Abstract
Zanamivir (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid; Relenza; GG167) is a potent and highly specific neuraminidase (sialidase) inhibitor with inhibitory activity in vivo against both influenza A and B viruses. This compound has been extensively tested in both mouse and ferret models of influenza and has recently been approved for the treatment of influenza in Europe and Australasia. The compound markedly reduces the clinical course of disease in humans when given therapeutically by inhalation directly into the respiratory tract. In addition, experimental influenza infections in phase I clinical trials have shown the benefit of giving a single prophylactic dose of zanamivir in addition to a therapeutic regime. The studies reported here were designed to determine the persistence of zanamivir, as assessed by its antiviral activity in vivo, in the respiratory tracts of infected animals. We have shown that the prophylactic administration of zanamivir, when the drug is given in a single dose by the intranasal route, can significantly reduce lung virus titers in the mouse and can reduce both viral titers and symptoms in the ferret. Whole-body autoradiographical analyses of mice have indicated a long retention time for this compound in respiratory tract tissues when it is given in a single dose by the intranasal route. These results indicate that zanamivir may have clinical value as a prophylactic agent in protecting at-risk groups from influenza virus infection. In addition, these data may be useful in the design of prophylactic protocols for humans, in that the dosing schedule may only need to be intermittent to provide protection.
The highly infectious nature of influenza virus has the potential to put whole communities at risk of infection. Particularly vulnerable are closed populations such as elderly nursing home residents (24) and armed forces. Influenza virus infections are primarily controlled by the prophylactic use of vaccines (4, 16). However, the constant antigenic change associated with influenza virus, coupled with the often inadequate protection afforded by vaccines, especially in those with inefficient immune responses (elderly, very young, and immunocompromised), highlights the need for an effective antiviral agent. In addition, the efficacy of available vaccines in the face of the emergence of new antigenic subtypes (as shown by the recent outbreaks of H5N1 [6] and H9N2 infections in humans) would be minimal. The identification of an anti-influenza virus chemotherapeutic agent that could be used prophylactically in high-risk patients with either a poor immune response to vaccines (e.g., the elderly) (3, 24, 29) or as an adjunct to vaccines in an outbreak scenario where cover is required while immunity develops is clearly of great importance (8, 17). In addition, such an agent would be invaluable on the emergence of a new antigenic subtype.
The utility of the currently available anti-influenza drugs, amantadine and rimantadine (7, 29), has been limited by the lack of activity against influenza B viruses (8, 32). Both drugs have been shown to be effective in preventing laboratory infections with influenza A viruses in volunteers (25, 28), and amantadine has proven effective in controlling an outbreak of influenza in a nursing home (1) when given prophylactically. However, the emergence of viruses resistant to these agents is both rapid and significant (14, 18). Thus the concomitant prophylactic and therapeutic use of these agents, in closed or semiclosed communities, may be contraindicated. In addition, these agents have unwanted neurological side effects (11).
The development of influenza virus-specific neuraminidase inhibitors has been a major breakthrough in the treatment of this disease. These may be typified by the inhaled inhibitor zanamivir and the oral inhibitor GS4104 (26, 31), which act by the inhibition of the viral surface glycoprotein neuraminidase (sialidase) and which were developed by a process of rational design (30). Zanamivir (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid; Relenza; GG167) has been shown to be effective for the duration and severity of illness in clinical trials (12, 13, 27). The efficacy data in these studies were predicted by the studies of animal models of influenza virus infection. Thus zanamivir has been shown to be an effective antiviral in both mouse (10, 22) and ferret (23) animal models of influenza virus infection. Early studies, however, showed that when doses of zanamivir were given up to 18 h before infection and for 4 days postinfection, the overall efficacy was enhanced compared to a postinfection regimen alone (22). Similar results were obtained in phase I and phase II clinical trials (12, 13), in which the addition of a single dose 4 h before influenza virus challenge, in conjunction with doses twice or six times daily given therapeutically, gave superior reductions in total symptom score and a nasal virus titer reduction. However, the efficacy of this compound as a prophylactic agent alone has not previously been examined. The experiments reported here investigate the prophylactic efficacy of a single intranasal dose of zanamivir in both mouse and ferret models of influenza A virus infection. These data suggest that the frequency of zanamivir administration could be reduced for prophylactic use compared with the frequency of administration for the treatment of symptomatic influenza. Clinical studies, in which daily administered zanamivir has shown a positive protective effect when given prophylactically, have now been reported (15).
