Abstract
The Indian laurel-leaf fig (Ficus microcarpa) is an important ornamental tree widely distributed in the urban areas of Italy. Surveys conducted in 2019 and 2020 on several tree-lined streets, squares, and public parks in Catania and Siracusa provinces (Sicily, southern Italy) revealed the presence of a new disease on mature trees. About 9% of approximately 450 mature plants showed extensive branch cankers and dieback. Isolations from woody tissues obtained from ten symptomatic plants consistently yielded species belonging to the Botryosphaeriaceae family. The identification of the recovered fungal isolates was based on a multi-loci phylogenetic (maximum parsimony and maximum likelihood) approach of the ITS, tef1-α, and tub2 gene regions. The results of the analyses confirmed the presence of three species: Botryosphaeria dothidea, Neofusicoccum mediterraneum, and N. parvum. Pathogenicity tests were conducted on potted, healthy, 4-year-old trees using the mycelial plug technique. The inoculation experiments revealed that all the Botryosphaeriaceae species identified in this study were pathogenic to this host. Previous studies conducted in California showed similar disease caused by Botryosphaeriaceae spp., and the pathogenic role of these fungi was demonstrated. To our knowledge, this is the first report of Botryosphaeriaceae affecting Ficus microcarpa in Europe.
Keywords: canker, dieback, Indian laurel-leaf fig, Ficus microcarpa, Botryosphaeriaceae, phylogeny
1. Introduction
Ficus microcarpa, commonly known as Chinese or Malayan banyan, Indian laurel-leaf fig, and curtain fig, is a widely distributed evergreen ornamental species belonging to the family Moraceae, native to Ceylon, India, southern China, the Ryukyu Islands, Australia, and New Caledonia [1]. It is considered one of the most common urban trees in warm climates worldwide [2]. Moreover, F. microcarpa is also well known as an invader species due to its ability to grow in inhospitable places, its large fruit production, and its numerous dispersal agents (birds, bats, rodents, and others) [3]. Many Ficus spp. were introduced in southern Italy as ornamental species; they are now common in many urban areas and viewed as an important form of historical heritage [4]. Parks and gardens in urban areas are of significant value for all people living their daily lives in the cities. Urban trees have a positive impact on reducing heat, providing a convenient shelter, reducing wind velocity, and increasing the aesthetic value of the landscape [5,6,7]. In addition, most people living in cities deal with schedules, work, appointments, meetings, etc., and urban parks and open spaces positively affect mental health [8]. Thus, it is important not to underestimate the health of urban trees.
According to Fungal Database 53 records of fungus association with this host have been reported worldwide [9]. Among these, particular attention is given to species belonging to Botryosphaeriaceae. In fact, diseases caused by Botryosphaeriaceae are drawing the attention of the researchers worldwide, since they are a significant threat to many crops, especially in Mediterranean climates [10]. Botryosphaeriaceae include a large group of diverse fungal species, distributed all over the world. These fungi are well known as plant pathogens, endophytes, and saprophytes of woody hosts [11]. Due to their role as plant pathogens, these fungal species have been studied for a long time, and their impact on forestry and agricultural production is well known [12]. Botryosphaeriaceae induce severe symptoms, such as branch, shoot, and trunk cankers, and blight fruits and leaves.
Botryosphaeriaceae disease studies on Ficus spp., including the cultivated common fig (F. carica), have been published worldwide, showing that Botryosphaeriaceae and Diaporthaceae spp. are involved in complex diseases [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Botryosphaeriaceae cause polyetic epidemics (2–3 cycles per season); thus, the progress of epidemics may extend for several years [10]. In addition, Botryosphaeriaceae, characterized by a wide host range, can easily jump from one host to another; this is particularly evident in Mediterranean landscapes, where different crops are cultivated nearby [10]. Especially in the case of urban environments, it must be remembered that dangerous situations are related to the health status of the trees. Therefore, it is important to monitor the health of trees before they become hazardous [30]. Surveys conducted in the metropolitan area of Catania and Siracusa (Sicily), during 2019 and 2020 revealed many F. microcarpa distributed among numerous metropolitan areas, including gardens, public parks, tree-lined streets, and squares, showing severe symptoms of branch cankers and dieback. The aims of this study were to (i) investigate the etiology of the disease by (ii) characterizing the fungal isolates recovered from diseased trees based on a multi-loci phylogenetic analysis and (iii) assess their pathogenicity.
2. Results
2.1. Surveys and Fungal Isolations
Ficus microcarpa growing in a wide range of site conditions (tree-lined streets, gardens, public parks, and squares) have suffered a widespread dieback in Catania and Siracusa provinces. In the public areas where the research was conducted, more than 40 mature F. microcarpa trees (20 to 50 years old) showed cankered twigs and branches on approximately 450 plants. The trees still appeared green in part of the canopy, although this was accompanied by parts of branches and shoots that were defoliated and dead (Figure 1A–D and Figure 2A). Sometimes, it was possible to observe new twigs growing below the damaged branches (Figure 1C). The sample consisted of large portions of branches showing severe internal wood discolouration, including of sapwood and the heartwood (Figure 2B–F). Often, the bark appeared cracked and split along the branches (Figure 2), and internal cankers were sharply demarcated from adjacent, healthy wood (Figure 2B–F). Isolations frequently (>70%) yielded Botryosphaeriaceae-like fungi, characterized, as reported by Slippers and Wingfield [11], by a ‘fluffy’ mycelium, either white-to-creamy, pigmented ‘greenish brown’, or gray-to-gray-black. Moreover, with lower frequencies, colonies of Eutypella-like species were also isolated from symptomatic tissues.
Figure 1.
Symptoms of Botryosphaeriaceae disease observed in urban areas on F. microcarpa. (A) Diseased (left) and healthy (right) plants. (B–D) Defoliation and shoot dieback all over the canopy. (C) New twigs growing below the dead shoots.
Figure 2.
Internal symptoms. (A) Dead branch showing cracking of the outer layers of the bark (upper), healthy branch (lower). (B–F) Internal cankers and bark cracked along the branch with diseased tissue sharply demarcated from adjacent, healthy wood. Scale bars: (B) = 15 cm; (C) = 50 cm; (D–F) = 20 cm.
