Abstract
Lipid- and lipoprotein-modifying therapies have expanded substantially in the last 25 years, resulting in reduction in the incidence of major adverse cardiovascular events. However, no specific lipoprotein(a) [Lp(a)]-targeting therapy has yet been shown to reduce cardiovascular disease risk. Many epidemiological and genetic studies have demonstrated that Lp(a) is an important genetically determined causal risk factor for coronary heart disease, aortic valve disease, stroke, heart failure, and peripheral vascular disease. Accordingly, the need for specific Lp(a)-lowering therapy has become a major public health priority. Approximately 20% of the global population (1.4 billion people) have elevated levels of Lp(a) associated with higher cardiovascular risk, though the threshold for determining ‘high risk’ is debated. Traditional lifestyle approaches to cardiovascular risk reduction are ineffective at lowering Lp(a). To address a lifelong risk factor unmodifiable by non-pharmacological means, Lp(a)-lowering therapy needs to be safe, highly effective, and tolerable for a patient population who will likely require several decades of treatment. N-acetylgalactosamine-conjugated gene silencing therapeutics, such as small interfering RNA (siRNA) and antisense oligonucleotide targeting LPA, are ideally suited for this application, offering a highly tissue- and target transcript-specific approach with the potential for safe and durable Lp(a) lowering with as few as three or four doses per year. In this review, we evaluate the causal role of Lp(a) across the cardiovascular disease spectrum, examine the role of established lipid-modifying therapies in lowering Lp(a), and focus on the anticipated role for siRNA therapeutics in treating and preventing Lp(a)-related disease.
Keywords: Lipoprotein(a), Cardiovascular disease, RNA interference, Therapeutics, Valvular disease
Graphical Abstract
1. Overview: the current status of Lp(a) in cardiovascular disease
1.1 Lipoprotein(a)—emergence as a risk factor
Lipoprotein(a) [Lp(a)] was first identified and reported in 19631 with evidence supporting its role as a cardiovascular (CV) risk factor accumulating steadily over the last five decades. However, the most informative insights into its causal role in risk for CV disease (CVD) have been generated during the last decade. Much of these data have been generated from large population-based observational studies2,3 and from post hoc analyses of trials studying other lipid-lowering agents.4,5 These analyses demonstrate a log-linear relationship between Lp(a) and risk of myocardial infarction (MI), stroke,3 and peripheral arterial disease (PAD).6 Although effective interventions are now available for many other lipid-related CV risk factors, elevated Lp(a) remains a largely untreatable dyslipidaemia.
Following the introduction of HMG-CoA reductase inhibitors (statins) in 1987, lipid-modifying therapies for the management of CVD have focused largely on modification of low- and high-density lipoprotein cholesterol (LDL-C and HDL-C) and triglycerides. Large randomized outcome studies have demonstrated important benefits of statins,7 ezetimibe,8 and fibrates9,10 for reducing risk of CV events, chiefly through lowering circulating levels of LDL-C. More recently, novel therapeutic targets, such as proprotein convertase subtilisin/kexin type 9 (PCSK9)11,12 and ATP citrate lyase, the target of bempedoic acid, have shown efficacy13 in lowering LDL-C. There have also been notable failures, most prominently niacin and cholesteryl ester transfer protein (CETP) inhibitors. While these approaches to modulation of HDL-C and other lipid fractions have not proved successful, HDL-C remains a potentially valuable route to reduction of CVD risk.14 This arsenal of lipid-modifying therapies offers excellent reduction in risk of CVD events through reduction in LDL-C and, possibly, triglycerides. However, existing therapies provide only modest reductions in Lp(a). Consequently, for many patients who have either effective control of other risk factors and persistently raised Lp(a), or for whom Lp(a) is their sole risk factor, a large portion of their risk remains unaddressed.
2. The Lp(a) particle and its role in health and disease
The biochemistry and pathophysiological properties of Lp(a) have been described in detail previously;15,16 here, we review the key features relevant to Lp(a)-lowering therapy. The Lp(a) particle is an apolipoprotein B (apoB)-containing lipoprotein similar to LDL, but with apolipoprotein(a) [apo(a)] covalently bound to apoB-100 on the surface of the particle.17 Apo(a) itself is a protein encoded by the LPA gene (chromosome 6q25.3) that bears substantial homology with plasminogen (PLG, chromosome 6q26). Lp(a) and PLG are characterized by kringle motifs, which are protein domains that fold into loops stabilized by three disulphide linkages. Kringle motifs I (KI)–V (KV) are present in PLG, whereas only KIV and KV are found in Lp(a).17 In contrast to kringle IV of PLG, in apo(a) KIV is present in 10 subtypes (KIV 1–10) with a single copy of each; the exception is KIV2, which can vary in copy number from 2 to more than 40 identical copies underlying the substantial size heterogeneity of apo(a) in the population. Thus, Lp(a) exists as multiple isoforms, the size of which is determined by the number of repeated KIV2 motifs. The number of repeats is genetically encoded by well-characterized variants at the LPA locus.18 Both alleles of these variants contribute to the kringle repeat number and serum levels of Lp(a) in individual patients. The Lp(a) isoform size has two important clinical implications. First, smaller isoforms (i.e. those with fewer KIV repeats) have greater atherogenic potential than the same circulating concentration of Lp(a) particles containing larger proteins with more KIV repeats.19 Second, the number of KIV repeats can lead mass-based assays (i.e. those reporting circulating concentration in mg/dL) to underestimate the true Lp(a) concentration in the presence of small Lp(a) isoforms. For this reason, the use of molar concentration-based assays (i.e. particle concentration-based reporting in nmol/L) has been increasingly advocated since these account adequately for isoform size, mitigating against underestimating levels of small Lp(a) isoforms or overestimating of large Lp(a) isoforms.20,21
Lp(a) has physiological and pathological roles. Its role in health is diverse and includes modulation of coagulation through its interactions with PLG and the fibrinolytic system, immune cell interaction with the vascular endothelium, and proliferation of vascular smooth muscle and adhesion molecules.22 Lp(a) is a major carrier of oxidized phospholipid (OxPL) in human plasma.23 OxPLs are mediators of potent proatherogenic and pro-inflammatory effects and are thought to account for a significant proportion of the CV risk attributable to Lp(a).24 LPA variants [genetically determining increased plasma Lp(a) levels and apo(a) isoform size] strongly associate with OxPL–apoB concentration.19 In disease, Lp(a) also has several roles, of which three are most prominent: (i) Lp(a) crosses the vascular endothelium and contributes to the formation and progression of atherosclerotic plaques.25 (ii) In the event of atheromatous plaque rupture, the prothrombotic properties of Lp(a)—including inhibition of plasminogen activation to inhibit fibrinolysis—potentiate thrombus formation and contribute to the onset of MI or ischaemic stroke. (iii) Lp(a) crosses the intima of the aortic valve leaflets, promoting inflammation, calcification, and, eventually, aortic valve stenosis.25 The role of Lp(a) in specific manifestations of CVD is discussed in more detail below.
3. Lp(a) epidemiology and relationship with CVD risk
3.1 The pathological relevance of Lp(a) for CVD
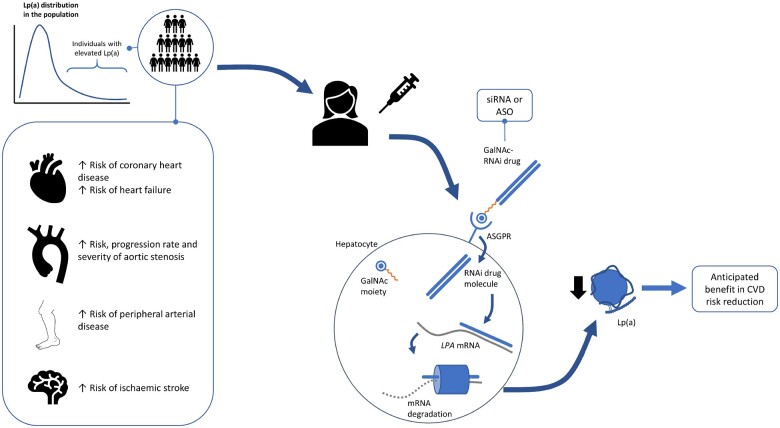
The profile of Lp(a) as a CV risk factor has several important differences compared with more established risk factors. Unlike LDL-C, blood pressure, and body mass index (BMI), the population distribution of circulating Lp(a) concentration is positively skewed rather than normally distributed. The majority of the population (∼80%) have concentrations of Lp(a) below ∼50 mg/dL (∼125 nmol/L),25 including a group of individuals with very low or undetectable circulating Lp(a).26 Importantly, those individuals are not known to experience any adverse consequences of their low Lp(a) thereby providing reassurance regarding the safety of large reductions using therapeutic interventions seeking to recapitulate this pharmacologically.27 The population distribution of Lp(a) also differs by ancestral group. People of African ancestry have, on average, higher circulating Lp(a) than people of European or Asian ancestry,28,29 raising important considerations for risk prediction and modification given the inter-ancestral variation of other risk factors, such as hypertension and dysglycaemia.
A person’s genotype regulates Lp(a) kringle number and isoform size; kringle IV repeats are the most important, but not the sole, determinant of serum concentrations, with LPA genotype accounting for >90% of circulating Lp(a) variance.30 Circulating Lp(a) concentration is determined at the time of conception and, with few exceptions, such as the influence of renal dysfunction and perimenopausal hormonal changes,31,32 varies little during their lifetime. In contrast, the development of other risk factors, such as LDL-C, blood pressure, and BMI, is predominantly a feature of middle age and beyond.33 These conventional risk factors often develop with aging and are subject to the influence of behaviour, diet, and concomitant disease. In the case of Lp(a), raised levels are detectable and fixed much earlier in life34,35 and the circulating concentration remains relatively stable as a person ages. The adverse effects of raised Lp(a) on disease risk are therefore present from an earlier stage in life than many other risk factors. Thus, earlier intervention to lower Lp(a) before the accumulation of additional risk later in life may offer greater benefit for prevention. Evidence suggests that the relationship of elevated Lp(a) with risk of recurrent CVD events may not be equivalent to that with risk of first CVD events.36–38 However, as discussed below, clinical trials are underway to provide deeper insights into those relationships.