MATERIALS AND METHODS
Reagents.
Unless stated otherwise, all reagents, media components, tissue culture cell lines and methods, and virus assays by enzyme-linked immunosorbent assay (ELISA), were as described previously (20–22, 33).
Influenza viruses.
Influenza viruses A/Singapore/1/57 (H2N2) and A/Mississippi/1/85 (H3N2) were supplied by A. J. Hay and were typed by A. Douglas (National Institute for Medical Research, Mill Hill, London, United Kingdom). Stock virus pools were generated in fertile hens’ eggs.
Influenza virus infection in mice.
The protocol for infecting mice has been described previously (30). Mice, anesthetized by the inhalation of ether, were inoculated in the external nares with 50 μl of a virus suspension containing 104.5 50% tissue culture infective doses (TCID50)/ml. This inoculum had previously been determined to give high titers of virus 24 h postinfection.
Treatment procedure and regimen.
A single dose of zanamivir was administered at time points between 3 and 240 h prior to infection. The compound (dissolved in phosphate-buffered saline [PBS]) was given intranasally in a volume of 50 μl to groups of 7 to 10 mice anesthetized by inhalation of ether. Sham-treated control animals received PBS only.
Assay of virus in lung homogenate samples.
Individual lung homogenates were prepared from mice culled 24 h postinfection. As described previously (2, 22, 30) the titers of lung virus were assayed by ELISA. Reductions in virus titer were expressed as a percentage of values from PBS-treated control animals. The Kruskal-Wallis rank sum test was used to determine the statistical significance of treatment regimens in reducing lung virus titers.
Autoradiography.
14C-radiolabelled zanamivir (5 MBq/mg; >99% radiopurity), labelled at the 4-guanidino position, was given intranasally to two anesthetized mice per group, in a volume of 50 μl. At 10-min, 45-min, 90-min, and 24-h time points following dosing, each group of mice was sacrificed and frozen in liquid nitrogen prior to analysis by whole-body autoradiography.
Influenza virus infection in ferrets.
While under light anesthesia (isofluorane), groups of four ferrets (female; 0.75 to 1.2 kg) were infected by intranasal instillation of 250 μl of influenza virus A/Mississippi/1/85 containing 104.5 TCID50/ml, as described previously (30).
Treatment procedure and regimen.
While under light anesthesia, animals received a single intranasal dose of 12.5 mg (0.25 ml/kg of body weight) of zanamivir/kg of body weight 48 h prior to infection. Virus-infected control animals were sham dosed with distilled water. Ferret weights were recorded daily for 8 days. Nasal washings were taken on days 1 to 8 following challenge, as described previously (19), and the washings were used for estimation of virus titer by ELISA and turbidity (19). The area under the curve (AUC) for virus titers for the samples taken on days 1 to 8 for each ferret was calculated from the antilog values obtained from the ELISA log10 values. This value was then converted back to a log10 value, and the log10 geometric mean AUC value for the group of ferrets was calculated. The Duncan multiple-range test was used to determine statistical significance of treatment regimens in reducing lung virus titres. Temperature profiles of ferrets were recorded every 10 min by implanted telemetric transmitters (Dataquest; Data Sciences, St. Paul, Minn.), prior to and up to 8 days following infection (30). AUC values were calculated for the period of pyrexic response (0 to 96 h); AUCs were computed as the area above and below the preinfection mean. Percentage reductions in pyrexia compared with controls were calculated from the AUC values. Pyrexia was also defined as the elevation of core body temperature by two standard deviations (or more) above the preinfection mean temperature for a period of at least 12 h during the postinfection period. Blood samples were taken 5 days preinfection and 21 days postinfection for the determination of neutralizing serum antibody levels to the infecting virus (19).
RESULTS
Efficacy of prophylactic regimens of zanamivir in mouse models of influenza A infection.
Single intranasal doses of between 12.5 and 1.56 mg of zanamivir/kg given 51 h prior to infection gave statistically significant reductions in lung virus titers (P ≤ 0.01), compared with titers in vehicle-treated control animals (Table 1). In addition a good dose response was evident (99.13 to 90.83% reductions compared with control titers). However, single intranasal doses of less than 12.5 mg/kg given 7 days prior to infection did not significantly reduce lung virus titers of mice (Table 2).
