Abstract
Objective:
The objective of the study was to determine incidence, risk factors, and short-term outcomes of young stroke in Ludhiana city, Northwest India.
Methods:
Data were collected on first-ever stroke in patients of age ≥18 years, from hospitals, diagnostic imaging centers, general practitioners, and municipal corporation during March 2011–March 2013 in Ludhiana city, using the World Health Organization Stepwise Approach to Surveillance (WHO STEPS). Outcome was documented using the modified Rankin Scale at 28 days.
Results:
Of 2948 patients, 700 (24%) were in the age group 18–49 years. Annual incidence in this age group was 46/100,000 person-years (95% confidence interval [CI], 41–51/100,000). Hypertension (84%), diabetes mellitus (48%), and atrial fibrillation (AF) (12%) were found more common in >49 years age group, as compared with 18–49 years age group. Drug abuse (8.7% vs. 6% in age >49 years; P = 0.04) and tobacco intake (8.7% vs. 5.6% in age >49 years; P = 0.02) was more common in young people, that is, 18–49 years age group in comparison to older patients, >49 years age group. Recovery was better in younger subjects (60% vs. 46% in age >49 years P < 0.001). In a multivariable analysis, younger people were more often literate (odds ratio [OR] 2.52; 95% CI, 1.68–3.77; P < 0.001), employed (OR 3.92; 95% CI, 2.20–5.21; P < 0.001), and 374 (60%) had good clinical outcome, modified Rankin Scale <2 at 28 days follow-up as compared with 938 (46%) older patients (OR 1.52; 95% CI, 1.15–2.00; P = 0.003).
Conclusion:
Hypertension, diabetes mellitus, drug addiction, and tobacco intake were significantly associated with young stroke. Outcome was also better in younger people.
Keywords: Population-based registry, lower-and middle-income countries, World Health Organization Stepwise Approach to Surveillance Approach (WHO STEP), young stroke
INTRODUCTION
Young stroke is defined as stroke affecting adults in the age group ≤49 years.[1] Its incidence is rising and it has become the third most common cause of death and fourth major cause of morbidity.[2] Approximately, 10–14% of ischemic strokes occur in young people,[3] although the incidence rate and causes of young stroke vary according to ethnicity and continent.[4] In the American population, the incidence of young stroke is low, that is, between 3.4 and 11.3/100,000 person-years, although the rate is as high as 22.8 per 100,000 people per year in the African population.[4,5] One study reported an incidence rate of 8 per 100,000 person-years, with the most common cause being rheumatic valvular heart disease.[6] Another study reported an incidence rate of 8.5% in 113 patients admitted over a period of 5 years, between 15 and 45 years of age group.[7] The North Manhattan stroke study revealed that 8% (74 of 924) of all stroke patients over a 4-year period were between the ages of 20 and 44.[8]
Stroke etiology in young people varies depending on methodological factors such as whether the study is hospital-based versus population-based, geographic region, the extent and availability of diagnostic evaluation at the time of the study, or the criteria used to determine the etiology and ascertain incident rates.[9,10] Most common causes of young stroke are hypertension, diabetes mellitus, coronary artery disease, dyslipidemia, valvular heart diseases, addictions, smoking, and hyper-homocysteinemia. In Switzerland, mitral valve prolapse and arterial dissection were amongst the most common causes for young strokes.[11]
Stroke has declined from the third to the fourth leading cause of death in the United States.[12] Improved stroke prevention with control of modifiable risk factors contributed greatly to this mortality decline, with a lesser but substantial contribution from improved acute stroke care.[13] The occurrence of stroke in young adults is of major concern as they are likely to live longer with stroke-related disability and it is associated with the significant financial burden for the caregivers and for society. Despite all these concerns, most studies of young stroke have small size. Overall, the incidence of stroke has increased in lower- and middle-income countries (LMIC) in age 20–64 years, over the last four decades.[14] India is a vast country with diverse economic and sociocultural settings. Most of the incidence studies have been undertaken in metropolitan cities and urban areas.[15] There is limited availability of information from the level 2 areas of India. In a study conducted in Gadchiroli village, stroke was found to be the leading cause of mortality with an age-adjusted stroke mortality rate of 192/100,000 persons. The study estimated that of 1599 deaths, nearly 14% deaths were caused by stroke. It was done through a verbal autopsy method over a 2-year period. Nearly, 9 out of 10 stroke deaths occurred at home, of which nearly half happened within the first 30 days.[16]
Young stroke has devastating effects on the patient, family, and caregivers as well as the society. Population-based epidemiological data on young stroke are scarce from LMIC. Therefore, we planned to undertake this study to evaluate the incidence, risk factors, and short-term outcomes of “young stroke” in Ludhiana city, Punjab, Northwest India, largely to supplant the lack of data from this part of the country.
