Abstract
Cancer cells may depend on exogenous serine, depletion of which results in slower growth and activation of adaptive metabolic changes. We previously demonstrated that serine and glycine (SG) deprivation causes loss of sphingosine kinase 1 (SK1) in cancer cells, thereby increasing the levels of its lipid substrate, sphingosine (Sph), which mediates several adaptive biological responses. However, the signaling molecules regulating SK1 and Sph levels in response to SG deprivation have yet to be defined. Here, we identify 1-deoxysphinganine (dSA), a noncanonical sphingoid base generated in the absence of serine from the alternative condensation of alanine and palmitoyl CoA by serine palmitoyl transferase, as a proximal mediator of SG deprivation in SK1 loss and Sph level elevation upon SG deprivation in cancer cells. SG starvation increased dSA levels in vitro and in vivo and in turn induced SK1 degradation through a serine palmitoyl transferase-dependent mechanism, thereby increasing Sph levels. Addition of exogenous dSA caused a moderate increase in intracellular reactive oxygen species, which in turn decreased pyruvate kinase PKM2 activity while increasing phosphoglycerate dehydrogenase levels, and thereby promoted serine synthesis. We further showed that increased dSA induces the adaptive cellular and metabolic functions in the response of cells to decreased availability of serine likely by increasing Sph levels. Thus, we conclude that dSA functions as an initial sensor of serine loss, SK1 functions as its direct target, and Sph functions as a downstream effector of cellular and metabolic adaptations. These studies define a previously unrecognized “physiological” nontoxic function for dSA.
Supplementary key words: sphingosine kinase, sphingosine, serine palmitoyl transferase (SPT), reactive oxygen species (ROS), pyruvate kinase (PKM2), phosphoglycerate dehydrogenase (PHGDH), serine biosynthesis, hereditary sensory and autonomic neuropathy (HSAN), ubiquitination, mass spectrometry
Abbreviations: deoxySL, deoxysphingolipid; dSA, 1-deoxysphinganine; HSAN, hereditary sensory and autonomic neuropathy; LC/MS/MS, liquid chromatography with tandem mass spectrometry; NAC, N-acetyl cysteine; OCR, oxygen consumption rate; PHGDH, phosphoglycerate dehydrogenase; PKM2, pyruvate kinase M2; ROS, reactive oxygen species; S1P, sphingosine 1 phosphate; SG, serine/glycine; SK1, sphingosine kinase 1; Sph, sphingosine; SPT, serine palmitoyl transferase; SSP, serine synthesis pathway
Regulation and metabolism of the nonessential amino acid serine have been the subject of several recent studies (1, 2, 3). Synthesized from glucose or taken up from exogenous sources, serine has vital roles in nucleotide synthesis, NADPH generation, formation of the antioxidant glutathione (through the interconversion of serine to glycine), methylation reactions, and lipid synthesis (sphingolipids and phospholipids). Serine also serves as a precursor to several amino acids such as cysteine and glycine (4, 5). The serine synthesis pathway (SSP) is initiated through phosphoglycerate dehydrogenase (PHGDH) activity, converting 3-phosphoglycerate (3PG) to phosphohydroxypyruvate, which is then transaminated to phosphoserine, and finally dephosphorylated to generate serine (6).
Published studies have shown that serine synthesis is dysregulated in different cancer cell types and that this dysregulation is important for cancer cell growth (7). Indeed, it has been shown that cancer cells often show a dependency on serine supply even though serine is considered a nonessential amino acid (3, 8, 9, 10). Moreover, high levels of PHGDH expression predict poor prognosis in non-small-cell lung cancer (11). Decreased intracellular serine levels can increase PHGDH expression and therefore increase substrate entry into the SSP (12). Increasing glycolytic substrates for SSP entry can also be achieved by decreasing pyruvate kinase M2 (PKM) activity (13). It has been shown that serine can bind to PKM2 and increase its activity, and therefore loss of serine decreases PKM2 activity and increases substrates for SSP. Furthermore, increased serine synthesis through PKM2 activity sustains mTORC1 activity and thus promotes increased cell proliferation (14).
Serine is also necessary for sphingolipid synthesis through a condensation reaction between serine and palmitoyl CoA, the 1st and committed step in sphingolipid synthesis, which is mediated by serine palmitoyl transferase (SPT) (5, 15). Sphingolipids are bioactive lipids with roles in apoptosis, mitophagy, cell survival, and inflammation (15). In particular, several studies have shown an effect of Sph kinase 1, which phosphorylates sphingosine (Sph) to form sphingosine-1-phosphate (S1P), on cell survival (16, 17). Studies examining SK1 expression in cancers have shown a correlation between high SK1 and worse disease outcomes (18, 19).
Interestingly, in the absence of serine, SPT can substitute alanine and glycine to form deoxysphinganine (dSA) and deoxymethylsphinganine, respectively (5). Elevated levels of 1-deoxysphingolipids have been seen in some human disease states such as hereditary sensory and autonomic neuropathy (HSAN), which has mutations in SPT that favor the use of alanine over serine (20). Elevated levels of deoxysphingolipids have also been seen in diabetes mellitus (21, 22), metabolic syndrome (23), nonalcoholic steatohepatitis (24), and macular telangiectasia type 2 (25). Increased deoxysphingolipids have been until now predominantly associated with toxic effects in a wide range of different cell types (21, 26, 27). From a lipid point of view, it appears that deoxy-dihydroceramide is the predominant sphingolipids mediating cytotoxicity (28, 29). However, it is unknown whether noncanonical sphingoid bases also play physiologic functions, and little is known about the function of the initial sphingoid base, dSA, in cell responses. It has been reported that there are increased levels of deoxysphingolipids in fibroblasts from mice with knockout of PHGDH and in tumors after serine/glycine starvation (28, 30). Thus, the possibility arose that serine deprivation may influence the levels of dSA.
We have recently shown that serine and glycine (SG) deprivation causes SK1 proteolysis in cancer cells (10) and that SK1 proteolysis in turn induces accumulation of Sph, which increases intracellular ROS. We did not detect any changes to SK2 levels, at either the protein or mRNA level. The SK1 changes are necessary for metabolic remodeling with an increase in de novo synthesis of serine, thus providing for a sphingolipid-mediated counter regulatory mechanism in response to serine starvation. Thus, with a link between sphingolipids and serine having been established, we evaluated whether serine starvation could increase the synthesis of noncanonical sphingolipid species and what effect these novel sphingolipids could have on SK1 loss, canonical sphingolipids, and cell growth. Here, we show that serine starvation causes a time- and dose-dependent increase in dSA synthesis in several cancer cell lines and in tumor xenografts. Increased dSA could bind to SK1 and cause its proteolysis and subsequent increase in Sph levels. Furthermore, SK1 proteolysis by dSA accumulation increases ROS, consequently allowing for increased PHGDH expression and decreased PKM2 activity. These results demonstrate a novel physiologic role for dSA in promoting cell survival during serine starvation.
Materials and methods
Cell lines and experiments
The human colon carcinoma cell line HCT 116 was obtained from the ATCC. MOLT-4 cells were grown in RPMI 1640 while HEK 293T cells were grown in DMEM. Cell lines were verified by ATCC STR profiling and checked monthly for Mycoplasma. For experiments, cells were initially seeded in high-glucose DMEM (11965) with 10% FBS and 2 mM L-glutamine and kept at 37°C in humidified 5% CO2 in air. For experiments on the effects of serine starvation, complete medium was formulated to closely match the nutrient composition of DMEM (which contains 0.4 mM serine and 0.4 mM glycine); thus, complete medium consisted of MEM (21090) supplemented with additional 1× MEM vitamins (11120), 1xMEM amino acids (11130), 10% dialyzed-FBS (HyClone, Thermo Scientific, Bridgewater, NJ), L-glutamine 2 mM, additional D-glucose (to 25 mM), serine 0.4 mM (42 mg/l), and glycine 0.4 mM. For starvation experiments, cells were fed the same medium formulation without serine and glycine. Removal of glycine is essential for serine starvation as the two amino acids are highly interchangeable. Myriocin was purchased from Sigma (Saint Louis, MO) and used at 100 nM. Bortezomib was purchased from Selleckchem. Caspase 2 (Z-VDVAD-FMK), and caspase-7 inhibitors (Z-DEVD-FMK) were obtained through R&D Systems (Minneapolis, MN). The PHGDH inhibitor CBR 5884 (Sigma) was used at 50 μM. Sphingolipids were purchased from Avanti Polar Lipids Inc. N-acetyl cysteine (NAC) (Sigma, Saint Louis, MO) was made up fresh before each usage, filter sterilized, and used at a final concentration of 0.5 mM.
