Abstract
We have examined the kinetics of the inhibition of human immunodeficiency virus type 1 (HIV-1) particle infectivity by protease inhibitors (PIs) in cell culture, using either transfected HeLa cells or infected peripheral blood mononuclear cells (PBMCs) as producers of infectious virions. Both the kinetics of the initiation of antiviral activity after addition of the PIs to these cultures and the kinetics of restoration of virion infectivity after removal of the PIs from the treated cultures were examined. We found that the kinetics of initiation of particle infectivity inhibition produced by a high extracellular concentration (5 μM) of the inhibitors were similar for all five inhibitors tested: loss of particle infectivity was perceptible as early as 1 h after the initiation of PI treatment and increased gradually thereafter. By contrast, the durability of this antiviral effect following removal of the drug from the culture varied dramatically according to the drug studied. In transfected HeLa cells, saquinavir and nelfinavir exerted the most prolonged inhibition, with the half-lives of their antiviral activities being greater than 24 h, while ritonavir exerted an intermediate length of inhibition (18 h) and indinavir and amprenavir exerted a reproducibly shorter length of inhibition (5 h). For all five tested PIs, these kinetics were significantly faster in PBMCs than in HeLa cells. The striking differences in antiviral kinetics observed among the different PIs appear mostly due to differences in their intracellular concentrations and/or rates of cellular clearance. Our observations, although limited to tissue culture conditions, may help delineate the cellular parameters of the antiviral activities of HIV-1 PIs and further optimize the efficiencies of these antiretrovirals in vivo.
Protease (PR) inhibitors (PIs) are among the most active compounds used in the therapy of human immunodeficiency virus type 1 (HIV-1) infection. These agents block the activity of a virally encoded aspartic PR required for the assembly of infectious viral particles (8, 12, 13). When used in combination with nucleosidic or nonnucleosidic inhibitors of reverse transcriptase, they can lead to long-term suppression of detectable viral replication in treated patients (2, 6, 9, 15, 16). However, because of the high risk of selection of resistant viral variants in the course of suboptimal therapeutic regimens, successful long-term HIV therapy has to be fully suppressive, with continuous inhibition of virus replication in spite of discontinuous drug intake. For most PIs, trough concentrations in plasma are generally above their 90% inhibitory concentrations (IC90s) for reference, drug-sensitive, HIV-1 strains (3, 10, 11, 17, 24, 26). However, little is known about the availability of PIs in some of the tissue compartments that may be essential for HIV replication in vivo. Therefore, one cannot exclude the possibility that in spite of apparently satisfactory plasma pharmacokinetics, important fluctuations in local drug concentrations can occur in other compartments, leading to subinhibitory trough levels of the drugs and to selection of resistant virus variants. This conclusion is supported by the observation of a significant correlation between the trough levels of some PIs and the duration of the antiviral effects of these molecules in vivo (25). In this context, the precise impact of short lapses in the maintenance of an extracellular concentration of a PI on the inhibition of HIV PR activity and on the inhibition of HIV infectivity in infected cells is not known. The delay in PI antiviral activity following addition of drug to virus-producing cells, as well as the delay in restoration of HIV infectivity following removal of the extracellular inhibitor from treated cells, has not been carefully examined. Furthermore, although it has been shown that the intracellular concentration of a PI is key to its antiviral activity (4), very little is known of the intracellular pharmacokinetic parameters of the antiviral activities of HIV-1 PIs. Here we report that although all currently used HIV-1 PIs have comparable kinetics of initiation of their antiviral activities on virus-producing cells, PIs can differ dramatically in the durability of their antiviral actions at the cellular level. We show that these differences are related to major differences in the intracellular concentration properties of the different compounds.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were maintained as subconfluent monolayers in Dulbecco’s modified minimal essential medium (DMEM) with 10% fetal calf serum. P4 indicator cells (HeLa CD4 LTR-LacZ cells) were cultured in DMEM with 10% fetal calf serum and 500 μg of G418 per ml as described previously (7). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy blood donors, cultivated in RPMI 1640 with 10% fetal calf serum, stimulated with 1 μg of phytohemagglutinin (Wellcome) per ml, and maintained in the presence of interleukin 2 (10% Lymphocult; Biotest Diagnostics). The virus used in all experiments was derived from the infectious proviral molecular clone pNL4-3 (1). Transfections were performed with HeLa cells and 15 μg of plasmid pNL4-3 in subconfluent 25-cm2 flasks in DMEM by the calcium-phosphate coprecipitation procedure. Virus production by the transfected and infected cultures was monitored by the HIV-1 p24 DuPont core profile enzyme-linked immunosorbent assay.
