Abstract
The molecular mechanism of the anti-inflammatory effect of erythromycin (EM) was investigated at the level of transcriptional regulation of cytokine gene expression in T cells. EM (>10−6 M) significantly inhibited interleukin-8 (IL-8) expression but not IL-2 expression from T cells induced with 20 ng of phorbol 12-myristate 13-acetate (PMA) per ml plus 2 μM calcium ionophore (P-I). In electrophoretic mobility shift assays EM at 10−7 to 10−5 M concentrations inhibited nuclear factor kappa B (NF-κB) DNA-binding activities induced by P-I. Reporter gene assays also showed that EM (10−5 M) inhibited IL-8 NF-κB transcription by 37%. The inhibitory effects of EM on transcriptional activation of IL-2 and DNA-binding activity of nuclear factor of activated T cells (NFAT) were not seen in T cells. On the other hand, FK506, which is also a macrolide derivative, inhibited transcriptional activation of both NF-κB and NFAT more strongly than EM did. The mechanism of EM inhibition of transactivation of NF-κB was further investigated in transiently transfected T cells that express calcineurin A and B subunits. Expression of calcineurin did not render transactivation of NF-κB in T cells more resistant to EM, while the inhibitory effect of FK506 on transactivation of NF-κB was attenuated. These findings indicate that EM is capable of inhibiting expression of the IL-8 gene in T cells through transcriptional inhibition and that this inhibition is mediated through a non-calcineurin-dependent signaling event in T lymphocytes.
Erythromycin (EM), first discovered by McGuire in the metabolic products of a strain of Streptomyces erythreus (31), is a 14-membered lactone ring macrolide antibiotic which has long been used for the treatment of infectious diseases caused by organisms that survive and multiply within host cells. There has recently been growing evidence of the effectiveness of EM in ameliorating airway inflammation irrespective of its antimicrobial action. It has been shown that EM can inhibit cytokine gene expression, such as interleukin-8 (IL-8) gene expression from polymorphonuclear leukocytes or IL-6 gene expression from airway epithelial cells (27, 37). Schultz et al. (33) have reported on EM inhibition of IL-6 and tumor necrosis factor alpha (TNF-α) production in whole blood. Given that regulation of cytokine gene expression is transcriptional, it is likely that transcriptional regulation is the target of the effect of EM. However, while there is some evidence of the biological effects of EM on the basis of cellular physiology (16, 38), the molecular mechanism of the effect of EM has not been elucidated at the level of transcription by testing in vitro the ability of EM to inhibit transcriptional activation preceding mRNA transcription.
A number of cis-acting trans-activating nuclear DNA-binding proteins that are induced and activated upon cell stimulation trigger transcriptional initiation which, in coordinate fashion, leads to optimal cytokine secretion from immune effector cells. Nuclear factor kappa B (NF-κB) is a nuclear transcription factor ubiquitously involved in gene expression for a variety of inflammatory mediators including IL-2, IL-6, IL-8, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), cell adhesion molecule, nitric oxide synthase, and other molecules (5). The nuclear factor of activated T cells (NFAT) plays a crucial role in transcriptional activation of cytokine gene expression of IL-2, TNF-α (24), and GM-CSF (9).
The objective of the present study was to investigate whether EM can inhibit the activation of these transcription factors, and since this was the case, the potency and the mechanism of the effects of EM were compared with those of FK506, which is a potent macrolide immunosuppressant (20) widely used in transplantation medicine. We have demonstrated using molecular biological methods that EM is capable of downregulating cytokine gene expression by inhibiting transcriptional activation of NF-κB through interference with non-calcineurin-dependent signaling. The study described in this report is the first to demonstrate that EM suppresses cytokine gene expression in T lymphocytes through inhibition of transcriptional activation.
MATERIALS AND METHODS
Cell culture, stimulation, and drug treatment.
Because of the restricted availability of primary human T cells for use in reporter gene assays and electrophoretic mobility shift assays (EMSAs), an adult human T-cell leukemic cell line (Jurkat) was used in this study. Jurkat T cells (clone E6-1; American Type Culture Collection, Manassas, Va.) were cultured in RPMI 1640 (Mediatech, Herndon, Va.) supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) (Biowhittaker, Walkersville, Md.) in 5% CO2 at 37°C. The cells were stimulated in culture medium containing 20 ng of phorbol 12-myristate 13-acetate (PMA; Calbiochem, La Jolla, Calif.) or PMA plus 2 μM ionomycin (Calbiochem) (P-I) dissolved in dimethyl sulfoxide for various periods of time, as indicated below. Modulation by EM (Sigma, St. Louis, Mo.) or FK506 (Fujisawa Pharmaceutical Co.) of transcriptional activation of gene expression was also investigated. Both of these drugs were initially dissolved in ethanol to a stock concentration of 100 mM, diluted with culture medium, and tested at clinically relevant concentrations. Under nonstimulated conditions or under conditions with no drug treatments, ethanol and/or dimethyl sulfoxide was added to the culture so that these solvents were used at the same final concentrations (percent [vol/vol]) in all experiments.
Transgenic T-cell lines stably expressing luciferase reporter gene.