Our understanding of the relevance of Lp(a) in many manifestations of CVD comes from observational studies, population genetic studies, and a few interventional trials. Observational studies, particularly those with longitudinal information on incident disease provide important epidemiological insights about the relationship between higher Lp(a) and higher risk of CV events, but do not permit causal inference. Genetic studies that evaluate associations of genetic variants at the LPA locus with Lp(a) concentrations enable causal inference using the Mendelian randomization paradigm39 and have been instrumental in elucidating the potential for Lp(a) lowering as a promising therapeutic strategy in CVD.40–42 Finally, randomized trials of lipid-modifying agents, such as statins and PCSK9 inhibitors with some effect on Lp(a) have offered some limited information on the value of Lp(a) lowering, although interpretation of these data is limited by the lack of specificity of the drugs tested for an effect on Lp(a).43–45
3.2 Coronary heart disease
The relationship between raised Lp(a) and risk of coronary heart disease (CHD) events is strongly supported by multiple observational studies. A large meta-analysis of longitudinal observational studies reported a risk ratio of 1.16 [95% confidence interval (95% CI) 1.11–1.22] for CHD for each 1 standard deviation higher Lp(a), which was equivalent to 3.5-fold higher Lp(a) in the population studied.3 This strong relationship was confirmed in a large Danish cohort with participants with Lp(a)≥20 mg/dL (∼300 nmol/L)—representing the 95th centile—demonstrating a 20% higher 10-year absolute risk of MI in female patients and 35% higher in males over 60 years in age who smoked and had hypertension.2
Genetic studies have supported a causal association underlying these observational estimates of the relationship between Lp(a) and CHD. Although circulating Lp(a) levels may differ markedly between ancestral groups,46 the Lp(a)–CHD risk association is observed in multiple populations.29 Genome-wide association studies (GWASs) have identified the LPA locus as strongly associated with risk of coronary disease. Two variants (rs10455872 and rs3798220) are both highly associated with increased plasma Lp(a), reduced LPA copy number (i.e. reduced KIV2 repeats), and smaller Lp(a) lipoprotein size—associated with an odds ratio for coronary artery disease of 1.5 for one variant and 2.57 for two or more variants.40 Mendelian randomization studies show a genetically determined doubling of Lp(a) plasma levels results in a 22% increased risk of MI during a maximum of 16 years of follow-up, consistent with a causal relationship between lifelong elevation in Lp(a) and MI.41 Two Mendelian randomization studies have sought to quantify the likely magnitude of Lp(a) reduction in a clinical trial equivalent to lower LDL-C by 1 mmol/L (38.67 mg/dL) with respect to CHD risk reduction.47,48 The estimates of the equivalent Lp(a) reduction ranged from a 65.7 mg/dL affording ∼22% relative risk reduction,48 to 101.5 mg/dL affording ∼24% CHD relative risk reduction.47 These estimates rest on the assumption that lifelong genetically determined circulating LDL-C and Lp(a) levels have approximately equivalent effects on CHD risks. While this assumption is supported by epidemiological data, it suggests that even large Lp(a) reductions in Lp(a) may confer only modest CHD risk reduction. However, the data used to generate these estimates came from general population samples with relatively low median baseline Lp(a) (range: 11–43.3 mg/dL). The potential for benefit in patients at the higher end of the skewed Lp(a) distribution (i.e. >50 mg/dL) may be greater. Furthermore, the findings of Mendelian randomization analyses reflect lifelong effects of genetic variants, which may differ in magnitude from the effects of drug treatment which is, in general, of relatively shorter duration and greater potency.
3.3 Aortic valve stenosis
Lp(a) shows a strong positive relationship with aortic stenosis (AS) in observational and genetic studies.49,50 Elevated Lp(a) and OxPL-apoB are independently associated with more rapid progression of stenosis in patients with mild-moderate AS, with patients in the top tertile of Lp(a) or OxPL-apoB exhibiting increased valvular calcification activity—measured by 18F-NaF PET/CT—compared with those in the lower tertiles, and a higher risk of aortic valve replacement (AVR) or cardiac death.49,51 Notably, the relationship between Lp(a) and OxPL levels with rate of AS progression appears to be linear rather than a threshold association.51 In a post hoc, exploratory analysis of the FOURIER trial of the PCSK9 inhibitor evolocumab, higher Lp(a), but not higher LDL-C, was associated with greater likelihood of new AS diagnoses or AS worsening over ∼2 years of follow-up.52 Genetic data also implicate Lp(a) as a key mediator in the pathogenesis and development of AS. A GWAS of aortic valve calcium burden detected by CT imaging identified a variant in LPA (rs10455872) 40 that was associated with approximately a two-fold increase in odds of aortic valve calcification. The same variant was significantly associated with incident AS.53 Using a Mendelian randomization approach, the same study identified a relationship between genetically determined higher Lp(a) levels and higher likelihood of the presence of aortic valve calcification. The association between this and other LPA variants, serum Lp(a) levels and AS has been replicated by other studies,54 including those employing a prospective Mendelian randomization design.55 Recent Mendelian randomization analyses have suggested a causal role for Lp(a) in other lesions of the aortic and mitral valves.42
3.4 Other manifestations of CVD
Beyond the two major disease associations described above, Lp(a) plays an important role in the risk of several other CVDs. Higher circulating Lp(a) levels associate with higher risk of ischaemic stroke in cohorts across ancestral groups.56–58 Notably, the association with ischaemic stroke was observed in individuals without atrial fibrillation (AF) but not in those with AF,59 which may suggest Lp(a) promotes an atheroembolic mechanism of cerebral infarction rather than a cardioembolic aetiology. Genetic studies also support a causal relationship between Lp(a) levels and ischaemic stroke risk.60 PAD associates strongly with higher Lp(a) levels, as does greater likelihood of peripheral revascularization in people with established PAD, suggesting Lp(a) has a role in not only the onset of disease, but also its progression.6,53,61,62 The role for Lp(a) in the epidemiological relationship with PAD is also supported by genetic studies. In a large GWAS of PAD risk, a single nucleotide polymorphism (SNP) in LPA was the variant most strongly associated with PAD risk.63 Heart failure is a disease phenotype closely related to CHD and AS and displays an observational relationship with Lp(a).64,65 Large-scale genetic studies support this relationship66 but the association between LPA genotype and heart failure is attenuated after adjustment for accompanying CHD, suggesting that the role of Lp(a) in heart failure is largely mediated through coronary atherothrombotic disease. LPA genotype and elevated Lp(a) were associated with younger age at death of participants’ parents in a large GWAS study, with the same genetic variant associated with higher Lp(a) levels and higher CHD risk.67
Taken together, the diseases in which Lp(a) plays an important causal role make a compelling case for the potential benefit to individual and population health of specific, safe, and effective Lp(a)-lowering therapy.
3.5 Lp(a) and familial hypercholesterolaemia
Familial hypercholesterolaemia (FH) is a collection of disorders characterized by very high circulating LDL-C levels largely caused by mutations at the LDLR, PCSK9, and APOB genetic loci. While high LDL-C in many patients with FH can be managed effectively with pharmacological and lifestyle measures, raised Lp(a) can confer a sizable burden of additional risk.68 In patients with heterozygous FH, median Lp(a) has been reported to be up to almost two-fold higher than the general population,68 although published findings on this subject vary,22,69,70 and raised Lp(a) is an independent predictor of CVD risk in patients with FH.70 These findings have led to recommendations to incorporate Lp(a) measurement in the systematic cascade screening of patients with molecularly defined FH, identifying 1 new case for every 2.4 screened.71 Patients with FH therefore carry two potent, genetically determined drivers of CVD risk,72 including risk of both CHD73 and AS.74 Patients with FH are therefore a group who may benefit from novel Lp(a)-lowering therapies.
4. Current options for management of Lp(a)-mediated CV risk
As summarized in Table 1, the effects of established lipid-modifying agents on Lp(a) are both modest and variable, with only trials of the monoclonal antibody PCSK9 inhibitors reporting a potentially clinically meaningful reduction in Lp(a). While PSCK9 inhibitors demonstrate some Lp(a)-lowering effect, the magnitude of the effect is likely insufficient to adequately reduce Lp(a)-mediated CVD risk in the population.47,48 The CETP inhibitors anacetrapib and evacetrapib reduced Lp(a) by 25% and 40%, respectively.75,76 These drugs did not demonstrate an overall benefit for CVD risk reduction but, importantly, were evaluated in trials that did not enrol patients on the basis of elevated Lp(a).
Table 1.
Effects of established lipid-modifying agents on Lp(a)
| Drug or drug class | Effect on Lp(a) |
|---|---|
| Statins | Substantial heterogeneity between statin drugs in a meta-analysis of RCTs. Effects ranged from 13% reduction (95% CI 10–15%) for atorvastatin in the CARDS study82 to 15% increase (95% CI 13–17%) for simvastatin in the 4S study.131 No overall effect when data meta-analysed leading to uncertainty of the effect of statins.43 |
| Ezetimibe | No significant effect in a meta-analysis of RCTs.44 |
| Niacin | Reduced by 22.9% (95% CI 18.5–22.9%) in a meta-analysis of RCTs.132 Effect was not dose-dependent. |
| Fibrates | No significant effect in a meta-analysis of RCTs.133 |
| Bempedoic acid | No significant effect on Lp(a) in phase 2 study.134 |
| PCSK9 inhibitor monoclonal antibodies | Median Lp(a) reduction with evolocumab was 26.9% [interquartile range (IQR) 6.2–46.7%) in the FOURIER study.5 Median reduction with alirocumab was 25.6% (IQR 7.2–42.7%) in pooled phase 3 trial data.135 This was confirmed in a pooled analysis.45 |
| Inclisiran | 18.6% reduction from baseline in the phase 3 ORION-11 study.136 |
| Mipomersen | Median Lp(a) reduction in pooled phase 3 trials was 26.4% (IQR 5.4–42.8%).137 |
| CETP inhibitors | Evacetrapib reduced Lp(a) by up to 40% in a phase 2 study.76 Anacetrapib reduced Lp(a) by 34.1% in a small phase 2 study.138 |
Lipoprotein apheresis offers an effective means of lowering apoB-containing lipoproteins, including LDL and Lp(a).77 Apheresis is an intensive treatment modality that requires patients to attend a specialist centre at 1–2 weekly intervals for treatment sessions lasting between 90 min and 4 h. Apheresis is successful in reducing both Lp(a) and LDL-C from baseline by ∼70% and 65%, respectively, although the reduction in Lp(a) follows a saw-tooth pattern with modest time-averaged reductions.78 Apheresis is recommended by clinical guidelines in only a small number of countries including Germany. In general, apheresis is offered to patients in the context of high Lp(a) (e.g. >60 mg/dL in Germany) with evidence of progressive CVD or recurrent CVD events despite optimal management. Apheresis has additional benefits beyond lipoprotein reduction, including lowering plasma viscosity79 and removal of pro-inflammatory mediators.80 While definitive data from randomized trials of apheresis in CVD event prevention are not available, prospective non-randomized studies, such as Pro(a)LiFe, which included 170 patients treated with apheresis, have suggested substantial reductions in event rates.78 In addition to the inconvenience for patients, apheresis carries some risk of adverse events. The majority of these are related to vascular access, and transient haemodynamic effects of extracorporeal circulation, such as dizziness.78,79
In addition to the treatment effects on Lp(a) reported in randomized trials of lipid-lowering drugs, these trials have shown interactions between baseline circulating Lp(a) levels and the effects of treatment on CV risk. In the JUPITER study, Lp(a) levels were associated with residual risk of CVD in participants treated with rosuvastatin, independently of LDL-C lowering.4 The ODYSSEY-OUTCOMES study reported that the effect of alirocumab mediated by Lp(a) reduction reduced major adverse cardiovascular events following an acute coronary syndrome independent of the effect mediated by LDL-C lowering.81 While encouraging, these post hoc findings from a trial of a drug principally targeting LDL-C will benefit from dedicated investigation in trials of specific Lp(a)-lowering agents. Pharmacogenetic analyses in statin RCTs have demonstrated an effect of genotype at the LPA locus (and for the rs10455872 variant in particular). In the CARDS and PROSPER studies, Lp(a)-raising alleles of the rs10455872 SNP were associated with a smaller reduction of LDL-C on atorvastatin treatment; in CARDS, however, there was no evidence of interaction between genotype and CVD end points.82 A similar finding was observed with simvastatin treatment in the Heart Protection Study,83 with rosuvastatin in the JUPITER study84 and in a population-based observational study in Scotland.85 Importantly, these observations may be confounded by the methods used to measure LDL-C, the results of which likely incorporate the cholesterol content of both LDL and Lp(a) particles (constituting ∼30–45% of the latter) and therefore do not entirely reflect the specific influence of LPA genotype on LDL-C.83 In a population-based GWAS of CHD events in individuals receiving statin treatment, the LPA rs10455872 SNP was the variant most significantly associated with higher CHD risk in statin-treated participants, an association that was independent of statin-induced LDL-C reduction.86
Risk for AS merits particular mention given the important role Lp(a) appears to play in the development and progression of this valve disorder. No pharmacotherapy is currently available to slow or reverse the progression of AS. Currently, the only option available for patients with severe symptomatic AS is surgical or transcatheter aortic valve replacement.87 In the stages of AS before disease progression necessitates AVR, the management of AS comprises longitudinal surveillance of valve function, management of left ventricular dysfunction and modification of CHD risk which often coexists with AS.88 RCTs investigating the effects of statin therapy in AS have not demonstrated improvements in aortic valve pressure gradient, jet velocity or valve area, or progression to valve replacement.89
Effective management of a lifelong risk factor, such as Lp(a) requires an intervention that is safe for long-term use, effective, and acceptable to the patients who will likely require many years of treatment. For existing oral lipid-modifying therapies administered daily, treatment adherence and persistence can be challenging for many patients. Available observational data suggest that lower treatment adherence and persistence has a strong association with higher risk of CV events.90–92 Monoclonal antibody PCSK9 inhibitors can potentially address this challenge through longer dosing intervals of 2–4 weeks.93,94 Long-term drug therapy must also take account of the risk of adverse effects. For statins, the risk of muscle pain and weakness95 and new-onset type 2 diabetes96 remains a concern to many patients and prescribers. The ideal specific Lp(a)-lowering therapy would have as benign a safety profile as possible and its administration would place the minimum burden on the patient. A major factor in determining that burden is the frequency of administration. Lp(a)-lowering therapy with a RNA interference (RNAi) agent [e.g. small interfering RNA (siRNA)] offers an opportunity for a particularly durable and effective therapy.
