Abstract
Because of the rising incidence of failures in the treatment of oropharyngeal candidosis in the case of severely immunosuppressed patients (mostly human immunodeficiency virus [HIV]-infected patients), there is need for the development of new, more effective agents and/or compounds that support the activity of the common antifungal agents. Since lactoferrin is one of the nonspecific host defense factors present in saliva that exhibit antifungal activity, we studied the antifungal effects of human, bovine, and iron-depleted lactoferrin in combination with fluconazole, amphotericin B, and 5-fluorocytosine in vitro against clinical isolates of Candida species. Distinct antifungal activities of lactoferrin were observed against clinical isolates of Candida. The MICs generally were determined to be in the range of 0.5 to 100 mg · ml−1. Interestingly, in the combination experiments we observed pronounced cooperative activity against the growth of Candida by using lactoferrin and the three antifungals tested. Only in a limited concentration range was minor antagonism detected. The use of lactoferrin and fluconazole appeared to be the most successful combination. Significant reductions in the minimal effective concentrations of fluconazole were found when it was combined with a relatively low lactoferrin concentration (1 mg/ml). Such combinations still resulted in complete growth inhibition, while synergy of up to 50% against several Candida species was observed. It is concluded that the combined use of lactoferrin and antifungals against severe infections with Candida is an attractive therapeutic option. Since fluconazole-resistant Candida species have frequently been reported, especially in HIV-infected patients, the addition of lactoferrin to the existing fluconazole therapy could postpone the occurrence of species resistance against fluconazole. Clinical studies to further elucidate the potential utility of this combination therapy have been initiated.
Since its discovery in 1839 by Langenbeck (16), the genus Candida has been shown to be the causative agent of many infections in an increasing range of anatomical sites and clinical settings. In normal healthy individuals, the yeast Candida is classified as a commensal organism that can colonize both internal and external surfaces. Under these circumstances, an equilibrium between the host and the yeast microflora ensures the avirulent, commensal status of this microorganism. This equilibrium is attained not only by specific immune responses but also by nonspecific factors that are secreted in saliva or mucosal secretions, such as immunoglobulin A, lysozyme, lactoferrin, and histatins (25, 27, 31). A range of predispositions, of which the most common is an immunocompromised status of the patient, can affect the equilibrium. For example the onset of candidosis in people infected with human immunodeficiency virus (HIV) is considered to be closely related to manifestation of AIDS and AIDS-related diseases. In fact, it represents one of the factors that is used by the Centers for Disease Control and Prevention to define AIDS (7). Although Candida albicans has been implicated in the early stages of AIDS, infections due to C. glabrata and C. krusei are becoming more widespread in late-stage AIDS (13).
Before the emergence of the HIV epidemic, oral mycotic infections were treated with polyene antifungals, such as amphotericin B or nystatin, and with azoles, such as clotrimazole or miconazole. The high relapse rate in HIV-positive patients and reported toxic side effects of amphotericin B led to the use of azoles as the first line of treatment (10, 17). Because ketoconazole and itraconazole are not as readily absorbed, their use has been limited. Although fluconazole has become the agent of choice in antimycotic therapy, its widespread use has resulted in an increase in resistance of Candida to its antifungal efficacy (1, 30). In addition, the use of 5-fluorocytosine as antimycotic has led to resistant strains of Candida being clinically significant as well (14). Because of the rising incidence of failures in the treatment of mycoses in the case of severely immunosuppressed patients, there is a need for the development of new therapeutic agents that support the antifungal activity of antimycotics (41).
As mentioned, saliva contains several nonspecific defense factors. Among them is lactoferrin, an iron-binding glycoprotein present at relatively high concentrations (up to 12 mg/ml) in many exocrine fluids (33). The protein has broad-spectrum antimicrobial properties against bacteria, yeasts, and viruses and is considered to play an important role in the host defense against infections on mucosal surfaces and in colostrum and milk (4, 6, 12, 15, 33, 37, 38).
The anti-Candida activity of lactoferrin, first reported by Kirkpatrick et al. (15), is commonly attributed to its ability to bind and sequester environmental iron (6, 33). Yet the iron-free form of lactoferrin (apo-lactoferrin) is able to kill both C. albicans and C. krusei, by mechanisms related to alterations in cell surface permeability (24, 37). Moreover, lactoferricin B, a peptide produced by enzymatic cleavage of bovine lactoferrin, was found to have a lethal effect on Candida species through direct interaction with the cell surface (3).
The need for reducing the development of microbial resistance has led to experiments in which the cooperative effects of lactoferrin and currently used treatments were assessed. This resulted in an increased resistance to apo-lactoferrin-mediated cell death when physiological concentrations of apo-lactoferrin and sub-MICs (concentrations causing substantial but incomplete growth inhibition) of antifungal drugs or agents were tested (23). However, others showed that combining lactoferrin and several antifungals led to a decrease in the concentration of lactoferrin required to inhibit the growth of Candida, whereas the combination of lactoferrin and clomitrazole even resulted in synergistic anti-Candida activity (41). In the present study we investigated in vitro the antifungal effect of lactoferrin combined with some common antifungal agents against several clinical isolates of Candida. Potential cooperative or synergistic anti-Candida activity with lactoferrin and antifungals would enable a lower dose of standard antimycotics during antimycotic therapy and might at the same time decrease the induction of drug-resistant species.
MATERIALS AND METHODS
Microorganisms.
Three C. albicans strains, four C. glabrata strains, and one C. tropicalis strain, isolated from the oral cavity and differing in their susceptibilities to the antifungal agents amphotericin B, fluconazole, and 5-flurocytocine, were obtained from the routine microbiology laboratory of the University Hospital Groningen, Groningen, The Netherlands. C. albicans ATCC 10231 was used as a control in all susceptibility tests. All strains were stored on Sabouraud dextrose agar (SDA) slopes (Oxoid, Unipath Ltd., Basingstoke, United Kingdom) at 4°C.