2.2. Morphological Characterization and Phylogenetic Analysis
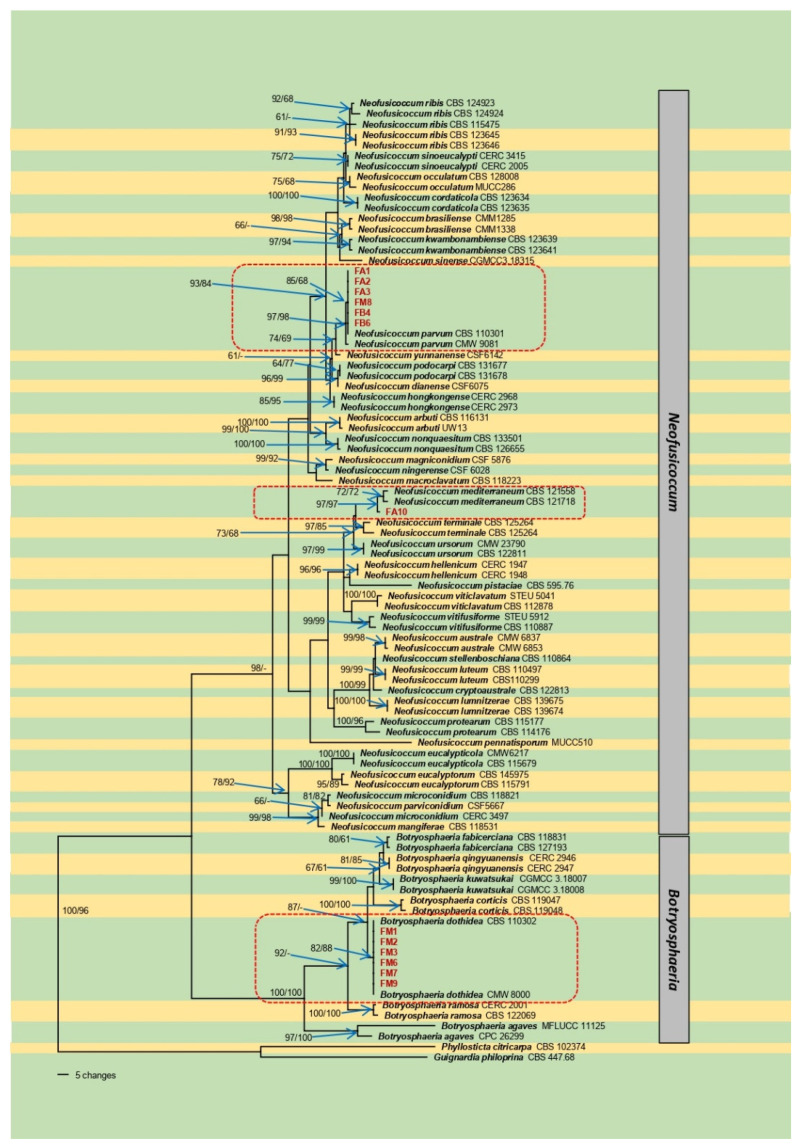
The PCR amplification of the ITS region, tef1-α, and tub2 generated 577 to 581, 273 to 288, and 422 to 446 bp fragments, respectively. The phylogenetic analyses were performed using a dataset of the three concatenated loci. The sequences generated in this study were deposited in GenBank (Table 1). A preliminary comparison of our sequences in GenBank showed our isolates belonging to the genera Botryosphaeria and Neofusicoccum. The Eutypella-like species showed high similarity with different Eutypella species submitted to GenBank. Since these isolates were excluded from the phylogenetic analyses due to their results in the pathogenicity test, they were identified as Eutypella spp. The phylogenetic analyses were then conducted only for the Botryosphaeriaceae. The results of the partition-homogeneity test indicated no (p = 1.00) significant differences in the three-gene dataset. The MP analysis of the combined dataset showed that of 2921 total characters, 391 were parsimony-informative, 220 were parsimony-uninformative, and 2310 were constant. In total, 100 trees were retained. Tree length was equal to 1098, CI = 0.707, RI = 0.912, RC = 0.644. The best-fit model of nucleotide evolution based on the AIC was GTR + I + G for ITS, GTR + G for tef1-α, and HKY + G for tub2. The ML analysis showed that of 2921 total characters, 2310 were constant, 475 were parsimony informative, and 136 were autapomorphic. The results of both analyses showed that the isolates FM1-3, FM6 and 7, and FM9 were grouped in the clade of B. dothidea (82/88, MP and ML bootstrap support %, respectively), the isolate FA10 grouped within N. mediterraneum clade (97/97), and FA1-3, FM8, FB4, and FB6 were grouped with the clade of N. parvum (97/98) (Figure 3). The conidia measurements were (18.66)–22.7–(28.34) × (3.61)–4.9–(6.38) for B. dothidea, (14.0)–20.0–(27.2) × (4.3)–5.8–(6.8) for N. mediterraneum, and (12.78)–15.1–(16.9) × (4.16)–5.3–(7.21) for N. parvum.
Table 1.
Information on fungal isolates used in the phylogenetic analyses and their corresponding GenBank accession numbers. Isolates in bold are from this study.
| Scheme 1. | Isolate ID | ITS | tef1-α | tub2 |
|---|---|---|---|---|
| Botryosphaeria agaves | CBS 133992 = MFLUCC 11-0125T | JX646791 | JX646856 | JX646841 |
| B. agaves | CBS 141505 = CPC 26299 | KX306750 | MT592030 | MT592463 |
| B. corticis | CBS 119047T | DQ299245 | EU017539 | EU673107 |
| B. corticis | CBS 119048 = CAP 198 | DQ299246 | EU017540 | MT592464 |
| B. dothidea | CBS 115476 = CMW 8000T | AY236949 | AY236898 | AY236927 |
| B. dothidea | CBS 110302 = CAP 007 | AY259092 | AY573218 | EU673106 |
| B. dothidea | FM1 | OM241975 | OM262426 | OM262439 |
| B. dothidea | FM2 | OM241976 | OM262427 | OM262440 |
| B. dothidea | FM3 | OM241977 | OM262428 | OM262441 |
| B. dothidea | FM6 | OM241978 | OM262429 | OM262442 |
| B. dothidea | FM7 | OM241979 | OM262430 | OM262443 |
| B. dothidea | FM9 | OM241980 | OM262431 | OM262444 |
| B. fabicerciana | CBS 118831 = CMW 14009 | DQ316084 | MT592032 | MT592468 |
| B. fabicerciana | CBS 127193 = CMW 27094T | HQ332197 | HQ332213 | KF779068 |
| B. kuwatsukai | CGMCC 3.18007 | KX197074 | KX197094 | KX197101 |
| B. kuwatsukai | CGMCC 3.18008 | KX197075 | KX197095 | KX197102 |
| B. qingyuanensis | CERC 2946 = CGMCC 3.18742T | KX278000 | KX278105 | KX278209 |
| B. qingyuanensis | CERC 2947 = CGMCC 3.18743 | KX278001 | KX278106 | KX278210 |
| B. ramosa | CERC 2001 = CGMCC 3.187396 | KX277989 | KX278094 | KX278198 |
| B. ramosa | CBS 122069 = CMW 26167T | EU144055 | EU144070 | KF766132 |
| Guignardia philoprina | CBS 447.68 | FJ824768 | FJ824773 | FJ824779 |
| Neofusicoccum arbuti | CBS 116131 = AR 4014T | AY819720 | KF531792 | KF531793 |
| N. arbuti | CBS 117090 = UW13 | AY819724 | KF531791 | KF531794 |
| N. australe | CBS 139662 = CMW 6837T | AY339262 | AY339270 | AY339254 |
| N. australe | CMW 6853 | AY339263 | AY339271 | AY339255 |
| N. brasiliense | CMM 1285 | JX513628 | JX513608 | KC794030 |
| N. brasiliense | CMM 1338T | JX513630 | JX513610 | KC794031 |
| N. cordaticola | CBS 123634 = CMW 13992T | EU821898 | EU821868 | EU821838 |
| N. cordaticola | CBS 123635 | EU821903 | EU821873 | EU821843 |
| N. cryptoaustrale | CBS 122813 = CMW 23785T | FJ752742 | FJ752713 | FJ752756 |
| N. dianense | CSF6075 = CGMCC3.20082T | MT028605 | MT028771 | MT028937 |
| N. eucalypticola | CBS 115679 = CMW 6539T | AY615141 | AY615133 | AY615125 |
| N. eucalypticola | CBS 115766 = CMW 6217 | AY615143 | AY615135 | AY615127 |
| N. eucalyptorum | CBS 115791 = CMW 10125 = BOT 24T | AF283686 | AY236891 | AY236920 |
