Abstract
The penicillin-binding proteins (PBPs) of helical (log-phase) Helicobacter pylori ATCC 43579 were identified by using biotinylated ampicillin. The major PBPs had apparent molecular masses of 47, 60, 63, and 66 kDa; an additional minor PBP of 95 to 100 kDa was also detected. The relative affinities of various β-lactams for these PBPs were tested by competitive-binding assays. Only PBP63 appeared to be significantly bound to each of the competing antibiotics, whereas PBP66 strongly bound mezlocillin, oxacillin, amoxicillin, and ceftriaxone. Whereas most of the β-lactams significantly bound two or more PBPs, aztreonam specifically targeted PBP63. The influence of sub-MICs of these β-lactams on the morphologies of log-phase H. pylori was observed at both the phase-contrast and transmission electron microscopy levels. Each of the eight β-lactams examined induced blebbing and sphere formation, whereas aztreonam was the only antibiotic studied which induced pronounced filamentation in H. pylori. Finally, studies comparing the PBPs of helical (log-phase) cultures with those of coccoid (7-, 14-, and 21-day-old) cultures of H. pylori revealed that the major PBPs at 60 and 63 kDa seen in the helical form were almost undetectable in the coccoid forms, whereas PBP66 remained the major PBP in the coccoid forms, although somewhat reduced in level compared to the helical form. PBP47 was present in both forms at approximately equal concentrations. These studies thus identified the major PBPs in both helical and coccoid forms of H. pylori and compared the relative affinities of seven different β-lactams for the PBPs in the helical forms and their effects on bacterial morphology.
Helicobacter pylori, a curved, microaerophilic, gram-negative bacterium which colonizes the mucus layer of the gastric epithelium, is the causative agent of chronic type B gastritis and has been linked to the development of peptic ulcers and gastric cancer (see reference 17 for a review). Epidemiologic studies estimate that at least a third of the world’s population is infected with H. pylori, including about 50% of Americans 60 years old or older, making this infection one of the most common in the world (11). Although there are a variety of therapeutic choices for H. pylori infections, most regimens employ various multidrug combinations, including one or more antibiotics and the addition of bismuth, an H2 receptor antagonist, or a proton pump inhibitor (17, 22). Therapy often includes the use of one or more β-lactams, including amoxicillin (4, 17, 20, 29). However, after initial clearance of the infection, there is often a relapse or recurrence of infection (6, 17). While this recurrence may be due to the emergence of antibiotic-resistant strains, such as seen in metronidazole resistance (38), some studies also point to the importance of coccoid forms of the bacterium (4, 39).
H. pylori has been found to occur in two morphologic forms: the actively replicating helical or rod form and the round or coccoid form, which some consider a degenerate form (28) while others consider the coccoid form to be viable but nonculturable (24). While the active helical form is thought to be responsible for disease production, the nonmotile coccoid form might be involved in the transmission of H. pylori (33, 39). This coccoid form has been found to be associated with tissue necrosis (9, 26) and with histopathologic changes in mouse stomachs (7) and can survive for prolonged periods in environmental samples such as water (33). The morphologic conversion from the helical or rod form to the coccoid form has been examined in various in vitro studies (1, 3, 8, 30, 31, 33, 35), but the pathogenicity of the coccoid form remains controversial (7, 10, 18, 39, 40).
To identify the antibiotic-binding sites for the various β-lactam antibiotics used in the treatment of H. pylori infection, as well as to investigate potential factors involved in the dramatic morphological conversion of the helical to the coccoid form, we characterized the penicillin-binding proteins (PBPs) expressed in helical versus fully coccoid H. pylori. The PBPs are a set of enzymes involved in the synthesis of the peptidoglycan layer of the bacterial cell wall and include transpeptidases, transglycosylases, endopeptidases, and carboxypeptidases (5, 21). It has been shown that β-lactam antibiotics bind covalently to the PBPs, and by using labeled β-lactams, these enzymes can be detected and analyzed by conventional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by fluorography (5, 16, 21, 32). Recently, a PBP detection method was developed by Dargis and Malouin (14), who used biotinylated aminopenicillins in place of radiolabeled penicillins, providing a method of detection of these proteins which is both faster and free of hazardous reagents. Using this new approach, we characterized the major PBPs of H. pylori ATCC 43579.