5. Current clinical guidance on management of raised Lp(a)
Recent revisions of clinical guidelines for CVD risk management and lipid modification in Europe, the USA, Canada, and the UK have addressed the issue of Lp(a) as a risk factor. Notably, the focus of these guidelines is chiefly atherosclerotic and atherothrombotic disease, while aortic valve disease is not directly addressed. The joint American Heart Association/American College of Cardiology Task Force guidelines in 2018 identify circulating Lp(a) over 50 mg/dL (124 nmol/L) as a ‘risk enhancer’ for atherosclerotic CVD (ASCVD), which should be measured particularly if a patient has a family history of premature ASCVD.97 The Canadian Cardiovascular Society 2016 guidelines take a similar position, noting that elevated Lp(a) may be of particular value for risk modification in younger patients for whom Lp(a) represents an important lifetime risk factor but might not meet standard risk criteria for treatment.98 The Japanese Atherosclerosis Society 2017 guidelines for ASCVD prevention acknowledge Lp(a) as a CVD risk factor but do not include specific recommendations for Lp(a) measurement or the management of risk in people with raised Lp(a).99 The European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) 2019 guidelines acknowledge the promise of RNA-based therapeutics for Lp(a)-lowering and recommend that a single measurement of Lp(a) may help to identify people with very high inherited levels who may have a substantial lifetime risk of ASCVD. A high Lp(a) plasma level may also be helpful in further risk stratification of patients at high risk of ASCVD, in patients with a family history of premature CVD, and to determine treatment strategies in people whose estimated risk falls on the border of risk categories.100 Since Lp(a) remains stable throughout adulthood, a single measurement is adequate for screening and therefore the burden on patients and health services of undertaking screening is minimal. Heart UK, a charity supporting patients with hyperlipidaemia in the UK, released a position statement on Lp(a) in 2019.101 The statement is broadly in line with the recommendations of the ESC/EAS guidelines and also advocates measurement of Lp(a) in patients with calcific AS and those with equivocal but <15% 10-year risk of CVD events. Further, Heart UK recommend overall reduction of CVD risk including non-HDL-C lowering and consideration of apheresis for the management of patients with elevated Lp(a) levels. The National Lipid Association (NLA) in the USA released a similar position paper in 2019.21 The NLA statement makes the important observation that measurement methods for Lp(a) are not yet standardized and that more evidence is needed to support Lp(a) thresholds for determining higher CVD risk in subpopulations defined by age, sex, ancestry, and comorbidity.
6. RNAi as an emerging treatment modality for lowering Lp(a)
RNAi is an emerging treatment modality with the potential to address Lp(a)-mediated disease risk. Several RNAi drugs, including siRNAs and antisense oligonucleotides (ASOs), against other therapeutic targets have demonstrated effectiveness in clinical studies and a few have received regulatory approval, such as the two Alnylam siRNA therapeutic agents Onpattro (patisiran; targeting hereditary ATTR amyloidosis, formulated as lipid nanoparticles) and Givlaari (givosiran; targeting acute hepatic porphyria, a GalNAc-conjugated siRNA, see below).
6.1 RNAi therapies: mechanism of action and utility in lipid-modifying therapy
Oligonucleotide therapeutics (OT) is a term that by now covers a broad range of modalities, most of which are synthetic oligonucleotides containing sequences of native and modified RNA and DNA nucleosides with phosphorothioate (PS) or modified internucleotide linkages. RNAi drugs, a subset of OT, exploit natural mechanisms for regulating gene expression in human cells.102,103 Unlike gene therapy (e.g. Luxturna for inherited retinal dystrophy104) which permanently modifies the patient’s genome, RNAi results in temporary and reversible down-regulation of gene expression. ASOs are single-stranded sequences of native and modified nucleotide molecules that bind to their target complementary RNA sequence via Watson–Crick base pairing. This binding leads to RNA degradation through RNase H enzyme activity, resulting in reduced protein production. Early generation ASOs were beset by challenges to their safety and effectiveness, including susceptibility to degradation by nucleases, poor tissue specific delivery, and a narrow therapeutic range due to class effect toxicities. Some of these challenges have been successfully overcome through chemical modifications to the oligonucleotide molecules and several ASO drugs have been successful in clinical trials. These include mipomersen105 for treatment of FH, volanesorsen for treatment of familial chylomicronaemia syndrome106 and notably an Lp(a)-lowering ASO, AKCEA-APO(a)-LRx.107
By contrast, siRNA drugs are double-stranded RNA molecules (Figures 1 and 2). Once inside the cell, the two RNA strands dissociate into sense and antisense strands by the action of the Argonaute 2 (AGO2) and the antisense strand gets inserted into, and ready to exert mRNA degrading activity in, RNA-induced silencing complex (RISC). The sense strand degrades and the antisense strand binds to its target mRNA sequence again using Watson–Crick base pairing. This binding induces cleavage of the target mRNA by AGO2 and degradation by exonucleases, resulting in reduced synthesis of its cognate protein. Regardless of whether the RNAi agent is a single-stranded ASO or a double-stranded siRNA, stretches of native RNA and DNA are quickly degraded in the circulation and inside cells, and much effort has been done to improve extra- and intracellular RNAi agent stability with retained or improved activity and safety profile. For siRNA, it has been clearly demonstrated that unless the siRNA is delivered encapsulated in a nanoparticle formulation, chemical modification of the siRNA sequence is required to avoid extracellular degradation and excessive renal clearance before the drug reaches its intended cell or tissue.103
Figure 1.
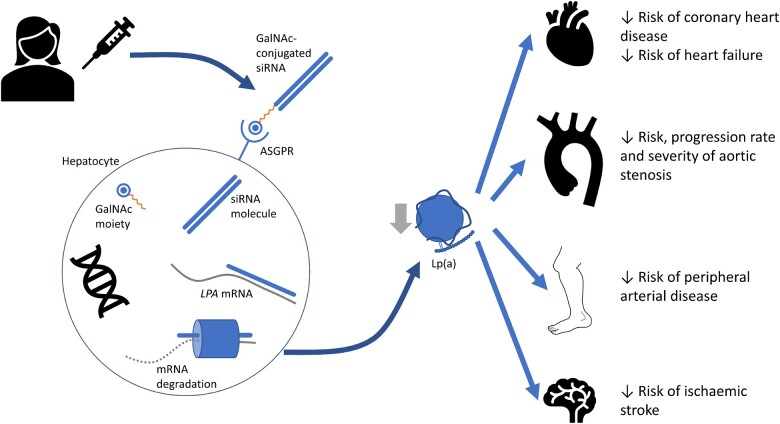
Mechanism of action of Lp(a)-lowering siRNA therapy, and potential clinical benefits. GalNAc–siRNA drugs lower circulating Lp(a) concentrations via the mechanisms described in more detail in Figure 2. Evidence from observational and genetic epidemiological studies supports a role for Lp(a) lowering as a means of reducing the risk of several types of cardiovascular disease, including atherosclerotic in the coronary and peripheral circulations, valvular disease and heart failure. siRNA, small interfering RNA; ASGPR, asialoglycoprotein receptor; LPA, apolipoprotein(a) gene; Lp(a), lipoprotein(a).
Figure 2.
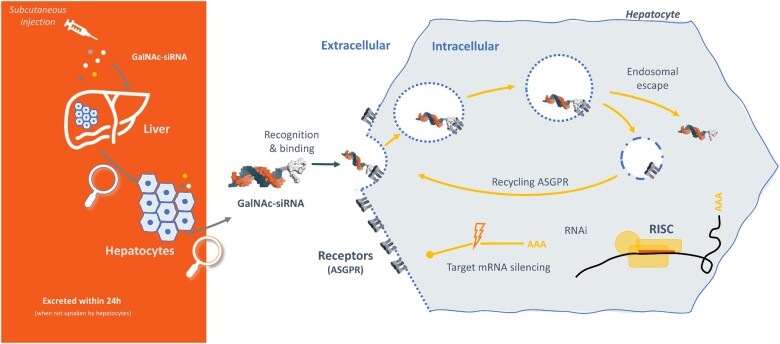
A detailed overview of GalNAc–siRNA mechanism of action in the hepatocyte. A GalNAc–siRNA drug is administered subcutaneously and the GalNAc moiety binds the ASGPR on the hepatocyte cell membrane. The drug conjugate enters the cell via an endosomal mechanism, following which it escapes into the cytosol and the ASGPR recycles to the cell surface. The siRNA sense and antisense strands dissociate, and the antisense strand binds its target sequence in the LPA gene mRNA. Binding of the antisense strand induces degradation of LPA mRNA, preventing its translation into apo(a) and thereby reducing the synthesis of Lp(a). GalNAc, N-acetyl-galactosamine; ASGPR, asialoglycoprotein receptor; RISC, RNA-induced silencing complex; RNAi, RNA interference; AAA, gene transcript.