In vivo experiments
In vivo experiments were performed as described by Maddocks et al. (31). Briefly, HCT-116 (5 × 106) cells were implanted into the left hind flank of female CD-1-Foxn1nu mice (Charles River, Willimantic, CT). Once the tumors became palpable, mice were fed either a control diet (containing serine and glycine as part of the amino acid mix) or diet deficient in serine and glycine (Mod TestDiet 5BQS and 5BQT, respectively, TestDiet, International Product Supplies, Saint Louis, MO). The diets had equal calorific value and equal total amino acid content. Mice were euthanized once tumors had reached a volume of 250 mm3. Tumors were excised and analyzed by LC/MS for sphingolipids as previously described (32), and by Western blot for protein. All animal procedures were approved by the Stony Brook University Institutional Animal Care and Use Committees (IACUC), and all studies conducted were based on NIH and the American Veterinary Medical Association guidelines.
Determination of ROS release
For quantification of ROS, cells were plated in a 96-well plate in triplicate. After loading with DCFH-DA (Cell Biolabs Inc, San Diego, CA) and washing, the cells were lysed and specific fluorescence (Ex/Em = 495/529 nm) measured using a Spectramax M5 (Molecular Devices, San Jose, CA).
Western blotting
Whole cell lysates were prepared in RIPA buffer containing protease inhibitors (Santa Cruz), and protein concentrations were quantified using the BCA Protein Assay (Thermo Scientific). Equal amounts of proteins were loaded and separated on precast Novex 4–20% SDS–PAGE midi gels (Life Technologies, Carlsbad, CA), transferred to nitrocellulose membranes, and probed with antibodies against SK1 (12071) and PHGDH (13428S) from Cell Signaling Technology (Danvers, MA); and β-actin (A5441) from Sigma. HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). Protein bands were revealed using Enhanced Chemiluminescence (ECL) reagent (Thermo Scientific), and their densities were measured by ImageJ. The density ratio of a protein of interest (POI) to the sample loading control, β-actin, is shown underneath the band of the POI.
Sphingolipid analysis
Cells were scraped and pelleted, and lipid extraction and analysis by LC/MS mass spectrometry were performed as described previously (33). DeoxySL was detected and analyzed as we have previously described (34). Lipids were normalized to total lipid phosphate levels of the selected sample.
Ubiquitination assay
This assay was performed as previously described (35). Briefly, cells were transfected with 1 μg of His-Ubiquitin for 24 h. Cells were then washed and treated for another 24 h before His-Ub was purified. A nonpurified sample was also kept as a control. SKi-II inhibitor, previously described as causing SK1 ubiquitination, was used as a positive control (36). SK1 protein was analyzed by Western blot. SK1 inhibitors PF543 (100 nM) and SKi-II (1 μM, both from Sigma Aldrich) were used as they have been reported to cause SK1 protein degradation (37) and data not shown.
Lipid protein overlay
Assays of SK1 binding to dSA were performed as previously described (38). Briefly, 5 μg of lipid was spotted onto a nitrocellulose (NC) membrane, which was subsequently blocked in 3% BSA in PBS-T before being incubated with 1 μg/ml SK1 protein (purified from SK1 overexpressing HCT-116 cells (10)) overnight. After being washed with PBS-T, the NC membrane was incubated for 1 h with anti-SK1 Ab, washed, and then incubated for 1 h with secondary HRP-conjugated Ab. Lipid-bound SK1 on the NC membrane was visualized using ECL.
Inhibition of SK1 proteolysis
ON-TARGETplus SMARTpool siRNAs against Kelch-like protein 5 (KLHL5) (Horizon Discovery, Lafayette, CO) or the negative control siRNA, AllStar (Qiagen) were transfected into cells using Lipofectamine RNAiMax according to the manufacturer’s protocol. KLHL5 knockdown was verified by Western blot as described in our previous study (10).
Experimental model and subject details
In vivo experiments were performed as described by Maddocks et al. (31). Briefly, HCT-116 (5 × 106) cells were implanted into the left hind flank of female CD-1-Foxn1nu mice (Charles River, Willimantic, CT). Once the tumors became palpable, mice were fed either a control diet (containing serine and glycine as part of the amino acid mix) or diet deficient in serine and glycine (Mod TestDiet 5BQS and 5BQT, respectively, TestDiet, International Product Supplies, Saint Louis, MO). The diets had equal calorific value and equal total amino acid content. Mice were euthanized once tumors had reached a volume of 250 mm3. Tumors were excised and analyzed by LC/MS for sphingolipids as previously described (32), and by Western blot for protein.
All animal procedures were approved by the Stony Brook University Institutional Animal Care and Use Committees (IACUC), and all studies conducted were based on NIH and the American Veterinary Medical Association guidelines.
Metabolite analysis
Cells grown in 60 mm dishes were prepared for metabolite analysis as previously described (39). Briefly, cells were plated at 2 × 106/ml and incubated overnight at 37°C. Cells were gently washed with sterile PBS, and the media replaced with serine or serine-free media. After a further 24 h, the media were removed, and the cells were incubated on dry ice for 30 min with 1 ml of 80% methanol at −80°C. Cells were scraped and centrifuged once at 14,000 g before removal of the supernatant. The pellet was washed once with 200 μl of −80°C, 80% methanol solution and pelleted a 2nd time at 14,000 g for 5 min, before removal of the methanol solution and mixing it with supernatant already obtained. The supernatants were divided equally into four aliquots and dried with a Savant SPD 2010 SpeedVac Concentrator (Thermo Scientific). The samples were stored for no more than 2 weeks at −80°C before analysis. Samples were randomized, and targeted analysis of metabolites by LC-MS/MS was performed using Agilent 6490 LC-Triple Quad. Metabolites were positively identified either by comparison to standard compounds or by identification of the largest selective reaction monitoring peak for selected Q1/Q3 transitions, further validated by concordance with standard retention times, and plotted as the peak area for each metabolite (39).
13C isotope tracing experiments
Cells were treated for 24 h before the medium was changed to one that was prewarmed to 37°C and containing [13C6]-glucose (Cambridge Isotope Laboratories, Tewksbury, MA). Cells were incubated for a further 12 h before the medium was removed, and cells were scraped into 50% ice-cold methanol. Before scraping, 1 μl of a 5 mM solution of Adonitol was added as an internal standard. Cells were lysed in methanol by three freeze/thaw cycles (transfer of samples from liquid nitrogen to 37°C water bath). The samples were dried under nitrogen and resuspended in 50 μl 2% MOX reagent (Pierce #45950). After 1 h incubation at 42°C, the samples were dried and TMS-derivatized in 100 μl N, O-Bistrifluoroacetamide (Sigma Aldrich) for 1 h at 75°C. Derivatized samples were analyzed by gas chromatography-electron impact mass spectrometry. Sample peaks were identified by matching retention times and EI fragmentation patterns to neat standards using Agilent MassHunter Software.
Pyruvate kinase (PK) activity
Cells were treated as described before being lysed in PK activity buffer (BioVision). The assay was performed as per the manufacturer’s instructions. Samples were incubated for 10 and 20 min at room temperature before activity was measured using a Spectramax M5 Multimode Plate Reader (Molecular Devices). Results were normalized according to protein levels as measured by BCA.
Real-time PCR
RNA extraction was performed using the Purelink RNA Kit (Life Technologies) according to the manufacturer's protocol. RNA quality and concentration were verified by nanodrop, after which 1 μg of RNA was transformed into cDNA using the SuperScript III cDNA Kit (Invitrogen) according to the manufacturer's protocol. For qRT-PCR, reactions were run in triplicates in 96-well plates with each reaction containing 10 μl of 2 × iTAQ mastermix, 5 μl of cDNA, 1 μl of Taqman primer probe, and 4 μl of water. The primer probes used were purchased from Life Technologies and amplified with PHGDH (cat. no. AM16708), or actin (cat. no. 4448484).
Statistical analysis
All experiments have been independently repeated at least three times. One-way ANOVA was used for three or more group comparisons, and Student's t test was used for two group comparisons. A P value less than 0.05 was considered significant.