We also compared the relative binding affinities of seven different β-lactams in comparison to biotin labelled ampicillin (Bio-Amp) for this H. pylori strain and determined the concentration of each antibiotic needed to inhibit the binding of Bio-Amp by 50% or greater (i.e., the IC50) for each major PBP. MICs were determined for each β-lactam on H. pylori, and the influence of sub-MICs of each antibiotic on bacterial morphology was observed by both phase-contrast microscopy and transmission electron microscopy. Finally, the PBP profiles of fully helical (48-h, log-phase) cultures were compared to those of broth cultures aged for 7 days (mostly coccoid), 14 days (>99% coccoid), and 21 days (100% coccoid).
MATERIALS AND METHODS
Bacterial culture conditions.
Stock cultures of H. pylori ATCC 43579 were streaked for isolation on brucella agar (Becton-Dickinson Microbiology, Cockeysville, Md.) supplemented with 10% defibrinated sheep blood (Colorado Serum Company, Denver) and 1% IsoVitalex (Becton-Dickinson Microbiology) and cultured at 37°C in a humidified 12% CO2 incubator. Liquid cultures were prepared by suspension of H. pylori colonies in brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with 10% fetal calf serum (Gibco Bethesda Research Laboratories, Grand Island, N.Y.) and 1% IsoVitalex and grown at 37°C in a humidified 12% CO2 incubator. Cultures were routinely passed by dilution into fresh medium at 48-h intervals; however, in some experiments, bacteria were cultured in the same medium for up to 21 days.
MIC and MBC determinations.
The following β-lactams were prepared in accordance with the manufacturer’s instructions and filter sterilized: amoxicillin (Sigma, St. Louis, Mo.), ampicillin (Fisher-Biotech, Fair Lawn, N.J.), penicillin G potassium (Marsham, Cherry Hill, N.J.), aztreonam (Azactam; Squibb, Princeton, N.J.), mezlocillin (Mezlin; Miles, West Haven, Conn.), oxacillin (Squibb), ceftriaxone (Rocephin; Roche, Nutley, N.J.), and cefuroxime (Zinacef; Glaxo, Research Triangle Park, N.C.). Twofold serial dilutions of each antibiotic ranging from 16 to 0.008 μg/ml were prepared in culture medium (brucella broth with 10% fetal calf serum and 1% IsoVitaleX), inoculated with a 100-fold dilution of a 48-h culture of H. pylori ATCC 43579, and incubated in 96-well Falcon tissue culture plates (Becton-Dickinson Microbiology) at 37°C in a humidified 12% CO2 incubator. The plates were examined for the presence of turbidity at 24 and 48 h, and the antibiotic concentration in the well containing the lowest concentration of antibiotic which was nonturbid was determined to be the MIC. Bacterial cultures grown in sub-MICs were visualized with a Zeiss phase-contrast microscope to characterize bacterial morphology. Aliquots of each bacterium-antibiotic mixture were then spread onto brucella-sheep blood agar plates in order to determine the MBC of each antibiotic. These plates were monitored for the presence of colonies at 24, 48, and 72 h of incubation, and the lowest antibiotic concentration which completely prevented bacterial growth was recorded as the MBC.
Bio-Amp labeling of PBPs.
