Abstract
Introduction
Psoriasis is an immune-mediated systemic disease. Neutrophils are enriched in psoriasis lesions and can form neutrophil extracellular traps (NETs) to activate keratinocytes. Receptor-interacting protein kinase RIPK1 and RIPK3 are involved in necroptosis and NET formation.
Aim
To elucidate whether RIPK1 regulates circulating neutrophils to form NETs and inflammation in psoriasis.
Material and methods
Blood samples of psoriasis patients (n = 20) and healthy controls (n = 20) were detected by flow cytometry. The expression level of RIPK1/3 in isolated circulating neutrophils from psoriasis patients (n = 17) and healthy controls (n = 17) was examined by quantitative real-time PCR. SYTOX Green dye and PicoGreen reagent were used to detect NET formation and DNA release in neutrophils under the stimulation of phorbol 12-myristate 13-acetate (PMA) and necrostain-1 (Nec-1). Correlation analysis was performed between RIPK1/3 expression and Psoriasis Area Severity Index (PASI), neutrophil-to-lymphocyte ratio (NLR).
Results
RIPK1 and RIPK3 expression in protein levels were decreased in monocytes and neutrophils from peripheral blood of psoriasis patients. In isolated psoriasis neutrophils, RIPK1 and Caspase8 mRNA were downregulated while RIPK3 and MLKL mRNA were elevated, leading to the necroptosis pathway. In addition, RIPK1-inhitor-necrostatin-1 (Nec-1) enhanced NETosis in psoriasis neutrophils in vitro. More importantly, there is a negative correlation between RIPK1 and psoriasis disease severity.
Conclusions
Our data demonstrated that downregulated RIPK1 expression in psoriasis neutrophils may enhance NET generation. RIPK1 may be identified as a novel therapeutic target in psoriasis.
Keywords: receptor-interacting protein kinase RIPK1, neutrophil, neutrophil extracellular traps, psoriasis
Introduction
Psoriasis is a chronic, immune-mediated, systemic disease characterized by skin lesions and other comorbidities [1]. The dysregulation of interplay between innate and adaptive immunity plays an essential role in the pathogenesis. Neutrophils are the most abundant cells in the innate immunity. They are enriched in psoriasis lesions and especially aggregate in Munro’s microabscesses, a hallmark of psoriasis histopathology [2]. Active neutrophil products in psoriasis, such as elastase and S100A8/A9, were reported to be high in skin lesions and peripheral blood [3–6]. Recently, the elevated neutrophil-to-lymphocyte ratio (NLR) was found to be correlated with disease severity and serum C-reactive protein (CRP) levels in psoriasis [7, 8]. The circulating low-density granulocytes (LDGs), a subset of neutrophils, were also increased in psoriasis and associated with the severity of the disease [9].
Neutrophils provide the first line of defence of immune response [10], and can undergo a distinct cell death process called NETosis, in which they form structures known as neutrophil extracellular traps (NETs) [11]. NETs are large, web-like structures that consist of decondensed DNA and granular proteins [12]. NETs have been shown to play a crucial role in infection, atherosclerosis [13] and autoimmune diseases including lupus [14], vasculitis [15, 16] and rheumatoid arthritis [17]. In psoriasis, NETs have been observed in the majority of psoriatic skin lesions from patients and imiquimod-induced mouse models [18, 19]. Furthermore, sera from psoriasis patients induced NETosis and the amount of NETs in blood sample were associated with psoriasis disease severity [19].
