Abstract
In a survey of resistance to amoxicillin among clinical isolates of Proteus mirabilis, 10 TEM-type β-lactamases were characterized: (i) the well-known penicillinases TEM-1 and TEM-2, the extended-spectrum β-lactamases (ESBLs) TEM-3 and TEM-24, and the inhibitor-resistant TEM (IRT) TEM-44 and (ii) five novel enzymes, a penicillinase TEM-57 similar to TEM-1, an ESBL TEM-66 similar to TEM-3, and three IRTs, TEM-65, TEM-73, and TEM-74. The penicillinase TEM-57 and the ESBL TEM-66 differed from TEM-1 and TEM-3, respectively, by the amino acid substitution Gly-92→Asp (nucleotide mutation G-477→A). This substitution could have accounted for the decrease in pIs (5.2 for TEM-57 and 6.0 for TEM-66) but did not necessarily affect the intrinsic activities of these enzymes. The IRT TEM-65 was an IRT-1-like IRT (Cys-244) related to TEM-2 (Lys-39). The two other IRTs, TEM-73 and TEM-74, were related to IRT-1 (Cys-244) and IRT-2 (Ser-244), respectively, and harbored the amino acid substitutions Leu-21→Phe and Thr-265→Met. In this study, the ESBLs TEM-66, TEM-24, and TEM-3 were encoded by large (170- to 180-kb) conjugative plasmids that exhibited similar patterns after digestion and hybridization with the TEM and AAC(6′)I probes. The three IRTs TEM-65, TEM-73, and TEM-74 were encoded by plasmids that ranged in size from 42 to 70 kb but for which no transfer was obtained. The characterization of five new plasmid-mediated TEM-type β-lactamases and the first report of TEM-24 in P. mirabilis are evidence of the wide diversity of β-lactamases produced in this species and of its possible role as a β-lactamase-encoding plasmid reservoir.
Proteus mirabilis is a species of the family Enterobacteriaceae commonly isolated in clinical laboratories during local and systemic infections; however, it has a predilection for the urinary tract (29). In studies performed at the Royal London Hospital in 1991 and, more recently, in French hospitals, P. mirabilis represented 15 and 8.3% of isolates of the family Enterobacteriaceae, respectively (21, 25). Thus, P. mirabilis is the second most often isolated species of the family Enterobacteriaceae, after Escherichia coli (64.6%) and before Klebsiella pneumoniae (5.9%) (25).
In hospitals the rate of amoxicillin resistance among P. mirabilis isolates is close to that among E. coli isolates (43 versus 46%) (25).
TEM penicillinases belong to Bush group 2b (7) and are the most common β-lactamases in P. mirabilis species. TEM-1 is the most common enzyme and is produced by 58.6% of penicillinase-producing P. mirabilis isolates. This species is distinguished by a high frequency of TEM-2 production (produced by 37.7% of penicillinase-producing P. mirabilis isolates) (9).
TEM-type extended-spectrum β-lactamases (ESBLs), which are members of group 2be (7) and which were initially observed in France in K. pneumoniae species (34), were also described in P. mirabilis strains by Mariotte et al. (24) in 1994. A study of the ESBLs produced by members of the family Enterobacteriaceae performed in Clermont-Ferrand, France, hospitals showed an increase in TEM-3 prevalence in P. mirabilis species between 1990 (0%; 0 of 338) and 1994 (6%; 15 of 244), making this enzyme the most often reported ESBL in the species (10). Since then, two other ESBLs, TEM-10 and TEM-26, have been characterized in P. mirabilis in the United States and South Africa, respectively (27, 28).
An inhibitor-resistant TEM (IRT), TEM-44 (IRT-13), which is a member of group 2br (7) and which is related to TEM-2, was recently observed in P. mirabilis (5).
In addition to the previously described TEM-1, TEM-2, TEM-3, TEM-24, and TEM-44 enzymes, which have been observed, five novel enzymes are described in this report.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Since 1996, amoxicillin-resistant strains of P. mirabilis isolated from patients hospitalized in different units of the teaching hospital of Clermont-Ferrand were screened for their resistance phenotypes: penicillinase, ESBL, and IRT producers. All ESBL and IRT enzymes and some penicillinases were studied by isoelectric focusing. One strain representative of each resistance phenotype and each isoelectric point value was retained for further analysis: three ESBL producers (CF39, CF249, and CF669), four IRT producers (CF449, CF659, CF739, and CF749), and one penicillinase producer (CF579). Penicillinase-producing strains P. mirabilis CF19 (TEM-1) and CF29 (TEM-2), isolated in 1994 at the Clermont-Ferrand hospital, were studied for comparison (blaTEM gene sequencing was performed). Rifampin- or nalidixic acid-resistant mutants of E. coli HB101 [supE44 hsdS20 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1] (30) and P. mirabilis ATCC 29906, obtained in vitro as described previously (30), were used as recipients during mating-out assays. Plasmids RSa (39.5 kb), TP114 (61 kb), pCFF04 (TEM-3-encoding plasmid of 85 kb) (34), pCFF74 (TEM-24-encoding plasmid of 85 kb) (11), pCFF14 (TEM-5-encoding plasmid of 180 kb) (11), and pCFF134 (TEM-3-encoding plasmid of K. pneumoniae CF34 isolated in our hospital) were used for comparison.
Mating-out assays and plasmid content.
Direct transfer of resistance into rifampin- or nalidixic acid-resistant strain E. coli HB101 or P. mirabilis ATCC 10381T was performed by overnight mating of logarithmic-phase cells at 37°C on drug-free liquid and solid Mueller-Hinton medium. Transconjugants were selected on Mueller-Hinton agar plates containing rifampin (300 μg/ml) or nalidixic acid (150 μg/ml) and either amoxicillin (100 μg/ml), ceftazidime (4 μg/ml), or cefotaxime (2 μg/ml).
