Abstract
Introduction:
Stress is a known trigger for the symptoms of irritable bowel syndrome (IBS), a gastrointestinal (GI) disorder that presents with abnormal bowel habits and abdominal pain due to visceral hypersensitivity. While behavioral therapies have been used to attenuate IBS symptoms, the underlying mechanisms by which these therapies interact with stress-induced pathology remains to be delineated. Here we use a rat model to test the hypothesis that exposure to environmental enrichment (EE) inhibits stress-induced changes within the brain-gut axis to prevent visceral and somatic hypersensitivity and colonic hyperpermeability.
Methods:
Female rats (n = 8/group) were housed in EE one week before and one week during exposure to water avoidance stress (WAS) while controls were housed in standard cages (SH). One day after the final WAS exposure, colonic and somatic sensitivity were assessed by the visceromotor response (VMR) to colorectal distension (CRD) and withdrawal threshold elicited by an electronic von Frey on the hind paw of the rats respectively. All rats were returned to SH for 3 weeks before colonic and somatic sensitivity were reassessed on day 28. The rats were then immediately euthanized and the spinal cord was collected to assess changes in neuronal activation (assessed via ERK phosphorylation) in response to noxious CRD. A separate cohort of animals (n = 8/group) that did not undergo behavioral assessments was euthanized the day after the final WAS exposure and the central nucleus of the amygdala (CeA) was collected to investigate WAS and EE induced epigenetic changes at the glucocorticoid receptor (GR) and corticotrophin releasing hormone (CRH) promoter. The colon from these rats was also collected to assess colonic permeability via changes in transepithelial electrical resistance (TEER) in vitro.
Results:
Exposure to stress persistently increased VMR to CRD (P < 0.01) and decreased the hind paw withdrawal threshold (P < 0.001) in female rats. WAS also decreased TEER in the colon tissue of female rats (p = 0.05). In the CeA, WAS induced a decrease in histone acetylation at the GR promoter but increased histone acetylation at the CRH promoter and reduced GR-CRH interactions in the CeA. Analysis of the spinal cord showed that WAS increased CRD-evoked ERK phosphorylation in the dorsal horn. Exposure to EE prevented WAS-induced changes in the CeA, dorsal horn and colon respectively to prevent visceral and somatic hypersensitivity.
Conclusion:
Our data reveals that behavioral therapies can produce long lasting molecular and epigenetic changes that can prevent stress-induced pathologies even after completion of the therapy. These results highlight the potential mechanisms by which behavioral therapies may ameliorate visceral pain associated stress-related pathologies such as the irritable bowel syndrome.
Keywords: Stress, Epigenetics, Visceral sensitivity, Brain-gut communication, colon permeability, Somatic sensitivity
1. Introduction
Psychotherapies and behavioral therapies have long been used to ameliorate symptoms of psychiatric diseases as well as other mood and anxiety disorders (David et al., 2018; Zimmermann et al., 2005; Bisson and Andrew, 2007; Chan, 2006) by educating patients on the use of cognitive and behavioral strategies that allow them to cope with their symptoms. Clinical studies show that behavioral therapies including cognitive behavioral therapy (CBT) stress-relief techniques, and mindfulness can lead to reduction in pain symptoms associated with multiple different disorders (Tang et al., 2013; Watkins et al., 2018; Orock et al., 2021; Garland et al., 2012) including musculoskeletal pain (Castro et al., 2012) and inflammatory pain (Otis et al., 2013). Behavioral therapies have also been effective in treating visceral pain in patients with irritable bowel syndrome (IBS) (Orock et al., 2021; Lackner et al., 2018). IBS is a common disorder of the brain-gut axis that affects up to 20% of the US population and is characterized by abnormal bowels habits and chronic abdominal pain due to visceral hypersensitivity (Dunphy et al., 2003; Peery et al., 2012). Furthermore, IBS is a female-predominant disorder, with roughly twice as many female patients as males, suggesting a sexually-dimorphic pathology. Epidemiological studies indicate the stress-related disorders including anxiety and depression are the most common comorbidities of IBS. Similarly, patients suffering from other pain disorders such as fibromyalgia and bladder pain syndrome also tend to display IBS symptomatology (Yang et al., 2015; Ustinova et al., 2006; Doiron et al., 2017). To date, the treatment of IBS focusses on alleviation of visceral pain and the normalization of bowel habits. Research has led to the development of pharmacological therapies for IBS patients, but due to the multifactorial nature of the disorder these drugs are not effective in all patients (Quigley et al., 2018), potentially highlighting the importance of more comprehensive treatment approaches. The role of stress in triggering or exacerbating the symptoms of IBS has led clinicians to investigate whether behavioral therapies, which can attenuate stress-induced pathology and pain in other disorders, are also capable of attenuating IBS symptoms.
Multiple studies have shown that psychotherapies such as CBT can relieve IBS symptoms in patients, however little is known about the underlying mechanisms by which these therapies improve patient outcomes. Clinical studies have shown that many IBS patients have an abnormal neuroendocrine stress response and patients often report that stress can trigger or exacerbate their symptoms (Chang et al., 2009). Therefore, the possibility exists that behavioral therapies may affect the stress-axis. The neuroendocrine response to stress is mediated by the hypothalamic-pituitary-adrenal (HPA) axis, which regulates the secretion of cortisol from the adrenal glands. Imaging studies revealed that the amygdala, a brain region that under normal circumstances facilitates HPA axis activity, is hyperactive in IBS patients during rectosigmoid distension when compared to healthy controls (Wilder-Smith et al., 2004). Our own preclinical studies have highlighted the importance of the amygdala in stress-induced visceral pain. In rats, stereotaxic implantation of corticosterone pellets on the central nucleus of the amygdala (CeA) contributes to visceral hypersensitivity by decreasing glucocorticoid receptor (GR) expression and increasing in corticotrophin-releasing hormone (CRH) expression within the CeA (Greenwood-Van Meerveld et al., 2001). Similarly, we also showed that repeated water avoidance stress (WAS), a model of chronic psychological stress, was sufficient to reduce GR expression and increase CRH expression in the CeA, leading to visceral hypersensitivity (Myers and Greenwood-Van Meerveld, 2012). Recently, we observed in rodent models of visceral hypersensitivity that stress causes dysregulation of the epigenome at the GR and CRH promoters. Specifically, epigenetic modifications such as DNA methylation and histone deacetylation are increased at the GR promoter following stress exposure, whereas DNA demethylation and histone acetylation occur at the CRH promoter (Louwies et al., 2019; Tran et al., 2015). In this way, stress as an environmental signal can cause long-lasting changes in GR and CRH expression. It should be noted that stress not only remodels supraspinal pathways but can also influence peripheral organs and the enteric nervous system. Our lab and others showed that chronic stress can increase gut permeability (Ligon et al., 2018). The increases in permeability favor translocation from the luminal content of the gut, which sensitize visceral afferents and lead to increases in pain perception (Zhou et al., 2009). The increased visceral afferent signaling may sensitize somatic nociceptors once these signaling pathways converge at the level of the dorsal root ganglia, or in the central nervous system (Sengupta, 2009). Thus, stress could lead to visceral and somatic hypersensitivity, as is often observed in patients. Additionally, stress can also directly affect neuronal signaling in the dorsal horn of the spinal cord (Xu et al., 2020). In support of this, we showed previously that chronic stress increases evoked ERK phosphorylation in the dorsal horn of the spinal cord in response to noxious colorectal distension (Mohammadi et al., 2018).