TABLE 1.
Efficacy of a single intranasal dose of zanamivir given intranasally to micea 51 h prior to infection
| Dose (mg/kg) | Mean lung virus titers (log10 TCID50) (SD) | % Reduction in mean lung virus titers vs controls | No. of virus-free animals/groupb |
|---|---|---|---|
| 12.5 | 3.09 (0.71) | 99.13c | 1 |
| 6.25 | 3.70 (0.30) | 96.45c | 0 |
| 3.125 | 3.92 (0.80) | 94.09c | 0 |
| 1.56 | 4.11 (0.94) | 90.83c | 0 |
| 0 (PBS controls) | 5.15 (0.96) | 0 |
n = 10 mice per group.
Below lower limit of detection (102.30 TCID50/ml).
Highly significantly different from untreated control (P ≤ 0.01; Kruskal-Wallis).
TABLE 2.
Efficacy of zanamivir given as a single intranasal dose of between 12.5 and 0.1 mg/kg to micea 7 days prior to infection
| Dose (mg/kg) | Mean lung virus titers (log10 TCID50) (SD) | % Reduction in mean lung virus titers | No. of virus-free animals/groupb |
|---|---|---|---|
| 12.5 | 3.34 (0.65) | 88.77c | 1 |
| 2.5 | 4.38 (0.51) | 0d | 0 |
| 0.5 | 4.47 (0.44) | 0d | 0 |
| 0.1 | 4.49 (0.43) | 0d | 0 |
| 0 (PBS controls) | 4.29 (1.00) | 0 |
n = 10 mice per group.
Below lower limit of detection (102.30 TCID50/ml).
Highly significantly different from untreated control (P ≤ 0.01; Kruskal-Wallis).
Not significant (P ≥ 0.05).
In order to investigate the activities of single intranasal doses of zanamivir, given at different time points prior to infection, a dose of 12.5 mg/kg was chosen. This dose, given at time points between 3 and 240 h prior to infection with influenza virus, resulted in statistically significant reductions in mean log10 lung virus titers (P ≤ 0.05 to 0.01), compared with those for vehicle-treated control animals (Table 3). Thus reductions in virus titer of from 1.41 to 3.74 log10 units were achieved with treatments administered up to 7 days (172 h) prior to infection and in many treated animals virus titers were below the level of detection.
TABLE 3.
Efficacy of zanamivir given as a single 12.5-mg/kg intranasal dose to mice at different time points prior to infectiona
| Time of prophylactic dose relative to time of infection (h) | Mean lung virus titers (log10 TCID50) ± SD in animals treated with:
|
Virus titer reduction (log10 units) | % Reduction vs control | No. of virus-free animals/no. of animals in treatment groupb | |
|---|---|---|---|---|---|
| Zanamivir | PBS | ||||
| −3 | 2.84 ± 0.87 | 6.58 ± 1.61 | 3.74 | 99.98 | 5/7 |
| −19 | 4.08 ± 1.52 | 6.58 ± 1.61 | 2.50 | 99.68c | 3/8 |
| −27 | 4.27 ± 1.25 | 6.58 ± 1.61 | 2.31 | 99.51c | 2/8 |
| −43 | 3.80 ± 1.2 | 6.58 ± 1.61 | 2.78 | 99.83c | 2/6 |
| −51 | 4.36 ± 1.48, 3.41 ± 0.91 | 6.58 ± 1.61, 5.30 ± 0.44 | 2.22, 1.89 | 99.40,c 98.71d | 3/9, 3/9 |
| −117 | 5.03 ± 1.25, 3.70 ± 0.55 | 7.03 ± 0.58, 5.30 ± 0.44 | 2.00, 1.60 | 99.00,d 97.49d | 1/10, 1/10 |
| −145 | 5.37 ± 0.98, 3.47 ± 0.95 | 7.03 ± 0.58, 5.30 ± 0.44 | 1.66, 1.83 | 97.79,d 98.53d | 0/10, 3/9 |
| −172 | 5.30 ± 1.87, 3.89 ± 0.80 | 7.03 ± 0.58, 5.30 ± 0.44 | 1.73, 1.41 | 98.12,c 96.08d | 2/8, 0/8 |
| −240 | 4.65 ± 0.72, 4.48 ± 1.03 | 5.30 ± 0.44, 5.24 ± 0.59 | 0.65, 0.76 | 77.61,c 83.00e | 0/10, 1/10 |
Duplicate values represent repeat experiments.