The sizes of the plasmids were estimated after plasmid DNA extraction by the method of Kado and Liu (19), and their electrophoretic migrations in a 1% agarose gel were compared to those of standard plasmids.
The study of plasmid restriction fragments was performed with plasmid DNA that was extracted by the alkaline lysis method and cesium chloride-ethidium bromide equilibrium centrifugation (30) and that was digested with restriction endonucleases HindIII, EcoRI, and SalI (Boehringer Mannheim), as recommended by the manufacturer. Restriction fragments were visualized after electrophoresis in 0.8% agarose gels with a 1-kb DNA ladder (Eurogentec).
Hybridization.
The DNA probes used for hybridization were PCR products which were labeled with 32P as described previously (30). The specific amplification was achieved under standard conditions with the primers TEM-A and TEM-B (22) and the primers AAC6′-A (5′-TTGTTACGGTACCTTGCCTC-3′) and AAC6′-B (5′-TTGCAATGCTGAATGGAGAG-3′) (temperature of annealing, 58°C), which are specific for the gene that encodes the enzyme AAC(6′)I (23). Hybridization and autoradiography were performed as described previously (30) with DNA that had been transferred to and immobilized on Nytran filters.
Susceptibility to β-lactams.
MICs were determined by a dilution method on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) with an inoculum of 104 CFU per spot. Antibiotics were provided as powders by SmithKline Beecham Pharmaceuticals (amoxicillin, ticarcillin, and clavulanate), Lederle Laboratories (piperacillin, tazobactam), Eli Lilly, Paris, France (cephalothin), Roussel-Uclaf (cefotaxime), Glaxo Wellcome Research and Development (ceftazidime), and Bristol-Myers Squibb (aztreonam).
Detection of ESBLs was performed by a modified double-disk synergy test as described previously (35). It was performed by the disk diffusion method on Mueller-Hinton agar. Antibiotic disks for agar tests were obtained from Sanofi Diagnostics Pasteur.
Isoelectric focusing.
Isoelectric focusing was performed with polyacrylamide gels containing ampholines with a pH range of 3.5 to 10 as described previously (32). β-Lactamases with known pIs were used as standards: TEM-1 (pI 5.4), TEM-2 (pI 5.6), TEM-3 (pI 6.3), and TEM-24 (pI 6.5).
Determination of β-lactamase kinetic constants (Km, Vmax and IC50).
The Km and Vmax constants of the β-lactamases were obtained by the computerized microacidimetric method described previously (20) with enzymes purified as follows. Bacterial strains were obtained from 4 liters of culture grown in brain heart infusion broth. The cells were harvested by centrifugation at 5,800 × g for 30 min. The pellets (weight, about 20 g) were washed by resuspension in 40 ml of a 0.85 mM NaCl solution (solution A), and the suspension was centrifuged as described above, and the supernatants were discarded. Then, the pellets were resuspended in 40 ml of the same solution and lysed by ultrasonic treatment. The crude extracts were cleared by centrifugation at 48,000 × g for 30 min at 40°C and then by filtration on microgranular cellulose (Sigma). Nucleic acids were precipitated by the addition of spermine (0.2 M) and centrifugation (30,000 × g for 30 min at 4°C), dialyzed one night against 5 liters of solution A, and concentrated. The enzyme was then chromatographed on a Bio-Rex 70 resin (weakly acidic cation exchanger) with an ammonium carbonate-bicarbonate buffer (pH 7.0) gradient. Active fractions were pooled. The relative Vmax values were compared with the Vmax for benzylpenicillin, which was taken as 100%. The concentrations of the inhibitors (clavulanate and tazobactam) required to inhibit enzyme activity by 50% (IC50s) were determined as described previously for penicillin G (33).
ASPCR.
The allele-specific PCR (ASPCR) (40) was used to detect the known mutations at nucleotide positions 32, 317, 512, 692, 911, 914, 917, and 929. Templates of DNA from the studied strains and standard strains were simultaneously amplified in different tubes, in an allele-specific manner, in a Gene Amp PCR System 2004 DNA thermal cycler (Perkin-Elmer Cetus Instruments). All PCR products were loaded on an agarose gel. Thus, the presence of an amplified fragment with an allele-specific primer and its absence with the other allele-specific primer provide a direct confirmation of detection of genotypes.
DNA sequencing.
All results obtained by ASPCR were confirmed by DNA sequencing. The sequences were determined by direct sequencing of specific amplified products obtained as described previously (22) with genomic template DNA prepared by boiling fresh overnight cultures grown in Luria-Bertani broth or with plasmid DNA extracted by the procedure of Kado and Liu (19) and purified (spin-X column; Costar, Cambridge, Mass.). It was performed by the dideoxy chain termination procedure of Sanger et al. (31) on an ABI 1377 automatic sequencer with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit with Ampli-Taq DNA polymerase FS (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.).
RESULTS
Resistance phenotypes (MICs) for P. mirabilis strains.
The P. mirabilis isolates formed three groups in relation to the β-lactam MICs for the strains. (i) The first group was represented by three strains: the TEM-1- and TEM-2-producing strains CF19 and CF29 and strain CF579, which produced a penicillinase with an atypical pI (see below). These three strains were susceptible to ticarcillin-clavulanate (MIC, 2 μg/ml), piperacillin-tazobactam (MICs, 0.5 to 1 μg/ml), and cephalothin (MIC, 8 μg/ml). Strain CF579 harbored a level of resistance to β-lactams comparable to that observed for the TEM-2-producing strain CF29 (Table 1), suggesting higher levels of penicillinase production in strains CF29 and CF579 than in strain CF19.