Environmental enrichment (EE) has been used as the rodent analogue of behavioral therapies in order to better understand the underlying physiological/molecular mechanisms by which behavioral therapies confer their effects. EE involves housing animals in an environment with sufficient physical and social cues to encourage brain stimulation and activity (Orock et al., 2021; Slater and Cao, 2015). Our previous data in male rats showed that exposure to EE before and during WAS prevented stress induced decrease in GR expression in the CeA and prevented WAS-induced visceral and somatic hypersensitivity (Orock et al., 2020). Here, we repeat these experiments in cycling female animals, while also investigating colonic permeability, spinal p-ERK expression and epigenetic changes in the CeA related to the expression of GR and CRH. We hypothesize that by beneficially altering the animals’ environment with EE, we can prevent the stress-induced changes in colonic permeability, spinal and central activation/expression of nociceptive signaling pathways/genes, and ultimately visceral and somatic hypersensitivity. By exposing the rats to EE, we beneficially change the environment. Therefore, we hypothesize that by changing the environment, we can keep the epigenome in a state that prevents the expression of nociceptive genes. Consequently, we hypothesize that the effects of EE are persistent and will last at least three weeks after female rats had undergone WAS.
2. Methods and expermental dedign
2.1. Ethical statement and assurances
All procedures were approved by the Veterans Affairs (VA) Health Care System (1604-001, 1809-001) and the University of Oklahoma Health Sciences Center (OUHSC) (15-070-SSHIL, 18-061-SSHIL) institutional animal care and use committees (IACUCs). Experiments were designed to minimize pain and distress to the animals per the Guide for Care and Use of Laboratory Animals (8th edition, 2011).
2.2. Animals
This study was performed with adult female (150–180 g; 74–90 days old) Fischer-344 (F344) rats (Charles River Laboratory, Wilmington MA, USA). The animals were maintained on a 12 h light/dark cycle at 21 °C with ad libitum access to food and water.
2.3. Environmental enrichment and standard housing procedures
Standard housing and enriched environment were set us as previously described (Orock et al., n.d.). Briefly, large cages (78 cm L x 52 cm W x 100 cm H) consisting of 3 levels and ramps with ample bedding, toys, food enrichment (sweetened cereal, seed/nut mix), and burrowing tunnels were used to house groups of 4 rats for environmental enrichment. The toys were changed every 2–3 days to maintain novelty. These cages also did not contain running wheels but the enriched diet and toys were placed at the top level of the cages to encourage movement. Standard housed animals remained in double-housed shoe-box cages (SH) with filter tops (43 cm L x 20 cm W x 21.5 cm H). Both housing conditions were kept in the same room in the animal facility. These animals were left to acclimate to the facility for 7 days before being moved to their designated housing conditions to habituate for another 7 days.
2.4. Experimental protocol
Experiments were carried out as previously described (Orock et al., n.d.). Briefly, Fisher 344 (F344) female rats were randomly divided into SH or EE housing conditions (randomization.com) and animals were acclimated to their experimenter, housing and laboratory environments for 7 days. The animals were further divided into 4 groups; SH + WAS, EE + WAS, SH + SH and EE + SH (n = 8 per group). WAS (1-h/day) for 7 days was carried out on rats in the WAS group while the SHAM group went through sham stress. Somatic sensitivity was measured on day 6 of WAS/SHAM. After the final WAS/SHAM test on day 7, animals were then fasted overnight and visceral sensitivity was assessed on day 8. Following visceral sensitivity assessment, all of the animal groups were placed in new SH cages and left undisturbed for 3 weeks. At the end of this period (day 28), visceral and somatic sensitivity were once again assessed.
2.5. Water avoidance stress (WAS)
The WAS procedure was performed as previously described (Orock et al., n.d.). Briefly, for 1 h a day (for 7 days), rats in the WAS group were placed on a platform (8 × 8 × 8 cm) mounted in the center of a white semi-transparent plastic container (50 × 35 × 33 cm). the container was filled with fresh, room temperature water to 1 cm below the surface of the platform. Animals in the sham group (SHAM) were placed in similar containers but without water. Animals were weighed every day before WAS/SHAM procedure. All animals were left undisturbed in the containers for 60 min before being returned to their home cages. The fecal pellet output (FPO) produced during WAS/SHAM was counted as a measure of stress-induced autonomic output.
2.6. Assessment of visceral sensitivity
Visceral sensitivity was assessed as previously described (Orock et al., n.d.; Myers and Greenwood-Van Meerveld, 2007). Briefly, visceral sensitivity was measured as the visceromotor response (VMR) to colorectal distensions (CRD) in freely moving rats. Rats were fasted overnight before being placed under anesthesia (2–2.5% isoflurane) and a latex balloon catheter (5 cm) was inserted into the colon via the anal cavity and fixed in place with surgical tape around the tail. Rats were placed into a clean cage and allowed to recover. After recovery, CRD was induced by inflating the balloon and maintaining an isobaric pressure using a barostat (G&J Electronics, Toronto, ON, Canada). CRD was performed at graded pressures of 0 (baseline), 20, 40, and 60 mmHg for 10 min each in a randomized order with a 10 min recovery period between distensions. VMR was measured by quantifying the number of abdominal contractions during each period of CRD.
2.7. Assessment of somatic sensitivity
Somatic sensitivity was measured as previously described (Orock et al., n.d.; Prusator and Greenwood-Van Meerveld, 2016; Johnson and Greenwood-Van Meerveld, 2015). Briefly, rats were placed in Plexiglas boxes with elevated wire mesh floor and allowed to acclimate for 10 min. Following acclimation, the IITC 2390 series Electronic von Frey anesthesiometer (IITC Life Science, Woodland Hills, CA) was used to assess somatic sensitivity. Somatic sensitivity was measured as the minimum force required to illicit a withdrawal reflex on the hind paw of the rats. This is achieved by applying the von Frey wand on the plantar region of the hind paw of the rats until a withdrawal reflex was observed. The von Frey anesthsiometer recorded the minimum force required to elicit this withdrawal reflex. The process was repeated three additional times on the plantar region of either hind paw with 5-min intervals between each testing session. The mean value of the 4 trials was used as the final withdrawal force for each animal.
2.8. Tissue processing
After the last 60 mmHg CRD for visceral sensitivity is complete on day 28, Rats were deeply anesthetized with 5% isoflurane before being euthanized and perfused with 4% paraformaldehyde in PBS. The spinal cord was removed and incubated overnight in 4% paraformaldehyde before being dehydrated in a 30% sucrose solution (in PBS). The T10-L1 sections of the spinal cord were isolated then frozen in cryogel and non-consecutive slides (10 μm) were collected from on Fisherbrand Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA) before being stored in −80 °C for pERK analysis at the dorsal horn.
A separate cohort of animals (n = 8/group) who did not undergo the CRD procedure were euthanized on day 8 following the WAS/SHAM. CeA punches were collected from these animals and used for epigenetic and CHiP assays. Colon tissue was also collected and used for TEER experiments to assess the effects of EE and WAS on colonic permeability.
2.9. Colonic permeability assay
Colonic permeability was measured as previously described (Mohammadi et al., 2018). Briefly, rat colon segments were isolated postmortem (between 8:00 AM and 10:00 AM) and placed into ice-cold Krebs buffer composed of 120 mM NaCl, 6 mM KCl, 1.2mMMgCl2, 1.2mMH2PO4, 2.5mMCaCl2, 14.4mMNaHCO3, and 11.5 mM glucose, aerated with 95% O2–5% CO2. The tissue was opened longitudinally and mounted into modified Ussing chambers. Tissues were bathed in oxygenated Kreb’s solution at 37 °C for 30–45 min before experimentation. Permeability was assessed electrophysiologically via measurement of transepithelial electrical resistance (TEER). To calculate TEER, the potential difference (PD) and short circuit current (Isc) were recorded and TEER was calculated using Ohm’s law as follows: I=PD/R.