HIV infectivity assays.
The single-cycle titers of the recombinant viruses on P4 cells were determined. P4 cells are HeLa CD4 LTR-LacZ cells in which the expression of β-galactosidase is strictly inducible by the HIV transactivator protein Tat and which allow precise quantitation of HIV-1 infectivity based on a single cycle of replication. Triplicate subconfluent P4 cells in 96-well plates were infected in the presence of 20 μg of DEAE-dextran per ml. Twenty-four hours after infection of P4 cells, the single-cycle titers of viruses were determined by quantification of the β-galactosidase activity in P4 lysates by a colorimetric assay (herein termed the CPRG assay) based on cleavage of chlorophenol red-β-d-galactopyranoside (CPRG) by β-galactosidase as described previously (14, 30).
PIs.
The following PIs were used: indinavir (IDV; Merck Pharmaceuticals), ritonavir (RTV; Abbott Laboratories), saquinavir (SQV; Roche Products), nelfinavir (NFV; Agouron Pharmaceuticals), and amprenavir (APV; Vertex Pharmaceuticals). All PIs were dissolved in dimethyl sulfoxide (final concentration, 1 mM) and stored at −20°C before use.
Effects of PIs on producer cells, target cells, or isolated viral particles.
Virus-producing HeLa cells, transfected with pNL4-3 and grown in 25-cm2 flasks, were trypsinized 24 h after transfection, suspended in 10 ml of fresh DMEM, and plated in separate wells of 24-well plates. These separate cultures, derived from the same transfected cell population and therefore assumed to be equivalent in their levels of virus production were then treated with increasing concentrations of the different PIs for a total of 24 h before supernatant harvest and titration of HIV infectivity on P4 cells as described above. To measure the effect of PIs on target cells, P4 cells were pretreated overnight in 96-well plates with serial dilutions of the inhibitors and infected with the supernatant from untreated, pNL4-3-transfected HeLa cells in the presence of the same amounts of the inhibitors. The effect of PI treatment on isolated particles was determined with particles freshly produced by transfected HeLa cells. Supernatant harvested at 48 h after transfection was subjected to treatment by the different PIs at a concentration of 5 μM for 0, 2, 4, and 8 h at 37°C. The infectious titers of these supernatants were then compared with those of mock-treated supernatants from the same cultures.
Kinetics of initiation of the antiviral effects of PIs.
At the peak of virus production, infected PBMCs or transfected HeLa cells were washed in phosphate-buffered saline (PBS) and trypsinized to remove residual, cell-associated, infectious virions. The cells were then suspended in fresh prewarmed medium and subcultured in six separate 24-well plates at 4 × 106 cells in a volume of 1 ml per well. Each well in these plates corresponded to an individual time point in the follow-up. The cultures from five of these six plates were then treated with one of the five PIs tested (final concentration of 5 μM). The sixth plate, used as an untreated control, was processed similarly to the other plates except that the PI was omitted from the medium. At each time point, the medium of the corresponding culture well in each of the plates was removed and stored at 4°C before titration. In parallel, all of the other 1-ml cultures of all the plates were washed and further incubated in PI-containing medium. At the end of the follow-up, all stored supernatants were analyzed for HIV infectivity on P4 cells by the CPRG protocol. The inhibition of HIV infectivity at each time point by the different PIs tested was expressed as a percentage of the HIV titer in the supernatant from the untreated culture.
Kinetics of the restoration of HIV infectivity after removal of PIs.
In a manner similar to that used in the experiment described above, infected PBMCs or transfected HeLa cells at the peak of virus production were washed in PBS, trypsinized, and split in the wells of six separate 24-well plates, with 4 × 106 cells/well in a final volume of 1 ml. Five of the plates were treated with a PI at 5 μM, while one plate was left untreated. Each well in these plates corresponds to an individual time point. After 6 h at 37°C, the culture medium was replaced by fresh inhibitor-containing medium and the cells were cultivated for an additional 14-h period. The cultures were then extensively washed and incubated in the absence of an inhibitor, which marked the start of the kinetic follow-up. At each time point, the supernatant from one of the wells in the plate was removed and stored at 4°C while the culture media were changed in all of the other wells. At the end of the follow-up, stored supernatants were analyzed for HIV infectivity on P4 cells by the CPRG protocol. Again, the inhibitory effect of a PI was expressed as a percentage of the inhibition level in the untreated control.
Quantitation of cell-associated PI concentrations.