TABLE 1.
β-Lactam MICs for TEM-producing P. mirabilis strains
| β-Lactam | MIC (μg/ml) for the following strains (enzymes):
|
||
|---|---|---|---|
| CF19 (TEM-1) | CF29 (TEM-2) | CF579 (TEM-57) | |
| Amoxicillin | 512 | 4,096 | 4,096 |
| Amoxicillin + CAa | 4 | 16 | 16 |
| Ticarcillin | 128 | 512 | 512 |
| Ticarcillin + CA | 2 | 2 | 2 |
| Piperacillin | 8 | 256 | 256 |
| Piperacillin + TAZb | 0.5 | 1 | 1 |
| Cephalothin | 8 | 8 | 8 |
CA, clavulanic acid (2 μg/ml).
TAZ, tazobactam (4 μg/ml).
(ii) The second group consisted of P. mirabilis CF39, CF249, and CF669. The modified double-disk synergy test was positive. Their β-lactam resistance phenotypes were characterized by high levels of resistance to amoxicillin (MIC, 1,024 μg/ml), ticarcillin (MIC, 256 μg/ml), and cephalothin (MIC, 32 μg/ml) (data not shown). The MICs of cefotaxime, ceftazidime, and aztreonam (Table 2), even though they were low, were appreciably higher than those obtained for TEM-producing P. mirabilis (0.06 to 8 versus 0.03 to 0.12 μg/ml). The MICs observed for E. coli transconjugants were higher than those observed for P. mirabilis (2- to 30-fold) except for that for strain CF669 (Table 2). Clavulanate (2 μg/ml) restored the MICs of cefotaxime, ceftazidime, and aztreonam (MICs, ≤0.25 μg/ml).
TABLE 2.
β-Lactam MICs for ESBL-producing P. mirabilis strains and their E. coli transconjugants
| β-Lactams | MIC (μg/ml) for the following strains (enzymes):
|
|||||
|---|---|---|---|---|---|---|
| P. mirabilis CF249 (TEM-24) | E. coli TrCF249 (TEM-24) | P. mirabilis CF39 (TEM-3) | E. coli TrCF39 (TEM-3) | P. mirabilis CF669 (TEM-66) | E. coli TrCF669 (TEM-66) | |
| Cefotaxime | 0.12 | 1 | 2 | 4 | 8 | 4 |
| Cefotaxime + CAa | 0.03 | 0.03 | 0.06 | 0.06 | 0.06 | 0.03 |
| Ceftazidime | 4 | 64 | 0.5 | 8 | 8 | 4 |
| Ceftazidime + CA | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 | 0.12 |
| Aztreonam | 0.06 | 4 | 0.06 | 2 | 0.25 | 2 |
| Aztreonam + CA | 0.01 | 0.03 | 0.01 | 0.06 | 0.06 | 0.003 |
CA, clavulanic acid (2 μg/ml).
(iii) P. mirabilis CF449, CF659, CF739, and CF749 constituted the third group (Table 3). They were characterized by similar high levels of resistance to amoxicillin and amoxicillin-clavulanate (MICs, 64 to 1,024 μg/ml) and low levels of resistance to ticarcillin (MICs, 1 to 32 μg/ml). These strains were susceptible to ticarcillin-clavulanate (MICs, 1 to 4 μg/ml) and were mainly susceptible to cephalosporins, including cephalothin (MICs, 2 to 4 μg/ml). These phenotypes of resistance to β-lactam antibiotics differed from those observed for TEM-1- and TEM-2-producing strains CF19 and CF29 by the higher MICs of amoxicillin-clavulanate (4 to 16 μg/ml) and lower MICs of ticarcillin (128 to 512 μg/ml) and, to a lesser extent, the MICs of cephalothin (8 μg/ml) (Table 1).
TABLE 3.
β-Lactam MICs for IRT-producing P. mirabilis strains
| β-Lactam | MIC (μg/ml) for the following strains (enzymes):
|
|||
|---|---|---|---|---|
| CF739 (TEM-73 [IRT-18]) | CF749 (TEM-74 [IRT-19]) | CF449 (TEM-44 [IRT-13]) | CF659 (TEM-65 [IRT-16]) | |
| Amoxicillin | 1,024 | 2,048 | 1,024 | 64 |
| Amoxicillin + CAa | 1,024 | 512 | 512 | 64 |
| Ticarcillin | 8 | 32 | 16 | 1 |
| Ticarcillin + CA | 4 | 4 | 2 | 1 |
| Piperacillin | 16 | 16 | 8 | 2 |
| Piperacillin + TAZb | 4 | 1 | 0.5 | 0.5 |
| Cephalothin | 2 | 4 | 4 | 2 |
CA, clavulanic acid (2 μg/ml).
TAZ, tazobactam (4 μg/ml).
β-Lactamase characterization.
For the first group of penicillinases, the β-lactamase of strain CF579 had kinetic parameters (Km and Vmax) similar to those obtained with TEM-1 and TEM-2 (Tables 4 and 5). These three enzymes had distinctly higher affinities (Km, ≤40 μM) for penicillins than those obtained for cephalothin (≥250 μM). The relative Vmax values for ticarcillin and cephalothin (≤25%) were clearly decreased compared to that for amoxicillin (≥73%). However, a band at pI 5.2 that was distinct from those of TEM-1 (pI 5.4) and TEM-2 (pI 5.6) was observed for strain CF579 (Table 4).