2.10. Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation (ChIP) assays were performed using the MAGnify ChIP assay kit (ThermoFisher Scientific). Chromatin was isolated from CeA micropunches and immunoprecipitated using one of the following antibodies: rabbit anti-acetyl-H3K9 (1:25, #9649, Cell Signal Technologies), rabbit anti-GR (1:25, #3360, Cell Signal Technologies), or normal non-immune rabbit IgG antibody (1:100, ThermoFisher Scientific). One tenth of the lysate was reserved as input control. After reverse crosslinking, the rat GR and CRH promoter regions were subjected to qPCR amplification using QuantiFAST SYBR Green Mastermix (Qiagen, Valencia, CA, USA). The GR promoter that was immunoprecipitated with H3K9 was amplified using the forward primer: 5′-TCAGTATGTTTTCCACACTTGGAT-3′ and reverse primer: 5′-TTTATCGCCTCCTTGGTGAC-3′. The CRH promoter that was immunoprecipitated with H3K9 antibodies was amplified using the forward primer 5′-TTCCATTTTAGGGCTCGTTG-3′ and the reverse primer 5′-CGACCCTCTTCAGAAAGCAC-3′, whereas the CRH promoter that was immunoprecipitated with GR antibodies was amplified using the forward primer5′-GGTCAGTATGTTTTCCACACTTG-3′ and the reverse primer 5′-GCCTCTGCTCCTGCATAAAT-3′. qPCR reactions were carried out in triplicate on the QuantStudio 5 (ThermoFischer) with the following protocol: initial denaturation cycle (5 min, 95 °C), 40 cycles of denaturation (10 s, 95 °C), annealing and extension (30 s, 60 °C) and final hold at 4 °C. Relative quantification of binding was calculated by normalizing the immunoprecipitated DNA Ct values to the input DNA Ct values (ΔCt) and transformed (2ΔCt) to show relative quantities.
2.11. CRD-induced neuronal activation (p-ERK expression) assay
p-ERK expression assay was performed as previously described in Mohammadi et al. (2018). Briefly, non-consecutive slides from the T10-L1 regions of the spinal cord were used to measure expression of the neuronal activator phospho-extracellular signal-related kinase (p-ERK). Sections were incubated with biocare’s Rodent Block R for 20 min at room temperature and then washed 3 times with Triton X (0.2%) before being incubated overnight with anti—phospho-p44/42 mitogen-activated protein kinase (Erk1/2) (Thr202/Tyr204) antibody (pERK; 1:400, no. 4370; Cell Signaling Technology, Danvers, MA) at 4 °C. The sections were then washed and incubated with secondary antibodies for 1 h at room temperature. Sections were washed again and incubated with Betazoid DAB chromogen kit (cat. no. BDB2004H; Biocare Medical) at room temperature for 5 min. The sections are then washed again before being counterstained with hematoxylin. Random sections were imaged using a Zeiss Axiovert epifluorescence microscope (Zeiss, Jena, Germany) and pERK immunoreactive cells in these regions were counted. We averaged the results from two non-consecutive sections and graphed the results.
2.12. Statistical methods
Data are represented as means ± SD and analyses were performed using PRISM 8. Cohort sizes were determined by power analysis based on previous studies involving similar experimental designs. VMR to CRD data was evaluated using a two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni’s post hoc test to determine if there were significant differences between multiple groups. One-way ANOVA followed by Bonferroni’s post-hoc tests were utilized to investigate differences in FPO, somatic sensitivity thresholds, p-ERK expression and epigenetic changes between the cohorts respectively. Grubbs’ test was used to identify and exclude outliers. Animals which did not participate in the visceral sensitivity assays or showed signs of a perforated colon during visceral sensitivity assessment were excluded from the study (~1 animal from each group was excluded from the final analyses).
2.13. Blinding and experimental rigor
The animals were randomized into the different groups at the start of the study (randomization.com). visceral sensitivity, colonic permeability and ChIP assays were performed by experimenters blinded to the animal treatments. For the p-ERK experiments on neuronal activity, the experimenter was partially blinded to the animal groups until after the p-ERK counting was completed.
3. Results
3.1. WAS induced visceral and somatic hypersensitivity in female rats that is blocked by exposure to EE
In order to induce chronic psychological stress, female rats were subjected to WAS for 7 days (n = 8/group). FPO was measured daily during the SHAM/WAS procedure as an indirect measure of autonomic nervous system output (Fig. 1A). 1-way ANOVA analysis revealed significant differences between the groups as WAS significantly increased the average daily FPO in female rats when compared to SHAM controls (Fig. 1B). The WAS-induced FPO was not significantly different throughout the procedure indicating the animals did not habituate to the stressor. Twenty-four hours after the last WAS (day 8), visceral sensitivity was assessed, and 2-way ANOVA revealed significant difference in visceral sensitivity between the groups. We observed a main group effect (F (4, 30) = 14.62, P < 0.001), a main pressure effect (F (2.574, 77.22) = 643.5; p < 0.001) and a group x pressure interaction effect (F (12, 90) = 8.550; p < 0.001). Post-hoc tests revealed that SH + WAS rats had more abdominal contractions at 40 mmHg (p < 0.05) and 60 mmHg (p < 0.001), when compared to controls indicating that WAS induces visceral hypersensitivity (Fig. 1C). To measure the effect of EE in females, a cohort of animals was also housed in EE 1 week before and 1 week during the WAS/SHAM procedure which did not prevent WAS-induced increase in FPO indicating that EE does not prevent the induction of psychological stress. However, the abdominal contractions in EE + WAS animals was similar to controls and significantly higher than SH + WAS at 40 mmHg (P < 0.05) and 60 mmHg (P < 0.001). Somatic sensitivity was also assessed and 1-way ANOVA revealed significant differences between the groups (F (4, 30) = 9.659; p < 0.001). SH + WAS animals had a significantly lower withdrawal threshold when compared to controls (p < 0.001). In contrast, the withdrawal threshold for EE + WAS rats was comparable to controls and significantly higher than SH + WAS rats (p < 0.001)(Fig. 1D).
Fig. 1.
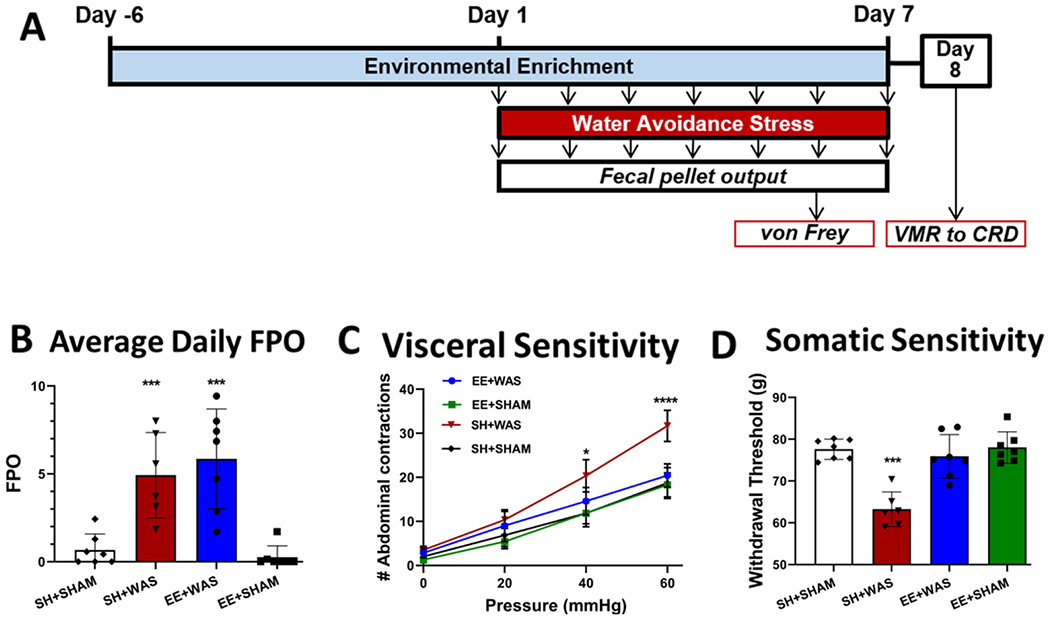
WAS induces visceral and somatic hypersensitivity in female rats that is blocked by exposure to EE. (A) Experimental design. (B) WAS increased the FPO of rats compared to SHAM for both SH and EE rats (p < 0.01). (C) EE for 1 week before and during WAS prevented the induction of visceral hypersensitivity at 40 mmHg (p < 0.05) and 60 mmHg (p < 0.001), and (D) also inhibited WAS-induced somatic hypersensitivity (p < 0.01) in female rats.
