgi itself92 when a bolus of secretory cargo enters the Golgi, although specific channels or pumps and their mode of activation were not identified.
Secretory pathway Ca2+-ATPase (SPCA1) is a primarily trans-Golgi-localized Ca2+ ATPase69,70, which, along with the ER SERCA pump, is responsible for the maintenance of Golgi Ca2+ levels. Mutations in SPCA1 (gene name: ATP2C1) usually manifest as a decrease in expression and elicit the autosomal dominant skin disorder Hailey-Hailey disease93. As we will see in the following examples, the role of SPCA1 in secretion seems to be mainly in providing the lumen of the Golgi and associated organelles with high Ca2+ that is used in sorting and processing of secretory cargoes en route to their final destinations. The role of SPCA1 in trafficking was first demonstrated in yeast, where its medial Golgi-localized ortholog PMR1 transports both Ca2+ and Mn2+ into secretory compartments94. In that early study, pmr1 mutants are both unable to sort carboxypeptidase properly to the vacuole and unable to degrade misfolded carboxypeptidase, a luminal ER protein—a trait that was later found to be reversible via addition of external calcium95. This suggested that low luminal calcium in secretory organelles is the primary cause of these sorting defects. Further studies reinforce this idea. For example, SPCA1 inhibition also produced a decrease in insulin-like growth factor receptor (IGF1R) at the cell surface, a phenotype caused by defective proteolytic processing and accumulation of previously undetectable TGN-localized pro-IGF1R96. One putative effector for the sorting role of SPCA1 in secretion arose later when it was discovered that the luminal, secreted Ca2+-binding protein Cab45, of the CREC family, preferentially accumulates near SPCA1, where it oligomerizes with secretory cargoes in response to the presumably high local concentration of Ca2+97. Cab45 does not play a general role in secretion but rather binds a few select secretory cargoes, including lysozyme C and cartilage oligomeric matrix protein (COMP), and presumably assists their secretion by forming condensates97,98. Curiously, Cab45 is secreted along with its client cargoes. This could imply that Cab45 has additional functions in the extracellular environment.
Nucleobindin-1 (NUCB1, also called CALNUC or NUC) appears to be a multi-functional soluble EF-hand-containing Ca2+ effector protein. NUCB1 is widely distributed within the cell. The protein contains both an ER signal sequence and an ER export signal and displays prominent localization to the cis Golgi72–74 prior to being constitutively secreted99. Surprisingly, NUCB1 is also present in the cytoplasm100. In the cytoplasm, it has been suggested to, among other things, undergo Ca2+-dependent interactions with G⍺i subunits on Golgi membranes101,102. Importantly, cytosolic NUCB1 acts as a regulator of endosomal recycling of lysosomal receptors such as the mannose-6-phosphate receptor and sortilin, which capture cargo at the TGN and ferry it to late endosomes for ultimate deposition in the lysosome100,103. In these studies, NUCB1 appeared to act in the cytosol and as a regulator of rab7 activation to bring about recruitment of the retrograde coat, retromer, that mediates return of the lysosomal receptors to the TGN. The role of Ca2+ in NUCB1 function here was not investigated.
In regard to NUCB1 that is targeted to the luminal cellular domain, some reports have suggested a chaperone-like activity due to its ability to bind Alzheimer’s amyloid precursor protein (APP) and assist in its folding and biogenesis104,105. In the Golgi, NUCB1 may function as a regulator of Golgi Ca2+ homeostasis since its OE increased Ca2+ uptake into the Golgi73 while NUCB1 KO induced a loss in Golgi luminal Ca2+106. Furthermore, NUCB1 KO and NUCB1 with mutant EF hands produced a delay in the trafficking of ECM constituents MMP2 and MT1-MMP at the cis-to-trans Golgi stage. Again, like Cab45, NUCB1 appeared to regulate the trafficking of only a subset of cargoes; for example, NUCB1 KO had no effect on the trafficking of lysozyme C106. This trafficking defect was a delay and not a block, possibly due to compensatory mechanisms mediated by other Ca2+-binding proteins. The trafficking function of NUCB1 was presumed to be mediated by direct interactions with the cargo, but a more detailed mechanism of how NUC1B facilitates anterograde transport while remaining in the cis-Golgi has not yet been established. Finally, NUCB1 is reported to, after a long period in the Golgi, be constitutively secreted into the extracellular space99, where it may have signaling functions via its capacity to bind Ca2+, such as in bone matrix maturation107.
Highlights of this section are summarized in Figure 3.
Late endosome/lysosome
The late endosomes/lysosomes (LELs) are central organelles responsible for macromolecule recycling. Importantly, they are also intracellular stores for Ca2+, reaching concentrations that approach that of the ER lumen108. We have known for quite some time that LEL trafficking requires luminal Ca2+ and that CaM is an important Ca2+ effector for this5,6,109. Resident in the lysosome is the TRPML1 channel that is critical in Ca2+-dependent lysosome trafficking; lack of TRPML1 activity causes the genetic disorder mucolipidosis and enlarged vacuole-like lysosomes110. TRPML1 belongs to the mucolipin subgroup of the TRP ion channel family whose members we have discussed in the sections on trafficking in the ER and the Golgi. Like other TRP channels, TRPML1 acts as a non-selective ion channel permeable to Ca2+. Some work has indicated that TRPML1 is an activator of lysosome-MVB fusion. In this context, TRPML1 was suggested to regulate exosome release since this process is inhibited when lysosomes fuse with MVBs, the source of exosomes111,112. Other work, however, has implicated TRPML1 in LEL fission113. TRPML1 has been shown to be regulated by pH108 and activated by the rare LEL phospholipid PI(3,5)P2. Deficiency of either TRPML1 or PI(3,5)P2 produced enlarged LEL, while OE of TRMPL1 both attenuated the defect observed with PI(3,5)P2 deficiency and enhanced vacuolar calcium release114. This local increase in juxta-organellar calcium could serve to recruit cytosolic complexes necessary for membrane fusion and fission. In fact, CaM was implicated as the specific Ca2+ effector for the role of TRPML1 in LEL fission113, and the mammalian target of rapamycin 1 (mTORC1) served as a required downstream target115. ALG-2 (discussed above in the ER section) has also been implicated as an effector of Ca2+ released by TRPML1 since it binds to TRPML1 in a Ca2+-dependent manner116. Furthermore, activation of TRPML1 via PI(3,5)P2 caused ALG-2 to Ca2+-dependently couple dynein-dynactin with the N-terminus of TRPML110. This ALG-2-mediated adaptor system ultimately permitted migration of lysosomes to the perinuclear region in response to Ca2+ signals. Distinct from the proposed role of CaM in LEL fission, this dynein-mediated trafficking implicates Ca2+ and ALG-2 in autolysosome formation, which occurs when the migrated lysosomes fuse with autophagosomes.