RNAi molecules need to overcome several barriers in order to be efficacious drugs. First, they need to be stable enough in circulation not to get degraded before they reach their target cell/tissues, second, they need to reach, and be endocytosed into, their target cell/tissue, and third, they need to escape the vesicles of the endo/lysosomal pathway in order to reach their final location—cytosol or nucleus, depending on modality. ASOs, in particular, ASOs stabilized with a PS ‘backbone’, can enter many cells and tissues relatively freely due to cell surface protein binding followed by endocytosis.108,109 In contrast, the larger, double stranded siRNA molecules have a size and charge that strongly reduces non-specific, or unassisted, uptake in target cells and tissues. Different nanoparticle formulations, such as used in Onpattro, will increase siRNA uptake and activity for instance in liver. However, over the last 5–6 years focus has shifted to using direct conjugation of siRNA and ASOs to targeting moieties designed for binding to and internalization through cell-specific receptors The most successful and broadly used conjugation strategy to date, and delivery method pertinent to lipid modification, is conjugation of siRNA or ASO molecule to a cluster of sugars, N-acetylgalactosamines, or GalNAc. GalNAc is a ligand for the asialoglycoprotein receptors, which are found in large concentrations on the surface of hepatocytes with a high degree of cell- type specificity.110 Conjugation of an RNAi drug molecule to GalNAc clusters therefore directs the oligonucleotide near-exclusively to the hepatocytes where it can exert its gene silencing activity without incurring risks of doing so in other, unintended cell types. Recent examples of clinically successful GalNAc–siRNAs are givosiran (Givlaari), approved for the treatment of acute hepatic porphyria111 and fitusiran for treatment of haemophilia A & B,112 with GalNAc-conjugated ASOs also in development.
6.2 Safety of GalNAc–RNAi therapy
Given the central role for the liver in lipid metabolism, GalNAc-conjugated siRNAs present a valuable opportunity for treating dyslipidaemia. Inclisiran, a GalNAc-conjugated siRNA inhibitor of PCSK9 is under consideration for approval in several countries following successful phase 3 trials.113 Hepatocyte specificity is just one of the features of GalNAc–siRNA drugs that make them attractive candidates for long-term Lp(a)-lowering therapy. The GalNAc–siRNA drugs evaluated in clinical trials to date have demonstrated reassuring safety profiles. Inclisiran demonstrated a modest increase in injection site reactions compared with placebo in two Phase-3 trials that included 1561 participants,113 but no excess adverse events in either of these trials or a Phase-2 trial in 482 patients with FH.114 A similar safety profile was observed in a Phase-1 trial of givosiran.111 Critical to these safety profiles are the chemical modifications to the siRNA molecule. These modifications not only help the drug to avoid premature degradation but also reduce the risk of activating an innate immune response leading to adverse effects related to systemic inflammation.115 Risk of off-target binding and interference in the expression of unintended target genes remains a potential concern with RNAi drugs. This risk is mitigated through a careful in silico design process during which sequence similarity to off-target genes guide chemical modifications optimized to reduce risk of off-target effects.116 These features have overcome many of the difficulties faced by earlier generation RNAi drugs and help establish siRNAs as promising approach suitable for long-term use.
6.3 Durability of RNAi treatment effects
A prominent feature of treatment with inclisiran during clinical development is its long duration of action. In the Phase 3 ORION-10 and ORION-11 studies, inclisiran was administered on day 1, day 90, and every 6 months thereafter, resulting in reductions in LDL-C of ∼50% maintained at 17 months of follow-up.113 Prolonged treatment durations were also observed with givosiran111 and fitusiran,112 although the effects were not as durable as observed with inclisiran. Although the pharmacodynamic effect of GalNAc–siRNA drugs persist for weeks or months, these drugs are rapidly cleared from the plasma compartment, chiefly by renal elimination such that circulating levels can be undetectable 24 h after dosing.117 The long, or very long, duration of action after single injections of GalNAc-conjugated siRNAs appears to at least in part be explained by chemical modifications leading to increased stability under acidic conditions.118 As mentioned above, RNAi molecules must undergo endocytosis followed by release from endo/lysosomal compartments in order to achieve their pharmacological effect. It has been demonstrated that chemical modifications that lead to increased siRNA stability under acidic conditions (such as in late endosomes) are connected with increased duration of action in animal studies.118,119 One possible explanation is that increased siRNA stability in an acidic vesicular compartment leads to formation of an intracellular depot from which a small amount of siRNA is released over time—a hypothesis supported by evidence that chemical modifications leading to a more stable siRNA molecule has no effect on RNAi activity, compared to a less stable version of the same siRNA, once the antisense strand has been inserted in the RISC complex.118
This extended duration of action represents an ideal profile for long-term CVD risk reduction. Adherence and persistence are major challenges in optimizing the benefit for CVD risk-modifying drugs. In the case of subcutaneously administered monoclonal antibody inhibitors of PSCK9, dosed at 2- or 4-weekly intervals, adherence has been shown in real-world datasets to be ∼70–80%,94,120 while for statins adherence has been reported as low as 17.8%.121 It is anticipated that adherence to long-acting RNAi lipid-modifying drugs, administered less frequently, may be higher than for monoclonal antibodies, resulting in greater clinical benefits. The long duration of action may also provide logistical and financial benefits to healthcare systems derived from the need for less frequent dosing.
7. Potential risks of Lp(a)-lowering therapy
The anticipated benefits of reducing Lp(a) are considerable, however, few treatments are without safety concerns. Available clinical studies of Lp(a)-lowering therapies have provided some insights into the potential risks of Lp(a) reduction. However, insufficient person-years of treatment have accumulated to provide a full understanding of the potential adverse effects of lowering Lp(a). Theoretical risks related to the sequence homology with plasminogen exist and thrombosis-related adverse events will require carefully monitored in clinical studies of Lp(a)-lowering agents. Large-scale genetic studies have utility in identifying target-related adverse effects in the same way that they can highlight beneficial therapeutic effects.122 A large phenome-wide association study examined the associations between variants at the LPA locus and risk of a range of diseases and related biomarkers.123 This study confirmed the predicted risk-lowering effects for CHD, AS, heart failure, and PAD. It also identified no associations with potential adverse effects on risk of 28 other disease phenotypes including gastrointestinal, endocrine, neurological, musculoskeletal, respiratory, and neoplastic disease. The analysis also examined relationships between LPA genotype and several biomarkers of cardiometabolic disease, demonstrating effects on circulating lipids while detecting no effects on blood pressure, or markers of adiposity or glycaemic control. Interestingly, there was a strong association between Lp(a)-lowering variants and higher estimated glomerular filtration rate, accompanied by a modest association with lower risk of chronic kidney disease. In an Icelandic study that included individuals homozygous for loss-of-function LPA mutations and therefore very low circulating Lp(a) concentrations, such variants were associated with a modestly increased risk of type 2 diabetes. While this association merits careful monitoring clinical trials of Lp(a)-lowering drugs, the weight of evidence from observational and genetic epidemiological studies suggests that, as for statins,124,125 the CV benefits Lp(a)-lowering are likely to outweigh any potential risks related to diabetes.
8. Emerging Lp(a)-reducing RNAi therapeutics
Novel, specific, RNAi-based Lp(a)-lowering therapies have entered clinical development in recent years. These include pelacarsen [formerly TQJ230 and AKCEA-APO(a)-LRx], an ASO targeting LPA originally developed by Akcea Therapeutics now being developed by Novartis under licence; olpasiran (formerly AMG890 and ARC-LPA), a GalNAc-conjugated siRNA originally developed by Arrowhead Pharmaceuticals now being developed by Amgen; and, SLN360, a GalNAc-conjugated siRNA being developed by Silence Therapeutics. The current status of these three molecules is summarized in Table 2.
Table 2.
Lp(a)-lowering RNAi therapeutics in development
| Drug | RNAi technology | Industrial sponsor | Stage of development | Route of delivery | Efficacy data to-date | Safety data to-date |
|---|---|---|---|---|---|---|
| AKCEA-APO(a)-LRx | ASO | Novartis—Ionis/Akcea | Phase 3 | Sub-cutaneous injection | Dose-dependent Lp(a) lowering in Phase 2 clinical trial in adults with Lp(a) ≥60 mg/dL (≈≥150 nmol/L) and established CVD. Up to 80% reduction (75.1 mg/dL; 187.8 nmol/L) at highest (20 mg weekly) dose in patients with median baseline Lp(a) 224.3 nmol/L (IQR 177.2–286.9).107 | Adverse events (AEs) more frequent in active arms (90%) than in placebo arm (83%); serious AEs followed a similar pattern (10% active arms; 2% placebo arm). Injection site reactions were the most common AE.107 |
| ARC-LPA/AMG890 | GalNAc–siRNA | Amgen-Arrowhead | Phase 2 | Sub-cutaneous injection |
Phase 1 clinical trial showed Lp(a) reduction of over 90% at doses ≥9 mg/kg that persisted 3–6 months.126 (https://clinicaltrials.gov/ct2/show/NCT03626662). Phase 2 clinical trial under way (https://clinicaltrials.gov/ct2/show/NCT04270760). In cynomolgus monkeys, 85–90% Lp(a) reduction at 29 days after 3 doses at 3 mg/kg Q1W; >75% reduction persisted at 6 weeks after third dose.139 |
In phase 1 study data, there was no excess of adverse events with AMG890.126 |
| SLN360 | GalNAc–siRNA | Silence Therapeutics | Phase 1 | Sub-cutaneous injection |
Phase 1 clinical trial starting in 2021 (https://clinicaltrials.gov/ct2/show/NCT04606602). In cynomolgus monkeys, >95% Lp(a) reduction at 29 days after 3 doses at 3 mg/kg Q1W; >95% reduction persisted at 7 weeks after third dose.140 |
Non-clinical safety studies in Cynomolgus monkey showed no observed adverse effect level was >60-fold greater than pharmacological active dose; <1% of peak liver exposure in tissues outside liver and kidney.141 |
All three molecules display reductions in Lp(a) that are likely to be clinically meaningful. The duration of effect appears to be substantially greater for the siRNA molecules than the ASO—data from the Phase-1 study of olpasiran show Lp(a) reduction of over 90%126 compared with reduction of up to 80% with pelacarsen in a Phase-2 study.107 Findings with inclisiran in a population of patients similar to those likely to be eligible for Lp(a)-lowering therapy suggest GalNAc-conjugated siRNAs are likely to perform well from a safety perspective. Injection site reactions were the most commonly reported adverse effect of pelacarsen in a Phase-2 study, while these were very rare in the Phase-1 study of olpasiran. If the subcutaneously delivered siRNA drugs, AMG890 and SLN360 prove both safe and effective in the ongoing and planned clinical studies, they stand to offer attractive options for CVD risk modification in patients with raised Lp(a).
9. Opportunities for precision medicine in managing raised Lp(a)
The evidence discussed above strongly suggests that reducing circulating Lp(a) is likely to have a beneficial impact on the onset and progression of CVD and risk of CVD events. Emerging drugs have shown promise in achieving safe and effective Lp(a) reduction. For these new medicines to be effective, they must be prescribed for patients most likely to gain the greatest clinical benefit.