Receptor-interacting protein kinase RIPK1 and RIPK3 are members of the receptor-interacting serine/threonine-protein kinase family. They have emerged as key regulators of inflammation, apoptosis and necroptosis pathways [20]. Necroptosis, a form of regulated cell death, has main characteristics of apoptosis and necrosis. Necroptosis requires activation of RIPK1, RIPK3 and mixed lineage kinase domain-like (MLKL) phosphorylation. Inhibition of caspase-8 is also necessary for induction of necroptosis, which shift apoptosis to necroptosis [21]. In addition, PMA-induced NETosis may involve RIPK1-RIPK3-MLKL necroptosis signalling, which was inhibited in RIPK3-/- mice neutrophils [22]. Necroptosis was also reported to control NET generation in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [16]. A recent study has shown that epidermis-specific-RIPK1 knock out mice exhibited psoriasis-like skin lesions, with epidermal thickening and neutrophil infiltration. RIPK3-MLKL-depedent and RIPK1-independent necroptosis contributed to skin inflammation [23]. Abnormal expressions of RIPK1 and RIPK3 have been observed in human psoriatic skin lesions [24–26]. However, neither the expression of RIPK1/3 in peripheral blood nor the relationship between RIPK1/3 and neutrophil extracellular traps (NETs) in psoriasis have been fully investigated.
Aim
In order to explore whether RIPK1/3 plays a role in pathogenesis of psoriasis, this study investigates the levels of RIPK1 and RIPK3 in peripheral blood from psoriasis patients.
Material and methods
Patients and samples
Patients with psoriasis vulgaris of Renji Hospital participated in this study. The disease severity of psoriasis was evaluated by using the Psoriasis Area Severity Index (PASI). Healthy donors matched by age and sex to patients were enrolled from the Medical Examination Centre of the Renji Hospital. We first collected samples from 20 psoriasis patients and 20 healthy controls for flow cytometry testing. However, the disease severity of these patients was not evaluated. We next collected blood samples from another 17 patients and calculated the PASI score. The study was performed according to the Declaration of Helsinki, and the collection of samples was approved by the Ethics Committee of the Renji Hospital. Patient characteristics are shown in Tables 1–3.
Table 1.
Demographics of healthy donors and psoriasis patients in this study
| Parameter | Healthy donors (n = 37) |
Psoriasis (n = 37) |
|---|---|---|
| Male (%) | 54.05% (20) | 59.46% (22) |
| Age [years] Mean ± SD | 46.59 ±12.68 | 48.43 ±14.58 |
Table 2.
Information for patients with psoriasis (n = 17) for neutrophil mRNA detection
| Sample ID | Age/Gender | PASI |
|---|---|---|
| 21 | 70/M | 5.2 |
| 22 | 36/M | 24 |
| 23 | 41/M | 21 |
| 24 | 45/M | 4.2 |
| 25 | 78/F | 1.8 |
| 26 | 47/M | 5.6 |
| 27 | 63/M | 21 |
| 28 | 61/F | 14 |
| 29 | 54/F | 0.3 |
| 30 | 39/F | 0.1 |
| 31 | 33/M | 18.6 |
| 32 | 36/M | 11 |
| 33 | 38/M | 0.2 |
| 34 | 66/M | 34.2 |
| 35 | 24//M | 0.8 |
| 36 | 45/F | 2.4 |
| 37 | 28/M | 11.8 |
Table 3.
Information for patients with psoriasis (n = 11) for neutrophil PicoGreen detection
| Sample ID | Age/Gender | PASI |
|---|---|---|
| 21 | 70/M | 5.2 |
| 22 | 36/M | 24 |
| 23 | 41/M | 21 |
| 24 | 45/M | 4.2 |
| 25 | 78/F | 1.8 |
| 26 | 47/M | 5.6 |
| 27 | 63/M | 21 |
| 28 | 61/F | 14 |
| 29 | 54/F | 0.3 |
| 30 | 39/F | 0.1 |
| 31 | 33/M | 18.6 |
Whole blood processing and flow cytometry
Peripheral blood from healthy individuals (n = 20) and psoriasis patients (n = 20) were collected. 200 ml whole blood was treated with 1× red blood cell lysis solution (BD Biosciences, USA). The resulting cells were washed with phosphate buffered saline PBS (HyClone, USA) and then fixed with fixation/permeabilization solution (BD Biosciences, USA) for 20 min at 4°C. 1× perm/wash buffer (BD Biosciences, USA) was used for washing cells two times. Fixed/permeabilized cells were individually stained with rabbit IgG1 anti-human RIPK1 or RIPK3 monoclonal antibody (Abcam, USA) and incubated for 30 min at 4°C. Cells were washed two times with PBS and stained with secondary PerCP-conjugated anti-rabbit IgG1 monoclonal antibody (Bioss, China). After incubation for 30 min at 4°C, samples were washed and analysed on BD FACS Aria II (BD Biosciences, USA). FCS data were evaluated on FlowJo software (Becton, Dickinson and Company, USA).