Also localized to LEL, purinergic receptor P2X4 is a Ca2+ release channel activated by luminal ATP in a pH-dependent manner. In a 2015 article, it was shown that P2X4 promotes LEL fusion in a cell-free assay and that this effect was blocked by various inhibitions of P2X4-mediated Ca2+ release117. Although inhibition of P2X4 activity by the low pH of LEL was overcome pharmacologically in these experiments, it is unknown whether and how this inhibition is overcome in cells. That study also showed that P2X4 activation recruits CaM and that together they form a complex at the LEL membrane. Finally, the fusion effect of P2X4 was shown to be suppressed by inhibiting CaM117. The authors do not specifically identify the downstream targets for CaM in this instance, although it may be relevant that CaM interacts with LEL SNARE proteins in a Ca2+-dependent manner118. It is interesting to note that the same research group also implicated CaM as the effector for TRPML1-mediated LEL fission113, but how two distinct channels could mediate opposing trafficking phenomena (fusion vs. fission) via the same Ca2+ effector was not resolved.
Highlights of this section are summarized in Figure 4.
Baseline exocytosis of secretory granules
Specialized epithelial goblet cells secrete gel-forming mucins, the first line of defense against pathogens or allergens. Following biogenesis at the Golgi, mucin granules undergo maturation and eventually release their contents at the PM. Although mucin granules undergo stimulated exocytosis in response to agonists such as ATP, it was discovered in 2015 that baseline secretion of mucins, which requires intracellular Ca2+ concentrations an order of magnitude lower than agonist-stimulated release119, can exceed stimulated release over long periods120. A high-affinity Ca2+ sensor for baseline mucin secretion is proposed to be K+ channel-interacting protein 3 (KChIP3), a member of the neuronal Ca2+ sensor (NCS) family of EF-hand-containing proteins121. KChIP3 inhibits baseline exocytosis, which was shown by an increase in baseline mucin secretion during KChIP3 KD and a decrease in baseline secretion during KChIP3 OE. This effect was demonstrated to be entirely dependent on intracellular Ca2+ oscillations, as increased intracellular calcium oscillations abrogated the inhibitory effect of KChIP3 OE. Finally, this Ca2+-dependent control of KChIP3 activity was discovered to be dependent on the ryanodine receptors, activity of which caused KChIP3 to dissociate from mucin granules121. Although this process sounds more akin to regulated secretion, it was able to proceed without stimulation by external agonists and did not require synaptotagmin, thus blurring the distinction between the regulated and constitutive secretory pathways. In vivo, the Ca2+ oscillations may be driven by fluid flow over the epithelium, as goblet cells were found to increase Ca2+ oscillations and mucin secretion in response to cell perfusion120.
Calcium and membranes
Secretory pathway organelles harbor significant stores of calcium. The ER is reported to be the highest with a total Ca2+ of up to 2 mM, equivalent to the extracellular Ca2+ concentration, whereas its free Ca2+ store is about 500 μM8. Meanwhile, the Golgi apparatus sits at an average of 250 μM free Ca2+8, although this number varies; Ca2+ steadily diminishes from the cis- to the trans-Golgi compartments122. Finally, the average free Ca2+ of lysosomes is comparable to the ER at about 500 μM8,10. Although so far we have discussed the influence of these stores primarily in the context of their capacity to regulate Ca2+-binding proteins that in turn regulate vesicle trafficking, here we discuss the possibility that Ca2+ directly modulates trafficking by influencing the structural and functional properties of membrane lipids. Indeed, experimental and theoretical work has shown that Ca2+ can quickly and concentration-dependently tighten and order lipid bilayers, primarily via coordination with anionic oxygens of acidic phospholipids such as phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2]123,124 and phosphatidylserine (PS)125–127. Together these studies suggested a role for cytosolic Ca2+ in promoting membrane fusion, possibly by masking the negative charge of anionic lipids and more readily allowing close association of membranes. Ca2+ concentrations on the order of 200 to 400 μM overcame DOPS (dioleoyl phosphatidylserine) inhibition of lipid or content mixing in prepared vesicles128. Importantly, this Ca2+ concentration is on the order one might expect for focal Ca2+ signals near release sites129,130. We note that, in a previously discussed study, elevations in cytosolic Ca2+ were observed to overcome a block in membrane tethering to allow fusion78. Since no Ca2+ effector was evident in that study, it could indicate that Ca2+ alone may have promoted membrane fusion in a physiological system.