TABLE 4.
pIs and kinetic parameters inhibitors for TEM-type and IRT-type β-lactamases
| Drug | Kinetic parameters for the following strains (enzymes; pIs):
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF19 (TEM-1; 5.4)
|
CF29 (TEM-2; 5.6)
|
CF579 (TEM-57; 5.2)
|
CF739 (TEM-73 [IRT-18]; 5.2)
|
CF749 (TEM-74 [IRT-19]; 5.2)
|
CF449 (TEM-44 [IRT-13]; 5.4)
|
CF659 (TEM-65 [IRT-16]; 5.4)
|
||||||||
| Vmaxa | Km (μM) | Vmax | Km (μM) | Vmax | Km (μM) | Vmax | Km (μM) | Vmax | Km (μM) | Vmax | Km (μM) | Vmax | Km (μM) | |
| Benzylpenicillin | 100 | 25 | 100 | 20 | 100 | 40 | 100 | 334 | 100 | 330 | 100 | 375 | 100 | 350 |
| Amoxicillin | 77 | 26 | 95 | 25 | 73 | 38 | 207 | 315 | 140 | 400 | 145 | 360 | 150 | 400 |
| Ticarcillin | 10 | 10 | 10 | 9 | 10 | 15 | 2 | 810 | 2 | 765 | 2.3 | 460 | 2 | 600 |
| Cephalothin | 10 | 250 | 25 | 500 | 17 | 900 | 1.5 | 1,000 | 1 | 300 | 2 | 312 | 2 | 400 |
Vmax values are given as a percentage of the Vmax for benzylpenicillin, which was taken as 100%.
TABLE 5.
IC50s of inhibitors for TEM-type and IRT-type β-lactamases
| Strain (enzyme) | IC50 (μM)
|
|
|---|---|---|
| Clavulanate | Tazobactam | |
| CF19 (TEM-1) | 0.18 | 0.05 |
| CF29 (TEM-2) | 0.09 | 0.04 |
| CF579 (TEM-57) | NDa | ND |
| CF739 (TEM-73 [IRT-18]) | 31 | 12 |
| CF749 (TEM-74 [IRT-19]) | 9.4 | 2.9 |
| CF449 (TEM-44 [IRT-13]) | 10 | 3.0 |
| CF65a [TEM-65 [IRT-16]) | 9.0 | 3.0 |
ND, not done.
The ESBL-type β-lactamases of the second group had much greater relative Vmax values (40 to 1,410%) and had lower affinities (Kms, 50 to 450 μM) for broad-spectrum cephalosporins than for amoxicillin and cephalothin (Kms, 5 to 43 μM; Table 6). The enzyme of strain CF249 (pI 6.5) had a Vmax value 10-fold higher for ceftazidime than for cefotaxime. Conversely, the β-lactamases of strains CF39 (pI 6.3) and CF669 (pI 6.0) had Vmax values 10-fold higher for cefotaxime than for ceftazidime (Table 6).
TABLE 6.
pI and kinetic parameters for ESBL-type β-lactamases
| Drug | Kinetic parameters for the following strains (enzymes; pIs):
|
|||||
|---|---|---|---|---|---|---|
| CF249 (TEM-24; 6.5)
|
CF39 (TEM-3; 6.3)
|
CF669 (TEM-66; 6.0)
|
||||
| Vmaxa | Km (μM) | Vmax | Km (μM) | Vmax | Km (μM) | |
| Benzylpenicillin | 100 | 5 | 100 | 4 | 100 | 5 |
| Amoxicillin | 62 | 43 | 72 | 5 | 70 | 5 |
| Cephalothin | 281 | 43 | 107 | 22 | 110 | 30 |
| Cefotaxime | 130 | 50 | 445 | 100 | 400 | 100 |
| Ceftazidime | 1,410 | 377 | 40 | 400 | 40 | 450 |
Vmax values are given as a percentage of the Vmax for benzylpenicillin, which was taken as 100%.
The Vmax values of β-lactamases of strains of the third group (Table 4) for amoxicillin were higher (≥140%) than those of the TEM penicillinases (≤95%) (Table 4). Conversely, the Vmax values of enzymes of the third group for ticarcillin and cephalothin were lower (≤2.3%) than those of TEM enzymes (≥10%). With regard to the Km values of TEM-1, the affinities of these enzymes were decreased about 10-fold for benzylpenicillin and amoxicillin and 26- to 74-fold for ticarcillin (Table 4). Clavulanate and tazobactam IC50s were 50- to 200-fold higher for β-lactamases of strains in this group than for penicillinases TEM-1 and TEM-2 (Table 5). Isoelectric focusing showed that the β-lactamases of these strains focused at pI 5.2 (strains CF739 and CF749) or pI 5.4 (strains CF449 and CF659).
DNA sequencing.
To confirm the mutations detected by ASPCR, the complete sequences of the blaTEM genes were obtained. Globally, the sequences were similar to those of blaTEM-1A, blaTEM-1B, and blaTEM-2. The nucleotide substitutions and deduced amino acid replacements in relation to the sequence of the blaTEM-1A gene are summarized in Table 7. The blaTEM genes of strains CF39, CF249, and CF449 code for previously described β-lactamases: TEM-3 (CTX-1), TEM-24 (CAZ-6), and TEM-44 (IRT-13), respectively.
TABLE 7.