| N. eucalyptorum | CBS 145975 = CPC 29337 | MT587477 | MT592190 | MT592682 |
| N. hellenicum | CERC 1947 = CFCC 50067T | KP217053 | KP217061 | KP217069 |
| N. hellenicum | CERC 1948 = CFCC 50068 | KP217054 | KP217062 | KP217070 |
| N. hongkongense | CERC2973 = CGMCC3.18749T | KX278052 | KX278157 | KX278261 |
| N. hongkongense | CERC 2968 = CGMCC 3.18748 | KX278051 | KX278156 | KX278260 |
| N. kwambonambiense | CBS 123639 = CMW 14023T | EU821900 | EU821870 | EU821840 |
| N. kwambonambiense | CBS 123641 = CMW 14140 | EU821919 | EU821889 | EU821859 |
| N. lumnitzerae | CBS 139674 = CMW 41469T | KP860881 | KP860724 | KP860801 |
| N. lumnitzerae | CBS 139675 = CMW 41228 | MT587480 | MT592193 | MT592685 |
| N. luteum | CBS 110497 = CPC 4594 = CAP 037 | EU673311 | EU673277 | EU673092 |
| N. luteum | CBS 110299 = LM 926 = CAP 002T | AY259091 | KX464688 | DQ458848 |
| N. macroclavatum | CBS 118223 = CMW 15955 = WAC 12444T | DQ093196 | DQ093217 | DQ093206 |
| N. magniconidium | CSF5876 = CGMCC3.20077T | MT028612 | MT028778 | MT028944 |
| N. mangiferae | CBS 118531 = CMW 7024T | AY615185 | DQ093221 | AY615173 |
| N. mediterraneum | CBS 121558 | GU799463 | GU799462 | GU799461 |
| N. mediterraneum | CBS 121718 = CPC 13137T | GU251176 | GU251308 | GU251836 |
| N. mediterraneum | FA10 | OM241968 | OM241976 | OM262432 |
| N. microconidium | CERC3497 = CGMCC3.18750T | KX278053 | KX278158 | KX278262 |
| N. microconidium | CBS 118821 = CMW 13998 | MT587497 | MT592212 | MT592704 |
| N. ningerense | CSF6028 = CGMCC3.20078T | MT028613 | MT028779 | MT028945 |
| N. nonquaesitum | CBS 126655 = L3IE1 = PD484T | GU251163 | GU251295 | GU251823 |
| N. nonquaesitum | CBS 133501 = UCR532 | MT587498 | MT592213 | MT592705 |
| N. occulatum | CBS 128008 = MUCC 227T | EU301030 | EU339509 | EU339472 |
| N. occulatum | MUCC 286 = WAC 12395 | EU736947 | EU339511 | EU339474 |
| N. parvum | CBS 138823 = ICMP 8003 = CMW 9081T | AY236943 | AY236888 | AY236917 |
| N. parvum | CBS 110301 = CAP 074 | AY259098 | AY573221 | EU673095 |
| N. parvum | FA1 | OM241969 | OM262420 | OM262433 |
| N. parvum | FA2 | OM241970 | OM262421 | OM262434 |
| N. parvum | FA3 | OM241971 | OM262422 | OM262435 |
| N. parvum | FM8 | OM241972 | OM262423 | OM262436 |
| N. parvum | FB4 | OM241973 | OM262424 | OM262437 |
| N. parvum | FB6 | OM241974 | OM262425 | OM262438 |
| N. parviconidium | CSF5667 = CGMCC3.20074T | MT028615 | MT028781 | MT028947 |
| N. pennatisporum | WAC 13153 = MUCC 510T | EF591925 | EF591976 | EF591959 |
| N. pistaciae | CBS 595.76T | KX464163 | KX464676 | KX464953 |
| N. podocarpi | CBS 131677 = CMW 35494 | MT587508 | MT592223 | MT592715 |
| N. podocarpi | CBS 131678 = CMW 35499 | MT587509 | MT592224 | MT592716 |
| N. protearum | CBS 114176 = CPC 1775 = JT 189T | AF452539 | KX464720 | KX465006 |
| N. protearum | CBS 115177 = CPC 4357 | FJ150703 | MT592239 | MT592731 |
| N. ribis | CBS 115475 = CMW 7772T | AY236935 | AY236877 | AY236906 |
| N. ribis | CBS 124923 = CMW 28320 | FJ900608 | FJ900654 | FJ900635 |
| N. ribis | CBS 124924T | FJ900607 | FJ900653 | FJ900634 |
| N. ribis | CBS 123645 = CMW 14058T | EU821904 | EU821874 | EU821844 |
| N. ribis | CBS 123646 = CMW 14060 | EU821905 | EU821875 | EU821845 |
| N. sinense | CGMCC3.18315T | KY350148 | KY817755 | KY350154 |
| N. sinoeucalypti | CERC2005 = CGMCC3.18752T | KX278061 | KX278166 | KX278270 |
| N. sinoeucalypti | CERC3415 | KX278063 | KX278168 | KX278272 |
| N. stellenboschiana | CBS 110864 = CPC 4598 | AY343407 | AY343348 | KX465047 |
| N. terminaliae | CBS 125263 = CMW 26679T | GQ471802 | GQ471780 | KX465052 |
| N. terminaliae | CBS 125264 = CMW 26683 | GQ471804 | GQ471782 | KX465053 |
| N. ursorum | CBS 122811 = CMW 24480T | FJ752746 | FJ752709 | KX465056 |
| N. ursorum | CBS 122812 = CMW 23790 | FJ752745 | FJ752708 | KX465057 |
| N. yunnanense | CSF6142 = CGMCC3.20083T | MT028667 | MT028833 | MT028999 |
| N. viticlavatum | CBS 112878 = CPC 5044 = JM 86T | AY343381 | AY343342 | KX465058 |
| N. viticlavatum | CBS 112977 = STE-U 5041 | AY343380 | AY343341 | KX465059 |
| N. vitifusiforme | CBS 110887 = CPC 5252 = JM5T | AY343383 | AY343343 | KX465061 |
| N. vitifusiforme | CBS 121112 = STE-U 5912 | EF445349 | EF445391 | KX465016 |
| Phyllosticta citricarpa | CBS 102374 | FJ824767 | FJ538371 | FJ824778 |
T: Type material.
Figure 3.
One of 100 equally parsimonious trees generated from maximum-parsimony analysis of the three-gene (ITS + tef1-α + tub2) combined dataset from Botryosphaeriaceae species. Numbers in front and after the slash represent parsimony and likelihood bootstrap values from 1000 replicates, respectively. Isolates in red were generated in this study. Bar indicates the number of nucleotide changes.