Assay media.
For the experiments we used Sabouraud liquid medium (SLM) (pH 5.6) (Oxoid, Unipath Ltd.) and RPMI 1640 medium (with l-glutamine, without NaHCO3, and supplemented with 2% glucose; pH 7.0) (Gibco BRL, Paisley, Scotland). These media were prepared according to the manufacturer’s instructions. RPMI 1640 was used when the antifungal activity of 5-fluorocytosine was studied. All other compounds were assayed in SLM.
Antifungal agents.
Bovine lactoferrin and human lactoferrin (both from Numico Research B.V., Wageningen, The Netherlands) were dissolved in assay medium at appropriate concentrations. Iron-free lactoferrin (apo-lactoferrin) was prepared from bovine lactoferrin by overnight dialysis against 0.1 M citric acid by the method earlier described by Masson et al. (19) and dissolved in assay medium at appropriate concentrations. Fluconazole (Diflucan I.V.; Pfizer B.V., Capelle aan den Yssel, The Netherlands) and 5-fluorocytosine (Ancotil; Roche Nederland B.V., Mijdrecht, The Netherlands) were dissolved in assay medium at appropriate concentrations. Amphotericin B (Fungizone; Bristol-Myers Squibb Company, Woerden, The Netherlands) was prepared to a concentration of 5 mg/ml in sterile water and was further diluted in assay medium. All suspensions were prepared in sterile glass tubes before addition to the microtiter plate.
In order to exclude the antifungal activity of endotoxins present in the lactoferrin preparations, the anti-Candida effect of lipopolysaccharide (LPS) (Biowhittaker, Inc. Walkerville, Md.) alone was tested in a range of 1 to 1,000 pg/ml in our assay system as well.
Inoculum.
The yeast isolates were grown on SDA for 24 h at 35°C in air. Suspensions were made by isolating five colonies from these cultures. These were suspended in 10 ml of SLM and mixed while being incubated for 18 h at 35°C in air. From this culture, a 1:10 dilution in SLM was incubated for 5 h, resulting in a culture in its growth phase. This was vortex mixed, and the turbidity was adjusted to the density of a 0.5 McFarland barium sulfate turbidity standard at 530 nm, resulting in a concentration of 1 × 106 to 5 × 106 cells per ml as previously described (29). From this, the test inoculum was prepared to a concentration of 1 × 104 to 5 × 104 cells per ml by a 1:100 dilution in SLM. Confirmation of the inoculum size was done by using a model C Spiral Plater (Spiral Systems, Inc., Cincinnati, Ohio). One hundred microliters was automatically plated out onto SDA and incubated for 18 h at 35°C in air, and the concentration was calculated according to the manufacturer’s specifications.
Assay format.
To each well of a sterile 96-well plastic flat-bottom tray with matching covers (Corning Costar, Cambridge, United Kingdom), 50 μl of test inoculum was added. Appropriate concentrations of the antifungal agents to be tested were added to the wells (75 to 150 μl). Controls were included for the determination of the growth characteristics of each Candida species without the presence of an antifungal agent. The final volume per well was adjusted to 200 μl with the assay medium used (SLM or RPMI).
Incubation, growth curves, and end point criteria.
After inoculation, plates were incubated for 24 h at 35°C in air without agitation. Turbidity measurements were performed at 0 h, hourly at 18 to 24 h, and 48 h at 630 nm in an automated microplate reader (ElX800; Bio-Tek Instruments, Inc., Winooski, Vt.) after resuspension of the contents of the wells with a multichannel pipette. Any bubbles were removed with the tip of a sterile needle. For any wells not producing a visual or spectrophotometrically measurable increase in turbidity after 48 h, the absence of growth was confirmed by inoculating 20 μl of the well contents onto SDA and then incubating for 5 days at 35°C in air.
The MIC was defined as the lowest concentration of antifungal agent that substantially inhibited the growth of Candida after 24 h, according to the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) for the antifungal agents used (22). All experiments were performed in quadruplicate.
Synergy experiments.
The combined effects of bovine lactoferrin and fluconazole, amphotericin B, or 5-fluorocytosine against the growth of Candida species were examined under the same experimental conditions used for determination of the MICs. A dilution matrix (eight by eight) with eightfold drug dilutions, which also included the drugs used individually, was prepared. The results of the turbidity measurements at 630 nm were used to calculate the inhibiting effects of the drug combinations on Candida growth. Maximum Candida growth was set at 0%, and complete inhibition of Candida growth was set at 100%. The results obtained are presented as growth inhibition curves. In addition, for characterization of drug-drug interactions, a three-dimensional analytical method was used according to the method described by Prichard and Shipman (32). This experimental design was used to identify regions at which significant drug interactions occurred. In brief, theoretical additive interactions were calculated from the dose-response curves of the individual drugs. The theoretical calculated additive interactions, representing the predicted additive interactions, were then subtracted from the experimental interactions, obtained via the effects of the drug combination on the turbidity measurements at 630 nm, to reveal dose-response ranges of greater-than-expected interactions (synergy). The resulting surface would appear as a horizontal plane at 0% inhibition above the calculated additive surface if the interactions were merely additive. Any peaks above this plane would be indicative of synergy. Similarly, any depressions below the plane would indicate antagonism. For example, a combination of 0.5 mg of lactoferrin per ml and 100 μg of fluconazole per ml resulting in a peak of +50% would indicate that the specified drug combination in this specified concentration range would cause a 50% synergistic antifungal effect. In other words, the growth of the Candida isolate is inhibited more efficiently (namely, 50% more efficiently) with the specified drug combination. Likewise, a peak of −25% would indicate an antagonistic antifungal effect of the specified drug combination, meaning that the growth of the Candida isolate is inhibited less than was expected on the basis of the individual dose-response curves (namely, 25% less efficiently).