Isolation of peripheral blood neutrophils
Blood neutrophils were purified from peripheral blood of healthy donors (n = 17) and psoriasis patients (n = 17) by density gradient centrifugation. In brief, peripheral blood mononuclear cells (PBMC) were separated by centrifugation with Lymphoprep (STEMCELL Technologies, Canada). The lower layer, consisting mainly of granulocytes and erythrocytes, was treated with 1× red blood cell lysis solution (BD Biosciences, USA). The remaining cells comprised > 95% neutrophils as confirmed by staining with anti-CD15 antibody staining (BD Biosciences, USA) and flow cytometry analysis. Purified neutrophils were used for RNA isolation or NET formation.
Quantitative real-time PCR
Total RNA extracted using TRIzol Reagent (Invitrogen, USA) and cDNA synthesis was applied for quantitative real-time PCR (RT-PCR) performed by Applied Biosystems Quantstudio 7 Flex (Applied Biosystems, USA). Primer sequences are as follows (5’->3’); RIPK1: Forward: TATCCCAGTGCCTGAGACCAAC; Reverse: GTAGGCTCCAATCTGAATGCCAG; RIPK3: Forward: ACTCCCGGCTTAGAAGGACT; Reverse: TCCTTT ACCGTGGAGACAGC; Mixed lineage kinase domain-like (MLKL): Forward: AAGAAGGTGGAAGAGCGA GC; Reverse: AGGACGATTCCAAAGACTGC; Caspase8: Forward: CTTCCTGCCTGCCTGTACC; Reverse: CGTGCCCAGAAAGTGGA; IL-1b: Forward: CCACAGACCTTCCAGGAGAA; Reverse: GTGATCGTACA GGTGCATCG; TNF-a: Forward: CAACCTCCTCTCTGCCATCAAGA; Reverse: CTGGAAGACCCCTCCC AGATAGA; GAPDH: Forward: CGCTGAGTACGTCGTGGAGTC; Reverse: GAGGCATTGCTGATGATC TTGAGG. Relative mRNA gene expression levels were performed by using the 2−ΔΔCt method and normalized to GAPDH.
NET generation
Purified neutrophils from healthy individuals (n = 12) and psoriasis patients (n = 11) were suspended in RPMI-1640 medium (Gibco, USA) at a density of 5 × 106 cells/ml and seeded into 6-well plates to settle for 30 min in a 5% carbon dioxide atmosphere at 37°C. Human neutrophils were exposed to 50 mM Nec-1 (Sigma-Aldrich, USA) for 30 min before being incubated with 10 ng/ml PMA (Sigma-Aldrich, USA) for 3 h. After centrifugation at 1500 rpm for 5 min, all cell supernatants were collected. 0.1 mM SYTOX Green dye (Life Technologies, USA) was added to detect cell death in 6 samples (3 psoriasis patients and 3 healthy donors).
Quantification of released dsDNA in culture supernatants
Released dsDNA was detected by adding 50 ml of PicoGreen (Invitrogen, USA) to 50 ml of cell supernatants. After 5 min, the relative fluorescence was read with excitation and emission wavelengths of 480 nm and 520 nm by using a fluorescence spectrometry (BioTek Gen5, USA). DNA concentration was calculated by standard curve.