Plasmids pIL-2/luc and pIL-8/luc contain the human IL-2 enhancer sequence (positions −326 to +45) (1) and the human IL-8 promoter sequence (positions −1451 to +44) (3), respectively. The sequences are linked to firefly luciferase cDNA. Plasmid pκB-2/luc contains three tandem copies of the human IL-2 NF-κB-binding site (positions −205 to −196; GGGATTTCAC) in the context of the minimal enhancer sequence of the IL-2 gene (2). Plasmid pκB-8/luc contains the IL-8 NF-κB element (positions −84 to +44) upstream of the luciferase cDNA. Briefly, a 128-bp length of the 3′ end of the promoter region was generated by PCR with a pair of primers (sense primer, 5′-CGCGCTCGAGTCGTGGAATTTCCTCTGAC-3′; antisense primer, 5′-TTGTCCTAGAAGCTTGTTGCTCTGCTGTC-3′; the underlined sequences represent XhoI and HindIII restriction sites, respectively) by using 1 μg of human genomic DNA as a template. The PCR-amplified IL-8 NF-κB element was sequenced in its entirety by the dideoxy method; the sequence proved identical to the published sequence (25). The PCR product was purified and was then cloned into the XhoI-HindIII site of the luciferase expression vector (1–3). Plasmid pNFAT/luc contains three tandem copies of the human IL-2 NFAT-1-binding site (positions −140 to −130; AAGAGGAAAAA) in the context of the minimal IL-2 enhancer, which drives a luciferase reporter gene (10). Plasmid pEF/lacZ constitutively expresses β-galactosidase and was used for transient cotransfection for standardization of possible differences in transfection efficiency. Transgenic Jurkat cell lines that express these luciferase reporter plasmids were generated by stable transfection under G418 selection, as has been described previously (1–4, 22). For each construct, two independent stable cell lines were generated. These cell lines enabled us to carry out experiments without consideration of differences in transfection efficiency.
Calcineurin experiments and transient transfection.
Plasmid pEF/CalA, which expresses a 60-kDa catalytic subunit of calcineurin, and pEF/CalB, which expresses a 19-kDa regulatory subunit of calcineurin (8, 19), were both generated by PCR amplification and cloning into the EcoRI site and the BamHI site of pEFX, which uses the human elongation factor 1α promoter (39). Plasmid pEF/ΔCaM-AI expresses the constitutively active form of calcineurin depleted of autoinhibitory and calmodulin-binding domains (14, 29, 30) and has been shown to act in synergy with PMA in the absence of intracellular calcium mobilization to induce transcriptional activation of NFAT and NF-κB (12). The deletion mutant was generated by PCR amplification and insertion of a fragment that encodes amino acids 1 to 398 into the BamHI and EcoRI sites of pEFX. The involvement of calcineurin in FK506 or EM inhibition of NF-κB and NFAT was analyzed by transient cotransfection of the plasmids. Briefly, 2 × 107 Jurkat T cells were resuspended in 0.3 ml of complete RPMI 1640 medium in a 4-mm-gap transfection cuvette and were then transfected with 1.5 μg each of the pEF/CalA, pEF/CalB, and reporter plasmids by use of an electroporator (250 V and 1,300 μF; BTX Electrocell Manipulator 600; BTX, San Diego, Calif.). For the control experiments, 3 μg of the control expression vector pEF(−) instead of pEF/CalA and pEF/CalB was used, and the method identical to that described above was used. Following 20 h of incubation at 37°C, the cells were subjected to the experiments.
Luciferase reporter gene assay and electrophoretic mobility shift assays.
For the luciferase reporter gene assay the cells were stimulated for 4 h in 12-well flat-bottom plates (Becton-Dickinson, Lincoln Park, N.J.) at a density of 2 × 106/ml. Preparation of whole-cell extracts and the luciferase reporter gene assay were carried out by previously described methods (2–4). β-Galactosidase activity was measured with a commercially available kit (β-Galactosidase Enzyme Assay System; Promega, Madison, Wis.).
The induction of NF-κB and NFAT DNA-binding activities was analyzed by EMSAs. In the experiments of drug modulation, Jurkat T cells were pretreated with either EM or FK506 for 1 h and were then stimulated for 2 h in the presence of the drugs. Preparation of nuclear extract (NE) and EMSA were carried out as described previously (2–4). For EMSA, 10 μg of NE was incubated at 4°C with binding buffer containing 1 μg of poly(dI-dC)–poly(dI-dC) and 2.5 pg of a 32P-labeled oligonucleotide probe for the NF-κB element of the 5′ flanking region of the human IL-8 gene (AGCTTCGTGGAATTTCCT) (25) or the IL-2 gene (5′-AGCTAAAGAGGGATTTCACCTAAA-3′) or the NFAT element of the 5′ flanking region of the human IL-2 gene (5′-AGCTAAGAAAGGAGGAAAAACTGTTTCATA-3′) (34). Protein-DNA complexes were resolved from free probe on 4% nondenaturing polyacrylamide gels and were visualized by fluorography. The intensity of retarded DNA-binding complex was measured semiquantitatively with a densitometer (Electronic Dual Light Transilluminator; Alpha Innotech Co., San Leandro, Calif.).
Statistical analysis.
The significance of the differences between the experimental conditions was determined by nonpaired Student’s t test (Microsoft Excel).