Concentrations of PIs in lysates of treated HeLa cells or PBMCs were determined for IDV, SQV, and RTV. Cells were treated as described above under “Kinetics of the restoration of HIV infectivity after removal of PIs,” except that at each of the time points, cells from the corresponding well were washed and suspended in 300 μl of culture medium. The resulting cell suspensions were diluted in sodium borate buffer (100 mM, pH 9.5) and sonicated for 10 min. For the determination of IDV concentration (29), the cell lysates were subjected to repeated liquid-liquid extraction with a mixture of isopropanol-chloroform, and IDV concentrations were determined by a high-performance liquid chromatography (HPLC) procedure with UV detection at 210 nm. The lower limit of detection was 5 ng/ml. The interassay coefficient of variation for quality control was 9.6%. SQV concentrations were determined after a double-step and back liquid-liquid extraction, evaporation, and injection in an HPLC system coupled to a photodiode array detector. The detection wavelength was 240 nm. The lower limit of detection was 10 ng/ml, and the interassay coefficient of variation was 10.4%. For the extraction of RTV, cell lysates were combined with a solution of ethyl acetate-hexane, sonicated for 10 min, and subjected to liquid-liquid extraction as previously described (23). RTV concentrations were determined by HPLC, with UV detection at 210 nm. The lower limit of detection was 30 ng/ml, and the interassay coefficient of variation was 6.7%. All methods of monitoring PI levels were found to be linear and specific, and results were reproducible. Standards and control samples were prepared in bovine serum and treated in the same way as the cell lysate samples. Quality-control samples were prepared by dissolving the stock sample in bovine serum to final concentrations of 5, 15, 150, and 1,000 ng/ml for IDV; 27, 135, and 360 ng/ml for SQV; and 1, 3, 9, and 12.5 mg/liter for RTV and by storing them at −80°C.
RESULTS
Evaluation of the antiviral activities of HIV-1 PIs on virus-producing cells versus those on target cells.
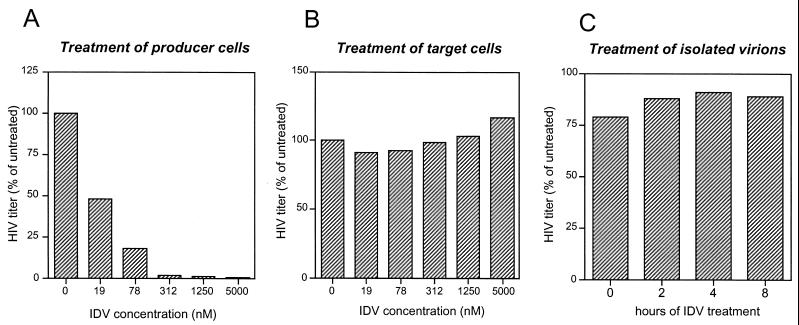
We first wished to ascertain, as previously shown by other authors with a different system (18, 20), that HIV PIs are active against HIV infectivity upon treatment of virion-producing cells but that they remain inactive upon treatment of target cells. To evaluate the effect of PI treatment of virus-producing cells, transfected HeLa cells were trypsinized and split in separate cultures that were subsequently treated with different concentrations of the five different PIs. Supernatants harvested 24 h later from these subcultures were titrated on β-galactosidase indicator P4 cells on the basis of a single cycle of HIV-1 replication. Since virus-producing cells in the different subcultures were derived from the same transfected culture, we observed that their levels of HIV-1 production were similar (not shown). As shown in Fig. 1A, a dramatic decrease in the infectious titers of the supernatants from IDV-treated HeLa cultures, relative to those of untreated cells, was observed. Similar results were obtained with the other tested PIs (data not shown). The effect of PI treatment of target cells was examined by treating P4 cells for 16 h before exposing them to a stock of infectious HIV-1 particles from transfected HeLa cultures and by maintaining the concentration of the inhibitor during the time of infection of the P4 cells. No significant change in HIV infectious titer was observed (Fig. 1B for the experiment with IDV; data not shown for the other PIs). Since the P4 titer of HIV-1 is a reflection of the early stages of viral replication up to the production of the Tat transactivator, this result indicates that the activity of the HIV-1 protease is not required during these stages. The effect of treatment of isolated particles was also examined. Particles harvested from transfected HeLa cells were subjected to treatment by a single concentration of PIs (5 μM) for different times at 37°C and then used to infect P4 cells. No significant effect of treatment of isolated particles on their infectious titer could be detected (Fig. 1C for results with IDV; data not shown for the other PIs). Overall, these results show that the protease of HIV-1 is accessible to inhibition by PIs only upon treatment of virus-producing cells.
FIG. 1.