METHODS
Our study was a population-based prospective cohort, a substudy of the Ludhiana population-based stroke registry which was conducted in Ludhiana city, situated in the northwestern state of Punjab from March 26, 2010 to March 25, 2013.[17,18] All patients who had first-ever stroke, age ≥18 years and resident of Ludhiana for more than 6 months using World Health Organization (WHO) Stepwise Approach to Surveillance methodology were included in the study. According to the 2011 census, the population of the city (≥18 years) was 1,065,127 of which 814,847 were in the age group 18–49 years.
This registry was conducted in two phases. Phase I was the feasibility study (March 26, 2010–March 25, 2011).[18] The first 5 months of phase 1 were preparatory months and the remaining 7 months were for data collection. All the major hospitals (public and private), diagnostic imaging centers, physiotherapy centers, and general practitioners (GP) were identified in phase I. Mortality data (hospital deaths and deaths at home) were collected from the municipal corporation (MC). Phase II was the main study (March 26, 2011–March 25, 2013).[18]
The separate questionnaire was used for collecting data from hospitals, diagnostic imaging centers, and MC. The demographic details, symptoms, risk factors, type of stroke, and diagnosis modalities were collected from hospitals, physiotherapy centers, and GP. Data available from diagnostic imaging centers included demographic details, type of stroke, and imaging modalities. The limited information was available from MC (demographic detail and cause of death). The short-term outcome was assessed at 28 days using the modified Rankin Scale (mRS) by telephonic interview of hospitalized and diagnostic imaging center's patients.
Phase II data collected from Ludhiana city were included in this study. Previous studies have used age criteria of 15–49 years to determine young stroke.[1,19] Our study population was above 18 years and above, therefore, we have used this age group as “young stroke.” In both the age groups, we planned to analyze the different types of stroke separately. Institutional ethics committees’ approval of Christian Medical College and Dayanand Medical College and Hospital, Ludhiana, Punjab was obtained.
Definition used
Stroke
WHO's the definition of stroke: “a focal (or at times global) neurological impairment of sudden onset, and lasting > 24 h (or leading to death), and of presumed vascular origin” with or without confirmation of imaging.[20]
Statistical analysis
Categorical variables were compared by Chi-square test and Fischer exact test. Multivariable analysis using binary logistic regression was performed for comparing young patients with patients over 49 years. Gender and all significant variables (P < 0.05) in univariate analysis were included in the model. The retrospective annual incidence rate was calculated for the preceding 1 year and 95% confidence intervals (CIs) were derived assuming Poisson distribution for observed rates. P value of <0.05 was considered significant. Statistical analysis was performed by using SPSS version 21 (IBM Corp., Armonk, NY).