Statistical analysis
In all figures, data are presented as mean ± SEM. The p-value was calculated with two-tailed Student’s t-test, and Pearson correlation was used in the correlation analysis. The statistical significance was calculated as follows: *p < 0.05, **p < 0.01, ***p < 0.001. All statistics were performed using GraphPad Prism 8 (GraphPad Software, USA).
Results
Decreased RIPK1 and RIPK3 expression in peripheral blood leukocytes
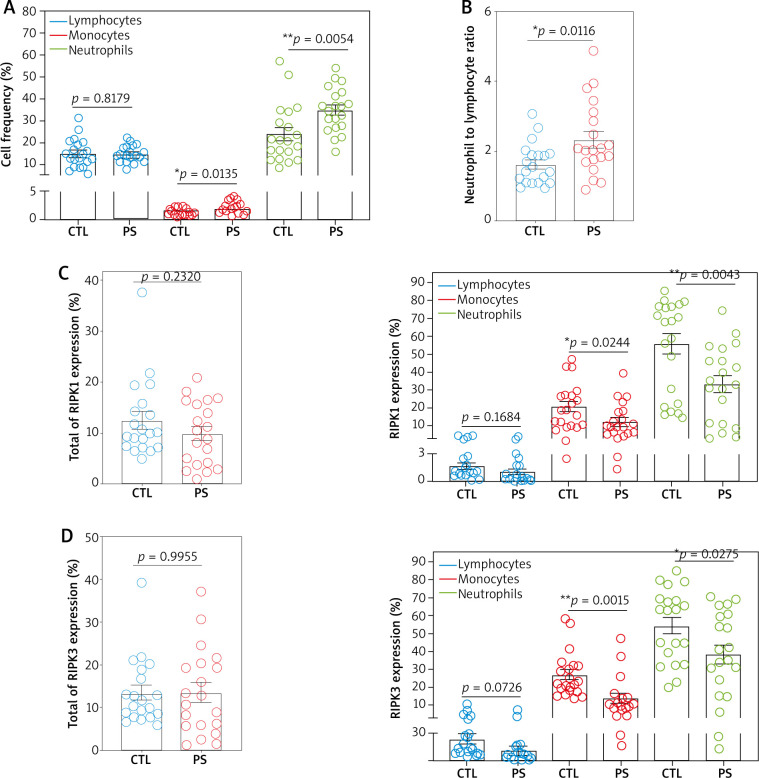
To clarify the expression of RIPK1 and RIPK3 in peripheral blood, we first performed flow cytometry for RIPK1 and RIPK3 in lymphocytes, monocytes and neutrophils from psoriasis patients and healthy controls. Patients had a higher frequency of neutrophils and monocytes than the controls (Figure 1 A). The neutrophil to lymphocyte ratio (NLR) was also significantly increased in patients (Figure 1 B). While the levels of RIPK1 and RIPK3 in whole blood had no difference, both RIPK1 and RIPK3 expression was significantly decreased in monocytes and neutrophils from psoriasis patients. Moreover, the expression levels of RIPK1 and RIPK3 were higher in neutrophils than those in monocytes and lymphocytes (Figures 1 C, D).
Figure 1.
The levels of RIPK1 and RIPK3 expression in peripheral blood leukocytes. A – The frequency of lymphocytes, monocytes and neutrophils were detected by flow cytometry in psoriasis patients (n = 20) and healthy controls (n = 20). B – Neutrophil-to-lymphocyte ratio (NLR) was calculated. C – RIPK1 expression in total of blood, lymphocytes, monocytes and neutrophils. D – RIPK3 expression in total of blood, lymphocytes, monocytes and neutrophils. E – Gating strategies for flow cytometry (FMO control is a sample not stained with RIPK1/3 antibody). Data are represented as mean ± SEM. Significance was established by two-tailed Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001.
CTL – healthy controls, PS – psoriasis patients.