Antiviral activities of IDV, an inhibitor of HIV-1 PR, on HIV-1 producer cells, target cells, and isolated virions. (A) Transfected, virus-producing HeLa cells were treated for 24 h with increasing concentrations of IDV, and the culture supernatants were evaluated for HIV infectivity on the basis of a single cycle of virus replication on indicator P4 cells. (B) Target indicator P4 cells were treated overnight with increasing concentrations of IDV and then infected with virus-containing supernatant from untreated, transfected HeLa cells in the presence of the same concentrations of IDV as those used in the pretreatment period. (C) Supernatant from transfected HeLa cells was subjected to treatment with 5 μM IDV for the indicated periods and used to infect P4 cells. The results are the means of results from at least two independent experiments and are expressed as percentages of results for untreated controls. Results are relative to those for untreated HeLa cells (A), untreated P4 cells (B), and untreated virus (C).
Kinetics of the initiation of antiviral activities by different PI molecules.
The kinetics of HIV inhibition by PIs was examined with two distinct virus production systems: transfected HeLa cells and infected PBMCs, which were treated at the peak of virus production. Although HeLa cells may lack some relevance to the in vivo situation, these cells allow synchronous production of virus, and because they are not reinfectible by HIV, no effect of PIs on the number of virus-producing cells is anticipated. On the other hand, infection of the more relevant PBMCs is dependent upon virus propagation within a heterogeneous cell population, in which treatment by PIs affects the number of infected cells as well as the viability parameters of the cultures, even upon short periods of treatment.
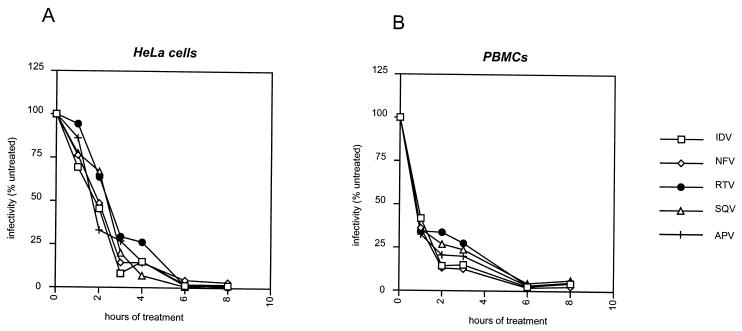
In HeLa cells a rapid inhibition of HIV particle infectivity was found for each of the PI molecules tested (Fig. 2A). A significant decrease in particle infectivity was perceptible as early as 1 h after the start of the treatment, less than 25% residual infectivity was measured after 4 h of treatment, and full inhibition was obtained in 12 h. In infected PBMCs (Fig. 2B), the kinetics of inhibition were even faster: a drop in virion infectivity of more than 50% was measured after 1 h of treatment, while near complete inhibition was seen after 6 h of treatment. It has to be noted, however, that in PBMCs, a small (less than 5%) but reproducible residual infectivity was still measurable at 8 and 12 h after the start of the treatment, with full inhibition visible only at 24 h (data not shown). This effect could be explained by small amounts of residual, trypsinization-resistant infectious particles that had assembled and matured before drug treatment.
FIG. 2.
Kinetics of initiation of the antiviral activities of five different HIV-1 PIs. Transfected HeLa cells (A) or infected PBMCs (B) were treated with 5 μM concentrations of each of the indicated PIs. Before treatment, all cells were trypsinized to remove unaffected, infectious virions. The infectivity of the newly assembled virions, produced in the presence of a PI, was determined by single-cycle tritration on P4 cells at different time intervals and is expressed as a percentage of the result for an untreated control. The results are means from three independent experiments with HeLa cells and two independent experiments with PBMCs.
Kinetics of the recovery of HIV-1 particle infectivity following removal of extracellular PIs.
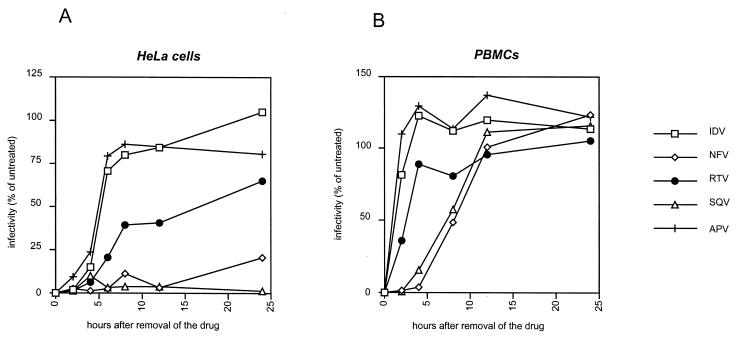
In these experiments, HIV-producing cells were treated with PI until full inhibition of infectivity was obtained; extracellular drugs were then removed from the culture medium, and particle infectivity was monitored over time. As shown in Fig. 3A, in HeLa cells, we observed dramatic differences in the kinetics of restoration of virion infectivity according to the inhibitor used. For IDV and APV, a rapid recovery of virus infectivity was observed, with more than 75% of virus infectivity being recovered at 8 h after removal of the drugs from the cultures. For RTV, the speed of restoration of infectivity was intermediate, with a 40% recovery at 8 h, which subsequently gradually increased. By contrast, for SQV and NFV, a prolonged inhibition of virus infectivity was measured: at 24 h after removal of the inhibitor from the culture, no virus infectivity was recovered in SQV-treated cultures and only 20% of the virus infectivity was recovered in NFV-treated cultures. Overall, in HeLa cells, the half-lives of the antiviral activities of the different PIs was 5 h for APV and IDV, between 12 and 24 h for RTV, and above 24 h for SQV and NFV.