3.2. Pre-exposure to an enriched environment (EE) prevented stress-induced colonic hyperpermeability in female rats
We assessed the effects WAS and EE exposure had on colonic permeability on day 8 post WAS in a cohort of animals that did not undergo the CRD procedure (Fig. 2A). One way Anova revealed significant differences between the groups (Fig. 2B)(F(3, 28) = 9.875; p < 0.001). WAS significantly decreased the TEER of colonic tissue compared to controls (SH + WAS = 172.5 ± 42.10 Ωcm2 vs SH + SHAM = 285.1 ± 44.19 Ωcm2; p = 0.05 vs SH + WAS), indicative of hyperpermeability. Pre-exposure to EE attenuated WAS-induced decrease in TEER (237.5 ± 61.25 Ωcm2).
Fig. 2.
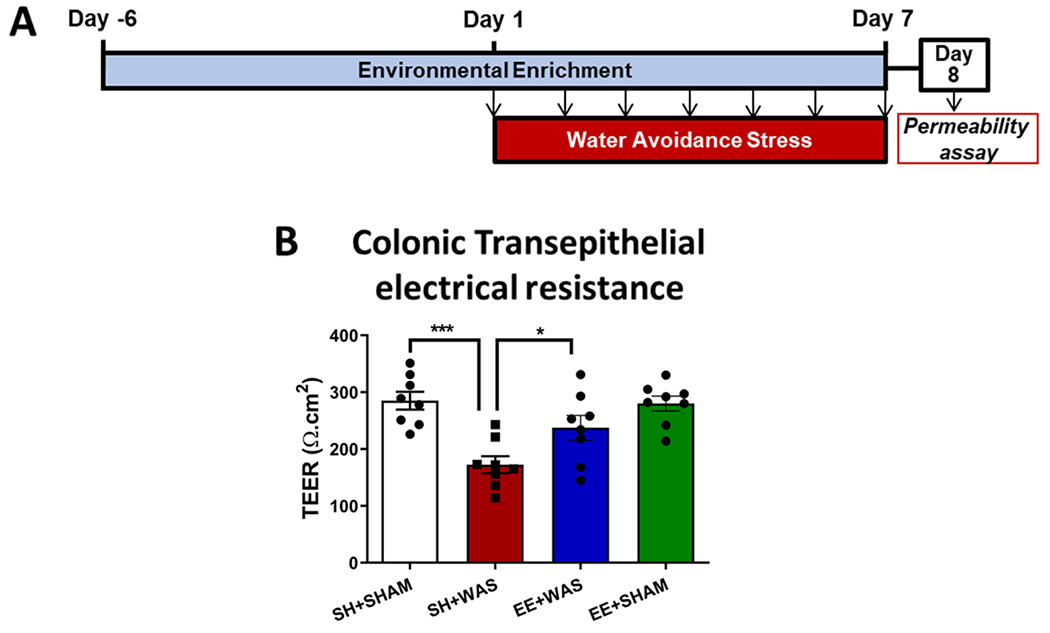
Pre-exposure to an enriched environment partially prevents stress-induced colonic hyperpermeability in female rats: (A)Experimental design. (B) WAS significantly decreased colonic resistance in female rats (P = 0.0003) indicative of colonic hyperpermeability. Pre-exposure to EE was able to moderately inhibit WAS-induced colonic hyperpermeability when compared to SH + WAS animals (P = 0.05).
3.3. Pre-exposure to EE prevented stress induced epigenetic changes at the GR and CRH promoter regions in the CeA
We next assessed whether EE acted at the level of the CeA to prevent stress-induced epigenetic changes on day 8 in a cohort of female animals who did not undergo the CRD procedure (Fig. 3A). One-way ANOVA revealed significant differences in relative H3K9 acetylation at the GR promoter between the groups (Fig. 3B)(F(3, 27) = 6.980; p = 0.0013). WAS significantly decreased H3K9 acetylation at the GR promoter relative to controls (SH + SHAM = 1.000 ± 0.162 vs SH + WAS = 0.4028 ± 0.20), however, pre-exposure to EE prevents the WAS-induced decrease in H3K9 acetylation at the GR promoter (EE + WAS = 0.8236 ± 0.1798). We also observed significant differences in H3K9 acetylation at the CRH promoter between the groups (Fig. 3C) (F(3, 25) = 12.05; p < 0.0001). WAS increased the H3K9 acetylation levels at the CRH promoter relative to controls (SH + SHAM = 1.000 ± 0.3165 vs SH + WAS = 4.721 ± 2.477) and pre-exposure to EE was also sufficient to prevent the stress-induced increase in H3K9 acetylation of the CRH promoter (1.364 ± 1.355; p = 0.001). Since GR is a negative regulator of CRH, ChIP assays were employed to measure GR binding to the CRH promoter. We observed significant effects of WAS and EE on GR-CRH interaction (Fig. 3D)(F(3, 25) = 4.562; p = 0.01). WAS caused a significant decrease in the amount of GR bound to the CRH promoter relative to SH + SHAM controls (SH + SHAM = 1.000 ± 0.1648 vs SH + WAS = 0.3902 ± 0.2044) which was absent in WAS animals pre-exposed to EE before and during WAS, who had similar levels of GR bound to the CRH promoter as controls (EE + WAS = 1.074 ± 0.7074).
Fig. 3.