Below the lower limit of detection (102.30 TCID50/ml).
Significantly different from untreated control (P < 0.05; Kruskal-Wallis).
Highly significantly different from untreated control (P < 0.01; Kruskal-Wallis).
Not significant.
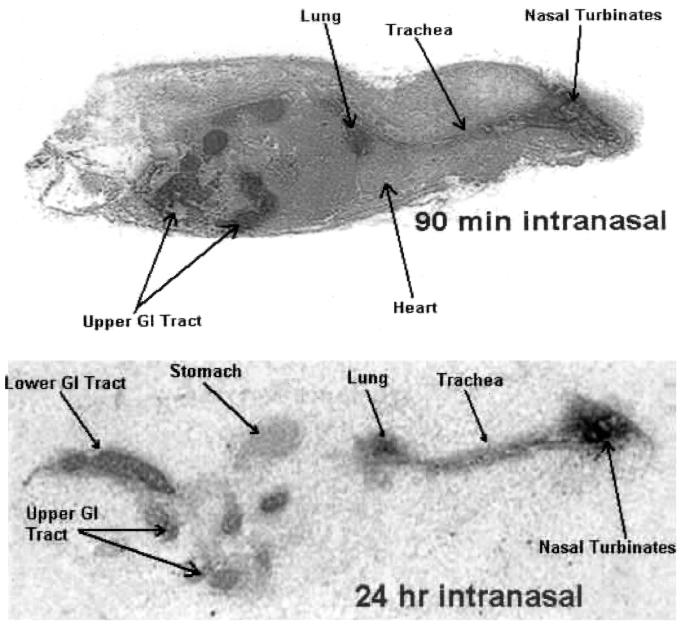
The elimination half-life of zanamivir in mice when given intravenously has previously been reported to be approximately 10 min (22). However, the data presented here suggest that the retention time of at least a proportion of the dose, in lungs of intranasally treated mice, is substantially longer than would be predicted from the half-life of the drug in plasma after intravenous dosing. To investigate this further, mice were given intranasal 14C-radiolabelled zanamivir at a dose of 12.5 mg/kg, and drug distribution was determined at various time points by whole-body autoradiography. It had previously been determined that the purity of the radiolabelled compound was in excess of 99% and that free radiolabel was undetectable. In addition, it has been shown that zanamivir is chemically stable and that it is not metabolized (5, 22). As can be seen in the autoradiographs, compound was clearly present in the nasal turbinates, trachea, and lung 90 min after administration (Fig. 1). In addition some of the dosed material had clearly been swallowed and entered the stomach and bowel. Involvement of the kidneys was also apparent, but to a much lesser degree. This observation is associated with the clearance, by glomerular filtration, of the low levels of compound present due to systemic absorption. Compound could also be detected in the lungs and trachea 24 h after the intranasal dose (Fig. 1). These observations, though not quantitative, further support the persistence of radiolabelled zanamivir in the respiratory tract following a single intranasal dose.
FIG. 1.
Autoradiography sections of mice dosed intranasally with 14C-labelled zanamivir 90 min and 24 h prior to sacrifice. GI, gastrointestinal.
Efficacy of prophylactic regimens of zanamivir in a ferret model of influenza A infection.
In ferrets treated with a single 12.5-mg/kg intranasal dose of zanamivir 48 h prior to infection, nasal wash virus titers were reduced by 60% (AUC between days 1 and 8) compared with those for vehicle-dosed animals (Table 4). However, although this value was not statistically significant (P = 0.051), statistically significant reductions in virus titers (P ≤ 0.0005) were obtained on days 1 and 2 postinfection (Table 4). These results are not surprising, as later samples from an animal given a single prophylactic dose prior to infection are less likely to show reductions due to clearance, albeit slow.
TABLE 4.