RESULTS
Effects of EM on expression of IL-2 and IL-8 genes in T cells.
The effects of EM and FK506 were first studied on T-cell secretion of two cytokines, IL-2 and IL-8. The cytokine concentrations in the supernatants from Jurkat T cells stimulated for 16 h with either PMA alone or P-I were measured by enzyme immunoassay (Table 1). Stimulation with PMA alone resulted in the apparent induction of the IL-8 protein (208 ± 33 pg/ml), which was additionally enhanced when PMA was combined with ionomycin treatment (480 ± 23 pg/ml). P-I-induced IL-8 protein expression was inhibited by 17% with 1 μM EM and by 31% with 10 μM EM (P < 0.05). FK506 at a 0.1 μM concentration suppressed P-I-induced IL-8 secretion by 66%, which was significant (P < 0.01); however, complete inhibition was not achieved, in agreement with the results presented in a previous report (28). Stimulation with P-I resulted in marked secretion of the IL-2 protein (2,877 ± 302 pg/ml). Treatment with EM at a dose range of 0.1 to 10 μM did not result in apparent inhibition of P-I-induced IL-2 secretion, while FK506 at a 0.1 μM concentration inhibited IL-2 secretion by 90% (261 ± 53 pg/ml; P < 0.01 by paired t test). These findings that EM is capable of inhibiting IL-8 protein expression but not IL-2 protein expression are consistent with those of previous investigators (18, 27).
TABLE 1.
Cytokine concentrations in culture supernatants of Jurkat T cells: inhibitory effects of EM and FK506
| Stimulant (concn [μM]) | Concn (pg/ml)a
|
|
|---|---|---|
| IL-8 | IL-2 | |
| NS | <1 | <1 |
| PMA | 208 ± 33 | <1 |
| P-I | 480 ± 66 | 2,877 ± 302 |
| P-I + EM (0.1) | 450 ± 23 | 2,901 ± 480 |
| P-I + EM (1) | 408 ± 66 | 2,657 ± 200 |
| P-I + EM (10) | 334 ± 79b | 2,520 ± 363 |
| P-I + FK506 (0.1) | 167 ± 26c | 261 ± 53c |
Data represent means ± standard deviations of IL-8 or IL-2 concentrations in culture supernatants from three independent experiments. NS, nonstimulated; P-I, PMA (20 ng/ml) plus ionomycin (2 μM).
P < 0.05.
P < 0.01.
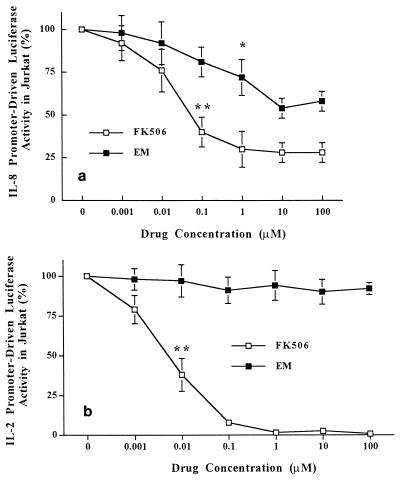
The effects of the drugs on transcriptional activation of IL-8 were then studied with the transgenic reporter cell line IL-8Luc/Jurkat (Fig. 1a). P-I-induced IL-8 transcriptional activation was inhibited by 19, 25 (P < 0.05), and 41% with 0.1, 1, and 10 μM EM, respectively. FK506 inhibited IL-8 transactivation more strongly than EM did. The inhibition was 60% (P < 0.01) with 0.1 μM FK506 and 70% with 1 μM FK506, but no further inhibition was seen with increasing doses of FK506. Transcriptional activation of IL-8 induced by PMA stimulation alone was 60% of that induced by P-I, against which neither EM nor FK506 treatment resulted in apparent inhibition (data not shown). The inhibitory effects of EM and FK506 on P-I-stimulated transcriptional activation of IL-2 in Jurkat cells were also studied (Fig. 1b). EM did not inhibit transcriptional activation of IL-2. On the other hand, FK506 had a very potent inhibitory effect on transcriptional activation of IL-2 (Fig. 1b). These results obtained by enzyme-linked immunosorbent assay demonstrate that EM is capable of inhibiting transcriptional activation of IL-8 but not IL-2, in contrast to the potent transcriptional inhibition of gene expression of these two cytokines by FK506.
FIG. 1.
Inhibitory effects of EM and FK506 on transcriptional activation of IL-8 (a) or IL-2 (b) gene expression. Transgenic Jurkat T-cell lines that stably express the firefly luciferase gene driven by full-length human IL-8 promoter (positions −1451 to +44) or human IL-2 enhancer (positions −326 to +45) were established (see Materials and Methods). These T-cell lines were pretreated with either EM or FK506 at a dose range of 10−9 to 10−4 M for 1 h and were then stimulated with PMA (20 ng/ml) plus ionomycin (2 μM) for 4 h in the presence of the drugs. Ten micrograms of whole-cell extracts was used for the luciferase assay. Transcriptional activation is shown as the enzymatic activities of luciferase contained in whole cells. Data represent means ± standard deviations for three independent experiments. *, P < 0.05; **, P < 0.01.
