Abstract
The topic of uremic toxicity has received broad attention from the nephrological community over the past few decades. An aspect that is much less often considered is the possibility that the metabolic pathways that generate uremic toxins also may produce molecules that benefit body functions. Here, we discuss this dualism based on the example of tryptophan-derived metabolites, which comprise elements that are mainly toxic, such as indoxyl sulfate, kynurenine and kynurenic acid, but also beneficial compounds, such as indole, melatonin and indole-3-propionic acid, and ambivalent (beneficial for some aspects and harmful for others) compounds such as serotonin. This dualism can also be perceived at the level of the main receptor of the tryptophan-derived metabolites, the aryl hydrocarbon receptor (AHR), which has also been linked to both harm and benefit. We hypothesize that these beneficial effects are the reason why uremic toxin generation remained preserved throughout evolution. This duality is also not unique for the tryptophan-derived metabolites, and in this broader context we discuss the remote sensing and signaling theory (RSST). The RSST proposes that transporters (e.g., organic anion transporter 1—OAT1; ATP-binding cassette transporter G—ABCG2) and drug metabolizing enzymes form a large network of proteins interacting to promote small molecule remote communication at the inter-organ (e.g., gut–liver–heart–brain–kidney) and inter-organismal (e.g., gut microbe–host) levels. These small molecules include gut microbe-derived uremic toxins as well as beneficial molecules such as those discussed here. We emphasize that this positive side of uremic metabolite production needs more attention, and that this dualism especially needs to be considered when assessing and conceiving of therapeutic interventions. These homeostatic considerations are central to the RSST and suggest that interventions be aimed at preserving or restoring the balance between positive and negative components rather than eliminating them all without distinction.
Keywords: uremia, uremic toxins, kidney disease, tryptophan, metabolism, indoles, kynurenines, aryl hydrocarbon receptor, remote sensing and signaling theory, gut microbiome
1. Introduction
The expected remaining lifetime for people with advanced chronic kidney disease (CKD) is more than halved across all age strata [1]. The average five-year survival of patients starting dialysis is lower than that of cancer, upon diagnosis [2]. The majority of CKD patients will die, mostly from cardio-vascular complications, before they reach the stage of dialysis or transplantation [3]. The calculated annual health care cost in Europe for CKD is higher than that of cancer or diabetes [2]. Acute kidney injury (AKI) only adds to outcome burden and cost [4,5].
This dismal picture can, to a considerable extent, be attributed to the accumulation of toxic metabolites (uremic toxins) that are eliminated by healthy kidneys via the urine [6]. These uremic toxins have been related to many of the lethal complications of kidney disease, especially cardio-vascular and infectious diseases and the progression of kidney insufficiency [6], but also to a number of distressing patient-related outcomes, such as cognitive dysfunction or itching [7,8], which are not fatal but affect quality of life substantially [2]. The pathophysiologic mechanisms defining uremic syndrome have received ample attention from the nephrologic community over the past few decades [6,9].
However, each shadow has a bright side. This duality exists throughout biology. Bacteria and yeasts cause killer diseases but are also used for the production of relatively benign foods, drinks or ingredients such as kombucha, yoghurt, soybean sauce and cheese. Duality has in antiquity been symbolized by the door deity Janus, who marked the separation between the inside and the outside of the house.
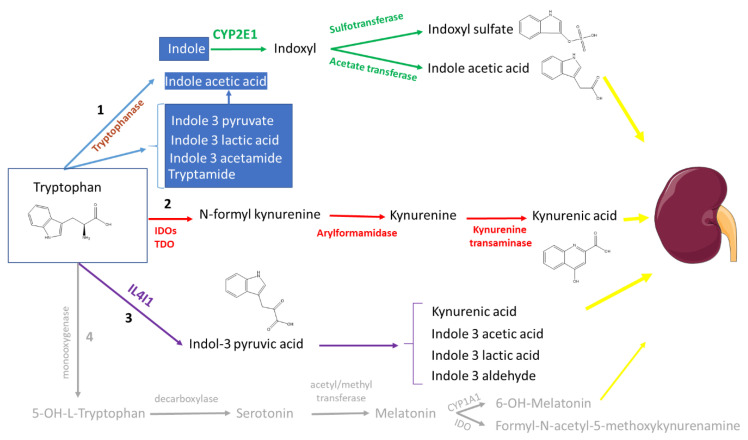
As we repetitively reviewed and ranked solutes for their toxicity [6,10,11], we realized that some metabolically related compounds were not toxic and even beneficial. However, the question as to how far the biologic ambiguity mentioned above also exists for uremic toxin metabolism has rarely been addressed or reviewed in depth: what if our tunnel vision disregards related compounds with beneficial impacts or if the so-called toxins are not unequivocally toxic? This question may be critical if successful efforts to reduce toxin concentrations have as a downside diminishing the benefits. We will consider this dilemma based on a detailed analysis of the functional effects of the metabolic cascade of tryptophan, including consideration of the recently described interleukin 4-induced-1 (IL4I1) pathway [12] (Figure 1). After having reviewed the functional properties of the main tryptophan metabolites, we will consider why the generation of potentially lethal molecules such as indoxyl sulfate, kynurenine, or indole-3-acetic acid [13,14,15] has not been switched off by natural selection or reproductive isolation.
Figure 1.
Global metabolic pathways of tryptophan according to the most recent insights. Different enzymes are involved in the generation of uremic toxins and activators of aryl hydrocarbon receptor (AhR). (1) Tryptophanase; (2) indoleamine-2,3-dioxygenases (IDOs) and tryptophan-2,3-dioxgenase (TDO) and (3) the newly identified interleukin 4-induced-1 (IL4I1). Indole is further metabolized in the liver (green arrows) by cytochrome P450 family 2 subfamily E member 1 (CYP2E1), resulting in indoxyl and indoxyl sulfate (sulfotransferase) and indole-3-acetic acid (acetate transferase). Both the IDOs/TDO (red arrows) and IL4I1-dependent pathways (purple arrows) are involved in the generation of kynurenic acid. The end-metabolites are excreted by the kidneys (yellow arrows). For the sake of completeness, in grey, (4) the serotonin pathway.
Our journey will lead us to consider biochemistry, (patho-)physiology, evolutionary biology, history, lifestyle and genetics, in order to propose a comprehensive hypothesis on solute metabolism in CKD. This will also lead to the consideration of the remote sensing and signaling theory (RSST) of small molecule inter-organ and inter-organismal communication mediated by drug transporters (e.g., organic anion transporter 1—OAT1; ATP-binding cassette transporter G—ABCG2) and drug metabolizing enzymes [16,17]. However, the field of uremic toxicity is rapidly advancing and encompasses many disciplines, and the viewpoint presented here might need to be fine-tuned in the future as novel information and interpretations emerge.
2. Tryptophan Metabolites: The Good, the Bad, and the Ambivalent
In what follows we will, based on the collected information, tentatively subdivide tryptophan metabolites (Table 1) into mainly toxic, mainly beneficial, and essentially ambivalent (or mixed) molecules (Figure 2). We make these categorizations with the understanding that simple subdivisions may need to be reconsidered; for instance, even those molecules we currently refer to as “toxic” are increasingly believed to also play roles in signaling through their influence upon nuclear receptors, G-protein coupled receptors (GPCRs) and kinases. After discussing selected molecules below, we provide a conceptual framework for a more nuanced interpretation of the roles of these molecules in local and systemic homeostasis and pathophysiology: the RSST. We then turn to evolutionary concerns, asking the question of why uremic toxins have persisted throughout evolution.
Table 1.
Metabolites of the tryptophan pathway with their chemical structures and the involved enzymes.
| Metabolite | Structure Formula | Enzymes Involved |
|---|---|---|
| Tryptophan |
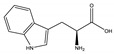
|
|
| Indole pathway | ||
| Indole |
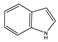
|
Tryptophanase |
| Indoxyl sulfate |
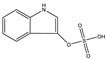
|
Sulfotransferase/CYP2E1 |
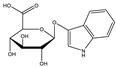
| Indoxyl glucuronide |
|
Glucuronyltransferase |
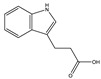
| Indole-3-propionic acid |
|
Tryptophanase (?) |
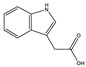
| Indole-3-acetic acid |
|
Aldehyde dehydrogenase (NAD+)/indole-3-acetaldehyde oxidase/IL4I1 |
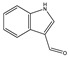
| Indole-3-(carbox)aldehyde |
|
Aromatic-L-amino-acid/L-tryptophan decarboxylase |
| Kynurenine pathway | ||
| L-Kynurenine |
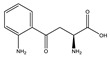
|
Tryptophan 2,3-dioxygenase or indoleamine 2,3-dioxygenase/arylformamidase |
| Kynurenic acid |
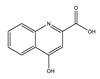
|
Kynurenine-oxoglutarate transaminase/cysteine-S-conjugate beta-lyase/glutamine-phenylpyruvate transaminase |
| Anthranilic acid |
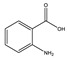
|
Kynureninase |
| Quinolinic acid |
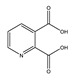
|
Anthranilate 3-monooxygenase (FAD)/4-hydroxyphenylacetate 3-monooxygenase/3-hydroxyanthranilate 3,4-dioxygenase |
| Nicotinic acid (niacin) (Nicotinate and Nicotinamide metabolism) |
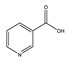
|
Nicotinate-nucleotide pyrophosphorylase (carboxylating)/nicotinate phosphoribosyltransferase |
| Nicotinamide (Nicotinate and Nicotinamide metabolism) |
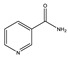
|
Nicotinate-nucleotide adenylyltransferase/nicotinamide adenine dinucleotide (NAD)+ synthase/NAD+ diphosphatase/5′-nucleotidase |
| N1-Methyl-2-pyridone-5-carboxamide (Nicotinate and Nicotinamide metabolism) |
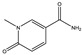
|
Nicotinamide methyl transferase/aldehyde oxidase |
| Serotonin Pathway | ||
| Serotonin |
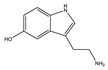
|
Tryptophan 5-monooxygenase/aromatic-L-amino-acid/L-tryptophan decarboxylase |
| Melatonin |
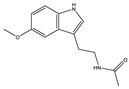
|
Arylalkylamine N-acetyltransferase/acetylserotonin O-methyltransferase |
IL4I1: interleukin 4-induced-1 (IL4I1); CYP2E1: cytochrome P450 family 2 subfamily E member 1.
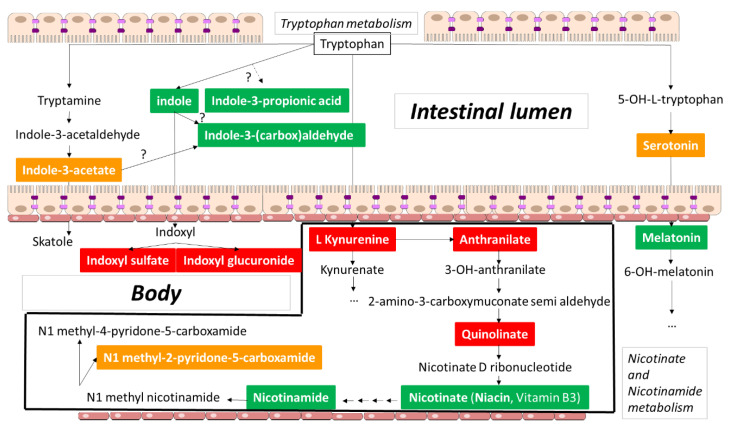
Figure 2.
Main metabolites of tryptophan. Extracted from Kyoto Encyclopedia of Genes and Genomes) (KEGG) pathways). The compounds with a colored background are discussed in the paper. Green background: mainly positive effect; red background: mainly negative effect; orange background: ambiguous data. Black frame: kynurenic acid pathway. Upper part: intestinal lumen; lower part: inside the body. Indole-3 propionic acid and Indole-3-(carbox)aldehyde are not mentioned in the KEGG pathways.
2.1. The (Mainly) Toxic Molecules
2.1.1. Indoxyl Sulfate
Few uremic retention solutes have received as much attention as indoxyl sulfate. Hundreds of studies have pointed to its effects contributing to uremic syndrome [6]. Although the correctness of the concentrations analyzed has been questioned for some studies [18,19], a considerable number of high-quality studies underscore its multifunctional burden [13], including its roles in cardio-vascular disease, kidney and heart fibrosis, thrombogenicity, metabolic and hormonal dysfunction, inflammation and chronic kidney disease-mineral and bone disorder (CKD-MBD) [6]. More recently reported functional defects include neurotoxicity [20,21,22], intestinal epithelial [23] and hematologic alterations [24,25,26], sarcopenia [27], loss of muscle mass [28], disturbed drug removal [29], and accelerated cell senescence [30]. Undeniably, indoxyl sulfate is responsible for a plethora of negative effects, and it is ranked among the most important uremic toxins [6]. However, these generally negative effects can be supportive in specific conditions such as breast cancer, in which tryptophan metabolism is suppressed. Supplementation of indoxyl sulfate to restore concentrations to the normal reference range exerted cytostatic properties via a reduction in cell proliferation and the induction of oxidative and nitrosative stress [31].