Efficacy of zanamivir given as a single 12.5-mg/kg intranasal dose to ferrets 48 h prior to infection
| Treatment | No. of animals with pyrexia/groupa | % Reduction in core body temperature, days 0–8 | Mean nasal wash virus AUC, days 1–8 (log10 TCID50) | % Reduction in mean nasal wash virus titer vs control (individual lung virus titers [log10 TCID50]) on day:
|
% Reduction in mean nasal wash turbidity (AUC for days 1–8) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1–8 | |||||
| Zanamivir | 0 | 71.1b | 6.04 | 99.72c (2.05, 2.80, 2.80, 3.80) | 94.37c (4.80, 5.05, 5.55, 4.55) | 0 (5.80, 5.80, 5.80, 5.05) | 60d | 55c |
| PBS (control) | 4 | 6.43 | NAe (5.80, 5.05, 5.80, 5.05) | NA (6.05, 6.80, 6.05, 6.05) | NA (5.55, 3.80, 4.55, 5.05) | |||
n = 4 ferrets per group.
Significantly different from untreated control (P ≤ 0.001; Duncan multiple-range test).
Significantly different from untreated control (P ≤ 0.0005; Duncan multiple-range test).
P = 0.051. Value is the AUC.
NA, not applicable.
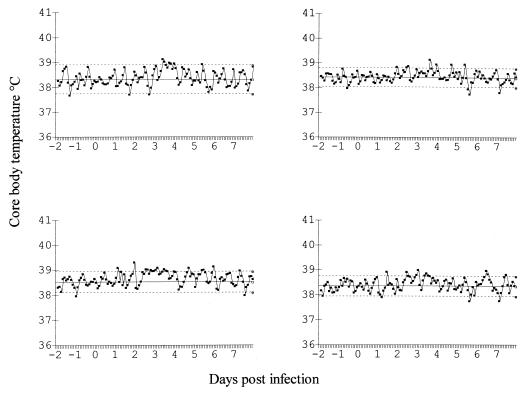
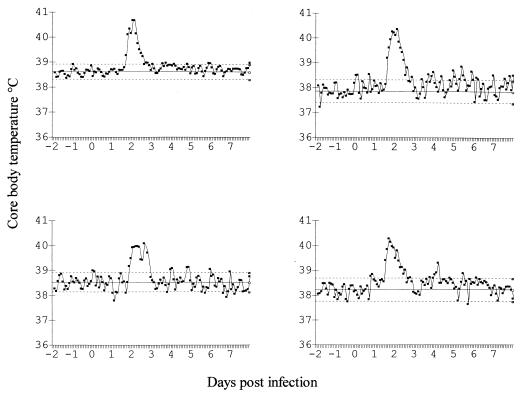
Significant reductions in pyrexia (71.1%; P < 0.001) in treated animals were also observed (Fig. 2; Table 4), compared with that for vehicle-treated controls (Fig. 3; Table 4). In addition, nasal wash turbidities were also significantly reduced in treated animals (55% reduction in AUC) compared with those for untreated controls (P < 0.01; Table 4). Slight body weight loss was apparent in all animals, typical of the anorexia associated with influenza infections of ferrets. A calculation of mean body weight change from day 0 to day 8 postinfection revealed that weight loss was 3.3 and 4.5% for treated and control groups, respectively; there was no significant difference in weight loss between these two groups. Serum titers of antibody specific for influenza virus A/Mississippi/1/85 in ferrets before infection were calculated as less than or equal to 1:10. Serum samples taken from each animal at 21 days postinfection indicated a serum antibody titer of at least 1:320. This result indicated that animals were naive prior to infection with influenza virus A/Mississippi/1/85 but seroconverted after viral challenge, irrespective of whether they had undergone successful chemoprophylaxis or had unabated influenza.
FIG. 2.
Core body temperatures of ferrets given a single intranasal dose of 12.5 mg of zanamivir/kg 48 h prior to infection with influenza virus A/Mississippi/1/85. Solid line, mean; dotted lines, 2 standard deviations.
FIG. 3.
Core body temperatures of control ferrets infected with influenza virus A/Mississippi/1/85. Solid line, mean; dotted lines, 2 standard deviations. Solid line, mean; dotted lines, 2 standard deviations.
