Abstract
Lumpy skin disease (LSD) is a transboundary viral disease of cattle and buffaloes transmitted by blood-feeding vectors and causes high morbidity and low-to-moderate mortality. Since the first observation of LSD in Zambia in 1929, it has spread in cattle populations across African countries, the Middle East, Europe, and Asia. Following the recent outbreaks of LSD in South Asian countries such as India and Bangladesh, the disease was first reported in cattle farms in Nepal in June 2020. This study investigated the Nepalese LSD outbreak and confirmed that the disease spread rapidly to three neighboring districts in a month, infecting 1300 animals. Both cattle and buffaloes showed common clinical signs of LSD, with the exception that the buffaloes presented small nodular lesions without centered ulcerations. The collected samples were first tested for the presence of LSDV by real-time PCR. We further applied molecular tools, RPO30, GPCR, EEV glycoprotein gene, and B22R, for additional characterization of the LSDV isolates circulating in Nepal. Using a PCR-based Snapback assay, we confirmed that samples collected from cattle and buffaloes were positive of LSDV. Furthermore, sequence analysis (phylogenetic and multiple sequence alignments) of four selected LSDV genes revealed that the Nepal LSDVs resemble the Bangladesh and Indian isolates and the historic isolates from Kenya. We also highlight the importance of a unique B22R gene region harboring single-nucleotide insertions in LSDV Neethling and LSDV KSGPO-240 vaccine strains, enabling us to differentiate them from the Nepalese isolates and other fields isolates. This study demonstrates the importance of disease surveillance and the need to determine the source of the disease introduction, the extent of spread, modes of transmission, and the necessary control measures.
Keywords: LSDV, Nepal, sequence, outbreak
1. Introduction
Lumpy skin disease (LSD) is a transboundary viral disease of cattle and buffaloes with a significant economic impact on the livestock industry, including financial losses [1,2]. The etiological agent is the LSD virus (LSDV), a DNA virus of the genus Capripoxvirus (CaPV) in the Poxviridae family. Sheeppox virus (SPPV) and goatpox virus (GTPV) are two other capripoxviruses that infect sheep and goats, respectively. They are known to be antigenically similar to LSDV [3].
LSDV is transmitted by blood-feeding vectors such as stable flies, mosquitoes, and ticks [4,5,6]. LSD causes high morbidity and low-to-moderate mortality. The disease may vary from subclinical infection to death based on the virus strain, vector prevalence, age, or the host’s immune status [7]. The clinically sick cattle present anorexia, enlarged superficial lymph nodes, nasal discharge, watery eyes, and firm flat-topped papules and nodules all over the body, including head, neck, udder, scrotum, and buccal mucosa [8,9].
LSD was first observed in Zambia in 1929, then spread rapidly in cattle population across African countries and became endemic. From 2012, the disease reached the Middle East, Europe, and South Asia [10] successively. While the initial LSDV genome in Africa was stable or had minor differences for decades, including for vaccines related field isolates which were only sequenced recently [11], the field isolates recovered after the transcontinental spread of LSD revealed significant genomic variabilities [12,13]. For instance, the molecular characterization of the field LSDV isolates from Russia and China showed vaccine-like profiles attributable to the recombination events between the Neethling vaccine strain and field isolates related to LSDV KSGPO-240 [14,15,16]. Notably, the recombinant LSDVs appeared more pathogenic than the typical field isolates [16,17].
Additionally, capripoxviruses are known to be cross-reactive. Thus, SPPV or GTPV based vaccines have also been used against LSDV [3,18]. Therefore, several molecular-based techniques were developed to correctly identify species within the Capripoxvirus genus and further differentiate live attenuated vaccines from field isolates and the recombinant LSDV strains [19,20,21,22,23,24,25]. Those assays target selected viral genomic regions, such as the G-protein-coupled receptor (GPCR), the RNA polymerase 30 kDa subunit (RPO30), the CaPV homolog of the variola virus B22R gene, and the EEV glycoprotein [26,27,28,29,30].
Nepal reported its first LSD case in cattle farms in June 2020 [31], following the recent outbreaks of LSD in South Asian countries such as China, India, and Bangladesh [32,33,34]. These first cases in Nepal occurred in a district near the Indian border. By the end of July 2020, the disease had spread to three more neighboring districts in Nepal, affecting 1300 animals. Samples collected from the suspected cases were confirmed to be LSD positive at the Central Veterinary Laboratory (CVL), Veterinary Complex, Kathmandu, Nepal, by Real-time polymerase chain reaction (RT-PCR) of the GPCR gene [31]. The current study aims to confirm the occurrence of LSDV in cattle and buffaloes in Nepal and provide a molecular characterization of the field LSDV isolates circulating in Nepal.
2. Materials and Methods
2.1. Study Area and Sample Collection
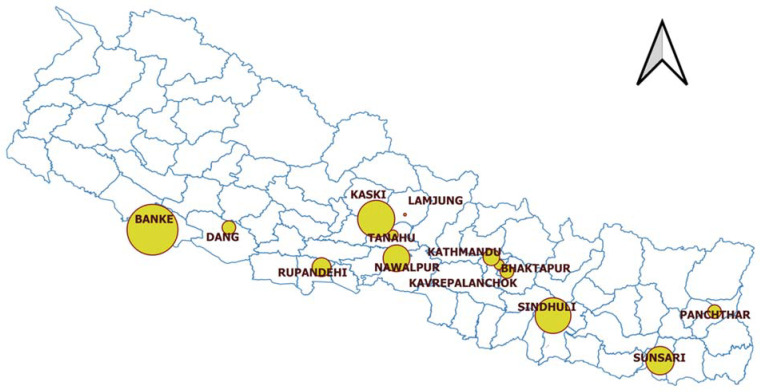
This study includes the samples collected following the spread of LSD after the first outbreak of the disease in Nepal. During the episode, suspected cases of LSD were noticed in cattle and captive buffaloes as of June 2020 in the Eastern part of Nepal. The suspected animals were sick and showed LSD-like symptoms, including high fever and nodular lesions on different body parts. Later, other provinces reported similar symptoms on cattle and buffaloes. The Central Veterinary Laboratory confirmed LSD, enabling the notification to OIE of the first LSD case in Nepal in July 2020. The disease then spread progressively throughout the country. For further investigation of the outbreak, samples from different tissues (scab, serum, nasal swab, ocular swab, and skin nodules) of the clinically infected cattle and buffaloes were collected from thirteen districts (representing four provinces) between August and October 2020 for further characterization (Figure 1). The total number of susceptible cattle and buffalo populations and the reported morbidities and mortalities in the thirteen districts are summarized in Table 1. The collected samples were transported to the Central Veterinary Laboratory (CVL).
Figure 1.