2.1.2. Indoxyl Glucuronide
Indoxyl glucuronide has rarely been studied. One study showed inhibition of hypoxia-inducible factor (HIF)-dependent erythropoietin expression [32]. Indoxyl glucuronide is also one of the uremic solutes inhibiting organic cation transporter-2 (OCT-2), which plays a role in the kidney excretion of drugs and environmental toxins [33].
2.1.3. Kynurenine and Kynurenic Acid
The kynurenine pathway follows a separate metabolic route from that of the indole derivatives (Figure 1 and Figure 2) [34]. This axis is mainly active in the liver but, especially after immune activation, also in other tissues [34]. Biologic studies essentially focused on kynurenine and kynurenic acid.
Kynurenine and/or kynurenic acid have been linked to cardio-vascular disease, inflammation, thrombogenicity, metabolic dysfunction and neurotoxicity [6]. Kynurenic acid has especially been studied as a regulator of neurologic function, whereby it acts as an N-methyl-D-aspartate (NMDA) receptor antagonist and functionally equilibrates with quinolinic acid, an NMDA receptor agonist [34]. A targeted metabolomic study in CKD found a significant relationship between kynurenine concentration and thrombotic events [14]. Other studies reported an impact on bone quality and strength [35,36]. Thus, kynurenine and kynurenic acid have a large array of negative effects, and have also been ranked among the most important uremic toxins [6].
2.1.4. Anthranilic Acid
Other metabolites of the kynurenic pathway have received less attention. One of these, anthranilic acid, has shown a positive correlation with fibrinolytic parameters in early CKD, while this relationship was inverted in more advanced stages [37]. Anthranilic acid is, at different stages of CKD, associated with markers of endothelial dysfunction [38]. However, direct causation has not been proven.
2.1.5. Quinolinic Acid
Quinolinic acid is especially known as a brain excitotoxin [39], but has also been linked to inflammation and inhibition of erythropoiesis [6], and more recently to a species-dependent effect on hemostasis, being prothrombotic in mice but the opposite in rats [40]. As with anthranilic acid (see above), in CKD, quinolinic acid is also associated with markers of endothelial dysfunction [38]. In addition, quinolinic acid is also related to intima-media thickness [38].
2.2. The (Mainly) Beneficial Molecules
2.2.1. Tryptophan
Tryptophan cannot be generated by the body and is thus an essential amino acid. There is limited evidence of an antidepressive effect of tryptophan-containing food supplements [41], although excessive intake has been linked to potentially lethal toxicity (eosinophilia-myalgia syndrome). Whether this complication is attributable to tryptophan as such or to a contaminant metabolite remains a matter of debate [41,42]. A diet rich in L-tryptophan preserved the secretion of insulin and delayed the progression of hereditary type 2 diabetes in rats [43]. It may, however, be questioned whether this effect should be attributed to tryptophan per se or to its downstream metabolites.
2.2.2. Indole
Indole is the mother compound of a large array of metabolites, which are generated in several organ systems, but mainly in the liver. Studies of intestinal indole generation observed no changes in generation at all stages of CKD, including kidney failure treated by dialysis [44]. Indole is known as a promotor of intestinal wall integrity and repair [45,46,47], and acts as an anti-inflammatory agent in enteropathy induced by non-steroidal anti-inflammatory drugs (NSAID) [47] and by counteracting the detrimental effects of lipopolysaccharides in the liver [48]. Indole also attenuates the virulence of several pathogens, such as Candida albicans, Staphyloccus aureus and Salmonella [49,50].
Alongside these positive effects, one single study reported portal hypertension, be it after supranormal doses [51], and in another study, indole administration to rats was associated with faster progression of kidney dysfunction and glomerular sclerosis [52]. As these two studies are isolated reports compared to a broad consensus on the advantages, we categorized indole as a beneficial compound. In addition, here also it can be questioned whether the responsible compound is indole or one of its downstream metabolites.
2.2.3. Indole-3-propionic Acid
Indole-3-propionic acid concentration in the serum is decreased in CKD [53]. This compound has mainly been labelled as neuroprotective [54,55], with stronger anti-oxidative capacities than melatonin, and the potential to counteract Alzheimer’s [54]. Furthermore, indole-3-propionic acid attenuates steatohepatitis [56], restrains the progression of cancer [57], improves the intestinal epithelial barrier [56,58], regulates endothelial function [59], and has been associated with a lower risk of type 2 diabetes [60]. Indole-3-propionic acid reduced weight gain in rats [61], but did not protect against the cardio-metabolic effects of Western diet in mice [62]. Indole-3-propionic acid was also identified as a modulator of cardiomyocyte mitochondrial function and overall cardiac function; however, it also showed an acute benefit but a negative chronic effect, thus exemplifying in this case setting-specific dualism [63].
2.2.4. Indole-3-(carbox)aldehyde
Indole-3-aldehyde is best known for its ability to promote gut epithelial barrier functions [64], but it also alters immune function, e.g., by the expression of interleukin-22, helping to stabilize gut mucosa immune homeostasis and intestinal microbial content [65]. It also modulates inflammatory injury induced by respiratory syncytial virus (RSV) in vitro [66], and displays antimicrobial activities, e.g., against both non methicillin-resistant and methicillin-resistant Staphylococcus aureus, and Candida [67]. Finally, studies in a murine model suggest protection against the metabolic syndrome [68].
2.2.5. Melatonin
Melatonin is essentially secreted at night by the pineal gland and controls the sleep–wake rhythm [69]. Several systematic reviews pointed to a positive effect of exogenous melatonin on sleep disturbances [70,71,72], although administration to healthy volunteers late in the evening when natural melatonin secretion was at its maximum had no impact, in contrast to administration early in the evening [73]. Additional attributed effects include immunoregulation [74,75] and analgesia [76]. Melatonin improved mitochondrial function, glycolytic metabolism and proliferation of mesenchymal stromal and stem cells collected in mice with CKD [77]. In a murine model of acute kidney injury, melatonin inhibited transition to chronic kidney disease [78]. In a randomized controlled cross-over study in hemodialysis patients, melatonin had an immunoregulatory and an anti-inflammatory effect [79].
2.2.6. Nicotinic Acid and Nicotinamide
Nicotinic acid (also referred to as niacin, although the term niacin is also used for both nicotinic acid and nicotinamide together) is generated downstream in the kynurenic pathway. About 60 mg of tryptophan is needed to generate 1 mg of nicotinic acid [80]. Also catalogued as vitamin B3, a niacin deficit has historically been linked to pellagra, a currently exceptional dermatologic disease, also characterized by diarrhea and dementia, that mainly occurs in impoverished populations with deficient meat consumption living on maize [81]. However, nicotinic acid and nicotinamide more recently gained new momentum as lipid metabolism regulators [82], although most randomized controlled trials could not demonstrate their benefit on hard outcomes [83,84]. Nicotinamide acts against endothelial oxidative stress, which links it to an anti-inflammatory and vasculoprotective effect [85]. In mice, nicotinamide supplementation prevented CKD progression by reducing kidney inflammation and fibrosis [86]. It also protected against acute kidney proximal tubule damage induced by metabolic acidosis [87] and against ischemic AKI [88]. In hemodialysis patients, nicotinic acid and nicotinamide have also been attributed a phosphate lowering effect [89,90]. However, one of the metabolites, 1-methyl-2-pyridone-5-carboxamide (2PY) [80,91], is dramatically increased in dialysis patients supplemented with these compounds, and has been linked to a number of both positive and negative effects [92] (see below).
2.3. The Ambivalent Molecules
2.3.1. Serotonin
Serotonin is primarily known as a neurotransmitter, low brain levels of which are linked to poor memory and depression [93]. However, most serotonin is found outside the central nervous system. Experimental data suggest that intestinally generated serotonin impacts central nervous serotonin levels and behavior [94]. Furthermore, serotonin also functions as a regulator of cardiovascular function, bowel motility, ejaculation, and bladder control [95]. However, it also has a pro-coagulant effect [96] and is linked to itching [97,98]. Circulating serotonin is increased in CKD patients and is dramatically high during dialysis [99,100]. In an experimental CKD study, serotonin was indirectly linked to loss of bone quality [100], and, in two clinical studies successfully administering a serotonin receptor antagonist, to the relief of itching [101,102]. However, the latter two studies were not placebo controlled and the used drug also neutralized histamine, making it impossible to attribute the drug effect to serotonin antagonism [101,102].
2.3.2. Indole-3-acetic Acid
Indole-3-acetic acid is directly produced by the intestinal microbiome. It has been linked to inflammation, cardiovascular disease, thrombogenicity, fibrosis and metabolic dysfunction [6], and more recently cognitive dysfunction [103]. On the other hand, indole-3-acetic acid has also been positively associated with the activation of stem cell factor (SCF), which plays a role in tissue repair, hematopoiesis and cell proliferation [104] and with anti-inflammatory and anti-oxidant activity on lipopolysaccharide stimulated macrophages [105], albeit at concentrations exceeding more than 30 times those observed in uremia. Indole-3-acetic acid is decreased in the feces of patients with inflammatory bowel disease and in mouse models of inflammatory bowel disease [106]. Inoculation of affected mice with microbiota generating tryptophan metabolites including indole-3-acetic acid improved inflammatory bowel disease in these mouse models [106]. Administration of indole-3-acetic acid to mice also attenuated non-alcoholic fatty liver induced by high-fat diet [107].
2.3.3. 1-Methyl-2-pyridone-5-carboxamide
1-Methyl-2-pyridone-5-carboxamide at uremic concentrations has been linked to genomic instability and anemia [92,108]. Another study, however, pointed to a protective effect against endothelial oxidative stress [85].
2.4. Remote Sensing and Signaling Theory (RSST) and Its Relation to Uremia
As discussed above, uremic molecules can be largely or somewhat toxic (especially in the setting of kidney dysfunction), largely or somewhat beneficial, or both (Figure 3). We have emphasized the theme of “balance” in normal local and/or systemic physiology, or in helping to restore the system after injury—in other words: homeostasis. Many tryptophan-derived molecules, often originating from gut microbes and labeled uremic solutes or toxins, play key roles in metabolism, redox state, and signaling, and act upon nuclear receptors (transcription factors), G-protein coupled receptors, kinases, or directly affect key metabolic pathways.
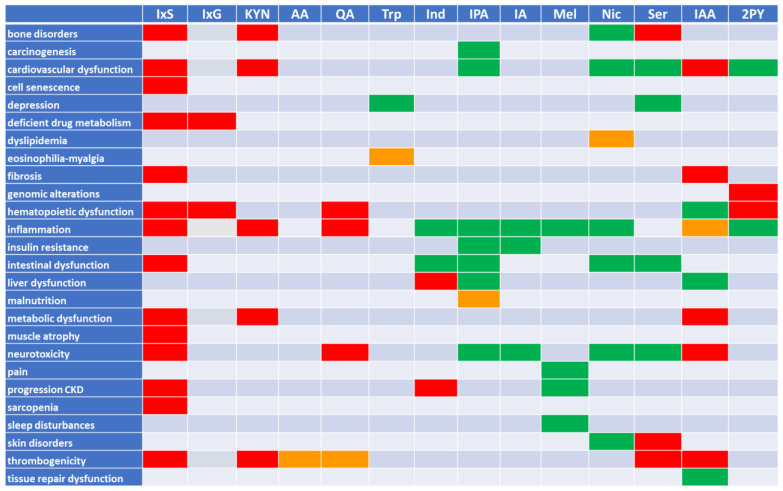
Figure 3.
Summary of the biological effects of the tryptophan metabolites discussed in this publication. Red: negative effect; green: positive effect; orange: conflicting data. CKD: chronic kidney disease. IxS: indoxyl sulfate; IxG: indoxyl glucuronide; KYN: kynurenine/kynurenic acid; AA: anthranilic acid; QA: quinolinic acid; Trp: tryptophan; Ind: indole; IPA: indole-3-propionic acid; IA: indole-3-(carbox)aldehyde; Mel: melatonin; Nic: nicotinic acid/nicotinamide; Ser: serotonin; IAA: indole-3-acetic acid; 2PY: 1-methyl-2-pyridone-5-carboxamide.