In addition to putative direct effects on fusion, Ca2+ may have additional roles in vesicular trafficking. Studies have shown, for example, induction of membrane tubulation in lipid vesicles in response to local Ca2+ addition at concentrations above 1 mM131. The authors of that study note that the observed inward spontaneous curvature and membrane bending are likely due to a large, Ca2+-facilitated reduction in the surface charge density of one membrane leaflet causing stress asymmetry and ultimately bending of the membrane. A study using a giant unilamellar vesicle transfer assay demonstrated that Ca2+ can trigger deformation of membranes containing PS or PI(4,5)P2 in a direction that points away from the ion source132, in a manner reminiscent of vesicle budding. It is important to note that the concentration of Ca2+ required to manifest the induced curvature of membranes appears to be on the order of a few hundred micromolar. Although this number falls well within the bounds of free luminal Ca2+ for secretory pathway organelles, it is highly unlikely that high levels of free Ca2+ alone could lead to a membrane budding event. Instead, it is much more plausible that luminal Ca2+ stores support induction of membrane curvature by working in concert with other proteins such as the annexins133. Finally, in support of a system wherein Ca2+ can facilitate membrane budding, we note that the membranes of organelles most enriched in the Ca2+-binding lipid PS are the luminal leaflets of the ER, Golgi, and mitochondria134,135, two of which are critical organelles for constitutive secretion.
Conclusions
Since our last review of this topic7, Ca2+ regulation of constitutive trafficking has expanded from examples employing mostly in vitro systems using Ca2+ addition or chelation into complex systems wherein specific Ca2+-related gene products, many implicated in human pathologies, fundamentally alter trafficking steps throughout secretory and LEL trafficking. Furthermore, whereas prior to this it was difficult to distinguish whether Ca2+ played only permissive, co-factor roles, now we know that Ca2+ signaling can play dynamic roles, altering trafficking rates or patterns in response to natural ligands. Furthermore, we note that Ca2+ signaling can have unexpectedly complex roles; for example, in the case of ER export, ALG-2, together with its multiple targets, can act to either restrict or expand entry of cargo into the secretory pathway, depending upon the intensity and duration of a Ca2+ signal. It is now clear that Ca2+ is a fundamental regulator of the cell’s trafficking toolkit but that each case is also mechanistically unique rather than following a universal pattern. Overall, constitutive trafficking is looking a lot more Ca2+-regulated and therefore less “constitutive” than once believed.
The peer reviewers who approve this article are:
Martin Lowe, Division of Molecular and Cellular Function, School of Biological Sciences, Faculty of Biology Medicine and Health, University of Manchester, Manchester, UK
Julia von Blume, Department of Cell Biology, Yale University School of Medicine, New Haven, CT, USA
Funding Statement
This work was supported by National Institutes of Health grant R15GM106323-03 (to JCH).
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lowe M, Rabouille C, Nakamura N, et al. : Cdc2 Kinase Directly Phosphorylates the cis-Golgi Matrix Protein GM130 and Is Required for Golgi Fragmentation in Mitosis. Cell. 1998; 94(6): 783–93. 10.1016/s0092-8674(00)81737-7 [DOI] [PubMed] [Google Scholar]
- 2. van Anken E, Romijn EP, Maggioni C, et al. : Sequential Waves of Functionally Related Proteins Are Expressed When B Cells Prepare for Antibody Secretion. Immunity. 2003; 18(2): 243–53. 10.1016/s1074-7613(03)00024-4 [DOI] [PubMed] [Google Scholar]
- 3. Berridge MJ, Lipp P, Bootman MD: The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000; 1(1): 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- 4. Chen JL, Ahluwalia JP, Stamnes M: Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J Biol Chem. 2002; 277(38): 35682–7. 10.1074/jbc.M204157200 [DOI] [PubMed] [Google Scholar]
- 5. Peters C, Mayer A: Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998; 396(6711): 575–80. 10.1038/25133 [DOI] [PubMed] [Google Scholar]
- 6. Pryor PR, Mullock BM, Bright NA, et al. : The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000; 149(5): 1053–62. 10.1083/jcb.149.5.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hay JC: Calcium: A fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007; 8(3): 236–40. 10.1038/sj.embor.7400921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bootman MD, Bultynck G: Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb Perspect Biol. 2020; 12(1): a038802. 10.1101/cshperspect.a038802 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 9. Wong AKC, Capitanio P, Lissandron V, et al. : Heterogeneity of Ca2+ handling among and within Golgi compartments. J Mol Cell Biol. 2013; 5(4): 266–76. 10.1093/jmcb/mjt024 [DOI] [PubMed] [Google Scholar]