Map showing the geographical locations and sample size of the LSD-affected districts in Nepal between August and October 2020.
Table 1.
Features of the LSD outbreak in Nepal in cattle and buffaloes between August and October 2020.
| District | Administration Region | No. of Susceptible Cases | No. of Clinically Affected Cases | No. of Dead Cases | Morbidity Rate (%) | Species | Type of Sample | Collection Date | Sample ID |
|---|---|---|---|---|---|---|---|---|---|
| Sunsari | Province I | 3780 | 8 | 0 | 0.21 | Cattle | Scab | October 2020 | Sn/C/Sb/10/2020 |
| Sunsari | 728 | 2 | 0 | 0.27 | Buffalo | Serum | October 2020 | Sn/B/S/15/2020 | |
| Panchthar | 238 | 3 | 0 | 1.26 | Cattle | Serum | October 2020 | P/C/S/7/2020 | |
| Kathmandu | Bagmati Province | 809 | 4 | 0 | 0.49 | Cattle | Nasal swab | August 2020 | Kt/C/NS/4/2020 |
| Kathmandu | Cattle | Nasal swab | August 2020 | Kt/C/NS/5/2020 | |||||
| Bhaktapur | 758 | 2 | 0 | 0.26 | Cattle | Scab | October 2020 | Bkt/C/Sb/6/2020 | |
| Sindhuli | 1969 | 15 | 0 | 0.76 | Cattle | Scab | September 2020 | S/C/Sb/9/2020 | |
| Sindhuli | Cattle | Nasal swab | September 2020 | S/C/NS/11/2020 | |||||
| Kavre | 3987 | 3 | 0 | 0.075 | Buffalo | Serum | September 2020 | Kr/B/S/8/2020 | |
| Kaski | Gandaki Province | 4682 | 4 | 0 | 0.08 | Buffalo | Scab | August 2020 | K/B/Sb/1/2020 |
| Kaski | Buffalo | Scab | August 2020 | K/B/Sb/2/2020 | |||||
| Kaski | 3170 | 12 | 0 | 0.38 | Cattle | Serum | September 2020 | K/C/S/16/2020 | |
| Tanahu | 4164 | 2 | 0 | 0.05 | Cattle | Scab | August 2020 | T/C/Sb/3/2020 | |
| Lamjung | 1100 | 1 | 0 | 0.09 | Cattle | Tissue | September 2020 | L/C/T/12/2020 | |
| Nawalpur | 1799 | 9 | 0 | 0.50 | Cattle | Serum | September 2020 | N/C/S/14/2020 | |
| Rupandehi | Lumbini Province | 5500 | 5 | 0 | 0.09 | Cattle | Ocular swab | September 2020 | R/C/OS/13/2020 |
| Banke | 1623 | 28 | 0 | 1.72 | Cattle | Scab swab | October 2020 | B/C/Sb/17/2020 | |
| Banke | Cattle | Scab swab | October 2020 | B/C/Sb/18/2020 | |||||
| Dang | 5958 | 3 | 0 | 0.05 | Cattle | Tissue (Skin nodules) | October 2020 | D/C/T/19/2020 | |
| Dang | Cattle | Tissue (Skin nodules) | October 2020 | D/C/T/20/2020 | |||||
| Total | 30868 | 92 | 0 | 0.3 | Cattle | ||||
| 9397 | 9 | 0 | 0.09 | Buffalo | |||||
2.2. Sample Processing and DNA Extraction
The samples (scab, nasal swab, skin nodules, serum) were processed in CVL. The skin nodules were cut into small pieces and ground in PBS. Scabs and nasal swabs were collected in PBS, centrifuged, and the supernatant was collected in sterile vials. 200 μL of the supernatant was used for DNA extraction using PureLinkTM Viral RNA/DNA Mini Kit (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. The DNA was eluted in 50 μL of elution buffer and stored at −80 °C until further use.
2.3. Molecular Detection and Differential Diagnosis
A previously developed Snapback assay was applied to detect and genotype the capripoxviruses found in the collected samples [29]. Table 2 presents the sequences of the primers used in this assay. This assay enables distinguishing the three genotypes of capripoxviruses: SPPV, GTPV, or LSDV based on the differences in the melting temperature (Tm) of the snapback stem and the PCR amplicons obtained following the fluorescence melting curve analysis. The PCR mixture was set up in a 20 μL reaction volume, containing 10 μL of the iQsupermix (Bio-Rad), 500 nM of the Snapback forward primer, 40 nM of the reverse primer, and 2 μL of template DNA. The PCR program consisted of an initial denaturation step at 95 °C for 3 min, followed by 45 cycles at 95 °C for 15 s and 58 °C for 80 s with the fluorescence reading at the end, followed by 95 °C for 1 min, 40 °C for 1 min, and melting between 40–85 °C at 10 s/0.5 °C with the fluorescence reading at each °C then 37 °C for 1 min.
Table 2.
List of the primers used for Snapback assay and amplification and sequencing of the targeted genes. The snapback tail of 16 bases is shown as underlined.
| Target Gene | Primer | Amplicon Size |
|---|---|---|
| RPO30 Snapback | Cp-HRM-SBF-5′-ggTGTAGTACGTATAAGATTATCGTATAGAAACAAGCCTTTA-3′ | SNAP BACK assay |
| Cp-HRM1R-5′-AATTTCTTTCTCTGTTCCATTTG-3′ | ||
| GPCR_ FRET | CpRt Forward- 5′-GATAGTATCGCTAAACAATGG-3′ | 200 bp |
| CpRt Reverse- 5′-ATCCAAACCACCATACTAAG-3′ | ||
| RPO30 | Cp-OL1F 5′-CAGCTGTTTGTTTACATTTGATTTTT-3′ | 554 bp |
| Cp-OL1R 5′-TCGTATAGAAACAAGCCTTTAATAGA-3′ | ||
| Cp-OL2F 5′-TTTGAACACATTTTATTCCAAAAAG-3′ | 520 bp | |
| Cp-OL2R 5′-AACCTACATGCATAAACAGAAGC-3′ | ||
| GPCR | CpGPCR-OL1F-5′-TGAAAAATTAATCCATTCTTCTAAACA-3′ | 684 bp |
| CpGPCR-OL1R-5′-TCATGTATTTTATAACGATAATGCAAA-3′ | ||
| CpGPCR-OL2F-5′-TTAGCGGTATAATCATTCCAAATA -3′ | 603 bp | |
| CpGPCR-OL2R-5′-GCGATGATTATGATGATTATGAAGTG-3‘ | ||
| CpGPCR-OL3F-5′-CACAATTATATTTCCAAATAATCCAA -3′ | 617 bp | |
| CpGPCR-OL3R-5′-TGTACATGTGTAATTTTAATGTTCGTA-3′ | ||
| EEV glycoprotein gene (ORF LSDV126) | EEVGly F- 5′-ATGGGAATAGTATCTGTTGTATACG-3′ | 250 bp |
| EEVGly R-5′-CGAACCCCTATTTACTTGAGAA-3′ | ||
| B22R | B22R F- 5′-TCATTTTCTTCTAGTTCCGACGA-3′ | 863 bp |
| B22R R- 5′-TTCGTTGATGATAAATAACTGGAAA-3′ |
2.4. Amplification of Selected Genes and Sequencing
To confirm the identity and further characterize the LSDV positive samples, four genes: the RPO30, GPCR, Extracellular enveloped virus (EEV) glycoprotein, and CaPV homolog of the variola virus B22R genes were amplified by PCR as previously described using the primers listed in Table 2. All PCRs were performed in a 20 μL reaction volume containing 500 nM of each of the forward and the reverse primers, 200 uM of dNTPs, 1x PCR buffer (Qiagen), 1.25 U of Taq DNA polymerase (Qiagen), and 2 μL template DNA. After amplification, the PCR products were analyzed on a 2% gel electrophoresis and visualized on a Gel Documentation System (Bio-Rad). The PCR products were purified using the PCR clean-up system kit (Promega), following the manufacturer’s instructions. The products were sequenced using both forward and reverse primers at LGC Genomics (Germany). The nucleotide sequences were deposited in the GenBank database under the accession numbers OL689584 to OL689619.