Two liters of a 48-h culture of H. pylori was centrifuged at 6,000 × g for 10 min, washed, and resuspended in ice-cold 0.01 M phosphate-buffered saline (PBS), pH 7.2, treated with lysozyme (1 mg/5 ml of bacterial suspension, 20 min at room temperature), and sonicated until most of the cells were disrupted as visualized microscopically. Unbroken cells were removed by centrifugation at 8,000 × g for 20 min. Inner and outer membranes were concentrated by centrifugation at 100,000 × g for 40 min and washed twice in ice-cold PBS. Membrane fractions from 5-ml aliquots were frozen at −20°C until analyzed.
Membrane pellets were thawed and resuspended in 500 μl of 0.1 M phosphate buffer, pH 7.2. Bio-Amp was prepared by the method of Dargis and Malouin (14). Briefly, solutions of biotinamidocaproic acid 3-sulfo-N-hydroxysuccinimide ester (Sigma) and ampicillin (Fisher-Biotech) in 0.1 M sodium phosphate buffer, pH 7.2, were incubated at a 5:1 ratio for 30 min at room temperature with gentle agitation. The reaction was then stopped by addition of a 10-fold molar excess of glycine and incubated for 30 min at room temperature with gentle agitation. The Bio-Amp was then frozen in aliquots at −80°C for up to 12 months at a final concentration of 800 μg/ml. This biotinylation procedure did not adversely affect the activity of ampicillin, since the MIC of Bio-Amp was comparable to that of ampicillin (data not shown).
Approximately 1 mg of total membrane proteins was incubated with Bio-Amp at a concentration of 40 μg/ml for 30 min at room temperature. The membranes were then washed in 0.1 M phosphate buffer, pH 7.2, and centrifuged at 40,000 × g for 40 min. The pellets were then resuspended in 0.01 M phosphate buffer, pH 7.2, and the inner membrane proteins were solubilized in 1% N-lauroylsarcosine (Sigma) for 20 min. The insoluble membranes were then removed by centrifugation at 40,000 × g for 40 min, and the supernatant was used as described below. In some experiments, the Bio-Amp was pretreated for 30 min at room temperature with increasing amounts of β-lactamase (0.005, 0.05, 0.5, 5 and 50 U/ml; Sigma) prior to incubation with H. pylori membranes. Pretreatment of Bio-Amp with β-lactamase cleaves the β-lactam ring of the ampicillin molecule, thus preventing ampicillin from binding to PBPs via the β-lactam ring. Bio-Amp binding not decreased by pretreatment with β-lactamase was considered to be due to non-PBP-specific binding of Bio-Amp.
The supernatant fractions, containing solubilized, labeled membrane proteins, were then separated by SDS–10% PAGE using ∼100 μg of protein per well. After electrophoresis, proteins were transferred to nitrocellulose by using SDS-PAGE running buffer with 5% methanol at 400 mA of constant current for 2 h. The nitrocellulose sheets were then blocked either for 1 h at room temperature or overnight at 4°C in 0.01 M PBS–Tween (0.1% Tween 20) containing 5% nonfat dry milk. Membranes were rinsed in PBS-Tween three times and then incubated in streptavidin-horseradish peroxidase (Amersham, Arlington Heights, Ill.) diluted 1:1,000 in PBS-Tween for 1 h at room temperature. The membranes were then rinsed three times in PBS-Tween and examined for luminescence by the ECL protocols described by the manufacturer (Amersham). Membranes were exposed to ECL Hyperfilm (Amersham) for 10 to 60 s until banding patterns appeared. Molecular weights were determined by comparison to ECL molecular weight standards (Amersham). Reported molecular weights were the averages determined from blots from at least three separate experiments. For comparison experiments, resulting protein patterns were read for absorbance intensities using densitometric tracings with an Ultroscan XL laser densitometer (LKB Products, Bromma, Sweden).
PBP patterns were also compared in aged versus log-phase H. pylori cultures. Membrane fractions were prepared as described above from 48-h-old (log-phase) and 7-, 14-, and 21-day-old cultures. Morphologies of bacteria at all of these time points were observed by phase-contrast microscopy.