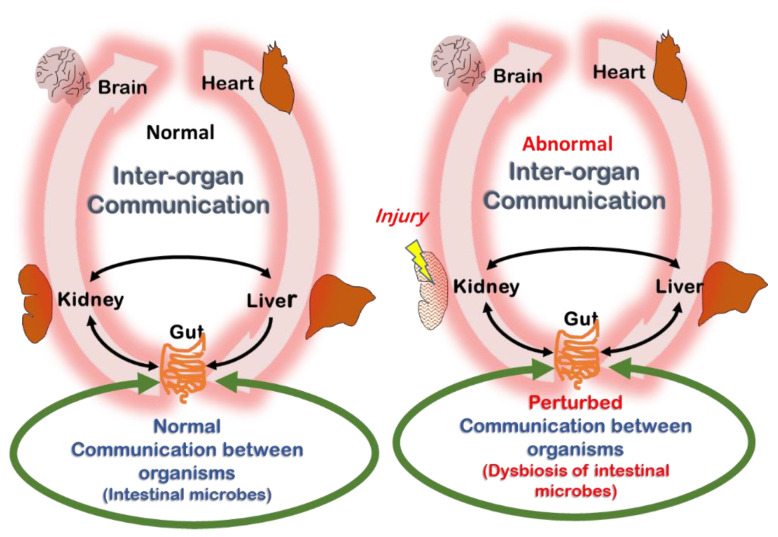
Nigam and coworkers proposed that about 600 transporters (e.g., organic anion transporters such as OAT1), “drug” metabolizing enzymes (e.g., cytochrome P450s—CYPs) and nuclear receptors (e.g., aryl hydrocarbon receptor—AHR (see below)) collaborate across organs and organisms (gut microbes–host) in a “remote sensing and signaling network” to maintain small molecule homeostasis (Figure 4) [16,17,109]. In this way, transporters, drug metabolizing enzymes (DMEs) and nuclear receptors regulate metabolic pathways, signaling, and oxidative state within and between cells, tissues, organs and organisms (e.g., gut microbes–host). Although the focus in this article as well as in other reviews on the RSST as applied to CKD [17,110] is on understanding the central role of transporters, DMEs and nuclear receptors in uremic metabolism, it is to be emphasized that the RSS theory is a general theory of small molecule communication between cells, tissues, organs, and organisms (Figure 4) [16].
Figure 4.
Normal and abnormal (uremic) remote sensing and signaling. Please see text for a detailed explanation of the remote sensing and signaling theory of interorgan and inter-organismal (gut microbe–host) communication via small molecules that regulate metabolism, signaling, and oxidative state. The proteins mediating these effects of small molecules include transporters, drug metabolizing enzymes, and nuclear receptors. Some of the regulated molecules include gut microbe-derived uremic toxins.
This transporter and DME-based small molecule remote communication system operates in parallel with, and supports, the neuroendocrine and other classic homeostatic systems. For example, thyroid hormones, sex steroids, bioactive lipids, tryptophan derivatives, and bile acids are key players in multiple homeostatic systems, including the RSS [16,111].
Many genes in the remote sensing and signaling network seem to have been conserved throughout evolution [112]. A large number of these genes or their homologs can be found in fish and flies. Many have been shown in model organisms to subserve key functions [113]. Most of these genes are highly expressed in ducts and tubules in organs throughout the body, or at other interfaces with body fluids.
If ducts or tubules fail in one organ, the ducts and tubules of other organs try to restore the system. This occurs through the regulation of genes in the remote sensing and signaling system (e.g., transporters, DMEs) in an attempt to bring gut microbe products, uremic toxins, bile acids, fatty acids, signaling molecules, urate, energy metabolites, and other molecules back into some kind of balance/compromise [16,17].
OAT1 (solute carrier 22A6—SLC22A6) and OAT3 (SLC22A8), multi-specific members of the SLC22 transporter family, are well-known for their role in kidney drug elimination [114]. They also provide examples of how the remote sensing and signaling system operates. OAT1 and OAT3 are not only the key proximal tubule transporters involved in kidney elimination of gut microbe-derived uremic toxins; they are also central to the kidney “remote sensing” of tryptophan-derivatives, which play important roles in local and systemic metabolism, signaling and redox state regulation [115,116,117].
In the case of indoxyl sulfate, there appears to be a pathway from gut microbes to liver DMEs to RSS in proximal tubule cells in the service of maintaining homeostasis via effects on metabolism, redox state and signaling events [17,118]. This suggests how uremic compounds, while potentially toxic at high concentrations in the setting of kidney disease, may otherwise subserve important normal physiological functions [14,110,111,112,113].
Declining kidney function is linked to a disturbance of the homeostatic balance maintained by the remote sensing and signaling system (Figure 4). Nevertheless, in the setting of kidney disease, an interesting example of a homeostatic correction by RSS has also been uncovered. As kidney function declines, intestinal ABCG2, an ABC transporter which extrudes urate and possibly indoxyl sulfate into the gut, becomes more active [119]. It has been suggested that this increased intestinal activity of the ATP-binding cassette G2—ABCG2 transporter is mediated by urate and/or indoxyl sulfate [120,121]. Thus, the kidney with declining function, unable to properly eliminate urate and indoxyl sulfate due in part to the loss of OAT function, may remotely signal the intestine to eliminate them via the ABCG2 transporter, to help restore homeostasis. This example of inter-organ communication in the face of injury is unlikely to be an isolated one, and the field awaits detailed studies of how transporters and DMEs in different organs help to stabilize multi-organ physiology in the face of kidney injury—and the resulting effect on uremic solutes and toxins [122]. Multi-omics analysis in humans and animal models will likely provide important clues.
2.5. Summary
Tryptophan has a substantial number of metabolites, some mainly toxic, some mainly beneficial (although to our knowledge not unequivocally supported by controlled studies), and some playing an ambivalent role. This is reviewed concisely in Figure 3, which allows to see at a glance that there are as many harmful (red background) as beneficial (green background) effects, while several molecules harbor both positive and negative effects. Apart from thrombogenicity, which is negatively impacted by most involved solutes, and insulin resistance, depression and intestinal alterations that are in the majority of cases positively influenced, most effects seem to be modified to more or less equivalent degrees in both directions (Figure 3). However, even some notorious pathogens, such as kynurenine, still may play a physiological role, e.g., by outbalancing quinolinic acid, whereas niacin at the end of the essentially negative kynurenic metabolic chain has a largely positive impact. In addition, several of the tryptophan metabolites exert their activities through transport via OAT1 and activation of the multifunctional AHR. Although one should be cautious in extrapolating animal data to humans [123], it is generally accepted that the AHR not only activates the thrombogenic tissue factor [124,125,126], but also is both a positive and negative immunomodulator [127,128], and has likely regulated the detoxification of xenobiotics since long before the appearance of humans [127,129,130] (see below).
Thus, the harms and benefits of the solutes generated by the tryptophan metabolic chain cannot be unequivocally classified as negative or positive. However, whereas the shadow side of this group of molecules has mainly drawn the attention of nephrology researchers and clinicians because of its involvement in uremic toxicity, the benefits have unfortunately often been overlooked. The RSST provides something of a remedial framework, as it considers the physiological impact of remote inter-organ (e.g., gut-liver-heart-brain-kidney) and inter-organismal (microbe-host) communication via transported small molecules, including those labeled as uremic toxins [16,17]. While the theory accepts the potential toxic nature of protein-bound uremic toxins handled by proximal tubule transporters such as OAT1 and OAT3, the RSST emphasizes their roles in the regulation of metabolism, signaling and redox events in normal and pathophysiological settings [17,109].
3. The Dualism of the Aryl Hydrocarbon Receptor
Aryl hydrocarbon receptor was first described as the dioxin receptor in the 1970s [131]. Since then, several ligands activating this transcription factor have been described [132]. AHR is an 848-amino acid protein with a nuclear localization sequence (NLS), a DNA-binding domain close to a basic helix-loop-helix (bHLH) domain, and two domains, per-Arnt-sim (PAS) A and B. PAS A plays an important role in AHR dimerization and PAS B functions as a ligand binder [133]. Finally, there is a Q-rich domain that plays a role in the activation of transcription. After ligand binding in the cytoplasm, AHR is released from its stabilizing complex (AHR-interacting protein (AIP), cellular-sarc (c-Src), P23 and heat shock protein (HSP90)) into the nucleus and heterodimerize with AHR nuclear translocator (ARNT) [134]. This heterodimer will bind to xenobiotic response element (XRE) regulatory sequences in the promoters of many genes, mainly molecules involved in detoxification such as cytochrome P450 family 1 subfamily A member 1 (CYP1A1), CYP1A2, and CYP1B1 [135]. AHR also has a ubiquitin ligase (E3), which may play a role in the negative feedback of AHR signaling [136]. In addition to the activation of the traditional genomic pathway, AHR can play the role of a signaling molecule and activate a so-called inflammatory or non-genomic pathway that will lead to the activation of the nuclear factor-kappa-light-chain enhancer of activated B cells (NF-kB) pathway [137].
From an evolutionary point of view, AHR is one of the rare genes with a variation in the coding phase between homo sapiens (Val381) and homo neanderthalensis (Ala381), with the sapiens variant responding less well to activation by xenobiotics, an effect proposed to protect modern humans from a too strong activation of AHR by environmental pollutants such as smoke [138]. However, this hypothesis has been contradicted by recent data [139].
Activation of AHR by dioxin is associated with multiple complications. These can be either acute complications, such as chloracne [140], or long-term complications, such as lymphoma and malformative syndrome [141]. The long-term effects of AHR activation by dioxin are well known from the massive use of the herbicide Agent Orange during the Vietnam War [141]. The activation by dioxin is peculiar, as it is not degraded by the organism and leads, contrary to other agonists, to a permanent activation of AHR which is not eliminated by the physiological response. Additionally, activation of AHR by benzo(a)pyrene (BaP) has deleterious effects because it promotes the formation of oncogenic adducts by activating the expression of CYP450 [135]. During chronic kidney disease, AHR is activated and plays an important role in the pro-thrombotic [142], vasculotoxic [143] and neurotoxic [21] activities of tryptophan-derived uremic toxins [123]. AHR also induces or aggravates autoimmune diseases [144] and cancer [135], and has more recently been implicated in infectious diseases affecting the central nervous system, such as Zika [145]. Inhibition of AHR could be a key to the management of many diseases [146].
Besides these negative aspects of AHR activation, mice lacking activity of this transcription factor show multiple phenotypic alterations, confirming its important physiological role outside the response to xenobiotics. Rodents that have lost AHR show vascular abnormalities, persistence of ductus venosus, a tendency towards hypertension, hepatic abnormalities, fibrosis, small liver abnormalities and immune system abnormalities mainly associated with the mucosa [147]. Animal models confirmed the necessary role of AHR in physiology. Loss of AHR studies in multiple species ranging from Caenorhabditis elegans to Drosophila and mice show that the loss of AHR elicits a decrease in healthy life expectancy [148]. A lack of activation reduces the physical capacity of these animals with age, whereas supplementation with indole has a beneficial effect by increasing healthy life expectancy. A defect of AHR activation plays an important role in ageing [149]. Hence, activation of AHR during CKD could protect against the complications related to uremic syndrome. Furthermore, it has been shown that AHR plays a crucial role in keeping the digestive epithelium healthy, and that the lack of activation of AHR by bacterial tryptophan metabolites is associated with an aggravation of inflammatory bowel diseases [150]. Similarly, defective activation of AHR in the digestive tract plays a role in the development of metabolic syndrome [151], in gluten intolerance [152] and in liver alcohol toxicity [153].
Thus, if tryptophan-derived uremic toxins are dual in their activity, this is also the case for their main receptor AHR, which can have both beneficial and deleterious effects (Table 2), depending on the cell type, the ligand [154] and the time in the life of the organism [147]. This duality of action justifies understanding the pathways activated by AHR signaling to specifically block the deleterious pathways while maintaining the beneficial ones, mainly in cardiovascular disease, the main complication of CKD [155]. In the same way as for uremic toxins, AHR could be the Pharmakon of chronic kidney disease, at the same time being a cure and a poison [156].
Table 2.
Duality in the response to activation of aryl hydrocarbon receptor.
| Positive | Negative |
|---|---|
| Detoxification | Pro-inflammatory effect |
| Preservation vascular structure | Chloracne |
| Closure ductus venosus (post-partum) | Lymphoma |
| Normalization blood pressure | Malformative syndrome |
| Preservation liver function | Carcinogenicity |
| Prevention fibrosis | Prothrombotic effect |
| Prolongation healthy life | Vascular toxicity |
| Forestalls ageing | Neurotoxicity |
| Preservation digestive epithelial function | Auto-immune diseases |
| Prevention inflammatory bowel disease | Zika infection |
| Prevention metabolic syndrome | |
| Prevention gluten enteropathy | |
| Prevention alcohol toxicity |
4. Why Did the Body Continue Producing Toxic Tryptophan Metabolites throughout Evolution?
An intriguing question is why the generation of the noxious uremic toxins has persisted despite control mechanisms such as natural selection, reproductive isolation, and developmental plasticity. Of note, in this process, humans are not an isolated entity, but closely interact with the intestinal microbiome that generates a large array of uremic toxins or their precursors [157]. Due to developmental symbiosis, symbionts can impact phenotypic adaptation that subsequently may lead to genotypic accommodation [158]. Whatever the mechanism, the metabolic cascade leading to uremic toxins has not been switched off, nor has this been prevented by adapting gut microbial composition or function during evolution.