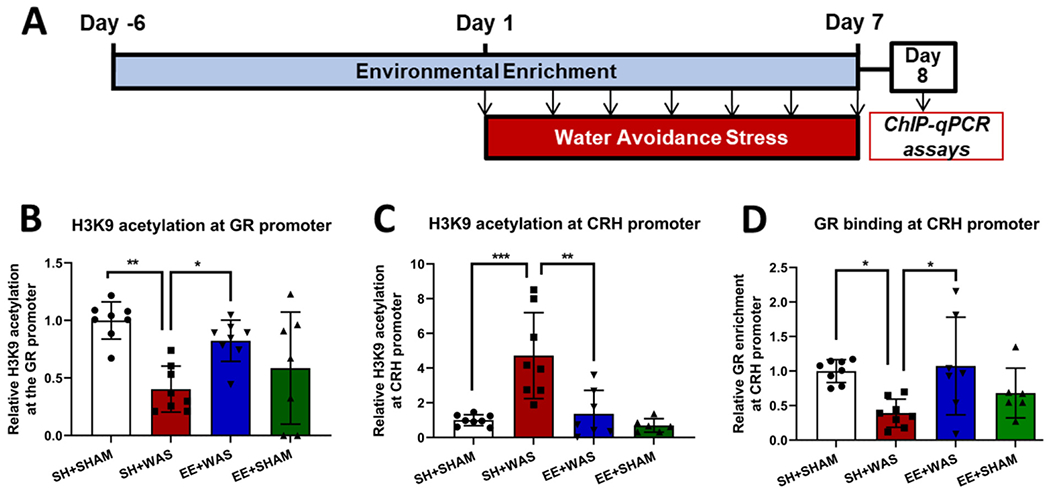
Pre-exposure to EE prevents stress induced epigenetic changes at the GR and CRH promoter regions in the CeA: (A) Experimental design (B) SH + WAS rats had significantly lower H3K9 acetylation at the GR promoter when compared to SH + SHAM (p = 0.02). Pre-exposure to EE maintained H3K9 acetylation of the GR promoter at control levels. (C) SH + WAS rats also had significantly higher H3K9 acetylation levels at the CRH promoter when compared to SH + SHAM (p = 0.0002), which was blocked by pre-exposure to EE. (D) The relative amount of GR bound to the CRH promoter was significantly reduced in WAS animals compared to SH + SHAM (p = 0.02) and pre-exposure to EE maintained GR binding at the CRH promoter at control levels.
3.4. Pre-exposure to EE is sufficient to prevent emergence of persistent effects of WAS on visceral and somatic hypersensitivity
Our previous studies showed that WAS-induced hypersensitivity in males was persistent long after the stressor had been removed. Three weeks after the final WAS and EE sessions, visceral and somatic sensitivity were once again assessed to reveal any persistent effects of WAS and EE in female rats (Fig. 4A). Similar to day 8, 2-way ANOVA of visceral sensitivity on day 28 showed a main group effect (F (4, 30) = 11.78; P < 0.001), a main pressure effect (F (2.228, 66.84) = 551.5; p < 0.001) and a main group x pressure interaction effect (F (12, 90) = 8.614; p < 0.001). Post hoc analysis revealed that SH + WAS animals continued to have significantly higher abdominal contractions at 40 mmHg (p < 0.001) and 60 mmHg (p < 0.01) when compared to controls while EE + WAS animals continued to have similar number of contractions to controls at all distension pressures (Fig. 4B). Assessing somatic sensitivity 3 weeks after WAS showed significant differences between the groups (F (4, 30) = 23.09; p < 0.001; 1-way ANOVA). SH + WAS animals displayed significantly lower withdrawal threshold when compared to controls (p < 0.001) which was not seen in the EE + WAS group (Fig. 4C).
Fig. 4.
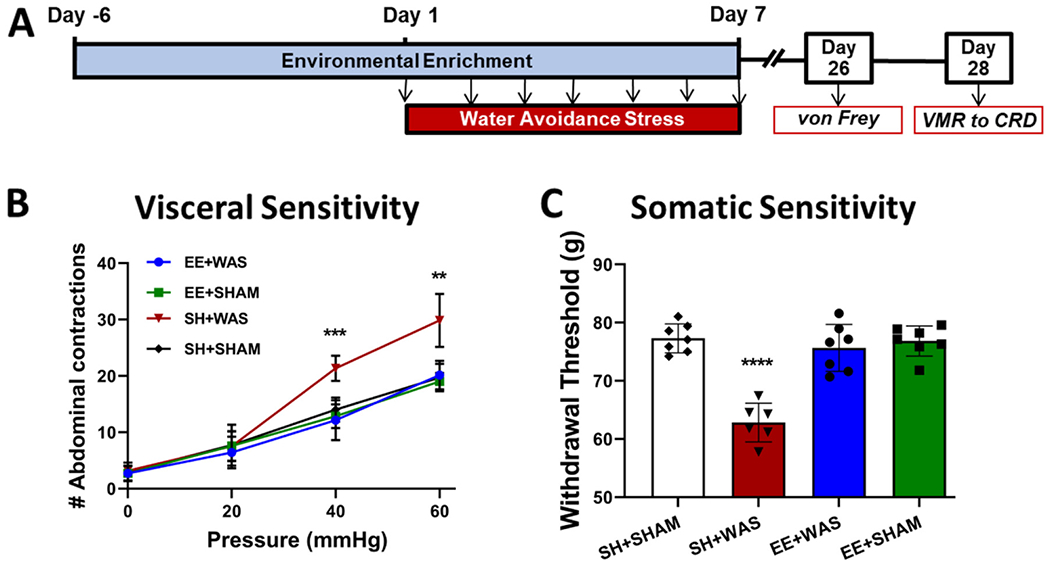
Pre-exposure to an enriched environment prevents the development of persistent WAS-induced visceral and somatic hypersensitivity in female rats. (A) Experimental design. At day 28, SH + WAS rats still displayed (B) visceral hypersensitivity (p < 0.01) and (C) somatic hypersensitivity (p < 0.01) when compared to SH + SHAM controls. EE + WAS animals were still similar to controls and did not display visceral or somatic hypersensitivity.
3.5. Pre-exposure to EE inhibited stress induced increase in neuronal activation at the dorsal horn in response to colorectal distension
Persistent visceral pain is associated with enhanced nociception which can be measured by neuronal activation of the dorsal horn in response to visceral stimuli. To assess whether pre-exposure to EE is sufficient to inhibit peripheral sensitization, sections of the dorsal horn of the spinal cord were isolated from a cohort of animals and stained for p-ERK activity immediately after the final 60 mmHg distension on day 28. One-way ANOVA revealed a significant difference in the number of p-ERK positive cells between the groups for sections T10-T12 (Fig. 5B)(F(2, 15) = 14.27; p = 0.0003) and T-13-L1 (Fig. 5D)(F(2,11) = 40.78). We confirmed that WAS increased the number of p-ERK immunoreactive cells in the dorsal horn compared to SHAM controls (T10-T12: SH + SHAM = 3.750 ± 1.508 vs. SH + WAS = 9.500 ± 3.033 cells; p = 0.001; T13-L1:SH + SHAM = 4.400 ± 1.597 vs. SH + WAS = 11.13 ± 0.8539 cells, p < 0.0001). Pre-exposure to EE prevents the WAS-induced increase in p-ERK activation (Fig. 5B: T10-T12: EE + WAS = 3.583 ± 1.686 cells; T13-L1: EE + WAS = 4.700 ± 1.037 cells, Fig. 5D) with these animals displaying levels of p-ERK immunoreactive cells comparable to controls.
Fig. 5.