Nucleotide and amino acid substitutions in the blaTEM genes from P. mirabilis strains
| β-Lactamase and strain or enzyme | Mutation at the following nucleotide (substituted amino acid)a:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32b | 226 (8) | 263 (21) | 317 (39) | 346 (48) | 436 (78) | 477 (92) | 512 (104) | 604 (134) | 682 (160) | 692 (164) | 911 (237) | 914 (238) | 917 (240) | 925 (242) | 929 (244) | 990 (265) | |
| TEM-like | |||||||||||||||||
| TEM-1 (blaTEM-1A)c | GCC-TA | C (Phe) | C (Leu) | C (Gln) | A (Glu) | C (Gly) | G (Gly) | G (Glu) | G (Ala) | T (Thr) | C (Arg) | G (Ala) | G (Gly) | G (Glu) | G (Gly) | C (Arg) | C (Thr) |
| TEM-1 (blaTEM-1B)c | GCC-TA | T | T | T | |||||||||||||
| TEM-2 (blaTEM-2)c | GCT-TA | A (Lys) | G | T | C | A | |||||||||||
| CF579 (TEM-57) | GCTATA | G | T | A (Asp) | C | A | |||||||||||
| ESBL | |||||||||||||||||
| CF249 (TEM-24) | GCT-TA | A (Lys) | G | T | A (Lys) | C | A (Ser) | A (Thr) | A (Lys) | A | |||||||
| CF39 (TEM-3) | GCT-TA | A (Lys) | G | T | A (Lys) | A (Ser) | A | ||||||||||
| CF669 (TEM-66) | GCT-TA | A (Lys) | G | T | A (Asp) | A (Lys) | A (Ser) | ||||||||||
| IRT-like | |||||||||||||||||
| CF449 (TEM-44 [IRT-13]) | GCT-TA | A (Lys) | G | T | C | A | A (Ser) | ||||||||||
| CF659 (TEM-65 [IRT-16]) | GCT-TA | A (Lys) | G | C | A | T (Cys) | |||||||||||
| CF739 (TEM-73 [IRT-18]) | GCT-TA | T (Phe) | C | G | T | C | A | T (Cys) | T (Met) | ||||||||
| CF749 (TEM-74 [IRT-19]) | GCT-TA | T (Phe) | C | G | T | C | A | A (Ser) | T (Met) | ||||||||
(i) In the first group of strains (TEM-like penicillinase production), the blaTEM gene of the strain CF579 harbored the nucleotide change C-477→A, which led to the new amino acid substitution Gly-92→Asp. This enzyme is therefore a new TEM-like β-lactamase, which we designate TEM-57.
(ii) In the second group, the blaTEM gene of ESBL-producing strain CF669 exhibited the same C-477→A mutation and, in addition, nucleotide changes at positions 317, 512, and 914, which led to amino acid substitutions at positions 39, 104, and 238, as in TEM-3. The enzyme produced by CF669 is therefore a new TEM-3-like ESBL, which we designate TEM-66.
(iii) In the third group, the blaTEM genes of IRT-producing strains CF739 and CF749 harbored mutations that led to the presence of Gln-39, as in TEM-1, and, in particular, the amino acids Phe-21 and Met-265. However, these two enzymes differed at position 244 by a Ser, as in IRT-2 (strain CF749), and a Cys, as in IRT-1 (strain CF739). These enzymes are therefore two new IRT-type β-lactamases, which we designate TEM-74 (IRT-2-like) and TEM-73 (IRT-1-like), respectively. For the other IRT-producing strain, strain CF659, the deduced amino acid sequence exhibited Lys-39, as in TEM-2, and Cys-244, as in IRT-1. This β-lactamase is therefore the first IRT-1-like enzyme related to TEM-2 and is named TEM-65 (IRT-16).
Known silent mutations at positions 346, 436, 604, 682, and 925 were also observed in the studied genes (Table 7). The gene sequences of TEM-24, TEM-44 (IRT-13), TEM-57, TEM-73, and TEM-74 showed a pattern of silent mutations identical to that in the sequence of gene blaTEM-2. The blaTEM genes of strains CF39, CF659, and CF669 differed from blaTEM-2, but only by nucleotide 436 for the gene of TEM-65, nucleotide 682 for the gene of TEM-3, and nucleotides 682 and 925 for the gene of TEM-66.
The results for position 32 and the sequences of blaTEM gene promoters obtained by ASPCR showed the presence of a T base at position 32 in all genes studied. The insertion of an A base immediately under this position was observed in the blaTEM gene promoter of strain CF579 (Table 7).
Plasmid study.
The mating-out assays permitted the transfer of ESBL-type resistance (strains CF39, CF249, and CF669) in E. coli and P. mirabilis species. The other antibiotics to which resistance was transferred were identical for these three strains and for TEM-3-producing strain K. pneumoniae CF34: kanamycin, tobramycin, amikacin, netilmicin, tetracyclines, trimethoprim, and sulfonamides. In contrast, no transconjugant was obtained under our conditions with the TEM-like- and IRT-like-producing strains.
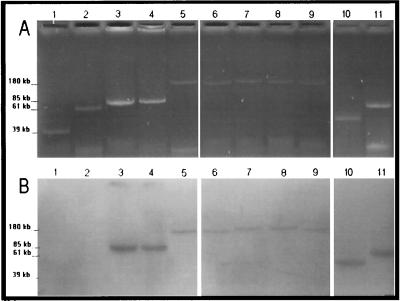
Plasmid visualization in agarose gels and hybridization with the TEM probe of ESBL-producing transconjugants showed one plasmid of about 170 to 180 kb for each of the strains P. mirabilis CF39, CF249, and CF669 and K. pneumoniae CF34 (Fig. 1). The digestion of these four plasmids and the hybridization with the TEM and AAC(6′)I probes showed a fragment of 11 kb that was common with the EcoRI-SalI digestion patterns. In the same way, two fragments of 11.5 and 13 kb were observed to be in common with the EcoRI-HindIII digestion patterns for all plasmids except the plasmid of strain CF39, which did not provide the 13-kb hybridized fragment (data not shown).
FIG. 1.