- 10. Li X, Rydzewski N, Hider A, et al. : A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016; 18(4): 404–17. 10.1038/ncb3324 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 11. Chakrabarti A, Chen AW, Varner JD: A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011; 108(12): 2777–93. 10.1002/bit.23282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hetz C, Zhang K, Kaufman RJ: Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020; 21(8): 421–38. 10.1038/s41580-020-0250-z [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 13. Burgoyne T, Patel S, Eden ER: Calcium signaling at ER membrane contact sites. Biochim Biophys Acta. 2015; 1853(9): 2012–7. 10.1016/j.bbamcr.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 14. Patel S: Getting close. Lysosome-ER contact sites tailor Ca2+ signals. Cell Calcium. 2019; 80: 194–6. 10.1016/j.ceca.2019.02.003 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 15. Lemos FO, Bultynck G, Parys JB: A comprehensive overview of the complex world of the endo- and sarcoplasmic reticulum Ca2+-leak channels. Biochim Biophys Acta Mol Cell Res. 2021; 1868(7): 119020. 10.1016/j.bbamcr.2021.119020 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 16. Le Corre S, Eyre D, Drummond IA: Modulation of the secretory pathway rescues zebrafish polycystic kidney disease pathology. J Am Soc Nephrol. 2014; 25(8): 1749–59. 10.1681/ASN.2013101060 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 17. Fu X, Wang Y, Schetle N, et al. : The subcellular localization of TRPP2 modulates its function. J Am Soc Nephrol. 2008; 19(7): 1342–51. 10.1681/ASN.2007070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wegierski T, Steffl D, Kopp C, et al. : TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 2009; 28(5): 490–9. 10.1038/emboj.2008.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sammels E, Devogelaere B, Mekahli D, et al. : Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J Biol Chem. 2010; 285(24): 18794–805. 10.1074/jbc.M109.090662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aguiari G, Banzi M, Gessi S, et al. : Deficiency of polycystin-2 reduces Ca2+ channel activity and cell proliferation in ADPKD lymphoblastoid cells. FASEB J. 2004; 18(7): 884–6. 10.1096/fj.03-0687fje [DOI] [PubMed] [Google Scholar]
- 21. Held A, Sargeant J, Hojanazarova J, et al. : Steady-State Regulation of Secretory Cargo Export by Inositol Trisphosphate Receptors and Penta EF Hand Proteins. bioRxiv. 2021; 2020.06.13.150144. 10.1101/2020.06.13.150144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinton P, Pozzan T, Rizzuto R: The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998; 17(18): 5298–308. 10.1093/emboj/17.18.5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerasimenko OV, Gerasimenko JV, Tepikin AV, et al. : ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995; 80(3): 439–44. 10.1016/0092-8674(95)90494-8 [DOI] [PubMed] [Google Scholar]
- 24. Huh YH, Yoo SH: Presence of the inositol 1,4,5-triphosphate receptor isoforms in the nucleoplasm. FEBS Lett. 2003; 555(2): 411–8. 10.1016/s0014-5793(03)01273-0 [DOI] [PubMed] [Google Scholar]
- 25. Stehno-Bittel L, Perez-Terzic C, Clapham DE: Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science. 1995; 270(5243): 1835–8. 10.1126/science.270.5243.1835 [DOI] [PubMed] [Google Scholar]
- 26. Fadool DA, Ache BW: Plasma membrane inositol 1,4,5-Trisphosphate-Activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992; 9(5): 907–18. 10.1016/0896-6273(92)90243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuno M, Maeda N, Mikoshiba K: IP3-activated calcium-permeable channels in the inside-out patches of cultured cerebellar Purkinje cells. Biochem Biophys Res Commun. 1994; 199(3): 1128–35. 10.1006/bbrc.1994.1348 [DOI] [PubMed] [Google Scholar]
- 28. Satoh T, Ross CA, Villa A, et al. : The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: Quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990; 111(2): 615–24. 10.1083/jcb.111.2.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibata H: Adaptor functions of the Ca2+-binding protein ALG-2 in protein transport from the endoplasmic reticulum. Biosci Biotechnol Biochem. 2019; 83(1): 20–32. 10.1080/09168451.2018.1525274 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 30. Tarabykina S, Møller AL, Durussel I, et al. : Two forms of the apoptosis-linked protein ALG-2 with different Ca(2+) affinities and target recognition. J Biol Chem. 2000; 275(14): 10514–8. 10.1074/jbc.275.14.10514 [DOI] [PubMed] [Google Scholar]
- 31. Shibata H, Suzuki H, Yoshida H, et al. : ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem Biophys Res Commun. 2007; 353(3): 756–63. 10.1016/j.bbrc.2006.12.101 [DOI] [PubMed] [Google Scholar]
- 32. Yamasaki A, Tani K, Yamamoto A, et al. : The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol Biol Cell. 2006; 17(11): 4876–87. 10.1091/mbc.e06-05-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. La Cour JM, Mollerup J, Berchtold MW: ALG-2 oscillates in subcellular localization, unitemporally with calcium oscillations. Biochem Biophys Res Commun. 2007; 353(4): 1063–7. 10.1016/j.bbrc.2006.12.143 [DOI] [PubMed] [Google Scholar]
- 34. Bentley M, Nycz DC, Joglekar A, et al. : Vesicular calcium regulates coat retention, fusogenicity, and size of pre-Golgi intermediates. Mol Biol Cell. 2010; 21(6): 1033–46. 10.1091/mbc.e09-10-0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. La Cour JM, Schindler AJ, Berchtold MW, et al. : ALG-2 attenuates COPII budding in vitro and stabilizes the Sec23/Sec31A complex. PLoS One. 2013; 8(9): e75309. 10.1371/journal.pone.0075309 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 36. Shibata H, Inuzuka T, Yoshida H, et al. : The ALG-2 binding site in Sec31A influences the retention kinetics of Sec31A at the endoplasmic reticulum exit sites as revealed by live-cell time-lapse imaging. Biosci Biotechnol Biochem. 2010; 74(9): 1819–26. 10.1271/bbb.100215 [DOI] [PubMed] [Google Scholar]