Results
Serine starvation induces a marked increase in 1-deoxysphinganine levels in cancer cells
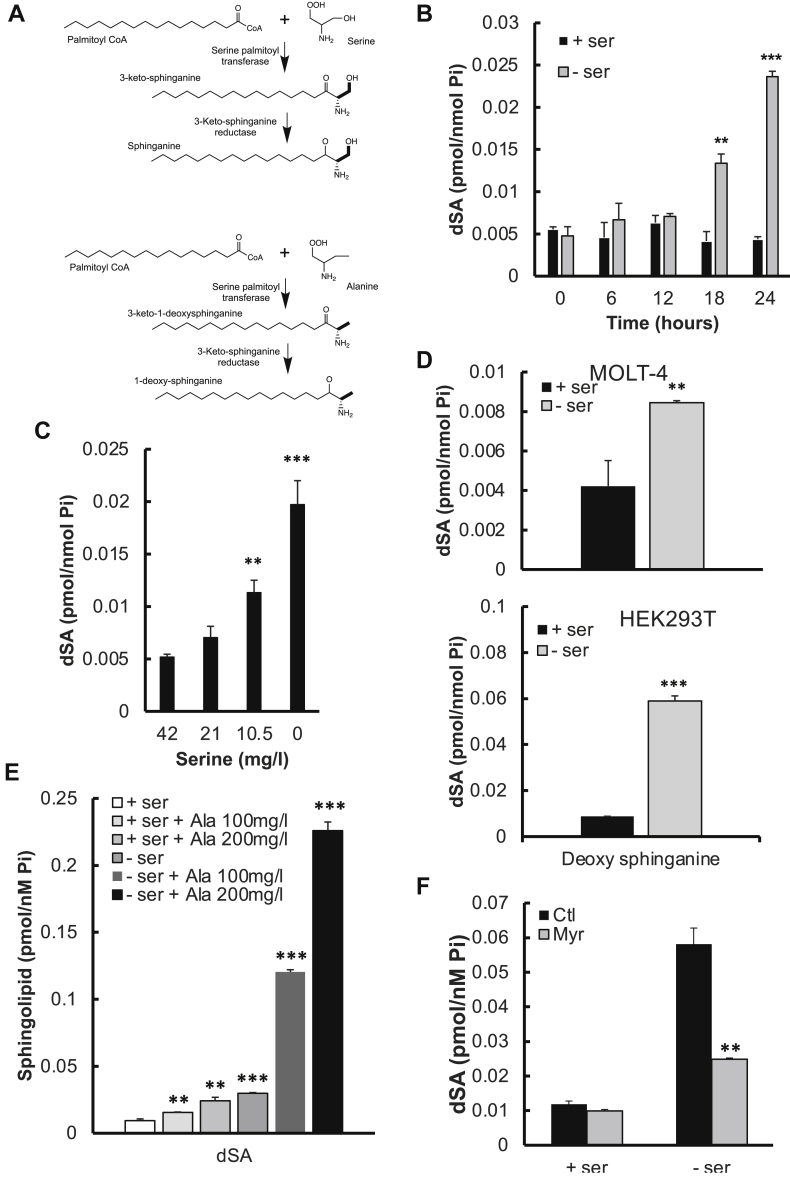
We recently documented a role for SK1 loss and increased Sph in the adaptive responses to SG starvation in cancer cells (10). It should be noted that SG dual starvation is employed because of the direct interconversion of Ser and Gly. However, the downstream mediator that regulates SK1 loss and Sph levels in response to SG deprivation remains unclear. Recent attention has been directed at novel sphingolipids, especially noncanonical sphingoid bases, which form in cells starved for both serine and glycine. When cellular serine is limited, SPT catalyzes the condensation of palmitoyl-CoA and glycine or L-alanine instead to yield 1-deoxysphinganine (dSA) and 1-de(oxymethyl)sphinganine, respectively (Fig. 1A) (30, 40, 41). Therefore, we employed LC/MS/MS to quantitate these noncanonical sphingolipids in HCT116 cancer cells in the absence or presence of exogenous serine and glycine. 1-Deoxy-methylsphingolipids were not detected during SG starvation (data not shown), suggesting that the levels of endogenously synthesized glycine were too low for incorporation into sphingolipids. On the other hand, levels of dSA were increased remarkably at 18–24 h following SG withdrawal (Fig. 1B). The levels of dSA generated in HCT116 cells after 24 h were inversely proportional to the concentration of serine in the medium (Fig. 1C), being the lowest when serine was at levels normally found in DMEM (42 mg/l) and the highest when serine was absent from the medium. These results were mirrored in HEK293T and MOLT-4 cells (Fig. 1D). These results suggest that the increase in dSA synthesis in the absence of serine is a universal phenomenon. It should be noted that L-alanine was not added to the medium, and media usually do not contain L-alanine. On the other hand, addition of exogenous L-alanine (100 mg/l and 200 mg/l) to the culture medium increased dSA synthesis in complete medium and more so in SG-deficient medium (Fig. 1E), suggesting that endogenous synthesis of L-alanine is sufficient to drive dSA synthesis but that exogenous alanine can further drive the process. Pretreatment with myriocin, a selective inhibitor of SPT (42), blocked the dSA increase in response to SG deprivation (Fig. 1F). These results demonstrate that the increased generation of dSA is the consequence of a lack of sufficient amounts of exogenous serine, consistent with the observation that SPT can use alanine as an alternative substrate under conditions of serine insufficiency.
Fig. 1.
1-Deoxysphinganine levels increase during serine starvation. A: Pathways showing the synthesis of both sphinganine and 1-deoxysphinganine (dSA) whereby SPT acts on serine versus alanine, respectively. B: Time course of dSA levels during SG starvation. Cells were incubated without serine and glycine for increasing times before dSA quantification by LC/MS/MS. C: Effects of exogenous serine concentration on intracellular dSA levels in HCT-116 cells. Cells were incubated with decreasing levels of serine for 24 h before dSA levels were analyzed. D: The effect of SG starvation on 1-deoxysphinganine levels. HEK293T or MOLT-4 cells were incubated for 24 h in either serine-replete or serine-deficient media, before dSA analysis. E: The effect of addition of L-alanine on dSA levels. L-alanine at the doses indicated was added and incubated for 24 h concurrently with either serine-replete or serine-deficient media, before analysis of 1-deoxysphinganine. F: Effect of myriocin on dSA levels following SG starvation. Cells were incubated for 24 h in either SG replete or deficient media. Myriocin (100 nM) was added at the same time as levels of 1-deoxyspghinganine were measured by HPLC MS/MS and normalized to levels of cellular phosphates. Statistical significance is shown compared with the + serine control. Data were normalized to total cellular phosphates and indicate mean ± SD, n = 3. ∗∗ P < 0.005, ∗∗∗ P < 0.0005.
dSA is the lipid mediator of serine deprivation in loss of SK1
Previous studies showed that pharmacologic analogs of the natural Sph substrate, such as N-N dimethylSph (36) and also dSA (43), result in loss of SK1. These results prompted us to test whether the increases in endogenous dSA mediate SK1 loss in response to SG deprivation. As we have previously shown, SK1 protein is proteolyzed during SG starvation (10). First, we tested whether inhibiting the formation of dSA using myriocin, a selective inhibitor of SPT, inhibits SK1 loss in response to SG deprivation. Indeed, pretreatment of HCT-116 cells with 100 nM myriocin inhibited the loss of SK1, indicating that de novo sphingolipid synthesis is required for SK1 loss (Fig. 2A). Addition of the proteasome inhibitor bortezomib (BTZ) inhibited SK1 loss during SG deprivation (Fig. 2B), reinforcing the role of the proteasome. To further establish the role of de novo synthesis of dSA as an effector of SK1 proteolysis in response to serine deprivation, exogenous Sph or dSA was added to HCT-116 cells for 24 h. Addition of dSA but not Sph, caused proteolysis of SK1, which could be inhibited by BTZ but not by caspase inhibitors (Fig. 2C). These results support the conclusion that increased dSA mediates loss of SK1 in response to SG deprivation and provide 1st evidence for “bioactivity” of endogenous dSA.
Fig. 2.
dSA regulates SK1 levels. A: Effect of inhibiting de novo sphingolipid synthesis on SK1 levels following serine starvation. Myriocin (100 nM) was added 6 h before the medium change, and cells were incubated for 24 h before processing and analysis of SK1 by Western blot. B: Effect of the proteasomal inhibitor bortezomib (BTZ) on SG starvation-induced SK1 proteolysis. C: SK1 protein levels following treatment with Sph and dSA. Both caspase (ZVAD-fmk) and proteosomal (BTZ) inhibitors were added concurrently with either Sph or dSA and incubated for 24 h before SK1 was analyzed using Western blot. D: Effect of SG starvation on SK1 ubiquitination. Cells were transfected with 1 μg of His-Ub containing plasmid for 24 h before the media was changed. Cells were incubated for a further 24 h before lysis and purification of His-Ub and detection of SK1 by Western blot. For comparison, treatment with SK1-inhibitors PF543 and SKi-II that cause SK1 ubiquitination is also shown. E: Protein lipid overlay comparing SK1 protein binding to Sph, dSA, or phosphatidylcholine (PC). Sph (positive control), dSA, and PC (negative control) were spotted onto nitrocellulose before blocking and overlay of SK1 protein. SK1 was identified as described for Western blotting.