(A) Agarose (0.7%) electrophoresis of plasmid DNA from ESBL-producing E. coli transconjugants and from IRT-producing P. mirabilis. (B) Hybridization with TEM-specific DNA probes. Lanes: 1, plasmid RSa (39 kb); 2, plasmid TP114 (61 kb); 3, pCFF04 (TEM-3; 85 kb); 4, pCFF74 (TEM-24; 85 kb); 5, pCFF14 (TEM-5; 180 kb); 6, pCFF134 (TEM-3); 7, TrCF669 (TEM-66); 8, TrCF39 (TEM-3); 9, TrCF249 (TEM-24); 10, CF449 (TEM-44); 11, CF659 (TEM-65).
The study of the plasmids of IRT-producing P. mirabilis strains showed for each strain the presence of a plasmid that hybridized with the TEM probe. The plasmid was about 42 kb for strains CF449, CF739, and CF749 and about 70 kb for strain CF659 (Fig. 1).
DISCUSSION
This work reports on five novel β-lactamases and provides the first description of TEM-24 in P. mirabilis. TEM-57 (CF579) is a novel penicillinase related to TEM-1 (Gln-39), which is characterized by the Gly→Asp substitution at position 92 and which constitutes a new polymorphic position of TEM-type enzymes. TEM-66 (CF669) is a novel ESBL that harbors the amino acid substitutions that have been described for TEM-3 (36) and that are associated with the substitution Gly-92→Asp, as in TEM-57.
The substitution at position 92 of the neutral glycine residue in TEM-1 and TEM-3 by the negatively charged aspartate residue in TEM-57 and TEM-66 could have accounted for the decrease in pI from 5.4 (TEM-1) to 5.2 (TEM-57) and from 6.3 (TEM-3) to 6.0 (TEM-66). Crystallographic analysis (18) shows that amino acid 92, located in the loop that joins helices H2b and H3, is far from the active site of the enzyme. Hence, it probably has no effect on the intrinsic activity of the enzyme, as suggested by the kinetic constants, which were similar to those of TEM-1 for TEM-57 and similar to those of TEM-3 for TEM-66. Although TEM-57 and TEM-66 had the same substitution, Gly-92→Asp, it seems unlikely that the latter derives from the former. Hence, nucleotide position 477, at which a nucleotide substitution led to the amino acid substitution at position 92, could be a potential hot spot of mutation.
TEM-65 (IRT-16) (CF659) is a novel IRT-1-type (Cys-244) (38) β-lactamase. This enzyme is related to TEM-2 (Lys-39), as in the previously described IRT-2-type (Ser-244) (38) enzyme TEM-44 (5). TEM-73 (IRT-18) (CF739) and TEM-74 (IRT-19) (CF749) are two novel IRT enzymes that harbor both the substitutions Leu-21→Phe and Thr-265→Met that were previously reported in TEM-like and ESBL β-lactamases. These two enzymes differ by the amino acid substitution at position 244: Cys in TEM-73 (like IRT-1) and Ser in TEM-74 (like IRT-2).
The study of nucleotide substitutions at positions 32, 226, 263, 317, 346, 436, 604, 682, and 925 of the TEM-24 and TEM-44 genes showed a nucleotide pattern identical to that of gene blaTEM-2. The nucleotide sequence patterns of the other genes differed from that of gene blaTEM-2 by only one or two nucleotides at position 317, 682, or 925. These other genes could therefore be considered TEM-2-like genes (8). The blaTEM genes described here could result from a complex evolutionary process that originated in gene blaTEM-2 and/or could reflect the diversity of the genes that encode the TEM penicillinases.
For all enzymes reported, the nucleotide sequence showed a T at position 32, as observed in strong promoters (12). Despite these strong promoters, the IRTs in P. mirabilis conferred low levels of resistance to ticarcillin compared to the levels of resistance of E. coli strains (32). Likewise, ESBL expression was weak, requiring a modified synergy test for routine detection (35). When the ESBL-encoding plasmids of P. mirabilis were transferred to E. coli, the level of resistance to broad-spectrum cephalosporins was higher in E. coli than in P. mirabilis, suggesting the existence of a factor that leads to weak expression of β-lactam resistance, despite the presence of a strong promoter. The reasons for this discrepancy are not known. It could be the result, as suggested by Wu et al. (41), of regulational phenomena such as mRNA transcription attenuation, protease, or export problems. The promoter of TEM-57 is singular in that it has an adenine immediately under the thymidine at position 32. This insertion may not affect the strength of the promoter that directs the enzyme gene since a level of resistance similar to that conferred by TEM-2 was observed.
The IRT-encoding plasmids were not transferable by conjugation under our conditions and were distinctly smaller (42 to 70 kb) than ESBL-encoding plasmids (170 to 180 kb), which can easily conjugate. Thus, ESBL-producing P. mirabilis could be a reservoir of β-lactamase-encoding plasmids. In contrast, IRT-producing P. mirabilis, as suggested previously for E. coli strains (16), may have been selected after mutations of the TEM gene as a result of pressure from penicillin-inhibitor associations instead of the acquisition of IRT-encoding plasmids.
In P. mirabilis strains, TEM-3, TEM-24, and TEM-66 were encoded by large plasmids which were similar in size, which were associated with similar resistance markers, and which had similar hybridization patterns. The same plasmid was observed in TEM-3-producing K. pneumoniae CF34, whereas previously it was reported that TEM-3 and TEM-24 are encoded by 85-kb plasmids (13, 17, 34).
In our hospital, a 2-year survey (1997 to 1998) revealed that 14.3% of the amoxicillin-resistant P. mirabilis strains produce an ESBL identified as TEM-3 in 73 of 74 isolates (9). In the present study, ESBLs TEM-24 and TEM-66 were encountered for the first time in this species. The findings of TEM-10 (27), TEM-26 (28), TEM-8 (26), and TEM-21 (14) in previous studies and our results show the great diversity of ESBLs in P. mirabilis species.