2.3. Pathogenicity Test
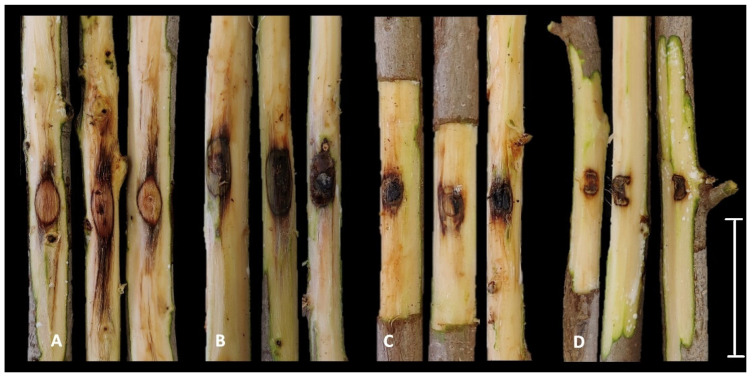
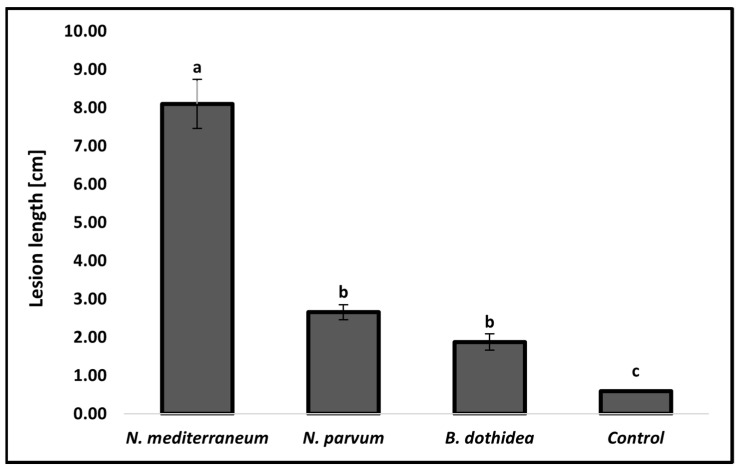
The results of the pathogenicity test showed that all three species of Botryosphaeriaceae identified in this study were pathogenic to F. microcarpa. Otherwise, the Eutypella sp. isolate inoculated did not induce any lesions on the woody tissues, which was similar to the control. For this reason, this species was excluded from the phylogenetic analyses. External discoloration out of the inoculation point was observed after 7 days and all the inoculated trees showed severe wood discoloration after the outer layer of bark was removed (Figure 4A–D) Moreover, young twigs close to the inoculation point rapidly wilted a few days after inoculation. Specifically, among the fungal species, the N. mediterraneum isolate FA10 induced the longest lesions (mean 8.10 cm), followed by N. parvum isolate FB4 (2.66 cm) and B. dothidea isolate FM2 (1.88 cm). All the inoculated species statistically differed from the control (p < 0.05) (Figure 5). The colonies that emerged from the re-isolations showed morphological characteristics (color, shape, and mycelium texture) that fulfilled the Koch’s postulates.
Figure 4.
Results of pathogenicity test after two weeks. (A) Neofusicoccum mediterraneum. (B) N. parvum. (C) Botryosphaeria dothidea. (D) Control. Scale bar = 10 cm.
Figure 5.
Comparisons of average lesion length (cm) resulting from pathogenicity test among B. dothidea, N. mediterraneum and N. parvum on potted plants. Columns are the means of 15 inoculation points (five per plant) for each fungal species. Control consisted of 12 inoculation points. Vertical bars represent the standard error of the means. Bars topped with different letters indicate treatments that were significantly different according to Fisher’s protected LSD test (α = 0.05).
3. Discussion
The results of our study confirm, for the first time, the presence of three species, B. dothidea, N. mediterraneum, and N. parvum, affecting F. microcarpa in Italy. Regarding Botryosphaeriaceae, little is known about its association with F. microcarpa. According to the U.S. National Fungus Collections Fungal Database [9], only a few, old reports describe the association of Lasiodiplodia theobromae (as Botryodiplodia theobromae) in Pakistan [31] and Egypt [29], and Diplodia fici-retusae in Taiwan on Ficus retusa (synonymous of F. microcarpa) [32,33]. A commonly reported disease of F. microcarpa, as well as other Ficus spp., is “sooty canker”, which is caused by Neoscytalidium dimidiatum (traditionally reported also as Hendersonula toruloidea and Natrassia mangiferae). The pathogen, as well as other Botryosphaeriaceae, induces cankers and dieback, often accompanied by a powdery mass of black spores (arthroconidia) produced by this species [14,18,19,20,21,26,28,34]. Recently, in California B. dothidea, N. luteum, N. mediterraneum, and N. parvum were reported as causing branch cankers and dieback on F. microcarpa trees in Los Angeles County [25]. In recent years, Botryosphaeriaceae spp. have been reported attacking many different crops in Italy, and, especially in Sicily, it is well known that these species spread from nurseries to the open field, from ornamental plants to the agricultural ones. Specifically, B. dothidea has recently been reported in Sicily on walnut and pistachio [35,36]. Moreover, N. mediterraneum and N. parvum have been reported as highly aggressive pathogens among the Botryosphaeriaceae in Sicily [13,35,37,38]. In addition, N. mediterraneum was the most encountered species in Sicilian pistachio orchards [36]. From this and previous studies conducted in Sicily, it emerged that Botryosphaeriaceae spp., and especially the species described in this study, are easily encountered in different hosts and landscapes. Regarding the ecology of these fungi, it is well known that they are also endophytes on many hosts [11], often coexisting in the same tissues [39] and forming long latent infections [40,41]. This must be taken into serious consideration, since many infections can spread from nurseries (as latent infections) to open fields. Recently, studies conducted in California on latent infections on nut crops helped us to properly quantify these pathogens using real-time PCR assays [40,42,43]. The ability of these fungi to disperse their spores (conidia) by wind, rain, and insects [10] in conjunction with intercontinental human movements with no adequate quarantine strategies led them to easily spread all over the world [44], as demonstrated for N. parvum, the most adapted organism, which is detected from the north to the south, excluding boreal forests and montane grasslands [45]. Many factors can be involved in the ability of some Botryosphaeriaceae species to jump from one host to another, meaning that they are more virulent than other species. Among these, a recent study [46] revealed how some groups of taxa, such as Botryosphaeria, Lasiodiplodia, and Neofusicoccum, show an expansion of certain clades of gene families involved in the pathogenesis. Specifically, in the Botryosphaeria and Neofusicoccum genomes, an expansion of secreted cell-wall-degrading enzymes (CAZymes) was observed [46]. It is no surprise that the species identified in this study also occurred on other taxonomically distant hosts in Sicily. Batista et al. [45] showed that B. dothidea is associated with 403 hosts in 66 countries, and N. parvum with 223 hosts in 50 countries. In recent decades, in Sicily, a relevant increase was observed in Botryosphaeriaceae in nurseries, as well as in open fields (Polizzi G., unpublished data). Botryosphaeriaceae disease expression is strongly related to stresses due to factors other than the Botryosphaeriaceae infection itself [47,48,49]. Related to this, it should be noticed that climate change contributes to additional stress or pressure on woody plants through extreme weather conditions or the expansion of pathogens’ host ranges [11]. In fact, climate change affects the dynamics of fungal populations, in terms of biology and ecology [49]. Gange et al. [50] conducted a study in the UK on the species Auricularia auricula-judae, demonstrating an alteration in the phenology (the earlier appearance of fruit bodies and a longer fruiting period) and an expansion of the host range consistent with a response to observed warming trends in the climate, also suggesting that climate change affected the interactions between wood-inhabiting fungi. Combative interactions are considered the main drivers of fungal community development in decaying wood [51,52], and these can be strongly affected by temperature, water potential, gaseous regime, and resource size [53,54,55]. All these factors contribute to making Botryosphaeriaceae disease severe and ubiquitous, compared with otherwise “mild diseases” [56]. Urban areas, which are even less investigated than agricultural ones, must be considered crucial routes of introduction and dissemination for Botryosphaeriaceae [57]. It is well known that stressed trees are much more predisposed to Botryosphaeriaceae disease [11,58], and this should be taken into careful consideration regarding ornamental trees in urban landscapes. In fact, trees grown in urban areas can also be considered more exposed to stress factors [59], and thus more susceptible to Botryosphaeriaceae disease. This could represent a serious threat in urban areas, not only in terms of aesthetic damage, but mostly in terms of public safety. In relation to these predisposing factors, we ascertained during our investigation that F. microcarpa trees grown in the urban areas of Catania and Siracusa provinces were severely and improperly pruned, especially during the humid seasons. In order to avoid the spread of