Abstract
The poor membrane permeability of oligonucleotides is one of the major problems of antisense technology. Here we report the construction of designer oligonucleotides for targeted delivery to macrophages. The oligonucleotides tethered to a 10-mer poly(G) sequence at their 3′ ends were recognized by scavenger receptors on macrophages and were taken up about 8- to 10-fold as efficiently as those oligonucleotides that either lacked a poly(G) tail or that contained a 10-mer poly(C) tail instead of the poly(G) tail. The enhanced uptake of poly(G) constructs was inhibited in the presence of poly(G) and other known ligands of the scavenger receptor. The bioefficacy of poly(G)-mediated targeting of antisense oligonucleotides (ANS) was demonstrated by using vesicular stomatitis virus (VSV) as a model system. The ability of ANS directed against the translation initiation site of N protein mRNA of VSV to inhibit virus replication was assessed. The ANS with the 10-mer poly(G) sequences (ANS-G) brought about significant inhibition of VSV replication in J774E cells (a murine monocyte/macrophage cell line) and Chinese hamster ovary (CHO) cell transfectants expressing scavenger receptors. The ANS lacking a 10-mer poly(G) stretch were ineffective. The inhibition of VSV replication due to ANS-G was completely abrogated in the presence of 10-mer poly(G), indicating that the antisense effect of the ANS-G molecule was a consequence of scavenger receptor-mediated enhanced uptake. Importantly, antisense molecules linked exclusively by natural phosphodiester bonds were as bioeffective as those synthesized with a mixed backbone of phosphodiester and phosphorothioate. Taken together, these results suggest that macrophage-directed designer ANS against infective agents may simply be obtained by adding a short stretch of guanylic acid sequence to the desired specific ANS during solid-phase synthesis. This nucleic acid-based strategy, which utilizes homogeneous preparation of ANS, may find applications in directed manipulation of macrophage metabolism for a variety of purposes as well as in therapy of a broad spectrum of macrophage-related disorders amenable to the antisense approach.
The conceptual simplicity of antisense design, its high theoretical specificity, the affinity of the antisense oligonucleotides for their targets, the ease of chemical synthesis, and the low systemic toxicity have endowed the antisense approach with considerable therapeutic potential, especially in the treatment of diseases such as cancers and viral infections (44). Antisense oligonucleotides are particularly attractive as antiviral agents as they can be designed to block viral replication within infected cells without affecting the metabolism of the host cells (2). However, prominent among the problems that limit the success of the antisense approach are (i) nuclease sensitivity of the oligonucleotide molecules and (ii) inefficient uptake by cells. While the problem of nuclease sensitivity of normal phosphodiester (PO) oligonucleotides has been circumvented to some extent by modifying the phosphate backbone of the oligonucleotides, as for analogs such as phosphorothioate (PS) and methylphosphonate oligonucleotides, the poor cellular uptake of these molecules remains a severe limitation (17).
The PO and PS oligonucleotides cannot passively diffuse across the cell membrane because of their polyanionic nature. Thus the processes of absorptive endocytosis and fluid phase endocytosis appear to be the principal routes of entry of these molecules into the cells (28, 48). However, there are reports suggesting the presence of cell surface receptors for oligonucleotides (5, 6, 21, 24, 39, 51). The putative cell membrane proteins vary in molecular size, exhibit moderate affinities, and bind to oligonucleotides in a specific as well as nonspecific fashion. These unrelated proteins have not been characterized fully, and hence their structural and functional significance is not readily apparent. At any rate the oligonucleotide delivery to cells remains a persistent problem considering that only 1 to 2% of the oligonucleotides become cell associated when added directly to cells in culture (40).
Current approaches to overcome the problem of poor cellular uptake of oligonucleotides include microinjection of oligonucleotides (41), delivery by the calcium phosphate precipitation method (47), and the use of cationic lipids such as N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-tetramethylammonium methyl sulfate and cytofectin (27). Lipid-oligonucleotide conjugates (42) and cholesteryl-oligonucleotide conjugates (26), antisense molecules conjugated either to fusogenic peptides (9) or poly(l-lysine) (25), have been shown to be bioeffective at concentrations at which the free oligonucleotides are ineffective. Liposome-mediated delivery (52) and receptor-mediated delivery of antisense oligonucleotides to cells have also been reported (14, 15, 49, 50).
Targeting of antisense oligonucleotides by using endocytic receptor systems specific to a given cell type is an attractive strategy for circumventing the intrinsic problems of nonpermeability and nonspecific cellular uptake of oligonucleotides. This strategy has been employed for delivery of antisense oligonucleotides with the asialoglycoprotein receptor (50), transferrin receptor (49), and epidermal growth factor receptor (15). In most of these cases, the oligonucleotide molecules are complexed to a poly(l-lysine)–ligand conjugate for delivery to cells expressing the particular receptor. The poly(l-lysine) is conjugated to the carrier molecule through a heterobifunctional cross-linker, and the conjugate is incubated with the oligonucleotide to allow for noncovalent association of the oligonucleotide with the poly(l-lysine). The resultant coacervates of receptor-recognizable macromolecules consist of chemically heterogeneous complex mixtures which cannot be easily formulated into pharmaceutical preparations for therapeutic applications. Thus antisense delivery strategies involving direct chemical manipulation of oligonucleotides and yielding a homogeneous product should be preferred over those that generate mixtures of covalently and/or noncovalently associated molecules (17).
In this study we report a simple strategy for designing antisense oligonucleotides for macrophage targeting. We show that the presence of a short poly(G) tail at the 3′ end of the antisense sequence is sufficient for scavenger receptor (SCR)-mediated targeting of oligonucleotides to macrophages. In a model system, we demonstrate the enhanced cellular uptake and bioefficacy of a vesicular stomatitis virus (VSV)-specific antisense oligonucleotide in VSV-infected J774E cells and CHO transfectants expressing SCR.
MATERIALS AND METHODS
Cell lines and virus.
J774E, a murine monocyte/macrophage cell line, was a gift from P. Stahl, Washington University, St. Louis, Mo. The Chinese hamster ovary (CHO) transfectant PJA28.C5 expressing the type I SCR and the parent CHO cell line were obtained from M. Krieger, Massachusetts Institute of Technology. All cell lines were cultured in medium A (RPMI 1640 containing 10% fetal bovine serum [FBS] and gentamicin [50 mg/liter]) at 37°C in a 5% CO2–95% air atmosphere. The transfectant was cultured in medium containing Geneticin (400 μg/ml) as a selection marker. VSV (Indiana strain) was a kind gift from Ranjit Ray (St. Louis University, St. Louis, Mo.). VSV was grown to high titers in J774E cells cultured in medium B (RPMI containing 2% FBS and 50 mg of gentamicin/ml).
Oligonucleotides.