2.5. Sequence and Phylogenetic Analysis
The raw sequences were assembled using the Vector NTI software (Invitrogen) version 11.5. The sequences for each targeted gene for all the samples, together with representative CaPV sequences retrieved from GenBank, were aligned with MEGA7 using the Muscle algorithm and the codon option [35]. The alignment files of the complete RPO30 and GPCR gene sequences were converted to a Nexus format using Seaview. The Bayesian phylogenetic inference was performed using BEAST v1.8.4 [36]. The log files generated by BEAST were inspected using the TRACER v1.7.1 program to determine the optimum burn-in value number, based on the effective sample sizes (ESS). TreeAnnotator was then used to generate the maximum clade credibility (MCC) file based on the burn-in value. The trees were visualized and annotated using the Interactive tree of life (iTOL) [37]. The LSDV clusters based on the presence/absence of insertion in the GPCR or EEV glycoprotein were visualized with the RPO30 tree. The multiple sequence alignments of the partial EEV glycoprotein and B22R genes were visualized using BioEdit (v7.2.6).
2.6. Targeted Nanopore Sequencing of Short PCR Fragments in the Buffalo Serum
Following the unsuccessful amplification and sequencing of the four targeted genes in the positive buffalo samples, we have undertaken to amplify and sequence short genome fragments to confirm the presence of the LSDV genome. Using the fluorescence resonance energy transfer assay (FRET) [31], and Snapback assay [29] primers, in the absence of probes or ds DNA intercalating dyes, we have amplified a short segment of each the RPO30 gene (96 bp) and GPCR gene (200 bp). The resulting amplicons were purified and sequenced using the MinION device of nanopore as described below: The purified PCR products were used to prepare the library using Amplicons by Ligation Kit (SQK-LSK109) according to Oxford Nanopore Technologies (ONT) standard protocols. The prepared library was sequenced on R9 flow cell. Data acquisition and real-time basecalling was performed with the MinKNOW v3.5.10.1 and the integrated Guppy v3.2.6 (ONT).
The raw sequences were cleaned to remove the adaptors, using porechop (v 0.2.4), and the low-quality and short reads, using NanoFilt (v2.8.0). The quality of the remaining reads was assessed with NanoStat (v1.6.0). After mapping the cleaned raw reads against the GPCR gene and the RPO30 gene of LSDV NI-2490 (NC_003027), using minimap2 (v2.24), SAMtools (v1.11) was used to generate Mpileup files and performed variant calling using BCFtools (v1.9). The sorted reads were displayed within the Integrated Genome Viewer (IGV, v2.8.0) browser for visualization. The consensus sequences were produced with vcfutils.pl (VCFtools v0.1.16) and seqtk (v1.3.106) and compared to the corresponding fragments in publicly available LSDVs sequences by multiple sequence alignments using BioEdit (v7.2.6).
3. Results
3.1. Clinical Signs and Symptoms and Outbreak Investigations
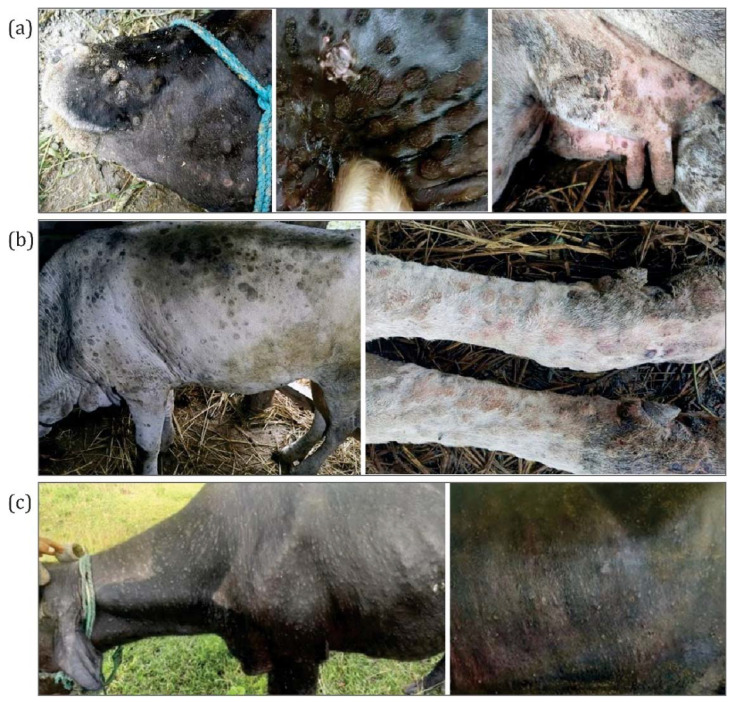
Most of the affected cattle and buffaloes showed common clinical signs and symptoms such as fever (40–41.5 °C), loss of appetite, nasal and ocular discharge, and generalized skin nodules all over the body. Besides, the sick animals presented enlarged sub-scapular and pre-femoral lymph nodes and a sharp drop in milk production. Some infected cattle also presented necrotic plaques in the oral and nasal mucous membranes, ulcerated nodules, and scabs. However, in the case of buffaloes, only small nodular lesions without centered ulcerations were observed. Figure 2 illustrates the nodular skin lesions over the face, neck, limbs, and all over the body of cattle and buffalo.