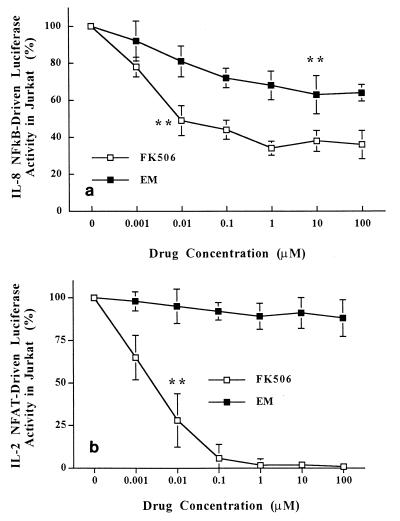
Inhibition of transcriptional activation of NF-κB and NFAT by EM and FK506.
NF-κB is a core transcription factor for IL-8 gene expression (25), and NFAT is a core transcription factor for IL-2 gene expression (34). Jurkat T cells stably transfected with either pκB-8/luc, pκB-2/luc, or pNFAT/luc were stimulated with P-I in the presence of EM or FK506. The inhibition of transcriptional activation of IL-8 NF-κB with 100 nM EM was 22%, and further inhibition was achieved with an increasing dose of EM, with maximum inhibition being 37% with 10 μM EM (P < 0.01) (Fig. 2a). The levels of FK506 inhibition of IL-8 NF-κB were 50% (P < 0.01) at a 10 nM concentration and 66% at a 100 nM concentration (Fig. 2a). A transgenic Jurkat T-cell line that stably expresses the IL-2 NF-κB luciferase gene was also used to test the inhibitory effects of EM and FK506. The results were similar to those obtained for IL-8 NF-κB inhibition. The levels of inhibition of IL-2 NF-κB by 100 μM EM and 100 μM FK506 were 42 and 63%, respectively (data not shown). P-I-induced transcriptional activation of NFAT in Jurkat T cells was dramatically inhibited by FK506 in a dose-dependent manner (Fig. 2b), as has been described previously (29). In contrast, transcriptional inhibition of NFAT was not seen even with 100 μM EM, a concentration at which FK506 completely inhibited NFAT. In any of these experiments, the protein concentrations in whole-cell extracts, as measured by the assay of Bradford (7), from the cells treated with EM did not differ under the various conditions, indicating no inhibition of protein synthesis by EM.
FIG. 2.
Inhibitory effects of EM and FK506 on transcriptional activation of cis-acting enhancer elements. Transgenic Jurkat T cells that stably express the firefly luciferase gene driven by IL-8 NF-κB (positions −84 to +44) (a) or three tandem copies of IL-2 NFAT (b) were established (see Materials and Methods). These T-cell lines were pretreated with either EM or FK506 at a dose range of 10−9 to 10−4 M for 1 h and were then stimulated with PMA (20 ng/ml) plus ionomycin (2 μM) for 4 h in the presence of the drugs. The cells were then subjected to the luciferase assay as described in the legend to Fig. 1.
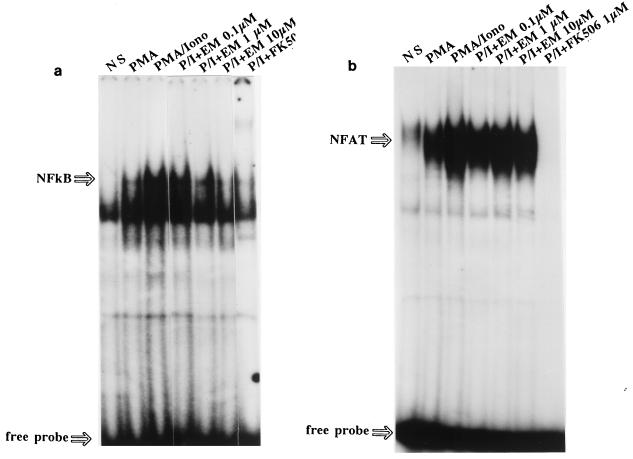
Inhibition of NF-κB and NFAT DNA-binding activities by EM and FK506.
Finally, modulation of the DNA-binding activities of transcription factors NF-κB and NFAT by EM and FK506 were studied by EMSA. The DNA-binding activities of these nuclear proteins thus investigated have been proved to be specific in our previous investigations (2, 4). NF-κB DNA-binding complex was detectable in nuclear extracts from Jurkat cells treated with PMA alone; the levels were further enhanced by treatment with P-I (Fig. 3a). Although EM at a 0.1 μM concentration very slightly inhibited the P-I-induced NF-κB DNA-binding complex, the inhibition was more significant with increasing doses of EM: the densitometer analyses showed that the NF-κB complex was inhibited by 20% with 1 μM EM and by 65% with 10 μM EM. No apparent inhibition by EM was seen for the NF-κB complex induced by treatment with PMA alone (data not shown). On the other hand, FK506 at 1 μM more significantly inhibited NF-κB DNA-binding activity.
FIG. 3.