Management of Lp(a)-mediated disease presents an opportunity for precision medicine on a large scale. Since circulating Lp(a) levels are largely determined by genotype and only modestly influenced by other modifiable factors, patients eligible for Lp(a)-lowering therapy can be identified in early adulthood. This information may be valuable as a prognostic indicator for CVD. However, like LDL-C, the utility of Lp(a) for risk prediction may differ between first and subsequent CVD events. Identification can be accomplished with a single blood test, as recommended by the ESC/EAS 2019 guidelines100 or through genotyping to identify variants at the LPA locus known to be associated with elevated Lp(a) levels. The utility of genetic data as a tool for predicting an individual’s future disease risk is an area of active research and debate.127,128 As genetic data, including LPA genotype, becomes more readily available to patients and clinicians, it may provide incremental information to supplement what can be offered from circulating Lp(a) measurement in the assessment of lifetime CVD risk. This genetic approach nonetheless needs close evaluation before being employed in clinical practice in order to ensure the most informative genetic variants are used, their impact on Lp(a) concentration and isoform size is well understood and such a test is applicable to all ancestral groups.129 In the nearer term, genetic information could prove valuable for evaluating pharmacogenetic interactions of RNAi therapeutics, and for identifying likely responder patients for inclusion in clinical trials.
10. Conclusion
Lp(a) is a well-identified risk factor for a range of CVDs. It has a potent causal effect on the onset and development of atherosclerotic, atherothrombotic, myocardial, and valvular disease, yet no specific and potent means of reducing Lp(a) is currently available for patients. Existing lipid-modifying therapies exert modest and variable effects on circulating Lp(a), while behavioural and dietary interventions130 are ineffective. RNAi therapeutics have considerable promise for treatment of long-term conditions with at least three experimental medicines for reducing circulating Lp(a). Preclinical and early clinical studies suggest that these emerging drugs may provide effective management of Lp(a)-mediated disease in a safe and acceptable manner. Although Lp(a) has previously been under-recognized as a CV risk factors, the work of investigators and of patient advocacy groups, such as the Lipoprotein(a) Association (www.familylipoproteina.org), the FH Foundation (www.thefhfounation.org), Heart UK (www.heartuk.org.uk), and FH Europe (www.fheurope.org) is ensuring that patients with raised Lp(a) are aware of their risk status and potential treatment options. If the novel Lp(a)-lowering agents prove safe and effective, they hold the potential to have an important beneficial impact on the health of patients, their families and of the wider population.
Acknowledgements
The authors acknowledge the contribution of Dr Rosamund J. Wilson to the preparation of the manuscript.
Conflict of interest: D.I.S., D.A.R., M.W.L., and G.V.C. are employees of Silence Therapeutics plc. A.Y. is a consultant to Silence Therapeutics plc.
Contributor Information
Daniel I Swerdlow, Silence Therapeutics plc, 72 Hammersmith Road, London W14 8TH, UK.
David A Rider, Silence Therapeutics plc, 72 Hammersmith Road, London W14 8TH, UK.
Arash Yavari, Experimental Therapeutics, Radcliffe Department of Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK.
Marie Wikström Lindholm, Silence Therapeutics plc, 72 Hammersmith Road, London W14 8TH, UK.
Giles V Campion, Silence Therapeutics plc, 72 Hammersmith Road, London W14 8TH, UK.
Steven E Nissen, Department of Cardiovascular Medicine, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
References
- 1. Berg K. A new serum type system in man-the Lp system. Acta Pathol Microbiol Scand 1963;59:369–382. [DOI] [PubMed] [Google Scholar]
- 2. Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation 2008;117:176–184. [DOI] [PubMed] [Google Scholar]
- 3. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J; Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial. Circulation 2014;129:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Donoghue M, Giugliano R, Keech A, Kanevsky E, Im K, Pineda AL, Somaratne R, Sever P, Pederson T, Sabatine M. Lipoprotein(a), PCSK9 Inhibition and cardiovascular risk: insights from the Fourier trial. Atherosclerosis 2018;275:e9–e10. [Google Scholar]
- 6. Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Wainwright NWJ, Pomilla C, Wareham NJ, Khaw K-T, Boekholdt SM, Sandhu MS. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 2012;32:3058–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet Lond Engl 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst Rev 2018;11:CD012502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-González I, Briel M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev 2016;11:CD009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Millan J, Pintó X, Brea A, Blasco M, Hernández-Mijares A, Ascaso J, Diaz A, Mantilla T, Pedro-Botet J. Fibrates in the secondary prevention of cardiovascular disease (infarction and stroke). Results of a systematic review and meta-analysis of the Cochrane collaboration. Clin Investig Arterioscler 2018;30:30–35. [DOI] [PubMed] [Google Scholar]
- 11. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJP; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 13. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM; CLEAR Harmony Trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 14. Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ 2014;349:g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jawi MM, Frohlich J, Chan SY. Lipoprotein(a) the insurgent: a new insight into the structure, function, metabolism, pathogenicity, and medications affecting lipoprotein(a) molecule. J Lipids 2020;2020:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu T, Yoon W-S, Lee S-R. Recent updates of lipoprotein(a) and cardiovascular disease. Chonnam Med J 2021;57:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maranhão RC, Carvalho PO, Strunz CC, Pileggi F. Lipoprotein (a): structure, pathophysiology and clinical implications. Arq Bras Cardiol 2014;103:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, Peloso GM, Manichaikul A, Yang C, Ryan KA, Fu M, Johnson WC, Tsai M, Budoff M, Vasan RS, Cupples LA, Rotter JI, Rich SS, Post W, Mitchell BD, Correa A, Metspalu A, Wilson JG, Salomaa V, Kellis M, Daly MJ, Neale BM, McCarroll S, Surakka I, Esko T, Ganna A, Ripatti S, Kathiresan S, Natarajan P; NHLBI TOPMed Lipids Working Group. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun 2018;9:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saleheen D, Haycock PC, Zhao W, Rasheed A, Taleb A, Imran A, Abbas S, Majeed F, Akhtar S, Qamar N, Zaman KS, Yaqoob Z, Saghir T, Rizvi SNH, Memon A, Mallick NH, Ishaq M, Rasheed SZ, Memon F-U-R, Mahmood K, Ahmed N, Frossard P, Tsimikas S, Witztum JL, Marcovina S, Sandhu M, Rader DJ, Danesh J. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol 2017;5:524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res 2016;57:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–392. [DOI] [PubMed] [Google Scholar]
- 22. Tada H, Takamura M, Kawashiri M-A. Lipoprotein(a) as an old and new causal risk factor of atherosclerotic cardiovascular disease. J Atheroscler Thromb 2019;26:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin M-J, Binder CJ, Hörkkö S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res 2008;49:2230–2239. [DOI] [PubMed] [Google Scholar]
- 24. Que X, Hung M-Y, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Määttä A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Ylä-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S, Witztum JL. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018;558:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 2016;57:1953–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coassin S, Erhart G, Weissensteiner H, Eca Guimarães de Araújo M, Lamina C, Schönherr S, Forer L, Haun M, Losso JL, Köttgen A, Schmidt K, Utermann G, Peters A, Gieger C, Strauch K, Finkenstedt A, Bale R, Zoller H, Paulweber B, Eckardt K-U, Hüttenhofer A, Huber LA, Kronenberg F. A novel but frequent variant in LPA KIV-2 is associated with a pronounced Lp(a) and cardiovascular risk reduction. Eur Heart J 2017;38:1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim ET, Würtz P, Havulinna AS, Palta P, Tukiainen T, Rehnström K, Esko T, Mägi R, Inouye M, Lappalainen T, Chan Y, Salem RM, Lek M, Flannick J, Sim X, Manning A, Ladenvall C, Bumpstead S, Hämäläinen E, Aalto K, Maksimow M, Salmi M, Blankenberg S, Ardissino D, Shah S, Horne B, McPherson R, Hovingh GK, Reilly MP, Watkins H, Goel A, Farrall M, Girelli D, Reiner AP, Stitziel NO, Kathiresan S, Gabriel S, Barrett JC, Lehtimäki T, Laakso M, Groop L, Kaprio J, Perola M, McCarthy MI, Boehnke M, Altshuler DM, Lindgren CM, Hirschhorn JN, Metspalu A, Freimer NB, Zeller T, Jalkanen S, Koskinen S, Raitakari O, Durbin R, MacArthur DG, Salomaa V, Ripatti S, Daly MJ, Palotie A; for the Sequencing Initiative Suomi (SISu) Project. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet 2014;10:e1004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Enkhmaa B, Anuurad E, Zhang W, Berglund L. Significant associations between lipoprotein(a) and corrected apolipoprotein B-100 levels in African–Americans. Atherosclerosis 2014;235:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paré G, Çaku A, McQueen M, Anand SS, Enas E, Clarke R, Boffa MB, Koschinsky M, Wang X, Yusuf S; INTERHEART Investigators. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation 2019;139:1472–1482. [DOI] [PubMed] [Google Scholar]
- 30. Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest 1992;90:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. Am J Epidemiol 2009;169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hopewell JC, Haynes R, Baigent C. The role of lipoprotein (a) in chronic kidney disease. J Lipid Res 2018;59:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duncan MS, Vasan RS, Xanthakis V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all‐cause mortality: observations from the Framingham study over 35 years. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2019;8:e011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McNeal CJ, Peterson AL. Lipoprotein (a) in youth. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (eds). Endotext. South Dartmouth (MA: ): MDText.com, Inc.; 2000, S57-S66. [Google Scholar]
- 35. McNeal CJ. Lipoprotein(a): its relevance to the pediatric population. J Clin Lipidol 2015;9:S57–S66. [DOI] [PubMed] [Google Scholar]