Figure 2.
Lesions in LSD suspected animals. (a) Local Terai cow with nodular skin lesions over the face, neck, and udder. (b) Local cow with nodular skin lesions all over the body and limbs. (c) Buffalo showing small skin nodules all over the body.
Ninety-two cattle and nine buffaloes out of 30,868 and 9397 susceptible cattle and buffaloes were clinically affected from the thirteen districts. The overall morbidity rate was 0.3% for cattle and 0.1% for buffaloes. The morbidity rate in different districts ranged from 0.05 to 1.7% for cattle and 0.08 to 0.3% for buffaloes (Table 1). No deaths were reported in the districts included in this study. The vectors known for transmitting LSDV, such as ticks and mosquitoes, were also observed within the farms.
3.2. Molecular Detection and Diagnosis of LSDV
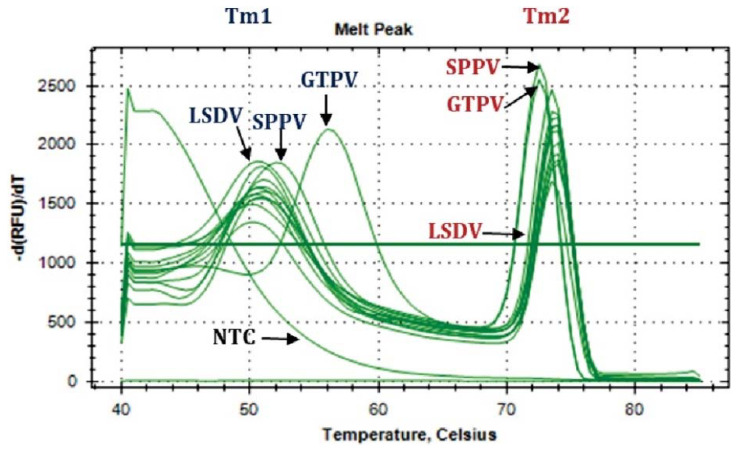
As summarized in Table 3, CaPV DNA was detected in 14 out of 20 samples (12 cattle and two buffalo samples) using the PCR-based Snapback assay. Furthermore, based on the melting temperature of the Snapback stem (Tm1) and that of the PCR amplicons (Tm2), the CaPV positive samples were confirmed to be LSDV positive, as shown by the corresponding melting temperatures (Figure 3 and Table 3). Subsequently, only samples with a Cq of 30.00 or less were sequenced for further analyses.
Table 3.
PCR-based snap back assay results for detection and genotyping of LSDV.
| S. No. | Sample ID | Host | SNAP BACK Assay Analysis | Remarks | ||
|---|---|---|---|---|---|---|
| Cq | Tm1 (°C) | Tm2 (°C) | ||||
| 1 | K/B/Sb/1/2020 | Buffalo | N/A | N/A | N/A | Negative |
| 2 | K/B/Sb/2/2020 | Buffalo | N/A | N/A | N/A | Negative |
| 3 | T/C/Sb/3/2020 | Cattle | 20.18 | 51.00 | 74.00 | LSDV |
| 4 | Kt/C/NS/4/2020 | Cattle | 28.73 | 50.50 | 74.00 | LSDV |
| 5 | Kt/C/NS/5/2020 | Cattle | N/A | N/A | N/A | Negative |
| 6 | Bkt/C/Sb/6/2020 | Cattle | 24.25 | 51.00 | 74.00 | LSDV |
| 7 | P/C/S/7/2020 | Cattle | 37.38 | 50.50 | 73.50 | LSDV |
| 8 | Kr/B/S/8/2020 | Buffalo | 24.86 | 50.00 | 73.50 | LSDV |
| 9 | S/C/Sb/9/2020 | Cattle | 22.48 | 51.00 | 74.00 | LSDV |
| 10 | Sn/C/Sb/10/2020 | Cattle | 19.83 | 51.00 | 74.00 | LSDV |
| 11 | S/C/NS/11/2020 | Cattle | 24.83 | 51.00 | 74.00 | LSDV |
| 12 | L/C/T/12/2020 | Cattle | 25.14 | 51.00 | 74.00 | LSDV |
| 13 | R/C/OS/13/2020 | Cattle | N/A | N/A | N/A | Negative |
| 14 | N/C/S/14/2020 | Cattle | N/A | N/A | N/A | Negative |
| 15 | Sn/B/S/15/2020 | Buffalo | 36.34 | 51.00 | 73.50 | LSDV |
| 16 | K/C/S/16/2020 | Cattle | N/A | N/A | N/A | Negative |
| 17 | B/C/Sb/17/2020 | Cattle | 24.97 | 51.00 | 74.00 | LSDV |
| 18 | B/C/Sb/18/2020 | Cattle | 29.28 | 50.50 | 74.00 | LSDV |
| 19 | D/C/T/19/2020 | Cattle | 31.02 | 50.00 | 73.50 | LSDV |
| 20 | D/C/T/20/2020 | Cattle | 22.87 | 50.50 | 74.00 | LSDV |
| GTPV | GTPV positive control | N/A | 27.82 | 56.00 | 72.50 | GTPV |
| SPPV | SPPV positive control | N/A | 27.88 | 52.00 | 72.50 | SPPV |
| LSDV | LSDV positive control | N/A | 24.90 | 50.50 | 73.50 | LSDV |
| NTC | RNAse free water | N/A | N/A | N/A | N/A | Negative |
Figure 3.
Melting peaks (Tm) of samples from Nepal compared to the positive control of GTPV, SPPV, and LSDV. Both melting temperatures for the snapback stem (Tm1) and the PCR amplicons (Tm2) are shown.
3.3. Amplification and Sequencing
Nine LSDV positive cattle samples were successfully amplified and sequenced for the complete RPO30 and GPCR genes and partial EEV glycoprotein and B22R genes. After the quality check and editing, the appropriate size, 606, 1146, 327, and 832 bp, for the RPO30, GPCR, EEV, and B22R genes, respectively, the sequences were deposited to GenBank. In addition, for one bufallo sample, we have successfully amplified and sequenced using nanopore technology two short fragments in the RPO30 and GPCR genes, corresponding to the target of the the snapback and FRET assays (Figure S1).