Antibiotic competition experiments.
In antibiotic competition experiments, log-phase H. pylori membrane fractions were prepared as described above and incubated with each antibiotic at 1, 10, and 100 times the MIC for 30 min at room temperature. These samples were then incubated with 40 μg of Bio-Amp per ml for 30 min at room temperature. Proteins were then prepared as described above and compared for banding pattern intensity using densitometric tracings, and the IC50s of each antibiotic for the major PBPs were approximated by using tracing data.
Transmission electron microscopy.
Cultures were grown in the presence or absence of antibiotics at sub-MICs for 24 to 48 h. After incubation in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 60 min, the bacteria were washed twice in sterile double-distilled H2O (ddH2O) with gentle centrifugation and resuspended in a final volume of 50 μl in sterile ddH2O. Copper grids (100-mesh thick bar) were prepared with a Formvar-coated support film, heavy carbon coated, and then glow discharged treated in an Edwards 306 vacuum evaporator (Edwards High Vacuum International, Wilmington, Mass.) to make the surfaces of the grids hydrophilic. After addition of samples to the grids, all of the liquid was drawn off, the samples were washed three times with sterile ddH2O, and all of the liquid was again drawn off. For unidirectional shadowing, samples were prepared by using a tungsten hairpin filament with 10 mm of 80% platinum–20% palladium metal wire at a 20°C angle in an Edwards 306 vacuum evaporator. Grids were observed with a Hitachi H-600 transmission electron microscope (Hitachi Instruments, Inc., San Jose, Calif.) operating at 75 kV at magnifications ranging from ×4,000 to ×40,000.
RESULTS
Identification of PBPs of H. pylori.
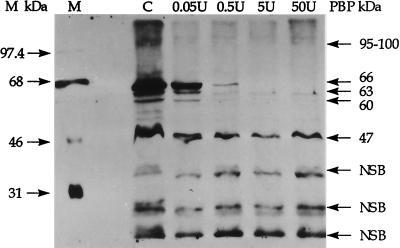
Treatment of H. pylori membrane fractions with Bio-Amp identified a number of membrane proteins which were potential PBPs (Fig. 1, lane C). Because of possible nonspecific binding of Bio-Amp to non-PBP membrane proteins, additional experiments were conducted with Bio-Amp pretreated with increasing concentrations of β-lactamase. As shown in Fig. 1, increasing concentrations of β-lactamase significantly decreased Bio-Amp labeling of proteins with molecular masses of 47, 60, 63, and 66 kDa. Although the protein band at 47 kDa did not completely disappear with β-lactamase treatment, the intensity of the band decreased by greater than 50% in this experiment and almost completely disappeared in other experiments and in antibiotic competition experiments (described later). A minor (i.e., less-abundant) PBP which was also affected by β-lactamase treatment was seen at 95 to 100 kDa. The protein bands seen at 51 and 54 kDa in this gel were not consistently observed in repeat studies and are unlikely to be major PBPs. Protein bands which were not significantly reduced in intensity by the addition of β-lactamase were considered to be the result of nonspecific Bio-Amp binding. Based on this analysis, the major PBPs identified by labeling with Bio-Amp in helical H. pylori had apparent molecular sizes of 47, 60, 63, and 66 kDa.
FIG. 1.
Log-phase H. pylori membrane fractions were labeled with either Bio-Amp (lane C) or Bio-Amp pretreated with increasing amounts of β-lactamase (lanes 0.05U, 0.5U, 5U, and 50U). Protein bands which were not decreased in intensity by the use of Bio-Amp pretreated with increasing β-lactamase concentrations were considered to be proteins nonspecifically bound with Bio-Amp (NSB). Lane M represents the ECL molecular size markers, while the molecular sizes on the right reflect estimates for PBPs determined as the averages from at least three separate blots.