- 36. Liu H-H, Cao Y-X, Jin J-L, Zhang H-W, Hua Q, Li Y-F, Guo Y-L, Zhu C-G, Wu N-Q, Gao Y, Xu R-X, Hong L-F, Li J-J. Association of lipoprotein(a) levels with recurrent events in patients with coronary artery disease. Heart 2020;106:1228–1235. [DOI] [PubMed] [Google Scholar]
- 37. Schwartz GG, Ballantyne CM, Barter PJ, Kallend D, Leiter LA, Leitersdorf E, McMurray JJV, Nicholls SJ, Olsson AG, Shah PK, Tardif J-C, Kittelson J. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol 2018;3:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szarek M, Bittner VA, Aylward P, Baccara-Dinet M, Bhatt DL, Diaz R, Fras Z, Goodman SG, Halvorsen S, Harrington RA, Jukema JW, Moriarty PM, Pordy R, Ray KK, Sinnaeve P, Tsimikas S, Vogel R, White HD, Zahger D, Zeiher AM, Steg PG, Schwartz GG; ODYSSEY OUTCOMES Investigators. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J 2020;41:4245–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swerdlow DI, Hingorani AD, Humphries SE. Genetic risk factors and Mendelian randomization in cardiovascular disease. Curr Cardiol Rep 2015;17:33. [DOI] [PubMed] [Google Scholar]
- 40. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, Faire U, de Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M; PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518–2528. [DOI] [PubMed] [Google Scholar]
- 41. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 42. Larsson SC, Gill D, Mason AM, Jiang T, Bäck M, Butterworth AS, Burgess S. Lipoprotein(a) in Alzheimer, atherosclerotic, cerebrovascular, thrombotic, and valvular disease: Mendelian randomization investigation. Circulation 2020;141:1826–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, Drechsler C, Wanner C, Mora S, Lesogor A, Tsimikas S. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 2018;392:1311–1320. [DOI] [PubMed] [Google Scholar]
- 44. Sahebkar A, Simental-Mendía LE, Pirro M, Banach M, Watts GF, Sirtori C, Al-Rasadi K, Atkin SL. Impact of ezetimibe on plasma lipoprotein(a) concentrations as monotherapy or in combination with statins: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 2018;8:17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toth PP, Jones SR, Monsalvo ML, Elliott-Davey M, López JAG, Banach M. Effect of evolocumab on non-high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a): a pooled analysis of phase 2 and phase 3 studies. J Am Heart Assoc 2020;9:e014129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hackler E, Lew J, Gore MO, Ayers CR, Atzler D, Khera A, Rohatgi A, Lewis A, Neeland I, Omland T, Lemos J. D. Racial differences in cardiovascular biomarkers in the general population. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2019;8:e012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, Bolton TR, Peters JE, Kamstrup PR, Tybjærg-Hansen A, Benn M, Langsted A, Schnohr P, Vedel-Krogh S, Kobylecki CJ, Ford I, Packard C, Trompet S, Jukema JW, Sattar N, Di Angelantonio E, Saleheen D, Howson JMM, Nordestgaard BG, Butterworth AS, Danesh J; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol 2018;3:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lamina C, Kronenberg F; Lp(a)-GWAS-Consortium. Estimation of the required lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes. JAMA Cardiol 2019;4:575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, White AC, Timmers NKLM, Hjortnaes J, Rogers MA, Aikawa E, Arsenault BJ, Witztum JL, Newby DE, Koschinsky ML, Fayad ZA, Stroes ESG, Boekholdt SM, Dweck MR. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol 2019;73:2150–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vassiliou VS, Flynn PD, Raphael CE, Newsome S, Khan T, Ali A, Halliday B, Studer Bruengger A, Malley T, Sharma P, Selvendran S, Aggarwal N, Sri A, Berry H, Donovan J, Lam W, Auger D, Cook SA, Pennell DJ, Prasad SK. Lipoprotein(a) in patients with aortic stenosis: insights from cardiovascular magnetic resonance. PLoS One 2017;12:e0181077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Capoulade R, Chan KL, Yeang C, Mathieu P, Bossé Y, Dumesnil JG, Tam JW, Teo KK, Mahmut A, Yang X, Witztum JL, Arsenault BJ, Després J-P, Pibarot P, Tsimikas S. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol 2015;66:1236–1246. [DOI] [PubMed] [Google Scholar]
- 52. Bergmark BA, O'Donoghue ML, Murphy SA, Kuder JF, Ezhov MV, Ceška R, Gouni-Berthold I, Jensen HK, Tokgozoglu SL, Mach F, Huber K, Gaciong Z, Lewis BS, Schiele F, Jukema JW, Pedersen TR, Giugliano RP, Sabatine MS. An exploratory analysis of proprotein convertase subtilisin/kexin type 9 inhibition and aortic stenosis in the FOURIER trial. JAMA Cardiol 2020;5:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang S-J, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O'Donnell CJ, Post WS. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen HY, Dufresne L, Burr H, Ambikkumar A, Yasui N, Luk K, Ranatunga DK, Whitmer RA, Lathrop M, Engert JC, Thanassoulis G. Association of LPA variants with aortic stenosis: a large-scale study using diagnostic and procedural codes from electronic health records. JAMA Cardiol 2018;3:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arsenault BJ, Boekholdt SM, Dubé M-P, Rhéaume E, Wareham NJ, Khaw K-T, Sandhu MS, Tardif J-C. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet 2014;7:304–310. [DOI] [PubMed] [Google Scholar]
- 56. Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol 2019;74:54–66. [DOI] [PubMed] [Google Scholar]
- 57. Arora P, Kalra R, Callas PW, Alexander KS, Zakai NA, Wadley V, Arora G, Kissela BM, Judd SE, Cushman M. Lipoprotein(a) and risk of ischemic stroke in the REGARDS study. ATVB 2019;39:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fu H, Zhang D, Zhu R, Cui L, Qiu L, Lin S, Peng B. Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: a retrospective case-control study. Ann Transl Med 2020;8:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aronis KN, Zhao D, Hoogeveen RC, Alonso A, Ballantyne CM, Guallar E, Jones SR, Martin SS, Nazarian S, Steffen BT, Virani SS, Michos ED. Associations of lipoprotein(a) levels with incident atrial fibrillation and ischemic stroke: the ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc 2017;6:e007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pan Y, Li H, Wang Y, Meng X, Wang Y. Causal effect of Lp(a) [lipoprotein(a)] level on ischemic stroke and Alzheimer disease: a Mendelian randomization study. Stroke 2019;50:3532–3539. [DOI] [PubMed] [Google Scholar]
- 61. Kosmas CE, Silverio D, Sourlas A, Peralta R, Montan PD, Guzman E, Garcia MJ. Role of lipoprotein (a) in peripheral arterial disease. Ann Transl Med 2019;7:S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Golledge J, Rowbotham S, Velu R, Quigley F, Jenkins J, Bourke M, Bourke B, Thanigaimani S, Chan DC, Watts GF. Association of serum lipoprotein (a) with the requirement for a peripheral artery operation and the incidence of major adverse cardiovascular events in people with peripheral artery disease. J Am Heart Assoc 2020;9:e015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, Lee KM, Shao Q, Huffman JE, Natarajan P, Arya S, Small A, Sun YV, Vujkovic M, Freiberg MS, Wang L, Chen J, Saleheen D, Lee JS, Miller DR, Reaven P, Alba PR, Patterson OV, DuVall SL, Boden WE, Beckman JA, Gaziano JM, Concato J, Rader DJ, Cho K, Chang K-M, Wilson PWF, O'Donnell CJ, Kathiresan S, Tsao PS, Damrauer SM; VA Million Veteran Program. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat Med 2019;25:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail 2016;4:78–87. [DOI] [PubMed] [Google Scholar]
- 65. Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, Ndumele C, Shahar E, Heiss G, Boerwinkle E, Konety S, Hoogeveen RC, Ballantyne CM. The association of lipoprotein(a) with incident heart failure hospitalization: atherosclerosis risk in communities study. Atherosclerosis 2017;262:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman ÅK, Wilk JB, Morley MP, Chaffin MD, Helgadottir A, Verweij N, Dehghan A, Almgren P, Andersson C, Aragam KG, Ärnlöv J, Backman JD, Biggs ML, Bloom HL, Brandimarto J, Brown MR, Buckbinder L, Carey DJ, Chasman DI, Chen X, Chen X, Chung J, Chutkow W, Cook JP, Delgado GE, Denaxas S, Doney AS, Dörr M, Dudley SC, Dunn ME, Engström G, Esko T, Felix SB, Finan C, Ford I, Ghanbari M, Ghasemi S, Giedraitis V, Giulianini F, Gottdiener JS, Gross S, Guðbjartsson DF, Gutmann R, Haggerty CM, van der Harst P, Hyde CL, Ingelsson E, Jukema JW, Kavousi M, Khaw K-T, Kleber ME, Køber L, Koekemoer A, Langenberg C, Lind L, Lindgren CM, London B, Lotta LA, Lovering RC, Luan J, Magnusson P, Mahajan A, Margulies KB, März W, Melander O, Mordi IR, Morgan T, Morris AD, Morris AP, Morrison AC, Nagle MW, Nelson CP, Niessner A, Niiranen T, O’Donoghue ML, Owens AT, Palmer CNA, Parry HM, Perola M, Portilla-Fernandez E, Psaty BM, Rice KM, Ridker PM, Romaine SPR, Rotter JI, Salo P, Salomaa V, van Setten J, Shalaby AA, Smelser DT, Smith NL, Stender S, Stott DJ, Svensson P, Tammesoo M-L, Taylor KD, Teder-Laving M, Teumer A, Thorgeirsson G, Thorsteinsdottir U, Torp-Pedersen C, Trompet S, Tyl B, Uitterlinden AG, Veluchamy A, Völker U, Voors AA, Wang X, Wareham NJ, Waterworth D, Weeke PE, Weiss R, Wiggins KL, Xing H, Yerges-Armstrong LM, Yu B, Zannad F, Zhao JH, Hemingway H, Samani NJ, McMurray JJV, Yang J, Visscher PM, Newton-Cheh C, Malarstig A, Holm H, Lubitz SA, Sattar N, Holmes MV, Cappola TP, Asselbergs FW, Hingorani AD, Kuchenbaecker K, Ellinor PT, Lang CC, Stefansson K, Smith JG, Vasan RS, Swerdlow DI, Lumbers RT; Regeneron Genetics Center. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun 2020;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joshi PK, Pirastu N, Kentistou KA, Fischer K, Hofer E, Schraut KE, Clark DW, Nutile T, Barnes CLK, Timmers PRHJ, Shen X, Gandin I, McDaid AF, Hansen TF, Gordon SD, Giulianini F, Boutin TS, Abdellaoui A, Zhao W, Medina-Gomez C, Bartz TM, Trompet S, Lange LA, Raffield L, van der Spek A, Galesloot TE, Proitsi P, Yanek LR, Bielak LF, Payton A, Murgia F, Concas MP, Biino G, Tajuddin SM, Seppälä I, Amin N, Boerwinkle E, Børglum AD, Campbell A, Demerath EW, Demuth I, Faul JD, Ford I, Gialluisi A, Gögele M, Graff M, Hingorani A, Hottenga J-J, Hougaard DM, Hurme MA, Ikram MA, Jylhä M, Kuh D, Ligthart L, Lill CM, Lindenberger U, Lumley T, Mägi R, Marques-Vidal P, Medland SE, Milani L, Nagy R, Ollier WER, Peyser PA, Pramstaller PP, Ridker PM, Rivadeneira F, Ruggiero D, Saba Y, Schmidt R, Schmidt H, Slagboom PE, Smith BH, Smith JA, Sotoodehnia N, Steinhagen-Thiessen E, van Rooij FJA, Verbeek AL, Vermeulen SH, Vollenweider P, Wang Y, Werge T, Whitfield JB, Zonderman AB, Lehtimäki T, Evans MK, Pirastu M, Fuchsberger C, Bertram L, Pendleton N, Kardia SLR, Ciullo M, Becker DM, Wong A, Psaty BM, van Duijn CM, Wilson JG, Jukema JW, Kiemeney L, Uitterlinden AG, Franceschini N, North KE, Weir DR, Metspalu A, Boomsma DI, Hayward C, Chasman D, Martin NG, Sattar N, Campbell H, Esko T, Kutalik Z, Wilson JF. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat Commun 2017;8:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vuorio A , Watts GF, Schneider WJ, Tsimikas S, Kovanen PT. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J Intern Med; https://onlinelibrary.wiley.com/doi/full/10.1111/joim.12981. 1 May 2020. [DOI] [PubMed] [Google Scholar]