DISCUSSION
Zanamivir, a potent inhibitor of influenza virus neuraminidase, has been shown in both animal models and clinical trials to be an effective therapeutic agent for influenza. Recent clinical studies of healthy adults have shown that once daily administration of zanamivir is an effective prophylaxis dosing strategy for the prevention of influenza (15). We report here the prophylactic activity of this compound in animal models of influenza virus infection and show that doses as low as 1.56 mg of zanamivir/kg given 51 h prior to infection significantly reduce lung virus titers in infected mice, compared with untreated animals. In addition, in mice, a single intranasal dose of zanamivir given up to 7 days prior to infection with influenza A virus was effective in reducing lung virus titers. This observation was unexpected since in this species the compound is rapidly cleared from plasma (an elimination half-life of 10 min following intravenous administration [22]), suggesting that there may be a substantial “depot” of at least part of the dose in the lung after topical administration. In humans, zanamivir is also rapidly eliminated when given intravenously (half-life = 1.6 h [9]). Following intranasal drops or inhaled administration, maximum serum concentrations were reached within 2 h postdose (9). However, the terminal-phase half-lives (3.4 and 2.9 h, respectively) suggested the presence of either a slow or complex absorption process in humans (9). On the basis of urine excretion data, the bioavailabilities following intranasal drops and inhaled administration were estimated to be approximately 10 and 25%, respectively (5, 9).
The retention of zanamivir in the respiratory tract tissues of mice is supported by whole-body autoradiography studies, in which radiolabelled zanamivir remained detectable in the lungs of mice up to 24 h after a single intranasal dose.
Similarly, in ferrets, a single intranasal dose 48 h prior to infection was able to significantly reduce the clinical signs of infection (pyrexia, nasal wash turbidity) and viral shedding, compared with those for vehicle-treated animals. This provides further evidence of the retention of compound in the respiratory tract, since elimination from the plasma in ferrets is again rapid (43-min elimination half-life after intravenous administration [unpublished in-house data]).
The significant prevention of symptoms in ferrets, however, did not affect the generation of a serum antibody response specific to the infecting virus. This is necessary since a good serum antibody response is important in protection from reinfection with homologous virus and has some protective value against other antigenically related subtypes.
In conclusion, the data suggest that infrequent administration of zanamivir may be a successful strategy for prophylaxis, in the clinical situation.
ACKNOWLEDGMENTS
We thank Richard Bethell and Peter Collins for their advice and intellectual input into this study, Nikki Yiannakis for data processing, and the Biosciences Support Unit for their excellent animal husbandry.
REFERENCES
- 1.Arden N H, Patriarch P A, Fasano M B, Lui K J, Harmon M W, Kendal A P, Rimland D. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza (H3N2) in a nursing home. Arch Intern Med. 1988;148:865–868. [PubMed] [Google Scholar]
- 2.Belshe R B, Smith M H, Hall C B, Betts R, Hay A J. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988;62:1508–1512. doi: 10.1128/jvi.62.5.1508-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts R F, Treanor J J, Graman P S, Bentley D W, Dolin R. Antiviral agents to prevent or treat influenza in the elderly. J Respir Dis. 1987;8(Suppl. 11A):S56–S59. [Google Scholar]
- 4.Carrat F, Tachet A, Rouzioux C, Housset B, Valleron A J. Influenza vaccine and morbidity. Field investigation of influenza vaccine effectiveness on morbidity. Vaccine. 1998;16:893–898. doi: 10.1016/s0264-410x(97)00307-1. [DOI] [PubMed] [Google Scholar]
- 5.Cass L M R, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36(Suppl. 1):1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Claas E C J, Osterhaus A D M E, Vanbeek R, Dejong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 7.Dolin R, Reichman R C, Madore H P, Maynard R, Linton P N, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982;307:580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- 8.Douglas R G., Jr Prophylaxis and treatment of influenza. N Engl J Med. 1990;322:443–450. doi: 10.1056/NEJM199002153220706. [DOI] [PubMed] [Google Scholar]
- 9.Efthymiopoulos C, Barrington P, Patel J, Harker A, Harris A, Hussey E K, Bye A. Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1994. Pharmacokinetics of the neuraminidase inhibitor 4-guanidino Neu5Ac2en (GG167) following intravenous, intranasal and inhaled administration in man, abstr. H70; p. 265. [Google Scholar]