RIPK1/3 leads to necroptosis and inflammation pathway in neutrophils
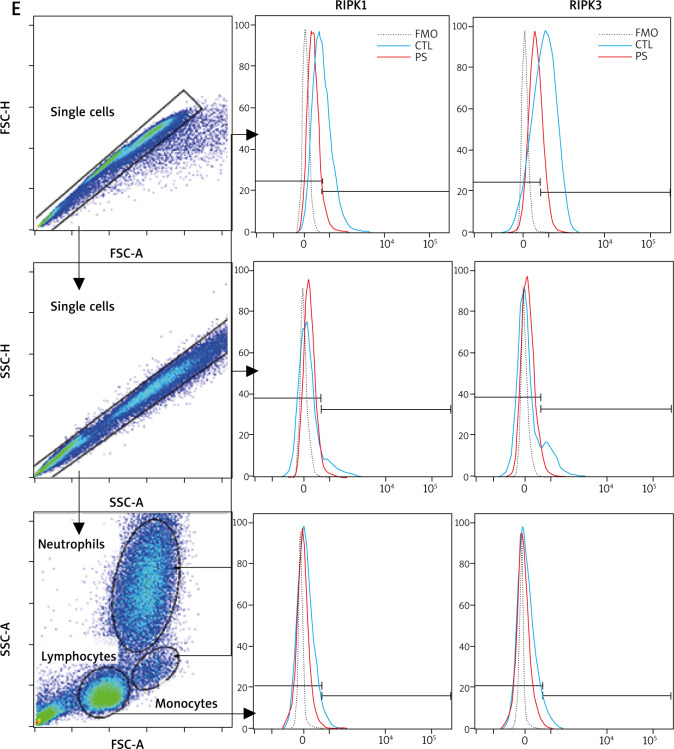
We detected mRNA expression of RIPK1, RIPK3, MLKL, Caspase8, IL-1β and TNF-α in human blood neutrophils. Consistent with the flow cytometry results, RIPK1 gene expression was decreased in psoriasis neutrophils. Unexpectedly, RIPK3 mRNA expression was increased in psoriasis neutrophils. Mixed lineage kinase domain-like (MLKL) expression was increased while caspase8 mRNA expression was inhibited, suggesting the dysregulated necroptosis may be involved in psoriasis neutrophils. The cytokines of IL-1β and TNF-α expressed by neutrophils were also significantly increased in psoriasis neutrophils (Figure 2).
Figure 2.
RIPK1 and RIPK3 expression in neutrophils. The mRNA levels of RIPK1, RIPK3, MLKL, Caspase8, IL-1b and TNF-a in neutrophils were compared between psoriasis patients (n = 17) and healthy controls (n = 17). Data are represented as mean ± SEM. Significance was established by two-tailed Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001
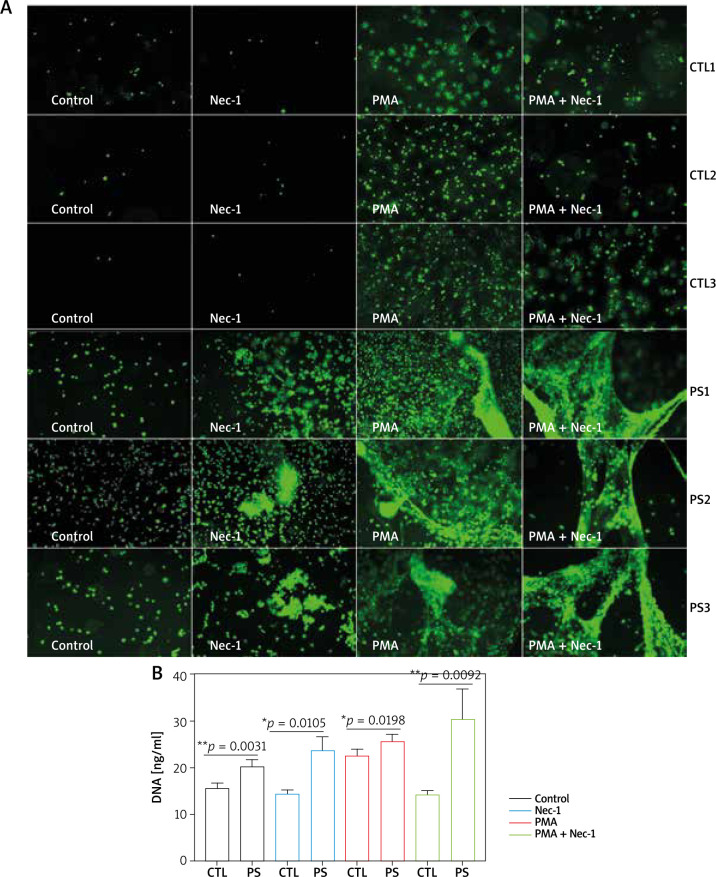
Necrostain-1 enhances NET formation in psoriasis
Neutrophils from psoriasis patients and healthy controls were subsequently stimulated with 50 mM Nec-1, which inhibits the activity of RIPK1. Nec-1 decreased NET formation and cell death in healthy controls. However, psoriasis neutrophils with Nec-1 enhanced NET formation in the same manner. Phorbol 12-myristate 13-acetate (PMA), a classic NETosis promoting agent, induced a higher count of NETs in psoriasis patients than healthy controls. Nec-1 inhibited PMA-induced NETosis in healthy neutrophils while enhanced it in psoriasis neutrophils (Figure 3 A). PicoGreen+ DNA release further verified those findings (Figure 3 B). Together, these data indicated that the inhibition of RIPK1 can significantly increase NET generation and cell death in psoriasis neutrophils.
Figure 3.