- 37. Helm JR, Bentley M, Thorsen KD, et al. : Apoptosis-linked gene-2 (ALG-2)/Sec31 interactions regulate endoplasmic reticulum (ER)-to-Golgi transport: A potential effector pathway for luminal calcium. J Biol Chem. 2014; 289(34): 23609–28. 10.1074/jbc.M114.561829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rayl M, Truitt M, Held A, et al. : Penta-EF-Hand Protein Peflin Is a Negative Regulator of ER-To-Golgi Transport. PLoS One. 2016; 11(6): e0157227. 10.1371/journal.pone.0157227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sargeant J, Seiler DK, Costain T, et al. : ALG-2 and peflin regulate COPII targeting and secretion in response to calcium signaling. J Biol Chem. 2021; 297(6): 101393. 10.1016/j.jbc.2021.101393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGourty CA, Akopian D, Walsh C, et al. : Regulation of the CUL3 Ubiquitin Ligase by a Calcium-Dependent Co-adaptor. Cell. 2016; 167(2): 525–538.e14. 10.1016/j.cell.2016.09.026 [DOI] [PubMed] [Google Scholar]
- 41. Kitaura Y, Matsumoto S, Satoh H, et al. : Peflin and ALG-2, members of the penta-EF-hand protein family, form a heterodimer that dissociates in a Ca2+-dependent manner. J Biol Chem. 2001; 276(17): 14053–8. 10.1074/jbc.M008649200 [DOI] [PubMed] [Google Scholar]
- 42. Konopka-Postupolska D, Clark G: Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells. Int J Mol Sci. 2017; 18(4): 863. 10.3390/ijms18040863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chasserot-Golaz S, Vitale N, Umbrecht-Jenck E, et al. : Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol Biol Cell. 2005; 16(3): 1108–19. 10.1091/mbc.e04-07-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lennon NJ, Kho A, Bacskai BJ, et al. : Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003; 278(50): 50466–73. 10.1074/jbc.M307247200 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 45. Bouter A, Gounou C, Bérat R, et al. : Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat Commun. 2011; 2: 270. 10.1038/ncomms1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shibata H, Kanadome T, Sugiura H, et al. : A new role for annexin A11 in the early secretory pathway via stabilizing Sec31A protein at the endoplasmic reticulum exit sites (ERES). J Biol Chem. 2015; 290(8): 4981–93. 10.1074/jbc.M114.592089 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 47. Kanadome T, Shibata H, Kuwata K, et al. : The calcium-binding protein ALG-2 promotes endoplasmic reticulum exit site localization and polymerization of Trk-fused gene (TFG) protein. FEBS J. 2017; 284(1): 56–76. 10.1111/febs.13949 [DOI] [PubMed] [Google Scholar]
- 48. Takahara T, Inoue K, Arai Y, et al. : The calcium-binding protein ALG-2 regulates protein secretion and trafficking via interactions with MISSL and MAP1B proteins. J Biol Chem. 2017; 292(41): 17057–72. 10.1074/jbc.M117.800201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rauter T, Burgstaller S, Gottschalk B, et al. : ER-to-Golgi Transport in HeLa Cells Displays High Resilience to Ca 2+ and Energy Stresses. Cells. 2020; 9(10): 2311. 10.3390/cells9102311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cho HJ, Mook-Jung I: Amyloid beta regulates ER exit sites formation through O-GlcNAcylation triggered by disrupted calcium homeostasis. Biol Cell. 2020; 112(12): 439–51. 10.1111/boc.201900062 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. Cho HJ, Mook-Jung I: O-GlcNAcylation regulates endoplasmic reticulum exit sites through Sec31A modification in conventional secretory pathway. FASEB J. 2018; 32(9): 4641–57. 10.1096/fj.201701523R [DOI] [PubMed] [Google Scholar]
- 52. Booth C, Koch GL: Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989; 59(4): 729–37. 10.1016/0092-8674(89)90019-6 [DOI] [PubMed] [Google Scholar]
- 53. Henderson MJ, Richie CT, Airavaara M, et al. : Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J Biol Chem. 2013; 288(6): 4209–25. 10.1074/jbc.M112.400648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Henderson MJ, Wires ES, Trychta KA, et al. : SERCaMP: A carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol Biol Cell. 2014; 25(18): 2828–39. 10.1091/mbc.E14-06-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trychta KA, Bäck S, Henderson MJ, et al. : KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency. Cell Rep. 2018; 25(7): 1829–1840.e6. 10.1016/j.celrep.2018.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sönnichsen B, Füllekrug J, Nguyen Van P, et al. : Retention and retrieval: Both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J Cell Sci. 1994; 107(Pt 10): 2705–17. 10.1242/jcs.107.10.2705 [DOI] [PubMed] [Google Scholar]
- 57. Ludhiadch A, Sharma R, Muriki A, et al. : Role of Calcium Homeostasis in Ischemic Stroke: A Review. CNS Neurol Disord Drug Targets. 2022; 21(1): 52–61. 10.2174/1871527320666210212141232 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 58. Appenzeller C, Andersson H, Kappeler F, et al. : The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat Cell Biol. 1999; 1(6): 330–4. 10.1038/14020 [DOI] [PubMed] [Google Scholar]
- 59. Velloso LM, Svensson K, Pettersson RF, et al. : The crystal structure of the carbohydrate-recognition domain of the glycoprotein sorting receptor p58/ERGIC-53 reveals an unpredicted metal-binding site and conformational changes associated with calcium ion binding. J Mol Biol. 2003; 334(5): 845–51. 10.1016/j.jmb.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 60. Nyfeler B, Zhang B, Ginsburg D, et al. : Cargo selectivity of the ERGIC-53/MCFD2 transport receptor complex. Traffic. 2006; 7(11): 1473–81. 10.1111/j.1600-0854.2006.00483.x [DOI] [PubMed] [Google Scholar]