In this context, it is important to note that many of the ~600 genes that have been identified as potentially critical to the remote sensing and signaling network are found in gene families that have high evolutionary conservation [112]. Indeed, SLC22 transporters—the family in which OAT1 (SLC22A6) and OAT3 (SLC22A8) are found—are conserved in lower organisms and play an essential role in protecting fruit flies from oxidative injury [113]. Thus, it is conceivable that if selection tended to preserve the functioning of the remote sensing and signaling network, a key aspect of that functioning may be to optimize interorgan and inter-organismal small molecule communication in normal and perturbed states. The molecules necessary for optimal remote sensing and signaling include key metabolites, signaling molecules and antioxidants. Although some of these molecules also happen to be uremic toxins or other uremic molecules of the sorts already discussed, they and/or their biosynthetic pathways might be expected to be conserved throughout evolution.
In addition, one must consider the possibility that the changes leading to uremic toxin generation came relatively late on the evolutionary timescale, so that there was not enough time for biologic correction. However, sulfotransferases, which are responsible for the conjugation of endo- and xenobiotics [159] including the sulfation of indoxyl to indoxyl sulfate, are present in mammals that made a much earlier evolutionary appearance than men [159,160], and even in more primitive organisms [161]. Thus, sulfotransferase has a long ancestral history originating many years before homo sapiens (Figure 5A).
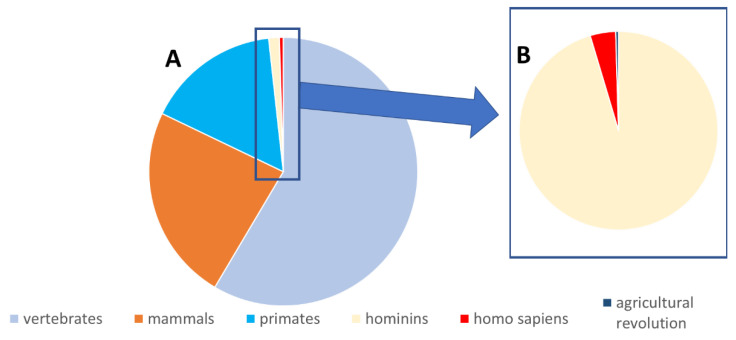
Figure 5.
Evolutionary time scale of the main elements at play in uremic toxin generation on a 24 h scale. (A) Evolution starting with the vertebrates up to now; (B) enlargement of the boxed section in (A), starting with the hominins until now. If the vertebrates appear at time 0, homo sapiens appear only during the last minutes, and the agricultural revolution is only a fraction of the homo sapiens period. Whereas sulfotransferases appear long before the animals and the intestinal microbiome appears with the invertebrates (i.e., for both before this scale starts), the current conditions leading to an epidemic propensity of CKD appear only very late, with the agricultural revolution. However, this period is long enough to cover several hundreds of generations. The red section in panel (A) and the blue section in panel (B) are enlarged out of proportion to the other sections to allow visibility.
Furthermore, symbionts, which include the intestinal microbiota, are ubiquitous across animals and plants and are in many cases essential for the functionality of the host [158]. The intestinal microbiome has been shown to be relatively consistent even across related species in conditions where dietary habits were similar [162], e.g., showing close similarities between non-human primates, Neanderthal men and hunter-gatherers [163], conforming to the thesis that symbiont microbiomes are relatively resilient [164]. The intestinal microbiome is also flexible depending on environment and lifestyle [165]. Additionally, CKD has an environmental impact [166,167,168], although the gut metabolome of hemodialysis patients also shows striking similarities with that of their household contacts without CKD [166], and the progression of CKD up to the stage of kidney failure treated by dialysis has no quantitative impact on the intestinal generation of toxins and their precursors [44]. Thus, also the intestinal microbiome processes generating uremic toxins seem to have been relatively well preserved over time.
Obviously, the risk of developing kidney disease itself, as we know it today [2], came latest of all. Although kidney disease must have existed since kidneys became functionally active, the circumstances promoting the current epidemical propensity were probably generated essentially with the agricultural revolution some 12,000 years ago, due to the transition to a more sedentary lifestyle [169,170,171] (Figure 5B), although the current exponential growth probably only started when obesity as a risk factor for diabetes overshadowed malnutrition as a nutritional problem [169,172]. However, such a period, although proportionally short compared to the other mechanisms at play in uremic toxin generation, still amounts to several generations. Given that adaptive variation due to environmental changes can occur relatively quickly [173,174,175], it might thus be assumed that, even when accounting for the history of CKD, timing may be less likely an explanation for the evolutionary persistence of uremic toxin production, and that other factors are more important.
It might also be that the involved mechanisms have other functions that are useful or even indispensable for preserving the hosting species, its symbionts or, according to the holobiont concept, the interaction between both. Conjugation mechanisms such as sulfation and glucuronidation are nonspecific and play a role not only in the generation of uremic toxins but also in the detoxification of xenobiotics, such as phenolic food components [159]. The gut microbiome is essential for the development of an efficacious immune system [176,177], gastro-intestinal motility [178], platelet function [178], brain functioning [179,180] and psychological health [179,180,181], and not only produces uremic toxins but also many beneficial compounds, as particularly exemplified by the tryptophan metabolites. In addition, some of these metabolites (indole-3-acetic acid, indole-3-propionic acid), which in plant physiology act as growth hormones [182,183] (auxins), may also stabilize the intestinal microbiome and preserve it against pathogen intrusion [56,184]. The AHR, which is activated by several toxic tryptophan metabolites resulting in a thrombogenic response [124,126], but also by non-toxic tryptophan metabolites [68], is also essential for detoxification [127,129,130] and immune regulation [127,128]. Thus, the metabolic system generating the tryptophan metabolites may be too valuable to be discarded, especially since the production of uremic toxins does not matter to most people, in whom kidney function is normal or close to normal. In addition, uremic retention solutes are probably only toxic at increased concentrations, causing no or only little harm for a host in good health. Finally, in most people developing kidney disease, this problem only occurs at a relatively advanced age, thus precluding reproductive isolation [158]. This does not, however, exclude the possibly of the occurrence of other, possibly more limited, phenotypic changes such as partial resistance to biological effects.
5. Is the Example of Tryptophan Metabolites Representative for Uremic Retention at Large?
Tryptophan metabolism may be unrepresentative for other uremic toxin families or mechanisms, but it may also be that other uremic patho-mechanisms are counterbalanced as well. This is biologically plausible, as it provides a fine-tuning mechanism preventing amplification or overly damaging effects.
For example, pro-inflammatory peptides including cytokines, which are retained and generated in uremia [185], are counteracted by their circulating receptors or anti-inflammatory cytokines (Table 3). The anorexigen desacyl-ghrelin counterbalances the orexigen ghrelin [186]. Despite many arguments in favor of a negative cardio-vascular impact [187], trimethyl amine-N-oxide is also known as a protein stabilizer [188]. The toxicity of urea [189] may be neutralized by another group of uremic retention solutes, the methylamines [190]. Uric acid can act both as a pro-oxidant and an antioxidant [191,192]. Finally, among the larger peptides (middle molecules) that are retained in uremia, alongside compounds with toxic potential, some, such as adrenomedullin, atrial natriuretic peptide, glomerulopressin and visfatin, have a positive rather than a negative biological impact [6,185]. Admittedly, the toxic effect of uremic retention solutes is supported by more scientific evidence than the potentially positive effects, but these alternative routes may have been explored insufficiently and the potential for publication bias cannot be ruled out.
Table 3.
Opposite mechanisms in families of peptidic uremic retention compounds.
| Toxic | Neutral or Non-Toxic |
|---|---|
| Complement factor D | Complement factor Ba |
| Interleukin-1β | Interleukin-1 receptor antagonist |
| Tumor necrosis factor-α | Soluble tumor necrosis factor receptor |
| Interleukin-6 | Interleukin-10 |
| Cholecystokinin | Ghrelin |
| Desacyl Ghrelin | Ghrelin |
| Leptin | Orexin A |
| Peptide YY | Neuropeptide Y |
Some may argue that this reasoning does not apply for many other groups of intestinally generated uremic toxins, such as the cresols and the phenols, which are largely classified as toxic [6,193]. However, to the best of our knowledge, very few metabolites of these pathways have been fully evaluated from the perspective of potentially beneficial effects. It is possible that if research were to be extended to a broader array of metabolites of the mother compounds in the same way as for tryptophan, this would allow for detecting some yet insufficiently understood substances. Interestingly, different steric variants of cresyl glucuronide had an opposite impact on human embryonic kidney cells [194]; whereas m- and p-cresyl glucuronide were toxic, o-cresyl glucuronide slightly stimulated cell growth [194]. Dihydroxy phenyl propionic acid has been characterized as an anti-inflammatory agent [195]. Phenyl aldehyde had a damaging effect on Candida albicans.
Whatever the pathophysiologic impact of a broader array of compounds than those we usually consider, the same argumentation as the one held above—in other words, that the mechanisms leading to uremic toxin production were not switched off because the preservation of the species was not (sufficiently) endangered—still applies.
6. Summary and Future Outlook
The message conveyed in this publication is that solutes emanating from the same origin as uremic toxins may be beneficial or even essential for body functioning. The RSST is a useful construct for thinking about small molecule communication between organs and organisms to preserve normal physiology and counteract pathophysiological states. Likewise, AHR, the main receptor of the tryptophan metabolites, also has been linked both to harms and benefits. In addition, as for other poisonous compounds, uremic poisons might also, under certain conditions and at certain concentrations, have positive effects, as exemplified in this text for the solutes that were labelled as ambivalent, but also for an accepted toxin such as indoxyl sulfate.
As this text proposes a hypothesis, it is by definition provocative and formulates a number of as yet not consolidated viewpoints, and parts of what is formulated may be refuted in the future when new knowledge appears. On the other hand, this text was formulated only after ample literature search and involves elements related to several different biological pathways and systems. Additionally, this publication essentially focuses on tryptophan metabolism, as acknowledged in Section 5, although also a limited number of other examples that are not related to tryptophan are illustrated. However, the focus on tryptophan is unavoidable in view of the scarcity of data on other pathways and factors.
More important than the question of why the production of uremic toxins has been preserved is to consider the consequences of this functional dualism for further analysis and treatment of uremic toxicity (Table 4). The baby should not be thrown away with the bath water. Specifically for tryptophan metabolites, a complete mapping of the evolution of concentrations of all involved compounds throughout the progression of kidney disease should offer a clear insight in the basic condition without therapeutic intervention. This infers that such analysis would not only include the usual suspects (presumed toxins), but also beneficial and ambiguous compounds, as depicted above, to assess in what direction their concentrations change as kidney dysfunction progresses. Additionally, research on biological effects in CKD should be extended to a broader array of metabolites. This implies that any study assessing treatment options to decrease uremic toxin concentration should consider the impact on the complete metabolite balance—with special attention to the molecules with a favorable effect, to clarify whether or not beneficial compounds are removed or affected in the same way as the toxins. Maybe a simultaneous removal of compounds with positive and negative effects explains the deceiving results of several controlled studies comparing high efficacy dialysis strategies such as on-line hemodiafiltration compared to standard treatment [196,197].
Table 4.
Recommendations for future consideration.
| Tryptophan metabolites |
|---|
| Provide a complete mapping of the evolution of compounds with positive and negative biological impacts throughout all CKD stages. |
| Extend biological research to a broader array of compounds than the usual suspects |
| Assess the effect of therapeutic strategies on molecules with as well positive as negative biological effects. |
| Consider choosing therapies that maintain or restore the balance between components with positive and negative impact, rather than removing toxins as well as beneficial compounds. |
| Promote the publication of suitable studies showing atypical results of uremic toxin actions. |
| Researchers should not shelve uremic toxin research with atypical results. |
| Other than tryptophan metabolites |
| Develop extensive reviews and studies on a broader array of metabolites than the ones frequently considered now. |
| Based on this knowledge, extend the analysis and development of therapeutic options. |
In the context of the RSST, one can envision a homeostatic system attempting to restore itself via small molecule interorgan (and inter-organismal) communication in the setting of CKD and between dialysis sessions. Dialytic removal of some small molecules that are beneficial to this endogenous restoration effort (remote sensing and signaling) may partly defeat the purpose, especially if these beneficial molecules tend to decrease inflammation, cardiovascular and endothelial damage, or fibrosis. It follows, then, that therapeutic options which maintain or restore the balance between positive and negative effects are to be preferred over those aimed only at eliminating toxins but at the expense of worsening the imbalance. In this sense, pre-, pro-, syn- or postbiotics [198] or strategies preserving kidney function may be more physiological than removal strategies; in terms of the RSST, such strategies may be more prone to promoting beneficial inter-organismal (e.g., gut microbe–host) communication via small molecules, and this question should be answered. Finally, it is desirable that scientific journals actively solicit reports of suitable quality on the neutral or beneficial effects of apparent uremic toxins, even if these results conflict with existing knowledge. Likewise, it is the responsibility of researchers not to shelve such atypical results.