RESULTS
Inhibition of Candida growth.
The activities of various forms of lactoferrin (bovine and human lactoferrins and bovine apo-lactoferrin) against several clinical isolates of C. albicans, C. glabrata, and C. tropicalis were determined and compared to the susceptibilities of Candida species to currently used antimycotics. The MICs were determined, according to the recommendations by the NCCLS (22), after 24 h of incubation of the Candida species with the antifungal agents and are presented in Table 1.
TABLE 1.
MICs of several antifungal agents against Candida species
| Isolateb | Species | MICa (mean ± SD)
|
||||
|---|---|---|---|---|---|---|
| Lactoferrin (bovine) (mg/ml) | Apo-lactoferrin (mg/ml) | Amphotericin B (μg/ml) | Fluconazole (μg/ml) | 5-Fluorocytosine (μg/ml) | ||
| 10231 | C. albicans | 97 ± 46 | 22 ± 13 | 0.06 ± 0.05 | —d | — |
| Y098 | C. albicans | 21 ± 1 | 54 ± 2 | 0.08 ± 0.03 | — | — |
| Y106 | C. albicans | 0.5 | 33 ± 7 | 0.08 ± 0.03 | 10 | — |
| Y127 | C. albicans | 98 ± 42 | 41 ± 11 | 0.2 ± 0.07 | 10 | — |
| Y110 | C. glabrata | 31 ± 14 | 57 ± 7 | 0.2 ± 0.06 | 156 ± 50 | — |
| Y111 | C. glabrata | 6 ± 5 | <5 | 0.1 ± 0.07 | 24 ± 7 | — |
| Y112 | C. glabrata | 21 ± 8 | 41 ± 14 | 0.4 ± 0.05 | — | — |
| Y110c | C. glabrata | — | — | — | — | 0.03 ± 0.005 |
| Y140c | C. tropicalis | — | — | — | — | 35 ± 7 |
The MIC was defined as the lowest concentration of the antifungal agent that substantially inhibited the growth of Candida after 24 h, according to the recommendations of the NCCLS for the antifungal agents tested (22). All experiments were performed in quadruplicate.
Isolate 10231 is an ATCC strain; all other isolates are clinical, mostly oral, Candida isolates.
The isolate was tested in RPMI medium instead of SLM.
—, not determined.
Since the antifungal activity of human lactoferrin was comparable to or even less than that of the bovine variant (results not shown), we continued all other experiments with bovine lactoferrin because of its better availability. Bovine lactoferrin and bovine apo-lactoferrin exhibited equivalent antifungal activities. The MICs found for these two variants were all in the same range and for the Candida species tested in SLM medium were in the range of 0.5 to 100 mg/ml (Table 1).
The antifungal activities of the other currently used antimycotics in our test system were comparable to those obtained earlier from the microbiology services of the University Hospital, which supports the accuracy of our test system (results not shown).
Because lactoferrin is able to bind LPS (8), we wanted to exclude the contribution of LPS to the antifungal activity of these milk proteins. We noted that at concentrations of up to 1,000 pg of LPS per ml no killing of Candida species occurred.
Cooperative or synergistic activity.
From the panel of clinical isolates we chose four Candida species, depending on their susceptibility to the tested antifungals, to be examined for the combined activity of lactoferrin and fluconazole, amphotericin B, and 5-fluorocytosine. For this we used a dilution matrix with eight- by eightfold drug dilutions and we measured the effects on Candida growth. The results are presented as calculations of the effects of both compounds on the inhibition of Candida species and also are represented by so-called synergy plots, in which the actual experimental dose-response values were compared with the theoretical dose-response values (see Materials and Methods). For an additive interaction of the two antifungals, the actual experimental dose-response curves should coincide with the theoretical ones (with no exclusion of Candida growth inhibition), but any peaks above or below these values (above or below baseline [0%]) would be indicative of synergistic or unwanted antagonistic interactions, respectively.
Combination of fluconazole and lactoferrin.
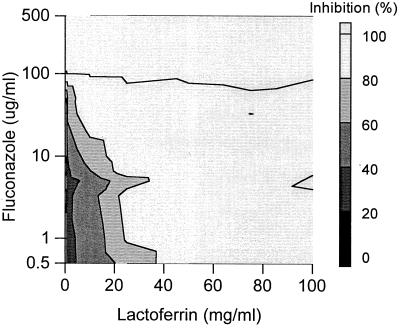
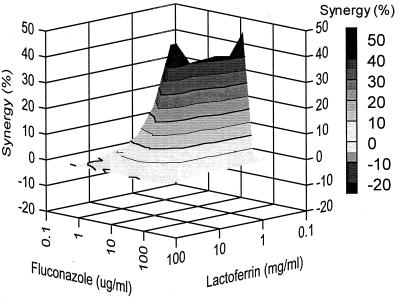
The combined effect of fluconazole and lactoferrin against the growth of Candida isolate Y110 is shown in Fig. 1. It was found that this Candida strain was completely inhibited in its growth by using 50 μg of fluconazole per ml in combination with 10 mg of lactoferrin per ml, whereas their MICs against this isolate were 156 μg/ml and 31 mg/ml, respectively (Table 1). This implies that a complete inhibition of growth is possible with lower concentrations of antimycotic than could be extrapolated from the MICs. This applies to other combinations of lactoferrin and fluconazole as well (Fig. 1). Several combinations of fluconazole and lactoferrin resulted in a clear synergistic anti-Candida effect against Y110, as shown in Fig. 2. Effects above baseline from +5 to +50% were observed. For example, a combination of 0.5 mg of lactoferrin per ml and 100 μg of fluconazole per ml resulted in 50% synergistic effects, whereas 25 mg of lactoferrin per ml in combination with 3.3 μg of fluconazole per ml induced only a 5% extra anti-Candida effect. In the range of the MICs of both compounds, synergistic effects were not evident. This was expected because concentrations of fluconazole in the range of its MIC should be capable in themselves of complete Candida growth inhibition. Importantly, no antagonistic anti-Candida activity between lactoferrin and fluconazole was observed for this isolate.