NET generation in neutrophils from psoriasis patients and healthy controls. A – Sytox green immunofluorescence 3 h after Nec-1, PMA and PMA in combination with Nec-1 exposure to neutrophils from psoriasis patients (n = 3) and healthy controls (n = 3). Images were shown at 10´ magnification. B – DNA release into the supernatant was quantified by PicoGreen reagent between psoriasis patients (n = 11) and healthy controls (n = 12). Data are represented as mean ± SEM. Significance was established by two-tailed Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001
RIPK1 expression in neutrophils was negatively associated with psoriasis disease activity
In patients with psoriasis, RIPK1 gene expression was negatively correlated with PASI score (r = –0.5039, p = 0.0196). However, there was no correlation between RIPK3 gene expression and PASI score (Figure 4 A). The frequency of RIPK1 and RIPK3 in neutrophils was found to be negatively correlated with NLR respectively (r = –0.4868, p = 0.0202; r = –0.4314, p = 0.0370) (Figure 4 B).
Figure 4.
The correlation between RIPK1/3 in neutrophils and psoriasis disease activity. A – The RIPK1 mRNA expression in neutrophils was associated with PASI score while RIPK3 mRNA had no association with PASI score in psoriasis patients (n = 17). B – The frequency of RIPK1 and RIPK3 in neutrophils correlated with NLR respectively in psoriasis patients (n = 18). The Pearson correlation coefficient was used in the correlation analysis. *p < 0.05, **p < 0.01, ***p < 0.001
PASI – psoriasis area severity index, NLR – neutrophil to lymphocyte ratio.
Discussion
In this study, we first showed the downregulation of RIPK1 and RIPK3 in monocytes and neutrophils from peripheral blood of psoriasis patients. We found that neutrophils from psoriasis patients might be involved in necroptosis pathways, supported by the data of RIPK1 and Caspase8 inhibition, with MLKL upregulation in the mRNA level. Moreover, Nec-1 was firstly found to induce NETosis in psoriasis neutrophils in vitro. Finally, we observed the correlation between RIPK1 and PASI score.