In addition, a more extended metabolic review and study of the metabolites of other intestinally generated precursors (cresol, phenol, hippurates) might also reveal as yet insufficiently explored perspectives. In particular, the dualism of the metabolites of other amino acids than tryptophan (e.g., tyrosine and phenylalanine for p-cresol) should be explored with an open mind for counterbalancing elements dampening toxic effects. For all metabolic pathways, determination of concentration thresholds determining toxicity vs. benefit might also be relevant. As our understanding of the remote sensing and signaling network becomes more detailed, it may also be possible to assess the impact of “non-normal” levels of these uremic small molecules on communication pathways between cells, organs, and organisms. The results might in turn lead to reconsidering therapeutic options, e.g., by stimulating certain specific pathways and inhibiting others, or by trying not only to affect intestinal generation but also absorption by intestinal epithelial cells.
Another intriguing but unanswered question is whether a relative increase in uremic toxins with normal kidney function has a clinical impact or not. Although observational, a study by Glorieux et al. including the entire spectrum of CKD stages whereby the link of free p-cresyl sulfate to cardio-vascular disease remained significant after adjustment for several risk factors, including estimated glomerular filtration rate [199], may point in this direction, but this should be further analyzed.
Acknowledgments
Griet Glorieux. and Stéphan Burtey are beneficiaries of the European Union’s Horizon 2020 research and innovation programme under grant agreement No [860329] (“STRATEGY-CKD”). Raymond Vanholder, Stéphan Burtey and Griet Glorieux are members of the European Uremic Toxins Workgroup (EUTox). Raymond Vanholder and Griet Glorieux thank Alain Goossens (VIB-UGent) and Anne De Paepe (UGent) for the discussion and for sharing their expertise in relation to this review.
Author Contributions
Conceptualization, writing and coordination, R.V.; writing and editing, S.K.N., S.B. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Based on the example of tryptophan, we illustrate that the pathways leading to the generation of uremic toxins also produce compounds that are beneficial. This duality of uremic toxicity may have a therapeutic impact, as treatment options maintaining or restoring the balance between beneficial and noxious compounds might be preferred to therapies removing both. This aspect deserves more in-depth analysis.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kramer A., Boenink R., Noordzij M., Bosdriesz J.R., Stel V.S., Beltrán P., Ruiz J.C., Seyahi N., Farnés J.C., Stendahl M., et al. The ERA-EDTA Registry Annual Report 2017: A summary. Clin. Kidney J. 2020;13:693–709. doi: 10.1093/ckj/sfaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanholder R., Annemans L., Bello A.K., Bikbov B., Gallego D., Gansevoort R.T., Lameire N., A Luyckx V., Noruisiene E., Oostrom T., et al. Fighting the unbearable lightness of neglecting kidney health: The decade of the kidney. Clin. Kidney J. 2021;14:1719–1730. doi: 10.1093/ckj/sfab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanholder R., Annemans L., Brown E., Gansevoort R., Gout-Zwart J.J., Lameire N., Morton R.L., Oberbauer R., Postma M.J., Tonelli M., et al. Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat. Rev. Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 4.A Silver S., Long J., Zheng Y., Chertow G.M. Cost of Acute Kidney Injury in Hospitalized Patients. J. Hosp. Med. 2017;12:70–76. doi: 10.12788/jhm.2683. [DOI] [PubMed] [Google Scholar]
- 5.Susantitaphong P., Cruz D.N., Cerda J., Abulfaraj M., Alqahtani F., Koulouridis I., Jaber B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. CJASN. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanholder R., Pletinck A., Schepers E., Glorieux G.L. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins. 2018;10:33. doi: 10.3390/toxins10010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugnicourt J.-M., Godefroy O., Chillon J.-M., Choukroun G., Massy Z.A. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J. Am. Soc. Nephrol. 2013;24:353–363. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]
- 8.Martin C.E., Clotet-Freixas S., Farragher J.F., Hundemer G.L. Have We Just Scratched the Surface? A Narrative Review of Uremic Pruritus in 2020. Can. J. Kidney Health Dis. 2020;7:2054358120954024. doi: 10.1177/2054358120954024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanholder R., Argiles A., Jankowski J., European Uraemic Toxin Work Group A history of uraemic toxicity and of the European Uraemic Toxin Work Group (EUTox) Clin. Kidney J. 2021;14:1514–1523. doi: 10.1093/ckj/sfab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanholder R., De Smet R., Glorieux G., Argilés A., Baurmeister U., Brunet P., Clark W., Cohen G., de Deyn P.P., Deppisch R., et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 11.Duranton F., Cohen G., De Smet R., Rodriguez M., Jankowski J., Vanholder R., Argiles A., European Uremic Toxin Work Group Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. JASN. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadik A., Patterson L.F.S., Öztürk S., Mohapatra S.R., Panitz V., Secker P.F., Pfänder P., Loth S., Salem H., Prentzell M.T., et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell. 2020;182:1252–1270.e34. doi: 10.1016/j.cell.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Vanholder R., Schepers E., Pletinck A., Nagler E.V., Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014;25:1897–1907. doi: 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolachalama V.B., Shashar M., Alousi F., Shivanna S., Rijal K., Belghasem M.E., Walker J., Matsuura S., Chang G.H., Gibson C.M., et al. Uremic Solute-Aryl Hydrocarbon Receptor-Tissue Factor Axis Associates with Thrombosis after Vascular Injury in Humans. J. Am. Soc. Nephrol. 2018;29:1063–1072. doi: 10.1681/ASN.2017080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondouin B., Cerini C., Dou L., Sallée M., Duval-Sabatier A., Pletinck A., Calaf R., Lacroix R., Jourde-Chiche N., Poitevin S., et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84:733–744. doi: 10.1038/ki.2013.133. [DOI] [PubMed] [Google Scholar]
- 16.Nigam S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2015;14:29–44. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nigam S.K., Bush K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019;15:301–316. doi: 10.1038/s41581-019-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirich T., Meyer T.W. Indoxyl sulfate: Long suspected but not yet proven guilty. Clin. J. Am. Soc. Nephrol. CJASN. 2011;6:3–4. doi: 10.2215/CJN.10141110. [DOI] [PubMed] [Google Scholar]
- 19.Leong S.C., Sirich T.L. Indoxyl Sulfate—Review of Toxicity and Therapeutic Strategies. Toxins. 2016;8:358. doi: 10.3390/toxins8120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adesso S., Magnus T., Cuzzocrea S., Campolo M., Rissiek B., Paciello O., Autore G., Pinto A., Marzocco S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017;8:370. doi: 10.3389/fphar.2017.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobot M., Thomas L., Moyon A., Fernandez S., McKay N., Balasse L., Garrigue P., Brige P., Chopinet S., Poitevin S., et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020;31:1509–1521. doi: 10.1681/ASN.2019070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun C.-Y., Li J.-R., Wang Y.-Y., Lin S.-Y., Ou Y.-C., Lin C.-J., Wang J.-D., Liao S.-L., Chen C.-J. Indoxyl sulfate caused behavioral abnormality and neurodegeneration in mice with unilateral nephrectomy. Aging. 2021;13:6681–6701. doi: 10.18632/aging.202523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adesso S., Ruocco M., Rapa S.F., Dal Piaz F., Di Iorio B.R., Popolo A., Autore G., Nishijima F., Pinto A., Marzocco S. Effect of Indoxyl Sulfate on the Repair and Intactness of Intestinal Epithelial Cells: Role of Reactive Oxygen Species’ Release. Int. J. Mol. Sci. 2019;20:2280. doi: 10.3390/ijms20092280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamano H., Ikeda Y., Watanabe H., Horinouchi Y., Izawa-Ishizawa Y., Imanishi M., Zamami Y., Takechi K., Miyamoto L., Ishizawa K., et al. The uremic toxin indoxyl sulfate interferes with iron metabolism by regulating hepcidin in chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2018;33:586–597. doi: 10.1093/ndt/gfx252. [DOI] [PubMed] [Google Scholar]
- 25.Dias G.F., Bonan N.B., Steiner T.M., Tozoni S.S., Rodrigues S., Nakao L.S., Kuntsevich V., Filho R.P., Kotanko P., Moreno-Amaral A.N. Indoxyl Sulfate, a Uremic Toxin, Stimulates Reactive Oxygen Species Production and Erythrocyte Cell Death Supposedly by an Organic Anion Transporter 2 (OAT2) and NADPH Oxidase Activity-Dependent Pathways. Toxins. 2018;10:280. doi: 10.3390/toxins10070280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C.-J., Chen C.-Y., Lai T.-S., Wu P.-C., Chuang C.-K., Sun F.-J., Liu H.-L., Chen H.-H., Yeh H.-I., Lin C.-S., et al. The role of indoxyl sulfate in renal anemia in patients with chronic kidney disease. Oncotarget. 2017;8:83030–83037. doi: 10.18632/oncotarget.18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues G.G.C., Dellê H., Brito R.B.O., Cardoso V.O., Fernandes K., Mesquita-Ferrari R.A., Cunha R.S., Stinghen A., Dalboni M.A., Barreto F.C. Indoxyl Sulfate Contributes to Uremic Sarcopenia by Inducing Apoptosis in Myoblasts. Arch. Med. Res. 2020;51:21–29. doi: 10.1016/j.arcmed.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y.-L., Liu C.-H., Lai Y.-H., Wang C.-H., Kuo C.-H., Liou H.-H., Hsu B.-G. Association of Serum Indoxyl Sulfate Levels with Skeletal Muscle Mass and Strength in Chronic Hemodialysis Patients: A 2-year Longitudinal Analysis. Calcif. Tissue Res. 2020;107:257–265. doi: 10.1007/s00223-020-00719-x. [DOI] [PubMed] [Google Scholar]
- 29.Santana Machado T., Poitevin S., Paul P., McKay N., Jourde-Chiche N., Legris T., Mouly-Bandini A., Dignat-George F., Brunet P., Masereeuw R. Indoxyl Sulfate Upregulates Liver P-Glycoprotein Expression and Activity through Aryl Hydrocarbon Receptor Signaling. J. Am. Soc. Nephrol. JASN. 2018;29:906–918. doi: 10.1681/ASN.2017030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Y.S., Kim S.M., Lee J.H., Lee S.H. Co-Administration of Melatonin Effectively Enhances the Therapeutic Effects of Pioglitazone on Mesenchymal Stem Cells Undergoing Indoxyl Sulfate-Induced Senescence through Modulation of Cellular Prion Protein Expression. Int. J. Mol. Sci. 2018;19:1367. doi: 10.3390/ijms19051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sári Z., Mikó E., Kovács T., Boratkó A., Ujlaki G., Jankó L., Kiss B., Uray K., Bai P. Indoxylsulfate, a Metabolite of the Microbiome, Has Cytostatic Effects in Breast Cancer via Activation of AHR and PXR Receptors and Induction of Oxidative Stress. Cancers. 2020;12:2915. doi: 10.3390/cancers12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asai H., Hirata J., Watanabe-Akanuma M. Indoxyl glucuronide, a protein-bound uremic toxin, inhibits hypoxia-inducible factordependent erythropoietin expression through activation of aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2018;504:538–544. doi: 10.1016/j.bbrc.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Cheung K.W.K., Hsueh C.-H., Zhao P., Meyer T.W., Zhang L., Huang S.-M., Giacomini K.M. The Effect of Uremic Solutes on the Organic Cation Transporter 2. J. Pharm. Sci. 2017;106:2551–2557. doi: 10.1016/j.xphs.2017.04.076. [DOI] [PubMed] [Google Scholar]
- 34.Badawy A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalaska B., Pawlak K., Domaniewski T., Oksztulska-Kolanek E., Znorko B., Roszczenko A., Rogalska J., Brzóska M.M., Lipowicz P., Doroszko M., et al. Elevated Levels of Peripheral Kynurenine Decrease Bone Strength in Rats with Chronic Kidney Disease. Front. Physiol. 2017;8:836. doi: 10.3389/fphys.2017.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mor A., Pawlak K., Kalaska B., Domaniewski T., Sieklucka B., Zieminska M., Cylwik B., Pawlak D. Modulation of the Paracrine Kynurenic System in Bone as a New Regulator of Osteoblastogenesis and Bone Mineral Status in an Animal Model of Chronic Kidney Disease Treated with LP533401. Int. J. Mol. Sci. 2020;21:5979. doi: 10.3390/ijms21175979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaminski T.W., Pawlak K., Karbowska M., Mysliwiec M., Grzegorzewski W., Kuna J., Pawlak D. Association between uremic toxin-anthranilic acid and fibrinolytic system activity in predialysis patients at different stages of chronic kidney disease. Int. Urol. Nephrol. 2017;50:127–135. doi: 10.1007/s11255-017-1729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawlak K., Myśliwiec M., Pawlak D. Kynurenine pathway—A new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv. Med. Sci. 2010;55:196–203. doi: 10.2478/v10039-010-0015-6. [DOI] [PubMed] [Google Scholar]