RESULTS
Over a period of 3 years, a total of 7,199 patients were recruited to the study. After excluding 713 duplicates, 49 with incomplete addresses, and transient ischemic attack patients, there remained 5713 patients. As the first year of study was the feasibility period, the 2965 patients enrolled during this feasibility period were excluded from final analysis. The total number of subjects from Ludhiana city was 2948, of whom 194 (7%) were from public hospitals, 1511 (51%) from private hospitals, 986 (33%) from diagnostic imaging centers, 23 (0.8%) contributed from general physicians, and 8 (0.2%) from physiotherapy centers. Research staff screened death certificates of MC including hospital deaths as well as deaths that occurred at home and identified the cause of death mentioned on certificates as stroke. Ninety-one (3%) such death certificates were obtained from MC. In addition, 135 (5%) verbal autopsies were conducted. Of 2948 patients, 700 (24%) were in 18–49 years age group and 2248 (76%) were >49 years [Figure 1].
Figure 1.
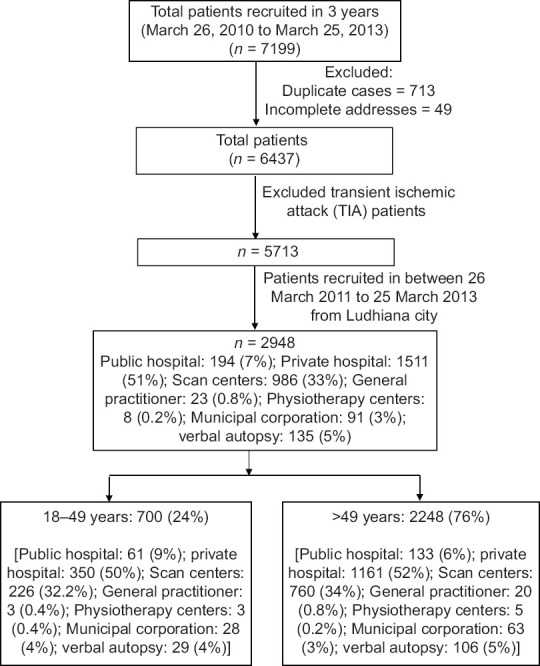
Recruitment algorithm
We calculated retrospective annual incidence over 1 year and during this period the total number of subjects in 18–49 years of age group were 374. The annual stroke incidence rate in the “young stroke” group was 46/100,000 (95% CI, 41–51/100,000). The case fatality rate for 18–49 years age group was 18% (127 deaths among 700 patients). In the age group >49 years, the case fatality rate was 21% (465 deaths among 2248 subjects).
Demographic characteristics
People with young stroke were more often literate (P < 0.001) and employed (P < 0.0001) compared with the older subgroup shown in Table 1.
Table 1.
Comparison of demographic details, stroke type, imaging modalities, and outcome between the two stroke groups, young (18-49 years) stroke patients with older (>49 years) patients with stroke
| Variables | 18-49 years (n=700) n (%)* | >49 years (n=2248) n (%)* | p value |
|---|---|---|---|
| Gender | 0.06 | ||
| Men | 451 (64) | 1360 (61) | |
| Women | 249 (36) | 888 (39) | |
| Education | <0.001 | ||
| Illiterate† | 51 (13) | 276 (22) | |
| Literate | 349 (87) | 967 (78) | |
| Occupation | <0.0001 | ||
| Employed | 205 (48) | 390 (30) | |
| Business | 53 (12) | 168 (13) | |
| Housewives | 135 (31) | 463 (35) | |
| Unemployed | 40 (9) | 287 (22) | |
| Religion | <0.001 | ||
| Sikh | 142 (34) | 579 (44) | |
| Hindu | 257 (62) | 712 (54) | |
| Others‡ | 18 (4) | 27 (2) | |
| Type of stroke | <0.001 | ||
| Ischemic stroke | 404 (63) | 1486 (72) | |
| Intracranial hemorrhage | 214 (34) | 573 (27) | |
| Cerebral venous thrombosis | 18 (3) | 9 (1) | |
| Diagnosis | <0.01 | ||
| Clinical Diagnosis alone | 32 (5) | 58 (3) | |
| CT scan | 294 (48) | 860 (43) | |
| MRI scan | 281 (46) | 1035 (52) | |
| CT and MRI scan both | 6 (1) | 28 (1) | |
| mRS† at 28 days follow-up | <0.001 | ||
| Good outcome‡ | 374 (60) | 938 (46) | |
| Poor outcome§ | 249 (40) | 1085 (54) |
* percentage calculated after excluding missing data, † less than primary school completed, ‡ Muslim, Jain, Christian * percentage calculated after excluding missing data, † modified Rankin Scale, ‡ mRS: 0-2, § mRS: 3-6
Type of stroke, diagnosis modalities, and outcome
A total of 404 (63%) patients had an ischemic stroke in the younger age group as compared with 1486 (72%) in the older adult group [Table 1]. Thirty-four percent of patients suffered from intracranial hemorrhage as compared with 27% older patients (P < 0.001) and 18 young patients (3%) had cerebral venous thrombosis compared with 1% in older stroke patients. Thirty-two young patients were diagnosed with only clinically and 58 older patients. More young patients, that is, 294 (48%) underwent computed tomography (CT) head compared with 860 (43%) older adults, and 1035 (52%) older patients had MRI brain for the initial diagnosis of stroke compared with 46% young patients.