Necroptosis is a regulated inflammatory mode of cell death, in which RIPK1 and RIPK3 are involved. It triggers inflammation through damage-associated molecular pattern (DAMP) secretion, cytokine production and inflammasome activation [21]. RIPK1 is a key regulator of keratinocyte survival and inflammation in the skin via inhibiting RIPK3-MLKL-mediated necroptosis [23]. RIPK1 expression was decreased in the psoriasis skin lesions of human and imiquimod-induced mouse models. RIPK1-knockdown keratinocytes triggered the release of cytokines, including IL-1β, IL-6, IL-8 and TNF-α. TNF-related apoptosis-inducing ligand (TRAIL)-neutralization alleviated psoriasis-like phenotype via reversing inhibition of RIPK1 in imiquimod-induced mouse models [25]. Furthermore, the upregulation of RIPK3 was also observed in human psoriasis and imiquimod-induced psoriasis-like lesions. Compared to controls, RIPK3–/– mice in daily application of imiquimod also showed significantly decreased ear swelling response, cytokine secretion and infiltrated neutrophils [23, 24]. In our study, we first explored the role of RIPK1 and RIPK3 in peripheral blood leukocytes of psoriasis. In psoriasis circulating neutrophils, we demonstrated that RIPK1 expression was reduced both in mRNA and protein levels while RIPK3 expression was increased in the mRNA level and decreased in the protein level. These findings suggested that abnormal expression of RIPK1 and RIPK3 might affect neutrophil survival and cytokine secretion.
PMA-induced neutrophil-forming web-like structures were first described as neutrophil extracellular traps [27]. Whether NETosis is a form of necroptosis or a distinct cell death category remains controversial. Desai et al. [22] reported that Nec-1 and MLKL-inhibitor necrosulfonamide (NSA) decreased PMA-induced NET formation in human and mouse neutrophils after 2 h. RIPK3 expression and phosphorylation of MLKL were also detected after PMA stimulation of neutrophils. PMA-induced neutrophils from RIPK3–/– mice were more susceptible to undergo NETosis compared to RIPK3+/+ neutrophils. However, a previous study demonstrated that Nec-1 can increase NET formation in SLE patients [28]. Antineutrophil cytoplasmic antibody (ANCA) triggered NET formation by RIPK1-RIPK3-MLKL dependent necroptosis [16]. Our data showed that Nec-1 could decrease NET formation and PMA-induced NETosis in normal neutrophils but enhance those in psoriasis neutrophils after 3 h. These findings suggested that the essence of NET generation varied in types of the disease. We elucidated that NET formation in psoriasis was related to the abnormal expression of RIPK1.
NLR has been used as a marker to monitor systemic inflammatory response [29]. NLR was increased in psoriasis patients and correlated with PASI score in 46 chronic plaque psoriasis [7]. These findings are also consistent with our data. NLR was elevated in psoriasis and RIPK1/3 in neutrophils was negatively correlated with NLR. These results implied that the frequency of RIPK1 and RIPK3 in neutrophils were also parameters to monitor psoriasis. In conclusion, we provide a novel idea that downregulated RIPK1 expression in neutrophils may affect NET formation in psoriasis. RIPK1 may be a therapeutic target of psoriasis in the future.
There are potential limitations to this study. First, as a prospective study, we designed two independent psoriasis patient groups, including 20 patients for flow cytometry analysis and 17 patients for neutrophil mRNA detection. We cannot combine those two groups for further verification in our study. Second, our limited sample size emphasizes the importance of validation in a multicentre cohort. Moreover, due to the shortened neutrophil survival in vitro, we did not further explore the mechanism of RIPK1 in psoriasis neutrophils and future studies on animal models are needed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82001707) and Shanghai Sailing Program (20YF1425700).