- 39.Guillemin G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356–1365. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 40.Leszczyńska A., Misztal T., Marcińczyk N., Kamiński T., Kramkowski K., Chabielska E., Pawlak D. Effect of quinolinic acid—A uremic toxin from tryptophan metabolism—On hemostatic profile in rat and mouse thrombosis models. Adv. Med. Sci. 2019;64:370–380. doi: 10.1016/j.advms.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Shaw K., Turner J., Del Mar C. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst. Rev. 2001:CD003198. doi: 10.1002/14651858.CD003198. [DOI] [PubMed] [Google Scholar]
- 42.Smith M.J., Garrett R.H. A heretofore undisclosed crux of eosinophilia-myalgia syndrome: Compromised histamine degradation. Agents Actions. 2005;54:435–450. doi: 10.1007/s00011-005-1380-7. [DOI] [PubMed] [Google Scholar]
- 43.Inubushi T., Kamemura N., Oda M., Sakurai J., Nakaya Y., Harada N., Suenaga M., Matsunaga Y., Ishidoh K., Katunuma N. L-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J. Nutr. Sci. Vitaminol. 2012;58:415–422. doi: 10.3177/jnsv.58.415. [DOI] [PubMed] [Google Scholar]
- 44.Gryp T., De Paepe K., Vanholder R., Kerckhof F.-M., Van Biesen W., Van de Wiele T., Verbeke F., Speeckaert M., Joossens M., Couttenye M.M., et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020;97:1230–1242. doi: 10.1016/j.kint.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 45.Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA. 2009;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berstad A., Raa J., Valeur J. Indole—The scent of a healthy ‘inner soil’. Microb. Ecol. Health Dis. 2015;26:27997. doi: 10.3402/mehd.v26.27997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitfield-Cargile C.M., Cohen N.D., Chapkin R.S., Weeks B.R., Davidson L.A., Goldsby J.S., Hunt C.L., Steinmeyer S.H., Menon R., Suchodolski J.S., et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7:246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beaumont M., Neyrinck A.M., Olivares M., Rodriguez J., de Rocca Serra A., Roumain M., Bindels L.B., Cani P.D., Evenepoel P., Muccioli G.G., et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018;32:fj201800544. doi: 10.1096/fj.201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh S., Go G., Mylonakis E., Kim Y. The bacterial signalling molecule indole attenuates the virulence of the fungal pathogen Candida albicans. J. Appl. Microbiol. 2012;113:622–628. doi: 10.1111/j.1365-2672.2012.05372.x. [DOI] [PubMed] [Google Scholar]
- 50.Lee J.-H., Cho H.S., Kim Y., Kim J.-A., Banskota S., Cho M.H., Lee J. Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013;97:4543–4552. doi: 10.1007/s00253-012-4674-z. [DOI] [PubMed] [Google Scholar]
- 51.Huc T., Konop M., Onyszkiewicz M., Podsadni P., Szczepańska A., Turło J., Ufnal M. Colonic indole, gut bacteria metabolite of tryptophan, increases portal blood pressure in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R646–R655. doi: 10.1152/ajpregu.00111.2018. [DOI] [PubMed] [Google Scholar]
- 52.Niwa T., Ise M., Miyazaki T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am. J. Nephrol. 1994;14:207–212. doi: 10.1159/000168716. [DOI] [PubMed] [Google Scholar]
- 53.Sun C.-Y., Lin C.-J., Pan H.-C., Lee C.-C., Lu S.-C., Hsieh Y.-T., Huang S.-Y., Huang H.-Y. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clin. Nutr. 2019;38:2945–2948. doi: 10.1016/j.clnu.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 54.Chyan Y.J., Poeggeler B., Omar R.A., Chain D.G., Frangione B., Ghiso J., Pappolla M.A. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem. 1999;274:21937–21942. doi: 10.1074/jbc.274.31.21937. [DOI] [PubMed] [Google Scholar]
- 55.Hwang I.K., Yoo K.-Y., Li H., Park O.K., Lee C.H., Choi J.H., Jeong Y.-G., Lee Y.L., Kim Y.-M., Kwon Y.-G., et al. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J. Neurosci. Res. 2009;87:2126–2137. doi: 10.1002/jnr.22030. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Z.-H., Xin F.-Z., Xue Y., Hu Z., Han Y., Ma F., Zhou D., Liu X.-L., Cui A., Liu Z., et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019;51:1–14. doi: 10.1038/s12276-019-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sári Z., Mikó E., Kovács T., Jankó L., Csonka T., Lente G., Sebő É., Tóth J., Tóth D., Árkosy P., et al. Indolepropionic Acid, a Metabolite of the Microbiome, Has Cytostatic Properties in Breast Cancer by Activating AHR and PXR Receptors and Inducing Oxidative Stress. Cancers. 2020;12:2411. doi: 10.3390/cancers12092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J., Zhang L., Wu T., Li Y., Zhou X., Ruan Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021;69:1487–1495. doi: 10.1021/acs.jafc.0c05205. [DOI] [PubMed] [Google Scholar]
- 59.Pulakazhi Venu V.K., Saifeddine M., Mihara K., Tsai Y., Nieves K., Alston L., Mani S., McCoy K.D., Hollenberg M.D., Hirota S.A. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am. J. Physiol. Endocrinol. Metab. 2019;317:E350–E361. doi: 10.1152/ajpendo.00572.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Mello V.D., Paananen J., Lindstrom J., Lankinen M.A., Shi L., Kuusisto J., Pihlajamäki J., Auriola S., Lehtonen M., Rolandsson O., et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017;7:46337. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konopelski P., Konop M., Gawrys-Kopczynska M., Podsadni P., Szczepanska A., Ufnal M. Indole-3-Propionic Acid, a Tryptophan-Derived Bacterial Metabolite, Reduces Weight Gain in Rats. Nutrients. 2019;11:591. doi: 10.3390/nu11030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee D.M., Ecton K.E., Trikha S.R., Wrigley S.D., Thomas K.N., Battson M.L., Wei Y., Johnson S.A., Weir T.L., Gentile C.L. Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am. J. Physiol. Liver Physiol. 2020;319:G51–G62. doi: 10.1152/ajpgi.00375.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gesper M., Nonnast A.B.H., Kumowski N., Stoehr R., Schuett K., Marx N., Kappel B.A. Gut-Derived Metabolite Indole-3-Propionic Acid Modulates Mitochondrial Function in Cardiomyocytes and Alters Cardiac Function. Front. Med. 2021;8:648259. doi: 10.3389/fmed.2021.648259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zelante T., Puccetti M., Giovagnoli S., Romani L. Regulation of host physiology and immunity by microbial indole-3-aldehyde. Curr. Opin. Immunol. 2021;70:27–32. doi: 10.1016/j.coi.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Hou X., Zhang X., Bi J., Zhu A., He L. Indole-3-carboxaldehyde regulates RSV-induced inflammatory response in RAW264.7 cells by moderate inhibition of the TLR7 signaling pathway. J. Nat. Med. 2021;75:602–611. doi: 10.1007/s11418-021-01506-0. [DOI] [PubMed] [Google Scholar]
- 67.Borghi M., Pariano M., Solito V., Puccetti M., Bellet M.M., Stincardini C., Renga G., Vacca C., Sellitto F., Mosci P., et al. Targeting the Aryl Hydrocarbon Receptor With Indole-3-Aldehyde Protects From Vulvovaginal Candidiasis via the IL-22-IL-18 Cross-Talk. Front. Immunol. 2019;10:2364. doi: 10.3389/fimmu.2019.02364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puccetti M., Pariano M., Borghi M., Barola C., Moretti S., Galarini R., Mosci P., Ricci M., Costantini C., Giovagnoli S. Enteric formulated indole-3-carboxaldehyde targets the aryl hydrocarbon receptor for protection in a murine model of metabolic syndrome. Int. J. Pharm. 2021;602:120610. doi: 10.1016/j.ijpharm.2021.120610. [DOI] [PubMed] [Google Scholar]
- 69.Arendt J. Melatonin, circadian rhythms, and sleep. N. Engl. J. Med. 2000;343:1114–1116. doi: 10.1056/NEJM200010123431510. [DOI] [PubMed] [Google Scholar]
- 70.Li T., Jiang S., Han M., Yang Z., Lv J., Deng C., Reiter R.J., Yang Y. Exogenous melatonin as a treatment for secondary sleep disorders: A systematic review and meta-analysis. Front. Neuroendocr. 2019;52:22–28. doi: 10.1016/j.yfrne.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Auld F., Maschauer E., Morrison I., Skene D., Riha R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017;34:10–22. doi: 10.1016/j.smrv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Fatemeh G., Sajjad M., Niloufar R., Neda S., Leila S., Khadijeh M. Effect of melatonin supplementation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2021;269:205–216. doi: 10.1007/s00415-020-10381-w. [DOI] [PubMed] [Google Scholar]
- 73.Stone B.M., Turner C., Mills S.L., Nicholson A.N. Hypnotic activity of melatonin. Sleep. 2000;23:663–669. doi: 10.1093/sleep/23.5.1i. [DOI] [PubMed] [Google Scholar]
- 74.Crespo I., Fernandez-Palanca P., San-Miguel B., Alvarez M., Gonzalez-Gallego J., Tunon M.J. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J. Cell Mol. Med. 2020;24:7625–7636. doi: 10.1111/jcmm.15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo J., Zhang Z., Sun H., Song J., Chen X., Huang J., Lin X., Zhou R. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci. 2020;242:117191. doi: 10.1016/j.lfs.2019.117191. [DOI] [PubMed] [Google Scholar]
- 76.Wilhelmsen M., Amirian I., Reiter R.J., Rosenberg J., Gögenur I. Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res. 2011;51:270–277. doi: 10.1111/j.1600-079X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 77.Go G., Yoon Y.M., Yoon S., Lee G., Lim J.H., Han S.-Y., Lee S.H. Melatonin Protects Chronic Kidney Disease Mesenchymal Stem/Stromal Cells against Accumulation of Methylglyoxal via Modulation of Hexokinase-2 Expression. Biomol. Ther. 2021;30:28–37. doi: 10.4062/biomolther.2021.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen D.Q., Feng Y.L., Chen L., Liu J.R., Wang M., Vaziri N.D., Zhao Y.-Y. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFkappaB/Nrf2 axis. Free Radic. Biol. Med. 2019;134:484–497. doi: 10.1016/j.freeradbiomed.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 79.Marzougui H., Hammouda O., Ben Dhia I., Maaloul R., Agrebi I., Chaker H., Kammoun K., Ben Hmida M., Ayadi F., Kallel C., et al. Melatonin ingestion before intradialytic exercise improves immune responses in hemodialysis patients. Int. Urol. Nephrol. 2021;53:553–562. doi: 10.1007/s11255-020-02643-3. [DOI] [PubMed] [Google Scholar]
- 80.Creeke P.I., Seal A.J. Quantitation of the niacin metabolites 1-methylnicotinamide and l-methyl-2-pyridone-5-carboxamide in random spot urine samples, by ion-pairing reverse-phase HPLC with UV detection, and the implications for the use of spot urine samples in the assessment of niacin status. J. Chromatogr. B. 2005;817:247–253. doi: 10.1016/j.jchromb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Hegyi J., Schwartz R.A., Hegyi V. Pellagra: Dermatitis, dementia, and diarrhea. Int. J. Dermatol. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 82.Kamanna V.S., Kashyap M.L. Mechanism of action of niacin. Am. J. Cardiol. 2008;101:20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 83.Garg A., Sharma A., Krishnamoorthy P., Garg J., Virmani D., Sharma T., Stefanini G., Kostis J.B., Mukherjee D., Sikorskaya E. Role of Niacin in Current Clinical Practice: A Systematic Review. Am. J. Med. 2017;130:173–187. doi: 10.1016/j.amjmed.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 84.D’Andrea E., Hey S.P., Ramirez C.L., Kesselheim A.S. Assessment of the Role of Niacin in Managing Cardiovascular Disease Outcomes: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2019;2:e192224. doi: 10.1001/jamanetworkopen.2019.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slominska E., Yuen A., Osman L., Gebicki J., Yacoub M.H., Smolenski R. Cytoprotective effects of nicotinamide derivatives in endothelial cells. Nucleosides Nucleotides Nucleic Acids. 2008;27:863–866. doi: 10.1080/15257770802146528. [DOI] [PubMed] [Google Scholar]
- 86.Kumakura S., Sato E., Sekimoto A., Hashizume Y., Yamakage S., Miyazaki M., Ito S., Harigae H., Takahashi N. Nicotinamide Attenuates the Progression of Renal Failure in a Mouse Model of Adenine-Induced Chronic Kidney Disease. Toxins. 2021;13:50. doi: 10.3390/toxins13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bugarski M., Ghazi S., Polesel M., Martins J.R., Hall A.M. Changes in NAD and Lipid Metabolism Drive Acidosis-Induced Acute Kidney Injury. J. Am. Soc. Nephrol. 2021;32:342–356. doi: 10.1681/ASN.2020071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faivre A., Katsyuba E., Verissimo T., Lindenmeyer M., Rajaram R.D., Naesens M., Heckenmeyer C., Mottis A., Feraille E., Cippà P. Differential role of nicotinamide adenine dinucleotide deficiency in acute and chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2021;36:60–68. doi: 10.1093/ndt/gfaa124. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi Y., Tanaka A., Nakamura T., Fukuwatari T., Shibata K., Shimada N., Ebihara I., Koide H. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 90.He Y.-M., Feng L., Huo D.-M., Yang Z.-H., Liao Y.-H. Benefits and harm of niacin and its analog for renal dialysis patients: A systematic review and meta-analysis. Int. Urol. Nephrol. 2014;46:433–442. doi: 10.1007/s11255-013-0559-z. [DOI] [PubMed] [Google Scholar]