Two IRTs related to TEM-2, TEM-44 (IRT-13) (5) and TEM-65 (IRT-16), have been found in P. mirabilis. The frequency of TEM-2 in P. mirabilis is high: 32.7% of penicillinase-producing strains (9). This high frequency could explain why the two IRTs derived from TEM-2 have been characterized in the species.
The description of TEM-24 in P. mirabilis and the characterization of five new TEM mutants, TEM-57 (TEM-like), TEM-66 (ESBL), and TEM-65, TEM-73, and TEM-74 (IRTs), show the diversity of TEM mutants in P. mirabilis. The chromosomal β-lactamase CMY-3 (6) and the plasmid-mediated β-lactamases CEP-1 (4), CTX-M-2 (3), PER-2 (2), and CMY-4 (39) have also been observed in this species. This diversity of β-lactamases associated with the high frequency of resistance to β-lactam antibiotics raises the fear that P. mirabilis-resistant strains could become entrenched in hospitals and could be involved in nosocomial infections, in which case surveillance of this species for susceptibility to β-lactam antibiotics would be warranted.
ACKNOWLEDGMENTS
We thank Rolande Perroux, Marlène Jan, and Dominique Rubio for technical assistance.
This work was supported in part by a grant from the Direction de la Recherche et des Etudes Doctorales of the Ministère de l’Education Nationale of France.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frere J-M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind A, Stemplinger I, Jungwirth R, Mangold P, Amann S, Akalin E, Ang Ö, Bal C, Casellas J M. Characterization of β-lactamase gene blaPER-2 which encodes an extended-spectrum class A β-lactamase. Antimicrob Agents Chemother. 1996;40:616–620. doi: 10.1128/aac.40.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequence of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequence with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobrowski M M, Matthew M, Barth P T, Datta N, Grinter N J, Jacob A E, Kontomichalou P, Dale J W, Smith J T. Plasmid-determined beta-lactamase indistinguishable from the chromosomal beta-lactamase of Escherichia coli. J Bacteriol. 1976;125:149–157. doi: 10.1128/jb.125.1.149-157.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bret L, Chanal C, Sirot D, Labia R, Sirot J. Characterization of an inhibitor-resistant enzyme IRT-2 derived from TEM-2 β-lactamase produced by Proteus mirabilis strains. J Antimicrob Chemother. 1996;38:183–191. doi: 10.1093/jac/38.2.183. [DOI] [PubMed] [Google Scholar]
- 6.Bret L, Chanal-Claris C, Sirot D, Chaibi E B, Labia R, Sirot J. Chromosomally encoded AmpC-type β-lactamase in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 1998;42:1110–1114. doi: 10.1128/aac.42.5.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1213. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canica M M, Lu C L, Krishnamoorthy R, Paul G. Molecular diversity and evolution of blaTEM genes encoding β-lactamases resistant to clavulanic acid in clinical E. coli. J Mol Evol. 1997;44:57–65. doi: 10.1007/pl00006121. [DOI] [PubMed] [Google Scholar]
- 9.Chanal C, Bonnet R, De Champs C, Romaszko J P, Sirot D, Sirot J. Surveillance de la resistance aux β-lactamases et diversite moleculaire des enzymes de type penicillinase chez Proteus mirabilis. In Program and abstracts of the 18th Interdisciplinary Meeting on Anti-Infections Chemotherapy. Paris, France: Société Française de Microbiologie; 1998. [Google Scholar]
- 10.Chanal C, Sirot D, Romaszko J P, Bret L, Sirot J. Survey of prevalence of extended spectrum β-lactamases among Enterobacteriaceae. J Antimicrob Chemother. 1996;38:127–132. doi: 10.1093/jac/38.1.127. [DOI] [PubMed] [Google Scholar]
- 11.Chanal C M, Sirot D L, Petit A, Labia R, Morand A, Sirot J L, Cluzel R A. Multiplicity of TEM-derived β-lactamases from Klebsiella pneumoniae strains isolated at the same hospital and relationships between the responsible plasmids. Antimicrob Agents Chemother. 1989;33:1915–1920. doi: 10.1128/aac.33.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S T, Clowes R C. Variation between the nucleotide sequences of Tn1 and Tn3 and expression of β-lactamase in Pseudomonas aeruginosa and Escherichia coli. J Bacteriol. 1987;169:913–926. doi: 10.1128/jb.169.2.913-916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Champs C, Sirot D, Chanal C, Poupart M C, Dumas M P, Sirot J. Concomitant dissemination of three extended-spectrum β-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991;27:441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein F W, Pean Y, Rosato A, Gertner J, Gutmann L the Vigil’Roc Study Group. Characterization of ceftriaxone-resistant Enterobacteriaceae: a multicentre study in 26 French hospitals. J Antimicrob Chemother. 1993;32:595–603. doi: 10.1093/jac/32.4.595. [DOI] [PubMed] [Google Scholar]
- 15.Goussard S, Courvalin P. Sequence of the genes blaT-1B and blaT-2. Gene. 1991;102:71–73. doi: 10.1016/0378-1119(91)90540-r. [DOI] [PubMed] [Google Scholar]
- 16.Henquell C, Sirot D, Chanal C, De Champs C, Chatron P, Lafeuille B, Texier P, Sirot J, Cluzel R. Frequency of inhibitor-resistant TEM β-lactamases in Escherichia coli isolates from urinary tract infections in France. J Antimicrob Chemother. 1994;34:707–714. doi: 10.1093/jac/34.5.707. [DOI] [PubMed] [Google Scholar]