Xinyu Meng and Ruru Guo contributed equally to this work.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers 2016; 2: 16083. [DOI] [PubMed] [Google Scholar]
- 2.Chowaniec O, Jablonska S, Beutner EH, et al. Earliest clinical and histological changes in psoriasis. Dermatologica 1981; 163: 42-51. [DOI] [PubMed] [Google Scholar]
- 3.Wiedow O, Wiese F, Christophers E. Lesional elastase activity in psoriasis. Diagnostic and prognostic significance. Arch Dermatol Res 1995; 287: 632-5. [DOI] [PubMed] [Google Scholar]
- 4.Örem A, Değer O, Çimşit G, et al. Plasma polymorphonuclear leukocyte elastase levels and its relation to disease activity in psoriasis. Clin Chim Acta 1997; 264: 49-56. [DOI] [PubMed] [Google Scholar]
- 5.Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and ne utrophil activation in a prospective observational study. Arterioscler Thromb Vascul Biol 2015; 35: 2667-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Huang K, Wu Y, et al. The expression of interleukin-22 and S100A7, A8, A9 mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technol 2007; 27: 605-7. [DOI] [PubMed] [Google Scholar]
- 7.Polat M, Bugdayci G, Kaya H, et al. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Turkish patients with chronic plaque psoriasis. Acta Dermatovenerol Alpina Pannon Adriat 2017; 26: 97-100. [DOI] [PubMed] [Google Scholar]
- 8.Sen BB, Rifaioglu EN, Ekiz O, et al. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol 2014; 33: 223-7. [DOI] [PubMed] [Google Scholar]
- 9.Teague HL, Varghese NJ, Tsoi LC, et al. Neutrophil subsets, platelets, and vascular disease in psoriasis. JACC Basic Transl Sci 2019; 4: 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascão R, Rosário HS, Fonseca JE. Neutrophils: warriors and commanders in immune mediated inflammatory diseases. Acta Reumatol Portug 2009; 34: 313-26. [PubMed] [Google Scholar]
- 11.Okubo K, Kamiya M, Urano Y, et al. Lactoferrin suppresses neutrophil extracellular traps release in inflammation. EBioMedicine 2016; 10: 204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018; 18: 134-47. [DOI] [PubMed] [Google Scholar]
- 13.Grayson PC, Kaplan MJ. At the bench: neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukocyte Biol 2016; 99: 253-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011; 187: 538-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15: 623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreiber A, Rousselle A, Becker JU, et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci USA 2017; 114: e9618-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demoruelle MK, Harrall KK, Ho L, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol 2017; 69: 1165-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao S, Fang H, Dang E, et al. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front Immunol 2019; 10: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu SC, Yu HS, Yen FL, et al. Neutrophil extracellular trap formation is increased in psoriasis and induces human beta-defensin-2 production in epidermal keratinocytes. Sci Rep 2016; 6: 31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Wang X. RIP kinases as modulators of inflammation and immunity. Nat Immunol 2018; 19: 912-22. [DOI] [PubMed] [Google Scholar]
- 21.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflam 2018; 15: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai J, Kumar SV, Mulay SR, et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol 2016; 46: 223-9. [DOI] [PubMed] [Google Scholar]
- 23.Dannappel M, Vlantis K, Kumari S, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014; 513: 90-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda T, Yamamoto O, Sawada Y, et al. Receptor-interacting protein kinase 3 controls keratinocyte activation in a necroptosis-independent manner and promotes psoriatic dermatitis in mice. J Allergy Clin Immunol 2017; 140: 619-22. [DOI] [PubMed] [Google Scholar]
- 25.Saito N, Honma M, Shibuya T, et al. RIPK1 downregulation in keratinocyte enhances TRAIL signaling in psoriasis. J Dermatol Sci 2018; 91: 79-86. [DOI] [PubMed] [Google Scholar]
- 26.Honda T, Kabashima K. Involvement of necroptosis in the development of imiquimod-induced psoriasis-like dermatitis (BA3P. 135). Am Assoc Immnol 2014; 192 (Suppl. 1): 44.5. [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532-5. [DOI] [PubMed] [Google Scholar]
- 28.Guo R, Tu Y, Xie S, et al. A role for receptor-interacting protein kinase-1 in neutrophil extracellular trap formation in patients with systemic lupus erythematosus: a preliminary study. Cell Physiol Biochem 2018; 45: 2317-28. [DOI] [PubMed] [Google Scholar]
- 29.Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]