- 91.Inamadugu J.K., Damaramadugu R., Mullangi R., Ponneri V. Simultaneous determination of niacin and its metabolites--nicotinamide, nicotinuric acid and N-methyl-2-pyridone-5-carboxamide--in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Biomed. Chromatogr. 2010;24:1059–1074. doi: 10.1002/bmc.1406. [DOI] [PubMed] [Google Scholar]
- 92.Lenglet A., Liabeuf S., El Esper N., Brisset S., Mansour J., Lemaire-Hurtel A.-S., Mary A., Brazier M., Kamel S., Mentaverri R., et al. Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2017;32:1597. doi: 10.1093/ndt/gfx249. [DOI] [PubMed] [Google Scholar]
- 93.Jenkins T.A., Nguyen J.C.D., Polglaze K.E., Bertrand P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016;8:56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berger M., Gray J.A., Roth B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez-Vilchez I., Diaz-Ricart M., White J.G., Escolar G., Galan A.M. Serotonin enhances platelet procoagulant properties and their activation induced during platelet tissue factor uptake. Cardiovasc. Res. 2009;84:309–316. doi: 10.1093/cvr/cvp205. [DOI] [PubMed] [Google Scholar]
- 97.Yamaguchi T., Nagasawa T., Satoh M., Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res. 1999;35:77–83. doi: 10.1016/S0168-0102(99)00070-X. [DOI] [PubMed] [Google Scholar]
- 98.Ständer S., Böckenholt B., Schürmeyer-Horst F., Weishaupt C., Heuft G., Schneider T. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: Results of an open-labelled, two-arm proof-of-concept study. Acta Derm. Venereol. 2009;89:45–51. doi: 10.2340/00015555-0553. [DOI] [PubMed] [Google Scholar]
- 99.Kerr P.G., Argiles A., Mion C. Whole blood serotonin levels are markedly elevated in patients on dialytic therapy. Am. J. Nephrol. 1992;12:14–18. doi: 10.1159/000168411. [DOI] [PubMed] [Google Scholar]
- 100.Pawlak D., Oksztulska-Kolanek E., Znorko B., Domaniewski T., Rogalska J., Roszczenko A., Brzóska M.M., Pryczynicz A., Kemona A., Pawlak K. The Association between Elevated Levels of Peripheral Serotonin and Its Metabolite—5-Hydroxyindoleacetic Acid and Bone Strength and Metabolism in Growing Rats with Mild Experimental Chronic Kidney Disease. PLoS ONE. 2016;11:e0163526. doi: 10.1371/journal.pone.0163526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehrpooya M., Gholyaf M., Yasrebifar F., Mohammadi Y., Sheikh V. Evaluation of Efficacy of Mirtazapine on Pruritus and Serum Histamine and Serotonin Levels in Patients Undergoing Hemodialysis: A Before—After Pilot Clinical Trial. Int. J. Nephrol. Renov. Dis. 2020;13:129–138. doi: 10.2147/IJNRD.S246393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balaskas E.V., Bamihas G.I., Karamouzis M., Voyiatzis G., Tourkantonis A. Histamine and serotonin in uremic pruritus: Effect of ondansetron in CAPD-pruritic patients. Nephron. 1998;78:395–402. doi: 10.1159/000044967. [DOI] [PubMed] [Google Scholar]
- 103.Lin Y.-T., Wu P.-H., Lee H.-H., Mubanga M., Chen C.-S., Kuo M.-C., Chiu Y.-W., Kuo P.-L., Hwang S.-J. Indole-3 acetic acid increased risk of impaired cognitive function in patients receiving hemodialysis. NeuroToxicology. 2019;73:85–91. doi: 10.1016/j.neuro.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 104.Wu P.-H., Lin Y.-T., Wu P.Y., Lee H.-H., Lee S.-C., Hung S.-C., Chen S.-C., Kuo M.-C., Chiu Y.-W. Association between Circulation Indole-3-Acetic Acid Levels and Stem Cell Factor in Maintenance Hemodialysis Patients: A Cross-Sectional Study. J. Clin. Med. 2020;9:124. doi: 10.3390/jcm9010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji Y., Yin W., Liang Y., Sun L., Yin Y., Zhang W. Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells. Int. J. Mol. Sci. 2020;21:1579. doi: 10.3390/ijms21051579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lamas B., Richard M.L., Leducq V., Pham H.-P., Michel M.-L., DA Costa G., Bridonneau C., Jegou S., Hoffmann T.W., Natividad J.M., et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji Y., Gao Y., Chen H., Yin Y., Zhang W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients. 2019;11:2062. doi: 10.3390/nu11092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutkowski B., Slominska E., Szolkiewicz M., Smolenski R.T., Striley C., Rutkowski P., Swierczynski J. N-methyl-2-pyridone-5-carboxamide: A novel uremic toxin? Kidney Int. Suppl. 2003:S19–S21. doi: 10.1046/j.1523-1755.63.s84.36.x. [DOI] [PubMed] [Google Scholar]
- 109.Rosenthal S.B., Bush K.T., Nigam S.K. A Network of SLC and ABC Transporter and DME Genes Involved in Remote Sensing and Signaling in the Gut-Liver-Kidney Axis. Sci. Rep. 2019;9:11879. doi: 10.1038/s41598-019-47798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lowenstein J., Nigam S.K. Uremic Toxins in Organ Crosstalk. Front. Med. 2021;8:592602. doi: 10.3389/fmed.2021.592602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang J., Wang H., Fan Y., Yu Z., You G. Regulation of organic anion transporters: Role in physiology, pathophysiology, and drug elimination. Pharmacol. Ther. 2021;217:107647. doi: 10.1016/j.pharmthera.2020.107647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu C., Nigam K.B., Date R.C., Bush K.T., Springer S.A., Saier M.H., Wu W., Nigam S.K. Evolutionary Analysis and Classification of OATs, OCTs, OCTNs, and Other SLC22 Transporters: Structure-Function Implications and Analysis of Sequence Motifs. PLoS ONE. 2015;10:e0140569. doi: 10.1371/journal.pone.0140569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engelhart D.C., Azad P., Ali S., Granados J.C., Haddad G.G., Nigam S.K. Drosophila SLC22 Orthologs Related to OATs, OCTs, and OCTNs Regulate Development and Responsiveness to Oxidative Stress. Int. J. Mol. Sci. 2020;21:2002. doi: 10.3390/ijms21062002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nigam S.K., Bush K.T., Martovetsky G., Ahn S.-Y., Liu H.C., Richard E., Bhatnagar V., Wu W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015;95:83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jansen J., Jansen K., Neven E., Poesen R., Othman A., van Mil A., Sluijter J., Toraño J.S., Zaal E.A., Berkers C.R., et al. Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc. Natl. Acad. Sci. USA. 2019;116:16105–16110. doi: 10.1073/pnas.1821809116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu W., Bush K.T., Nigam S.K. Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in vivo Handling of Uremic Toxins and Solutes. Sci. Rep. 2017;7:4939. doi: 10.1038/s41598-017-04949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Granados J.C., Richelle A., Gutierrez J.M., Zhang P., Zhang X., Bhatnagar V., Lewis N.E., Nigam S.K. Coordinate regulation of systemic and kidney tryptophan metabolism by the drug transporters OAT1 and OAT3. J. Biol. Chem. 2021;296:100575. doi: 10.1016/j.jbc.2021.100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bush K.T., Singh P., Nigam S.K. Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. JCI Insight. 2020;5:e133817. doi: 10.1172/jci.insight.133817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhatnagar V., Richard E.L., Wu W., Nievergelt C.M., Lipkowitz M.S., Jeff J., Maihofer A.X., Nigam S.K. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: Potential role of remote sensing and signaling. Clin. Kidney J. 2016;9:444–453. doi: 10.1093/ckj/sfw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Komori H., Yamada K., Tamai I. Hyperuricemia enhances intracellular urate accumulation via down-regulation of cell-surface BCRP/ABCG2 expression in vascular endothelial cells. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2018;1860:973–980. doi: 10.1016/j.bbamem.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 121.Lu Y., Nakanishi T., Hosomi A., Komori H., Tamai I. In-vitro evidence of enhanced breast cancer resistance protein-mediated intestinal urate secretion by uremic toxins in Caco-2 cells. J. Pharm. Pharmacol. 2015;67:170–177. doi: 10.1111/jphp.12328. [DOI] [PubMed] [Google Scholar]
- 122.Torres A.M., Dnyanmote A.V., Granados J.C., Nigam S.K. Renal and non-renal response of ABC and SLC transporters in chronic kidney disease. Expert Opin. Drug Metab. Toxicol. 2021;17:515–542. doi: 10.1080/17425255.2021.1899159. [DOI] [PubMed] [Google Scholar]
- 123.Ravid J.D., Kamel M.H., Chitalia V.C. Uraemic solutes as therapeutic targets in CKD-associated cardiovascular disease. Nat. Rev. Nephrol. 2021;17:402–416. doi: 10.1038/s41581-021-00408-4. [DOI] [PubMed] [Google Scholar]
- 124.Shivanna S., Kolandaivelu K., Shashar M., Belghasim M., Al-Rabadi L., Balcells M., Zhang A., Weinberg J., Francis J., Pollastri M., et al. The Aryl Hydrocarbon Receptor is a Critical Regulator of Tissue Factor Stability and an Antithrombotic Target in Uremia. J. Am. Soc. Nephrol. 2016;27:189–201. doi: 10.1681/ASN.2014121241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sallée M., Dou L., Cerini C., Poitevin S., Brunet P., Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins. 2014;6:934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stevens E.A., Mezrich J.D., Bradfield C.A. The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neavin D.R., Liu D., Ray B., Weinshilboum R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018;19:3851. doi: 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tian J., Feng Y., Fu H., Xie H.Q., Jiang J.X., Zhao B. The Aryl Hydrocarbon Receptor: A Key Bridging Molecule of External and Internal Chemical Signals. Environ. Sci. Technol. 2015;49:9518–9531. doi: 10.1021/acs.est.5b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hahn M.E. Aryl hydrocarbon receptors: Diversity and evolution. Chem. Interact. 2002;141:131–160. doi: 10.1016/S0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 131.Poland A.P., Glover E., Robinson J.R., Nebert D.W. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically “nonresponsive” to other aromatic hydrocarbons. J. Biol. Chem. 1974;249:5599–5606. doi: 10.1016/S0021-9258(20)79769-3. [DOI] [PubMed] [Google Scholar]
- 132.Gutiérrez-Vázquez C., Quintana F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barroso A., Mahler J.V., Fonseca-Castro P.H., Quintana F.J. The aryl hydrocarbon receptor and the gut–brain axis. Cell. Mol. Immunol. 2021;18:259–268. doi: 10.1038/s41423-020-00585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19:184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 135.Murray I.A., Patterson A., Perdew G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luecke-Johansson S., Gralla M., Rundqvist H., Ho J.C., Johnson R.S., Gradin K., Poellinger L. A Molecular Mechanism To Switch the Aryl Hydrocarbon Receptor from a Transcription Factor to an E3 Ubiquitin Ligase. Mol. Cell. Biol. 2017;37:e00630-16. doi: 10.1128/MCB.00630-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Addi T., Poitevin S., McKay N., El Mecherfi K.E., Kheroua O., Jourde-Chiche N., De Macedo A., Gondouin B., Cerini C., Brunet P., et al. Mechanisms of tissue factor induction by the uremic toxin indole-3 acetic acid through aryl hydrocarbon receptor/nuclear factor-kappa B signaling pathway in human endothelial cells. Arch. Toxicol. 2019;93:121–136. doi: 10.1007/s00204-018-2328-3. [DOI] [PubMed] [Google Scholar]
- 138.Hubbard T.D., Murray I.A., Bisson W.H., Sullivan A.P., Sebastian A., Perry G.H., Jablonski N.G., Perdew G.H. Divergent Ah Receptor Ligand Selectivity during Hominin Evolution. Mol. Biol. Evol. 2016;33:2648–2658. doi: 10.1093/molbev/msw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aarts J.M.M.J.G., Alink G.M., Franssen H.J., Roebroeks W. Evolution of Hominin Detoxification: Neanderthal and Modern Human Ah Receptor Respond Similarly to TCDD. Mol. Biol. Evol. 2021;38:1292–1305. doi: 10.1093/molbev/msaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Furue M., Tsuji G. Chloracne and Hyperpigmentation Caused by Exposure to Hazardous Aryl Hydrocarbon Receptor Ligands. Int. J. Environ. Res. Public Health. 2019;16:4864. doi: 10.3390/ijerph16234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. [(accessed on 13 February 2022)]; Available online: https://www.publichealth.va.gov/exposures/agentorange/index.asp.