3.4. Sequence and Phylogenetic Analysis
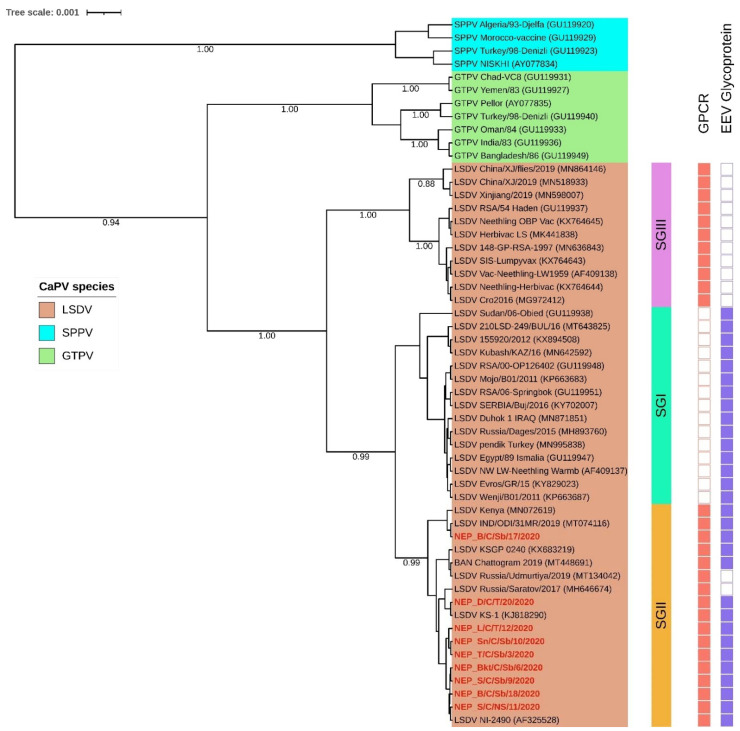
The multiple sequence alignments showed 100% similarity among the nine Nepalese LSDV positive cattle samples for all the four targeted genes. Besides, sequence alignment of the short segments of the RPO30 and GPCR sequences of the LSDV positive buffalo sample, with other Nepalese LSDVs, showed 100% similarity to the LSDV isolate in cattle (Figure S2). In the phylogenetic analysis based on the complete RPO30 gene sequences, Nepal isolates clustered in the LSDV subgroup SG II. SG II also included recent LSDV isolates from Bangladesh and India (2019), two historical LSDVs, LSDV Kenya and LSDV NI-2490. In addition, the vaccine strain LSDV KSGPO-240 and two recombinant LSDVs from Russia (LSDV Udmurtiya/2019, and LSDV Saratov/2017) also belonged to SG II (Figure 4). The multiple sequence alignment of the complete GPCR gene sequences showed a 12-nucleotide insertion in Nepal isolates similar to the recombinant LSDVs from Russia, LSDVs from Bangladesh and India, LSDV NI-2490, LSDV Kenya, and Chinese LSDVs. This marker is typically present in LSDV vaccine strains such as LSDV KSGPO-240 and LSDV Neethling vaccine strain as well as LSDVs in SG II and SG III (Figure 4). However, in the GPCR phylogenetic analysis, the Nepalese isolates clustered in sub-group SG I with only LSDVs from Bangladesh and India, and LSDV KSGPO-240, LSDV NI-2490, and LSDV Kenya and not with the recombinant LSDVs from Russia (Figure S3).
Figure 4.
Maximum clade credibility (MCC) tree based on the complete RPO30 gene sequences of CaPVs with LSDVs from Nepal (in red) visualized on iTOL, together with isolates clustering based on the presence (filled box) or absence (empty box) of sequence insertion in the GPCR and the EEV glycoprotein genes.
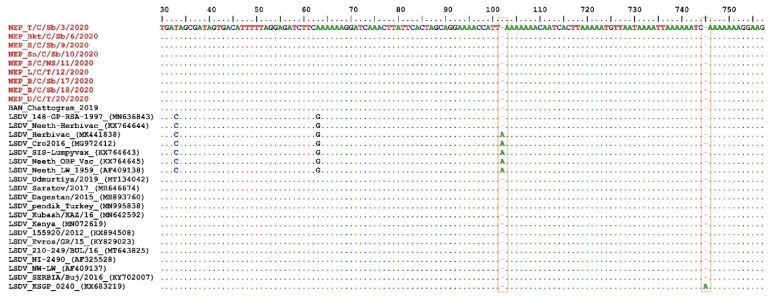
The multiple sequence alignment of the EEV glycoprotein gene showed a 27-nucleotide insertion (175-201) in Nepalese LSDVs, similar to LSDVs from Bangladesh, LSDV KSGPO-240, LSDV NI-2490, and LSDV Kenya. Interestingly, this 27-nucleotide insertion differentiated SGI and SGII from SGIII, which include LSDV Neethling derived vaccines and Chinese LSDVs, and from the recombinant LSDVs from Russia (Figure 4 and Figure S4). Moreover, the multiple sequence alignment of the targeted B22R fragment reiterated that the Nepalese isolates were like the LSDV NI-2490 and LSDV Kenya and LSDVs from Bangladesh. However, they differed from LSDV Neethling and LSDV KSGPO-240 derived vaccines with nucleotide insertion at nucleotide alignment positions 102 and 745, respectively (Figure 5).
Figure 5.
Multiple sequence alignments of the partial nucleotide sequences of the B22R gene. The Nepal isolates (in red) were aligned with representative LSDV sequences retrieved from GenBank. The nucleotide insertion in LSDV Neethling and LSDV KSGPO-240 vaccines that are absent in Nepal isolates are shown in the blocks. The dots indicate the identical nucleotides in the alignment.
4. Discussion
Although LSDV has spread from Africa to the Middle East and East Europe since 2012, the disease only reached East and South Asia in 2019, affecting China, India, Bangladesh, Bhutan, Vietnam, Thailand, Hong Kong, and Myanmar [38,39]. The first suspected LSD cases in Nepal were reported in June 2020 in the Morang district near the Indian border and were confirmed by molecular techniques [31]. The disease spread within four weeks to three neighboring districts, with a series of the suspected diseased animals showing typical LSD clinical signs such as generalized nodular skin disease, sharp drop in milk production, and infertility [8]. The overall morbidity rate observed in this study was low, and no mortality cases were reported. Low mortality rate to a total absence of mortality was also reported in recent LSD outbreaks in India (0%), Bangladesh (0.002%), Russia (0%), and Greece (0.40%) [32,34,40,41]. The main explanation for the low to the absence of mortality could be the low virulence of this LSDV strain or the influence of host factors such as the species, breed, age, immune status, and stage of production of the host [7].