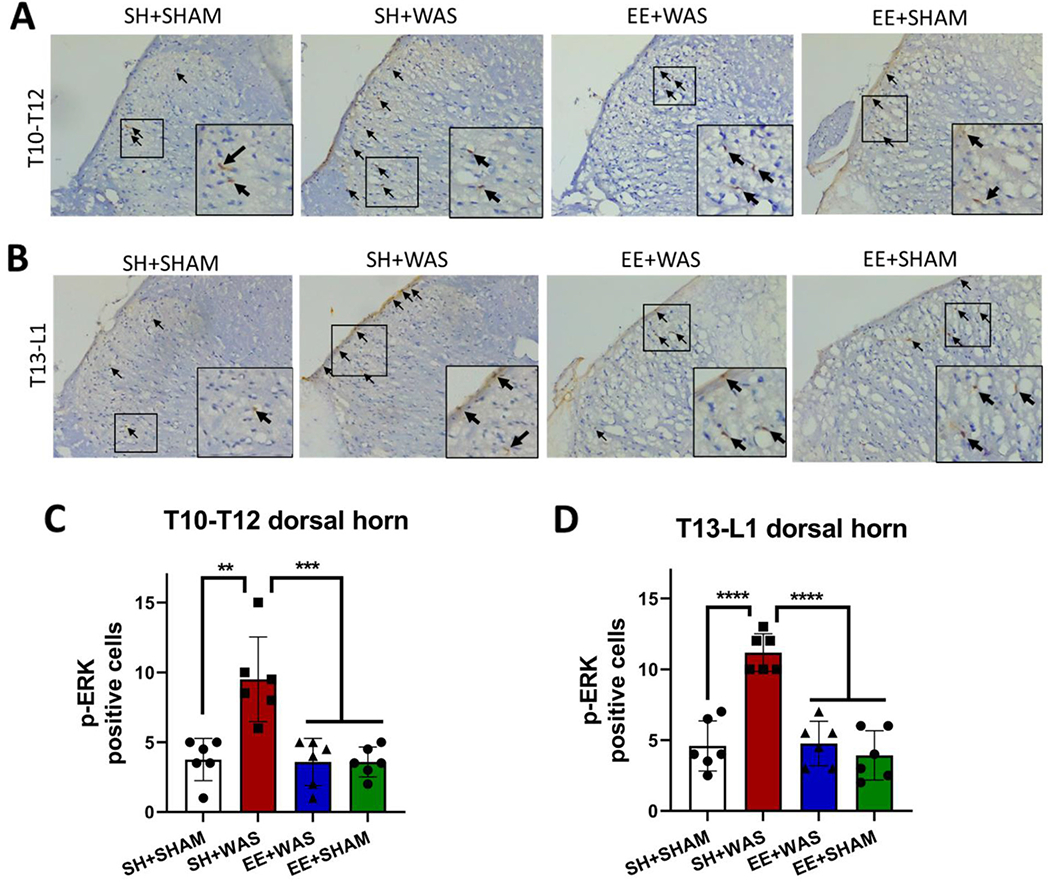
Pre-exposure to EE inhibits stress induced increase in neuronal activation at the dorsal horn in response to CRD.
(A) T10-T12 representative slices showing p-ERK positive cells in SH + SHAM, SH + WAS, EE + WAS and EE + SHAM groups. (B) T13-L1 representative slices showing p-ERK positive cells in SH + SHAM, SH + WAS, EE + WAS and EE + SHAM groups. (C) summarized dorsal horn data (T10-T12) from SH + WAS animals had significantly higher numbers of p-ERK cells when compared to EE + WAS, EE + SHAM and SH + SHAM controls (p = 0.001). (D) summarized dorsal horn data (T13-L1 slices) showing SH + WAS animals had significantly higher numbers of p-ERK cells when compared to EE + WAS, EE + SHAM and SH + SHAM controls (p < 0.0001) in response to CRD.
4. Discussion
In this study, we found that exposing female animals to EE before and during repeated WAS is sufficient to inhibit the development of stress-induced visceral and somatic hypersensitivity, reproducing the phenotypical effects of EE that we observed previously in male rats (Orock et al., n.d.). Since the molecular mechanisms underlying the therapeutic effects of EE on visceral sensitivity are largely unknown, we investigated the effect of EE on three different components known to be relevant to the visceral afferent sensitization: colonic permeability, spinal nociceptive signaling and epigenetic modulation of gene expression in the CeA. Our data revealed that EE is able to prevent stress-induced increases in colonic permeability and evoked ERK phosphorylation in the spinal cord as well as maladaptive epigenetic changes at the GR and CRH promoters in the CeA. By inhibiting these molecular and epigenetic changes induced by stress, EE prevented the development of visceral and somatic hypersensitivity, an effect that persisted long after cessation of EE. Chronic stress can lead to visceral hypersensitivity through “top-down” initiated central and peripheral sensitization (Greenwood-Van Meerveld et al., 2001; Prusator and Greenwood-Van Meerveld, 2017; Ligon et al., 2021). Studies have shown that psychological stress-induced changes in GR and CRH expression in the amygdala is sufficient to induce visceral hypersensitivity (Myers and Greenwood-Van Meerveld, 2012; Hong et al., 2015). In addition, we also observed that chronic stress also increased colonic permeability (Ligon et al., 2018; Zhou et al., 2009). Chronic stress induces changes in expression of GR and CRH leading to central sensitization of the pain pathway which in turns precipitates visceral afferent sensitization and induces neurogenic inflammation and increased colonic permeability. Increased permeability allows luminal mediators to contact visceral afferents leading to further sensitization in a feed forward cycle. For example, increased colonic permeability can allow luminal mediators, such as serotonin (5-HT), to make direct contact with visceral sensory afferents that express 5-HT receptors. In addition, translocation of luminal content can trigger an immune response in the gut wall that can lead to further peripheral sensitization (Nakajima et al., 2009). Taken together, we see there are multiple potential pathways through which chronic stress can induce visceral hypersensitivity. We have shown previously that changes in the expression of GR and CRH in the CeA can precipitate the development of visceral hypersensitivity (Johnson and Greenwood-Van Meerveld, 2015; Johnson et al., 2015). Our results also revealed that chronic WAS decreases histone acetylation at the GR promoter and increases histone acetylation at the CRH promoter. In addition, after chronic WAS, the amount of GR bound to the CRH promoter was decreased. In our previous work, we showed that these changes in histone acetylation are associated with decreases in GR and increases in CRH expression (Tran et al., 2015; Tran et al., 2013). Since GR is an important negative regulator of CRH, the loss of GR leads to the increase in CRH in the CeA, which is important for the development of stress-induced visceral hypersensitivity. It is important to note that GR expression not limited to the brain, but is found throughout the body, and so we cannot rule out the possibility of other potential GR-mediated mechanisms contributing to visceral hypersensitivity. Zheng et al. showed that GR expression in the colon is downregulated after chronic stress, which decreases tight junction proteins expression and increases intestinal permeability (Zheng et al., 2017). In addition, Wiley et al. showed that chronic WAS decreased GR binding and increased H3K9 methylation at the occluding and claudin-1 gene promoters (Wiley et al., 2020). As a result, increased intestinal permeability may favor peripheral sensitization of visceral afferents. Similarly, Hong et al. showed that decreased GR expression in the L6-S2 dorsal root ganglia (DRG) was pivotal in stress-induced visceral hypersensitivity (Hong et al., 2015). Interestingly, the WAS-induced changes in GR expression in the DRGs were mediated by increases in DNA methylation at the GR promoter. However, these changes were only observed in the L6-S2 DRGs innervating the colon. In this study, we report WAS-induced epigenetic changes at the level of the CeA. Taken together, we and others observed epigenetic changes in the periphery, peripheral and central part of the pain pathway, whereas regions that were not involved in pain transmission from the colon were unaffected (Hong et al., 2015). In all aforementioned studies, chronic stress activates GR in the periphery and in central regions. Repeated activation of GR triggers a signaling cascade that ultimately leads to a permanent decrease in GR expression. The loss of GR in both peripheral and central pathways contributes to stress-induced visceral pain. The novelty of our findings is that EE can prevent these aberrant molecular changes in female rats exposed to chronic WAS. Here, we observed that EE prevented the chronic WAS-induced increase in colonic permeability, prevented the upregulation of the nociceptive marker p-ERK in the dorsal horn of the spinal cord, and prevented the epigenetic changes at the GR and CRH promoters in the amygdala. That EE can also prevent epigenetic changes at the CRH promoter was probably due to its preventative effects on the GR promoter. Maintaining normal histone acetylation levels at the GR promoter, despite chronic WAS, could have prevented the decrease in GR expression and the loss of negative CRH regulation. In our previous work, we found that the histone deacetylase Sirtuin-6 was recruited to the GR promoter after elevated CORT exposure (Tran et al., 2015). It is likely that EE prevented this recruitment of Sirtuin-6, which may have prevented the deacetylation of the GR promoter. In our future studies, we will investigate the effect of EE on the activity of this and other histone deacetylases. In this way, we hope to uncover the epigenetic enzymes that are involved in the protective effects of EE.