Botryosphaeriaceae species, some recommendations should be taken into serious consideration. Since it is known that both rainfall and fog [60,61] positively affect the release of Botryosphaeriaceae spores, farmers or pruning crews should not prune when rain is forecasted or with dense fog to avoid the contamination of fresh wounds by Botryosphaeriaceae [62]. Moreover, recommendations as to pruning type depend on the tree species, which is why trained pruning crews should be selected for this crucial practice. As demonstrated on pistachio, Botryosphaeria panicle and shoot blight were reduced by 50–60% by trained pruning crews compared to the disease levels in trees pruned by unspecialized crews [63]. Furthermore, in California, field experiments conducted on F. carica affected by fig limb dieback demonstrated that pruning 5 cm below the canker successfully removed the pathogen from the tissues [36]. Regarding trained pruning crews, it is crucial that workers disinfect their pruning tools, since these could easily transmit inoculum (spores, mycelium, and fruit bodies) from one tree to another. As demonstrated on walnut, pathogen spores were transferred from the chainsaws to the agar media, whereas Botryosphaeriaceae species were not found when the chainsaws were disinfected with a 2% dilution of vinegar or commercial household bleach (T.J. Michailides, unpublished data/personal communication). In addition, the usage of biological control agents as protectants for pruning wounds, especially in urban areas, should be considered. Encouraging results have been obtained on other crops, such as almond and grapevine treated with Trichoderma-based formulants against canker pathogens [64,65,66]. Further investigations need to be conducted in this direction. Good agronomic practices and, possibly, the usage of biocontrol agents, can help us to control Botryosphaeriaceae disease in urban areas. To our knowledge, this is the first study of Botryosphaeriaceae disease on F. microcarpa in Europe.
4. Materials and Methods
4.1. Surveys and Fungal Isolations
During the years between 2019 and 2020, surveys were carried out in numerous urban areas of the cities of Catania (Catania province), and Siracusa (Siracusa province), Sicily, where F. microcarpa were the most prevalent ornamental trees, including tree-lined streets, gardens, public parks, and squares. Several symptomatic samples obtained from ten plants were collected and brought to the laboratory of the Dipartimento di Agricoltura, Alimentazione e Ambiente, University of Catania, for further investigations. For culture isolation, small sections (0.2 to 0.3 cm2) of symptomatic tissues (branches and shoots) were surface-disinfected for 1 min in 1.5% sodium hypochlorite, rinsed in sterile water, dried on sterile absorbent paper under laminar hood and placed on potato dextrose agar (PDA, Lickson, Vicari, Italy) amended with 100 mg/liter of streptomycin sulfate (Sigma-Aldrich, St. Louis, MO, USA) (PDAS) to prevent bacterial growth, and then incubated at 25 ± 1 °C for 3–5 days until fungal colonies were large enough to be examined. Subsequently, colonies of interest were transferred to fresh PDAS to make pure cultures, and then single-hyphal tip cultures were obtained and maintained on PDAS at 25 ± 1 °C. Isolates characterized in this study were stored in the fungal collection of the laboratory with the labels FA, FB, and FM.
4.2. Morphological and Molecular Characterization
For the morphological characterization of the pathogens, the length and width of 50 conidia from the 21-day-old colonies of the isolates FM1, FA10, and FA1 grown on PDA were measured using a fluorescence microscope (Olympus-BX61) coupled to an Olympus DP70 digital camera; measurements were captured using software analysis 3.2 (Soft Imaging System GmbH, Münster, Germany). Dimensions are reported as the minimum and maximum in parentheses and the average. Total fungal DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA), scraping the mycelium with a sterile scalpel from 5-day-old fungal cultures grown on PDA or malt extract agar (MEA, Oxoid LTD. Basingstoke, Hampshire, England) media. The genomic DNA extracted was visualized on 1% agarose gels (90 V for 40 min) stained with GelRed® (Biotium, Fremont, CA, USA). The quality of the DNA was determined through Nanodrop Lite Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The internal transcriber spacer region (ITS) of the nuclear ribosomal RNA operon was amplified with primers ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) [67]; the primers EF1-728F (5′-CAT CGA GAA GTT CGA GAA GG-3′) and EF1-986R (5′-TAC TTG AAG GAA CCC TTA CC-3′) [68] were used to amplify part of the translation elongation factor 1alpha gene (tef1-α); and primer sets Bt2a (5′-GGT AAC CAA ATC GGT GCT TTC-3′) and Bt2b (5′-ACC CTC AGT GTA GTG ACC CTT GGC-3′) [69] were used for the partial beta tubulin (tub2). Amplification by polymerase chain reaction (PCR) was performed in a total volume of 25 µL using One Taq® 2X Master Mix with Standard Buffer (BioLabs, New England, NEB), according to the manufacturer’s instructions, on an Eppendorf Mastercycler (AG 22331 Hamburg, Germany). The thermal cycle consisted of initial 30 s at 94 °C, followed by 35 cycles at 94 °C for 30 s, 49 °C (ITS), 57–59 °C (tef1-α), or 52 °C (tub2) for 1 min, 68 °C for 1 min, and 5 min at 68 °C. Regarding Botryosphaeriaceae, in total, 45 isolates were sequenced (tub2) and only 13 representative isolates were considered for further gene sequencing and phylogenetic analyses. Concerning the Eutypella-like species, a total of 7 isolates were sequenced (ITS and tub2). PCR products were visualized on 1% agarose gels (90 V for 40 min), purified, and sequenced by Macrogen Inc. (Seoul, South Korea). Forward and reverse DNA sequences were assembled and edited using MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms [70] and submitted to GenBank.
4.3. Phylogenetic Analysis
Chromatograms were viewed using FinchTV Version 1.4.0 (Geospiza, Inc.; Seattle, WA, USA; http://www.geospiza.com (accessed on 16 February 2022)). Sequences were read and edited using MEGAX. Before constructing the phylogenetic tree, BLAST searches were performed using the NCBI nucleotide database [71]. ITS, tef1-α, and tub2 DNA sequence datasets were aligned using MEGAX, and manual alignments were performed when necessary. A partition-homogeneity test with heuristic search and 1000 homogeneity replicates was performed using PAUP* (Phylogenetic Analysis Using Parsimony) version 4.0a (Sinauer Associates, Sunderland, MA, USA) [72] to test for discrepancies in the three-gene dataset. For comparison, 79 additional sequences were selected according to the recent literature on the Botryosphaeriaceae [73,74] to be included in the alignment (Table 1). Maximum parsimony analysis (MP) was performed in PAUP v.4.0a. The analysis of the combined dataset (ITS + tef1-α + tub2) was performed with the heuristic search function and tree bisection and reconstruction (TBR) as branch-swapping algorithms with the branch-swapping option set to ‘best trees’ only. Gaps were treated as ‘missing’, the characters were unordered and of equal weight, and Maxtrees were limited to 100. Tree length (TL), consistency index (CI), retention index (RI), and rescaled consistency index (RC) were calculated. To identify the best-fit model of nucleotide evolution for each gene according to the Akaike information criterion (AIC), MrModeltest v. 2.4 [75] was used. The maximum likelihood analysis (ML) of the combined genes was performed in GARLI v.0.951 [76]. For both analyses, clade support was assessed by 1000 bootstrap replicates. Guignardia philoprina (CBS 447.68) and Phyllosticta citricarpa (CBS 102374) served as the outgroup in both analyses.