- 142.Addi T., Dou L., Burtey S. Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease. Toxins. 2018;10:412. doi: 10.3390/toxins10100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lano G., Burtey S., Sallée M. Indoxyl Sulfate, a Uremic Endotheliotoxin. Toxins. 2020;12:229. doi: 10.3390/toxins12040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang X.-S., Cao F., Zhang Y., Pan H.-F. Therapeutic potential of aryl hydrocarbon receptor in autoimmunity. Inflammopharmacology. 2020;28:63–81. doi: 10.1007/s10787-019-00651-z. [DOI] [PubMed] [Google Scholar]
- 145.Giovannoni F., Bosch I., Polonio C.M., Torti M.F., Wheeler M.A., Li Z., Romorini L., Varela M.S.R., Rothhammer V., Barroso A., et al. AHR is a Zika virus host factor and a candidate target for antiviral therapy. Nat. Neurosci. 2020;23:939–951. doi: 10.1038/s41593-020-0664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bock K.W. From TCDD-mediated toxicity to searches of physiologic AHR functions. Biochem. Pharmacol. 2018;155:419–424. doi: 10.1016/j.bcp.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 147.Wilson R.H., Bradfield C.A. Rodent genetic models of Ah receptor signaling. Drug Metab. Rev. 2021;53:350–374. doi: 10.1080/03602532.2021.1955916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sonowal R., Swimm A., Sahoo A., Luo L., Matsunaga Y., Wu Z., Bhingarde J.A., Ejzak E.A., Ranawade A., Qadota H., et al. Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA. 2017;114:E7506–E7515. doi: 10.1073/pnas.1706464114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serna E., Cespedes C., Vina J. Anti-Aging Physiological Roles of Aryl Hydrocarbon Receptor and Its Dietary Regulators. Int. J. Mol. Sci. 2020;22:374. doi: 10.3390/ijms22010374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lavelle A., Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 151.Natividad J.M., Agus A., Planchais J., Lamas B., Jarry A.C., Martin R., Michel M.-L., Chong-Nguyen C., Roussel R., Straube M., et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018;28:737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 152.Lamas B., Hernandez-Galan L., Galipeau H.J., Constante M., Clarizio A., Jury J., Breyner N.M., Caminero A., Rueda G., Hayes C.L., et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 2020;12:eaba0624. doi: 10.1126/scitranslmed.aba0624. [DOI] [PubMed] [Google Scholar]
- 153.Wrzosek L., Ciocan D., Hugot C., Spatz M., Dupeux M., Houron C., Moal V.L.-L., Puchois V., Ferrere G., Trainel N., et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2020;70:1299–1308. doi: 10.1136/gutjnl-2020-321565. [DOI] [PubMed] [Google Scholar]
- 154.Murray I.A., Perdew G.H. How Ah Receptor Ligand Specificity Became Important in Understanding Its Physiological Function. Int. J. Mol. Sci. 2020;21:9614. doi: 10.3390/ijms21249614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bock K.W. Human AHR functions in vascular tissue: Pro- and anti-inflammatory responses of AHR agonists in atherosclerosis. Biochem. Pharmacol. 2019;159:116–120. doi: 10.1016/j.bcp.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 156. [(accessed on 13 February 2022)]. Available online: https://arsindustrialis.org/pharmakon.
- 157.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gilbert S.F., Bosch T.C.G., Ledón-Rettig C. Eco-Evo-Devo: Developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 2015;16:611–622. doi: 10.1038/nrg3982. [DOI] [PubMed] [Google Scholar]
- 159.Alnouti Y., Klaassen C.D. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol. Sci. 2006;93:242–255. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- 160.Coughtrie M.W.H. Sulfation through the looking glass—Recent advances in sulfotransferase research for the curious. Pharm. J. 2002;2:297–308. doi: 10.1038/sj.tpj.6500117. [DOI] [PubMed] [Google Scholar]
- 161.Yamazoe Y., Nagata K., Ozawa S., Kato R. Structural similarity and diversity of sulfotransferases. Chem. Interact. 1994;92:107–117. doi: 10.1016/0009-2797(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 162.Davenport E.R., Sanders J.G., Song S.J., Amato K.R., Clark A.G., Knight R. The human microbiome in evolution. BMC Biol. 2017;15:127. doi: 10.1186/s12915-017-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weyrich L.S., Duchene S., Soubrier J., Arriola L., Llamas B., Breen J., Morris A.G., Alt K.W., Caramelli D., Dresely V., et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544:357–361. doi: 10.1038/nature21674. [DOI] [PubMed] [Google Scholar]
- 164.Sommer F., Anderson J.M., Bharti R., Raes J., Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017;15:630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 165.Monda V., Villano I., Messina A., Valenzano A., Esposito T., Moscatelli F., Viggiano A., Cibelli G., Chieffi S., Monda M., et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017;2017:3831972. doi: 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Poesen R., Windey K., Neven E., Kuypers D., de Preter V., Augustijns P., D’Haese P., Evenepoel P., Verbeke K., Meijers B. The Influence of CKD on Colonic Microbial Metabolism. J. Am. Soc. Nephrol. JASN. 2016;27:1389–1399. doi: 10.1681/ASN.2015030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vaziri N.D., Wong J., Pahl M., Piceno Y.M., Yuan J., DeSantis T.Z., Ni Z., Nguyen T.-H., Andersen G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 168.Gryp T., Huys G.R., Joossens M., Van Biesen W., Glorieux G., Vaneechoutte M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020;21:1986. doi: 10.3390/ijms21061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pontzer H., Wood B.M., Raichlen D.A. Hunter-gatherers as models in public health. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018;19((Suppl. 1)):24–35. doi: 10.1111/obr.12785. [DOI] [PubMed] [Google Scholar]
- 170.Oparil S., Acelajado M.C., Bakris G.L. Hypertension. Nat. Rev. Dis. Primers. 2018;4:18014. doi: 10.1038/nrdp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weller O.D.G. [(accessed on 13 February 2022)]. Available online: http://www.antiquity.ac.uk/projgall/weller306/
- 172.Eknoyan G. A history of obesity, or how what was good became ugly and then bad. Adv. Chronic Kidney Dis. 2006;13:421–427. doi: 10.1053/j.ackd.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 173.Mc Guigan K., Nishimura N., Currey M., Hurwit D., Cresko W.A. Cryptic genetic variation and body size evolution in threespine stickleback. Evolution. 2010;65:1203–1211. doi: 10.1111/j.1558-5646.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 174.Scoville A.G., Pfrender M.E. Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl. Acad. Sci. USA. 2010;107:4260–4263. doi: 10.1073/pnas.0912748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Aubret F., Shine R. Genetic assimilation and the postcolonization erosion of phenotypic plasticity in island tiger snakes. Curr. Biol. 2009;19:1932–1936. doi: 10.1016/j.cub.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 176.O’Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dobber R., Hertogh-Huijbregts A., Rozing J., Bottomly K., Nagelkerken L. The involvement of the intestinal microflora in the expansion of CD4+ T cells with a-naive phenotype in the periphery. Dev. Immunol. 1992;2:141–150. doi: 10.1155/1992/57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays. 2011;33:574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 180.Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.-N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhao Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant. 2012;5:334–338. doi: 10.1093/mp/ssr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fu S.-F., Wei J.-Y., Chen H.-W., Liu Y.-Y., Lu H.-Y., Chou J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015;10:e1048052. doi: 10.1080/15592324.2015.1048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Castillo-Rodriguez E., Pizarro-Sanchez S., Sanz A.B., Ramos A.M., Sanchez-Niño M.D., Martin-Cleary C., Fernandez-Fernandez B., Ortiz A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins. 2017;9:114. doi: 10.3390/toxins9040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Asakawa A., Inui A., Fujimiya M., Sakamaki R., Shinfuku N., Ueta Y., Meguid M.M., Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Velasquez M.T., Ramezani A., Manal A., Raj D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins. 2016;8:326. doi: 10.3390/toxins8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ma J., Pazos I.M., Gai F. Microscopic insights into the protein-stabilizing effect of trimethylamine N-oxide (TMAO) Proc. Natl. Acad. Sci. USA. 2014;111:8476–8481. doi: 10.1073/pnas.1403224111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vanholder R., Gryp T., Glorieux G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research) Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2018;33:4–12. doi: 10.1093/ndt/gfx039. [DOI] [PubMed] [Google Scholar]
- 190.Lee J.A., Lee H.A., Sadler P.J. Uraemia: Is urea more important than we think? Lancet. 1991;338:1438–1440. doi: 10.1016/0140-6736(91)92733-I. [DOI] [PubMed] [Google Scholar]
- 191.Spiga R., Marini M.A., Mancuso E., di Fatta C., Fuoco A., Perticone F., Andreozzi F., Mannino G.C., Sesti G. Uric Acid Is Associated With Inflammatory Biomarkers and Induces Inflammation via Activating the NF-kappaB Signaling Pathway in HepG2 Cells. Arterioscler. Thromb. Vasc. Biol. 2017;37:1241–1249. doi: 10.1161/ATVBAHA.117.309128. [DOI] [PubMed] [Google Scholar]
- 192.Sautin Y.Y., Johnson R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gryp T., Vanholder R., Vaneechoutte M., Glorieux G. p-Cresyl Sulfate. Toxins. 2017;9:52. doi: 10.3390/toxins9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.London J.A., Wang E.C.S., Barsukov I.L., Yates E.A., Stachulski A.V. Synthesis and toxicity profile in 293 human embryonic kidney cells of the beta D-glucuronide derivatives of ortho-, meta- and para-cresol. Carbohydr. Res. 2021;499:108225. doi: 10.1016/j.carres.2020.108225. [DOI] [PubMed] [Google Scholar]
- 195.Wang J., Blaze J., Haghighi F., Kim-Schulze S., Raval U., Trageser K.J., Pasinetti G.M. Characterization of 3(3,4-dihydroxy-phenyl) propionic acid as a novel microbiome-derived epigenetic modifier in attenuation of immune inflammatory response in human monocytes. Mol. Immunol. 2020;125:172–177. doi: 10.1016/j.molimm.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Susantitaphong P., Siribamrungwong M., Jaber B.L. Convective therapies versus low-flux hemodialysis for chronic kidney failure: A meta-analysis of randomized controlled trials. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2013;28:2859–2874. doi: 10.1093/ndt/gft396. [DOI] [PubMed] [Google Scholar]
- 197.Nistor I., Palmer S.C., Craig J., Saglimbene V., Vecchio M., Covic A., Strippoli G.F. Convective versus diffusive dialysis therapies for chronic kidney failure: An updated systematic review of randomized controlled trials. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014;63:954–967. doi: 10.1053/j.ajkd.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 198.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Glorieux G., Vanholder R., Van Biesen W., Pletinck A., Schepers E., Neirynck N., Speeckaert M., de Bacquer D., Verbeke F. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2021;36:998–1005. doi: 10.1093/ndt/gfab004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.