Induction and drug modulation of DNA-binding activities of nuclear transcriptional proteins NF-κB and NFAT in plain Jurkat T cells. The cells were either nonstimulated (NS) or stimulated with PMA (20 ng/ml) or PMA plus ionomycin (2 μM) (PMA/Iono) for 2 h. Inhibition by EM (10−7 to 10−5 M) or FK506 (10−6 M) of P-I-induced specific DNA-binding activities was also tested. Ten micrograms of NEs was incubated with the 32P-labeled consensus sequence of IL-8 NF-κB (a) or IL-2 NFAT (b) oligonucleotides in the presence of 1 μg of poly(dI-dC), and the NE-DNA complexes were resolved by EMSAs.
Substantial induction of NFAT DNA-binding activity was found in NEs prepared from P-I-induced Jurkat T cells (Fig. 3b). The inhibitory effect of EM on NFAT DNA-binding activity was tested. Again, P-I-induced NFAT DNA-binding activities were not inhibited by EM, a finding that is consistent with the results of the reporter gene assay. In marked contrast, FK506 at a 1 μM concentration completely inhibited NFAT DNA-binding activity.
EM inhibition of NF-κB through non-calcineurin-dependent signaling.
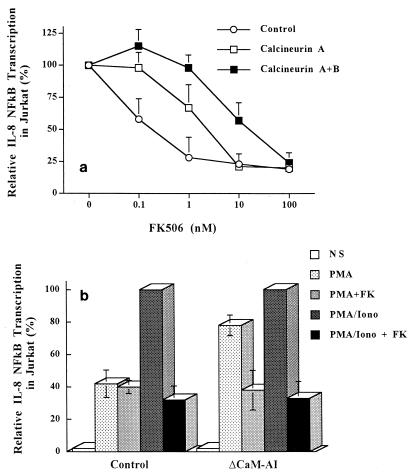
Since EM had inhibitory effects on transcriptional activation and the DNA-binding activity of NF-κB induced with P-I (Fig. 2 and 3) but not PMA alone (data not shown), it was suggested that EM inhibition of NF-κB could target signaling events elicited by intracellular calcium mobilization. The interest in this study was, then, to see if the molecular mechanism of EM inhibition on NF-κB is mediated through an interaction with calcineurin, which is a key enzyme for the immunosuppressive effect of FK506 (23).
Jurkat T cells transiently cotransfected with pEF/CalA, pEF/CalB, and pκB-8/luc were stimulated by P-I and treated with increasing doses of FK506 (Fig. 4a). In studies of calcineurin A expression, the inhibitory effects of FK506 on NF-κB transactivation were markedly attenuated compared to those for the controls. This attenuation of FK506 inhibition was further enhanced by coexpression of the calcineurin B subunit. The 50% inhibitory concentration of FK506 was 0.2 nM in the control experiments, whereas it was 20 μM with calcineurin A and B expression. In another set of experiments, Jurkat T cells were transiently cotransfected with pκB-8/luc and pEF/ΔCaM-AI (Fig. 4b). PMA treatment induced 40% of the transcriptional activation of IL-8 NF-κB transactivation induced by P-I (Fig. 4b, control). With ΔCaM-AI expression, this activation by PMA was markedly enhanced to 78% of that induced by P-I in a FK506-sensitive manner (Fig. 4b, ΔCaM-AI). Thus, calcineurin proved to upregulate IL-8 NF-κB in T cells. However, the level of P-I-induced transcriptional activation of IL-8 NF-κB exceeded that induced by PMA plus ΔCaM-AI, indicating that calcineurin does not totally substitute for the calcium-dependent signaling required for full activation of IL-8 NF-κB. These results showing that expression of calcineurin makes T cells resistant to FK506 inhibition of IL-8 NF-κB (Fig. 4a) and that constitutively active calcineurin is capable of transactivating IL-8 NF-κB (Fig. 4b) indicate that calcineurin is partly involved in the transcriptional activation of IL-8 NF-κB in T cells.
FIG. 4.
Involvement of calcineurin in transcriptional activation of IL-8 NF-κB. (a) Attenuation of IL-8 NF-κB sensitivity to FK506 inhibition by calcineurin expression in plain Jurkat T cells. All transfections include the IL-8 NF-κB luciferase reporter plasmid and either the pEF/CaA-expressing catalytic subunit of calcineurin (closed squares), the pEF/CaA- plus pEF/CaB-expressing regulatory subunits of calcineurin (open squares), or mock plasmid pEF(−) (open circles). At 15 h following transient cotransfection, the cells which had already been treated for 1 h with FK506 were stimulated with PMA (20 ng/ml) plus ionomycin (2 μM) (PMA/Iono) for 4 h in the presence of the drug. (b) Upregulation of transcriptional activation of IL-8 NF-κB by constitutively active calcineurin in an FK506-sensitive manner. Plain Jurkat T cells were transiently cotransfected with reporter plasmid pκB-8/luc and either a control expression vector, pEF(−) (Control) or pEF/ΔCaM-AI, that constitutively expresses the catalytic subunit of calcineurin (ΔCaM-AI). Following 15 h of recovery, the cells were pretreated with FK506 and were then stimulated as described above for panel a. Whole-cell extracts were prepared, and luciferase assays were performed as described in Materials and Methods. NS, nonstimulated; FK, FK506. In each experiment, the luciferase activity produced was normalized to the amount of β-galactosidase activity and protein concentrations. Data represent means ± standard deviations for three independent experiments.