The Snapback assay confirmed that all the CaPV positive samples from cattle or buffaloes contained LSDV and not GTPV or SPPV [29]. Furthermore, the multiple sequence alignments of each of the four targeted LSDV genes showed that all Nepalese LSDVs were identical. Although we have failed to amplify and sequence the full length of the four targeted genes of the LSDV from buffalo, we successfully obtained short RPO30 and GPCR fragments by nanopore sequencing. The multiple sequence alignment of these short fragments confirmed the presence of the LSDV genome in the buffaloes and also suggested that the buffalo LSDV isolates could be similar to the cattle LSDV isolates in Nepal. It has been reported that LSDV viremia is relatively short-lived in blood, 4–11 days post-infection [42]. Therefore, it is possible that the LSDV DNA in buffalo blood samples was degraded, and only short fragments targeted by the LSDV Snapback and FRET primers could be amplified and not the long PCR fragments targeted for Sanger sequencing in this study. This agrees with Zeynalova et al., 2016 [43], who previously concluded that skin nodules were better-suited samples for LSDV PCR detection than blood samples.
In the RPO30 gene tree, the Nepalese LSDVs clustered within LSDV subgroup SG II, together with LSDV isolates from the neighboring Bangladesh and India, as well as LSDV Kenya and LSDV NI-2490, two historical LSDVs collected in Kenya before the 1960s, two recombinant LSDV field isolates from Russia and the LSDV KSGPO-240 derived vaccines [14,32]. However, the Nepalese isolates clustered separately from LSDVs from neighboring China located within LSDV subgroup SG III. In addition, the GPCR sequences of the Nepalese LSDVs presented a 12-nucleotide insertion like LSDV NI-2490, LSDV Kenya, LSDVs from Bangladesh, India, and China, LSDV vaccines, and recombinant LSDVs from Russia. This insertion differentiates the Nepalese isolates from other GPCR LSDV subgroup SG I members from the Middle East, Europe, and Africa [27].
Furthermore, the 27-nucleotide deletion in the EEV glycoprotein gene in Nepalese LSDVs, similar to LSDVs from Bangladesh and LSDV NI-2490 and LSDV Kenya, two historical LSDVs from Kenya, distinguished them from the Chinese LSDV isolates and the recombinant LSDVs from Russia. Lastly, we have also exploited a unique region of the B22R gene to differentiate the Nepalese isolates from LSDV Neethling and LSDV KSGPO-240 vaccine, using single nucleotide insertions present in each of the two vaccine strains (positions 102 and 745, respectively) [28]. Therefore, based on sequence analysis for all the four selected LSDV genes: the RPO30, GPCR, EEV glycoprotein, and the B22R, the Nepal LSDV isolates resemble the Bangladesh isolates and the historic isolates NI-2490 and LSDV Kenya [32]. Although only the full-length RPO30 and GPCR gene sequences of the Indian isolate are available, both are 100% identical to the Nepal isolate, which suggests that they are very closely related [34]. Notably, the LSD outbreak in Nepal was reported soon after the LSD outbreak in the neighboring countries, Bangladesh and India. The fact that the strains circulating in these three countries are similar suggests that they may have a common source of infection for these countries. Although the Nepalese isolates were similar to the KSGPO-240 vaccine strain, there was no report of a vaccination program against LSD in Nepal before the outbreak. Previous studies have shown that using the KSGPO-240 vaccine could lead to lesions in vaccinated animals similar to the LSD symptoms [44,45]. However, since the disease is transmitted by arthropod vectors capable of moving across countries such as those reported in the farms in Nepal, it is likely that the LSD virus was introduced to Nepal by animal importation from neighboring countries or by mechanical transmission [4,5]. It was recently suggested that the introduction of LSD in Nepal in June 2020 was likely due to the continuous flow of informal cross-border movements of cattle from India to districts in eastern Nepal, usually by foot, given there are no official records of live cattle or buffalo imports from India in the fiscal year 2019 [46]. Currently, the Department of Livestock Services (DLS) has enforced LSD control measures such as quarantine at the border, control of vectors (flies, mosquitoes and ticks) that play an important role in LSDV transmission as well as continuous surveillance [31]. Regardless, the source of the strains currently circulating in South Asia with genetic features resembling “historic” field African strains and vaccine strains (KSGPO-240) remains obscure and deserves further investigation.
5. Conclusions
In conclusion, based on both clinical signs and molecular characterization, this study confirmed an outbreak of LSDV in both cattle and buffaloes in Nepal. In addition, the circulating Nepalese LSDV isolates were different from the common LSDV field isolates encountered in Africa, the Middle East, and Europe and the newly emerged LSDV variants in Russia and China. However, the Nepalese LSDV isolates were closely related to the historic LSDVs from Kenya and those circulating in the neighboring countries, Bangladesh and India. Therefore, following the current LSDV outbreak in Nepal and the neighboring countries successively, this study reiterates the importance of surveillance and early detection of diseases as a crucial control measure for disease outbreaks.
Acknowledgments
The authors thank the Central Veterinary Laboratory and other Veterinary Laboratories Nepal staff for collecting and providing the samples for this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms10030539/s1, Figure S1: Visualization of aligned nanopore sequencing reads for two targeted fragments in the LSDV positive Buffalo sample (Kr/B/S/8/2020) from Nepal. The reads were mapped against (a) RPO30 and (b) GPCR genes of the LSDV NI-2490 (NC_003027) and displayed in the IGV browser. Figure S2: Multiple sequence alignments of the partial nucleotide sequences of (a) RPO30 and (b) GPCR genes of the Nepal LSDV isolates from cattle (in red) and buffalo (in green) aligned with representative LSDV sequences retrieved from GenBank. The dots indicate the identical nucleotides in the alignment. Figure S3: Maximum clade credibility (MCC) tree based on the complete GPCR gene sequences of CaPVs, with LSDVs from Nepal (in red), visualized on iTOL. Figure S4: Multiple sequence alignments of the partial nucleotide sequences of the EEV glycoprotein gene. The Nepal isolates (in red) were aligned with representative LSDV sequences retrieved from GenBank. A 27-nucleotide deletion absent in Nepal isolates is highlighted in the box. The dots indicate the identical nucleotides in the alignment.
Author Contributions
Conceptualization, P.K., I.K.M. and C.E.L.; methodology, P.K., M.M. and B.K.S.; software, I.K.M.; formal analysis, P.K., I.K.M. and B.K.S.; investigation, P.K., I.K.M., M.M., S.M. and S.K.Y.; resources, S.M., S.K.Y. and G.C; writing—original draft preparation, I.K.M., P.K. and C.E.L.; writing—review and editing, P.K., I.K.M., M.M., S.M., S.K.Y., G.C. and C.E.L.; supervision, S.M., S.K.Y., G.C. and C.E.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by VETLAB network initiative of the Joint FAO/IAEA Division, funded through the Peaceful Uses Initiatives (PUI) by Japan and the United States of America.