FIG. 3.
Kinetics of the restoration of the infectivity of HIV-1 after removal of PIs from the producer cell cultures. Transfected HeLa cells (A) or infected PBMCs (B) that had been treated with 5 μM concentrations of each of the indicated PIs for 20 h, resulting in the production of noninfectious virus, were washed and incubated in drug-free medium. The infectivity of the newly released virus in the supernatant was measured by single-cycle titration on P4 cells at different time intervals and is expressed as a percentage of the result for an untreated control. The results are means from three independent experiments with HeLa cells and two independent experiments with PBMCs.
In infected PBMCs, the kinetics of restoration of virus infectivity was significantly faster than in transfected HeLa cells. However, the striking differences observed in the HeLa cells were readily perceptible in PBMCs, with the different PIs ranking in the same order as in HeLa cells. Indeed, recovery from the inhibition by IDV and APV was the fastest, recovery from RTV was intermediate, and recovery from SQV and NFV inhibition was the slowest. The half-lives of the antiviral activities of PIs in PBMCs were calculated as 1 to 2 h for APV and IDV, 3 h for RTV, and approximately 8 h for SQV and NFV. Unlike in the HeLa cell experiments, we found that with all inhibitors, virus infectivity recovered at 24 h from treated PBMCs was greater than that from the untreated control. This result is likely to stem from the effect of PIs on the propagation of virus within the PBMC population, which should result in differences in the number and/or in the viability of virus-producing cells between the treated and untreated cultures.
Intracellular pharmacokinetics of PIs.
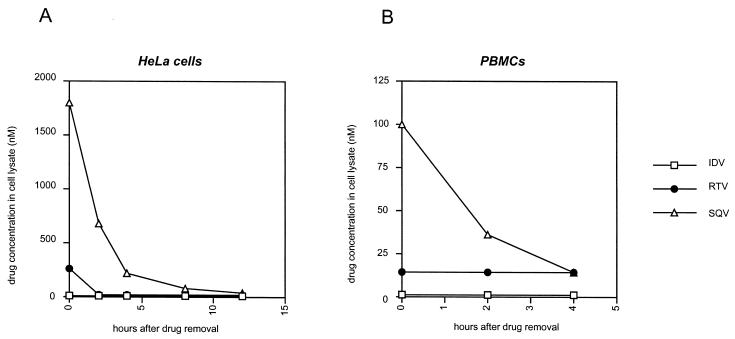
To evaluate whether the observed differences between the kinetics of the antiviral activities of distinct PI molecules were due to differences affecting their intrinsic antiviral activities or to differences in their intracellular pharmacokinetic properties, we measured the kinetics of decay of cell-associated PIs in cells treated with inhibitors and further cultivated in drug-free medium. As shown in Fig. 4A, we found that the concentration of PI in lysates of treated HeLa cells, immediately after removal of drug from the medium and trypsinization of the cells, differed dramatically according to the drug studied. The concentration of SQV at time zero was the highest of the three studied molecules (1,800 nM/106 cells in 300 μl), followed by RTV (260 nM), while the concentration of IDV in cell lysates was below the limit of detection by the HPLC method used in this study. The concentration of PI in the lysates decreased rapidly over time for both RTV and SQV. However, since the initial concentration of drug in the cell lysates was much higher for SQV, and in spite of the rapid clearance of the drug from the treated cells, the amounts of SQV in the lysates were still significantly above the limit of detection at 4 and 8 h after drug removal, which was not the case for RTV and for IDV. The striking differences observed here in the concentrations of cell-associated SQV, RTV, and IDV appear to fully correlate with our findings on the half-lives of the antiviral activities of these three PIs, for which it was found that SQV has the longest antiviral half-life, RTV has an intermediate antiviral half-life, and IDV has the shortest antiviral half-life.