Risk factors
On comparing common risk factors for stroke in both young and old subgroups, hypertension (72% vs. 84% in older patients, P < 0.001), diabetes mellitus (23% vs. 48% in older patients, P < 0.001), atrial fibrillation (8% vs. 12% in older patients, P = 0.02), dyslipidemia (15% vs. 20% in older patients, P = 0.02), and coronary artery disease (6% vs. 13% in older patients, P < 0.001) were found to be more common in older patients. Drug abuse (9% vs. 6%, P = 0.05) and past tobacco use (22% vs. 15%, P < 0.001%) was found to be more common and significant risk factor in stroke in young. Past and present tobacco (22% and 9%, respectively) use was more common in younger patients, although the past tobacco use (22%) was significantly associated with stroke (P < 0.001) [Table 2].
Table 2.
Univariate comparison of risk factors in young (18-49 years) stroke subjects with older (>49 years) patients with stroke
| Variables | 18-49 years (n=417) n (%)* | >49 years (n=1319) n (%)* | P |
|---|---|---|---|
| Hypertension | 317 (72) | 1189 (84) | <0.001 |
| Atrial fibrillation | 32 (8) | 152 (12) | 0.02 |
| Hyperlipidemia | 66 (15) | 281 (20) | 0.02 |
| Drug Addiction | 36 (9) | 78 (6) | 0.05 |
| Rheumatic Heart Disease | 13 (3) | 45 (3) | 0.75 |
| Tobacco (current use) | 40 (9) | 90 (6) | 0.06 |
| Tobacco (past) | 93 (22) | 194 (15) | <0.001 |
| Alcohol (current use) | 67 (15) | 196 (14) | 0.51 |
| Alcohol (Past) | 151 (36) | 473 (36) | 0.90 |
| Diabetes Mellitus | 102 (23) | 675 (48) | <0.001 |
| Carotid Stenosis | 11 (3) | 44 (4) | 0.48 |
| Previous TIA | 54 (13) | 147 (11) | 0.32 |
| Coronary Artery Disease | 27 (6) | 176 (13) | <0.001 |
| Pregnancy/Postpartum | 6 (1) | 1 (0.1) | <0.001 |
* percentages calculated excluding missing values
Multivariable analysis
To find the cause and effects relationships between different variables, multivariate regression analyses were planned. In this analysis, differences were observed in both groups in terms of literacy, employment, and clinical recovery. Younger patients were more often literate (odds ratio [OR] 2.52; 95% CI, 1.68–3.77; P < 0.001), employed (OR 3.92; 95% CI, 2.20–5.21; P < 0.001) and had a good clinical outcome (OR 1.52; 95% CI, 1.15–2.00; P = 0.003) as compared with older patients [Table 3].
Table 3.