FIG. 1.
Combined inhibitory effects of lactoferrin and fluconazole on the growth of C. glabrata isolate Y110. Presented is the top elevation of a three-dimensional dose-response graph (concentration of fluconazole versus concentration of lactoferrin versus percent growth inhibition). The amount of inhibition of the Candida growth is indicated by the bar at the right.
FIG. 2.
Combined inhibitory effects of lactoferrin and fluconazole on the growth of C. glabrata isolate Y110. The graph demonstrates the amount of synergy (i.e., potentiation of inhibition above expected additivity) observed with the combination of the two compounds. Presented is the front elevation of the synergy plot. The amount of synergy is indicated by the bar at the right.
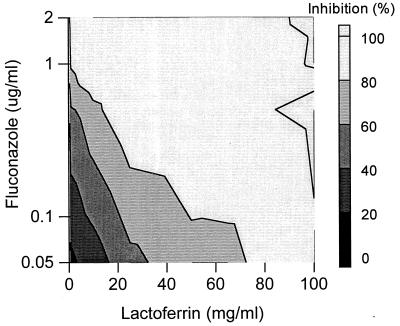
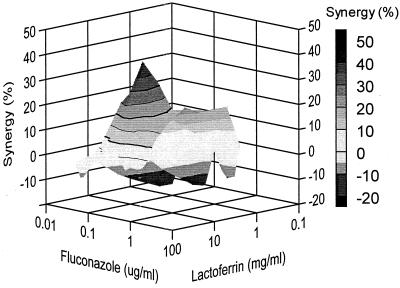
Y127, a rather lactoferrin-insensitive Candida isolate (MIC of 98 mg/ml), was completely inhibited by using 10 mg of lactoferrin per ml in combination with 1 μg of fluconazole per ml, while the MIC of fluconazole was 10 μg/ml (Fig. 3). For this species, antagonistic effects along with synergistic effects on Candida growth inhibition were found, in the range of −20 to +40% (20% antagonism to 40% synergism). For example, a combination of 1 mg of lactoferrin per ml and 0.05 μg of fluconazole per ml resulted in about 20% antagonistic effects; in other words, 20% less inhibition of Candida growth was found than theoretically could be expected on basis of the individual inhibitory effects of lactoferrin and fluconazole. In contrast, 25 mg of lactoferrin per ml in combination with 0.5 μg of fluconazole per ml induced as much as 40% extra growth inhibition (Fig. 4).
FIG. 3.
Combined inhibitory effects of lactoferrin and fluconazole on the growth of C. albicans isolate Y127. Presented is the top elevation of a three-dimensional dose-response graph (see the legend to Fig. 1). The amount of inhibition of the Candida growth is indicated by the bar at the right.
FIG. 4.
Combined inhibitory effects of lactoferrin and fluconazole on the growth of C. albicans isolate Y127. The graph demonstrates the amount of synergy (i.e., potentiation of inhibition above expected additivity) observed with the combination of the two compounds. Presented is the front elevation of the synergy plot. The amount of synergy is indicated by the bar at the right.
Likewise, Y111, one of the more lactoferrin-sensitive strains, was efficiently inhibited by combinations of lactoferrin and fluconazole. A reduction of more than 50% of the MICs of both compounds against this isolate (1 mg of lactoferrin per ml with 10 μg of fluconazole per ml) resulted in a complete inhibition of Candida growth. The cooperative activities of fluconazole and lactoferrin tended to be slightly antagonistic (10%) with minor amounts of both compounds (0.005 mg of lactoferrin per ml and 0.3 μg of fluconazole per ml) but were clearly synergistic (50%) with concentrations of 0.5 mg of lactoferrin per ml and 10 μg of fluconazole per ml.
Combination of amphotericin B and lactoferrin.
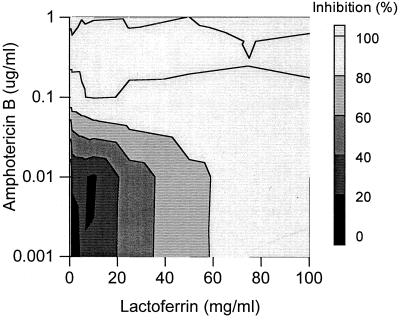
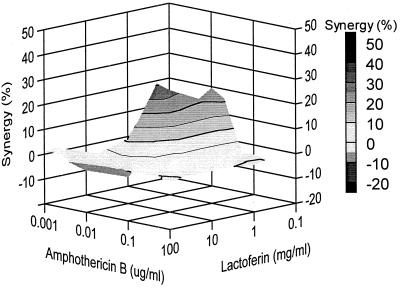
Efficient inhibition of the Candida growth was also observed with the combination of amphotericin B and lactoferrin against isolate Y110. A complete inhibition of the Candida growth was observed with 0.5 mg of lactoferrin per ml and 0.1 μg of amphotericin B per ml (Fig. 5). In addition, both moderate antagonistic (10%) and also synergistic (30%) inhibition of the Candida growth was demonstrated with certain concentration ranges (Fig. 6).