- 10.Gubareva L V, McCullers J A, Bethell R C, Webster R G. Characterisation of influenza A/Hong Kong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 11.Hayden F G, Hoffman H E, Spyker D A. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob Agents Chemother. 1983;23:458–464. doi: 10.1128/aac.23.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden F G, Treanor J J, Betts R F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 13.Hayden F G, Osterhaus A D M E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 14.Monto A S, Arden N H. Implications of viral resistance to amantadine in control of influenza A. Clin Infect Dis. 1992;15:362–369. doi: 10.1093/clinids/15.2.362. [DOI] [PubMed] [Google Scholar]
- 15.Monto A S, Robinson D P, Herlocher M L, Hinson J M, Elliott M J, Crisp A. Zanamivir in the prevention of influenza among healthy adults. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Oates J A, Wood A J J. Prophylaxis and treatment of influenza. N Engl J Med. 1990;322:443–450. doi: 10.1056/NEJM199002153220706. [DOI] [PubMed] [Google Scholar]
- 17.Palache A M. Influenza vaccines—a reappraisal of their use. Drugs. 1997;54:841–856. doi: 10.2165/00003495-199754060-00004. [DOI] [PubMed] [Google Scholar]
- 18.Peng G, Jennings R, Potter C W, Oxford J S. Amantadine resistance in clinical influenza (H3N2 and H1N1) virus isolates. J Antimicrob Chemother. 1986;18(Suppl. B):135–140. doi: 10.1093/jac/18.supplement_b.135. [DOI] [PubMed] [Google Scholar]
- 19.Potter C W, Oxford J S, Shore S L, McLaren C, Stuart-Harris C H. Immunity to influenza in ferrets. I. Response to live and killed virus. Br J Exp Pathol. 1972;53:153–167. [PMC free article] [PubMed] [Google Scholar]
- 20.Reed L J, Muench H. A simple method of estimating fifty per cent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 21.Reuman P D, Bernstein D I, Keefer M C, Young E C, Sherwood J R, Schiff G M. Efficacy and safety of low dosage amantadine hydrochloride as prophylaxis for influenza A. Antiviral Res. 1989;11:27–40. doi: 10.1016/0166-3542(89)90018-1. [DOI] [PubMed] [Google Scholar]
- 22.Ryan D M, Ticehurst J, Dempsey M H, Penn C R. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase) Antimicrob Agents Chemother. 1994;38:2270–2275. doi: 10.1128/aac.38.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan D M, Ticehurst J, Dempsey M H. GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is a potent inhibitor of influenza virus in ferrets. Antimicrob Agents Chemother. 1995;39:2583–2584. doi: 10.1128/aac.39.11.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilling M, Povinelli L, Krause P, Gravenstein M, Ambrozaitis A, Jones H H, Drinka P, Shult P, Powers D, Gravenstein S. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza outbreaks. Vaccine. 1998;16:1771–1774. doi: 10.1016/s0264-410x(98)00141-8. [DOI] [PubMed] [Google Scholar]
- 25.Sears S D, Clements M L. Protective efficacy of low-dose amantadine in adults challenged with wild-type influenza A virus. Antimicrob Agents Chemother. 1987;31:1470–1473. doi: 10.1128/aac.31.10.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M-H, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuramnidase inhibitor. Antivir Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 27.Silagy C, Campion K, Gardiner S, Gummer M. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 28.Smorodintsev A A, Zlydnikov D M, Kiseleva A M, Romanov J A, Kazantsev A P, Rumovsky V I. Evaluation of amantadine in artificially induced A2 and B influenza. JAMA. 1970;213:1448–1454. [PubMed] [Google Scholar]
- 29.Tominack R L, Hayden F G. Rimantadine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect Dis Clin North Am. 1985;1:459–478. [PubMed] [Google Scholar]
- 30.von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Dyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature (London) 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 31.Weixing L I, Escarpe P A, Eisenberg E J, Cundy K C, Sweet C, Jakeman K J, Merson J, Lew W, Zhang L, Kim C U, Bischofberger N, Chen M S, Mendel D B. Identification of GS4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS4071. Antimicrob Agents Chemother. 1998;42:647–653. doi: 10.1128/aac.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson S Z, Knight V, Wyde P R, Drake S, Couch R B. Amantadine and ribavirin aerosol treatment of influenza A and B infection in mice. Antimicrob Agents Chemother. 1980;17:642–648. doi: 10.1128/aac.17.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods J M, Bethell R C, Coates J A V, Healey N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S A, Penn C R. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]