Comparison of young (18-49 years) stroke subjects with older (>49 years) stroke subjects in multivariate analysis (data presented as odds ratio with 95% CI)
| Variables | Regression coefficient | Adjusted odds ratio (95% Confidence interval) | P |
|---|---|---|---|
| Gender (Reference: women) | |||
| Men | -0.58 | 0.56 (0.32-0.97) | 0.04 |
| Education (Reference: Illiterate) | |||
| Literate | 0.92 | 2.52 (1.68-3.77) | <0.001 |
| Occupation (Reference: unemployed) | |||
| Employed | 1.22 | 3.39 (2.20-5.21) | <0.001 |
| Business | 0.84 | 2.32 (1.35-4.00) | 0.002 |
| Housewives | 0.68 | 1.97 (1.08-3.59) | 0.03 |
| Religion | |||
| Sikh | -1.20 | 0.30 (0.16-0.58) | 0.001 |
| Hindu | -0.90 | 0.41 (0.21-0.79) | 0.007 |
| Type of stroke (Reference: Hemorhagic stroke) | |||
| Ischemic stroke | -0.32 | 0.73 (0.55-0.96) | 0.02 |
| Diagnosis (Reference: Clinical diagnosis) | |||
| Imaging (CT/MRI)† | -0.54 | 0.58 (0.33-1.04) | 0.07 |
| Hypertension (Yes) | -0.54 | 0.58 (0.42-0.82) | 0.002 |
| Atrial fibrillation Yes) | -0.46 | 0.63 (0.39-1.01) | 0.05 |
| Tobacco past (Yes) | 0.25 | 1.28 (0.88-1.88) | 0.20 |
| Hyperlipidemia (Yes) | -0.21 | 0.81 (0.57-1.14) | 0.23 |
| Diabetes Mellitus (Yes) | -0.98 | 0.37 (0.28-0.50) | <0.001 |
| CAD (Yes) | -0.33 | 0.72 (0.44-1.17) | 0.18 |
| modified Rankin Scale (Reference: poor outcome) | |||
| Good outcome | 0.42 | 1.52 (1.15-2.00) | 0.003 |
*Dependent variable is coded as 18-49 years: 1; >49 years: 0, †CT, MRI and CT/MRI both grouped as Imaging
DISCUSSION
Stroke in young has a huge long-term impact in terms of disease burden and socioeconomic burden over the caregivers of patient and society. It is very important to estimate the disease burden and to recognize its risk factors so as to optimize the treatment and improve young stroke outcome. Our study was a population-based registry of all first-ever strokes, over 18 years of age. Only a few incidence studies have been undertaken in younger people over the past two decades. The incidence rate of young stroke calculated in our study was high (46/100,000) compared with previous studies[7,21,22] [Table 4]. A study by Putaala et al.,[1] reported a very high incidence in their study, that is, 70.4%.
Table 4.
Comparison of Incidence rate of young stroke of various studies
In our study, the WHO STEPS approach was followed to diagnose stroke, 32 (5%) young patients and 58 (3%) older were diagnosed clinically. Invariably majority of the patients underwent neuroimaging CT head/MRI brain in our study. MRI brain was done more commonly in older people, incidentally. The incidence of ischemic stroke was found to be high in older people. The plausible explanation for the same may be that a CT scan was done more frequently in younger subjects and it may miss small ischemic infarcts.[23]
Young people with stroke were more often literate and employed compared with the older population in our study. They usually have a more productive lifestyle and are likely to be working as compared with older people. More people from the young stroke group had an intracranial hemorrhage and hemorrhagic stroke. The reason for the same may be because of accelerated and undiagnosed hypertension. The various reasons contributing to a higher incidence of intracranial hemorrhage and hemorrhagic stroke in the younger people besides hypertension may be cortical venous thrombosis, peripartum stroke, drug abuse, and cardioembolic stroke. The use of recreational drugs and drug abuse is alarming in our part of the country. Near border areas of Punjab, the rate of drug abuse (especially heroin and cocaine) is as high as 75% in 15–25 years of age group.[24] Cocaine abuse is associated with a high risk of ischemic stroke in about 25–60% of people as well as hemorrhagic stroke.[24] Crack cocaine is associated with both ischemic strokes and hemorrhage strokes, whereas cocaine hydrochloride results more often in hemorrhagic events.[24,25]