FIG. 4.
Drug concentrations in lysates of PI-treated cells after removal of the drugs from the culture medium. HeLa cells (A) or PBMCs (B) were treated with 5 μM IDV, RTV, or SQV for 20 h, washed, and incubated in drug-free medium. At the indicated times, cells were washed and lysed and the concentration of each of the drugs in the cell lysates was determined by HPLC. Results are from one experiment that was representative of three independent experiments and are expressed in nanomolar units in a 300-μl lysate, corresponding to 106 cells. The limits of the sensitivity of the measurement method are 0.5 nM for IDV and 16 nM for RTV and SQV.
The same findings were made, on a different scale, when we analyzed the kinetics of the amounts of PBMC-associated PIs in culture. As shown in Fig. 4B, SQV was again the molecule for which the highest concentration was detectable at the earliest time point after removal of the drug from the medium. In PBMCs, we could not detect either cell-associated RTV or IDV at any of the time points. The lower amounts of cell-associated PIs in PBMCs appear to correlate with the shorter antiviral activities of PIs in these cells, compared to values obtained with HeLa cells.
Time of recovery of HIV infectivity as a function of the initial dose of PI.
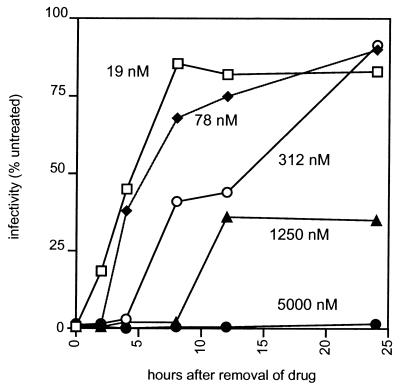
To further demonstrate that the recovery of HIV infectivity following removal of a PI from the culture is essentially a function of the clearance of the drug from treated cells, we measured the recovery of virus infectivity over time after removal of SQV from cultures treated for 24 h with different concentrations of the drug. As shown in Fig. 5, there was a strong relationship between the amount of drug used to initiate inhibition of HIV infectivity and the duration of that inhibition. After an initial treatment of 19 nM SQV, the recovery of virus infectivity was rapid, with an antiviral half-life of approximately 4 h; at 78 nm, it was around 6 h, and at 312 nM, it was around 15 h. After a 1,250 nM SQV initial treatment, the recovery of HIV infectivity at 24 h after removal of the drug was 35% of that of untreated virus and at 5,000 nM, as found in previous experiments, there was no recovery of virus infectivity over the 24-h follow-up period. These results emphasize that the durability of the inhibition of HIV infectivity by PIs at the cellular level is essentially dependent upon the concentration of inhibitor targeted to the infected cell.
FIG. 5.
Kinetics of the restoration of HIV infectivity from cultures treated with different concentrations of SQV and further incubated in the absence of drug. Transfected HeLa cells that had been treated with SQV at the indicated concentrations for 20 h, all of which treatments resulted in the production of noninfectious virus, were washed and incubated in drug-free medium. The infectivity of the newly released virus in the supernatant was measured by single-cycle titration on P4 cells at different time intervals and is expressed as a percentage of the result with an untreated control. The results are the means of values from three independent experiments.
DISCUSSION
The goal of antiretroviral therapy of HIV disease is long-term suppression of HIV replication, which should prevent the emergence of resistant variants. Therefore, many attempts have been made to maximize the potency, the stability, and the durability of the antiviral effects of drugs used in HIV therapy. In vivo, the concentration of antivirals in plasma or tissues is subjected to temporal fluctuations, which are largely dependent upon the general pharmacokinetic properties of the drug used (3, 17, 22, 24, 26). In treated HIV-infected subjects, the amplitudes of these fluctuations can differ widely from one inhibitor to another, from one tissue compartment to another, and perhaps even from one subject to another. However, the virological impact of such fluctuations is not known. Therefore, our study was designed to evaluate the kinetics of viral infectivity inhibition by PIs in the context of large and severe fluctuations of extracellular drug concentrations. We found that HIV-1 infectivity drops rapidly following exposure of virus-producing cells to a high concentration of a PI, which was true for all tested PIs. Conversely, we found that the kinetics of restoration of virion infectivity following removal of PIs from producer cell cultures vary dramatically from one inhibitor to another. In brief, APV and IDV exerted the shortest inhibition, SQV and NFV exerted prolonged inhibition, while RTV exerted intermediate inhibition.