FIG. 5.
Combined inhibitory effects of lactoferrin and amphotericin B on the growth of C. glabrata isolate Y110. Presented is the top elevation of a three-dimensional dose-response graph (see the legend to Fig. 1). The amount of inhibition of the Candida growth is indicated by the bar at the right.
FIG. 6.
Combined inhibitory effects of lactoferrin and amphotericin B on the growth of C. glabrata isolate Y110. The graph demonstrates the amount of synergy (i.e., potentiation of inhibition above expected additivity) observed with the combination of the two compounds. Presented is the front elevation of the synergy plot. The amount of synergy is indicated by the bar at the right.
In the case of isolate Y127, the combination of lactoferrin and amphotericin B did not result in a pronounced decrease in the required concentrations of lactoferrin or amphotericin B to obtain complete Candida inhibition. Complete inhibition of the Candida growth could be obtained only with concentrations of amphotericin B or lactoferrin close to their MICs. Yet, slightly antagonistic (10%) as well as synergistic (30%) effects of this combination against Y127 could be observed. The 30% synergistic effects were detected with 30 mg of lactoferrin per ml in combination with 0.1 μg of amphotericin B per ml, which is only a small decrease from the MIC of amphotericin B itself (0.15 μg/ml).
Combination of 5-fluorocytosine and lactoferrin.
A combination of 5-fluorocytosine and lactoferrin resulted in an effective inhibition of the growth of isolate Y110. A 100% growth inhibition was observed with 0.0008 μg of 5-fluorocytosine per ml (a decrease of 30-fold compared to its established MIC) in combination with 0.02 mg of lactoferrin per ml. This combination of antifungals did demonstrate a clear synergistic activity against Y110 of 50% with 0.025 μg of 5-fluorocytosine per ml in combination with 0.01 mg of lactoferrin per ml. Yet, minor antagonistic effects of 5% on the growth inhibition were also observed with 0.001 μg of 5-fluorocytosine per ml in combination with 0.01 mg of lactoferrin per ml.
The C. tropicalis isolate Y140 is a 5-fluorocytosine-resistant isolate (see also Table 1). We investigated antifungal effects against Y140 with only a combination of 5-fluorocytosine and lactoferrin. Unfortunately, a complete inhibition of Candida growth could not be observed; only 90% inhibition was reached. In addition, only moderate synergistic effects of 15% with 10 mg of lactoferrin per ml and 10 μg of 5-fluorocytosine per ml and antagonistic effects of 10% with 0.001 mg of lactoferrin per ml and 45 μg of 5-fluorocytosine per ml were seen.
DISCUSSION
C. albicans and other Candida species are common pathogens that frequently cause oral infections in immunocompetent and immunocompromised individuals due to the suppression of local as well as systemic defense mechanisms (34). Due to the increased incidence of fungal infections, a collection of broad-spectrum antifungal agents has been introduced. For example, HIV-infected patients who undergo several episodes of oral thrush have been extensively treated with fluconazole, one of the available triazoles. Yet, fluconazole-resistant Candida species have been observed even in patients who did not have an azole treatment history. These patients were rather severely immunocompromised (low CD4 count or C2 or -3 category of HIV infection) (39). An attractive therapeutic option in these circumstances might be a combination of active agents with different modes of action.
Earlier studies already showed that intrinsic components of the host defense system are capable of killing several Candida species, such as histatins and several saliva proteins, like lysozyme and lactoferrin. These products therefore represent attractive choices to be used in combination with the common antifungal drugs, since the availability and toxicity of the endogenous agents are of no concern.
We focused our attention on lactoferrin, since it was demonstrated that this milk protein not only is able to inhibit the growth of several bacteria and Candida species but also has important activity against several viruses (HIV, herpes simplex virus, and human cytomegalovirus) (12, 18, 38).
Prior to the combination experiments, we tested the antifungal activities of the various lactoferrins and some common antifungals alone. We found that lactoferrin is able to inhibit the growth of several Candida species and that the MICs of this milk protein commonly varied between 0.5 and 100 mg/ml. The potential mechanisms by which lactoferrin inhibits the growth of yeasts were discussed by Nikawa et al. (24) earlier. Structural changes in the microbial cell wall, indirect effects on enzyme activation, an increased generation of metabolic by-products of aerobic metabolism, iron deprivation, and combinations of these factors were mentioned as possible explanations for the fungicidal activity of lactoferrin. The differences in activity against different Candida species detected in the present study can be explained by an unequal effectiveness of lactoferrin due to differences in cell wall composition, sensitivity for enzyme activation, or the need for iron in the distinct Candida species (24).
Several studies demonstrated the antifungal activity of the iron-free from of lactoferrin, apo-lactoferrin, whereas in the same studies the native form of lactoferrin did not show any effect on Candida growth inhibition (15, 24). However, in our present study we showed that both apo-lactoferrin and lactoferrin were able to inhibit the growth of several clinical isolates of Candida, albeit to different extents. Since the potential mechanism for the antifungal activity of lactoferrin, as well as that of apo-lactoferrin, is not likely to be directed to a single phenomenon (24), the observed differences in activity between these two proteins can also be explained by unequal effectivities of the proteins against different Candida species, due to differences in cell wall composition, sensitivity for enzyme activation, or the need for iron in the distinct Candida species.
In an earlier experiment we determined the LPS content of lactoferrin (5 pg/mg of protein). In our present experiment we found that 1,000 pg of LPS per ml was not able to kill the Candida species. Because concentrations of lactoferrin of up to 100 mg/ml do contain 500 pg of LPS per ml, we can therefore assume that the milk protein itself predominantly causes the inhibition of Candida species.