Data Availability Statement
The DNA sequences generated and used in the analysis for this study are available in the GenBank under the accession numbers OL689584 to OL689619.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethical Statement
The authors confirm that the ethical policies of the journal, as stated on the journal’s ethical guidelines, have been adhered to. The samples used in this study were those collected and submitted to Central Veterinary Laboratory, Nepal, for LSDV diagnosis. Ethical approval was not required.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gari G., Bonnet P., Roger F., Waret-Szkuta A. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev. Vet. Med. 2011;102:274–283. doi: 10.1016/j.prevetmed.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Davies F.G. Lumpy skin disease, an African capripox virus disease of cattle. Br. Vet. J. 1991;147:489–503. doi: 10.1016/0007-1935(91)90019-J. [DOI] [PubMed] [Google Scholar]
- 3.Abutarbush S.M., Tuppurainen E.S.M. Serological and clinical evaluation of the Yugoslavian RM65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transbound. Emerg. Dis. 2018;65:1657–1663. doi: 10.1111/tbed.12923. [DOI] [PubMed] [Google Scholar]
- 4.Gelaye E., Lamien C.E. Transboundary Animal Diseases in Sahelian Africa and Connected Regions. Springer; Cham, Switzerland: 2019. Lumpy skin disease and vectors of LSDV; pp. 267–288. [Google Scholar]
- 5.Sprygin A., Pestova Y., Wallace D.B., Tuppurainen E., Kononov A.V. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019;269:197637. doi: 10.1016/j.virusres.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Tuppurainen E.S.M., Stoltsz W.H., Troskie M., Wallace D.B., Oura C.A.L., Mellor P.S., Coetzer J.A.W., Venter E.H. A Potential Role for Ixodid (Hard) Tick Vectors in the Transmission of Lumpy Skin Disease Virus in Cattle. Transbound. Emerg. Dis. 2011;58:93–104. doi: 10.1111/j.1865-1682.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- 7.Tuppurainen E.S.M., Venter E.H., Shisler J.L., Gari G., Mekonnen G.A., Juleff N., Lyons N.A., De Clercq K., Upton C., Bowden T.R., et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017;64:729–745. doi: 10.1111/tbed.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babiuk S., Bowden T.R., Boyle D.B., Wallace D.B., Kitching R.P. Capripoxviruses: An emerging worldwide threat to sheep, goats and cattle. Transbound. Emerg. Dis. 2008;55:263–272. doi: 10.1111/j.1865-1682.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 9.Molla W., de Jong M.C.M., Gari G., Frankena K. Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Prev. Vet. Med. 2017;147:100–107. doi: 10.1016/j.prevetmed.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Alkhamis M.A., VanderWaal K. Spatial and temporal epidemiology of lumpy skin disease in the Middle East, 2012–2015. Front. Vet. Sci. 2016;3:19. doi: 10.3389/fvets.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Schalkwyk A., Byadovskaya O., Shumilova I., Wallace D.B., Sprygin A. Estimating evolutionary changes between highly passaged and original parental lumpy skin disease virus strains. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14326. in press . [DOI] [PubMed] [Google Scholar]
- 12.Tulman E.R., Afonso C.L., Lu Z., Zsak L., Kutish G.F., Rock D.L. Genome of Lumpy Skin Disease Virus. J. Virol. 2001;75:7122–7130. doi: 10.1128/JVI.75.15.7122-7130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglass N., Van Der Walt A., Omar R., Munyanduki H., Williamson A.L. The complete genome sequence of the lumpy skin disease virus vaccine Herbivac LS reveals a mutation in the superoxide dismutase gene homolog. Arch. Virol. 2019;164:3107–3109. doi: 10.1007/s00705-019-04405-8. [DOI] [PubMed] [Google Scholar]
- 14.Sprygin A., Babin Y., Pestova Y., Kononova S., Wallace D.B., Van Schalkwyk A., Byadovskaya O., Diev V., Lozovoy D., Kononov A. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS ONE. 2018;13:e0207480. doi: 10.1371/journal.pone.0207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprygin A., Pestova Y., Bjadovskaya O., Prutnikov P., Zinyakov N., Kononova S., Ruchnova O., Lozovoy D., Chvala I., Kononov A. Evidence of recombination of vaccine strains of lumpy skin disease virus with field strains, causing disease. PLoS ONE. 2020;15:e0207480. doi: 10.1371/journal.pone.0232584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprygin A., Van Schalkwyk A., Shumilova I., Nesterov A., Kononova S., Prutnikov P., Byadovskaya O., Kononov A. Full-length genome characterization of a novel recombinant vaccine-like lumpy skin disease virus strain detected during the climatic winter in Russia, 2019. Arch. Virol. 2020;165:2675–2677. doi: 10.1007/s00705-020-04756-7. [DOI] [PubMed] [Google Scholar]
- 17.Kononova S., Kononov A., Shumilova I., Byadovskaya O., Nesterov A., Prutnikov P., Babiuk S., Sprygin A. A lumpy skin disease virus which underwent a recombination event demonstrates more aggressive growth in primary cells and cattle than the classical field isolate. Transbound. Emerg. Dis. 2021;68:1377–1383. doi: 10.1111/tbed.13798. [DOI] [PubMed] [Google Scholar]
- 18.Tuppurainen E.S.M., Oura C.A.L. Review: Lumpy Skin Disease: An Emerging Threat to Europe, the Middle East and Asia. Transbound. Emerg. Dis. 2012;59:40–48. doi: 10.1111/j.1865-1682.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 19.Gelaye E., Mach L., Kolodziejek J., Grabherr R., Loitsch A., Achenbach J.E., Nowotny N., Diallo A., Lamien C.E. A novel HRM assay for the simultaneous detection and differentiation of eight poxviruses of medical and veterinary importance. Sci. Rep. 2017;7:42892. doi: 10.1038/srep42892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamien C.E., Lelenta M., Goger W., Silber R., Tuppurainen E., Matijevic M., Luckins A.G., Diallo A. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. J. Virol. Methods. 2011;171:134–140. doi: 10.1016/j.jviromet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Haegeman A., Zro K., Vandenbussche F., Demeestere L., Van Campe W., Ennaji M.M., De Clercq K. Development and validation of three Capripoxvirus real-time PCRs for parallel testing. J. Virol. Methods. 2013;193:446–451. doi: 10.1016/j.jviromet.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Stubbs S., Oura C.A.L., Henstock M., Bowden T.R., King D.P., Tuppurainen E.S.M. Validation of a high-throughput real-time polymerase chain reaction assay for the detection of capripoxviral DNA. J. Virol. Methods. 