The mechanism of how EE can prevent all these changes is currently unclear. EE may be acting directly on the CeA, preventing the epigenetic changes that cause visceral hypersensitivity and increased colonic permeability. Conversely, by preventing the increase in colonic permeability, EE may have prevented bottom-up sensitization which may have resulted in spinal ERK phosphorylation and amygdalar epigenetic changes, as observed in chronic WAS animals. On the other hand, EE may have had global effects and affected these mechanisms independently of each other. Several reports suggest that EE can lower the (re)activity of the HPA axis (Skwara et al., 2012). For instance, exposure to EE can confer stress resiliency via increased neuronal activation in the PFC (Lehmann and Herkenham, 2011) in rodents. Similarly, EE promotes significant changes in gene expression and neuronal proliferation in the hippocampus (Hüttenrauch et al., 2016; Dandi et al., 2018) which plays a regulatory role on the HPA axis (Jankord and Herman, 2008) and is also involved in the pathophysiology of visceral hypersensitivity (Zhang et al., 2016). In this way, it is possible that EE blunted the HPA response to chronic WAS, which may have resulted in lower CORT levels. As a result, peripheral and central CORT-GR signaling may have been insufficient to increase colonic permeability or central remodeling of the pain pathways in EE animals. Other investigations have shown that EE can stimulate the endogenous opioid system or increase opioid receptor expression (Stagg et al., 2011; Hoffmann et al., 1990). Studies have shown that blocking the endogenous opioid system can negatively affect HPA activity through a GR-mediated mechanism (Szklarczyk et al., 2016). If, in the current study, EE engaged the endogenous opioid system, it may have counterbalanced the detrimental effects of chronic WAS. Some studies have shown that removing animals from long term EE can cause HPA dysregulation leading psychological disorders (Morano et al., 2019). This raised the concern that the effects of EE were only temporary and symptoms may return after completion of the therapy. Three weeks after the cessation of EE, females from the EE + WAS cohort still showed normal visceral and somatic sensitivity. Similarly, 3 weeks after the cessation of EE, the animals in the EE + WAS group had reduced ERK1/2 phosphorylation at the dorsal horn in response to CRD when compared to females in the SH + WAS group indicative of normal spinal nociception in the EE + WAS cohort. These results indicate that the positive effects of EE are persistent and do not ebb after the cessation of the therapy.
One caveat of our study is that it focuses on the prevention as opposed to reversal of stress induced pathology. While our results show that EE can prevent WAS induced changes in the CeA, dorsal horn and colon permeability, it is unclear whether EE will still have the same beneficial effects if administered after the stressor. Our future research will be designed to investigate whether EE is also sufficient to reverse stress-induced molecular and epigenetic changes to attenuate visceral hypersensitivity when administered after the stressor.
There are also multiple different paradigms and methods employed in EE and the literature shows the results are not always consistent. Indeed some studies show that EE may lead to increased stress reactivity (Girbovan and Plamondon, 2013). Our EE protocol before and during a stressor has consistently shown to be protective against the development of visceral hypersensitivity in both males and females although the exact etiology of EE in these cases is still unclear. EE also influences multiple pathways in the brain and body making it more difficult to untangle the exact role of EE. For this study, we took a more targeted approach and looked mainly at regions that are known to play a role in visceral pain to reveal how exposure to EE affects them. Our lab has previously shown the role stress, the CeA and epigenetic regulation (Tran et al., 2013; Meerveld and Johnson, 2018) in the pathophysiology of visceral pain and here we showed that EE can inhibit these effects. It should be noted that EE did not prevent the induction of stress and the FPO was not significantly reduced in the WAS procedure between EE and SH groups. We also looked at the dorsal horn as activation of these fibers are known to contribute to chronic pain (Mohammadi et al., 2018). Also, the literature suggests that EE can inhibit activity in the dorsal horn to relieve neuropathic pain (Tai et al., 2021), leading us to investigate whether it can produce the same effects for visceral pain. We showed that exposure to EE inhibits neuronal activation to colorectal distension at the dorsal horn. Chronic stress is also known to downregulate intestinal tight junction proteins leading to increased intestinal permeability (Zheng et al., 2017). Here we showed that stress does cause increased colonic permeability and exposure to EE can prevent this effect. However, the mechanisms behind this interaction are still unclear. EE could be acting directly on the dorsal horn and colon tissue to prevent visceral hypersensitivity, or EE could also be acting only in the brain and reverses stress-induced CeA hyperactivity, which in-turn lowers HPA-axis activity and reverses central and enteric neuron sensitization, hence reduced p-ERK activity, which in turn causes inhibits stress-mediated increases in colonic permeability. Further studies will be required to understand the exact etiology and role of EE in the dorsal horn and colon tissue. Estrogen can also have an effect on pain sensation and so we measured the estrous cycle of these females at the time of the VMR measurements but we did not see any significant effects of estrous cycle on stress-induced visceral or somatic hypersensitivity which is consistent with other studies in the literature (Terner et al., 2005).
In conclusion, our results indicate that pre-exposure to EE before and during chronic stress is sufficient to prevent stress-induced changes in the colon, spinal cord and amygdala that are associated with visceral and somatic hypersensitivity. The translational relevance of this study indicates that behavioral therapies could counteract the stress-induced physiological changes that can trigger symptoms in IBS patients. Our data reveals multiple pathways via which EE can be preventing stress induced pathology, suggesting cognitive and behavioral therapies could be beneficial for individuals at risk for developing chronic stress-induced visceral pain or people who anticipate recurring exposure to stressful situations.
Acknowledgements
Dr. Greenwood-Van Meerveld would like to acknowledge the funding provided by her Department of Veterans Affairs Merit Grant – I01BX002188-03. Dr. Greenwood-Van Meerveld is also the recipient of a Senior Research Career Scientist award (1IK6BX003610-01) from the Department of Veterans Affairs.
Footnotes
Declaration of Competing Interest
None of the authors have any conflicts of interest.