4.4. Pathogenicity Test
Pathogenicity tests were conducted on potted, healthy, 4-year-old F. microcarpa plants maintained at room temperature. For each fungal species, one representative isolate was inoculated. Specifically, three plants were used for each isolate, and five inoculation points were chosen along the trunk on each plant (~30 cm distant one from each other). The inoculation site was first surface-disinfected by spraying with 70% ethanol solution, and wounds were made with a sterilized 6-millimeter cork borer after removing the bark, and a mycelium plug (6 mm in diameter) was placed upside down into the plant tissue wound. Wounds were sealed with Parafilm® (Pechney Plastic Packaging Inc., Chicago, IL, USA). In total, 12 additional wounds were inoculated with sterile PDA plugs as controls. Plants were regularly watered. The presence and length of the resulting lesions were recorded two weeks after the inoculation. Lesion length measurements were analyzed in Statistix 10 [77] via analysis of variance (ANOVA), and mean differences were compared with the Fisher’s protected least significant difference (LSD) test at α = 0.05. In order to fulfil Koch’s postulates, re-isolations were carried out on PDAS following the procedure described above. Each re-isolated fungus was identified through the observation of colony characteristics.
5. Conclusions
In the present study, three species of Botryosphaeriaceae were isolated from symptomatic samples of F. microcarpa showing severe symptoms of cankers, wood discolourations, bark cracking, and dieback. Morphological and molecular tools identified B. dothidea, N. mediterraneum, and N. parvum. Pathogenicity tests fulfilled Koch’s postulates. The results of this study provide new information on this important family of phytopathogenic fungi and its wide host range. This is the first report in Europe of Botryosphaeriaceae affecting F. microcarpa.
Author Contributions
Conceptualization, D.A., A.F. and G.P.; methodology, A.F., M.B.C. and G.G.; software, G.G.; validation, G.P.; formal analysis, G.G.; investigation, G.P.; resources, G.P.; data curation, G.G.; writing—original draft preparation, G.G.; writing—review and editing, D.A., A.F., G.G. and G.P.; visualization, G.P.; supervision, D.A. and G.P.; project administration, G.P.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
Programma Ricerca di Ateneo MEDIT-ECO UNICT 2020–2022 Linea 2-University of Catania (Italy); Starting Grant 2020, University of Catania (Italy); Fondi di Ateneo 2020–2022, University of Catania (Italy), Linea Open Access. Research Project 201–62018, University of Catania 5A722192134.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wagner W.L., Herbst D.R., Sohmer S.H. Manual of the Flowering Plants of Hawai’i, 2 Vols. Volume 83 University of Hawai’i and Bishop Museum Press; Honolulu, HI, USA: 1999. Bishop Museum Special Publication. [Google Scholar]
- 2.Riffle R.L. The Tropical Look. Timber Press; Portland, OR, USA: 1998. [Google Scholar]
- 3.Starr F., Starr K., Loope L. Ficus microcarpa. Chinese Banyan, Moraceae. United States Geological Survey-Biological Resources Division Haleakala Field Station, Maui, Hawai’i. 2003. [(accessed on 16 February 2022)]. Available online: http://www.hear.org/starr/hiplants/reports/pdf/ficus_microcarpa.
- 4.Fici S., Raimondo F.M. On the real identity of Ficus magnolioides. Curtis’s Bot. Mag. 1996;13:105–107. doi: 10.1111/j.1467-8748.1996.tb00549.x. [DOI] [Google Scholar]
- 5.Heisler G.M. Trees Modify Metropolitan Climate and Noise. J. Arboric. 1977;311:201–207. [Google Scholar]
- 6.Scott K., Simpson J., McPherson E. Effects of Tree Cover on Parking Lot Microclimate and Vehicle Emissions. J. Arboric. 1999;25:129–142. doi: 10.48044/jauf.1999.019. [DOI] [Google Scholar]
- 7.Tyznik A. Trees as Design Elements in the Landscape. J. Arboric. 1981;7:53–55. [Google Scholar]
- 8.Wolf K. Urban Nature Benefits: Psycho-Social Dimensions of People and Plants. University of Washington-Center for Urban Horticulture; Seattle, WA, USA: 1998. [Google Scholar]
- 9.Farr D.F., Rossman A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. [(accessed on 10 January 2022)]; Available online: https://nt.ars-grin.gov/fungaldatabases/
- 10.Moral J., Morgan D., Trapero A., Michailides T.J. Ecology and epidemiology of diseases of nut crops and olives caused by Botryosphaeriaceae fungi in California and Spain. Plant Dis. 2019;103:1809–1827. doi: 10.1094/PDIS-03-19-0622-FE. [DOI] [PubMed] [Google Scholar]
- 11.Slippers B., Wingfield M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007;21:90–106. doi: 10.1016/j.fbr.2007.06.002. [DOI] [Google Scholar]
- 12.Phillips A.J.L., Alves A., Abdollahzadeh J., Slippers B., Wingfield M.J., Groenewald J.Z., Crous P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013;76:51–167. doi: 10.3114/sim0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiello D., Gusella G., Fiorenza A., Guarnaccia V., Polizzi G. Identification of Neofusicoccum parvum causing canker and twig blight on Ficus carica in Italy. Phytopathol. Mediterr. 2020;59:147–153. doi: 10.36253/phyto-10798. [DOI] [Google Scholar]
- 14.Al-Bedak O.A., Mohamed R.A., Seddek N.H. First detection of Neoscytalidium dimidiatum associated with canker disease in Egyptian Ficus trees. For. Pathol. 2018;48:e12411. doi: 10.1111/efp.12411. [DOI] [Google Scholar]
- 15.Banihashemi Z., Javadi A.R. Further investigations on the biology of Phomopsis cinerascens, the cause of fig canker in Iran. Phytopathol. Mediterr. 2009;48:454–460. doi: 10.14601/Phytopathol_Mediterr-2947. [DOI] [Google Scholar]
- 16.Çeliker N.M., Michailides T.J. First report of Lasiodiplodia theobromae causing canker and shoot blight of fig in Turkey. New Dis. Rep. 2012;25:12. doi: 10.5197/j.2044-0588.2012.025.012. [DOI] [Google Scholar]
- 17.El-Atta H.A., Aref I.M. Pathogenic mortality of Ficus spp. Int. J. Plant Animal Environ. Sci. 2013;3:204–210. [Google Scholar]
- 18.Elshafie A.E., Ba-Omar T. First report of Albizia lebbeck dieback caused by Scytalidium dimidiatum in Oman. Mycopathologia. 2002;154:37–40. doi: 10.1023/A:1015200707971. [DOI] [PubMed] [Google Scholar]