The kinetics of recovery of HIV infectivity from inhibition of PR function by PIs should be essentially dependent upon three factors: (i) the reversibility of PR inhibition at the molecular level, (ii) the kinetics of de novo production of inhibitor-free PR, and (iii) the kinetics of a decrease in the local concentration of an inhibitor at the site of virus assembly. We consider it unlikely that the first of these three parameters explains the marked differences in the antiviral kinetics of the tested drugs. Although there exist slight differences in the IC50s and IC90s of different PIs in tissue culture, their molecular parameters of HIV-1 PR inhibition in vitro are comparable (11, 13, 19, 21, 27). De novo PR synthesis, which is certainly a factor in virus recovery from PI treatment, cannot explain the differences in kinetics between the drugs since all PIs were tested on the same cell systems. In fact, we found that the differences in antiviral kinetics of PIs could be essentially attributed to striking differences in the intracellular pharmacokinetics of these molecules. Indeed, the correlation between the durability of HIV inhibition by PIs and the concentration of drug in cell lysates is particularly striking. SQV, the drug that exhibited the most prolonged antiviral activity, also displayed the highest cell lysate concentration immediately after removal of the drug from medium, and although the clearance of SQV from the cells was rapid, we surmise that there was still enough cell-associated drug for full inhibition of HIV infectivity in HeLa cells after 24 h. IDV, on the other hand, had the shortest antiviral half-life and displayed undetectable concentrations in HeLa and PBMC lysates. We cannot provide here a satisfactory explanation for the dramatic differences observed in cell-associated amounts of SQV and IDV. Whether they relate to differences in entry or efflux, or to some other phenomenon involving the affinities of the drugs towards particular cell structures or proteins, remains to be resolved. Because the cell lysates in which drug concentrations were measured had to be prepared after several washes, the absolute values that were measured in the lysates are not actual cytoplasmic concentrations and have to be interpreted with caution. Our experiments were comparative in essence and aimed at delineating differences in the antiviral and intracellular behaviors of different molecules. Clearly, further studies of the kinetics of cell association of HIV-1 PIs and of the kinetics of their clearance from treated cells are required. Overall, our findings are reminiscent of the postantibiotic effect described for some antibacterial agents (5, 28). Several mechanisms are invoked in postantibiotic effects, including persistent nonlethal inhibition of bacterial growth following removal of the antibiotic from the culture and persistence of the drug within bacterial cells. This persistence is comparable to what we can conclude from our experiments, where the prolonged antiviral activities of PIs appeared related to high intracellular concentrations of drugs. Recalling the effort that has been devoted to improving the general pharmacokinetic properties of HIV PIs, we believe that a similar effort should now be devoted to carefully evaluating the parameters of the intracellular pharmacokinetics of existing and future antivirals of this and other classes.
ACKNOWLEDGMENTS
We thank John Leonard and Fabrizio Mammano for their interest in this work and for helpful suggestions.
This study was supported by grants from the Agence Nationale de Recherche sur le Sida (ANRS). M.N. is the recipient of a predoctoral fellowship from the ANRS.
REFERENCES
- 1.Adachi A, Gendelmann H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Barry M, Gibbons S, Back D, Mulcahy F. Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet. 1997;32:194–209. doi: 10.2165/00003088-199732030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bilello J A, Bilello P A, Stellrecht K, Leonard J, Norbeck D W, Kempf D J, Robins T, Drusano G L. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1996;40:1491–1497. doi: 10.1128/aac.40.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundtzen R W, Gerber A U, Cohn D L, Craig W A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981;3:28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society—USA Panel. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 7.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription: a termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 8.Coffin J M. Retroviridae and their replication. In: Fields B, editor. Virology. 2nd ed. New York, N.Y: Raven Press; 1991. pp. 645–708. [Google Scholar]
- 9.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 10.Denissen J F, Grabowski B A, Johnson M K, Buko A M, Kempf D J, Thomas S B, Surber B W. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug Metab Dispos. 1997;25:489–501. [PubMed] [Google Scholar]