In our previous study, we showed that serine starvation induced the proteolysis of SK1 via a Kelch-like protein 5 (KLHL5)-mediated mechanism (10), suggesting that the SK1 loss is carried out by the ubiquitin-proteosome pathway. We therefore investigated whether dSA could initiate proteolysis of SK1 via this pathway. Indeed, we demonstrated that similar to SG starvation, treatment of cells with dSA but not Sph induced the ubiquitination of SK1 (Fig. 2D), a phenomenon previously described to occur with substrate-based inhibitors of SK1 such as PF543 and SKi-II (43), to which dSA belongs as a naturally produced one. To determine whether dSA could directly bind to SK1, lipid protein overlay assays were performed. SK1 protein was detected binding to either Sph or dSA (Fig. 2E). For a negative control, phosphatidylcholine was also used, to which SK1 did not bind (Fig. 2E). These data demonstrate that SK1 can directly bind to dSA, Together, these data demonstrate that dSA may interact with SK1 and lead to proteasomal degradation.
Depletion of exogenous serine and glycine induces dSA accumulation and SK1 loss in tumor xenografts in mice
In order to evaluate the effects of SG starvation on SK1 and dSA levels in vivo, we investigated whether SG starvation induces SK1 loss and increases in dSA and Sph levels in tumor xenografts implanted in mice. HCT116 cells were implanted subcutaneously in female CD-1-Foxn1nu mice that were fed a control diet (normal chow) or a diet deficient in both serine and glycine. As described by Maddocks et al., mouse xenografts in mice fed an SG-deficient diet grew slower than in mice fed the control diet containing serine and glycine (Fig. 3A) (31). Levels of SK1 in HCT-116 xenografts were decreased in mice fed the SG-deficient diet as compared with mice fed the control diet (Fig. 3B), and conversely, the opposite was true with the levels of dSA (Fig. 3C) and dihydroSph and Sph, the substrates of SK1 (Fig. 3D). Expectedly, the tumor levels of dihydroS1P and S1P, the products of SK1, were lower in the SG-deficient diet group compared with the control diet group (Fig. 3E). These results show that loss of SK1 and dSA accumulation also occurs in tumors under serine-deficient conditions.
Fig. 3.
Serine-deficient diet alters the levels of SK1 and sphingolipids in tumor xenografts implanted in mice. Mice were implanted subcutaneously with HCT116 cells (5 × 106) in the left hind flank and fed either control or SG-deficient diet until the control tumors had reached their maximum allowable volume. Tumors were analyzed for (A) growth, (B) SK1 levels, (C) levels of dSA, (D) levels of dihydrosphingosine (dhSph) and Sph, and (E) levels of dihydroS1P(dhS1P) and S1P via LC/MS and normalized to total lipid phosphate. Results represent mean ± SEM (n = 10). ∗ P < 0.05, ∗∗ P < 0.005, ∗∗∗ P < 0.0005.
dSA mediates production of ROS and the reprogramming of the glycolytic pathway in response to SG deprivation
As we have previously demonstrated that Sph, either generated endogenously as a result of SG starvation or added exogenously, increased intracellular ROS (10), the effect of exogenously added dSA on ROS was examined. Addition of exogenous dSA increased intracellular ROS in HCT116 cells to a level similar to SG starvation (Fig. 4A).
Fig. 4.
dSA or Sph accumulation inhibits PKM2 activity and remodels glycolysis via ROS production. A: Effect of exogenous dSA and Sph on intracellular ROS levels. Cells were treated with either 0.5 μM dSA or 1 μM Sph and incubated in medium either SG replete or deficient for 24 h. ROS was then measured as described in the Materials and methods. Statistical analysis compared treatments to its respective untreated control. Results represent mean ± SD, n = 3. ∗ P < 0.05, ∗∗ P < 0.005. B|B, Increased phosphoenolpyruvate (PEP) and decreased pyruvate levels after 24 h treatment with Sph or dSA. Pyruvate and phosphoenolpyruvate were measured by Triple Quad HPLC, from cells incubated 24 h with either Sph or dSA. Peak area corresponding to the metabolite spectrum was divided by peak area of the metabolite in control samples. Results represent mean ± SD, n = 5. C: PKM activity after myriocin treatment and sphingolipid addition. Myriocin (100 nM) was added at the same time as the medium change and addition of exogenous sphingolipid. Cells were lysed, and PKM activity was measured using a colorimetric assay and normalized to total cellular protein. D: NAC inhibits Sph and dSA effects on PKM2 activity. Cells were treated for 24 h before processing. Pyruvate kinase activity was measured using a kit and normalized to the total concentration of protein present within the sample. Results represent mean ± SD, n = 3.
Having demonstrated that dSA regulates ROS production, we investigated its role in mediating the reprogramming of central carbon metabolism as ROS have been implicated in regulating metabolic reprogramming (13, 44). A survey of multiple pathway intermediates in central carbon metabolism by LC-MS metabolomics showed an increase in phosphoenolpyruvate (PEP) and a corresponding decrease in pyruvate in Sph or dSA-treated cells (Fig. 4B), consistent with the effects reported by Maddocks et al. on the effects of serine starvation (31), and thus functionally linking dSA and Sph to serine starvation.
Since these results suggest a decrease in PKM2 activity, which is thought to divert glycolysis toward anabolic metabolism including the SSP (13, 14), PKM activity was measured in cells treated for 24 h with either Sph or dSA. The results showed that PKM activity was significantly decreased in SG-starved cells (Fig. 4C). Likewise, dSA or Sph also reduced PKM2 activity (Fig. 4C). Furthermore, pretreatment with myriocin partially inhibited the PKM2 activity decrease by SG starvation but not by treatment with exogenous Sph or dSA, suggesting that increased Sph or dSA mediates the reduction of PKM activity in response to SG deprivation.
Since we had shown that a moderate increase in the amounts of ROS, inhibitable by NAC, mediated the effects of SG deprivation on cell growth and metabolism, we evaluated the role of ROS as mediators downstream of dSA. Addition of NAC inhibited the Sph or dSA-induced suppression of PKM2 activity (Fig. 4D), indicating that the intracellular rise of ROS was responsible for mediating the effects of the bioactive sphingoid bases on PKM2. These results show that the small rise in intracellular ROS observed after increased dSA and Sph during serine starvation can modify metabolic pathways as an adaptive response.
dSA mediates the reprogramming of the serine synthesis pathway in response to SG deprivation
To compensate for the reduced serine levels during serine starvation, the cell upregulates the de novo synthesis of serine (31). To determine whether dSA (as a 1st responder to SG deprivation and inducer of SK1 loss) or Sph (as a downstream effector of SK1 loss) could mediate adaptive changes in serine synthesis, the levels of serine generated endogenously from unlabeled glucose and from other cellular substrates not directly related to exogenous glucose (m + 0) and serine synthesized through the SSP from the labeled glucose (m + 3) were measured after [13C6]-glucose labeling. Levels of both endogenous (m + 0, left panel) and de novo serine synthesized from labeled glucose (m + 3, right panel) were increased in Sph and dSA-treated cells (Fig. 5A), demonstrating a key role for these bioactive lipids in driving flux through the SSP. Since increased expression of PHGDH, the 1st committed enzyme in the SSP (45), has been reported in response to serine starvation (13, 14), levels of the enzyme were examined. Changes to PHGDH protein levels were minimal after dSA treatment of cells cultured in the presence of serine (data not shown). As HCT-116 cells highly express PHGDH and have been shown to have a high intake of serine (31), we postulated that a high concentration (42 mg/l) of serine in the medium (as formulated in DMEM) may compensate for any changes in glycolytically derived serine. Therefore, we conducted the experiment with cells cultured in media containing 11 mg/l serine (one-quarter the normal culture conditions), which did not elicit any significant changes in PHGDH levels. Under this culture condition, we observed a significant increase in PHGDH expression upon addition of dSA (Fig. 5B). Pretreatment of HCT-116 cells with myriocin inhibited SG starvation-induced PHGDH upregulation (Fig. 5C), supporting the requirement for the de novo formation of a bioactive sphingolipid mediator. In addition, treatment of MOLT-4 (Fig. 5D, left panel) and HEK293T cells (Fig. 5D, right panel) with exogenous Sph or dSA also increased PHGDH levels, once again confirming that this phenomenon is not restricted to a single cell line. Furthermore, pretreatment of MOLT-4 or HEK293 cells with myriocin inhibited the increased expression of PHGDH in response to SG deprivation (Fig. 5E), once again underlining the importance of sphingolipid metabolism in the response of cells to SG starvation. Interestingly, levels of glycine (Fig. 5F), either background (m + 0, left panel) or derived from the cleavage of glycolytically generated serine (m + 2, right panel), were also significantly elevated after treatment with Sph or dSA, again underscoring the ability of these bioactive sphingolipids to drive the increase in serine synthesis through the SSP.