Usually, the modifiable risk factors are the same for both younger and older subgroups; however, their prevalence is not the same in these two age groups. Hypertension, heart disease (including atrial fibrillation), and diabetes mellitus are the most common risk factors among the elderly people. In our study, hypertension (72%), diabetes mellitus (23%), dyslipidemia (15%), and drug addiction (9%) were the common risk factors in people with young stroke. Eight percent of young stroke patients were detected to have atrial fibrillation, of which 3% had rheumatic heart disease and the remaining 5% had nonvalvular AF. It may be caused by underlying coronary artery disease as 6% had underlying coronary artery disease. Past and present tobacco use (22% and 9%, respectively) was more common in young stroke people; though past tobacco use was found statistically significant in young stroke. In another study conducted in Finland, among 1008 young stroke patients, the most common vascular risk factors were dyslipidemia (60%), smoking (44%), and hypertension (39%).[1] As per Helsinki young stroke registry, the distribution of vascular risk factors in 3944 young stroke patients from three distinct geographic regions found was current smoking (49%), dyslipidemia (46%), and hypertension (36%).[26]
We had a short-term follow-up in our study and mRS was measured in all the patients on 28 days of stroke. Short-term outcome at 28 days was found to be better in the young stroke subgroup. A better outcome at a young age can be explained by various reasons, which may be because of their age, chances of more recovery at this age as there is a better collateral reserve in young subjects; there is more scope for functional recovery as compared to older people. Despite improvements in diagnosis and treatment, from the patient's perspective, ischemic stroke in young adults remains a catastrophic event.
CONCLUSION
In our population study, hypertension, diabetes mellitus, and dyslipidemia were found to be common risk factors in younger people similar to older patients; however, drug abuse and tobacco past and present use were also among the risk factors. Stroke recovery was better in younger people at 28 days. As the burden of stroke is increasing, especially in the younger people, therefore, there is a need to conduct larger population-based studies to determine the precise incidence, risk factors, to predict outcome, and to ascertain etiologies.
Financial support and sponsorship
ICMR: The population-based stroke registry was supported by the Indian Council of Medical Research, New Delhi (grant ICMR/SWG/22Neuro/2008-NCD-1 (2009–0956) A and B).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: The Helsinki young stroke registry. Stroke. 2009;40:1195–203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 2.Dzevdet S. Strokes in young adults: Epidemiology and prevention. Vasc Health Risk Manage. 2015;11:157–64. doi: 10.2147/VHRM.S53203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renna R, Pilato F, Profice P, Della Marca G, Broccolini A, Morosetti R, et al. Risk factor and etiology analysis of ischemic stroke in young adult patients. J Stroke Cerebrovasc Dis. 2014;23:e221–7. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: The excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 5.Putaala J. Ischemic stroke in the young: Current perspectives on incidence, risk factors, and cardiovascular prognosis. European Stroke J. 2016;1:28–40. doi: 10.1177/2396987316629860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghandehari K, Izadi-Mood Z. Etiology of young adult onset brain infarction in Iran. Arch Iran Med. 2006;9:240–3. [PubMed] [Google Scholar]
- 7.Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke. 1990;21:382–6. doi: 10.1161/01.str.21.3.382. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the northern Manhattan stroke study. Stroke. 2002;33:2789–93. doi: 10.1161/01.str.0000038988.64376.3a. [DOI] [PubMed] [Google Scholar]