In the literature there is no consensus with regard to the inhibitory potency of lactoferrin against Candida species. Inhibition of Candida growth has been tested with protein concentrations of as low as 20 μg/ml, while sub-MICs (concentrations of antifungal agents causing substantial but incomplete growth inhibition of Candida) of 100 μg/ml have been reported (41). However, exact MICs often were not determined. In addition, the varying experimental conditions, such as differences in medium composition, pH, incubation temperature, incubation time, and end point criteria, as well as the variable use of human versus bovine lactoferrin and of iron-free lactoferrin (apo-lactoferrin) versus iron-containing lactoferrin, make a useful comparison of the present results with those reported earlier difficult.
The multiple fungistatic mechanisms of lactoferrin make this protein a promising compound for combination therapy. A synergistic fungistatic activity of a combination of drugs can be anticipated in particular when the drugs used have different mechanisms of action. The drugs tested in combination with lactoferrin in this study have distinct modes of activity. Fluconazole inhibits ergosterol synthesis by the inhibition of microsomal cytochrome P450. 5-Fluorocytosine is converted either to 5-fluorouridine triphosphate, a precursor for cellular RNA, or to 5-fluorodeoxyuridylic acid, a potent inhibitor of thymidylate synthase, both of which inhibit DNA synthesis by the fungus. Amphotericin B, on the other hand, interacts with ergosterol in the plasma membranes. In addition, recent publications suggested that the antifungal activity of amphotericin B might also be mediated by oxidative damage (28, 36). At first sight, the interaction with ergosterol makes amphotericin B a less favorable candidate for combination with lactoferrin, which also interacts with the cell membrane. However, different sites of interaction at the membrane are possible and can even lead to additive effects.
In the present study we show a synergistic activity between lactoferrin and several antifungal agents. Even though the claim of synergism is definition dependent, it can be clearly stated that a substantial cooperative effect of lactoferrin with fluconazole, amphotericin B, and 5-fluorocytosine was observed. The combination of lactoferrin and fluconazole appeared to be the most successful combination. Significant reductions in necessary fluconazole concentrations with addition of lactoferrin still resulted in complete growth inhibition, and synergy of up to 50% against several Candida species was noted. This result is in line with several studies that reported at least some synergistic antifungal activity of fluconazole and combinations of other compounds. Barchiesi et al. reported a twofold reduction in MICs by using a combination of terbinafine and fluconazole (2). Scott et al. described synergistic antifungal activities with fluconazole and ibuprofen (35). The combination of neutrophils or macrophages with fluconazole also showed a synergistic antifungal effect (5, 21, 26). Also, the presence of even 5% human serum in the assay medium was demonstrated to be enough for a significant enhancement of fluconazole activity, probably caused by the presence of a low-molecular-weight component in the serum (20). In this respect, we expanded our present work by adding saliva to the assay medium. We observed only minimal changes in the antifungal activities of the compounds tested, whereas changes in the assay medium or in the pH of the assay medium resulted in considerable variation of antifungal activity (15a).
In addition, cooperative effects of lactoferrin with azole agents against Candida growth were reported by Wakabayashi et al. (41). Those authors reported on a synergistic activity between lactoferrin and clomitrazole. They demonstrated that 200 μg of lactoferrin per ml alone completely inhibited the growth of Candida during incubation for 17 h, whereas the addition of clomitrazole (3 to 12 ng/ml) reduced the MIC of lactoferrin to 50 to 100 μg/ml. It was suggested that the interference with proper membrane synthesis by clomitrazole combined with membrane interactions of lactoferrin explains the observed cooperative inhibitory effects of clomitrazole and lactoferrin. In a more recent study the same group also described an increased activity of fluconazole against fluconazole-resistant C. albicans strains in the presence of lactoferrin (42). In these experiments the authors observed a synergistic activity against the growth of fluconazole-resistant Candida strains with 25 to 400 μg of lactoferrin per ml in combination with 0.12 to 0.25 μg of fluconazole per ml after incubation for 15 h in RPMI medium at pH 7.0. These observations compare well with the results of our study with C. albicans, C. glabratas, and C. tropicalis species. On the other hand, in the earlier study this group did not find cooperative effects with the combinations of lactoferrin with amphotericin B or 5-fluorocytosine (41). Of note, they used only concentrations of up to 100 μg of lactoferrin per ml in their assays to test influences on the MICs of amphotericin B or 5-fluorocytosine. However, in case of amphotericin B, the concentration of 100 μg of lactoferrin per ml might be too low to observe significant changes in MICs (we had to use 500 μg of lactoferrin per ml to observe any effects on the antifungal activity of amphotericin B). In the case of 5-fluorocytosine, the lack of a cooperative effect might be caused by differences in sensitivity of the Candida strains used to the tested antifungals.
Our study shows that the combination of lactoferrin and amphotericin B can be synergistic up to 30% but may also exhibit a moderate antagonistic activity of 10% against both Candida species tested. In an earlier study by Nikawa et al. (23), it was shown that preexposure of C. albicans to amphotericin B resulted in an increased resistance to apo-lactoferrin-mediated cell death. This observation points to similarities in the antifungal mechanisms of apo-lactoferrin and amphotericin B. It should be realized, however, that the mechanism of Candida growth inhibition by lactoferrin is not necessarily explained by actions directed at cell surfaces only. In addition, other studies suggested that amphotericin B might also exert its antifungal activity by interference with cellular oxidation (28, 36). Therefore, differences in the mechanisms of action of lactoferrin and amphotericin B can explain the observed synergistic activity.