- 61. Nyfeler B, Reiterer V, Wendeler MW, et al. : Identification of ERGIC-53 as an intracellular transport receptor of alpha1-antitrypsin. J Cell Biol. 2008; 180(4): 705–12. 10.1083/jcb.200709100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fu YL, Zhang B, Mu TW: LMAN1 (ERGIC-53) promotes trafficking of neuroreceptors. Biochem Biophys Res Commun. 2019; 511(2): 356–62. 10.1016/j.bbrc.2019.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 63. Zheng C, Liu HH, Yuan S, et al. : Molecular basis of LMAN1 in coordinating LMAN1-MCFD2 cargo receptor formation and ER-to-Golgi transport of FV/FVIII. Blood. 2010; 116(25): 5698–706. 10.1182/blood-2010-04-278325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang B, McGee B, Yamaoka JS, et al. : Combined deficiency of factor V and factor VIII is due to mutations in either LMAN1 or MCFD2. Blood. 2006; 107(5): 1903–7. 10.1182/blood-2005-09-3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Appenzeller-Herzog C, Roche AC, Nufer O, et al. : pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J Biol Chem. 2004; 279(13): 12943–50. 10.1074/jbc.M313245200 [DOI] [PubMed] [Google Scholar]
- 66. Zheng C, Page RC, Das V, et al. : Structural characterization of carbohydrate binding by LMAN1 protein provides new insight into the endoplasmic reticulum export of factors V (FV) and VIII (FVIII). J Biol Chem. 2013; 288(28): 20499–509. 10.1074/jbc.M113.461434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sahoo SK, Kim T, Kang GB, et al. : Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem. 2009; 284(45): 31109–21. 10.1074/jbc.M109.031989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hansen GA, Vorum H, Jacobsen C, et al. : Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis. Mol Cell Biochem. 2009; 320(1–2): 25–33. 10.1007/s11010-008-9895-1 [DOI] [PubMed] [Google Scholar]
- 69. Pizzo P, Lissandron V, Capitanio P, et al. : Ca(2+) signalling in the Golgi apparatus. Cell Calcium. 2011; 50(2): 184–92. 10.1016/j.ceca.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 70. Lissandron V, Podini P, Pizzo P, et al. : Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci U S A. 2010; 107(20): 9198–203. 10.1073/pnas.1004702107 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 71. van Baelen K, Dode L, Vanoevelen J, et al. : The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004; 1742(1–3): 103–12. 10.1016/j.bbamcr.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 72. Lin P, Le-Niculescu H, Hofmeister R, et al. : The mammalian calcium-binding protein, nucleobindin (CALNUC), is a Golgi resident protein. J Cell Biol. 1998; 141(7): 1515–27. 10.1083/jcb.141.7.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lin P, Yao Y, Hofmeister R, et al. : Overexpression of CALNUC (nucleobindin) increases agonist and thapsigargin releasable Ca2+ storage in the Golgi. J Cell Biol. 1999; 145(2): 279–89. 10.1083/jcb.145.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aradhyam GK, Balivada LM, Kanuru M, et al. : Calnuc: Emerging roles in calcium signaling and human diseases. IUBMB Life. 2010; 62(6): 436–46. 10.1002/iub.341 [DOI] [PubMed] [Google Scholar]
- 75. Morel-Huaux VM, Pypaert M, Wouters S, et al. : The calcium-binding protein p54/NEFA is a novel luminal resident of medial Golgi cisternae that traffics independently of mannosidase II. Eur J Cell Biol. 2002; 81(2): 87–100. 10.1078/0171-9335-00224 [DOI] [PubMed] [Google Scholar]
- 76. Crevenna AH, Blank B, Maiser A, et al. : Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J Cell Biol. 2016; 213(3): 305–14. 10.1083/jcb.201601089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Scherer PE, Lederkremer GZ, Williams S, et al. : Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol. 1996; 133(2): 257–68. 10.1083/jcb.133.2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mukherjee I, Barlowe C: Overexpression of Sly41 suppresses COPII vesicle-tethering deficiencies by elevating intracellular calcium levels. Mol Biol Cell. 2016; 27(10): 1635–49. 10.1091/mbc.E15-10-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chia PZC, Gleeson PA: Membrane tethering. F1000Prime Rep. 2014; 6: 74. 10.12703/P6-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ireland S, Ramnarayanan S, Fu M, et al. : Cytosolic Ca2+ Modulates Golgi Structure Through PKCα-Mediated GRASP55 Phosphorylation. iScience. 2020; 23(3): 100952. 10.1016/j.isci.2020.100952 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 81. Miller M, Wu M, Xu J, et al. : High-Throughput Screening of TRPC Channel Ligands Using Cell-Based Assays. TRP Channels. 2011; 1–20. [PubMed] [Google Scholar]
- 82. Lavender V, Chong S, Ralphs K, et al. : Increasing the expression of calcium-permeable TRPC3 and TRPC7 channels enhances constitutive secretion. Biochem J. 2008; 413(3): 437–46. 10.1042/BJ20071488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahluwalia JP, Topp JD, Weirather K, et al. : A role for calcium in stabilizing transport vesicle coats. J Biol Chem. 2001; 276(36): 34148–55. 10.1074/jbc.M105398200 [DOI] [PubMed] [Google Scholar]