When decreasing concentrations of H. pylori membrane fractions labeled with Bio-Amp were analyzed densitometrically for PBP band intensities, PBP66 labeling remained the most intense throughout the dilution series (data not shown). Although less than that of PBP66, Bio-Amp labeling of PBP60 and that of PBP63 were roughly equivalent in intensity and labeling of PBP47 was clearly less than that of the other three PBPs. Therefore, PBP66 appears to be the most abundant Bio-Amp-labeled PBP in H. pylori.
PBP binding study—competition between various β-lactams and Bio-Amp.
The MIC of each antibiotic used was first determined as described in Materials and Methods, and the results are shown in Table 1. Aztreonam, the only monobactam examined, had the highest MIC, twice that of oxacillin and more than 10 to 100 times greater than those of the other antibiotics used. As expected, the MBCs of each of these antibiotics were either the same or 2 to 4 dilutions above the MIC. In the following experiments, each antibiotic was preincubated with H. pylori membrane fractions at a concentration equal to 10 or 100 times the MIC for 30 min prior to the addition of Bio-Amp. Reduction in Bio-Amp labeling of PBPs with increasing concentrations of each β-lactam represents competition due to prebinding of these PBPs by that specific antibiotic. A representative experiment illustrating the results of this type of analysis using penicillin G is shown in Fig. 2; in order to demonstrate quantitative differences in PBP binding, laser densitometric tracings were performed and a summary of these studies is shown in Fig. 3.
TABLE 1.
Determination of the MIC and MBC of each β-lactam antibiotic for H. pylori ATCC 43579 and the effects of sub-MICs of these antibiotics on bacterial morphology
| β-Lactam | MIC (μg/ml)a | MBC (μg/ml)a | Morphologiesb |
|---|---|---|---|
| Penicillin G | 0.03–0.06 | 0.03–0.06 | Spheres, blebs |
| Ampicillin | 0.03–0.06 | 0.03–0.06 | Spheres, blebs |
| Amoxicillin | 0.03–0.06 | 0.125–0.5 | Spheres, blebs |
| Oxacillin | 2–4 | 4–8 | Spheres, blebs |
| Mezlocillin | 0.125–0.25 | 0.25–1.0 | Spheres, blebs |
| Cefuroxime | 0.03–0.06 | 0.06–0.5 | Spheres, blebs |
| Ceftriaxone | 0.125–0.25 | 0.125–1.0 | Spheres, blebs |
| Aztreonam | 4–8 | 4–8 | Filaments, spheres, blebs |
MICs of each β-lactam were determined after 24 to 48 h of incubation at 37°C by a microbroth dilution method. MBCs were determined by examination of agar plate subcultures after 72 h of incubation at 37°C (see Materials and Methods for details). The data shown are averages of three separate experiments.
Morphologies of H. pylori were observed between 24 and 48 h of incubation at 37°C in the presence of antibiotic concentrations equal to one-half to one-fourth of the MIC. Although they are not listed here, some residual normal-appearing helical or rod forms were also present in each culture.
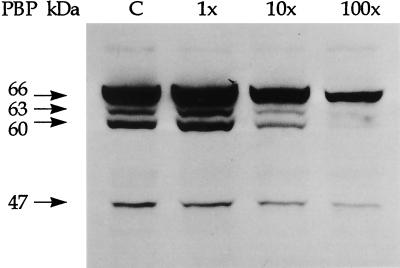
FIG. 2.
H. pylori membrane fractions were preincubated with penicillin G at 1, 10, or 100 times the MIC prior to labeling with Bio-Amp, separated by SDS-PAGE, and visualized on Western blots by chemiluminescence. The control lane (C) represents PBPs labeled by Bio-Amp in the absence of penicillin G. The locations of major PBPs at 66, 63, 60, and 47 kDa are shown on the left. The decreased Bio-Amp labeling intensity of these PBPs in the presence of increasing concentrations of penicillin G reflects competition between penicillin G and Bio-Amp for binding sites on these PBPs. Densitometric tracings of these protein bands are shown in Fig. 3A.