References
- Bisson J, Andrew M, 2007. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst. Rev 3, Cd003388. [DOI] [PubMed] [Google Scholar]
- Castro MM, et al. , 2012. The cognitive behavioral therapy causes an improvement in quality of life in patients with chronic musculoskeletal pain. Arq. Neuropsiquiatr 70 (11), 864–868. [DOI] [PubMed] [Google Scholar]
- Chan EK-H, 2006. Efficacy of cognitive-behavioral, pharmacological, and combined treatments of depression: A meta-analysis. In: ProQuest Information & Learning: US, p. 2218. [Google Scholar]
- Chang L, et al. , 2009. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil 21 (2), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandi E, et al. , 2018. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int. J. Dev. Neurosci 67, 19–32. [DOI] [PubMed] [Google Scholar]
- David D, Cristea I, Hofmann SG, 2018. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front. Psychiatry 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doiron RC, et al. , 2017. Childhood bladder and bowel dysfunction predicts irritable bowel syndrome phenotype in adult interstitial cystitis/bladder pain syndrome patients. Can. Urol. Assoc. J 11 (8), 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy RC, et al. , 2003. Visceral and cutaneous hypersensitivity in Persian gulf war veterans with chronic gastrointestinal symptoms. Pain 102 (1–2), 79–85. [DOI] [PubMed] [Google Scholar]
- Garland EL, et al. , 2012. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J. Behav. Med 35 (6), 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbovan C, Plamondon H, 2013. Environmental enrichment in female rodents: considerations in the effects on behavior and biochemical markers. Behav. Brain Res 253, 178–190. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, et al. , 2001. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 893 (1–2), 135–142. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Terenius L, Thorén P, 1990. Cerebrospinal fluid immunoreactive beta-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat. Regul. Pept 28 (2), 233–239. [DOI] [PubMed] [Google Scholar]
- Hong S, Zheng G, Wiley JW, 2015. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology 148 (1), p. 148–157.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttenrauch M, Salinas G, Wirths O, 2016. Effects of long-term environmental enrichment on anxiety, memory, hippocampal plasticity and overall brain gene expression in C57BL6 mice. Front. Mol. Neurosci 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Herman JP, 2008. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. N. Y. Acad. Sci 1148, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Greenwood-Van Meerveld B, 2015. Knockdown of steroid receptors in the central nucleus of the amygdala induces heightened pain behaviors in the rat. Neuropharmacology 93, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Tran L, Greenwood-Van Meerveld B, 2015. Knockdown of corticotropin-releasing factor in the central amygdala reverses persistent viscerosomatic hyperalgesia. Transl. Psychiatry 5 (3), e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, et al. , 2018. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology 155 (1), 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M, 2011. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J. Neurosci 31 (16), 6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon C, et al. , 2018. Linaclotide inhibits colonic and urinary bladder hypersensitivity in adult female rats following unpredictable neonatal stress. Neurogastroenterol. Motil 30 (10), e13375. [DOI] [PubMed] [Google Scholar]
- Ligon CO, Hannig G, Greenwood-Van Meerveld B, 2021. Peripheral guanylate cyclase-C modulation of corticolimbic activation and corticotropin-releasing factor signaling in a rat model of stress-induced colonic hypersensitivity. Neurogastroenterol. Motil 33 (3), e14076. [DOI] [PubMed] [Google Scholar]
- Louwies T, et al. , 2019. Targeting epigenetic mechanisms for chronic visceral pain: a valid approach for the development of novel therapeutics. Neurogastroenterol. Motil 31 (3), e13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerveld BG-V, Johnson AC, 2018. Mechanisms of stress-induced visceral pain. J. Neurogastroenterol. Motil 24 (1), 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi EN, et al. , 2018. Linaclotide attenuates visceral organ crosstalk: role of guanylate cyclase-C activation in reversing bladder-colon cross-sensitization. J. Pharmacol. Exp. Ther 366 (2), 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano R, et al. , 2019. Loss of environmental enrichment elicits behavioral and physiological dysregulation in female rats. Front. Behav. Neurosci 12, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B, 2007. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am. J. Physiol. Gastrointest. Liver Physiol 292 (6), G1622–G1629. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B, 2012. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am. J. Physiol. Gastrointest. Liver Physiol 302 (2), G260–G266. [DOI] [PubMed] [Google Scholar]
- Nakajima K, et al. , 2009. The nociceptive mechanism of 5-hydroxytryptamine released into the peripheral tissue in acute inflammatory pain in rats. Eur. J. Pain 13 (5), 441–447. [DOI] [PubMed] [Google Scholar]
- Orock A, et al. , 2020. Environmental enrichment prevents chronic stress-induced brain-gut axis dysfunction through a GR-mediated mechanism in the central nucleus of the amygdala. Neurogastroenterol. Motil 32 (6), e13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orock A, Yuan T, Greenwood-Van Meerveld B, 2021. Importance of non-pharmacological approaches for treating irritable bowel syndrome: mechanisms and clinical relevance. Front. Pain Res 1(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orock A, et al. , Environmental enrichment prevents chronic stress-induced brain-gut axis dysfunction through a GR-mediated mechanism in the central nucleus of the amygdala. Neurogastroenterol. Motil n/a(n/a): p. e13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JD, et al. , 2013. A randomized controlled pilot study of a cognitive-behavioral therapy approach for painful diabetic peripheral neuropathy. J. Pain 14 (5), 475–482. [DOI] [PubMed] [Google Scholar]
- Peery AF, et al. , 2012. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143 (5), p. 1179–1187.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusator DK, Greenwood-Van Meerveld B, 2016. Sex-related differences in pain behaviors following three early life stress paradigms. Biol. Sex Differ 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusator DK, Greenwood-Van Meerveld B, 2017. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain 158 (2), 296–305. [DOI] [PubMed] [Google Scholar]
- Quigley EMM, et al. , 2018. Better understanding and recognition of the disconnects, experiences, and needs of patients with irritable bowel syndrome with constipation (BURDEN IBS-C) study: results of an online questionnaire. Adv. Ther 35 (7), 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, 2009. Visceral pain: the neurophysiological mechanism. Handb. Exp. Pharmacol 194, 31–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwara AJ, et al. , 2012. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behav. Brain Res 234 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater AM, Cao L, 2015. A protocol for housing mice in an enriched environment. J. Vis. Exp 100, e52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg NJ, et al. , 2011. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology 114 (4), 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk K, et al. , 2016. Endogenous opioids regulate glucocorticoid-dependent stress-coping strategies in mice. Neuroscience 330, 121–137. [DOI] [PubMed] [Google Scholar]
- Tai WL, et al. , 2021. Additive effects of environmental enrichment and ketamine on neuropathic pain relief by reducing glutamatergic activation in spinal cord injury in rats. Front. Neurosci 15 (176). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QL, Lin GY, Zhang MQ, 2013. Cognitive-behavioral therapy for the management of irritable bowel syndrome. World J. Gastroenterol 19 (46), 8605–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner JM, Lomas LM, Picker MJ, 2005. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J. Pain 6 (6), 372–383. [DOI] [PubMed] [Google Scholar]
- Tran L, et al. , 2013. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 38 (6), 898–906. [DOI] [PubMed] [Google Scholar]
- Tran L, et al. , 2015. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol. Psychiatry 20 (10), 1219–1231. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA, 2006. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am. J. Phys. Renal Phys 290 (6), F1478–F1487. [DOI] [PubMed] [Google Scholar]
- Watkins LE, Sprang KR, Rothbaum BO, 2018. Treating PTSD: a review of evidence-based psychotherapy interventions. Front. Behav. Neurosci 12, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH, et al. , 2004. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 53 (11), 1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JW, et al. , 2020. Histone H3K9 methylation regulates chronic stress and IL-6-induced colon epithelial permeability and visceral pain. Neurogastroenterol. Motil 32 (12), e13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GZ, et al. , 2020. Valproate reverses stress-induced somatic hyperalgesia and visceral hypersensitivity by up-regulating spinal 5-HT(2C) receptor expression in female rats. Neuropharmacology 165, 107926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T-Y, et al. , 2015. Risk for irritable bowel syndrome in fibromyalgia patients: a national database study. Medicine 94 (10), e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, et al. , 2016. Hippocampal microglial activation and glucocorticoid receptor down-regulation precipitate visceral hypersensitivity induced by colorectal distension in rats. Neuropharmacology 102, 295–303. [DOI] [PubMed] [Google Scholar]
- Zheng G, et al. , 2017. Chronic stress and intestinal barrier dysfunction: glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci. Rep 7 (1), 4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhang B, Verne GN, 2009. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146 (1–2), 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G, et al. , 2005. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr. Res 77 (1), 1–9. [DOI] [PubMed] [Google Scholar]