- 84. Burke JE, Dennis EA: Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009; 50 Suppl(Suppl): S237–42. 10.1194/jlr.R800033-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bechler ME, de Figueiredo P, Brown WJ: A PLA1-2 punch regulates the Golgi complex. Trends Cell Biol. 2012; 22(2): 116–24. 10.1016/j.tcb.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bechler ME, Doody AM, Racoosin E, et al. : The phospholipase complex PAFAH Ib regulates the functional organization of the Golgi complex. J Cell Biol. 2010; 190(1): 45–53. 10.1083/jcb.200908105 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 87. Ben-Tekaya H, Kahn RA, Hauri HP: ADP ribosylation factors 1 and 4 and group VIA phospholipase A2 regulate morphology and intraorganellar traffic in the endoplasmic reticulum-Golgi intermediate compartment. Mol Biol Cell. 2010; 21(23): 4130–40. 10.1091/mbc.E10-01-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. San Pietro E, Capestrano M, Polishchuk EV, et al. : Group IV phospholipase A(2)alpha controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol. 2009; 7(9): e1000194. 10.1371/journal.pbio.1000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bechler ME, Brown WJ: Gβ1γ2 activates phospholipase A 2-dependent Golgi membrane tubule formation. Front Cell Dev Biol. 2014; 2(4): 0004. 10.3389/fcell.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Evans JH, Gerber SH, Murray D, et al. : The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells. Mol Biol Cell. 2004; 15(1): 371–83. 10.1091/mbc.e03-05-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Regan-Klapisz E, Krouwer V, Langelaar-Makkinje M, et al. : Golgi-associated cPLA2alpha regulates endothelial cell-cell junction integrity by controlling the trafficking of transmembrane junction proteins. Mol Biol Cell. 2009; 20(19): 4225–34. 10.1091/mbc.e08-02-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Micaroni M, Perinetti G, Di Giandomenico D, et al. : Synchronous intra-Golgi transport induces the release of Ca2+ from the Golgi apparatus. Exp Cell Res. 2010; 316(13): 2071–86. 10.1016/j.yexcr.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 93. Missiaen L, Raeymaekers L, Dode L, et al. : SPCA1 pumps and Hailey-Hailey disease. Biochem Biophys Res Commun. 2004; 322(4): 1204–13. 10.1016/j.bbrc.2004.07.128 [DOI] [PubMed] [Google Scholar]
- 94. Dürr G, Strayle J, Plemper R, et al. : The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998; 9(5): 1149–62. 10.1091/mbc.9.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. D'hooge P, Coun C, van Eyck V, et al. : Ca(2+) homeostasis in the budding yeast Saccharomyces cerevisiae: Impact of ER/Golgi Ca(2+) storage. Cell Calcium. 2015; 58(2): 226–35. 10.1016/j.ceca.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 96. Grice DM, Vetter I, Faddy HM, et al. : Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J Biol Chem. 2010; 285(48): 37458–66. 10.1074/jbc.M110.163329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Deng Y, Pakdel M, Blank B, et al. : Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network. Dev Cell. 2018; 47(4): 464–478.e8. 10.1016/j.devcel.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. von Blume J, Alleaume AM, Kienzle C, et al. : Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol. 2012; 199(7): 1057–66. 10.1083/jcb.201207180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lavoie C, Meerloo T, Lin P, et al. : Calnuc, an EF-hand Ca(2+)-binding protein, is stored and processed in the Golgi and secreted by the constitutive-like pathway in AtT20 cells. Mol Endocrinol. 2002; 16(11): 2462–74. 10.1210/me.2002-0079 [DOI] [PubMed] [Google Scholar]
- 100. Brodeur J, Larkin H, Boucher R, et al. : Calnuc binds to LRP9 and affects its endosomal sorting. Traffic. 2009; 10(8): 1098–114. 10.1111/j.1600-0854.2009.00933.x [DOI] [PubMed] [Google Scholar]
- 101. Garcia-Marcos M, Kietrsunthorn PS, Wang H, et al. : G Protein binding sites on Calnuc (nucleobindin 1) and NUCB2 (nucleobindin 2) define a new class of G(alpha)i-regulatory motifs. J Biol Chem. 2011; 286(32): 28138–49. 10.1074/jbc.M110.204099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lin P, Fischer T, Lavoie C, et al. : Calnuc plays a role in dynamic distribution of Galphai but not Gbeta subunits and modulates ACTH secretion in AtT-20 neuroendocrine secretory cells. Mol Neurodegener. 2009; 4: 15. 10.1186/1750-1326-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Larkin H, Costantino S, Seaman MNJ, et al. : Calnuc Function in Endosomal Sorting of Lysosomal Receptors. Traffic. 2016; 17(4): 416–32. 10.1111/tra.12374 [DOI] [PubMed] [Google Scholar]
- 104. Lin P, Li F, Zhang YW, et al. : Calnuc binds to Alzheimer's beta-amyloid precursor protein and affects its biogenesis. J Neurochem. 2007; 100(6): 1505–14. 10.1111/j.1471-4159.2006.04336.x [DOI] [PubMed] [Google Scholar]
- 105. Kanuru M, Aradhyam GK: Chaperone-like Activity of Calnuc Prevents Amyloid Aggregation. Biochemistry. 2017; 56(1): 149–59. 10.1021/acs.biochem.6b00660 [DOI] [PubMed] [Google Scholar]
- 106. Pacheco-Fernandez N, Pakdel M, Blank B, et al. : Nucleobindin-1 regulates ECM degradation by promoting intra-Golgi trafficking of MMPs. J Cell Biol. 2020; 219(8): e201907058. 10.1083/jcb.201907058 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 107. Petersson U, Somogyi E, Reinholt FP, et al. : Nucleobindin is produced by bone cells and secreted into the osteoid, with a potential role as a modulator of matrix maturation. Bone. 2004; 34(6): 949–60. 10.1016/j.bone.2004.01.019 [DOI] [PubMed] [Google Scholar]
- 108. Feng X, Yang J: Lysosomal Calcium in Neurodegeneration. Messenger (Los Angel). 2016; 5(1–2): 56–66. 10.1166/msr.2016.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Colombo MI, Beron W, Stahl PD: Calmodulin regulates endosome fusion. J Biol Chem. 1997; 272(12): 7707–12. 10.1074/jbc.272.12.7707 [DOI] [PubMed] [Google Scholar]