- 19.Giha O.H. Hendersonula toruloidea associated with serious wilt disease of shade trees in the Sudan. Plant Dis. Rep. 1975;59:899–902. [Google Scholar]
- 20.Güney İ.G., Bozoğlu T., Özer G., Türkölmez Ş., Derviş S. First report of Neoscytalidium dimidiatum associated with dieback and canker of common fig (Ficus carica L.) in Turkey. J. Plant Dis. Prot. 2022:1–5. doi: 10.1007/s41348-022-00586-8. [DOI] [Google Scholar]
- 21.Gusella G., Morgan D.P., ad Michailides T.J. Further Investigation on Limb Dieback of Fig (Ficus carica) Caused by Neoscytalidium dimidiatum in California. Plant Dis. 2020;105:324–330. doi: 10.1094/PDIS-06-20-1226-RE. [DOI] [PubMed] [Google Scholar]
- 22.Hampson M.C. Phomopsis canker on weeping fig in Newfoundland. Can. Plant. Dis. Surv. 1981;61:3–5. [Google Scholar]
- 23.Javadi A.R., Banihashemi Z. III International Symposium on Fig. Volume 798. ISHS Acta Horticulturae, Vilamoura; Algarve, Portugal: 2005. Biology and pathogenicity of Phomopsis cinerascens, the causal agent of fig canker in Fars Province of Iran; pp. 219–222. [DOI] [Google Scholar]
- 24.Lima M.L.P., Uesugi C.H., Santos G.R. First record of dieback of Ficus benjamina caused by Phomopsis cinerescens in the States of Tocantins and Minas Gerais. Fitopatol. Bras. 2005;30:91. doi: 10.1590/S0100-41582005000100019. [DOI] [Google Scholar]
- 25.Mayorquin J.S., Eskalen A., Downer A.J., Hodel D.R., Liu A. First report of multiple species of the Botryosphaeriaceae causing bot canker disease of Indian laurel-lea fig in California. Plant Dis. 2012;96:459. doi: 10.1094/PDIS-08-11-0714. [DOI] [PubMed] [Google Scholar]
- 26.Mirzaee M.R., Mohammadi M., Rahimian H. Nattrassia mangiferae, the cause of die-back and trunk cankers of Ficus religiosa and branch wilt of Psidium guajava in Iran. J. Phytopathol. 2002;150:244–247. doi: 10.1046/j.1439-0434.2002.00748.x. [DOI] [Google Scholar]
- 27.Mohali S.R., Castro Medina F., Úrbez-Torres J.R., Gubler W.D. First report of Lasiodiplodia theobromae and L. venezuelensis associated with blue stain on Ficus insipida wood from the Natural Forest of Venezuela. Forest Pathol. 2017;47:e12355. doi: 10.1111/efp.12355. [DOI] [Google Scholar]
- 28.Ray J.D., Burgess T., Lanoiselet V.M. First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australas. Plant Dis. Notes. 2010;5:48–50. doi: 10.1071/DN10018. [DOI] [Google Scholar]
- 29.Rehab M.A., Rashed M.F., Ammar M.I., El-Morsy S.A. Dieback and sooty canker of Ficus trees in Egypt and its control. Pak. J. Biol. Sci. 2014;17:364–371. doi: 10.3923/pjbs.2014.364.371. [DOI] [PubMed] [Google Scholar]
- 30.Penerbit U.M.T. Health of trees in Titiwangsa recreational park, Kuala Lumpur, Malaysia. J. Sustain. Sci. Manag. 2013;8:191–196. [Google Scholar]
- 31.Ahmad S., Iqbal S.H., Khalid A.N. Fungi of Pakistan. Sultan Ahmad Mycological Society of Pakistan Lahore; Lahore, Pakistan: 1997. p. 248. [Google Scholar]
- 32.Anonymous. List of plant diseases in Taiwan. Pl. Protect. Soc. 1979;404:238–245. [Google Scholar]
- 33.Sawada K. Descriptive catalogue of Taiwan (Formosan) fungi. Part XI. Special Publ. Coll. Agric. Natl. Taiwan Univ. 1959;8:268. [Google Scholar]
- 34.Hodel D.R., Downer A.J., Mathews D.M. Sooty canker, a devastating disease of Indian laurel-leaf fig trees. West. Arb. 2009;35:28–32. [Google Scholar]
- 35.Gusella G., Giambra S., Conigliaro G., Burruano S., Polizzi G. Botryosphaeriaceae species causing canker and dieback of English walnut (Juglans regia) in Italy. Forest Pathol. 2020;51:e12661. doi: 10.1111/efp.12661. [DOI] [Google Scholar]
- 36.Gusella G., Lawrence D.P., Aiello D., Luo Y., Polizzi G., Michailides T. Etiology of Botryosphaeria Panicle and Shoot Blight of Pistachio (Pistacia vera) caused by Botryosphaeriaceae in Italy. Plant Dis. 2021 doi: 10.1094/PDIS-08-21-1672-RE. in press. [DOI] [PubMed] [Google Scholar]
- 37.Gusella G., Costanzo M.B., Aiello D., Polizzi G. Characterization of Neofusicoccum parvum causing canker and dieback on Brachychiton species. Eur. J. Plant Pathol. 2021;161:999–1005. doi: 10.1007/s10658-021-02379-5. [DOI] [Google Scholar]
- 38.Ismail A.M., Cirvilleri G., Lombard L., Crous P.W., Groenewald J.Z., Polizzi G. Characterisation of Neofusicoccum species causing mango dieback in Italy. J. Plant Pathol. 2013;95:549–557. doi: 10.4454/JPP.V95I3.008. [DOI] [Google Scholar]
- 39.Luo Y., Niederholzer F., Lightle D., Felts D., Lake J., Michailides T.J. Limited Evidence for Accumulation of Latent Infections of Canker-Causing Pathogens in Shoots of Stone Fruit and Nut Crops in California. Phytopathology. 2021;111:1963–1971. doi: 10.1094/PHYTO-01-21-0009-R. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y., Lichtemberg P.S.F., Niederholzer F.J.A., Lightle D.M., Felts D.G., Michailides T.J. Understanding the process of latent infection of canker-causing pathogens in stone fruit and nut crops in California. Plant Dis. 2019;103:2374–2384. doi: 10.1094/PDIS-11-18-1963-RE. [DOI] [PubMed] [Google Scholar]
- 41.Marsberg A., Kemler M., Jami F., Nagel J.H., Postma-smidt A., Naidoo S., Wingfield M.J., Crous P.W., Spatafora J.W., Hesse C.N., et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017;18:477–488. doi: 10.1111/mpp.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo Y., Gu S., Felts D., Puckett R.D., Morgan D.P., Michailides T.J. Development of qPCR systems to quantify shoot infections by canker-causing pathogens in stone fruits and nut crops. J. Appl. Microbiol. 2017;122:416–428. doi: 10.1111/jam.13350. [DOI] [PubMed] [Google Scholar]
- 43.Luo Y., Niederholzer F.J.A., Felts D.G., Puckett R.D., Michailides T.J. Inoculum quantification of canker-causing pathogens in prune and walnut orchards using real-time PCR. J. Appl. Microbiol. 2020;129:1337–1348. doi: 10.1111/jam.14702. [DOI] [PubMed] [Google Scholar]
- 44.Slippers B., Crous P.W., Jami F., Groenewald J.Z., Wingfield M.J. Diversity in the Botryosphaeriales: Looking back, looking forward. Fungal Biol. 2017;121:307–321. doi: 10.1016/j.funbio.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Batista E., Lopes A., Alves A. What Do We Know about Botryosphaeriaceae? An Overview of a Worldwide Cured Dataset. Forests. 2021;12:313. doi: 10.3390/f12030313. [DOI] [Google Scholar]