- 9.Smajlović D. Strokes in young adults: Epidemiology and prevention. Vas Health Risk Manag. 2015;11:157–64. doi: 10.2147/VHRM.S53203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiamkao S, Sawanyawisuth K, Silaruks S, Kiatchoosakun S, Tatsanavivat P, Chotmongkol V, et al. Correlation of causes and outcomes in stroke in the young. J Stroke Cerebrovasc Dis. 2013;22:55–7. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Orencia AJ, Petty GW, Khandheria BK, Annegers JF, Ballard DJ, Sicks JD, et al. Risk of stroke with mitral valve prolapse in population-based cohort study. Stroke. 1995;26:7–13. doi: 10.1161/01.str.26.1.7. [DOI] [PubMed] [Google Scholar]
- 12.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: Historical perspective and challenges ahead. Stroke. 2011;42:2351–5. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American heart association prevention conference. IV. Prevention and rehabilitation of stroke. Risk factors. Stroke. 1997;28:1507–17. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 14.Kamalakannan S, Gudlavalleti AS, Gudlavalleti VS, Goenka S, Kuper H. Incidence and prevalence of stroke in India: A systematic review. Indian J Med Res. 2017;146:175–85. doi: 10.4103/ijmr.IJMR_516_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee TK, Mukherjee CS, Sarkhel A. Stroke in the urban population of Calcutta-An epidemiological study. Neuroepidemiology. 2001;20:201–7. doi: 10.1159/000054788. [DOI] [PubMed] [Google Scholar]
- 16.Kalkonde YV, Deshmukh MD, Sahane V, Puthran J, Kakarmath S, Agavane V, et al. Stroke is the leading cause of death in rural Gadchiroli, India: A prospective community-based study. Stroke. 2015;46:1764–8. doi: 10.1161/STROKEAHA.115.008918. [DOI] [PubMed] [Google Scholar]
- 17.Pandian JD, Singh G, Bansal RK, Paul BS, Singla M, Singh S, et al. Establishment of population based stroke registry in Ludhiana city, northwest India: Feasibility and methodology. Neuroepidemiology. 2015;44:69–77. doi: 10.1159/000371520. [DOI] [PubMed] [Google Scholar]
- 18.Pandian JD, Singh G, Kaur P, Bansal R, Paul BS, Singla M, et al. Incidence, short-term outcome and spatial distribution of stroke patients in Ludhiana, India. Neurology. 2016;86:425–33. doi: 10.1212/WNL.0000000000002335. [DOI] [PubMed] [Google Scholar]
- 19.Prasad K, Singhal KK. Stroke in young: An Indian perspective. Neurol India. 2010;58:343–50. doi: 10.4103/0028-3886.65531. [DOI] [PubMed] [Google Scholar]
- 20.WHO STEPS Stroke Manual Version 1.2. The WHO STEP wise approach to stroke surveillance. [Last accessed on 2010 Jul 02]. Available from: http://www.who.int/ ncd_surveillance/en/steps_stroke_manual_v1.2.pdf .
- 21.Das SK, Banerjee TK, Biswas A, Roy T, Raut DK, Mukherjee CS, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38:906–10. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 22.Dalal P, Bhattacharjee M, Vairale J, Bhat P. International stroke society-WHO global stroke initiative: A report on population-based Mumbai stroke registry (2005-2006), India. Int J Stroke. 2009;4:239–40. doi: 10.1111/j.1747-4949.2009.00313.x. [DOI] [PubMed] [Google Scholar]
- 23.Smajlović D, Sinanović O. Sensitivity of the neuroimaging techniques in ischemic stroke. Med Arh. 2004;58:282–4. [PubMed] [Google Scholar]
- 24.Fonseca AC, Ferro JM. Drug abuse and stroke. Curr Neurol Neurosci Rep. 2013;13:325. doi: 10.1007/s11910-012-0325-0. [DOI] [PubMed] [Google Scholar]
- 25.Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad Med J. 2007;83:389–94. doi: 10.1136/pgmj.2006.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putaala J, Yesilot N, Waje-Andreassen U, Pitkäniemi J, Vassilopoulou S, Nardi K, et al. Demographic and geographic vascular risk factor differences in European young adults with ischemic stroke: The 15 cities young stroke study. Stroke. 2012;43:2624–30. doi: 10.1161/STROKEAHA.112.662866. [DOI] [PubMed] [Google Scholar]