Fig. 5.
dSA or Sph accumulation increases PHGDH expression and serine biosynthesis via ROS production. A: Generation of intracellular serine from glycolytic intermediates after 24 h treatment with dSA. The left panel shows intracellular levels of endogenously derived serine (m + 0), the right panel shows serine (m + 3) synthesized from labeled glucose. B: Increased levels of PHGDH in Sph or dSA-treated cells. Cells were cultured for 24 h in serine-reduced media (11 mg/l) containing increasing levels of dSA. PHGDH levels were then characterized by Western blot. The 1st two lanes show the effect of SG starvation on PHGDH levels and are included as a positive control. Also shown is the densitometry of the PHGDH band normalized to its corresponding actin band and presented as a percentage of the control, serine-replete cultured cells. C: Effect of myriocin on PHGDH upregulation. Myriocin (100 nM) was added at the same time as the medium was changed. Cells were processed 24 h later, and PHGDH levels were examined by Western blot. D: Addition of either dSA or Sph on PHGDH expression in both MOLT-4 and HEK293T. E: Role of de novo synthesis of sphingolipids on induction of PHGDH in response to serine deprivation. Myriocin (100 nM) was added concurrently with either serine-replete or serine-deficient media and incubated for 24 h before PHGDH levels were analyzed by Western blot. F: Increased levels of glycolytically derived glycine after treatment with Sph or dSA. The left panel shows intracellular levels of exogenously derived glycine, the right panel shows glycine synthesized from serine hydrolysis. Serine and glycine levels were measured by GC/MS after labeling cells with [13C6]-glucose for 24 h. G: Levels of PHGDH mRNA after treatment with NAC. Cells were medium changed to one containing reduced serine (labeled as < ser). At the same time, Sph, dSA, and NAC were added. After 24 h incubation, cells were prepared for qRT-PCR for PHGDH. Samples were analyzed in triplicate, and the mean average ± SD is shown. Statistical analysis for non-NAC treated cells was compared with the non-NAC treated control, while NAC-treated cells were compared with NAC-treated control. H: Effects of NAC on PHGDH protein levels. Cells were cultured for 24 h in serine-reduced media, containing either NAC or Sph or dSA. I: Effect of NAC treatment on the abundance of glucose-derived serine. NAC was added to cells for 3 h before the medium was changed to one containing [13C6]-glucose. The cells were incubated for a further 24 h before analysis of serine by HPLC GC/MS. HPLC GC/MS data are the mean average of five separate measurements, ± SD. ∗ P < 0.05, ∗∗ P < 0.005, ∗∗∗ P < 0.0005.
To determine whether ROS play a role in regulating serine synthesis, cells were preincubated with NAC for 3 h before serine starvation. Pretreatment with NAC in serine-starved cells markedly blunted the upregulation of PHGDH, both at the mRNA and protein levels (Fig. 5G, H). Additionally, analysis of glucose-derived serine showed reduced levels of m + 3 serine after NAC treatment (Fig. 5I), further confirming that the generation of ROS during serine starvation is important in potentiating any increase in serine synthesis. Furthermore, loss of SK1 during serine starvation is necessary for the increase in intracellular ROS and therefore allows for full metabolic adaptation.
Blocking SK1 loss inhibits adaptive responses to SG deprivation or addition of exogenous dSA
As we have previously shown that SK1 degradation during SG starvation is mediated via a KLHL5-dependent mechanism (10), we wondered whether knocking down KLHL5 can inhibit adaptive responses to SG deprivation. Importantly, manipulating KLHL5 can distinguish between the upstream action of dSA (upstream of SK1) or the action of Sph downstream of SK1; that is, knocking down of KLHL5 should prevent actions of dSA mediated by loss of SK1, but it should not prevent the actions of Sph. First, we verified that SK1 proteolysis is necessary for Sph accumulation. Cells were treated with KLHL5 siRNA (siKLHL5) or a control siRNA (siCON) before serine starvation or addition of exogenous dSA, and Sph was then measured. As predicted, SG starvation or addition of exogenous dSA increased Sph levels in HCT116 treated with siCON, and the Sph increase was inhibited in HCT116 cells treated with siKLHL5 (Fig. 6A). Thus, KLHL5 is required for the action of SG deprivation and dSA on Sph accumulation.
Fig. 6.
Determination of the effector lipid. A: HCT-116 cells were treated with either control siRNA (siCON) or siRNA against KLHL5 (siKLHL5). After 24 h the cells were placed in either SG free medium or control medium either with or without exogenous dSA addition, before Sph levels were measured. B: Effect of sphingolipids in ROS generation. Cells were treated with either siCON or siKLHL5. After 24 h, the cells were placed in either SG-free medium or control medium either with or without exogenous dSA or Sph addition. ROS was measured after a further 24 h by DCFH-DA fluorescence. Statistics compared treatment with its relevant control. C: HCT-116 cells were transfected with either siCON or siKLHL5 for 24 h before being cultured in either SG deficient or replete media supplemented with exogenous Sph or dSA. Every 24 h post medium switch, cell growth was assessed in triplicate by trypan blue exclusion. Data shown represent the mean average ± SD. Statistics compared treatment with its relevant control. D: PHGDH protein expression in siKLHL5-treated cells and (E) PKM2 activity. Cells were cultured for 24 h in either SG deficient or replete media and either with or without exogenous dSA or Sph addition, before the samples were either prepared for Western blot or for PKM2 activity assay. ∗ P < 0.05, ∗∗ P < 0.005.
Subsequently, the effect of KLHL5 knockdown was examined in the context of ROS generation (Fig. 6B). We observed that treatment with siCON had a minimal effect on ROS generated during SG starvation. On the other hand, siKLHL5 inhibited the increase of ROS following SG starvation, supporting that SK1 loss is required for ROS increase. siKLHL5 treatment also inhibited the increase in ROS levels in response to treatment with exogenous dSA but not Sph (Fig. 6B), thus distinguishing the upstream action of dSA from the downstream effects of Sph (upstream and downstream of KLHL5/SK1). These results support the notion that Sph accumulation due to SK1 loss is required for increase ROS during SG starvation.
Next, we examined the effect of KLHL5 knockdown on cell growth. The results showed that as expected, dSA and Sph, at the concentrations used, had little effects on growth of HCT116 cells grown in SG replete medium and transfected siCON or siKLHL (Fig. 6C panel 1). As previously described, SG starvation caused growth suppression in HCT-116 transfected with siCON (compare panels 1 and 2 in Fig. 6C). In this case, addition of either dSA or Sph was able to moderately enhance growth (Fig. 6C panel 2), supporting the overall adaptive role of this pathway in the response to SG starvation. Knocking down KLHL5 worsened the growth arrest of HCT116 cells in response to SG deprivation (compare panels 1 and 3 in Fig. 6C). Importantly, the growth inhibition by knockdown of KLHL5 was rescued by addition of exogenous Sph but not dSA (Fig. 6C, panel 4). Moreover, addition of dihydrosphingosine could also increase cell growth during SG starvation, but at a lower efficiency than Sph (supplemental Fig. S1). These results demonstrate a protective role for KLHL5 mediated proteolysis of SK1 in SG starvation. The results are also consistent with dSA acting upstream of KLHL5 and with Sph acting downstream.
We next examined the effect of KLHL5 knockdown on PHGDH expression. The results showed that knocking down KLHL5 in HCT116 cells inhibited the increase in PHGDH protein levels in response to either SG deprivation or addition of exogenous dSA, which was rescued by addition of Sph but not dSA (Fig. 6D), suggesting that Sph accumulation is necessary for increased PHDGH expression during SG starvation. Finally, the effect of KLHL5 knockdown on PKM2 activity was examined. Knocking down KLHL5 rescued the decrease in PKM2 activity in response to either SG deprivation or addition of dSA in HCT116 cells (Fig. 6E). However, addition of Sph reinstated the decrease in PKM2 activity, again showing that Sph is required.