- 46.Garcia J.F., Lawrence D.P., Morales-Cruz A., Travadon R., Minio A., Hernandez-Martinez R., Rolshausen P.E., Baumgartner K., Cantu D. Phylogenomics of plant-associated Botryosphaeriaceae species. Front. Microbiol. 2021;12:587. doi: 10.3389/fmicb.2021.652802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blodgett J.T., Stanosz G.R. Sphaeropsis sapinea and host water stress in a red pine plantation in central Wisconsin. Phytopathology. 1995;85:1044. doi: 10.1094/PHYTO.1997.87.4.429. [DOI] [PubMed] [Google Scholar]
- 48.Schoeneweiss D.F. The role of environmental stress in diseases of woody plants. Plant Dis. 1981;65:308–314. doi: 10.1094/PD-65-308. [DOI] [Google Scholar]
- 49.Swart W.J., Wingfield M.J. Biology and control of Sphaeropsis sapinea on Pinus species in South Africa. Plant Dis. 1991;75:761–766. doi: 10.1094/PD-75-0761. [DOI] [Google Scholar]
- 50.Gange A.C., Gange E.G., Mohammad A.B., Boddy L. Host shifts in fungi caused by climate change? Fungal Ecol. 2011;4:184–190. doi: 10.1016/j.funeco.2010.09.004. [DOI] [Google Scholar]
- 51.Boddy L. Fungal community ecology and wood decomposition processes: From standing tree to complete decay of coarse woody debris. Ecol. Bull. 2001;49:43–56. [Google Scholar]
- 52.Boddy L., Heilmann-Clausen J. Basidiomycete community development in temperate angiosperm wood. In: Boddy L., Frankland J., van West P., editors. Ecology of Saprotrophic Basidiomycetes. Academic Press; London, UK: 2008. pp. 211–237. [Google Scholar]
- 53.Boddy L. Interspecific combative interactions between wood decaying basidiomycetes. FEMS Microbiol. Ecol. 2000;31:185–194. doi: 10.1111/j.1574-6941.2000.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 54.Toljander Y.K., Lindahl B.D., Holmer L., Hogberg N.O.S. Environmental fluctuations facilitate species co-existence and increase decomposition in communities of wood-decay fungi. Oecologia. 2006;148:625–631. doi: 10.1007/s00442-006-0406-3. [DOI] [PubMed] [Google Scholar]
- 55.Woodward S., Boddy L. Interactions between saprotrophic fungi. In: Boddy L., Frankland J., van West P., editors. Ecology of Saprotrophic Basidiomycetes. Academic Press; London, UK: 2008. pp. 125–141. [Google Scholar]
- 56.Desprez-Loustau M.L., Marcais B., Nageleisen L.M., Piou D., Vannini A. Interactive effects of drought and pathogens in forest trees. Ann. Forest Sci. 2006;63:597–612. doi: 10.1051/forest:2006040. [DOI] [Google Scholar]
- 57.Lopes A., Barradas C., Phillips A.J.L., Alves A. Diversity and phylogeny of Neofusicoccum species occurring in forest and urban environments in Portugal. Mycosphere. 2016;7:906–920. doi: 10.5943/mycosphere/si/1b/10. [DOI] [Google Scholar]
- 58.Mehl J.W.M., Slippers B., Roux J., Wingfield M.J. Cankers and other diseases caused by Botryosphaeriaceae. In: Paolo Gonthier P., Nicolotti G., editors. Infectious Forest Diseases. CAB International; Boston, MS, USA: 2013. pp. 298–317. [Google Scholar]
- 59.Tubby K.V., Webber J.F. Pests and diseases threatening urban trees under a changing climate. Forestry. 2010;83:451–459. doi: 10.1093/forestry/cpq027. [DOI] [Google Scholar]
- 60.Kuntzmann P., Villaume S., Bertsch C. Conidia dispersal of Diplodia species in a French vineyard. Phytopathol. Mediterr. 2009;48:150–154. [Google Scholar]
- 61.Urbez-Torres J.R., Battany M., Bettiga L.J., Gispert C., McGourty G., Roncoroni J., Smith R.J., Verdegaal P., Gubler W.D. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010;94:717–724. doi: 10.1094/PDIS-94-6-0717. [DOI] [PubMed] [Google Scholar]
- 62.Moral J., Morgan D., Michailides T.J. Management of Botryosphaeria canker and blight diseases of temperate zone nut crops. Crop Prot. 2019;126:104927. doi: 10.1016/j.cropro.2019.104927. [DOI] [Google Scholar]
- 63.Holtz B.A. Plant protection for pistachio. HortTechnology. 2002;12:626–632. doi: 10.21273/HORTTECH.12.4.626. [DOI] [Google Scholar]
- 64.Berbegal M., Ramón-Albalat A., León M., Armengol J. Evaluation of long-term protection from nursery to vineyard provided by Trichoderma atroviride SC1 against fungal grapevine trunk pathogens. Pest Manag Sci. 2020;76:967–977. doi: 10.1002/ps.5605. [DOI] [PubMed] [Google Scholar]
- 65.Holland L.A., Travadon R., Lawrence D.P., Nouri M.T., Trouillas F.P. Evaluation of pruning wound protection products for the management of almond canker diseases in California. Plant Dis. 2021;105:3368–3375. doi: 10.1094/PDIS-11-20-2371-RE. [DOI] [PubMed] [Google Scholar]
- 66.Pertot I., Prodorutti D., Colombini A., Pasini L. Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. Biocontrol. 2016;61:257–267. doi: 10.1007/s10526-016-9723-6. [DOI] [Google Scholar]
- 67.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Michael A., Innis D.H., Gelfand J.J., Sninsky T.J., editors. PCR Protocols: A Guide to Methods and Applications. White Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 68.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.2307/3761358. [DOI] [Google Scholar]
- 69.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 72.Swofford D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sinauer Associates; Sunderland, MA, USA: 2002. [(accessed on 16 February 2022)]. v 4.0a, build 169. Available online: https://paup.phylosolutions.com. [Google Scholar]
- 73.Bezerra J.D.P., Crous P.W., Aiello D., Gullino M.L., Polizzi G., Guarnaccia V. Genetic Diversity and Pathogenicity of Botryosphaeriaceae Species Associated with Symptomatic Citrus Plants in Europe. Plants. 2021;10:492. doi: 10.3390/plants10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W., Groenewald J.Z., Lombard L., Schumacher R.K., Phillips A.J.L., Crous P.W. Evaluating species in Botryosphaeriales. Persoonia. 2021;46:63–115. doi: 10.3767/persoonia.2021.46.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nylander J.A.A. MrModeltest v2. Program distributed by the author; Evolutionary Biology Centre, Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- 76.Zwickl D.J. Ph.D. Thesis. The University of Texas; Austin, TX, USA: 2006. Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets under the Maximum Likelihood Criterion. [Google Scholar]
- 77.Analytical Software . Statistix 10. User’s Manual. Analytical Software; Tallahassee, FL, USA: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.