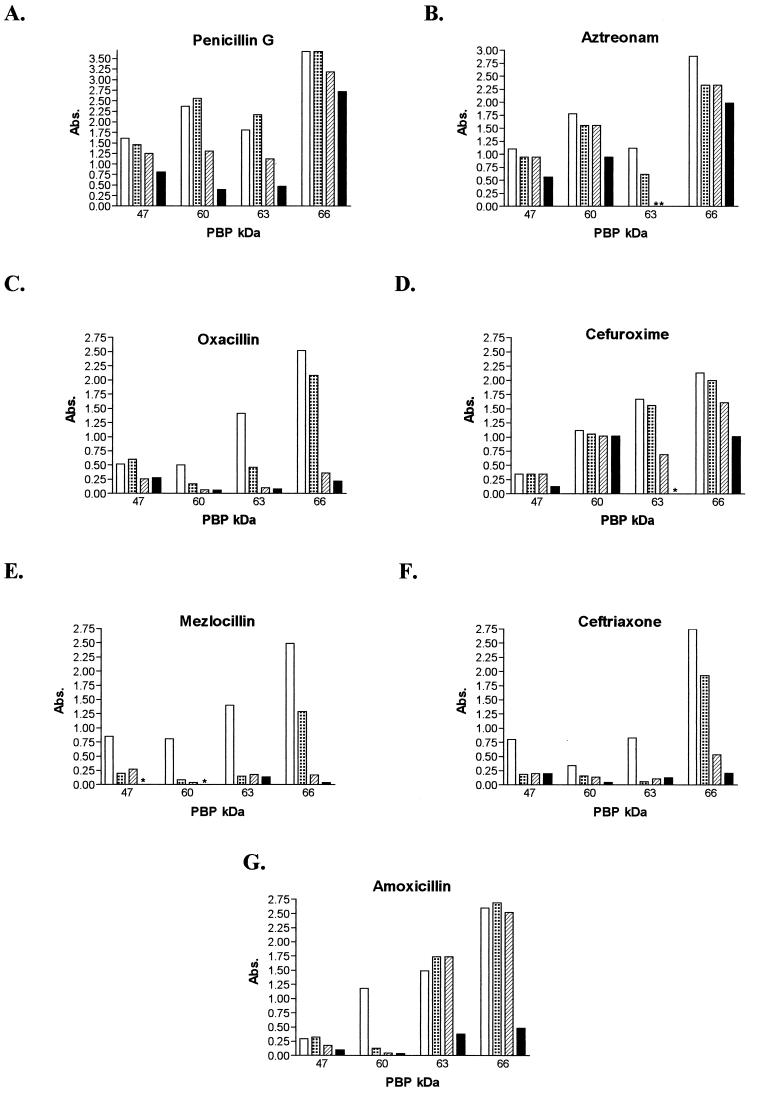
FIG. 3.
H. pylori membrane fractions were preincubated with each of the antibiotics shown at 1, 10, or 100 times the MIC prior to labeling with Bio-Amp, separated by SDS-PAGE, and visualized on Western blots. These blots were then quantitatively examined by laser densitometry. Control (Bio-Amp labeling without preincubation with a competing antibiotic), open bars; MIC, dotted bars; 10 times the MIC, hatched bars; 100 times the MIC, solid bars. Abs., absorbance.