Discussion
This study advances our understanding of physiologic and regulated functions of dSA as a novel bioactive lipid by demonstrating that dSA levels respond to SG starvation and that increased dSA causes loss of SK1, with attendant increases in SK1 substrates (dhSph and Sph) and a decrease in its products (dhS1P and S1P, respectively). The results also show that dSA regulates an adaptive response to SG starvation, including production of modest/moderate amounts of ROS, and regulation of PKM2 and the levels of PEP and pyruvate. This results in increased de novo synthesis of serine from glycolysis through the SSP. These result in an enhanced growth of tumor cells. Together, these results define specific bioactivity of dSA, and they help establish a novel pathway in regulation of cancer cell metabolism.
The deoxysphingolipids, which primarily include dSa and its acylated form deoxy-dihydroceramide, have been the subject of recent studies; however, their physiologic functions have remained rather enigmatic. Levels of 1-deoxysphingolipids are increased hereditary sensory and autonomic neuropathy (HSAN), which harbors mutations in SPT that favor (relative to wild-type enzyme) the use of alanine over serine (20). Cells cultured at high density or treated with the ceramide synthase inhibitor fumonisin B1 both showed increased dSA levels (41). In addition, prediabetic and diabetic monkeys fed high-fructose and high-fat diets also generate more plasma deoxysphingolipids (46). A recent work has shown that prolonged increases in deoxysphingolipids were detrimental to tumor growth (28). This paper focused on deoxy-dihydroceramide, and it showed that inhibition of deoxy-dihydroceramide synthesis could rescue cells during prolonged serine starvation, and they implicated deoxy-dihydroceramide as the key lipid mediating the growth suppressive effects, consistent with other work with HSAN (20).
The current study defines a physiologic function for dSA in driving adaptive responses to serine deprivation in cells. Indeed, these results demonstrate that dSA fulfills key requirements as a novel bioactive lipid mediator. First, dSA levels are directly induced by deprivation of exogenous serine, and dSA levels are inversely related to the amount of exogenous serine. That is dSA functions as an “indicator” of serine status in the cell, at least its availability for synthesis of sphingolipids. Second, the results reveal that SK1 is at least one direct target of dSA, which induces the proteasomal degradation of SK1. Third, changes in levels of dSA result in specific downstream events. These include induction of an adaptive ROS response, regulation of PKM2, regulation of PHGDH, and culminating in the induction of the SSP (Scheme, Fig. 7).
Fig. 7.
Scheme representing the biological effects of serine starvation on sphingolipid synthesis and their consequences. Serine deprivation leads to substitution of alanine by SPT to generate 1-dSA. Binding of dSA to SK1 leads to KLHL5 mediated proteolysis of SK1, allowing for Sph accumulation. Increased Sph leads to increased ROS, which increases PHGDH expression while decreasing PKM2 activity. These lead to increased serine synthesis, allowing for increased cell growth. Serine palmitoyl transferase (SPT), deoxysphinganine (dSA), sphingosine (Sph), Sph kinase 1 (SK1), Sph 1-phosphate (S1P), oxygen consumption rate (OCR), reactive oxygen species (ROS), phosphoglycerate dehydrogenase (PHGDH), pyruvate kinase m2 (PKM2). Dotted line represents speculated consequence.
The current work, coupled with our recent study on the role of SK1 in serine deprivation (10), demonstrates that loss of SK1 plays an adaptive response, most likely through the accumulation of its substrate Sph, which then drives specific adaptive responses, including a decrease in PKM2 enzymatic activity and increase in ROS levels, PHGDH expression, and de novo serine synthesis (Scheme, Fig. 7).
Given the low levels of dSA measured in SG starved cells, SK1 displays a high sensitivity to dSA levels. Mechanistically, dSA, which binds SK1, causes ubiquitination and proteolysis of SK1 via a KLHL5-mediated pathway. Deoxysphingolipids lack a 1-hydroxyl group and are therefore unable to be phosphorylated by SK1 (47). This is a process known to operate with inhibitors, especially substrate-based inhibitors, of the enzyme, including dSA analogues (43). Presumably, binding of dSA (and other substrate-based inhibitors of SK1) causes some degree of conformational change, leading to proteasomal degradation. In addition, the biophysical properties of dSA make it poorly miscible with other lipids, making the disruption of lipid membranes, presumably leading to SK1 proteolysis, another pathway by which dSA could disrupt SK1 activity (48). It is tempting to speculate that the remarkable sensitivity of SK1 to dSA levels serves as a mechanism to ensure that metabolic changes are initiated quickly in order to keep dSA levels to a minimum, nontoxic level. Our data demonstrate for the 1st time that endogenous dSA induces SK1 degradation.
The results also implicate Sph as a downstream effector to induce adaptive responses to serine deficiency. Low concentrations of Sph, and in a stereospecific manner (10) induce ROS and PHGDH and suppress PKM2, resulting in increased de novo synthesis of serine. Importantly, the current results clearly distinguish the upstream role of dSA from the downstream role of Sph. As shown in the Scheme in Fig. 7, downregulation of KLHL5, which we show, is required for the proteolysis of SK1 during SG starvation, prevents the actions of dSA but not those of Sph (e.g., on induction of PHGDH, growth of HCT-116 cells under SG starvation). Thus, we propose that SPT functions as the sensor of availability of serine and dSA as the proximal signal/responder for serine starvation, whereas Sph acts as an effector of the adaptive response. Interestingly, myriocin treatment decreased PHGDH levels in HCT-116 cells but not in either MOLT4 or HEK293T cells (Fig. 5C, E). Previous measurement of Sph levels in SG replete media has shown a higher basal level of Sph in HCT-116 cells when compared with MOLT4 or HEK293T (supplemental Fig. S2). These results may suggest that high basal levels of Sph have a role in mediating PHGDH expression in HCT-116 cells and therefore bestow a growth advantage. It is interesting to note that dihydrosphingosine (dhSph) levels also increase during SG starvation, but are present at a much lower levels than Sph (supplemental Fig. S2, (10)). We also showed that exogenous Sph was also more efficient at increasing cell growth compared to exogenous dhSph during SG starvation. These results suggest that Sph plays a more prominent role than dhSPH in mediating biological responses to SG deprivation. This is the reason why exogenous Sph but not dhSph was used in our experiments.
The current results implicate ROS formation as an adaptive response to SG starvation, acting downstream of dSA, including reversing effects of dSA on serine formation by NAC. In our previous study, we also showed that ROS (prevented by NAC) acted downstream of Sph, but upstream of PHGDH and serine synthesis (Fig. 7). It has been shown that loss of SK1 can lead to increases in ROS (49), and conversely, increased SK1 expression could protect cardiac cells from oxidative-stress-induced apoptosis (50). ROS have been shown to exert many biological effects affecting metabolism (51). Regulation of PKM2 activity by hypoxia (thereby increasing ROS through mitochondrial complex III) was shown to decrease PKM2 activity (52). Interestingly, this was correlated with an increase in flux into the pentose phosphate pathway and an increase in NADPH necessary for glutathione synthesis (52). Additionally, hypoxic conditions in breast cancer stem cells were shown to induce PHGDH expression (53). Again, this finding was correlated with mitochondrial redox homeostasis. Given these findings, it is hypothesized that loss of SK1 and the accumulation of Sph due to SG starvation lead to mitochondrial release of ROS. Compensatory metabolic mechanisms to mitigate ROS damage also lead to increased serine synthesis. Moderate elevations in ROS have also been implicated in mediating mitogenic responses in contrast to high levels of ROS production, which can be cytotoxic (54, 55, 56). Interestingly, our results showed that NAC worsened the growth of cells under SG starvation (implying a beneficial role for ROS).
Interestingly, NAC did not completely inhibit the PKM2-activity decrease in serine-starved cells, indicating an ROS-independent mechanism for the reduction of PKM2 activity, which is not mediated by dSA or Sph likely in this case a lack of serine binding to the active site of PKM2 (13). Levels of intracellular serine have been shown to upregulate PHGDH through a p53-dependent mechanism (31). Also, ROS may increase serine synthesis through the activation and SUMOylation of NRF2 (44), resulting in increased PHGDH expression. To maintain a lower level of intracellular ROS, serine promotes the production of the antioxidant glutathione (57), a carefully regulated rise in ROS but not crossing the threshold of toxicity appears to be important in serine synthesis. Reduction of intracellular amino acid levels (including serine) has been shown to activate the transcription factor-4 pathway and to increase transcription of the enzymes of the SSP (58, 59). Additionally, serine starvation can increase the metabolism of glutamine to glutamate by increasing glutaminase 2 expression through the upregulation of Tap73 (60). Therefore, regulating intracellular ROS can activate pathways that can lead to various compensatory mechanisms, including serine synthesis.
Given the centrality of serine to cancer metabolism, recent studies have explored inhibition of the SSP as a mechanism for cancer treatment (4, 9, 61). However, it is still unclear which cancers would be most susceptible to SSP inhibition (9). Our study suggests that SK1 expression, a marker for tumor survival and chemoresistance, may function as a biomarker in predicting which tumors would respond well to treatments involving SSP inhibition (18, 19).
In summary, dSA can be now considered as a novel bioactive sphingolipid, and in this work, we propose that it functions as a Sphingoid Serine Responder. Subsequent to dSA accumulation, loss of SK1 leads to accumulation of Sph, which the current results show to function as a regulator of ROS production, adaptive cell growth, and metabolic reprogramming in response to serine starvation (Fig. 7). Thus, the results identify a novel sphingolipid-mediated pathway in the adaptation to serine starvation.
Data availability
All data are contained within the manuscript.
Supplemental data
This article contains supplemental data.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgments
The authors thank the Stony Brook Lipidomics Core for measurement and analysis of sphingolipids and the Stony Brook Cancer Center Biological Mass Spectrometry Shared Resource and the Lipidomics Shared Resource.
Author contributions
Y. A. H., L. M. O., and C. M. conceptualization; J.-P. T. and I. M. formal analysis; L. M. O. and C. M. funding acquisition; J.-P. T., C. F. R., E. M., M. G.-B., I. M., and A. J. S. investigation; L. M. O. and C. M. project administration; L. M. O. and C. M. supervision; J.-P. T. validation; J.-P. T. visualization; J.-P. T. writing–original draft; Y. A. H. and C. M. writing–review and editing.
Funding and additional information
This work was supported in part by NIH grants GM130878, P01 CA097132, and 5R01GM130878-02. The content is solely the responsibility of the authors/author and do/does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This work is dedicated to the memory of Lina M. Obeid.
Contributor Information
Yusuf A. Hannun, Email: yusuf.hannun@stonybrookmedicine.edu.
Cungui Mao, Email: cungui.mao@stonybrook.edu.
Supplemental data
References
- 1.Metcalf J.S., Dunlop R.A., Powell J.T., Banack S.A., Cox P.A. L-Serine: a naturally-occurring amino acid with therapeutic potential. Neurotox Res. 2018;33:213–221. doi: 10.1007/s12640-017-9814-x. [DOI] [PubMed] [Google Scholar]
- 2.Sun L., Suo C., Li S.T., Zhang H., Gao P. Metabolic reprogramming for cancer cells and their microenvironment: beyond the Warburg effect. Biochim. Biophys. Acta Rev. Cancer. 2018;1870:51–66. doi: 10.1016/j.bbcan.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Mattaini K.R., Sullivan M.R., Vander Heiden M.G. The importance of serine metabolism in cancer. J. Cell Biol. 2016;214:249–257. doi: 10.1083/jcb.201604085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amelio I., Cutruzzola F., Antonov A., Agostini M., Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan J., Merrill A.H., Jr. 1-Deoxysphingolipids encountered exogenously and made de novo: dangerous mysteries inside an enigma. J. Biol. Chem. 2015;290:15380–15389. doi: 10.1074/jbc.R115.658823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant G.A. D-3-Phosphoglycerate dehydrogenase. Front. Mol. Biosci. 2018;5:110. doi: 10.3389/fmolb.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 8.Maddocks O.D.K., Athineos D., Cheung E.C., Lee P., Zhang T., van den Broek N.J.F., Mackay G.M., Labuschagne C.F., Gay D., Kruiswijk F., Blagih J., Vincent D.F., Campbell K.J., Ceteci F., Sansom O.J., et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan M.R., Mattaini K.R., Dennstedt E.A., Nguyen A.A., Sivanand S., Reilly M.F., Meeth K., Muir A., Darnell A.M., Bosenberg M.W., Lewis C.A., Vander Heiden M.G. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 2019;29:1410–1421 e1414. doi: 10.1016/j.cmet.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truman J.P., Ruiz C.F., Trayssac M., Mao C., Hannun Y.A., Obeid L.M. Sphingosine kinase 1 downregulation is required for adaptation to serine deprivation. FASEB J. 2021;35 doi: 10.1096/fj.202001814RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J., Ma J., Wang X., Ma T., Zhang S., Wang W., Zhou X., Shi J. High expression of PHGDH predicts poor prognosis in non-small cell lung cancer. Transl. Oncol. 2016;9:592–599. doi: 10.1016/j.tranon.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid M.A., Allen A.E., Liu S., Liberti M.V., Liu P., Liu X., Dai Z., Gao X., Wang Q., Liu Y., Lai L., Locasale J.W. Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat. Commun. 2018;9:5442. doi: 10.1038/s41467-018-07868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaneton B., Hillmann P., Zheng L., Martin A.C.L., Maddocks O.D.K., Chokkathukalam A., Coyle J.E., Jankevics A., Holding F.P., Vousden K.H., Frezza C., O'Reilly M., Gottlieb E. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J., Mancuso A., Tong X., Ward P.S., Fan J., Rabinowitz J.D., Thompson C.B. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannun Y.A., Obeid L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gault C.R., Obeid L.M. Still benched on its way to the bedside: sphingosine kinase 1 as an emerging target in cancer chemotherapy. Crit. Rev. Biochem. Mol. Biol. 2011;46:342–351. doi: 10.3109/10409238.2011.597737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hait N.C., Oskeritzian C.A., Paugh S.W., Milstien S., Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Wang Y., Wan Z., Liu S., Cao Y., Zeng Z. Sphingosine kinase 1 and cancer: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffernan-Stroud L.A., Obeid L.M. Sphingosine kinase 1 in cancer. Adv. Cancer Res. 2013;117:201–235. doi: 10.1016/B978-0-12-394274-6.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotthier A., Auer-Grumbach M., Janssens K., Baets J., Penno A., Almeida-Souza L., Van Hoof K., Jacobs A., De Vriendt E., Schlotter-Weigel B., Löscher W., Vondráček P., Seeman P., De Jonghe P., Van Dijck P., et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am. J. Hum. Genet. 2010;87:513–522. doi: 10.1016/j.ajhg.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuellig R.A., Hornemann T., Othman A., Hehl A.B., Bode H., Guntert T., Ogunshola O.O., Saponara E., Grabliauskaite K., Jang J.H., Ungethuem U., Wei Y., von Eckardstein A., Graf R., Sonda S. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes. 2014;63:1326–1339. doi: 10.2337/db13-1042. [DOI] [PubMed] [Google Scholar]
- 22.Dohrn M.F., Othman A., Hirshman S.K., Bode H., Alecu I., Fahndrich E., Karges W., Weis J., Schulz J.B., Hornemann T., Claeys K.G. Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? Eur. J. Neurol. 2015;22:806–814. doi: 10.1111/ene.12663. e55. [DOI] [PubMed] [Google Scholar]
- 23.Othman A., Rutti M.F., Ernst D., Saely C.H., Rein P., Drexel H., Porretta-Serapiglia C., Lauria G., Bianchi R., von Eckardstein A., Hornemann T. Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia. 2012;55:421–431. doi: 10.1007/s00125-011-2384-1. [DOI] [PubMed] [Google Scholar]
- 24.Gorden D.L., Myers D.S., Ivanova P.T., Fahy E., Maurya M.R., Gupta S., Min J., Spann N.J., McDonald J.G., Kelly S.L., Duan J., Sullards M.C., Leiker T.J., Barkley R.M., Quehenberger O., et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 2015;56:722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eade K., Gantner M.L., Hostyk J.A., Nagasaki T., Giles S., Fallon R., Harkins-Perry S., Baldini M., Lim E.W., Scheppke L., Dorrell M.I., Cai C., Baugh E.H., Wolock C.J., Wallace M., et al. Serine biosynthesis defect due to haploinsufficiency of PHGDH causes retinal disease. Nat. Metab. 2021;3:366–377. doi: 10.1038/s42255-021-00361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garofalo K., Penno A., Schmidt B.P., Lee H.J., Frosch M.P., von Eckardstein A., Brown R.H., Hornemann T., Eichler F.S. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 2011;121:4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer R., Bielawski J., Kistner-Griffin E., Othman A., Alecu I., Ernst D., Kornhauser D., Hornemann T., Spassieva S. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB J. 2015;29:4461–4472. doi: 10.1096/fj.15-272567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthusamy T., Cordes T., Handzlik M.K., You L., Lim E.W., Gengatharan J., Pinto A.F.M., Badur M.G., Kolar M.J., Wallace M., Saghatelian A., Metallo C.M. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature. 2020;586:790–795. doi: 10.1038/s41586-020-2609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannich J.T., Haribowo A.G., Gentina S., Paillard M., Gomez L., Pillot B., Thibault H., Abegg D., Guex N., Zumbuehl A., Adibekian A., Ovize M., Martinou J.C., Riezman H. 1-Deoxydihydroceramide causes anoxic death by impairing chaperonin-mediated protein folding. Nat. Metab. 2019;1:996–1008. doi: 10.1038/s42255-019-0123-y. [DOI] [PubMed] [Google Scholar]
- 30.Esaki K., Sayano T., Sonoda C., Akagi T., Suzuki T., Ogawa T., Okamoto M., Yoshikawa T., Hirabayashi Y., Furuya S. L-Serine deficiency elicits intracellular accumulation of cytotoxic deoxysphingolipids and lipid body formation. J. Biol. Chem. 2015;290:14595–14609. doi: 10.1074/jbc.M114.603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddocks O.D., Berkers C.R., Mason S.M., Zheng L., Blyth K., Gottlieb E., Vousden K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang K., Xu R., Schrandt J., Shah P., Gong Y.Z., Preston C., Wang L., Yi J.K., Lin C.L., Sun W., Spyropoulos D.D., Rhee S., Li M., Zhou J., Ge S., et al. Correction: alkaline ceramidase 3 deficiency results in purkinje cell degeneration and cerebellar ataxia due to dyshomeostasis of sphingolipids in the brain. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielawski J., Pierce J.S., Snider J., Rembiesa B., Szulc Z.M., Bielawska A. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) Adv. Exp. Med. Biol. 2010;688:46–59. doi: 10.1007/978-1-4419-6741-1_3. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz N.U., Mileva I., Gurevich M., Snider J., Hannun Y.A., Obeid L.M. Quantifying 1-deoxydihydroceramides and 1-deoxyceramides in mouse nervous system tissue. Prostaglandins Other Lipid Mediat. 2019;141:40–48. doi: 10.1016/j.prostaglandins.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Y., Wang E., Peng G., Lin S.-Y. Ubiquitination assay for mammalian cells. Bio Protocol. 2016;6 [Google Scholar]
- 36.Loveridge C., Tonelli F., Leclercq T., Lim K.G., Long J.S., Berdyshev E., Tate R.J., Natarajan V., Pitson S.M., Pyne N.J., Pyne S. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J. Biol. Chem. 2010;285:38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson D.G., Tonelli F., Alossaimi M., Williamson L., Chan E., Gorshkova I., Berdyshev E., Bittman R., Pyne N.J., Pyne S. The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cell Signal. 2013;25:1011–1017. doi: 10.1016/j.cellsig.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulkoski-Gross M.J., Jenkins M.L., Truman J.P., Salama M.F., Clarke C.J., Burke J.E., Hannun Y.A., Obeid L.M. An intrinsic lipid-binding interface controls sphingosine kinase 1 function. J. Lipid Res. 2018;59:462–474. doi: 10.1194/jlr.M081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan M., Breitkopf S.B., Yang X., Asara J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruett S.T., Bushnev A., Hagedorn K., Adiga M., Haynes C.A., Sullards M.C., Liotta D.C., Merrill A.H., Jr. Biodiversity of sphingoid bases ("sphingosines") and related amino alcohols. J. Lipid Res. 2008;49:1621–1639. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitomer N.C., Mitchell T., Voss K.A., Bondy G.S., Pruett S.T., Garnier-Amblard E.C., Liebeskind L.S., Park H., Wang E., Sullards M.C., Merrill A.H., Jr., Riley R.T. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyake Y., Kozutsumi Y., Nakamura S., Fujita T., Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 43.Byun H.S., Pyne S., Macritchie N., Pyne N.J., Bittman R. Novel sphingosine-containing analogues selectively inhibit sphingosine kinase (SK) isozymes, induce SK1 proteasomal degradation and reduce DNA synthesis in human pulmonary arterial smooth muscle cells. Medchemcomm. 2013 doi: 10.1039/C3MD00201B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H., Xu J., Zheng Q., He J., Zhou W., Wang K., Huang X., Fan Q., Ma J., Cheng J., Mei W., Xing R., Cai R. NRF2 SUMOylation promotes de novo serine synthesis and maintains HCC tumorigenesis. Cancer Lett. 2019;466:39–48. doi: 10.1016/j.canlet.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Sun L., Song L., Wan Q., Wu G., Li X., Wang Y., Wang J., Liu Z., Zhong X., He X., Shen S., Pan X., Li A., Wang Y., Gao P., et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–444. doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brozinick J.T., Hawkins E., Hoang Bui H., Kuo M.S., Tan B., Kievit P., Grove K. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. (Lond) 2013;37:1064–1070. doi: 10.1038/ijo.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symolon H., Bushnev A., Peng Q., Ramaraju H., Mays S.G., Allegood J.C., Pruett S.T., Sullards M.C., Dillehay D.L., Liotta D.C., Merrill A.H., Jr. Enigmol: a novel sphingolipid analogue with anticancer activity against cancer cell lines and in vivo models for intestinal and prostate cancer. Mol. Cancer Ther. 2011;10:648–657. doi: 10.1158/1535-7163.MCT-10-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jimenez-Rojo N., Sot J., Busto J.V., Shaw W.A., Duan J., Merrill A.H., Jr., Alonso A., Goni F.M. Biophysical properties of novel 1-deoxy-(dihydro)ceramides occurring in mammalian cells. Biophys. J. 2014;107:2850–2859. doi: 10.1016/j.bpj.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huwiler A., Kotelevets N., Xin C., Pastukhov O., Pfeilschifter J., Zangemeister-Wittke U. Loss of sphingosine kinase-1 in carcinoma cells increases formation of reactive oxygen species and sensitivity to doxorubicin-induced DNA damage. Br. J. Pharmacol. 2011;162:532–543. doi: 10.1111/j.1476-5381.2010.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pchejetski D., Kunduzova O., Dayon A., Calise D., Seguelas M.H., Leducq N., Seif I., Parini A., Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ. Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 51.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., Thomas C.J., Vander Heiden M.G., Cantley L.C. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samanta D., Park Y., Andrabi S.A., Shelton L.M., Gilkes D.M., Semenza G.L. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 2016;76:4430–4442. doi: 10.1158/0008-5472.CAN-16-0530. [DOI] [PubMed] [Google Scholar]
- 54.Lu W., Hu Y., Chen G., Chen Z., Zhang H., Wang F., Feng L., Pelicano H., Wang H., Keating M.J., Liu J., McKeehan W., Wang H., Luo Y., Huang P. Correction: novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diebold L., Chandel N.S. Mitochondrial ROS regulation of proliferating cells. Free Radic. Biol. Med. 2016;100:86–93. doi: 10.1016/j.freeradbiomed.2016.04.198. [DOI] [PubMed] [Google Scholar]
- 56.Son Y., Cheong Y.K., Kim N.H., Chung H.T., Kang D.G., Pae H.O. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He L., Long J., Zhou X., Liu Y., Li T., Wu X. Serine is required for the maintenance of redox balance and proliferation in the intestine under oxidative stress. FASEB J. 2020;34:4702–4717. doi: 10.1096/fj.201902690R. [DOI] [PubMed] [Google Scholar]
- 58.Adams C.M. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J. Biol. Chem. 2007;282:16744–16753. doi: 10.1074/jbc.M610510200. [DOI] [PubMed] [Google Scholar]
- 59.Seo J., Fortuno E.S., 3rd, Suh J.M., Stenesen D., Tang W., Parks E.J., Adams C.M., Townes T., Graff J.M. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58:2565–2573. doi: 10.2337/db09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amelio I., Markert E.K., Rufini A., Antonov A.V., Sayan B.S., Tucci P., Agostini M., Mineo T.C., Levine A.J., Melino G. p73 regulates serine biosynthesis in cancer. Oncogene. 2014;33:5039–5046. doi: 10.1038/onc.2013.456. [DOI] [PubMed] [Google Scholar]
- 61.Locasale J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.