PS and chimeric antisense oligodeoxyribonucleotides were purchased from Biosynthesis Inc., Louisville, Tex. The PO oligonucleotides were purchased from Rama Biotechnologies, New Delhi, India. The oligonucleotide samples were radiolabeled, and their purity was determined by polyacrylamide gel electrophoresis. The commercially procured oligonucleotides were purified by ion exchange chromatography using standard procedures.
CD spectroscopy.
Circular dichroism (CD) spectra were recorded on a Jasco-J710 instrument equipped with a Peltier-type constant-temperature cell holder (PTC-348W). The instrument was calibrated with (+)-10-camphorsulfonic acid. The spectra of oligonucleotides were recorded at 37°C, and data were presented as mean residue ellipticity expressed in units of degrees times centimeters squared divided by decimoles. The spectra were smoothed with the built-in algorithm of the Jasco program.
Radiolabeling.
Oligonucleotides were end labeled with a T4 polynucleotide kinase kit (Promega, Madison, Wis.). The kination reaction was carried out on 10 pmol of the oligonucleotide at 37°C for 1 h in a total volume of 10 μl containing 10 U of the enzyme, 1 μl of 10× polynucleotide kinase buffer, and 30 μCi of [γ-32P]ATP. The labeled oligonucleotides were purified on a DE52 cellulose matrix, and specific activity was determined.
Maleylated bovine serum albumin (MBSA) was prepared as described previously (13) and radiolabeled by a modification of the iodine monochloride method (19). Briefly, about 2 mg of protein was added to 250 μl of 10 mM phosphate buffer containing 150 mM NaCl (PBS; pH 7.4), 250 μl of 1 M glycine-NaOH buffer (pH 10) was added, and the mixture was placed on ice. To this, 1 mCi of Na125I was added, along with 125 μl of freshly made iodine monochloride (2.64 mM) solution. The reaction mixture was vortexed and incubated on ice for 10 min. The iodinated protein (125I-MBSA) was separated from the free iodine by gel filtration with a G-25 prepacked column (10 by 1.25 cm) equilibrated with PBS. The radiolabeled protein was extensively dialyzed at 4°C against PBS.
Binding assays.
Cells (0.5 × 106 cells/well) were plated in six-well culture dishes in medium A and incubated for 18 h at 37°C in a humidified 5% CO2–95% air atmosphere. The growth medium was replaced with 1 ml of ice-cold medium C (RPMI 1640 containing BSA [1 mg/ml]) containing various concentrations of 125I-MBSA, and the cells were incubated at 4°C for 2 h. The cells were then washed three times with ice-cold PBS containing BSA (5 mg/ml), followed by three washes with ice-cold PBS to remove unbound radioactivity. The cells were then lysed in 0.1 N NaOH (1 ml per well), and cell-associated radioactivity was determined with a gamma counter.
Degradation assays.
The assays were carried out as described previously (19). Briefly, cells were plated as described above. Each well received 1 ml of prewarmed medium C containing 125I-MBSA alone or with putative competitors, and the cells were incubated at 37°C for 5 h. The supernatant (500 μl) from each well was collected, 200 μl of 50% trichloroacetic acid (TCA) was added, and the solutions were placed at 4°C overnight. The supernatants were spun at 1,000 × g for 10 min to pellet out the precipitated protein. To 400 μl of the TCA-soluble supernatant, 7 μl of 40% KI and 15 μl of 30% H2O2 were added, and the mixture was vortexed and kept at room temperature for 10 min. To each sample, 1 ml of chloroform was added, and the samples were vortexed and kept at room temperature for 10 min to allow for extraction of free iodine. The 125I content in the aqueous phase was determined with a gamma counter. The TCA-soluble radioactivity obtained from the “no cell blank” was subtracted from the experimental readings to calculate cell-specific degradation, the results being expressed as nanograms of 125I-MBSA degraded per milligram of cell protein.
Uptake assays.
Cells were plated as described above. Each well received 1 ml of medium C containing [γ-32P]ATP-labeled oligonucleotide alone or with putative competitors, and the cells were incubated for different periods of time. After this, the cells were processed as described for binding assays and cell-associated radioactivity was determined.
Virus replication inhibition assay.
Cells (0.125 × 106 cells/well) were plated in a 24-well culture plate and were incubated at 37°C for 6 to 8 h. The cells were washed in serum-free RPMI 1640 and incubated with the desired concentration of oligonucleotide in 250 μl of serum-free RPMI 1640 at 37°C for 4 h. The oligonucleotide-containing medium was then removed, and the cells were infected with VSV at a multiplicity of infection (MOI) of 0.5 for 30 min in 300 μl of serum-free RPMI 1640. The cells were then washed three times with serum-free RPMI 1640, and the oligonucleotide-containing medium was readded along with 50 μl of 10% FBS. The cells were now incubated at 37°C for 10 h. The cell supernatants were collected, and viral titers were determined by plaque assays. The assays were carried out on CHO cell monolayers by the agar overlay method. Plates were flushed with 0.07% neutral red solution to help count the plaques, and viral titers were calculated.
RESULTS
Design of oligonucleotides for targeted delivery to macrophages.
VSV, which infects a variety of cell types including macrophages, was chosen as a candidate virus for the study. The translation initiation region of the mRNA corresponding to the N protein of VSV was selected as a target sequence since the antisense oligonucleotide sequence (5′CATTTTGATTACTGT3′) directed against this site has been earlier shown to inhibit viral replication by an antisense-specific mechanism in VSV-infected L929 cells (25).
The SCR on macrophages recognize a wide variety of polyanionic macromolecules including maleylated and acetylated proteins, sulfated polysaccharides such as fucoidan, lipopolysaccharides, and certain polynucleotides such as poly(G) and poly(I) (11, 18). Short stretches (6- to 10-mer) of poly(G) or poly(I) were also shown to be effective ligands of the receptor (11, 36). The acquisition of a four-stranded helical structure (quadruplex structure) by poly(G) and poly(I) has been suggested as a crucial structural element for recognition by SCR (36). Therefore, we surmised that oligonucleotides in contiguity with poly(G) tails would be recognized by SCR if the innate “tetraplex”-forming ability of the poly(G) carrier is not compromised. Although a poly(G) quartet (GGGG) is the minimum stretch required for the acquisition of a quadruplex structure, the G tetramers may not assume this conformation due to steric or other conformational constraints when placed in the context of a relatively longer antisense sequence. Thus, it is necessary to ascertain the optimum length of guanylic acid in contiguity with the 15-mer antisense sequence that can take up a tetraplex structure.