- 11.Dorsey B D, Levin R B, McDaniel S L, Vacca J P, Guare J P, Darke P L, Zugay J A, Emini E A, Schleif W A, Quintero J C. L-735,524: the design of a potent and orally bioavailable HIV protease inhibitor. J Med Chem. 1994;37:3443–3451. doi: 10.1021/jm00047a001. [DOI] [PubMed] [Google Scholar]
- 12.Dreyer G B, Metcalf B W, Tomaszek T A, Jr, Carr T J, Chandler A C D, Hyland L, Fakhoury S A, Magaard V W, Moore M L, Strickler J E, et al. Inhibition of human immunodeficiency virus 1 protease in vitro: rational design of substrate analogue inhibitors. Proc Natl Acad Sci USA. 1989;86:9752–9756. doi: 10.1073/pnas.86.24.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson J, Neidhart D, VanDrie J, Kempf D, Wang X, Norbeck D, Plattner J, Rittenhouse J, Turon M, Wideburg N, Kohlbrenner W, Simmer R, Helfrick R, Paul D, Knigge M. Design, activity, and 2.8 Å crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990;249:527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- 14.Eustice D, Feldman P, Colberg-Poley A, Buckery R, Neubauer R. A sensitive method for the detection of β-galactosidase in transfected mammalian cells. BioTechniques. 1991;11:739–743. [PubMed] [Google Scholar]
- 15.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 16.Havlir D V, Lange J M. New antiretrovirals and new combinations. AIDS. 1998;12:S165–S174. [PubMed] [Google Scholar]
- 17.Hoetelmans R M, Meenhorst P L, Mulder J W, Burger D M, Koks C H, Beijnen J H. Clinical pharmacology of HIV protease inhibitors: focus on saquinavir, indinavir, and ritonavir. Pharm World Sci. 1997;19:159–175. doi: 10.1023/a:1008629608556. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen H, Ahlborn-Laake L, Gugel R, Mous J. Progression of early steps of human immunodeficiency virus type 1 replication in the presence of an inhibitor of viral protease. J Virol. 1992;66:5087–5091. doi: 10.1128/jvi.66.8.5087-5091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kageyama S, Hoekzema D T, Murakawa Y, Kojima E, Shirasaka T, Kempf D J, Norbeck D W, Erickson J, Mitsuya H. A C2 symmetry-based HIV protease inhibitor, A77003, irreversibly inhibits infectivity of HIV-1 in vitro. AIDS Res Hum Retroviruses. 1994;10:735–743. doi: 10.1089/aid.1994.10.735. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan A H, Manchester M, Smith T, Yang Y L, Swanstrom R. Conditional human immunodeficiency virus type 1 protease mutants show no role for the viral protease early in virus replication. J Virol. 1996;70:5840–5844. doi: 10.1128/jvi.70.9.5840-5844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempf D J, Marsh K C, Denissen J F, McDonald E, Vasavanonda S, Flentge C A, Green B E, Fino L, Park C H, Kong X P, Norbeck D W. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci USA. 1995;92:2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz M, Conant M, Hurley A, Schluger R, Duran M, Peterkin J, Chapman S, Patick A, Hendricks A, Yuen G J, Hoskins W, Clendeninn N, Ho D D. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV)-1 protease, to treat HIV infection. J Infect Dis. 1998;177:1533–1540. doi: 10.1086/515312. [DOI] [PubMed] [Google Scholar]
- 23.Marsh K C, Eiden E, McDonald E. Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography. J Chromatogr B. 1997;704:307–313. doi: 10.1016/s0378-4347(97)00454-4. [DOI] [PubMed] [Google Scholar]
- 24.Merry C, Barry M G, Mulcahy F, Ryan M, Heavey J, Tjia J F, Gibbons S E, Breckenridge A M, Back D J. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS. 1997;11:F29–F33. doi: 10.1097/00002030-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 26.Moyle G J, Youle M, Higgs C, Monaghan J, Prince W, Chapman S, Clendeninn N, Nelson M R. Safety, pharmacokinetics, and antiretroviral activity of the potent, specific human immunodeficiency virus protease inhibitor nelfinavir: results of a phase I/II trial and extended follow-up in patients infected with human immunodeficiency virus. J Clin Pharmacol. 1998;38:736–743. doi: 10.1002/j.1552-4604.1998.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 27.Patick A, Mo H, Markowitz M, Appelt K, Wu B, Musick L, Kalish V, Kaldor S, Reich S, Ho D, Webber S. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob Agents Chemother. 1996;40:292–297. doi: 10.1128/aac.40.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spivey J M. The postantibiotic effect. Clin Pharm. 1992;11:865–875. [PubMed] [Google Scholar]
- 29.Woolf E, Au T, Haddix H, Matuszewski B. Determination of L-735 524, an human immunodeficiency virus protease inhibitor, in human plasma and urine via high-performance liquid chromatography with column switching. J Chromatogr A. 1995;692:45–52. doi: 10.1016/0021-9673(94)00608-c. [DOI] [PubMed] [Google Scholar]
- 30.Zennou V, Mammano F, Paulous S, Mathez D, Clavel F. Loss of viral fitness associated with multiple Gag and Gag-Pol processing defects in human immunodeficiency virus type 1 variants selected for resistance to protease inhibitors in vivo. J Virol. 1998;72:3300–3306. doi: 10.1128/jvi.72.4.3300-3306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]