- 69. Laskowska-Klita T, Szymczak E, Radomyska B. Serum homocysteine and lipoprotein (a) concentrations in hypercholesterolemic and normocholesterolemic children. Clin Pediatr (Phila) 2001;40:149–154. [DOI] [PubMed] [Google Scholar]
- 70. Alonso R, Andres E, Mata N, Fuentes-Jiménez F, Badimón L, López-Miranda J, Padró T, Muñiz O, Díaz-Díaz JL, Mauri M, Ordovás JM, Mata P; SAFEHEART Investigators. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol 2014;63:1982–1989. [DOI] [PubMed] [Google Scholar]
- 71. Ellis KL, Pérez de Isla L, Alonso R, Fuentes F, Watts GF, Mata P. Value of measuring lipoprotein(a) during cascade testing for familial hypercholesterolemia. J Am Coll Cardiol 2019;73:1029–1039. [DOI] [PubMed] [Google Scholar]
- 72. Holmes DT, Schick BA, Humphries KH, Frohlich J. Lipoprotein(a) is an independent risk factor for cardiovascular disease in heterozygous familial hypercholesterolemia. Clin Chem 2005;51:2067–2073. [DOI] [PubMed] [Google Scholar]
- 73. Paquette M, Bernard S, Thanassoulis G, Baass A. LPA genotype is associated with premature cardiovascular disease in familial hypercholesterolemia. J Clin Lipidol 2019;13:627–633.e1. [DOI] [PubMed] [Google Scholar]
- 74. Vuorio A, Watts GF, Kovanen PT. Lipoprotein(a) as a risk factor for calcific aortic valvulopathy in heterozygous familial hypercholesterolemia. Atherosclerosis 2019;281:25–30. [DOI] [PubMed] [Google Scholar]
- 75.Hps3 /Timi55–Reveal Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 76. Nicholls SJ, Ruotolo G, Brewer HB, Wang M-D, Liu L, Willey MB, Deeg MA, Krueger KA, Nissen SE. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol 2016;10:519–527.e4. [DOI] [PubMed] [Google Scholar]
- 77. Waldmann E, Parhofer KG. Apheresis for severe hypercholesterolaemia and elevated lipoprotein(a). Pathology (Phila) 2019;51:227–232. [DOI] [PubMed] [Google Scholar]
- 78. Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Maerz W, Lehmacher W, Heibges A, Klingel R. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation 2013;128:2567–2576. [DOI] [PubMed] [Google Scholar]
- 79. Mickiewicz A, Borowiec-Wolna J, Bachorski W, Gilis-Malinowska N, Gałąska R, Raczak G, Chmara M, Wasąg B, Jaguszewski MJ, Fijałkowski M, Gruchała M. Long-term lipoprotein apheresis in the treatment of severe familial hypercholesterolemia refractory to high intensity statin therapy: three year experience at a lipoprotein apheresis center. Cardiol J 2019;26:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pokrovsky SN, Afanasieva OI, Safarova MS, Balakhonova TV, Matchin YG, Adamova I. U, Konovalov GA, Ezhov MV. Specific Lp(a) apheresis: a tool to prove lipoprotein(a) atherogenicity. Atheroscler Suppl 2017;30:166–173. [DOI] [PubMed] [Google Scholar]
- 81. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, Harrington RA, Jukema JW, Loizeau V, Moriarty PM, Moryusef A, Pordy R, Roe MT, Sinnaeve P, Tsimikas S, Vogel R, White HD, Zahger D, Zeiher AM, Steg PG, Schwartz GG; ODYSSEY OUTCOMES Committees and Investigators. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol 2020;75:133–144. [DOI] [PubMed] [Google Scholar]
- 82. Deshmukh HA, Colhoun HM, Johnson T, McKeigue PM, Betteridge DJ, Durrington PN, Fuller JH, Livingstone S, Charlton-Menys V, Neil A, Poulter N, Sever P, Shields DC, Stanton AV, Chatterjee A, Hyde C, Calle RA, Demicco DA, Trompet S, Postmus I, Ford I, Jukema JW, Caulfield M, Hitman GA; CARDS, ASCOT, and PROSPER Investigators. Genome-wide association study of genetic determinants of LDL-c response to atorvastatin therapy: importance of Lp(a). J Lipid Res 2012;53:1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hopewell JC, Parish S, Offer A, Link E, Clarke R, Lathrop M, Armitage J, Collins R; MRC/BHF Heart Protection Study Collaborative Group. Impact of common genetic variation on response to simvastatin therapy among 18 705 participants in the Heart Protection Study. Eur Heart J 2013;34:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction. Circ Cardiovasc Genet 2012;5:257–264. [DOI] [PubMed] [Google Scholar]
- 85. Donnelly LA, Zuydam NR, van Zhou K, Tavendale R, Carr F, Maitland-van der Zee AH, Leusink M, Boer A, de Doevendans PA, Asselbergs FW, Morris AD, Pearson ER, Klungel OH, Doney ASF, Palmer CNA. Robust association of the LPA locus with low-density lipoprotein cholesterol lowering response to statin treatment in a meta-analysis of 30 467 individuals from both randomized control trials and observational studies and association with coronary artery disease outcome during statin treatment. Pharmacogenet Genomics 2013;23:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wei W-Q, Li X, Feng Q, Kubo M, Kullo IJ, Peissig PL, Karlson EW, Jarvik GP, Lee MTM, Shang N, Larson EA, Edwards T, Shaffer CM, Mosley JD, Maeda S, Horikoshi M, Ritchie M, Williams MS, Larson EB, Crosslin DR, Bland ST, Pacheco JA, Rasmussen-Torvik LJ, Cronkite D, Hripcsak G, Cox NJ, Wilke RA, Stein CM, Rotter JI, Momozawa Y, Roden DM, Krauss RM, Denny JC. LPA variants are associated with residual cardiovascular risk in patients receiving statins. Circulation 2018;138:1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017;70:252–289. [DOI] [PubMed] [Google Scholar]
- 88. Marquis-Gravel G, Redfors B, Leon MB, Généreux P. Medical treatment of aortic stenosis. Circulation 2016;134:1766–1784. [DOI] [PubMed] [Google Scholar]
- 89. Thiago L, Tsuji SR, Nyong J, Puga ME, Gois AF, Macedo CR, Valente O, Atallah ÁN; Cochrane Heart Group (eds). Statins for aortic valve stenosis. Cochrane Database Syst Rev. 2016;9:CD009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khunti K, Danese MD, Kutikova L, Catterick D, Sorio-Vilela F, Gleeson M, Kondapally Seshasai SR, Brownrigg J, Ray KK. Association of a combined measure of adherence and treatment intensity with cardiovascular outcomes in patients with atherosclerosis or other cardiovascular risk factors treated with statins and/or ezetimibe. JAMA Netw Open 2018;1:e185554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nielsen SF, Nordestgaard BG. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J 2016;37:908–916. [DOI] [PubMed] [Google Scholar]
- 92. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2019;4:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Piccinni C, Antonazzo IC, Maggioni AP, Pedrini A, Calabria S, Ronconi G, Dondi L, Martini N, Roberto G, Sampietro T, Sbrana F, Pino BD, Bigazzi F, Surdo GL, Volpi E, Biagini S, Gini R. PCSK9 inhibitors’ new users: analysis of prescription patterns and patients’ characteristics from an Italian real-world study. Clin Drug Investig 2020;40:173–181. [DOI] [PubMed] [Google Scholar]
- 94. Kosmas CE, Silverio D, Ovalle J, Montan PD, Guzman E. Patient adherence, compliance, and perspectives on evolocumab for the management of resistant hypercholesterolemia. Patient Prefer Adherence 2018;12:2263–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, Virani SS, Louie MJ, Lee LV, Peterson ED, Navar AM. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. J Am Heart Assoc 2019;8:e011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JEL, Shah T, Sofat R, Stender S, Johnson PCD, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li KWah, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MCW, Tzoulaki I, Buxbaum SG, van der A DL, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RGJ, de Borst GJ, de Jong PA, Algra A, Spiering W, der Zee A. H M-V, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D, Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJV, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SRK, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WMM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg-Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimäki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP, Keating BJ, Hingorani AD, Sattar N. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet Lond Engl 2015;385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, S de F, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1046–e1081. [DOI] [PubMed] [Google Scholar]
- 98. Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GBJ, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian Cardiovascular Society Guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–1282. [DOI] [PubMed] [Google Scholar]
- 99. Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S; Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018;25:846–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Mueller C, Drexel H, Aboyans V, Corsini A, Doehner W, Farnier M, Gigante B, Kayikcioglu M; ESC Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 101. Cegla J, Neely RDG, France M, Ferns G, Byrne CD, Halcox J, Datta D, Capps N, Shoulders C, Qureshi N, Rees A, Main L, Cramb R, Viljoen A, Payne J, Soran H. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis 2019;291:62–70. [DOI] [PubMed] [Google Scholar]
- 102. Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab 2018;27:714–739. [DOI] [PubMed] [Google Scholar]
- 103. Laina A, Gatsiou A, Georgiopoulos G, Stamatelopoulos K, Stellos K. RNA therapeutics in cardiovascular precision medicine. Front Physiol 2018;9:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Russell S, Bennett J, Wellman JA, Chung DC, Yu Z-F, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, Zhu X, Haller JA, Sohn EH, Mahajan VB, Pfeifer W, Weckmann M, Johnson C, Gewaily D, Drack A, Stone E, Wachtel K, Simonelli F, Leroy BP, Wright JF, High KA, Maguire AM. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet Lond Engl 2017;390:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Raal FJ, Santos RD, Blom DJ, Marais AD, Charng M-J, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl 2010;375:998–1006. [DOI] [PubMed] [Google Scholar]
- 106. Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, Yang Q, Hughes SG, Geary RS, Arca M, Stroes ESG, Bergeron J, Soran H, Civeira F, Hemphill L, Tsimikas S, Blom DJ, O’Dea L, Bruckert E. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019;381:531–542. [DOI] [PubMed] [Google Scholar]