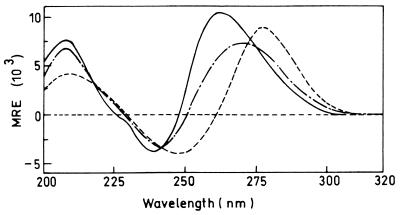
CD spectroscopy can be a useful tool for probing the presence of a tetraplex structure of deoxyguanylic acid oligomers since the tetraplex structure exhibits a characteristic spectrum (20, 22, 23, 29). In order to establish the minimum length of guanylic acid tail for the poly(G) antisense oligonucleotide constructs, we studied the CD spectra of the antisense constructs in contiguity with a 4-mer, 7-mer, and 10-mer poly(G) at the 3′ end of the antisense sequence (Fig. 1). All three spectra show two maxima and a minimum but at significantly different wavelengths, suggesting the presence of differences in the conformer populations. The two maxima for the 4-mer construct are centered at 275 and 209 nm, while the minimum is centered at 249 nm. The maximum at 275 nm and the minimum at 249 nm of the 4-mer construct are blue shifted in the 7-mer and 10-mer constructs. The spectra of the 10-mer construct exhibit maxima near 261 and 209 nm and a minimum near 241 nm. In addition there is a distinctive shoulder at 230 nm, which is absent in the spectra of lower oligomeric constructs. The spectra of the 10-mer construct are similar to the previously described spectra of quadruplex-forming oligonucleotides (20, 22, 23, 29) and are almost identical to the reported spectra of a 6- or 12-mer deoxyguanylic acid (36). Thus, the CD studies suggest that a 10-mer poly(G) stretch at the 3′ end of the antisense sequence is able to maintain the tetraplex structure just as in the isolated 6-mer or a 12-mer poly(G). We therefore used 10-mer poly(G) tails at the 3′ ends of the oligonucleotides for their recognition by SCR present on macrophages.
FIG. 1.
CD spectra of poly(G) antisense oligonucleotides. The respective oligonucleotides were dissolved in water at a nucleotide residue concentration of about 60 μM, and the spectra were recorded with a 10-mm cell at 37°C. Each spectrum represents an average of five scans. ANS with 4-mer poly(G) at the 3′ end (––––), ANS with 7-mer poly(G) (—–—), and ANS with 10-mer poly(G) (——) are shown.
The 15-mer antisense oligonucleotide directed against the translation initiation site of VSV was synthesized as a native phosphodiester (ANS), as a PS (sANS), or as a chimeric molecule (cANS) where only the first and last internucleotide linkages in the antisense portion of the oligonucleotide sequence were PS (Table 1). The 10-mer poly(G) tail at the 3′ end of the oligonucleotide constructs was retained as PO to facilitate degradation of the SCR recognition element [poly(G) portion] on internalization. The antisense oligonucleotide was also synthesized with a 10-mer poly(C) tail at the 3′ end (ANS-C) as a control sequence since poly(C) is not recognized by the SCR (11). Two other control oligonucleotides, in which the 15-mer sequence complementary to ANS (sense-G) and a scrambled sequence with the same composition as ANS (scram-G) were synthesized with poly(G) tails at the 3′ ends as shown in Table 1, were designed.
TABLE 1.
Oligonucleotide sequencesa
| Oligomer | Sequence |
|---|---|
| ANS | CAT TTT GAT TAC TGT |
| sANS | CAT TTT GAT TAC TGT |
| cANS | CAT TTT GAT TAC TGT |
| Sense | ACA GTA ATC AAA ATG |
| Scram | ATC GTA TTA CTT GTT |
| ANS-G | CAT TTT GAT TAC TGT GGG GGG GGG G |
| sANS-G | CAT TTT GAT TAC TGT GGG GGG GGG G |
| cANS-G | CAT TTT GAT TAC TGT GGG GGG GGG G |
| Sense-G | ACA GTA ATC AAA ATG GGG GGG GGG G |
| Scram-G | ATC GTA TTA CTT GTT GGG GGG GGG G |
| ANS-C | CAT TTT GAT TAC TGT CCC CCC CCC C |
The 15-mer antisense oligonucleotide (ANS) was directed against the translation initiation site of the VSV N protein mRNA. sANS, PS analog of ANS; cANS, chimeric antisense oligomer with the first and last internucleotide linkages modified by PS chemistry; sense, control sequence complementary to ANS; scram, scrambled control sequence with the same composition as ANS; ANS-G, sANS-G, cANS-G, sense-G, and scram-G are the respective oligonucleotides, each containing a 10-mer poly(G) tail at the 3′ end; ANS-C, control oligonucleotide sequence with ANS linked to a 10-mer poly(C) tail at the 3′ end. The internucleotide linkages for underlined sequences are linked through PS bonds.
Recognition of oligonucleotide sequences by SCR.
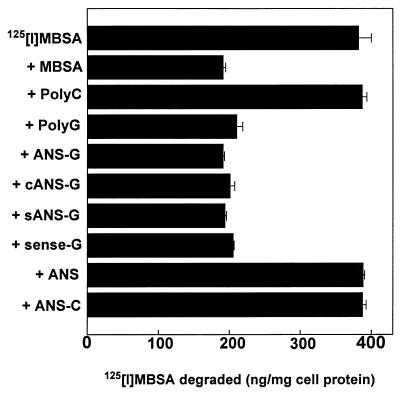
The specificity of recognition of the oligonucleotide-poly(G) constructs by SCR was examined with J774E, a murine monocyte/macrophage cell line. For this purpose, first the status of SCR on these cells was assessed. The binding of 125I-MBSA to J774E cells at 4°C exhibited saturation kinetics with a half-maximal binding of 2 μg/ml (data not shown). The degradation of 125I-MBSA by J774E cells in the presence or absence of different polyanionic molecules as competitors exhibited the characteristic SCR profile; cells that received 2 μg of 125I-MBSA/ml alone degraded 333 ± 9.2 ng of 125I-MBSA/mg of cell protein in 5 h. This value fell to 71 ± 11 ng in the presence of unlabeled MBSA (40 μg/ml), 90 ± 8 ng in the presence of fucoidan (20 μg/ml), and 33 ± 5 ng in the presence of poly(G) (2 μg/ml). Fetuin (20 μg/ml) and poly(C) (2 μg/ml) did not compete for the degradation of 125I-MBSA. These results suggest the presence of normal SCR activity on J774E cells.
We investigated if the SCR on these cells recognized oligonucleotides with 10-mer poly(G) tails at their 3′ ends. To establish this, the abilities of various oligonucleotides to compete for the degradation of 125I-MBSA were tested. The 10-mer poly(G) and oligonucleotides containing 10-mer poly(G) tails at their 3′ ends competed for the degradation of the radiolabeled MBSA, while a 10-mer poly(C) and oligonucleotides containing 10-mer poly(C) tails did not interfere with the degradation of MBSA (Fig. 2). These results suggest that oligonucleotides with poly(G) tails are efficiently recognized by SCR. Furthermore, recognition of the poly(G) constructs by the SCR was independent of both the sequence of the oligonucleotides and chemical modifications to the backbone of the oligonucleotides because Sense-G, Scram-G, cANS-G and sANS-G competed for the degradation of 125I-MBSA as efficiently as ANS-G itself.
FIG. 2.
Recognition of poly(G) constructs by SCR; competition for degradation of 125I-MBSA. Cells (0.5 × 106 cells/well) were plated in six-well culture vessels and incubated for 18 h at 37°C. Each cell monolayer received 1 ml of RPMI 1640 containing BSA (1 mg/ml) with 2 μg of 125I-MBSA (specific activity: 100 cpm/ng)/ml alone or along with different competitors and was incubated at 37°C. After 5 h of incubation, the medium in each well was processed to determine the amount of 125I-MBSA degraded, as described in Materials and Methods. The molar ratio of 125I-MBSA to different oligonucleotides was 1:20. Unlabeled MBSA was used as a competitor at a concentration of 2 μg/ml. Both poly(G) and poly(C) were 10-nucleotide-long molecules. Results shown are means ± standard errors of three independent determinations.
Enhanced SCR-mediated uptake of oligonucleotides.
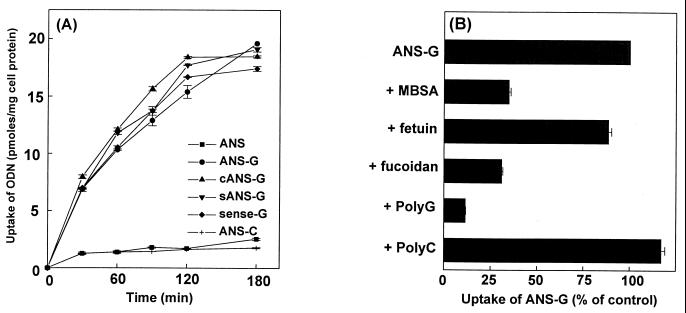
Next we examined the uptake of oligonucleotides with or without poly(G) tails or containing poly(C) tails to assess the extent of SCR-mediated facilitated delivery to the J774E cell line. The data shown in Fig. 3A indicate that uptake of the oligonucleotide constructs with a poly(G) tail was 8- 12-fold higher than uptake of constructs without the poly(G) extension. ANS-G, cANS-G, sANS-G, and sense-G were taken up with comparable efficiencies, whereas the uptake of ANS-C was around the same level as that of ANS. This is consistent with the results of the recognition studies described earlier in that poly(G)-containing oligonucleotide sequences competed for the degradation of 125I-MBSA to similar extents while ANS and ANS-C did not.
FIG. 3.
Enhanced uptake of poly(G) constructs by J774E cells through the SCR. (A) Cells (0.5 × 106 cells/well) were plated in six-well culture vessels and were incubated for 18 h at 37°C. Each cell monolayer received 1 ml of RPMI 1640 containing BSA (1 mg/ml), with 1 μM 32P-labeled oligonucleotide and was incubated at 37°C for the indicated periods of time. The cells were then processed as described in Materials and Methods, and cell-associated radioactivity was determined. Results are means ± standard errors of three independent determinations. (B) The assay was set up as described above. Cells received 1 μM 32P-labeled ANS-G (specific activity: 555 cpm/pmol) either alone or along with different polyanionic macromolecules. The competitors used were MBSA (500 μg/ml), fetuin (100 μg/ml), fucoidan (100 μg/ml), poly(G) (7 μg/ml), and poly(C) (7 μg/ml). After incubation for 4 h at 37°C, cell-associated radioactivity was determined. Uptake of ANS-G in the control cells was 43.63 ± 1.5 pmol/mg of cell protein. Results are expressed as means ± standard errors of three independent determinations.
To further confirm that the enhanced uptake of the antisense oligonucleotide containing a 10-mer poly(G) sequence was mediated by SCR, the uptake of ANS-G by J774E cells was competed by different polyanionic macromolecules. The results of this assay (Fig. 3B) showed that the uptake of ANS-G was competed by known SCR ligands such as MBSA, fucoidan, and poly(G). Fetuin and poly(C) did not show any effect. Thus the observed enhanced uptake of the poly(G) constructs as against that of the free oligonucleotides occurred predominantly through the SCR.
Bioefficacy of the poly(G)-containing oligonucleotides in VSV-infected J774E cells.
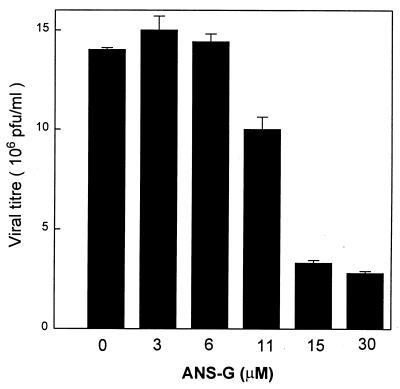
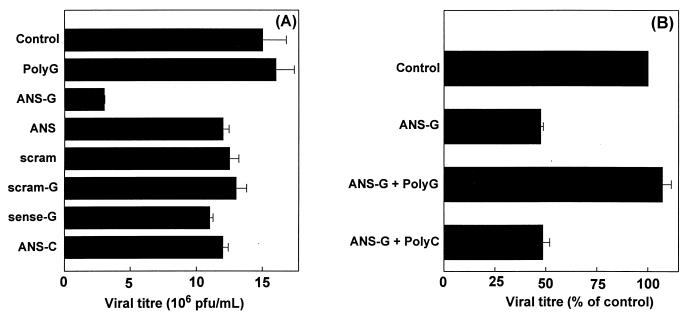
In order to test if the SCR-mediated uptake led to enhanced bioefficacy, the ability of the antisense oligonucleotide to inhibit VSV replication in SCR-bearing J774E cells was investigated. VSV infected the J774E cell line, and the virus was able to replicate in this cell line. The viral titer generated 10 h postinfection, with a MOI of 0.5, was 15 × 106 PFU/ml. Cells were treated with increasing concentrations of the ANS-G before being infected with VSV at a MOI of 0.5 as described in Materials and Methods. Under these conditions significant inhibition of VSV replication was not observed up to a concentration of 11 μM ANS-G. At 15 μM, the ANS-G treatment brought about 80% inhibition of VSV replication (Fig. 4). The cANS-G treatment yielded a similar profile of inhibition of VSV replication. The antisense effects due to ANS-G and cANS-G were not further enhanced when the concentration was increased to 30 μM. In contrast, the free antisense oligonucleotide (ANS), under assay conditions similar to those employed for the ANS-G treatment, did not significantly inhibit the VSV replication up to a concentration of 30 μM. Representative data in Fig. 5A show that the control oligonucleotides, sense-G, scram-G, and ANS-C, exhibited behavior similar to that of ANS. The carrier molecule [10-mer poly(G)] itself did not interfere with the viral replication.