- 110. Di Paola S, Scotto-Rosato A, Medina DL: TRPML1: The Ca(2+) retaker of the lysosome. Cell Calcium. 2018; 69: 112–21. 10.1016/j.ceca.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 111. Wong CO, Li R, Montell C, et al. : Drosophila TRPML is required for TORC1 activation. Curr Biol. 2012; 22(17): 1616–21. 10.1016/j.cub.2012.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li G, Huang D, Hong J, et al. : Control of lysosomal TRPML1 channel activity and exosome release by acid ceramidase in mouse podocytes. Am J Physiol Cell Physiol. 2019; 317(3): C481–C491. 10.1152/ajpcell.00150.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 113. Cao Q, Yang Y, Zhong XZ, et al. : The lysosomal Ca2+ release channel TRPML1 regulates lysosome size by activating calmodulin. J Biol Chem. 2017; 292(20): 8424–35. 10.1074/jbc.M116.772160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dong XP, Shen D, Wang X, et al. : PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010; 1(4): 38. 10.1038/ncomms1037 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 115. Yang Y, Xu M, Zhu X, et al. : Lysosomal Ca2+ release channel TRPML1 regulates lysosome size by promoting mTORC1 activity. Eur J Cell Biol. 2019; 98(2–4): 116–23. 10.1016/j.ejcb.2019.05.001 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 116. Vergarajauregui S, Martina JA, Puertollano R: Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009; 284(52): 36357–66. 10.1074/jbc.M109.047241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cao Q, Zhong XZ, Zou Y, et al. : Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J Cell Biol. 2015; 209(6): 879–94. 10.1083/jcb.201409071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Luzio JP, Bright NA, Pryor PR: The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans. 2007; 35(Pt 5): 1088–91. 10.1042/BST0351088 [DOI] [PubMed] [Google Scholar]
- 119. Rossi AH, Sears PR, Davis CW: Ca2+ dependency of 'Ca2+-independent' exocytosis in SPOC1 airway goblet cells. J Physiol. 2004; 559(Pt 2): 555–65. 10.1113/jphysiol.2004.070433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhu Y, Abdullah LH, Doyle SP, et al. : Baseline Goblet Cell Mucin Secretion in the Airways Exceeds Stimulated Secretion over Extended Time Periods, and Is Sensitive to Shear Stress and Intracellular Mucin Stores. PLoS One. 2015; 10(5): e0127267. 10.1371/journal.pone.0127267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cantero-Recasens G, Butnaru CM, Valverde MA, et al. : KChIP3 coupled to Ca2+ oscillations exerts a tonic brake on baseline mucin release in the colon. eLife. 2018; 7: e39729. 10.7554/eLife.39729 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 122. Micaroni M: Calcium around the Golgi apparatus: Implications for intracellular membrane trafficking. Adv Exp Med Biol. 2012; 740: 439–60. 10.1007/978-94-007-2888-2_18 [DOI] [PubMed] [Google Scholar]
- 123. Sarmento MJ, Coutinho A, Fedorov A, et al. : Ca(2+) induces PI(4,5)P2 clusters on lipid bilayers at physiological PI(4,5)P2 and Ca(2+) concentrations. Biochim Biophys Acta. 2014; 1838(3): 822–30. 10.1016/j.bbamem.2013.11.020 [DOI] [PubMed] [Google Scholar]
- 124. Graber ZT, Wang W, Singh G, et al. : Competitive cation binding to phosphatidylinositol-4,5-bisphosphate domains revealed by X-ray fluorescence. RSC Adv. 2015; 5(129): 106536–42. 10.1039/C5RA19023A [DOI] [Google Scholar]
- 125. Sinn CG, Antonietti M, Dimova R: Binding of calcium to phosphatidylcholine-phosphatidylserine membranes. Colloids Surf A Physicochem Eng Asp. 2006; 282–283: 410–9. 10.1016/j.colsurfa.2005.10.014 [DOI] [Google Scholar]
- 126. Pedersen UR, Leidy C, Westh P, et al. : The effect of calcium on the properties of charged phospholipid bilayers. Biochim Biophys Acta. 2006; 1758(5): 573–82. 10.1016/j.bbamem.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 127. Mao Y, Du Y, Cang X, et al. : Binding competition to the POPG lipid bilayer of Ca2+, Mg2+, Na+, and K+ in different ion mixtures and biological implication. J Phys Chem B. 2013; 117(3): 850–8. 10.1021/jp310163z [DOI] [PubMed] [Google Scholar]
- 128. Tarafdar PK, Chakraborty H, Dennison SM, et al. : Phosphatidylserine inhibits and calcium promotes model membrane fusion. Biophys J. 2012; 103(9): 1880–9. 10.1016/j.bpj.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bootman MD, Collins TJ, Peppiatt CM, et al. : Calcium signalling--an overview. Semin Cell Dev Biol. 2001; 12(1): 3–10. 10.1006/scdb.2000.0211 [DOI] [PubMed] [Google Scholar]
- 130. Duan J, Navarro-Dorado J, Clark JH, et al. : The cell-wide web coordinates cellular processes by directing site-specific Ca2+ flux across cytoplasmic nanocourses. Nat Commun. 2019; 10(1): 2299. 10.1038/s41467-019-10055-w [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 131. Ali Doosti B, Pezeshkian W, Bruhn DS, et al. : Membrane Tubulation in Lipid Vesicles Triggered by the Local Application of Calcium Ions. Langmuir. 2017; 33(41): 11010–7. 10.1021/acs.langmuir.7b01461 [DOI] [PubMed] [Google Scholar]
- 132. Graber ZT, Shi Z, Baumgart T: Cations induce shape remodeling of negatively charged phospholipid membranes. Phys Chem Chem Phys. 2017; 19(23): 15285–95. 10.1039/c7cp00718c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Boye TL, Jeppesen JC, Maeda K, et al. : Annexins induce curvature on free-edge membranes displaying distinct morphologies. Sci Rep. 2018; 8(1): 10309. 10.1038/s41598-018-28481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hullin-Matsuda F, Taguchi T, Greimel P, et al. : Lipid compartmentalization in the endosome system. Semin Cell Dev Biol. 2014; 31: 48–56. 10.1016/j.semcdb.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 135. Fairn GD, Schieber NL, Ariotti N, et al. : High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol. 2011; 194(2): 257–75. 10.1083/jcb.201012028 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation