Abstract
Congestion is the main cause of hospitalization in patients with acute heart failure (AHF), however its precise assessment by simple clinical evaluation remains elusive. The recent introduction of the lung ultrasound scan (LUS) allowed to physicians to more precisely quantify pulmonary congestion. The aim of this study was to compare clinical congestion (CC) with LUS and B-type natriuretic peptide (BNP) in order to achieve a more complete evaluation and to evaluate the prognostic power of each measurement. Methods: All patients were submitted to clinical evaluation for blood sample analysis and LUS at admission and before discharge. LUS protocol evaluated the number of B-lines for each chest zone by standardized eight site protocol. CC was measured following ESC criteria. The mean difference between admission and discharge congestion logBNP and B-lines values were calculated. Combined end points of death and rehospitalization was calculated over 180 days. Results: 213 patients were included in the protocol; 133 experienced heart failure with reduced ejection fraction (HFrEF), and 83 presented with heart failure with preserved ejection fraction (HFpEF). Patients with HFrEF had a more increased level of BNP (1150 (812–1790) vs. 851 (694–1196); p = 0.002) and B lines total number (32 (27–38) vs. 30 (25–36); p = 0.05). A positive correlation was found between log BNP and Blines number in both HFrEF (r = 0.57; p < 0.001) and HFpEF (r = 0.36; p = 0.001). Similarly, dividing B-lines among tertiles the upper group (B-lines ≥ 36) had an increased clinical congestion score. Among three variables at admission only B-lines were predictive for outcome (AUC 0.68 p < 0.001) but not LogBNP and CC score. During 180 days of follow-up, univariate analysis showed that persistent ΔB-lines <−32.3% (HR 6.54 (4.19–10.20); p < 0.001), persistent ΔBNP < −43.8% (HR 2.48 (1.69–3.63); p < 0.001) and persistent ΔCC < 50% (HR 4.25 (2.90–6.21); p < 0.001) were all significantly related to adverse outcome. Multivariable analysis confirmed that persistent ΔB-lines (HR 4.38 (2.64–7.29); p < 0.001), ΔBNP (HR 1.74 (1.11–2.74); p = 0.016) and ΔCC (HR 3.38 (2.10–5.44); p < 0.001 were associated with the combined end point. Conclusions: a complete clinical laboratory and LUS assessment better recognized different congestion occurrence in AHF. The difference between admission and discharge B-lines provides useful prognostic information compared to traditional clinical evaluation.
Keywords: heart failure, congestion, BNP, LUS
1. Introduction
Congestion is the main cause of hospital admission in patients with acute decompensated heart failure (ADHF). ADHF recurrence is often the consequence of fluid retention, which leads to both systemic and pulmonary congestion [1,2,3]. Moreover, the lack of congestion resolution during HF hospitalization should be related to poor prognosis. In this scenario, a multiparametric congestion evaluation in order to better stratify ADHF patients at high risk for re-hospitalization would be useful [4,5]. Despite the residual clinical congestion after discharge remains a strong predictor of worse outcomes, any congestion scores are not sensitive enough to detect the real cardiac filling pressure, and they do not precisely reflect the real intravascular and extravascular fluid overload. Traditionally, the most used strategy is the clinical evaluation, which is associated with chest radiography (CRx) and natriuretic peptides (NPs) measurement [6,7]. However, this approach is not readily available in a primary care setting and at the patient’s home. In this context, a precise and non-invasive evaluation of congestion by clinical, ultrasound, and laboratory tools appears to be mandatory, although up to now, there does not exist a precise algorithm evaluating systemic and pulmonary congestion all at once. Therefore, systemic congestion is not strictly related to cardiac congestion, and the simple absence of clinical signs of fluid overload cannot exclude an increased left ventricular filling pressure (LVEDP) [8]. Although natriuretic peptides (NP) are included in the diagnostic HF algorithm, recent data are cause for doubt about their prognostic significance, and NP guided therapy has not demonstrated significant benefit compared with the standard approach which is based on clinical signs [9,10]. Interestingly, lung ultrasound (LUS) examination has been proposed as a simple, available, and economic tool to recognize pulmonary congestion in ADHF. LUS is an integrated non-invasive tool based on the recognition of artefact reverberations arising from extravascular lung water and inter-lobar septal imbibition due to lung fluid accumulation. The number of ultrasound lung comets (B-lines) is directly related to the lung congestion degree [11,12]. Recently, some reports demonstrated the prognostic power of B-lines counts in acute HF, as well as the importance of this tool for guiding to a tailored treatment to achieve adequate decongestion [13,14,15]. Therefore, LUS analysis could be accounted for together with clinical evaluation and B-type natriuretic peptide (BNP) measurement in order to better identify the congestion status and degree during hospitalization in patients affected by ADHF. Accordingly, we aim to evaluate the congestion at admission and at discharge through three different methods as clinical examination, BNP measurement, and LUS assessment in heart failure with reduced or preserved ejection fraction; we assess the prognostic impact of different congestion evaluation during a mean follow-up of 180 days after discharge.
2. Methods
A prospective cohort of 248 patients admitted with a main diagnosis of ADHF were enrolled within 12 h of hospital admission. Consecutive patients were admitted to the Cardiovascular Diseases Unit of the Le Scotte Hospital in Siena. The inclusion criteria were the presence of signs and/or symptoms of AHF, irrespective of the aetiology, associated with chest radiography suggestive for HF. Exclusion criteria were (1) patients with a poor acoustic chest and cardiac window; (2) pulmonary disease as the main cause of dyspnoea (e.g., pulmonary asthma, chronic obstructive pulmonary disease interstitial lung disease); (3) a history of pneumothorax, lobectomy or lung cancer; (4) ST elevation myocardial infarction within the last two months; (5) patients with unstable cardiogenic shock (blood pressure < 90 mmHg); (6) patients with infection, inflammatory, autoimmune or neoplastic diseases.
3. Physical Examination and Blood Tests
Patients were evaluated by two physicians at admission and discharge to assess the grade of clinical congestion (CC) score, giving 1 point for each of following signs: pulmonary rales, third heart sound, jugular venous distention, peripheral edema and hepatomegaly (5 total points) [13].
Blood tests were performed, and hemocrome, electrolytes, renal function glycemia, uricemia, plasma osmolarity, troponin, and B-type natriuretic peptide were measured. The BNP assay was evaluated at admission and before discharge by Biosite Inc., San Diego, CA, USA.
4. Echocardiography
Left ventricular ejection fraction (LVEF) was measured using the biplane Simpson’s method. Diastolic function was assessed from the pattern of mitral inflow by pulsed-wave Doppler. Mitral annular early diastolic velocity (e’) was assessed at the septal and lateral sites of the mitral annulus using tissue Doppler imaging. E/A ratio, e’ wave peak velocity, E/e’ were calculated. Inferior vena cava (IVC) and tricuspid annular plane systolic excursion (TAPSE) were obtained as recommended. Pulmonary arterial systolic pressure (PASP) was estimated using tricuspid rigurgitation velocity, and ICV diameter and collapse which were analyzed by epigastric scan according to recent guidelines [16]. Patients were classified according to LVEF, reduced EF (LVEF < 50%) and preserved EF (LVEF ≥ 50%).
5. Lung Ultrasound
Measurements were averaged for each LUS zone. Eight LUS zones were analysed for each patient. LUS was performed, placing patients in a semi-supine position (45°), using the same probe used for echocardiography. We evaluated eight chest zones [12], two parasternal chest scans and two scans of the anterior and lateral basal chest was obtained on the right and left hemi thoraxes. LUS was performed twice in all patients: the first evaluation was done at admission at the end of standard echocardiography. The maximum number of B-lines was counted in each single scan and the sum of all eight zones was calculated as total number of B-lines counts. The second evaluation was performed before discharge. Inter- and intra-observer variability ranges from 5 to 8%.
5.1. Follow-Up
The composite primary outcome was considered as death for cardiovascular causes and hospitalisations primarily due to heart failure within 180 days after discharge. All patients were followed by direct clinical check-up or contacted by phone or remotely for six months after discharge and were reviewed by different clinicians who provided the local HF service. Clinical follow-up was obtained through information from subsequent patient visits or by telephone or internet contacts.
5.2. Statistical Analysis
Continuous variables were expressed as median and interquartile range (IQR) and categorical variables as count or percentage. Differences for patients with HFrEF compared to HFpEF were tested using a Mann-Whitney non-parametric test and X2 tests. Spearman’s rho correlation coefficient was used to assess relationships for continuous variables; BNP was analysed after logarithmic transformation. A receiving operating characteristic (ROC) curve was used to assess the relationship between variables and outcomes. Mean differences between admission and discharge for CC score BNP and B-lines were calculated. Cox regression analysis was used to assess the independent and the dependent relationship between variables (B-lines; BNP; congestion score; age; sex; cardiovascular risk factors, including hypertension, diabetes, dyslipidaemia, smoking, and coronary artery disease) and 180 days outcome. All reported probability values were two-tailed, and a p value ≤ 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 20.0 statistical software package (SPSS Inc., Chicago, IL, USA).
6. Results
Baseline Characteristics
Of 216 patients enrolled, 133 experienced heart failure with reduced ejection fraction (HFrEF), and 83 presented heart failure with preserved ejection fraction (HFpEF). Compared to patients with HFpEF, patients with HFrEF were more likely to be male (54% vs. 36%; p = 0.01), have lower BMI values (27.4 (24.5–29.7) vs. 28.7 (26.0–30.5) kg/m2; p = 0.02) and to have higher BNP levels at baseline (1150 (812–1790) vs. 851 (694–1196); p = 0.002). Patients with HFrEF experienced a higher percentage of CAD (66% vs. 23%; p < 0.001) and diabetes (50% vs. 32%; p = 0.01), and a lower prevalence of hypertension (81% vs. 51%; p < 0.001). No difference was observed regarding the incidence of dyslipidaemias, AF, smoke, and signs of congestion between HFrEF and HFpEF patients.
Compared to patients with HFpEF, at echocardiographic assessment the HFrEF population revealed higher LVEDVi (160 (140–190) vs. 115 (100–145) mL/m2; p < 0.001), LVESVi (100 (80–130) vs. 55 (45–70) mL/m2; p < 0.001), LA area (28 (24–31) vs. 25 (22–27) cm2; p < 0.001) and lower TAPSE (18 (16–21) vs. 20 (17–22) mm; p = 0.02). Overall, adverse events occurred in 53 patients; no differences were observed in terms of all cause mortality and HF hospitalization between HFrEF and HFpEF (Table 1).
Table 1.
Clinical risk factors and echocardiographic features of enrolled patients divided by ejection fraction (EF).
| Characteristic | HFrEF n = 133 |
HFpEF n = 83 |
p-Value |
|---|---|---|---|
| Age (years) | 82 (77–87) | 79 (77–83) | 0.04 |
| Men—n. (%) | 72 (54) | 30 (36) | 0.01 |
| BMI (kg/m2) | 27.4 (24.5–29.7) | 28.7 (26.0–30.5) | 0.02 |
| Risk factors—n. (%) | |||
| CAD | 88 (66) | 19 (23) | <0.001 |
| Diabetes | 66 (50) | 27 (32) | 0.01 |
| Dyslipidaemia | 69 (52) | 37 (46) | 0.30 |
| Hypertension | 68 (51) | 67 (81) | <0.001 |
| Smoking | 38 (29) | 29 (35) | 0.32 |
| AF | 38 (29) | 16 (19) | 0.12 |
| LVEF | 33 (25–44) | 56 (50–62) | <0.01 |
| Clinical examination & BNP | |||
| Heart rate (beats/min) | 90 (87–97) | 89 (86–94) | 0.36 |
| Systolic blood pressure (mmHg) | 125 (110–135) | 140 (130–150) | <0.001 |
| Diastolic blood pressure (mmHg) | 70 (55–80) | 85 (80–95) | <0.001 |
| Respiratory rate (n./min) | 29 (27–32) | 29 (26–33) | 0.58 |
| Rales n./(%) | 103 (77) | 72 (86) | 0.09 |
| Peripheral oedema n./(%) | 78 (58) | 44 (53) | 0.42 |
| Hepatomegaly n./(%) | 50 (38) | 24 (29) | 0.19 |
| Jugular vein distention n./(%) | 38 (28) | 24 (29) | 0.95 |
| Third heart sound n./(%) | 42 (31) | 21 (25) | 0.32 |
| BNP (pg/mL) | 1150 (812–1790) | 851 (694–1196) | 0.002 |
| Echocardiography and LUS | |||
| LVEDD (mm) | 61 (56–66) | 51 (45–55) | <0.001 |
| LVESD (mm) | 46 (41–52) | 34 (29–37) | <0.001 |
| LVEDVi (mL/min²) | 160 (140–190) | 115 (100–145) | <0.001 |
| LVESVi (mL/min²) | 100 (80–130) | 55 (45–70) | <0.001 |
| Left atrial Area (cm2) | 28 (24–31) | 25 (22–27) | <0.001 |
| PASP (mmHg) | 45 (40–50) | 45 (40–55) | 0.92 |
| Septal thickness (mm) | 11 (10–13) | 12 (11–14) | 0.001 |
| Posterior wall (mm) | 11 (9–12) | 12 (11–13) | 0.002 |
| TAPSE (mm) | 18 (16–21) | 20 (17–22) | 0.02 |
| Inferior cave vein diameter (mm) | 23 (22–24) | 22 (21–25) | 0.95 |
| E/e’ | 16 (14–18) | 16 (14–18) | 0.63 |
| B-lines (n) | 32 (27–38) | 30 (25–36) | 0.07 |
| Outcome | |||
| 60 days adverse events—n. (%) | 36 (27) | 17 (21) | 0.27 |
Abbreviations: Atrial Fibrillation (AF); Bpdy Mass Index (BMI); B-type Natriuretic Peptide (BNP); Coronary Artery Disease (CAD); Heart Failure with preserved ejection fraction (HFpEF); Heart Failure with reduced ejection fraction (HFrEF); Left Ventricular End-Diastolic Diameter (LVEDD); Left Ventricular End-Diastolic Volume indexed (LVEDVi); Left Ventricular Ejection Fraction (LVEF); Left Ventricular End-Systolic Diameter (LVESD); Left Ventricular End-Systolic Volume indexed (LVESVi); Lung Ultrasound (LUS); Pulmonary Artery Systolic Pressure (PASP); Tricuspid Anular Plane Systolic Excursion (TAPSE).
B-lines at hospital admission were slightly increased in HFrEF vs. HFpEF without statistical significance (32 (27–38) vs. 30 (25–36); p = 0.07). We divided our sample into three subgroups (1° tertile: B-lines ≤ 27, n = 63; 2° tertile: B-lines between 28 and 35, n = 83; 3° tertile: B-lines ≥ 36, n = 70). Statistical analyses highlighted a prominent clinical congestion in patients with B-lines ≥ 36 compared to other tertiles in terms of jugular vein distention (17% vs. 25% vs. 43%; p = 0.004), hepatomegaly (21% vs. 37% vs. 43%; p = 0.02), third heart sound (22% vs. 23% vs. 43%; p = 0.009), and BNP (822 (586–1130) vs. 890 (694–1354) vs. 1740 (982–2577)) (Table 2).
Table 2.
Clinical, biochemical and ultrasound prevalence of congestion according to tertiles of B-lines.
| B-Lines Tertile 1 (Range: ≤27) n = 63 |
B-Lines Tertile 2 (Range: 28–35) n = 83 |
B-Lines Tertile 3 (Range: ≥36) n = 70 |
p-Value | |
|---|---|---|---|---|
| Clinical congestion | ||||
| Rales (yes)—n. (%) | 51 (81) | 64 (77) | 60 (86) | 0.40 |
| Peripheral oedema—n. (%) | 31 (49) | 47 (57) | 44 (63) | 0.28 |
| JV distention—n. (%) | 11 (17) | 21 (25) | 30 (43) | 0.004 |
| Hepatomegaly—n. (%) | 13 (21) | 31 (37) | 30 (43) | 0.02 |
| Third heart sound—n. (%) | 14 (22) | 19 (23) | 30 (43) | 0.009 |
| Biochemical or ultrasound congestion | ||||
| BNP—pg/mL | 822 (586–1130) | 890 (694–1354) | 1740 (982–2577) | <0.001 |
| BNP—pg/mL (if in SR) | 836 (672–1131) | 974 (759–1383) | 1525 (915–2595) | <0.001 |
| BNP—pg/mL (if in AF) | 586 (408–1110) | 681 (473–815) | 1900 (1410–2572) | <0.001 |
| ICV—mm | 22 (21–24) | 22 (20–25) | 24 (22–26) | 0.002 |
Abbreviations: B-type natriuretic peptide (BNP); Inferior cave vein (ICV); Jugular vein (JV).
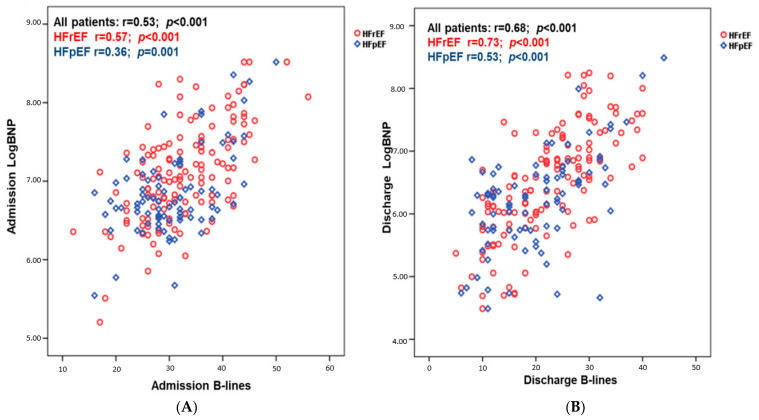
Overall, at admission there was a positive correlation among logBNP and B-lines both in HFrEF (r = 0.57; p < 0.001) and HFpEF (r = 0.36; p = 0.001) and at discharge among logBNP and B-lines in HFrEF (r = 0.73; p < 0.001) and HFpEF patients (r = 0.53; p < 0.001) (Figure 1).
Figure 1.
Correlation existing between LogBNP and B-lines number at admission (A) and at discharge (B) dividing patients according to ejection fraction.
7. Predictors of Outcome at Admission
Univariate and multivariate logistic regression analyses were performed to evaluate the prognostic role of congestion parameter at hospital admission and discharge. At admission, on univariate analyses, CC score > 3 at admission (HR 8.20 (4.74–14.16); p < 0.001), B-lines (HR 1.07 (1.03–1.11); p < 0.001), peripheral oedema (HR 3.44 (1.77–6.68); p < 0.001), hepatomegaly (HR 3.18 (1.84–5.50); p < 0.001), jugular vein distention (HR 3.48 (2.03–5.98); p < 0.001) and third heart sound (HR 2.66 (1.55–4.56); p < 0.001) were related to all cause mortality and HF hospitalization at 60 days follow-up. Conversely, logBNP (HR 1.47 (0.95–2.29), p = 0.08) did not correlate with outcome. Multivariate analyses demonstrated congestion score > 3 was an independent predictor of all-cause mortality and HF hospitalization and after adjustment for logBNP (HR 9.83 (5.27–18.31); p < 0.001), B-lines (HR 6.81 (3.82–12.13); p < 0.001) and for logBNP and B-lines (HR 8.26 (4.46–15.26); p < 0.001). Conversely, Congestion score > 2 was not related to outcome also after adjustment for logBNP and B-lines (Table 3).
Table 3.
Univariate and Multivariable analysis for admission clinical congestion, B-lines, and BNP including three combined models.
| Variables | Association with the Composite of First HFH or Death Multivariable Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Model including LogBNP | Model Including B-Lines | Model Including LogBNP and B-Lines | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Admission Congestion score > 3 | 8.20 (4.74–14.16) | <0.001 | 9.83 (5.27–18.31) | <0.001 | 6.81 (3.82–12.13) | <0.001 | 8.26 (4.46–15.26) | <0.001 |
| Admission Congestion score ≥ 2 | 2.11 (0.95–4.67) | 0.07 | 2.03 (0.91–4.50) | 0.08 | 1.81 (0.81–4.04) | 0.14 | 1.81 (0.81–4.03) | 0.15 |
| LogBNP | 1.47 (0.95–2.29) | 0.08 | / | / | / | / | / | / |
| B-lines | 1.07 (1.03–1.10) | <0.001 | / | / | / | / | / | / |
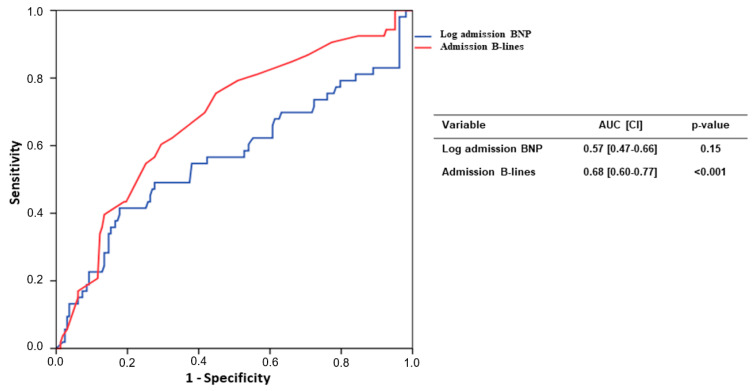
An ROC curve showed the relationship between the primary outcome and B-lines at admission (AUC: 0.68 (0.60–0.77); p < 0.001); conversely, logBNP at admission was not associated to the composite outcome (AUC: 0.57 (0.47–0.66); p = 0.15) (Figure 2).
Figure 2.
ROC curve analysis of admission BNP values and B-lines number.
8. Congestion Differences between Admission and Discharge
We calculate the mean difference between admission and discharge congestion logBNP and B-lines values and we analysed these cutoffs in relation to outcome. Based on our analysis and median values, we considered a CC reduction < 50%, ΔBNP < −43.8% and ΔB-lines < −32.3% as significant improvement. Conversely, subjects with mean clinical laboratory and LUS values above the mean reduction were defined as the persistent group. Mortality at 60 days occurs in 69% of subjects with persistent congestion, vs. 8% of patients with improved congestion. (p < 0.0001); 37% in those with unsolved ΔBNP vs. 11% in those with more significant BNP decrease (p < 0.001); 44% of patients with persistent B-lines vs. 5% of patients with significant improvement (p < 0.0001). Relative risk calculation demonstrates an excellent performance for the all Δ at both 60 and 180 days: persistent ΔCC RR 7.7 (IC 4.1–14.5) at 60 days and RR 2.0 at 180 days (IC 1.6–2.6 p < 0.001); persistent ΔBNP RR 3.4 (IC 1.9–6.1) at 60 days and RR 1.7 (IC 1.3–2.2) at 180 days (p < 0.001); persistent ΔB-lines RR 9.6 (IC 3.9–23) at 60 days and RR 3.3 (IC 2.3–4.7) p < 0.001 at 180 days compared to the resolved group.
During 180 days of follow-up, univariate analysis showed that persistent ΔB-lines (HR 6.54 (4.19–10.20); p < 0.001), persistent ΔBNP (HR 2.48 (1.69–3.63); p < 0.001) and persistent ΔCC (HR 4.25 (2.90–6.21); p < 0.001) were all significantly related to adverse outcome. Multivariable analysis confirmed that persistent ΔB-lines (HR 4.38 (2.64–7.29); p < 0.001), persistent ΔBNP (HR 1.74 (1.11–2.74); p = 0.016) and persistent ΔCC (HR 3.38 (2.10–5.44); p < 0.001) were associated with composite outcome (Table 4).
Table 4.
Univariate and multivariable analysis for 60 days and 180 days outcome regarding the mean Δ values of clinical congestion (CC) score, Log BNP and B-lines calculated as the differences between admission and discharge.
| Variables | 60 Days | 180 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate HR (CI) | p-Value | Multivariable HR (CI) * | p-Value | Univariate HR (CI) | p-Value | Multivariable HR (CI) * | p-Value | |
| Persistent ΔB-lines (<−32.3%) | 12.36 (4.92–31.07) | <0.001 | 7.52 (2.16–26.21) | 0.002 | 6.54 (4.19–10.20) | <0.001 | 4.38 (2.64–7.29) | <0.001 |
| Persistent ΔBNP (<−43.8%) | 4.26 (2.23–8.10) | <0.001 | 1.54 (0.69–3.41) | 0.29 | 2.48 (1.69–3.63) | <0.001 | 1.74 (1.11–2.74) | 0.016 |
| Persistent ΔCC (<50%) | 12.13 (5.87–25.06) | <0.001 | 11.64 (4.65–29.10) | <0.001 | 4.25 (2.90–6.21) | <0.001 | 3.38 (2.10–5.44) | <0.001 |
* Adjusted for age, gender, smoking, hypertension, diabetes mellitus, dyslipidaemia, CAD, LVEF < 50%, AF.
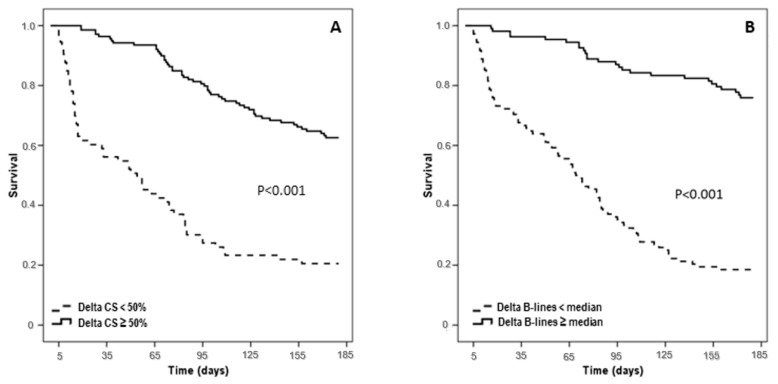
A Kaplan Meier survival curve confirmed the additional role of Δ congestion and ΔB-Lines in risk stratification (Figure 3).
Figure 3.
Kaplan-Meyer curve of median Δ Congestion score (CS) (A) and Δ B-lines (B) during follow-up (180 days).
9. Discussion
The current analysis confirmed the prognostic role of congestion evaluation at admission and discharge, however the combined analysis including B-lines count and BNP measurement is much more accurate for outcome prediction. More specifically, a high CC score at admission and discharge as evaluated by the application of the Gheorghiade scale demonstrated a good accuracy, confirming that clinical assessment remains an important feature during acute HF management [5]. In contrast, BNP measurement at admission was not related with outcome, confirming some results of the GUIDE trial [16]. Indeed, BNP is prone to several forms of bias not strictly related to congestion: intrinsic cardiac hemodynamic conditions such as wall stretching, associated mitral valve disease, atrial fibrillation, and right ventricular dysfunction may influence laboratory values. Therefore, systemic conditions such as high BMI, the presence of CKD, sarcopenia and inflammatory status are further biases. The serial LUS evaluation confirmed its diagnostic and prognostic role: subjects with a poor decrease in B-lines numbers from admission to discharge are much more prone to develop adverse events; conversely, significant reduction of B-lines during hospitalization is associated with good outcomes [17]. These findings highlight two factors: a significant percentage of patients hospitalized for Acute HF do not reach an optimal decongestion at discharge; and secondly, B-lines assessment and repeated LUS evaluation is an important approach for pulmonary congestion monitoring across the hospitalization period, and it could be inserted as ab additional tool during AHF management. Many hospitalized patients are usually discharged after symptom recovery without precise decongestion evaluation just to reduce hospitalization length and health costs. However, this approach demonstrated several weaknesses in terms of early hospital readmission and treatment efficacy [18]. Our findings are in line with previous studies evaluating the prognostic relevance of clinical congestion, and they appear to be integrative, suggesting that a residual congestion evaluated with different methods is related with increased risk [19,20]. Overall, the multiparametric diagnostic congestion approach combining clinical score with BNP assay and LUS evaluation showed a better accuracy compared with each method singly applied, and it is associated with a more accurate risk stratification [21]. Additionally, current policy may avoid invasive monitoring and become integrative for the congestion evaluation in different sites. It is well known that the best strategy for congestion evaluation remains right heart catheterization with direct measurement of wedge and right atrial pressures, although the invasive method limits any extensive application. Furthermore, the ESCAPE trial did not demonstrate any significant benefit in invasive monitoring assessment with respect with a clinical evaluation [22]. This is probably due to different mechanisms contributing to cardiac and systemic congestion: the former is closely related with cardiac invasive measurement, whereas the latter are due to hydrostatic and oncotic pressure, interstitial tissue condition, and Na tubular resorption directly related to systemic water retention. Interestingly, serial and repetitive screening during hospitalization is helpful for reducing hospitalization and congestion resolution identification [8]. Accordingly, in ambulatory patients with advanced HFrEF, the continued remote monitorization of pulmonary pressure by a Cardio-Mems system demonstrated a significant reduction in HF related hospitalization by a customized therapy [23]. Previous reports confirmed the importance of LUS assessment at admission and discharge: in a single center study, Gargani et al., showed discharge B-lines numbering < 15 were associated with reduced risk of HF hospitalization [24]. Similarly, Coiro revealed that a residual B-line > 30 independently predicts outcome, and an algorithm including LUS with BNP and NYHA class identifies patients with higher risk for hospitalization recurrence [25]. In a larger sample size of patients with both HF with reduced or preserved EF, Palazzuoli et al. revealed a discharge cut-off >22 b-lines for HFrEF and 18 for HFpEF were predictive for adverse outcome [26]. In a simplified model assessing LUS in four chest zones, Platz et al. stratified patients by mean B-lines reduction over six days from hospital admission [9]. Similarly, same authors in a retrospective study revealed that in both acute and chronic conditions, B-lines numbers change rapidly, and an LUS exam is an appropriate strategy for treatment monitoring and response [27]. Finally, our findings are in accordance with a recent position paper suggesting that a multiple ultrasound scan evaluation including imaging of the heart, lung, venous system and kidney is the optimal approach for cardiac pulmonary and systemic congestion evaluation, respectively [28].
10. Study Limitations
This is a single centre study with a relatively small sample size, but this is one of the larger studies with contemporary clinical, biochemical and LUS evaluation in patients hospitalised for acute HF. This is a post-hoc analysis, despite the fact that the patients were prospectively recruited and only patients with complete clinical, echocardiographic and follow-up data were included.
We used a simplified eight-zone protocol which is more practical for clinical use in the emergency department, but other studies applied a different scan protocol ranging from 4 to 28 chest zones. Current differences may partially explain the different B-lines counts compared with previous analyses. Other conditions, such as interstitial lung diseases, acute respiratory distress syndrome and interstitial pneumonia can confound B-lines analysis. Patient selection were performed by chest radiography and BNP level associated with dyspnoea in accordance with ESC guidelines, but some respiratory or extra cardiac conditions may have influenced the enrolment criteria. We did not divide our patients according to the ESC classification in HFrEF, HFpEF, and heart failure with mild reduced EF because only few patients (n. 32) had EF ranging from 41 to 49%, thus we included this group into HFrEF. In addition, physicians conducting clinical examinations and echocardiography were aware of the CRx and BNP results. This might have introduced further bias. Our results may partially depend on decongestion treatment, intensity of care, and diuretic amount adopted before and during hospitalization. Finally, the persistence of pre-discharge B-lines may imply sub-optimal decongestion treatment. However, we cannot assert that a more elevated diuretic dose could drive towards relevant B-lines reduction and better outcomes. Therefore, a multi-centre analysis comparing a larger sample size LUS with various congestion scores is welcome and might support or refute our findings.
11. Conclusions
Our data confirmed the importance of achieving congestion status resolution before discharge by an integrated clinical laboratory and LUS evaluation. The reduction of the B-lines number from admission to discharge appears to be the most powerful predictor for HF recurrence and death in patients hospitalized for HF. Future studies with larger sample would shed light on the efficacy of the current strategy in optimizing time discharge and in reducing adverse events.
Author Contributions
Conceptualization, design, writing and revision A.P. and G.R.; methodology, I.E., L.G. and G.R.; validation, A.P., L.G. and G.R.; formal analysis, L.G. and G.R.; investigation, M.B., M.C.T. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local ethic committee (C.E.A.V.S.E.).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from Dr. Alberto Palazzuoli after official request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boorsma E.M., Ter Maaten J.M., Damman K., Dinh W., Gustafsson F., Goldsmith S., Burkhoff D., Zannad F., Udelson J.E., Voors A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020;17:641–655. doi: 10.1038/s41569-020-0379-7. [DOI] [PubMed] [Google Scholar]
- 2.Mullens W., Damman K., Harjola V.P., Mebazaa A., Brunner-La Rocca H.P., Martens P., Testani J.M., Tang W.H.W., Orso F., Rossignol P., et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019;21:137–155. doi: 10.1002/ejhf.1369. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M., Filippatos G., De Luca L., Burnett J. Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. Am. J. Med. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Chioncel O., Mebazaa A., Maggioni A.P., Harjola V.P., Rosano G., Laroche C., Piepoli M.F., Crespo-Leiro M.G., Lainscak M., Ponikowski P., et al. ESC-EORP-HFA Heart Failure Long-Term Registry Investigators. Acute heart failure congestion and perfusion status—Impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2019;21:1338–1352. doi: 10.1002/ejhf.1492. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M., Follath F., Ponikowski P., Barsuk J.H., Blair J.E., Cleland J.G., Dickstein K., Drazner M.H., Fonarow G.C., Jaarsma T., et al. European Society of Cardiology; European Society of Intensive Care Medicine. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010;12:423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 6.Chakko S., Woska D., Martinez H., De Marchena E., Futterman L., Kessler K.M., Myerburg R.J. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: Conflicting results may lead to inappropriate care. Am. J. Med. 1991;90:353–359. doi: 10.1016/0002-9343(91)90576-J. [DOI] [PubMed] [Google Scholar]
- 7.Collins S.P., Lindsell C.J., Storrow A.B., Abraham W.T. ADHERE Scientific Advisory Committee, Investigators and Study Group. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann. Emerg. Med. 2006;47:13–18. doi: 10.1016/j.annemergmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Palazzuoli A., Evangelista I., Nuti R. Congestion occurrence and evaluation in acute heart failure scenario: Time to reconsider different pathways of volume overload. Heart Fail. Rev. 2020;25:119–131. doi: 10.1007/s10741-019-09868-0. [DOI] [PubMed] [Google Scholar]
- 9.Stienen S., Salah K., Moons A.H., Bakx A.L., van Pol P., Kortz R.A.M., Ferreira J.P., Marques I., Schroeder-Tanka J.M., Keijer J.T., et al. NT-proBNP (N-Terminal pro-B-Type Natriuretic Peptide)-Guided Therapy in Acute Decompensated Heart Failure: PRIMA II Randomized Controlled Trial (Can NT-ProBNP-Guided Therapy During Hospital Admission for Acute Decompensated Heart Failure Reduce Mortality and Readmissions?) Circulation. 2018;137:1671–1683. doi: 10.1161/CIRCULATIONAHA.117.029882. [DOI] [PubMed] [Google Scholar]
- 10.Pfisterer M., Buser P., Rickli H., Gutmann M., Erne P., Rickenbacher P., Vuillomenet A., Jeker U., Dubach P., Beer H., et al. TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: The Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 11.Gargani L., Ferre R.M., Pang P.S. B-lines in heart failure: Will comets guide us? Eur. J. Heart Fail. 2019;21:1616–1618. doi: 10.1002/ejhf.1641. [DOI] [PubMed] [Google Scholar]
- 12.Öhman J., Harjola V.P., Karjalainen P., Lassus J. Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Fail. 2018;5:120–128. doi: 10.1002/ehf2.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maw A.M., Hassanin A., Ho P.M., McInnes M.D., Moss A., Juarez-Colunga E., Soni N.J., Miglioranza M.H., Platz E., DeSanto K., et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: A systematic review and meta-analysis. JAMA Netw. Open. 2019;2:e190703. doi: 10.1001/jamanetworkopen.2019.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platz E., Campbell R.T., Claggett B., Lewis E.F., Groarke J.D., Docherty K.F., Lee M.M., Merz A.A., Silverman M., Swamy V., et al. Lung Ultrasound in Acute Heart Failure: Prevalence of Pulmonary Congestion and Short- and Long-Term Outcomes. JACC Heart Fail. 2019;7:849–858. doi: 10.1016/j.jchf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platz E., Campbell R.T., Claggett B., Lewis E.F., Groarke J.D., Docherty K.F., Lee M.M., Merz A.A., Silverman M., Swamy V., et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: A SIMEU multicenter study. Chest. 2015;148:202–210. doi: 10.1378/chest.14-2608. [DOI] [PubMed] [Google Scholar]
- 16.Felker G.M., Anstrom K.J., Adams K.F., Ezekowitz J.A., Fiuzat M., Houston-Miller N., Januzzi J.L., Jr., Mark D.B., Piña I.L., Passmore G., et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318:713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morvai-Illés B., Polestyuk-Németh N., Szabó I.A., Monoki M., Gargani L., Picano E., Varga A., Ágoston G. The Prognostic Value of Lung Ultrasound in Patients with Newly Diagnosed Heart Failure with Preserved Ejection Fraction in the Ambulatory Setting. Front. Cardiovasc. Med. 2021;8:758147. doi: 10.3389/fcvm.2021.758147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosy A.P., Pang P.S., Khan S., Konstam M.A., Fonarow G.C., Traver B., Maggioni A.P., Cook T., Swedberg K., Burnett J.C., Jr., et al. EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 19.Cogliati C., Casazza G., Ceriani E., Torzillo D., Furlotti S., Bossi I., Vago T., Costantino G., Montano N. Lung ultrasound and short-term prognosis in heart failure patients. Int. J. Cardiol. 2016;218:104–108. doi: 10.1016/j.ijcard.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Pang P.S., Russell F.M., Ehrman R., Ferre R., Gargani L., Levy P.D., Noble V., Lane K.A., Li X., Collins S.P. Lung Ultrasound-Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF): A Randomized Controlled Pilot Trial. JACC Heart Fail. 2021;9:638–648. doi: 10.1016/j.jchf.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellicori P., Platz E., Dauw J., Ter Maaten J.M., Martens P., Pivetta E., Cleland J.G.F., McMurray J.J.V., Mullens W., Solomon S.D., et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021;23:703–712. doi: 10.1002/ejhf.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binanay C., Califf R.M., Hasselblad V., O’Connor C.M., Shah M.R., Sopko G., Stevenson L.W., Francis G.S., Leier C.V., Miller L.W. ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 23.Givertz M.M., Stevenson L.W., Costanzo M.R., Bourge R.C., Bauman J.G., Ginn G., Abraham W.T. CHAMPION Trial Investigators. Pulmonary Artery Pressure-Guided Management of Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017;70:1875–1886. doi: 10.1016/j.jacc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Gargani L., Pang P.S., Frassi F., Miglioranza M.H., Dini F.L., Landi P., Picano E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound. 2015;13:40. doi: 10.1186/s12947-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coiro S., Rossignol P., Ambrosio G., Carluccio E., Alunni G., Murrone A., Tritto I., Zannad F., Girerd N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015;17:1172–1181. doi: 10.1002/ejhf.344. [DOI] [PubMed] [Google Scholar]
- 26.Palazzuoli A., Ruocco G., Beltrami M., Nuti R., Cleland J.G. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin. Res. Cardiol. 2018;107:586–596. doi: 10.1007/s00392-018-1221-7. [DOI] [PubMed] [Google Scholar]
- 27.Platz E., Merz A.A., Jhund P.S., Vazir A., Campbell R., McMurray J.J. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: A systematic review. Eur. J. Heart Fail. 2017;19:1154–1163. doi: 10.1002/ejhf.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platz E., Jhund P.S., Girerd N., Pivetta E., McMurray J.J.V., Peacock W.F., Masip J., Martin Sanchez F.J., Miró Ò., Price S., et al. Study Group on Acute Heart Failure of the Acute Cardiovascular Care Association and the Heart Failure Association of the European Society of Cardiology. Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur. J. Heart Fail. 2019;21:844–851. doi: 10.1002/ejhf.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from Dr. Alberto Palazzuoli after official request.