FIG. 4.
Dose dependence of ANS-G on inhibition of VSV replication. Cells (0.125 × 106 cells/well) were treated with various concentrations of ANS-G in 250 μl of serum-free RPMI 1640 for 4 h at 37°C. The cells were then infected with VSV at a MOI of 0.5 for 30 min after which they were given three rapid washes. The cells were incubated at 37°C for 10 h postinfection in medium containing the oligonucleotides with 2% FBS. Culture supernatants were collected, and viral titers were estimated by plaque assays of CHO cell monolayers. Results shown are means ± standard errors of three independent determinations.
FIG. 5.
Inhibition of VSV replication in J774E cells by different oligonucleotide constructs. (A) Cells (0.125 × 106 cells/well) were treated with 15 μM concentrations of oligonucleotides, and assays were carried out as described in the legend for Fig. 4. Results shown are means ± standard errors of three independent determinations. (B) Reversal of antisense effects by competing polyanionic macromolecules. The assay was performed as described above. Cells received, in addition to ANS-G, 7 μg of either poly(G) or poly(C) as competitors. The viral titer in the control group was 16 × 106 PFU/ml. Results shown are means ± standard errors of three independent determinations.
To establish that the observed inhibition of VSV replication was a result of SCR-mediated delivery, cells were incubated with the ANS-G conjugate in the presence or absence of polyanionic macromolecules known to be ligands of the SCR or otherwise. The coincubation with poly(G), an SCR ligand, resulted in complete abrogation of the antisense effect, whereas poly(C), which is not recognized by the SCR, caused no significant reversal of the antisense-mediated effect (Fig. 5B).
Bioefficacy in CHO transfectants.
One of the enduring problems faced in antisense research has been the variability of antisense effects in different cell lines against the same target. Therefore, it was pertinent to examine the bioefficacy of the SCR-mediated delivery of antisense oligonucleotides in other SCR-bearing cell lines. CHO cells that were stably transfected with the SCR type I (PJA28.C5 cells) were chosen for this purpose. The PJA28.C5 cells bound 125I-MBSA at 4°C with saturation kinetics (data not shown). Also, degradation of 125I-MBSA by these cells (64 ± 1.47 ng/mg of cell protein in 5 h) was effectively competed by MBSA and poly(G) but not by poly(C) or fetuin, indicating expression of characteristic SCR activity. However, in comparison to that for the J774E cell line, the level of degradation of 125I-MBSA in PJA28.C5 was about four- to fivefold lower.
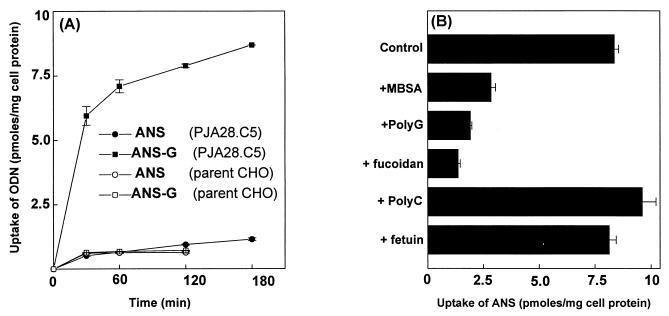
The levels of uptake of ANS and ANS-G were similar in the parental CHO cells that did not express SCR. In contrast, SCR-transfected PJA28C.5 cells took up the ANS-G oligonucleotide about 8- to 10-fold more efficiently than ANS (Fig. 6A). This amount of enhancement of uptake of ANS-G by PJA28C.5 cells was similar to that observed for J774E cells. However, the absolute amount of oligonucleotide taken up by the PJA28.C5 cells was about twofold less than the uptake by J774E cells. These results are consistent with the lower SCR activity in the CHO transfectants. The enhanced uptake of the poly(G) conjugate occurred predominantly through the SCR, as demonstrated in the results shown in Fig. 6B. The uptake was competed by known SCR ligands such as MBSA, poly(G), and fucoidan but was not affected by other polyanionic macromolecules such as poly(C) and fetuin, which are not recognized by the SCR.
FIG. 6.
Enhanced uptake of ANS-G by parent CHO cells and PJA28.C5 cells expressing SCR. (A) The assay was performed as described for Fig. 3A. Results shown are means ± standard errors for three independent determinations. ODN, oligonucleotide. (B) Assay was carried out as described for Fig. 3B. Cells received 1 μM 32P-labeled ANS-G (specific activity: 390 cpm/pmol) either alone or along with different competitors, viz., MBSA (500 μg/ml), poly(G) (7 μg/ml), fucoidan (100 μg/ml), poly(C) (7 μg/ml), and fetuin (100 μg/ml). Results shown are means ± standard errors of three independent determinations.
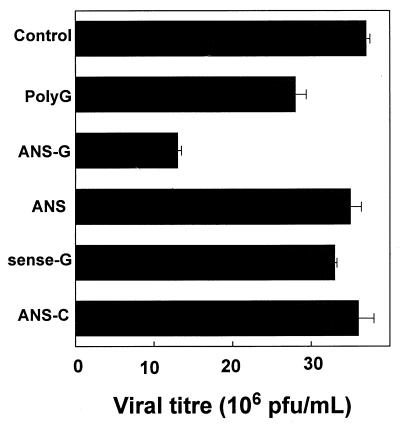
VSV infected and replicated in PJA28.C5 cells. At a MOI of 0.5, 10 h postinfection, a viral titer of 37 × 106 was generated. VSV was therefore able to replicate more efficiently in the CHO transfectant than in the J774E cell line where only 15 × 106 PFU/ml was generated under identical conditions of infection. Treatment with ANS-G at a concentration of 15 μM caused 65% inhibition of VSV replication (Fig. 7), whereas ANS, sense-G, and ANS-C, at the same concentration, did not cause inhibition of VSV replication. However, 10-mer poly(G) elicited a small antiviral activity. This is in contrast to the bioefficacy results for J774E-infected cells, where poly(G) was completely ineffective.
FIG. 7.
Inhibition of VSV replication in PJA28.C5 cells treated with ANS-G. The experiment was performed as described for Fig. 4. All oligonucleotides were used at a concentration of 15 μM. Results shown are means ± standard errors of three independent determinations.
DISCUSSION
The poor cellular uptake of oligonucleotides remains a major stumbling block in the development of antisense therapy. Although the problem has been circumvented to some extent by utilizing cationic and other lipids (27, 42) and certain receptor systems (15, 49, 50), most of these delivery systems are complicated by multistep preparation procedures yielding poorly defined heterogeneous mixtures. Here we report a simple approach to augment the antisense oligonucleotide uptake in macrophages that express SCR. We have exploited the innate tetraplex-forming propensity of poly(G) sequences and the ability of SCR to recognize this feature of the poly(G) conformation for targeting antisense oligonucleotides to macrophages. We show that oligonucleotides containing 10-mer poly(G) stretches at the 3′ termini are recognized and efficiently taken up by SCR and that this receptor-mediated uptake of antisense oligonucleotides directed against the N protein mRNA of VSV leads to inhibition of VSV replication in J774E macrophage cells as well as in CHO cells transfected with SCR.
In designing the poly(G)-antisense oligonucleotide constructs for SCR-mediated targeted delivery to cells, the nuclease sensitivity of antisense molecules was kept in mind. Thus antisense molecules using PS chemistry (sANS) were synthesized. However, in standard trypan blue exclusion studies, sANS was found to be extremely toxic to cells at concentrations of 15 μM; nearly 90 to 95% of the cells became permeable to the dye after incubation with sANS for 5 h at 37°C. However, chimeric molecules (cANS) in which only the first and last internucleotide linkages were modified by PS chemistry were not toxic to cells at concentrations as high as 100 μM. Nonetheless, the poly(G) constructs of PO oligonucleotides (ANS-G) were as bioeffective as chimeric oligonucleotides (cANS-G). The ability of ANS-G to inhibit VSV replication with efficiency almost identical to that of the cANS-G is an interesting result that suggests longer intracellular survival of the poly(G)-tethered antisense oligonucleotides. The reasons for the enhancement in the stability of oligonucleotides delivered through the SCR pathway are not readily apparent. The trafficking into specific intracellular compartments, which ensures a longer half-life of the antisense molecules, may be one of the reasons. It is also likely that the quadruplex structures acquired by the poly(G) tail may offer resistance to degradation and increase the half-life of the molecule. If this be so, then the fact that we have used antisense oligonucleotides with poly(G) tails at the 3′ ends may be important because the degradation of oligonucleotides is believed to occur predominantly due to 3′-exonuclease activity (37). It would also mean that using poly(G) as a carrier for PO antisense oligonucleotides may increase their stability due to the intrinsic properties of the carrier molecule. These results are important in the light of several recent reports questioning the use of PS oligonucleotides (43), which are now known to have serious drawbacks which include non-sequence-specific effects (37, 38), inhibition of important cellular enzymes such as human DNA polymerases and RNase H (16), and binding to several cytosolic proteins leading to cellular toxicity (10, 21).
The optimum concentration (15 μM) required to achieve 80% inhibition of VSV replication in J774E-infected cells compares favorably with the previously published data. Agris et al. (3) used methylphosphonate oligonucleotides at 150 μM for inhibiting VSV replication in L929 cells, while 50 μM was required for in vitro specific inhibition of hepatitis B viral gene expression by asialoglycoprotein-mediated delivery of antisense compounds to virus-infected HepG2 cells that possess the asialoglycoprotein receptors (50). Thus the results of the present study are significant in the light of the above observations. However, oligonucleotides are known to effect the inhibition of replication of certain viruses even at nanomolar concentrations. The use of a relatively higher oligonucleotide concentration for VSV in the present study may be related to the intrinsic high growth rate of this virus. In a recent study, Tackas and Banerjee (45) could demonstrate the inhibition of VSV in cells constitutively expressing an antisense RNA targeted against the virus L protein gene only at a very low MOI of 0.01 to 0.1 and concluded that the “robust growth rate of VSV eventually overwhelms the available antisense RNA and leads to delayed cell death.”
MBSA, a ligand of the SCR, has been used to target a variety of chemotherapeutic agents (14, 30–34) to macrophage cells. Chaudhury (14) has used liposome coated with MBSA to deliver antisense oligonucleotides to macrophage cells. We have also noted SCR-mediated enhanced uptake of oligonucleotides conjugated to MBSA by macrophages (data not shown). However, the immunogenicity of maleylated proteins (1) and the multistep, complex procedure for preparation of conjugates of an antisense oligonucleotide with the maleylated protein complicate its utility as a carrier of oligonucleotides to macrophages. In contrast, poly(G) molecules are not likely to induce an immune response and using poly(G) as a carrier eliminates the need for complex conjugation procedures because the addition of a poly(G) tail to the antisense sequence is very much a part of the solid-phase synthesis of oligonucleotides. However, there have been reports suggesting that the presence of G tetrads comprising both PS and PO oligonucleotides does cause sequence-independent effects (43); some examples include inhibition of human immunodeficiency virus (35, 46) and the biological activity of RelA (7) and c-myb (12). Binding of G tetrads to fibroblast growth factor has also been reported (21). Nonetheless, we did not observe any effect of 10-mer poly(G) on cell proliferation and viability up to concentrations of 100 μM. Poly(G) also did not elicit antiviral activity in VSV-infected J774E cells. However, VSV replication was marginally inhibited in CHO transfectants, suggesting that the antiviral activity of poly(G) might be intrinsic to the nature of the cell lines.
In some recent studies, SCR has been suggested to mediate oligonucleotide binding. SCR present on endothelial liver cells are implicated in liver uptake of oligonucleotides (8). Also Kimura et al. (24) reported the blockage of oligomer induction of interferon production on NK cells by ligands of SCR and suggested that SCR bound to oligonucleotides. However, these results are not in agreement with the findings of Benimetskaya et al. (7), who showed that binding of oligonucleotides to macrophages and microglia cells was unaffected by ligands of the SCR. The findings of Benimetskaya et al. are consistent with our results in that only those oligonucleotides that are compatible with the SCR ligand specificity are recognized. Furthermore, SCR recognition of poly(G) constructs and bioefficacy are consistent with the results of Chaudhury (14), who showed enhanced efficacy of SCR-mediated delivery of antileishmanial oligonucleotides encapsulated in MBSA-coated liposome.
Macrophages are an important component of the cellular arm of the immune system and are infected by many viral pathogens including human immunodeficiency virus and dengue virus. The idea of targeting oligonucleotides specifically to macrophages is, therefore, of considerable therapeutic significance. The SCR system lends itself admirably to this purpose, as the SCR receptors are predominantly expressed on cells of the macrophage lineage. Further, the levels of expression are high and the receptors are not down regulated on association with ligands and are recycled rapidly back to the surface after internalization (4). The SCR system has been used extensively for the targeted delivery of a variety of chemotherapeutic agents to macrophages to eliminate intracellular infectious agents as well as certain forms of cancer involving macrophage lineage cells both in vitro and in vivo (30–34). The present studies describing a simple strategy of SCR-mediated targeting of antisense oligonucleotides to macrophages is likely to facilitate the antisense-mediated therapy of macrophage-related intracellular infections of viral, bacterial, or protozoal etiology and would also serve as a facile way for modulating or controlling certain metabolic responses of macrophages that might have therapeutic implications. Furthermore, it would be of interest to explore if poly(G)-mediated targeting could be adapted for delivery of genes to SCR-bearing cells.
ACKNOWLEDGMENTS
We thank S. Vrati for helpful discussions. The technical assistance of S. Ramakrishna is gratefully acknowledged. Monty Krieger (Massachusetts Institute of Technology) generously provided the CHO cells that were transfected with SCR.
This work was supported by grants from the Department of Science and Technology, Government of India (SP/SO/B-58/95), the Department of Biotechnology, Government of India, and the Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India.
REFERENCES
- 1.Abraham R, Singh N, Mukhopadhyay A, Basu S K, Bal V, Rath S. Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptor on macrophages. J Immunol. 1995;154:1–8. [PubMed] [Google Scholar]
- 2.Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992;10:152–157. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- 3.Agris C H, Blake K R, Miller P S, Reddy M P, T’so P O P. Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence specific oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986;25:6268–6275. doi: 10.1021/bi00368a065. [DOI] [PubMed] [Google Scholar]
- 4.Basu S K. Receptor mediated endocytosis of macromolecular conjugates in selective drug delivery. Biochem Pharmacol. 1990;40:1941–1946. doi: 10.1016/0006-2952(90)90222-7. [DOI] [PubMed] [Google Scholar]
- 5.Beltinger C, Saragovi H U, Smith R M, LeSauteur L, Shah N, DeDionisio L, Christensen L, Raible A, Jarett L, Gerwitz A M. Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J Clin Investig. 1995;95:1814–1823. doi: 10.1172/JCI117860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benimatskaya L, Loike J D, Khaled Z, Loike G, Silverstein S C, Cao L, el Khoury J, Cai T Q, Stein C A. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997;3:414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 7.Benimetskaya L, Berton M, Kolbanovsky A, Benimetsky S, Stein C A. Formation of a G-tetrad and higher order structures correlates with biological activity of the RelA (NF-kB p65) ‘antisense’ oligodeoxynucleotide. Nucleic Acids Res. 1997;25:2648–2656. doi: 10.1093/nar/25.13.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biessen E A, Vietsch H, Kuiper J, Bijsterbosch M K, van Berkel T J. Liver uptake by phosphodiester oligodeoxynucleotides is mediated by scavenger receptor. Mol Pharmacol. 1998;53:262–269. doi: 10.1124/mol.53.2.262. [DOI] [PubMed] [Google Scholar]
- 9.Bongartz J P, Aubertin A M, Milhaud P G, Lebleu B. Improved biological activity of antisense oligonucleotides conjugated to a fusogenic peptide. Nucleic Acids Res. 1994;22:4681–4688. doi: 10.1093/nar/22.22.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown D A, Kang S H, Gryaznov S M, DeDionisio L, Heidenreich O, Sullivan S, Xu X, Nerenberg M I. Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J Biol Chem. 1994;269:26801–26805. [PubMed] [Google Scholar]
- 11.Brown M S, Basu S K, Falck J R, Ho Y K, Goldstein J L. The scavenger cell pathway for protein degradation: specificity of the binding site that mediates the uptake of negatively charged LDL by macrophages. J Supramol Struct. 1980;13:67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- 12.Burgess T, Fischer E F, Ross S L, Brady J V, Quin Y, Bayewitch L A, Cohen A M, Herrera C J, Hu S S, Kramer T B, Lott F D, Martin F H, Pierce G F, Simonet L, Ferrell C L. The antiproliferative activity of c-myb and c-myc antisense oligonucleotides in smooth muscle cells is caused by a nonantisense mechanism. Proc Natl Acad Sci USA. 1995;92:4051–4055. doi: 10.1073/pnas.92.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler P J G, Hartley B S. Maleylation of amino groups. Methods Enzymol. 1972;25:191. doi: 10.1016/S0076-6879(72)25016-9. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhury G. Scavenger receptor-mediated delivery of antisense mini-exon phosphorothioate oligonucleotides to leishmania-infected macrophages: selective and efficient elimination of the parasite. Biochem Pharmacol. 1997;53:385–391. doi: 10.1016/s0006-2952(96)00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande D, Toledo-Velasquez D, Thakkar D, Liang W, Rojanasakul Y. Enhanced cellular uptake of oligonucleotides by EGF receptor-mediated endocytosis in A549 cells. Pharm Res. 1996;13:57–61. doi: 10.1023/a:1016073132320. [DOI] [PubMed] [Google Scholar]
- 16.Gao W Y, Han F S, Storm C, Egan W, Cheng Y C. Phosphorothioate oligodeoxynucleotides are inhibitors of DNA polymerases and RNase H: implications for antisense technology. Mol Pharmacol. 1992;41:223–229. [PubMed] [Google Scholar]
- 17.Gewirtz A M, Stein C A, Glazer P M. Facilitating oligonucleotide delivery: helping antisense deliver on its promise. Proc Natl Acad Sci USA. 1996;93:3161–3163. doi: 10.1073/pnas.93.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein J L, Ho Y K, Basu S K, Brown M S. Stimulation of cholestryl ester synthesis in macrophages by extract of atherosclerotic human aortas and complexes of albumin/cholestryl esters. Proc Natl Acad Sci USA. 1979;76:333–337. [Google Scholar]
- 19.Goldstein J L, Basu S K, Brown M S. Receptor mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 20.Guo Q, Lu M, Marky M A, Kallenbach N R. Interaction of dye ethidium bromide with DNA containing guanine repeats. Biochemistry. 1992;31:2451–2455. doi: 10.1021/bi00124a002. [DOI] [PubMed] [Google Scholar]
- 21.Guvakova M A, Yakubov L A, Vlodavsky I, Tonkinson J L, Stein C A. Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors and remove it from low affinity binding sites on extracellular matrix. J Biol Chem. 1995;270:2620–2627. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- 22.Hardin C C, Henderson E, Watson T, Prosser J K. Monovalent cation induced structural transitions in telomeric DNAs: G-DNA folding intermediates. Biochemistry. 1991;30:4460–4472. doi: 10.1021/bi00232a013. [DOI] [PubMed] [Google Scholar]
- 23.Jin R, Breslauer K J, Jones R A, Gaffney B L. Tetraplex formation of a guanine-containing nanomeric DNA fragment. Science. 1990;250:543–546. doi: 10.1126/science.2237404. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Sonehara K, Kuramoto E, Makino T, Yamamoto T, Kataoka T, Tokunaga T. Binding of oligoguanylate to scavenger receptor is required for oligonucleotides to augment NK cell activity and induce IFN. J Biochem. 1994;116:991–994. doi: 10.1093/oxfordjournals.jbchem.a124658. [DOI] [PubMed] [Google Scholar]
- 25.Lemaitre M, Bayard B, Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci USA. 1987;84:648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letsinger R L, Zhang G, Sun D K, Ikeuchi T, Sarin P S. Cholesteryl conjugated oligonucleotides: synthesis, properties and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci USA. 1989;86:6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis J G, Lin K Y, Kothavale A, Flanagan W M, Matteucci M D, DePrince R B, Mook R A, Jr, Hendren R W, Wagner R W. A serum resistant cytofectin for cellular delivery of antisense oligonucleotides and plasmid DNA. Proc Natl Acad Sci USA. 1996;93:3176–3181. doi: 10.1073/pnas.93.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loke S L, Stein C A, Zhang X H, Mori K, Nakanishi M, Subasinghe C, Cohen J S, Neckers L M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci USA. 1989;86:3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M, Guo Q, Kallenbach N R. Structure and stability of sodium and potassium complexes of dT4G4 and dT4G4T. Biochemistry. 1992;31:2455–2459. doi: 10.1021/bi00124a003. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar S, Basu S K. Killing of Mycobacterium tuberculosis by receptor-mediated drug delivery. Antimicrob Agents Chemother. 1991;35:135–140. doi: 10.1128/aac.35.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay A, Mukhopadhyay B, Basu S K. Circumvention of multidrug resistance in neoplastic cells through scavenger receptor-mediated drug delivery. FEBS Lett. 1995;376:95–98. doi: 10.1016/0014-5793(95)01250-6. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay A, Mukhopadhyay B, Srivastava R K, Basu S K. Scavenger receptor mediated delivery of daunamycin elicits selective toxicity towards neoplastic cells of macrophage lineage. Biochem J. 1992;284:237–241. doi: 10.1042/bj2840237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay A, Chaudhuri G, Arora S K, Sehgal S, Basu S K. Receptor mediated delivery to macrophages in chemotherapy of leishmaniasis. Science. 1989;244:705–707. doi: 10.1126/science.2717947. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay B, Mukhopadhyay A, Basu S K. Enhancement of tumoricidal activity of daunomycin by receptor mediated delivery: in vivo studies. Biochem Pharmacol. 1993;46:919–924. doi: 10.1016/0006-2952(93)90502-n. [DOI] [PubMed] [Google Scholar]
- 35.Ojwang J, Elbaggari A, Marshall H B, Jayaraman K, McGarth M S, Rando R F. Inhibition of human immunodeficiency virus type I activity in vitro by oligonucleotides composed entirely of guanosine and thymidine. J Acquired Immune Defic Syndr. 1994;7:560–570. [PubMed] [Google Scholar]
- 36.Pearson A M, Rich A, Krieger M. Polynucleotide binding to macrophage scavenger receptors depends on the formation of base-quartet stabilized four stranded helices. J Biol Chem. 1993;268:3546–3554. [PubMed] [Google Scholar]
- 37.Perez R R, Yuling L, Stein C A, Majumdar S, Oorschot A V, Narayanan R. Sequence independent induction of Sp1 transcription factor activity by phosphorothioate oligodeoxynucleotides. Proc Natl Acad Sci USA. 1994;91:5957–5961. doi: 10.1073/pnas.91.13.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Probst J C, Skutella T. G-tetrads in antisense targeting. Trends Genet. 1996;12:290–291. doi: 10.1016/0168-9525(96)20009-5. [DOI] [PubMed] [Google Scholar]
- 39.Rappaport J, Hans B, Kopp J B, Copeland T D, Bruggeman I A, Coffman T M, Klotman P E. Transport of phosphorothioate oligonucleotides in kidney: implications for molecular therapy. Kidney Int. 1995;47:1462–1469. doi: 10.1038/ki.1995.205. [DOI] [PubMed] [Google Scholar]
- 40.Rojanasakul Y. Antisense oligonucleotide therapeutics: drug delivery and targeting. Adv Drug Delivery Rev. 1996;18:115–131. [Google Scholar]
- 41.Saxena S K, Ackerman E J. Microinjected oligonucleotides complementary to the α-sarcin loop of the 28 S RNA abolish protein synthesis in xenopus oocyte. J Biol Chem. 1990;265:3263–3269. [PubMed] [Google Scholar]
- 42.Shea R G, Marsters J C, Bischofberger N. Synthesis, hybridization properties and antiviral activity of lipid-oligodeoxynucleotide conjugates. Nucleic Acids Res. 1990;18:3777–3783. doi: 10.1093/nar/18.13.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein C A. Phosphorothioate oligodeoxyribonucleotides: questions of specificity. Trends Biotechnol. 1996;14:147–149. doi: 10.1016/0167-7799(96)20006-X. [DOI] [PubMed] [Google Scholar]
- 44.Stein C A, Cheng Y C. Antisense oligonucleotides as therapeutic agents—is the bullet really magical? Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 45.Tackas A M, Banerjee A K. Inhibition of vesicular stomatitis virus in cells constitutively expressing an antisense RNA targeted against the virus RNA polymerase gene. J Gen Virol. 1997;78:125–130. doi: 10.1099/0022-1317-78-1-125. [DOI] [PubMed] [Google Scholar]
- 46.Tang J Y, Temsamani J, Agrawal S. Self-stabilized antisense oligodeoxynucleotide phosphorothioates: properties and anti-HIV activity. Nucleic Acids Res. 1993;21:2729–2735. doi: 10.1093/nar/21.11.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tolou H. Administration of oligonucleotides to cultured cells by calcium phosphate precipitation method. Anal Biochem. 1993;215:156–158. doi: 10.1006/abio.1993.1568. [DOI] [PubMed] [Google Scholar]
- 48.Tonkinson J L, Stein C A. Patterns of intracellular compartmentalization, trafficking and acidification of 5′ fluorescein labelled phosphodiester and phosphorothioate oligodeoxynucleotides in HL60 cells. Nucleic Acids Res. 1994;22:4268–4275. doi: 10.1093/nar/22.20.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner E, Zenke M, Cotten M, Beug H, Birnstiel M L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci USA. 1990;87:3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu G Y, Wu C H. Specific inhibition of hepatitis B viral gene expression in vitro by targeted antisense oligonucleotides. J Biol Chem. 1992;267:12436–12439. [PubMed] [Google Scholar]
- 51.Yakubov L A, Deeva E A, Zarytova V F, Ivanova E M, Ryte A S, Yurchenko L V, Vlassov V V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci USA. 1989;86:6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zelphati O, Imbach J L, Signoret N, Zon G, Rayner B, Leserman L. Antisense oligonucleotides in solution or encapsulated in immunoliposomes inhibit replication of HIV-I by several different mechanisms. Nucleic Acids Res. 1994;22:4307–4314. doi: 10.1093/nar/22.20.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]