- 107. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif J-C, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, Xia S, Guerriero J, Viney NJ, O’Dea L, Witztum JL; AKCEA-APO(a)-LRx Study Investigators. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 108. Crooke ST, Vickers TA, Liang X. Phosphorothioate modified oligonucleotide–protein interactions. Nucleic Acids Res 2020;48:5235–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 2015;87:46–51. [DOI] [PubMed] [Google Scholar]
- 110. Springer AD, Dowdy SF. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther 2018;28:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sardh E, Harper P, Balwani M, Stein P, Rees D, Bissell DM, Desnick R, Parker C, Phillips J, Bonkovsky HL, Vassiliou D, Penz C, Chan-Daniels A, He Q, Querbes W, Fitzgerald K, Kim JB, Garg P, Vaishnaw A, Simon AR, Anderson KE. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med 2019;380:549–558. [DOI] [PubMed] [Google Scholar]
- 112. Pasi KJ, Rangarajan S, Georgiev P, Mant T, Creagh MD, Lissitchkov T, Bevan D, Austin S, Hay CR, Hegemann I, Kazmi R, Chowdary P, Gercheva-Kyuchukova L, Mamonov V, Timofeeva M, Soh C-H, Garg P, Vaishnaw A, Akinc A, Sørensen B, Ragni MV. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med 2017;377:819–828. [DOI] [PubMed] [Google Scholar]
- 113. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–1519. [DOI] [PubMed] [Google Scholar]
- 114. Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, Kastelein JJP; ORION-9 Investigators. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med 2020;382:1520–1530. [DOI] [PubMed] [Google Scholar]
- 115. Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res 2018;46:1584–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat Rev Genet 2015;16:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nair JK, Attarwala H, Sehgal A, Wang Q, Aluri K, Zhang X, Gao M, Liu J, Indrakanti R, Schofield S, Kretschmer P, Brown CR, Gupta S, Willoughby JLS, Boshar JA, Jadhav V, Charisse K, Zimmermann T, Fitzgerald K, Manoharan M, Rajeev KG, Akinc A, Hutabarat R, Maier MA. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc–siRNA conjugates. Nucleic Acids Res 2017;45:10969–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Brown CR, Gupta S, Qin J, Racie T, He G, Lentini S, Malone R, Yu M, Matsuda S, Shulga-Morskaya S, Nair AV, Theile CS, Schmidt K, Shahraz A, Goel V, Parmar RG, Zlatev I, Schlegel MK, Nair JK, Jayaraman M, Manoharan M, Brown D, Maier MA, Jadhav V. Investigating the pharmacodynamic durability of GalNAc–siRNA conjugates. Nucleic Acids Res 2020;48:11827–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Weingärtner A, Bethge L, Weiss L, Sternberger M, Lindholm MW. Less is more: novel hepatocyte-targeted siRNA conjugates for treatment of liver-related disorders. Mol Ther Nucleic Acids 2020;21:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Saborowski M, Dölle M, Manns MP, Leitolf H, Zender S. Lipid-lowering therapy with PCSK9-inhibitors in the management of cardiovascular high-risk patients: effectiveness, therapy adherence and safety in a real world cohort. Cardiol J 2018;25:32–41. [DOI] [PubMed] [Google Scholar]
- 121. Hope HF, Binkley GM, Fenton S, Kitas GD, Verstappen SMM, Symmons DPM. Systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease. PLoS One 2019;14:e0201196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Schmidt AF, Holmes MV, Preiss D, Swerdlow DI, Denaxas S, Fatemifar G, Faraway R, Finan C, Valentine D, Fairhurst-Hunter Z, Hartwig FP, Horta BL, Hypponen E, Power C, Moldovan M, van Iperen E, Hovingh K, Demuth I, Norman K, Steinhagen-Thiessen E, Demuth J, Bertram L, Lill CM, Coassin S, Willeit J, Kiechl S, Willeit K, Mason D, Wright J, Morris R, Wanamethee G, Whincup P, Ben-Shlomo Y, McLachlan S, Price JF, Kivimaki M, Welch C, Sanchez-Galvez A, Marques-Vidal P, Nicolaides A, Panayiotou AG, Onland-Moret NC, van der Schouw YT, Matullo G, Fiorito G, Guarrera S, Sacerdote C, Wareham NJ, Langenberg C, Scott RA, Luan J, Bobak M, Malyutina S, Pająk A, Kubinova R, Tamosiunas A, Pikhart H, Grarup N, Pedersen O, Hansen T, Linneberg A, Jess T, Cooper J, Humphries SE, Brilliant M, Kitchner T, Hakonarson H, Carrell DS, McCarty CA, Lester KH, Larson EB, Crosslin DR, de Andrade M, Roden DM, Denny JC, Carty C, Hancock S, Attia J, Holliday E, Scott R, Schofield P, O'Donnell M, Yusuf S, Chong M, Pare G, van der Harst P, Said MA, Eppinga RN, Verweij N, Snieder H, Christen T, Mook-Kanamori DO, Gustafsson S, Lind L, Ingelsson E, Pazoki R, Franco O, Hofman A, Uitterlinden A, Dehghan A, Teumer A, Baumeister S, Dörr M, Lerch MM, Völker U, Völzke H, Ward J, Pell JP, Meade T, Christophersen IE, Maitland-van der Zee AH, Baranova EV, Young R, Ford I, Campbell A, Padmanabhan S, Bots ML, Grobbee DE, Froguel P, Thuillier D, Roussel R, Bonnefond A, Cariou B, Smart M, Bao Y, Kumari M, Mahajan A, Hopewell JC, Seshadri S, Dale C, Costa RPE, Ridker PM, Chasman DI, Reiner AP, Ritchie MD, Lange LA, Cornish AJ, Dobbins SE, Hemminki K, Kinnersley B, Sanson M, Labreche K, Simon M, Bondy M, Law P, Speedy H, Allan J, Li N, Went M, Weinhold N, Morgan G, Sonneveld P, Nilsson B, Goldschmidt H, Sud A, Engert A, Hansson M, Hemingway H, Asselbergs FW, Patel RS, Keating BJ, Sattar N, Houlston R, Casas JP, Hingorani AD; METASTROKE Consortium of the ISGC. Phenome-wide association analysis of LDL-cholesterol lowering genetic variants in PCSK9. BMC Cardiovasc Disord 2019;19:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Emdin CA, Khera AV, Natarajan P, Klarin D, Won H-H, Peloso GM, Stitziel NO, Nomura A, Zekavat SM, Bick AG, Gupta N, Asselta R, Duga S, Merlini PA, Correa A, Kessler T, Wilson JG, Bown MJ, Hall AS, Braund PS, Samani NJ, Schunkert H, Marrugat J, Elosua R, McPherson R, Farrall M, Watkins H, Willer C, Abecasis GR, Felix JF, Vasan RS, Lander E, Rader DJ, Danesh J, Ardissino D, Gabriel S, Saleheen D, Kathiresan S; CARDIoGRAM Exome Consortium. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol 2016;68:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet Lond Engl 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Swerdlow DI, Preiss D. Genetic insights into statin-associated diabetes risk. Curr Opin Lipidol 2016;27:125–130. [DOI] [PubMed] [Google Scholar]
- 126. Koren MJ, Moriarty PM, Neutel J, Baum Seth J, Hernandez-Illas M, Weintraub HS, Hellawell J, Varrieur T, Sohn W, Wang H, Elliott-Davey M, Kassahun H, Watts GF. Abstract 13951: safety, tolerability and efficacy of single-dose Amg 890, a novel Sirna targeting Lp(a), in healthy subjects and subjects with elevated Lp(a). Circulation 2020;142:A13951. [Google Scholar]
- 127. Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, Ye S, Webb TR, Rutter MK, Tzoulaki I, Patel RS, Loos RJF, Keavney B, Hemingway H, Thompson J, Watkins H, Deloukas P, Di Angelantonio E, Butterworth AS, Danesh J, Samani NJ; UK Biobank CardioMetabolic Consortium CHD Working Group. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mosley JD, Gupta DK, Tan J, Yao J, Wells QS, Shaffer CM, Kundu S, Robinson-Cohen C, Psaty BM, Rich SS, Post WS, Guo X, Rotter JI, Roden DM, Gerszten RE, Wang TJ. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA 2020;323:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kronenberg F. Prediction of cardiovascular risk by Lp(a) concentrations or genetic variants within the LPA gene region. Clin Res Cardiol Suppl 2019;14:5–12. [DOI] [PubMed] [Google Scholar]
- 130. Fitó M, Estruch R, Salas-Salvadó J, Martínez-Gonzalez MA, Arós F, Vila J, Corella D, Díaz O, Sáez G, Torre R, de la Mitjavila M-T, Muñoz MA, Lamuela-Raventós R-M, Ruiz-Gutierrez V, Fiol M, Gómez-Gracia E, Lapetra J, Ros E, Serra-Majem L, Covas M-I; on behalf of the PREDIMED Study Investigators. Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. Eur J Heart Fail 2014;16:543–550. [DOI] [PubMed] [Google Scholar]
- 131. Berg K, Dahlén G, Christophersen B, Cook T, Kjekshus J, Pedersen T. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin Genet 1997;52:254–261. [DOI] [PubMed] [Google Scholar]
- 132. Sahebkar A, Reiner Ž, Simental-Mendía LE, Ferretti G, Cicero AFG. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism 2016;65:1664–1678. [DOI] [PubMed] [Google Scholar]
- 133. Sahebkar A, Simental-Mendía LE, Watts GF, Serban M-C, Banach M; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Comparison of the effects of fibrates versus statins on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of head-to-head randomized controlled trials. BMC Med 2017;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Thompson PD, Rubino J, Janik MJ, MacDougall DE, McBride SJ, Margulies JR, Newton RS. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol Elsevier 2015;9:295–304. [DOI] [PubMed] [Google Scholar]
- 135. Ray KK, Vallejo-Vaz AJ, Ginsberg HN, Davidson MH, Louie MJ, Bujas-Bobanovic M, Minini P, Eckel RH, Cannon CP. Lipoprotein(a) reductions from PCSK9 inhibition and major adverse cardiovascular events: pooled analysis of alirocumab phase 3 trials. Atherosclerosis 2019;288:194–202. [DOI] [PubMed] [Google Scholar]
- 136. Ray KK, Kallend D, Koenig W, Leiter L, Raal F, Wright S, Wijngaard P, Kastelein JJP. 6 monthly inclisiran and atherogenic lipoprotein reductions in orion-11. J Am Coll Cardiol 2020;75:1853. [Google Scholar]
- 137. Santos RD, Raal FJ, Catapano AL, Witztum JL, Steinhagen-Thiessen E, Tsimikas S. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia. Arterioscler Thromb Vasc Biol 2015;35:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Thomas T, Zhou H, Karmally W, Ramakrishnan R, Holleran S, Liu Y, Jumes P, Wagner JA, Hubbard B, Previs SF, Roddy T, Johnson-Levonas AO, Gutstein DE, Marcovina SM, Rader DJ, Ginsberg HN, Millar JS, Reyes-Soffer G. CETP (cholesteryl ester transfer protein) inhibition with anacetrapib decreases production of lipoprotein(a) in mildly hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol 2017;37:1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melquist S, Wakefield D, Hamilton H, Chapman C, Grondolsky J, Schienebeck C, Almeida L, Klas C, Hagen C, Almeida A, Hegge J, Chu Q, Trubetskoy V, Doss E, Hoover-Plow J, Rozema D, Lewis D, Steven K. Abstract 17167: Targeting apolipoprotein(a) with a novel RNAi delivery platform as a prophylactic treatment to reduce risk of cardiovascular events in individuals with elevated lipoprotein (a). Circulation 2016;134:A17167–A17167.
- 140.Aleku M, Eisermann M, Löffler K, Dames S, Frauendorf, Marie wikstrom Lindholm, Schubert S, Rider D. Abstract 9538: A novel short interfering ribonucleic acid shows potent and sustained reduction of serum lipoprotein (a) in cynomolgus monkeys. Circulation 2019;140:A9538–A9538. ,
- 141. Rider D, Swerdlow D, Eisermann M, Loeffler K, Hauptmann J, Morrison E, Wikstrom Lindholm M, Schubert S, Campion G. Abstract 14720: pre-clinical safety assessment of SLN360, a novel short interfering ribonucleic acid targeting LPA. Circulation 2020;142:A14720–A14720. [Google Scholar]