2012;179:419–422. doi: 10.1016/j.jviromet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Bowden T.R., Babiuk S.L., Parkyn G.R., Copps J.S., Boyle D.B. Capripoxvirus tissue tropism and shedding: A quantitative study in experimentally infected sheep and goats. Virology. 2008;371:380–393. doi: 10.1016/j.virol.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ireland D.C., Binepal Y.S. Improved detection of capripoxvirus in biopsy samples by PCR. J. Virol. Methods. 1998;74:1–7. doi: 10.1016/S0166-0934(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 25.Heine H.G., Stevens M.P., Foord A.J., Boyle D.B. A capripoxvirus detection PCR and antibody ELISA based on the major antigen P32, the homolog of the vaccinia virus H3L gene. J. Immunol. Methods. 1999;227:187–196. doi: 10.1016/S0022-1759(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 26.Lamien C.E., Le Goff C., Silber R., Wallace D.B., Gulyaz V., Tuppurainen E., Madani H., Caufour P., Adam T., El Harrak M., et al. Use of the Capripoxvirus homologue of Vaccinia virus 30kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: Development of a classical PCR method to differentiate Goat poxvirus from Sheep poxvirus. Vet. Microbiol. 2011;149:30–39. doi: 10.1016/j.vetmic.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Le Goff C., Lamien C.E., Fakhfakh E., Chadeyras A., Aba-Adulugba E., Libeau G., Tuppurainen E., Wallace D.B., Adam T., Silber R., et al. Capripoxvirus G-protein-coupled chemokine receptor: A host-range gene suitable for virus animal origin discrimination. J. Gen. Virol. 2009;90:1967–1977. doi: 10.1099/vir.0.010686-0. [DOI] [PubMed] [Google Scholar]
- 28.Chibssa T.R., Settypalli T.B.K., Berguido F.J., Grabherr R., Loitsch A., Tuppurainen E., Nwankpa N., Tounkara K., Madani H., Omani A., et al. An HRM Assay to Differentiate Sheeppox Virus Vaccine Strains from Sheeppox Virus Field Isolates and other Capripoxvirus Species. Sci. Rep. 2019;9:6646. doi: 10.1038/s41598-019-43158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelaye E., Lamien C.E., Silber R., Tuppurainen E.S.M., Grabherr R., Diallo A. Development of a Cost-Effective Method for Capripoxvirus Genotyping Using Snapback Primer and dsDNA Intercalating Dye. PLoS ONE. 2013;8:e75971. doi: 10.1371/journal.pone.0075971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menasherow S., Erster O., Rubinstein-Giuni M., Kovtunenko A., Eyngor E., Gelman B., Khinich E., Stram Y. A high-resolution melting (HRM) assay for the differentiation between Israeli field and Neethling vaccine lumpy skin disease viruses. J. Virol. Methods. 2016;232:12–15. doi: 10.1016/j.jviromet.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Acharya K.P., Subedi D. First outbreak of lumpy skin disease in Nepal. Transbound. Emerg. Dis. 2020;67:2280–2281. doi: 10.1111/tbed.13815. [DOI] [PubMed] [Google Scholar]
- 32.Badhy S.C., Chowdhury M.G.A., Settypalli T.B.K., Cattoli G., Lamien C.E., Fakir M.A.U., Akter S., Osmani M.G., Talukdar F., Begum N., et al. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet. Res. 2021;17:61. doi: 10.1186/s12917-021-02751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu G., Xie J., Luo J., Shao R., Jia K., Li S. Lumpy skin disease outbreaks in China, since 3 August 2019. Transbound. Emerg. Dis. 2021;68:216–219. doi: 10.1111/tbed.13898. [DOI] [PubMed] [Google Scholar]
- 34.Sudhakar S.B., Mishra N., Kalaiyarasu S., Jhade S.K., Hemadri D., Sood R., Bal G.C., Nayak M.K., Pradhan S.K., Singh V.P. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transbound. Emerg. Dis. 2020;67:2408–2422. doi: 10.1111/tbed.13579. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond A.J., Suchard M.A., Xie D., Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letunic I., Bork P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das M., Chowdhury M.S.R., Akter S., Mondal A.K., Uddin M.J., Rahman M.M., Rahman M.M. An updated review on lumpy skin disease: Perspective of southeast asian countries. J. Adv. Biotechnol. Exp. Ther. 2021;4:322–333. doi: 10.5455/jabet.2021.d133. [DOI] [Google Scholar]
- 39.Arjkumpa O., Suwannaboon M., Boonrod M., Punyawan I., Liangchaisiri S., Laobannue P., Lapchareonwong C., Sansri C., Kuatako N., Panyasomboonying P., et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front. Vet. Sci. 2022;8:799065. doi: 10.3389/fvets.2021.799065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aleksandr K., Pavel P., Olga B., Svetlana K., Vladimir R., Yana P., Alexander S. Emergence of a new lumpy skin disease virus variant in Kurgan Oblast, Russia, in 2018. Arch. Virol. 2020;165:1343–1356. doi: 10.1007/s00705-020-04607-5. [DOI] [PubMed] [Google Scholar]
- 41.Tasioudi K.E., Antoniou S.E., Iliadou P., Sachpatzidis A., Plevraki E., Agianniotaki E.I., Fouki C., Mangana-Vougiouka O., Chondrokouki E., Dile C. Emergence of Lumpy Skin Disease in Greece, 2015. Transbound. Emerg. Dis. 2016;63:260–265. doi: 10.1111/tbed.12497. [DOI] [PubMed] [Google Scholar]
- 42.Tuppurainen E.S.M., Venter E.H., Coetzer J.A.W. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort J. Vet. Res. 2005;72:153–164. doi: 10.4102/ojvr.v72i2.213. [DOI] [PubMed] [Google Scholar]
- 43.Zeynalova S., Asadov K., Guliyev F., Vatani M., Aliyev V. Epizootology and molecular diagnosis of lumpy skin disease among livestock in Azerbaijan. Front. Microbiol. 2016;7:1022. doi: 10.3389/fmicb.2016.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeruham I., Perl S., Nyska A., Abraham A., Davidson M., Haymovitch M., Zamir O., Grinstein H. Adverse reactions in cattle to a capripox vaccine. Vet. Rec. 1994;135:330–332. doi: 10.1136/vr.135.14.330. [DOI] [PubMed] [Google Scholar]
- 45.Abutarbush S.M. Adverse reactions to field vaccination against lumpy skin disease in cattle. Int. J. Infect. Dis. 2016;45:164. doi: 10.1016/j.ijid.2016.02.390. [DOI] [PubMed] [Google Scholar]
- 46.Roche X., Rozstalnyy A., TagoPacheco D., Kamata A., Pittiglio C., Beltran-alcrudo D., Bisht K., Karki S., Kayamori J., Larfaoui F., et al. Introduction and Spread of Lumpy Skin Disease in South, East and Southeast Asia: Qualitative Risk Assessment and Management, FAO Animal Production and Health Papers. FAO; Rome, Italy: 2020. Paper 183. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DNA sequences generated and used in the analysis for this study are available in the GenBank under the accession numbers OL689584 to OL689619.