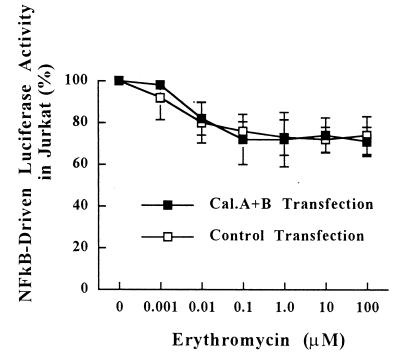
Having confirmed the role of calcineurin in IL-8 NF-κB activation, we tested whether the inhibitory effect of EM on IL-8 NF-κB is mediated through an interaction with calcineurin. As shown in Fig. 5, the overall level of inhibition by EM (1 nM to 100 μM) of transcriptional activation of NF-κB when cells are cotransfected with calcineurin A and B subunits did not differ from those in control experiments, although the suppressive effect of EM at 1 nM under conditions of calcineurin cotransfection appeared to be less than that in the control experiment (2% inhibition for pEF/CaA plus pEF/CaB cotransfection versus 8% inhibition for control transfection). These results indicate that the inhibitory effects of EM on transcriptional activation of NF-κB are not mediated through the interaction with calcineurin.
FIG. 5.
Effects of calcineurin expression on sensitivity of IL-8 NF-κB transactivation by EM in T cells. All transfections include the IL-8 NF-κB luciferase reporter plasmid and either pEF/CaA plus pEF/CaB (closed squares) or the control expression vector pEF(−) (open squares). Following 15 h of incubation, Jurkat T cells were pretreated for 1 h with various concentrations of EM and were then stimulated with PMA (20 ng/ml) plus ionomycin (2 μM) for 4 h in the presence of EM. Luciferase activities were analyzed as described in Materials and Methods. Data represent means ± standard deviations for three independent experiments.
DISCUSSION
Previous studies on diffuse panbronchiolitis seen in Asian and Caucasian patients have reported improvements to air flow limitations, excessive airway secretions, and arterial oxygen tension following long-term daily EM therapy (11, 13). Indeed, EM has proved to reduce the number of neutrophils, elastase activities, IL-8 concentration, and neutrophil chemotactic activity in the lung microenvironment (15, 17, 27). Importantly, the effects of EM have been noted in these patients whether or not chronic bacterial infections are present (13, 27), indicating that the effects of EM as a biological response modifier are exerted through an anti-inflammatory mechanism but not an antimicrobial mechanism. T lymphocytes are circulating immunocompetent cells in the lungs and play a major role in the integrated host defense by amplifying proinflammatory signals initiated by airway epithelial cells (AEC) or macrophages (35). Therefore, in view of airway inflammation, it would be important to ask if EM exerts its anti-inflammatory effects on T cells.
The present study showed that EM is capable of inhibiting IL-8 but not IL-2 protein expression from P-I-induced T lymphocytes. It has previously been reported that IL-8 production from Pseudomonas aeruginosa-stimulated neutrophils is inhibited by EM (27), whereas expression of the IL-2 and the IL-2 receptor genes in T cells is resistant to the effect of EM (18). The reporter gene assay with transgenic Jurkat T cells that stably express the luciferase gene demonstrated dose-dependent transcriptional inhibition of IL-8 gene expression. Moreover, the extent to which EM inhibited P-I-induced IL-8 transcription showed good correlations with the results of EM inhibition of IL-8 protein expression. These findings from the reporter gene assay and the enzyme-linked immunosorbent assay strongly suggest that the inhibitory effect of EM on IL-8 gene expression in T cells is due at least in part to transcriptional inhibition. The results of EMSA further clarified that transcriptional inhibition of P-I-induced IL-8 gene expression is caused by EM inhibition of the induction of IL-8 NF-κB DNA-binding activities (Fig. 3). Although the question of whether high concentrations of antibiotics contained in culture (100 U of benzylpenicillin per ml plus 100 mg of streptomycin per ml) may have influenced the results was not addressed in independent experiments in the present study, Takizawa et al. (37) have found no significant inhibition of cytokine gene expression by 1 mM aminobenzylpenicillin in a similar study on the anti-inflammatory effects of EM on airway epithelial cells.
Since FK506, a potent, macrocyclic lactone immunosuppressant, is also a substance from a strain of Streptomyces, as is EM (20), we then tested whether the underlying molecular mechanism for transcriptional inhibition could be analogous between the two drugs. The molecular mechanism of action of FK506 that takes place intracellularly has been studied extensively. The fundamental mechanism of FK506 is the inhibition of phosphatase activity of calcium- and calmodulin-dependent calcineurin, which plays an essential role in the activation of NFAT and NF-κB (12, 19, 23). In the present study, having confirmed that calcineurin is an upstream signal of transactivation of IL-8 NF-κB, EM was tested in an experiment involving the expression of a calcineurin subunit(s) and was found to inhibit IL-8 NF-κB, but not through the interaction with calcineurin. In this context, the molecular mechanism of EM inhibition of transcriptional activation is similar to that of rapamycin, which is also a macrolide immunosuppressant that interferes with non-calcineurin-dependent pathways (29). EM likely functions intracellularly since this drug is generally known to diffuse readily into intracellular fluids (31). Previous investigations with alveolar macrophages have shown that EM preferentially concentrates intracellularly (36), with the intracellular concentration/extracellular concentration ratio of EM being 17 (for a review, see reference 6). What is, then, the intracellular mechanism of EM inhibition of NF-κB? Fourteen-membered-ring macrolides have been reported to be capable of inhibiting oxidant production by mammalian cells in vitro at therapeutically achievable concentrations (21). Additionally, given that reactive oxygen intermediates serve as messengers that mediate the activation of NF-κB (32), a possible mechanism of EM inhibition on NF-κB activation could be due to the inhibition of reactive oxygen intermediates generation in the cytoplasm. The mechanism of action of EM that underlies NF-κB inhibition, however, awaits further investigations.