- 17.Jacoby G A, Sutton L. Properties of plasmids responsible for production of extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelsch C, Monrey L, Masson J M, Samama J P. Crystal structure of Escherichia coli TEM-1 beta-lactamase at 1.8 Å resolution. Proteins. 1993;16:364–383. doi: 10.1002/prot.340160406. [DOI] [PubMed] [Google Scholar]
- 19.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labia R, Andrillon J, Le Goffic F. Computerized microacidimetric determination of β-lactamase Michaelis-Menten constants. FEBS Lett. 1973;33:42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu P Y F, Gur D, Hall L M C, Livermore D M. Survey of the prevalence of β-lactamases amongst 1000 gram-negative bacilli isolated consecutively at the Royal London Hospital. J Antimicrob Chemother. 1992;30:429–447. doi: 10.1093/jac/30.4.429. [DOI] [PubMed] [Google Scholar]
- 22.Mabilat C, Goussard S, Sougakoff W, Spencer R C, Courvalin P. Direct sequencing of the amplified structural gene and promoter for the extended broad-spectrum β-lactamase TEM-9 (RHH-1) of Klebsiella pneumoniae. Plasmid. 1990;23:27–34. doi: 10.1016/0147-619x(90)90041-a. [DOI] [PubMed] [Google Scholar]
- 23.Mabilat C, Lourencao-Vital J, Goussard S, Courvalin P. A new example of physical linkage between Tn1 and Tn21: the antibiotic multiple resistance region of plasmid pCFF04 encoding extended-spectrum beta-lactamase TEM-3. Mol Gen Genet. 1992;235:113–121. doi: 10.1007/BF00286188. [DOI] [PubMed] [Google Scholar]
- 24.Mariotte S, Nordmann P, Nicolas M H. Extended-spectrum β-lactamase in Proteus mirabilis. J Antimicrob Chemother. 1994;33:925–935. doi: 10.1093/jac/33.5.925. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas-Chanoine M H, Chardon H, Avril J L, Cattoen Y, Croix J C, Dabernat H, Etienne J, Fosse T, Ghnassia J C, Lecaillon E, Marmonier A, Roussel-Delvallez M, Soussy J C, Trevoux A, Sirot J. Susceptibility of Enterobacteriaceae to betalactams and fluoroquinolones: a French multicentre study. Clin Microbiol Infect. 1997;3(Suppl. 2):74–75. doi: 10.1046/j.1469-0691.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 26.Pagani L, Luzzaro F, Migliavacca R, Perilli M G, Daturi R, Lombardi G, Matti C, Giacobone E, Amicosante G. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1997. Detection and characterization of extended spectrum β-lactamases in clinical isolates of Proteus mirabilis from North Italian Hospitals, abstr. D-14; p. 85. [Google Scholar]
- 27.Palzkill T, Thomson K S, Sanders C C, Moland E S, Huang W, Milligan T W. New variant of TEM-10 β-lactamase gene produced by a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 1995;39:1199–1200. doi: 10.1128/aac.39.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitout J D D, Thomson K S, Hanson N D, Ehrhardt A F, Moland E S, Sanders C C. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob Agents Chemother. 1998;42:1350–1354. doi: 10.1128/aac.42.6.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozalski A, Sidorczyk Z, Kotelko K. Potential virulence factors of Proteus bacilli. Microbiol Mol Biol Rev. 1997;61:65–89. doi: 10.1128/mmbr.61.1.65-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirot D, Chanal C, Henquell C, Labia R, Sirot J, Cluzel R. Clinical isolates of Escherichia coli producing multiple TEM-mutants resistant to β-lactamase inhibitors. J Antimicrob Chemother. 1994;33:1117–1126. doi: 10.1093/jac/33.6.1117. [DOI] [PubMed] [Google Scholar]
- 33.Sirot D, Recule C, Chaibi E B, Bret L, Croize J, Chanal-Claris C, Labia R, Sirot J. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 1997;41:1322–1325. doi: 10.1128/aac.41.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirot D, Sirot J, Labia R, Morand A, Courvalin P, Darfeuille-Michaud A, Perroux R, Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. J Antimicrob Chemother. 1987;20:323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- 35.Sirot J. Detection of extended-spectrum plasmid-mediated β-lactamases by disk diffusion. Clin Microbiol Infect. 1996;2(Suppl. 1):35–39. doi: 10.1111/j.1469-0691.1996.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 36.Sougakoff W, Goussard S, Courvalin P. The TEM-3 β-lactamase, which hydrolyzes broad-spectrum cephalosporins, is derived from the TEM-2 penicillinase by two amino acid substitutions. FEMS Microbiol Lett. 1988;56:343–348. [Google Scholar]
- 37.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vedel G, Belaaouaj A, Gilly L, Labia R, Philippon A, Nevot P, Paul G. Clinical isolates of Escherichia coli producing TRI β-lactamases: novel TEM-enzymes conferring resistance to β-lactamase inhibitors. J Antimicrob Chemother. 1992;30:449–462. doi: 10.1093/jac/30.4.449. [DOI] [PubMed] [Google Scholar]
- 39.Verdet C, Arlet G, Ben Redjeb S, Ben Hassen A, Lagrange P H, Philippon A. Characterization of CMY-4, an AmpC-type plasmid-mediated β-lactamase in a Tunisian clinical isolate of Proteus mirabilis. FEMS Microbiol Lett. 1998;169:235–240. doi: 10.1111/j.1574-6968.1998.tb13323.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu D Y, Ugozzoli L, Pal K B, Wallace R B. Allele-specific enzymatic amplification of beta-globin DNA for diagnosis of sickle cell anemia. Proc Natl Acad Sci USA. 1989;86:2757–2760. doi: 10.1073/pnas.86.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu P J, Shannon K, Phillips I. Mechanisms of hyperproduction of TEM-1 beta-lactamase by clinical isolates of Escherichia coli. J Antimicrob Chemother. 1995;36:927–939. doi: 10.1093/jac/36.6.927. [DOI] [PubMed] [Google Scholar]