Abstract
Klebsiella pneumoniae endotoxin has been found to decrease hepatic P450-mediated drug-metabolizing enzyme activity in a time-dependent manner. In this study, we investigated the role of nitric oxide (NO) in the decrease in hepatic drug-metabolizing enzyme activity caused by endotoxin in vivo. We measured in vivo pharmacokinetic parameters of antipyrine in rats treated with endotoxin and/or a selective inhibitor of inducible NO synthase (iNOS), S-methylisothiourea. Intraperitoneal injection of endotoxin (1 mg/kg of body weight) dramatically decreased the systemic clearance of antipyrine, reflecting reduced hepatic drug-metabolizing enzyme activity, and significantly increased the level of nitrite and nitrate (NOx) in the plasma. S-Methylisothiourea (10 mg/kg) reversed this decreasing antipyrine clearance and reduced the level of NOx in plasma. Repeated injections of an NO donor, (±)-(E)-4-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide (FK-409; 10 mg/kg), at a dose which maintained plasma NOx at the same levels as those caused by endotoxin injection, also decreased the systemic clearance of antipyrine. These findings suggest that the overproduction of NO observed in this animal model is at least partially responsible for the significant reduction in the hepatic drug-metabolizing enzyme activity that may happen in a gram-negative bacterial infection.
It is well known that bacterial infections impair hepatic drug metabolism in humans (28) and that endotoxin, a component of the cell wall of gram-negative bacteria, plays a key role in this phenomenon (18). That is, endotoxin reduces the clearance of hepatically metabolized drugs in humans (27), as well as in experimental animals (21), and decreases the total cytochrome P450 (CYP) content and catalytic activity (18).
Endotoxin stimulates the release of a variety of mediators, including interleukins, gamma interferon, tumor necrosis factor alpha, and nitric oxide (NO). Among them, NO is significantly released following endotoxin administration, subsequent to the expression of inducible NO synthase (iNOS) (1, 12, 17, 20, 25). NO may be involved in decreasing hepatic drug-metabolizing activity by endotoxin via at least two mechanisms: (i) overproduction of NO, followed by binding to the heme moiety of CYP, resulting in decreased catalytic activity (5, 11, 16), and (ii) reduction of CYP activity and mRNA expression by NO itself (2, 11, 29, 32). However, it is difficult to distinguish which mediator(s) is important in causing hepatic CYP down-regulation since some cytokines produced by endotoxin have an ability to decrease hepatic enzymatic activity. That is, the injection of interleukin-1 (6, 15, 19, 33), gamma interferon (2, 9), or tumor necrosis factor alpha (6, 22) itself decreases certain CYP activities and their mRNA expression. Furthermore, according to recent studies using primary cultured hepatocytes (26) and iNOS knockout mice (25), endotoxin itself decreases the mRNA expression and protein content of CYP.
Almost all of these studies have used in vitro or ex vivo methods such as primary cultured hepatic cell systems (2, 6, 19, 26), V79 Chinese hamster cells expressing CYP subtypes (29), and liver microsome preparations (5, 9, 11, 15, 16, 19, 22, 25, 32, 33) to investigate hepatic CYP activity, as well as protein content. There have been no experiments analyzing the net drug-metabolizing enzyme activity and the impact of the three above-mentioned mechanisms in endotoxemic animals, other than one study investigating levels of l-alanine aminotransferase and l-aspartate aminotransferase in plasma, parameters of hepatic injury (30). A recent study in our laboratory demonstrated that Klebsiella pneumoniae endotoxin (1 mg/kg) dramatically reduces the systemic clearance of antipyrine (21), a drug metabolized mainly in the liver (10). The endotoxin effect reached a maximum 24 h after the injection, and the control level was regained 96 h after the injection with no toxic damage to the liver (21). These studies also have demonstrated that endotoxin down-regulated some hepatic enzyme activities, including CYPs (21), suggesting that decreasing antipyrine clearance caused by endotoxin may be, in part, due to dysfunction of these hepatic enzymes. However, details of the mechanism were not known.
In the present study, the role of NO in endotoxin-induced decreases in antipyrine clearance was investigated in rats by using a potent iNOS inhibitor, S-methylisothiourea (SMT) (30), and an NO donor, (±)-(E)-4-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide (FK-409) (4, 13, 14). The results suggest that overproduction of NO by endotoxin contributes significantly to decreases in hepatic CYP-mediated drug-metabolizing enzyme activity.
MATERIALS AND METHODS
The procedures involving animals and their care were in accordance with the guidelines of the Nagoya University Animal Care Committee and the Animal Welfare Act (U.S. Department of Agriculture).
Animals.
Eight-week-old male Wistar rats (Japan SLC Inc., Hamamatsu, Japan) were used in all experiments. The animals were maintained in a temperature- and humidity-regulated room (22 to 24°C and 55% ± 5%, respectively) with food and water supplied ad libitum under controlled lighting (lights on from 0800 to 200 h) for at least 3 days before the experiment and surgery.
Chemicals.
Antipyrine and phenacetin were purchased from Sigma Chemical Company (St. Louis, Mo.), and SMT was obtained from Research Biochemical Institute (Natick, Mass.). FK-409 was kindly donated by Fujisawa Pharmaceutical Co. Ltd. (Tsukuba, Japan). Endotoxin was isolated from a culture supernatant of K. pneumoniae LEN-1 (O3:K1−) (7, 8), a decapsulated mutant strain derived from K. pneumoniae Kasuya (O3:K1) (23), which successfully decreased the clearance of antipyrine at a dose of 1 mg/kg in the previous study (21). All of the other chemicals used were obtained commercially and were used without further purification. Endotoxin, antipyrine, SMT, and FK-409 were dissolved in sterilized isotonic saline.
Pharmacokinetic experiments.
One day before the start of the experiments, rats were anesthetized with sodium pentobarbital (25 mg/kg of body weight) and the right jugular vein was cannulated with a sterilized polyethylene tube for drug administration and blood sampling. The peritoneal cavity was also cannulated with a sterilized polyethylene tube for repeated FK-409 or saline injections in order to reduce handling stress.
In the experiments, rats received a bolus intravenous injection of antipyrine (20 mg/kg of body weight) 24 h after an intraperitoneal injection of isotonic saline or endotoxin (1.0 mg/kg) and 22 h after an intraperitoneal injection of saline or SMT (5 mg/kg). In the experiments using FK-409, rats received 14 intraperitoneal injections of FK-409 (10 mg/kg) or saline 30 or 60 min apart (0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5.5, 6.5, 7.5, and 8.5 h after the first injection) over 8.5 h in a pattern determined to mimic the effects of an endotoxin injection (1 mg/kg) on plasma nitrite and nitrate (NOx) (see Fig. 1 and 3). This regimen was selected by simulation of the NO-producing effects of FK-409 in a one-shot study (see Fig. 3, inset). It should also be noted that since FK-409 causes hypotension in the first 15 min after the injection, we administered FK-409 at 30- or 60-min intervals, not as a continuous infusion. Rats then received a bolus intravenous injection of antipyrine (20 mg/kg) 7 h after the final dose of FK-409.
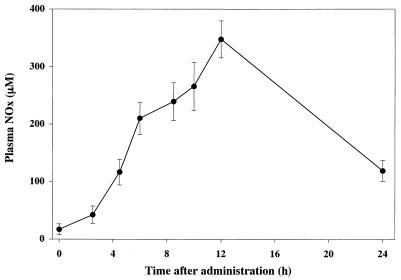
FIG. 1.
Typical plasma concentration-time course of NOx after endotoxin injection (1 mg/kg given intraperitoneally). The data are means and the standard errors of the means of three animals.
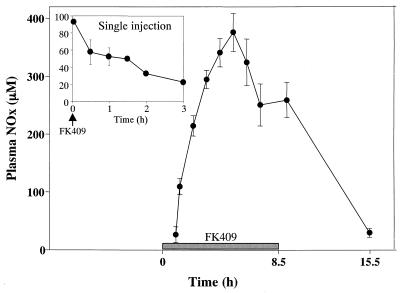
FIG. 3.
Typical plasma concentration-time course of NOx after a single injection and repeated injections of FK-409 (10 mg/kg given intraperitoneally). Each datum point represents the mean of five animals, and the error bars show the standard errors of the means.
In order to analyze plasma antipyrine concentrations, blood samples (approximately 0.25 ml each) were collected 30, 60, 90, 120, 180, 240, and 300 min after antipyrine administration. Plasma samples were immediately centrifuged at 6,000 × g for 5 min and stored at −40°C until analyzed.
Biochemical determinations.
Concentrations of NOx in plasma were measured with a commercial kit (Nitrate/Nitrite Colorimetric Assay Kit; Cayman Chemical, Ann Arbor, Mich.). Briefly, plasma samples collected at appropriate time points were ultrafiltered (molecular cutoff of 10,000) at 6,000 × g for 50 min. Filtered samples were allowed to incubate for 3 h with nitrate reductase and its cofactor and to react with Griess reagents for 20 min. The A540 was measured with a microplate reader (Molecular Devices Ltd., Crawley, United Kingdom) and converted to NOx concentrations by using a nitrate standard curve. Recovery of nitrate in this assay was over 95%.
Drug analysis.
Concentrations of antipyrine in plasma were measured by high-performance liquid chromatography (HPLC) with a slight modification of a previously described method (24). The HPLC apparatus was an LC-6A system (Shimadzu, Kyoto, Japan) consisting of an LC-6A liquid pump, an SPD-6A UV-VIS spectrophotometric detector, and an SIL-6A autoinjector. A Cosmosil 5C18 column (4.6 by 150 mm; Nacalai Tesque, Kyoto, Japan) was used with a column oven (OTC-6A) heated to 40°C. The UV detector was set at 254 nm. The mobile phase was 30% (vol/vol) methanol in distilled water, and the flow rate was 1.5 ml/min. Phenacetin was used as an internal standard. Standard curves for measuring antipyrine in plasma proved to be linear for concentrations ranging from 0.5 to 50 μg/ml with a correlation coefficient of 0.999. The intra- and interassay coefficients of variation for the HPLC assay were less than 6% at concentrations of 5 and 20 μg/ml. The detection limit of antipyrine was 0.2 μg/ml.
Data analysis.
Plasma concentration-time data for antipyrine in each rat were analyzed individually by noncompartmental methods. The area under the plasma concentration-time curve (AUC) and the area under the first-moment curve (AUMC) were calculated by the trapezoidal rule method up to the last measured plasma concentration and were extrapolated to infinity by adding the value of the last measured plasma concentration divided by the terminal elimination rate constant, which was calculated by determining the slope of the least-squares regression line from the terminal portion of the log concentration-time data. Systemic clearance (CLsys) was calculated by dividing the dose by the AUC. The steady-state volume of distribution (Vss) was calculated as Vss = CLsys × MRT, where MRT represents the mean residence time, which was calculated as MRT = AUMC/AUC. All computer analyses were performed by using a nonlinear least-squares regression program (MULTI), written by Yamaoka et al. (34), by weighting the data with the reciprocal of the concentration.
Statistical analysis.
Results were expressed as means ± standard errors for the indicated number of experiments. Statistical comparisons among the groups were assessed by one-way analysis of variance (ANOVA). When F ratios were significant (P < 0.05), Scheffe’s post-hoc tests between two groups were done and P values of <0.05 were considered statistically significant post-hoc differences.
RESULTS
Endotoxin increases plasma NOx concentration.
A typical plasma concentration-time curve for NOx after intraperitoneal injection of endotoxin at a dose of 1 mg/kg is presented in Fig. 1. Endotoxin dramatically increased the level of NOx in the plasma. NOx in plasma started to increase 4 to 6 h after endotoxin injection, reached a maximum level (20 times higher than at 0 h) approximately 12 h after endotoxin injection, and returned to close to normal by 24 h.
Effect of SMT on endotoxin-induced delayed metabolism of antipyrine.
Mean semilogarithmic plasma concentration-time curves for antipyrine in rats treated with saline, endotoxin alone, endotoxin-SMT, and SMT alone are illustrated in Fig. 2. Endotoxin injection increased the level of antipyrine in the plasma and markedly delayed the disappearance of antipyrine from plasma. Plasma concentrations of antipyrine in rats pretreated with SMT (10 mg/kg) and endotoxin were lower than those in rats pretreated with endotoxin alone, indicating that the inhibitory effect of endotoxin on antipyrine metabolism was reversed by coadministration of SMT. The corresponding pharmacokinetic parameter, CLsys of antipyrine, is summarized in Table 1. Endotoxin significantly decreased the CLsys of antipyrine to approximately 50% of the control (Table 1) without any changes in the volume of distribution (data not shown). The effect of endotoxin was significantly reversed by coadministration of SMT. No effect of SMT itself on the CLsys of antipyrine was observed.
FIG. 2.
Mean semilogarithmic plots of plasma concentration-time data for antipyrine in untreated rats and in rats pretreated with endotoxin (1 mg/kg given intraperitoneally), and/or SMT (10 mg/kg given intraperitoneally). Each symbol represents the mean ± the standard error (n = 6). Symbols: ○, control; ●, endotoxin only; ▾, endotoxin plus SMT; ▿, SMT only.
TABLE 1.
Effect of SMT on endotoxin-induced decrease in CLsys of antipyrine and endotoxin-stimulated NOx levels in ratsa
| Treatmentb | Antipyrine CLsys (liters/h/kg of body wt)c | NOx concn (μM) in plasmad |
|---|---|---|
| Control | 0.641 ± 0.037 (6) | 11.6 ± 3.03 (18) |
| Endotoxin | 0.300 ± 0.015e (6) | 394.3 ± 25.2e (7) |
| SMT + endotoxin | 0.455 ± 0.035ef (6) | 172.5 ± 29.9ef (8) |
| SMT | 0.517 ± 0.031g (6) | 9.1 ± 8.1g (3) |
The values shown are means ± the standard errors, and the number of animals per group is in parentheses. NOx levels were measured 12 h after endotoxin injection.
SMT was given at 10 mg/kg of body weight intraperitoneally, and endotoxin was given at 1 mg/kg of body weight intraperitoneally.
ANOVA revealed a statistically significant difference between groups [F(3, 19) = 21.23; P < 0.0001].
ANOVA revealed a statistically significant difference between groups [F(3, 32) = 110.13; P < 0.0001].
Significantly different from the control group (P < 0.01).
Significantly different from the endotoxin-treated group (P < 0.01).
Not significantly different from the control group (P > 0.05; Scheffe’s post-hoc test).
Effect of SMT on endotoxin-stimulated plasma NOx levels.
The level of plasma NOx was measured 12 h after endotoxin injection in order to assess the effect of SMT on NO production. Plasma NOx concentrations in rats treated with saline, endotoxin, endotoxin-SMT, and SMT alone are summarized in Table 1. Endotoxin dramatically stimulated NO release, and the plasma NOx concentration reached approximately 400 μM, which is a 35-fold increase over the basal plasma NOx concentrations. Pretreatment with SMT significantly decreased the endotoxin-induced increase in the plasma NOx concentrations by approximately 60%, whereas SMT itself did not show any effect on NO release.
Plasma NOx levels stimulated by single and repeated administrations of FK-409.
The concentration of NOx in plasma was measured after a single intraperitoneal injection and after repeated administrations of FK-409 (10 mg/kg). As shown in Fig. 3 (inset), a single administration of FK-409 immediately released NO into the plasma and increased plasma NOx concentrations, which returned to basal values within 3 h. The high plasma concentration of NOx observed after a single injection of FK-409 is not likely due to be an artifact of drug interference with the NOx assay, because high concentrations of FK-409 alone did not show any absorbance in our assay (data not shown). In the repeated-administration regimen, FK-409 was injected in a pattern (see Materials and Methods) which was designed to mimic endotoxin-induced plasma NOx concentrations over time. This pattern was based on the plasma concentration-time data of NOx after a single injection of FK-409, and as desired, plasma NOx concentrations induced by this regimen were maintained at more than 250 μM for 8 h and reached a maximum 12 h before the start of antipyrine pharmacokinetic studies (Fig. 3). Indeed, this NOx profile is very similar to that seen after endotoxin injection (Fig. 1).
Effect of repeated administration FK-409 on metabolism of antipyrine.
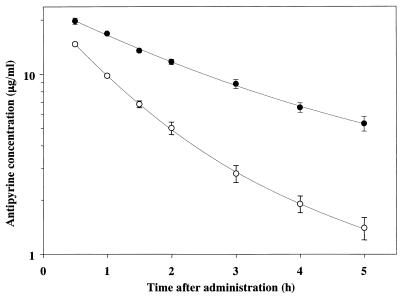
The mean semilogarithmic plasma concentration-time curves for antipyrine in rats pretreated with FK-409 or saline are illustrated in Fig. 4. The corresponding pharmacokinetic parameter, CLsys, of antipyrine is as follows. The CLsys of antipyrine in rats repeatedly administered saline or FK-409 (10 mg/kg, intraperitoneally) was 0.645 ± 0.064 or 0.268 ± 0.016 liters/h/kg of body weight, respectively. These values are the mean ± standard error of six rats. ANOVA revealed a statistically significant difference between the groups [F(1, 10) = 101.422; P < 0.0001]. The FK-409-treated group differed significantly (P < 0.01) from the saline-treated group (Scheffe’s post-hoc test). Thus, repeated doses of FK-409 significantly delayed the disappearance of antipyrine from plasma and dramatically decreased the CLsys of antipyrine by 60% without any change in the Vss (data not shown).
FIG. 4.
Mean semilogarithmic plots of plasma concentration-time data for antipyrine in untreated rats and rats pretreated with repeated injections of FK-409 (10 mg/kg given intraperitoneally). Each symbol represents the mean ± the standard error (n = 6). Symbols: ○, control; ●, FK-409.
DISCUSSION
We have recently reported that K. pneumoniae endotoxin nonselectively suppresses the activity of hepatic CYP-mediated drug-metabolizing enzymes without causing severe liver tissue damage (21). In the present study, we investigated the role of NO in the endotoxin-induced reduction of hepatic CYP-mediated drug-metabolizing enzyme activity by using antipyrine as a model substrate in rats. Intraperitoneal injection of endotoxin (1 mg/kg) caused prolonged overproduction of NO. The selective iNOS inhibitor SMT reversed the decreasing antipyrine clearance and inhibited the overproduction of NO in endotoxemic rats. Repeated injections of the NO donor FK-409, causing an elevation of the NOx level that mimicked the endotoxin-induced overproduction of NO, also produced delayed antipyrine metabolism. These findings clearly show that overproduction of NO contributes to the endotoxin-induced decrease in the activity of hepatic CYP-mediated drug-metabolizing enzymes in rats. It is noteworthy that the present experiments are the first to use an NO donor to demonstrate the inhibitory effect of excessive NO on drug metabolism in living animals. In addition, it is likely that the inhibition of iNOS activity is important in the decrease in antipyrine clearance caused by endotoxin since a nonselective endothelial NOS/iNOS inhibitor, NG-nitro-l-arginine methyl ester, aggravates endotoxin-induced liver damage, as expressed by l-alanine aminotransferase and l-aspartate aminotransferase levels (31), differently from SMT (31).
The results reported here suggest an interesting relationship between plasma NOx concentrations and decreases in CYP enzyme activities. As presented in the studies with endotoxin treatment and repeated FK-409 administration, the CLsys of antipyrine was dramatically decreased in the presence of peak NOx plasma concentrations of over 300 μM, whereas the protective effect of SMT on decreasing antipyrine clearance (hepatic drug-metabolizing enzyme activity) was evident in the presence of NOx at a maximum plasma concentration of approximately 170 μM (Table 1). Likewise, no significant changes in antipyrine clearance were observed in rats treated with seven intraperitoneal injections of FK-409 although peak plasma concentrations of NOx reached approximately 150 μM (data not shown). On the basis of these observations, we surmise that a certain minimum plasma NOx concentration maintained over a certain length of time is apparently necessary to affect drug metabolism. Furthermore, it should be noted that FK-409’s effect is not caused by the direct action of FK-409 and/or its metabolites, because the chemically degraded products of FK-409 neither produced NO nor changed antipyrine metabolism (data not shown).
The current results of experiments using an iNOS inhibitor (SMT) and an NO donor (FK-409) appear to imply that the mechanism involving endotoxin-induced overproduction of NO is more influential than the mechanisms involving either endotoxin-induced cytokines (2, 5, 6, 9, 11, 15, 19, 22, 29, 33) or endotoxin acting directly (25, 26). There are two possible mechanisms by which NO may decrease CYP enzyme activity. Either (i) NO binds to the heme moiety of CYP, resulting in its inactivation (5, 11, 16), or (ii) NO decreases CYP mRNA expression (2, 11, 29, 32). The studies reported here do not attempt to distinguish between these two possible mechanisms, and further studies are required to address this issue. Moreover, since antipyrine is completely metabolized by at least six hepatic CYP isozymes in humans (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C18, and CYP3A4) (3), decreased antipyrine clearance caused by endotoxin reflects the reduction of the activity of the sum of these enzymes. However, it has been reported that endotoxin suppresses the expression of different CYP mRNAs (CYP2C29 and CYP3A11) in iNOS knockout mice (25), suggesting that more than one mechanism regulates CYP down-regulation by endotoxin. Thus, it will also be of interest to investigate the expression of CYP mRNAs and their protein contents under our experimental conditions in detail.
In conclusion, these experiments strongly suggest that excess NO plays a key role in the endotoxin-induced decrease in hepatic CYP-mediated drug-metabolizing enzyme activity. These results caution that gram-negative bacterial infection may increase the risk of the side effects of some drugs, especially those which are metabolized mainly by the liver, and suggest that more selective iNOS inhibitors may be useful drugs for ameliorating these endotoxin-induced changes.
ACKNOWLEDGMENTS
This work was supported by research grant 11672296 from the Ministry of Education, Science, Sports and Culture and grant 10044 from the Daiko Foundation.
REFERENCES
- 1.Bredt D S, Snyder S H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 2.Donato M T, Guillen M I, Jover R, Castell J V, Gomez-Lechon M J. Nitric oxide-mediated inhibition of cytochrome P450 by interferon-γ in human hepatocytes. J Pharmacol Exp Ther. 1997;281:484–490. [PubMed] [Google Scholar]
- 3.Engel G, Hofmann U, Heidemann H, Cosme J, Eichelbaum M. Antipyrine as a probe for human oxidative drug metabolism: identification of the cytochrome P450 enzymes catalyzing 4-hydroxyantipyrine, 3-hydroxymethylantipyrine, and norantipyrine formation. Clin Pharmacol Ther. 1996;59:613–623. doi: 10.1016/S0009-9236(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 4.Fukuyama S, Azuma T, Hirasawa Y, Morokoshi N, Akama T, Koda S, Kita Y. Nitric oxide (NO)-releasing pathway of FK409 in the presence of sulfhydryl-bearing compounds. Pharm Res. 1996;13:1238–1242. doi: 10.1023/a:1016076623060. [DOI] [PubMed] [Google Scholar]
- 5.Gergel D, Misik V, Riesz P, Cederbaum A I. Inhibition of rat and human cytochrome P4502E1 catalytic activity and reactive oxygen radical formation by nitric oxide. Arch Biochem Biophys. 1997;337:239–250. doi: 10.1006/abbi.1996.9765. [DOI] [PubMed] [Google Scholar]
- 6.Ghezzi P, Saccardo B, Villa P, Rossi V, Bianchi M, Dinarello C A. Role of interleukin-1 in the depression of liver drug metabolism by endotoxin. Infect Immun. 1986;54:837–840. doi: 10.1128/iai.54.3.837-840.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa T, Ohta M, Mori M, Nakashima I, Kato N. The Klebsiella O3 lipopolysaccharide isolated from culture fluid: structure of the polysaccharide moiety. Microbiol Immunol. 1983;27:683–694. doi: 10.1111/j.1348-0421.1983.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa T, Ohta M, Nakashima I, Kato N, Morikawa K, Harada T, Okuyama T. Structure of the polysaccharide moiety of the Klebsiella O3 lipopolysaccharide isolated from culture supernatant of decapsulated mutant (Klebsiella O3:K1−) Chem Pharm Bull (Tokyo) 1985;33:333–339. doi: 10.1248/cpb.33.333. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson P D, Renton K W. The role of nitric oxide generation in interferon-evoked cytochrome P450 down-regulation. Int J Immunopharmacol. 1995;17:995–1000. doi: 10.1016/0192-0561(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 10.Inaba T, Otton S V, Kalow W. Deficient metabolism of debrisoquine and sparteine. Clin Pharmacol Ther. 1980;27:547–549. doi: 10.1038/clpt.1980.77. [DOI] [PubMed] [Google Scholar]
- 11.Khatsenko O, Kikkawa Y. Nitric oxide differentially affects constitutive cytochrome P450 isoforms in rat liver. J Pharmacol Exp Ther. 1997;280:1463–1470. [PubMed] [Google Scholar]
- 12.Khatsenko O G, Gross S S, Rifkind A B, Vane J R. Nitric oxide is a mediator of the decrease in cytochrome P450-dependent metabolism caused by immunostimulants. Proc Natl Acad Sci USA. 1993;90:11147–11151. doi: 10.1073/pnas.90.23.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kita Y, Fukuyama S, Hirasawa Y. Close correlation between nitric oxide (NO) formation from NO releasers and the biological activities of these agents in rats. Jpn J Pharmacol. 1995;69:69–74. doi: 10.1254/jjp.69.69. [DOI] [PubMed] [Google Scholar]
- 14.Kita Y, Hirasawa Y, Fukuyama S, Ohkubo K, Kato Y, Takamatsu H, Ohno M, Nishino S, Kato M, Seki J. Oral biological activities of spontaneous nitric oxide releasers are accounted for by their nitric oxide-releasing rates and oral absorption manners. J Pharmacol Exp Ther. 1996;276:421–425. [PubMed] [Google Scholar]
- 15.Kurokohchi K, Yoneyama H, Matsuo Y, Nishioka M, Ichikawa Y. Effects of interleukin 1α on the activities and gene expressions of the cytochrome P450IID subfamily. Biochem Pharmacol. 1992;44:1669–1674. doi: 10.1016/0006-2952(92)90485-2. [DOI] [PubMed] [Google Scholar]
- 16.Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, Inoue M. Irreversible inhibition of cytochrome P450 by nitric oxide. J Pharmacol Exp Ther. 1997;283:1479–1485. [PubMed] [Google Scholar]
- 17.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 18.Morgan E T. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 19.Morgan E T, Thomas K B, Swanson R, Vales T, Hwang J, Wright K. Selective suppression of cytochrome P-450 gene expression by interleukins 1 and 6 in rat liver. Biochim Biophys Acta. 1994;1219:475–483. doi: 10.1016/0167-4781(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 20.Morris S M, Jr, Billiar T R. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994;266:E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- 21.Nadai M, Sekido T, Matsuda I, Wang L, Kitaichi K, Itoh A, Nabeshima T, Hasegawa T. Time-dependent effects of Klebsiella pneumoniae endotoxin on drug-metabolizing enzyme activity in rats. J Pharm Pharmacol. 1998;50:871–879. doi: 10.1111/j.2042-7158.1998.tb04002.x. [DOI] [PubMed] [Google Scholar]
- 22.Nadin L, Butler A M, Farrell G C, Murray M. Pretranslational down-regulation of cytochromes P450 2C11 and 3A2 in male rat liver by tumor necrosis factor alpha. Gastroenterology. 1995;109:198–205. doi: 10.1016/0016-5085(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 23.Ohta M, Mori M, Hasegawa T, Nagase F, Nakashima I, Naito S, Kato N. Further studies of the polysaccharide of Klebsiella pneumoniae possessing strong adjuvanticity. I. Production of the adjuvant polysaccharide by noncapsulated mutant. Microbiol Immunol. 1981;25:939–948. doi: 10.1111/j.1348-0421.1981.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 24.Pilsgaard H, Poulsen H E. A one-sample method for antipyrine clearance determination in rats. Pharmacology. 1984;29:110–116. doi: 10.1159/000137999. [DOI] [PubMed] [Google Scholar]
- 25.Sewer M B, Barclay T B, Morgan E T. Down-regulation of cytochrome P450 mRNAs and proteins in mice lacking a functional NOS2 gene. Mol Pharmacol. 1998;54:273–279. doi: 10.1124/mol.54.2.273. [DOI] [PubMed] [Google Scholar]
- 26.Sewer M B, Morgan E T. Nitric oxide-independent suppression of P450 2C11 expression by interleukin-1β and endotoxin in primary rat hepatocytes. Biochem Pharmacol. 1997;54:729–737. doi: 10.1016/s0006-2952(97)00226-8. [DOI] [PubMed] [Google Scholar]
- 27.Shedlofsky S I, Israel B C, McClain C J, Hill D B, Blouin R A. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Investig. 1994;94:2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonne J, Dossing M, Loft S, Andreasen P B. Antipyrine clearance in pneumonia. Clin Pharmacol Ther. 1985;37:701–704. doi: 10.1038/clpt.1985.117. [DOI] [PubMed] [Google Scholar]
- 29.Stadler J, Trockfeld J, Schmalix W A, Brill T, Siewert J R, Greim H, Doehmer J. Inhibition of cytochromes P4501A by nitric oxide. Proc Natl Acad Sci USA. 1994;91:3559–3563. doi: 10.1073/pnas.91.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo C, Southan G J, Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci USA. 1994;91:12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vos T A, Gouw A S, Klok P A, Havinga R, van Goor H, Huitema S, Roelofsen H, Kuipers F, Jansen P L, Moshage H. Differential effects of nitric oxide synthase inhibitors on endotoxin-induced liver damage in rats. Gastroenterology. 1997;113:1323–1333. doi: 10.1053/gast.1997.v113.pm9322528. [DOI] [PubMed] [Google Scholar]
- 32.Wink D A, Osawa Y, Darbyshire J F, Jones C R, Eshenaur S C, Nims R W. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 33.Wright K, Morgan E T. Regulation of cytochrome P450IIC12 expression by interleukin-1α, interleukin-6, and dexamethasone. Mol Pharmacol. 1991;39:468–474. [PubMed] [Google Scholar]
- 34.Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. A pharmacokinetic analysis program (MULTI) for microcomputer. J Pharmacobiodyn. 1981;4:879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]