Although EM suppression of transcriptional activation of IL-8 NF-κB is significant, it was less significant than that of FK506. Since an effective host defense is maintained by well-balanced anti-inflammatory and proinflammatory responses of the host immune system, EM seems to be a clinically useful biological response modifier in that it provides a moderate immunomodulating effect against inflammation. It should also be taken into account, however, that the EM-induced downregulation of cytokine gene expression may have a negative impact on host defense mechanism, as indicated in previous studies. Nelson et al. (26) showed the ablation by EM of the defense against bacterial multiplication in the lung, and Schultz et al. (33) recently reported on the EM inhibition of TNF-α and IL-6 production in whole blood stimulated by heat-killed Streptococcus pneumoniae. We have reported that, unlike in T cells, calcium signaling pathways in epithelial cells exert suppressive effects on NF-κB activation and that this suppression is reversed by FK506, which suggests a mechanism through which FK506 can enhance the expression of inflammatory cytokines in nonlymphoid organs (2). In this context, it will be interesting to investigate whether macrolide antibiotics such as EM suppress or enhance cytokine gene expression from AEC through transcriptional modulation. Additionally, it would also be important to test whether EM inhibits transcriptional activation in primary T cells in a fashion similar to that observed in Jurkat T cells.
In conclusion, the mechanism of EM inhibition of cytokine gene expression in T cells is at the level of transcriptional regulation. This is the first report of a study in which it has been demonstrated that EM inhibits the induction and transcriptional activation of NF-κB through interference with calcineurin-independent calcium signaling. These findings suggest that EM possesses anti-inflammatory properties and lend support to the use of this drug in the treatment of airway inflammation.
ACKNOWLEDGMENTS
We thank Thomas A. Raffin (Pulmonary and Critical Care Medicine, Stanford University Medical Center) for continuous encouragement.
This work was supported by grants from the California Affiliate of the American Lung Association, the Donald E. and Delia B. Baxter Foundation, and National Institute of Allergy and Infectious Disease (grants KO4-AI-01147 and RO1-AI-39624 to Peter N. Kao).
REFERENCES
- 1.Aoki Y, Qiu D, Uyei A, Kao P N. Human airway epithelial cells express interleukin-2 in vitro. Am J Physiol. 1997;272:L276–L286. doi: 10.1152/ajplung.1997.272.2.L276. [DOI] [PubMed] [Google Scholar]
- 2.Aoki Y, Kao P N. CyclosporinA-sensitive calcium signaling represses NFκB activation in human bronchial epithelial cells and enhances NFκB activation in Jurkat T-cells. Biochem Biophys Res Commun. 1997;234:424–431. doi: 10.1006/bbrc.1997.6658. [DOI] [PubMed] [Google Scholar]
- 3.Aoki Y, Qiu D, Zhao G, Kao P N. Leukotriene B mediates histamine induction of NF-κB and IL-8 in human bronchial epithelial cells. Am J Physiol. 1998;274:L1030–L1039. doi: 10.1152/ajplung.1998.274.6.L1030. [DOI] [PubMed] [Google Scholar]
- 4.Aoki Y, Zhao G, Qiu D, Shi L, Kao P N. CsA-sensitive purine-box transcriptional regulator in bronchial epithelial cells contains NF45, NF90, and Ku. Am J Physiol. 1998;275:L1164–L1172. doi: 10.1152/ajplung.1998.275.6.L1164. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle P A, Henkel T. Function and activation of NFκB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 6.Bergogne-Berezin E. Pharmacokinetics of antibiotics in the respiratory tract: clinical significance. Clin Pulm Med. 1998;5:211–220. [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Clipstone N A, Crabtree G R. Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 9.Cockerill P N, Shannon M F, Bert A G, Ryan G R. The granulocyte-macrophage colony-stimulating factor/interleukin 3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci USA. 1993;90:2466–2470. doi: 10.1073/pnas.90.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emmel E A, Verweij C L, Durand D B, Higgins K M, Lacy E, Crabtree G R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald J E, King T E, Lynch D A, Tuder R M, Schwartz M I. Diffuse panbronchiolitis in the United States. Am J Respir Crit Care Med. 1996;154:497–503. doi: 10.1164/ajrccm.154.2.8756828. [DOI] [PubMed] [Google Scholar]
- 12.Frantz B, Nordby E C, Bren G, Steffan N, Paya C V, Kincaid R L, Tocci M J, O’Keefe F J, O’Neill E A. Calcineurin acts in synergy with PMA to inactivate IkB/MAD3, an inhibitor of NF-κB. EMBO J. 1994;13:861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii T, Kadota J, Kawakami K, Iida K, Shirai R, Kaseda M, Kawamoto S, Kohno S. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax. 1995;50:1246–1252. doi: 10.1136/thx.50.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Perrino B A, Soderling T R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990;265:1924–1927. [PubMed] [Google Scholar]