Similar reasoning can be put forward for the synergistic activity that we found with the combination of 5-fluorocytosine and lactoferrin. Nikawa et al. (23) showed that apo-lactoferrin interacts with cell membrane constituents of C. albicans. Although the details of this mechanism need clarification, the interaction of apo-lactoferrin with the cell membrane might antagonize the effect of antifungals such as 5-fluorocytosine. In our study we observed a pronounced synergistic antifungal effect only with 5-fluorocytosine in combination with lactoferrin. These results might indicate that the antifungal activity of lactoferrin was not likely to be caused by interactions with the cell membrane alone.
Combinations of drugs that show both antagonistic and cooperative activities are difficult to manage in clinical practice, since fluctuations in their levels in plasma and tissue are difficult to control. It should be attractive, therefore, to use lactoferrin in combination with fluconazole, not only because fluconazole is commonly used by patients but also because this combination is likely to exhibit cooperative or even synergistic antifungal activities over the entire range of potential concentrations. During the usual dosage regimens, a mean salivary concentration of 2.6 μg of fluconazole per ml can be reached, which is considerably higher than its MIC against most clinical isolates (9). In such a situation, the addition of lactoferrin to the existing therapy of fluconazole seems to be of no extra value. However, in case of in vitro resistance of the isolate to fluconazole, resulting in MICs higher than 2.6 μg/ml (Table 1) (11), the addition of lactoferrin can be useful. Our results show that both fluconazole-sensitive and -resistant strains react to the addition of lactoferrin. Therefore, the addition of lactoferrin to the existing therapy with fluconazole may enable a significant lowering of the fluconazole intake and/or may postpone the usual increase in the daily dosage of fluconazole through a delay in induction of resistance against fluconazole.
In an earlier study in our laboratory it was determined that the lactoferrin concentration in saliva was in the range of 10 μg/ml and was not significantly different in healthy and HIV type 1-infected persons. However, it was also demonstrated that larger amounts of Candida were prevalent in HIV type 1-infected persons with relatively small amounts of lactoferrin in their saliva. On the basis of this observation, it was argued that prophylactic treatment of these patients with additional amounts of lactoferrin might be worthwhile to consider (40).
In conclusion, in this study we report on a synergistic activity of fluconazole combined with lactoferrin in vitro against several Candida species. Clinical studies with the aim to elucidate the potential utility of this combination in antimycotic therapy are in progress.
ACKNOWLEDGMENTS
This work was sponsored by a research grant from Numico Research BV, Wageningen, The Netherlands, and in part by a research grant (95011) from the Council for Health Research (RGO/PccAO) and financed by Stichting AIDS funds.
REFERENCES
- 1.Baily G G, Perry F M, Denning D W, Mandal B K. Fluconazole-resistant candidosis in an HIV cohort. AIDS. 1994;8:787–792. doi: 10.1097/00002030-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi F, DiFrancesco L F, Compagnucci P, Arzeni D, Giacometti A, Scalise G. In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J Antimicrob Chemother. 1998;41:59–65. doi: 10.1093/jac/41.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy W, Wakabayashi H, Takase M, Kawase K, Shimamura S, Tomita M. Killing of Candida albicans by lactoferrin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med Microbiol Immunol. 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- 4.Brock J H. Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in the newborn infant. Arch Dis Child. 1980;55:417–421. doi: 10.1136/adc.55.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummer E, Stevens D A. Synergy of human neutrophils with fluconazole in killing Candida species. Mycopathologia. 1996;134:115–120. doi: 10.1007/BF00436717. [DOI] [PubMed] [Google Scholar]
- 6.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists and AIDS Program, Center for Infectious Diseases. Morbid Mortal Weekly Rep. 1997;36:1S–15S. [PubMed] [Google Scholar]
- 8.Cohen M S, Mao J, Rasmussen G T, Serody J S, Britigan B E. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J Infect Dis. 1992;166:1375–1378. doi: 10.1093/infdis/166.6.1375. [DOI] [PubMed] [Google Scholar]
- 9.Force R W, Nahata M C. Salivary concentrations of ketoconazole and fluconazole: implications for drug efficacy in oropharyngeal and esophageal candidiasis. Ann Pharmacother. 1995;29:10–15. doi: 10.1177/106002809502900102. [DOI] [PubMed] [Google Scholar]
- 10.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 11.Garcia Hermoso D, Dromer F, Improvisi L, Provost F, Dupont B. Fluconazole concentrations in saliva from AIDS patients with oropharyngeal candidosis refractory to treatment with fluconazole. Antimicrob Agents Chemother. 1995;39:656–660. doi: 10.1128/AAC.39.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmsen M C, Swart P J, de Béthune M-P, Pauwels R, De Clercq E, The T H, Meijer D K F. Antiviral effects of plasma and milk proteins: lactoferrin shows potent antiviral activity on both human immunodeficiency virus and human cytomegalovirus. J Infect Dis. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 13.Hood S, Denning D W. Treatment of fungal infection in AIDS. J Antimicrob Chemother. 1996;37(Suppl. B):71–85. doi: 10.1093/jac/37.suppl_b.71. [DOI] [PubMed] [Google Scholar]
- 14.Kerridge D, Nicholas R O. Drug resistance in the opportunistic pathogens Candida albicans and Candida glabrata. J Antimicrob Chemother. 1986;18:39–49. doi: 10.1093/jac/18.supplement_b.39. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick C H, Green I, Rich R R, Schade A L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971;124:539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- 15a.Kuipers, M. E., H. G. de Vries, J. H. Heegsma, J. J. M. van den Berg, D. K. F. Meyer, and P. J. Swart. Conditions influencing the antifungal activity of lactoferrin and antifungal drugs against clinical isolates of Candida. Submitted for publication. [DOI] [PubMed]
- 16.Langenbeck B. Auffingung von Pilzen aus der Schleimhaut der Speiseröhre einer Typhus-Leiche. Neue Not Geb Natur-u-Heilk (Froriep) 1839;12:145–147. [Google Scholar]
- 17.Lyman C A, Walsh T J. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs. 1992;44:9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti M, Longhi C, Conte M P, Pisani S, Valenti P, Seganti L. Lactoferrin inhibits herpes simplex virus type 1 adsorption to Vero cells. Antiviral Res. 1996;29:221–231. doi: 10.1016/0166-3542(95)00840-3. [DOI] [PubMed] [Google Scholar]
- 19.Masson P L, Heremans J F. Metal-combining properties of human lactoferrin (red milk protein): the involvement of bicarbonate in the reaction. Eur J Biochem. 1968;6:579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 20.Nassar F, Brummer E, Stevens D A. Different components in human serum inhibit multiplication of Cryptococcus neoformans and enhance fluconazole activity. Antimicrob Agents Chemother. 1995;39:2490–2493. doi: 10.1128/aac.39.11.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan U, Randhawa N, Brummer E, Stevens D A. Effect of granulocyte-macrophage colony-stimulating factor on candidacidal activity of neutrophil, monocytes or monocyte-derived macrophages and synergy with fluconazole. J Med Microbiol. 1998;47:359–363. doi: 10.1099/00222615-47-4-359. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Tentative standard M27-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 23.Nikawa H, Samaranayake L P, Tenovuo J, Hamada T. The effect of antifungal agents on the in vitro susceptibility of Candida albicans to apo-lactoferrin. Arch Oral Biol. 1994;39:921–923. doi: 10.1016/0003-9969(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 24.Nikawa H, Samaranayake L P, Tenovuo J, Pang K M, Hamada T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch Oral Biol. 1993;38:1057–1063. doi: 10.1016/0003-9969(93)90167-k. [DOI] [PubMed] [Google Scholar]
- 25.Odds F C. Candida and candidosis. A review and bibliography. London, United Kingdom: Baillière Tindall; 1988. pp. 93–114. [Google Scholar]
- 26.Okutomi T, Abe S, Tansho S, Wakabayashi H, Kawase K, Yamaguchi H. Augmented inhibition of growth of Candida albicans by neutrophils in the presence of lactoferrin. FEMS Immunol Med Microbiol. 1997;18:105–112. doi: 10.1111/j.1574-695X.1997.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheim F G, Xu T, McMillian F M, Levitz S M, Diamond R D, Offner G D, Troxler R F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 28.Osaka K, Ritov V B, Bernardo J F, Branch R A, Kagan V E. Amphothericin B protects cis-parinaric acid against peroxyl radical-induced oxidation: amphotericin B as an antioxidant. Antimicrob Agents Chemother. 1997;41:743–747. doi: 10.1128/aac.41.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller M A, Burmeister L, Bartlett M S, Rinaldi M G. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988;26:1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plettenberg A, Stoehr A, Hoffken G, Bergs C, Tschechne B, Ruhnke M, Heise W, Dieckmann S, Meigel W. Fluconazole therapy of oral candidiasis in HIV-infected patients: results of a multicentre study. Infection. 1994;22:118–123. doi: 10.1007/BF01739022. [DOI] [PubMed] [Google Scholar]
- 31.Pollock J J, Denepitiya L, Mackay B J, Iacona V J. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun. 1984;44:702–707. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prichard M N, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 33.Reiter B. The biological significance of lactoferrin. Int J Tissue React. 1983;5:87–96. [PubMed] [Google Scholar]
- 34.Samaranayake L P. Host factors and oral candidosis. In: Samaranayake L P, MacFarlane T W, editors. Oral candidosis. London, United Kingdom: Wright; 1990. pp. 66–103. [Google Scholar]
- 35.Scott E M, Tariq V N, McCrory R M. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob Agents Chemother. 1995;39:2610–2614. doi: 10.1128/aac.39.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokol-Anderson M L, Brajtburg J, Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis. 1986;154:76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- 37.Soukka T, Tenovuo J, Lenander Lumikari M. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol Lett. 1992;69:223–228. doi: 10.1016/0378-1097(92)90650-d. [DOI] [PubMed] [Google Scholar]
- 38.Swart P J, Kuipers M E, Smit C, van der Strate B W A, Harmsen M C, Meijer D K F. Lactoferrin: antiviral activity of lactoferrin. In: Spik G, Legrand D, Mazurier J, Pierce A, Perraudin J, editors. Advances in lactoferrin research. New York, N.Y: Plenum Press; 1998. pp. 205–213. [PubMed] [Google Scholar]
- 39.Tumbarello M, Caldarola G, Tacconelli E, Morace G, Posteraro B, Cauda R, Ortona L. Analysis of the risk factors associated with the emergence of azole resistant oral candidosis in the course of HIV infection. J Antimicrob Chemother. 1996;38:691–699. doi: 10.1093/jac/38.4.691. [DOI] [PubMed] [Google Scholar]
- 40.van der Strate, B. W. A., M. C. Harmsen, T. H. The, H. Sprenger, M. C. Eikelboom, M. E. Kuipers, D. K. F. Meijer, and P. J. Swart. Plasma lactoferrin levels are decreased in end-stage AIDS patients. Viral Immunol., in press. [DOI] [PubMed]
- 41.Wakabayashi H, Abe S, Okutomi T, Tansho S, Kawase K, Yamaguchi H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol Immunol. 1996;40:821–825. doi: 10.1111/j.1348-0421.1996.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi H, Abe S, Teraguchi S, Hayasawa H, Yamaguchi H. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob Agents Chemother. 1998;42:1587–1591. doi: 10.1128/aac.42.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]