- 15.Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, Oizumi K. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis. 1991;146:196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K, Wu D, Takasaka T. Inhibition of acetylcholine-evoked Cl− currents by 14-membered macrolide antibiotics in isolated acinar cells of the guinea pig nasal gland. Am J Respir Cell Mol Biol. 1995;13:449–454. doi: 10.1165/ajrcmb.13.4.7546775. [DOI] [PubMed] [Google Scholar]
- 17.Kadota J, Sakito O, Kohno S, Sawa H, Mukae H, Oda H, Kawakami K, Fukushima K, Hiratani K, Hara K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–159. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 18.Keicho N, Kudoh S, Yotsumoto H, Akagawa K S. Antilymphocytic activity of erythromycin distinct from that of FK506 and cyclosporin A. J Antibiot. 1993;46:1406–1413. doi: 10.7164/antibiotics.46.1406. [DOI] [PubMed] [Google Scholar]
- 19.Kincaid R L. The role of calcineurin in immune system response. J Allergy Clin Immunol. 1995;96:1170–1177. doi: 10.1016/s0091-6749(95)70202-4. [DOI] [PubMed] [Google Scholar]
- 20.Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, Goto T, Okuhara M, Kohsaka M, Aoki H, Ochiai T. FK506, a novel immunosuppressant isolated from a Streptomyces. J Antibiot. 1987;40:1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 21.Labro M T, Benna J E, Abdelghaffar H. Modulation of human polymorphonuclear neutrophil function by macrolides: preliminary data concerning dirithromycin. J Antimicrob Chemother. 1993;31(Suppl. C):51–64. doi: 10.1093/jac/31.suppl_c.51. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Handshumacher R E. Regulation of nuclear factor of activated T-cells in stably transfected Jurkat cell clones. Biochem Biophys Res Commun. 1996;219:96–99. doi: 10.1006/bbrc.1996.0187. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 24.McCaffrey P G, Goldfeld A E, Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-a gene transcription. J Biol Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- 25.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 26.Nelson S, Summer W R, Terry P B, Warr G A, Jakab G J. Erythromycin-induced suppression of pulmonary antibacterial defenses. Am Rev Respir Dis. 1987;136:1207–1212. doi: 10.1164/ajrccm/136.5.1207. [DOI] [PubMed] [Google Scholar]
- 27.Ohishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto S, Mukaida N, Yasumoto K, Rice N, Ishikawa Y, Horiguchi H, Murakami S, Matsushima K. The interleukin-8 AP-1 and κB-like sites are genetic end targets of FK-506 sensitive pathway accompanied by calcium mobilization. J Biol Chem. 1994;269:8582–8589. [PubMed] [Google Scholar]
- 29.O’Keefe S J, Tamura J, Kincaid R L, Tocci M J, O’Neill E A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 30.Parsons J N, Wiederrecht G J, Salowe S, Burbaum J J, Rokosz L L, Kincaid R L, O’Keefe S J. Regulation of calcineurin phosphatase activity and interaction with the FK506-FK506 binding protein complex. J Biol Chem. 1994;269:19610–19616. [PubMed] [Google Scholar]
- 31.Sanda M A, Mandell G L. Antimicrobial agents. Tetracyclines, chloramphenicol, erythromycin, and miscellaneous antibacterial agents. In: Goodman L S, Gilman A, editors. The pharmacological basis of therapeutics. 8th ed. New York, N.Y: Macmillan Publishing Company; 1985. pp. 1170–1198. [Google Scholar]
- 32.Schreck R, Rieber P, Baeuerle P A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz M J, Speelman P, Zaat S, van Deventer S J H, van der Poll T. Erythromycin inhibits tumor necrosis factor alpha and interleukin-6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother. 1998;42:1605–1609. doi: 10.1128/aac.42.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim Biophys Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 35.Shelhamer J H, Levine S J, Wu T, Jacoby D B, Kaliner M A, Rennard S I. Airway inflammation. Ann Intern Med. 1995;123:288–304. doi: 10.7326/0003-4819-123-4-199508150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Stamlar D A, Edelstein M A, Edelstein P H. Azithromycin pharmacokinetics and intracellular concentrations in Legionella pneumophila-infected and uninfected guinea pigs and their alveolar macrophages. Antimicrob Agents Chemother. 1994;38:217–222. doi: 10.1128/aac.38.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takizawa H, Desaki M, Ohtoshi T, Kikutani H, Okazaki M, Sato N, Akiyama S, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin-6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 38.Tamaoki J, Tagaya E, Yamawaki I, Sakai N, Nagai A, Konno K. Effect of erythromycin on endotoxin-induced microvascular leakage in the rat trachea and lung. Am J Respir Crit Care Med. 1995;151:1582–1588. doi: 10.1164/ajrccm.151.5.7735618. [DOI] [PubMed] [Google Scholar]
- 39.Uetsuki T, Naito A, Nagata S, Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1α. J Biol Chem. 1989;264:5791–5798. [PubMed] [Google Scholar]