In every experiment, PBP66 was clearly the protein which reacted most intensely with Bio-Amp whereas the relative concentrations of the other three major PBPs varied between membrane preparations. Of the major H. pylori PBPs identified, only PBP63 appeared to be significantly bound (≥75% reduction in Bio-Amp binding) by each of the antibiotics examined in this study. Aztreonam, the only monobactam used, showed preferential saturation of PBP63 by as little as 10 times the MIC, with less preference for PBP66 (31% reduction with 100 times the MIC), PBP60 (47% with 100 times the MIC), and PBP47 (48% with 100 times the MIC). Of the four penicillins studied, only mezlocillin showed almost complete saturation of each PBP, with PBP63 being 90% saturated with a concentration as low as the MIC. Oxacillin showed a similar pattern, although with somewhat less affinity for PBP47. Amoxicillin appeared to bind preferentially to PBP60, even at the MIC, and was near saturation at 100 times the MIC while significantly binding to PBP63 and PBP66 at 100 times the MIC. Penicillin G appeared to bind with some affinity to all of the PBPs but was less effective at competing with Bio-Amp than was oxacillin or mezlocillin and did not completely saturate any of the PBPs, even at 100 times the MIC. Ceftriaxone appeared to bind to all of the PBPs, with near saturation of all of the proteins except PBP47, although Bio-Amp binding to this protein was reduced by 75% by the MIC. Cefuroxime showed more specificity for H. pylori PBPs than did ceftriaxone, with preferential binding to PBP63 (saturation using 100 times the MIC) and PBP66 (53% saturation at 100 times the MIC). Bio-Amp labeling of PBP60 was not significantly affected by cefuroxime, and PBP47 was only affected (63% reduction) at 100 times the MIC.
The IC50s of each antibiotic for the four major PBPs were estimated by densitometry, and the results are shown in Table 2. Each value represents the concentration of β-lactam (at 1, 10, or 100 times the MIC) required to inhibit ≥50% of the binding of Bio-Amp at 40 μg/ml. Mezlocillin was most effective at inhibiting Bio-Amp binding to each major PBP, with an IC50 of 0.125 μg/ml for each PBP, while ceftriaxone inhibited the binding of Bio-Amp to PBP63, PBP60, and PBP47 at 0.25 μg/ml. Aztreonam, with the highest MIC, inhibited the binding of Bio-Amp to PBP63 at the MIC (8 μg/ml) but required ≥800 μg/ml to inhibit the binding of Bio-Amp to the other PBPs. Amoxicillin competed very well with Bio-Amp for each of the PBPs, with only 0.03 μg/ml required to inhibit Bio-Amp binding to PBP60 and only 3 μg/ml required for PBP66, PBP63, and PBP47. Penicillin G showed a similar pattern; however, greater than 3 μg of this antibiotic per ml was required to inhibit the binding of Bio-Amp to PBP66. Greater concentrations of oxacillin and cefuroxime were required to inhibit Bio-Amp binding to the major PBP of H. pylori, with 20 μg of oxacillin per ml and 6 μg of cefuroxime per ml required to inhibit Bio-Amp binding to PBP66 and PBP47.
TABLE 2.
IC50s of various β-lactam antibiotics specific for PBPs of H. pylori ATCC 43579
| PBP molecular size (kDa) | IC50 (μg/ml)a
|
||||||
|---|---|---|---|---|---|---|---|
| Pen G | Oxac | Mezl | Cefu | Ceft | Aztr | Amox | |
| 66 | >3 | 20 | 0.125 | 6 | 2.5 | >800 | 3 |
| 63 | ∼0.3 | 2 | 0.125 | 0.6 | 0.25 | ∼8 | 3 |
| 60 | ∼0.3 | 2 | 0.125 | >6 | 0.25 | 800 | 0.03 |
| 47 | 3 | 20 | 0.125 | 6 | 0.25 | ∼800 | 3 |
Abbreviations: Pen G, penicillin G; Oxac, oxacillin; Mezl, mezlocillin; Cefu, cefuroxime; Ceft, ceftriaxone; Aztr, aztreonam; Amox, amoxicillin. The IC50 of each antibiotic for the binding of 40 μg of Bio-Amp per ml to each major PBP was determined by using either 1, 10, or 100 times the MIC of each antibiotic. The values shown are estimates based on densitometric tracing data.
Effects of sub-MICs on H. pylori morphology.
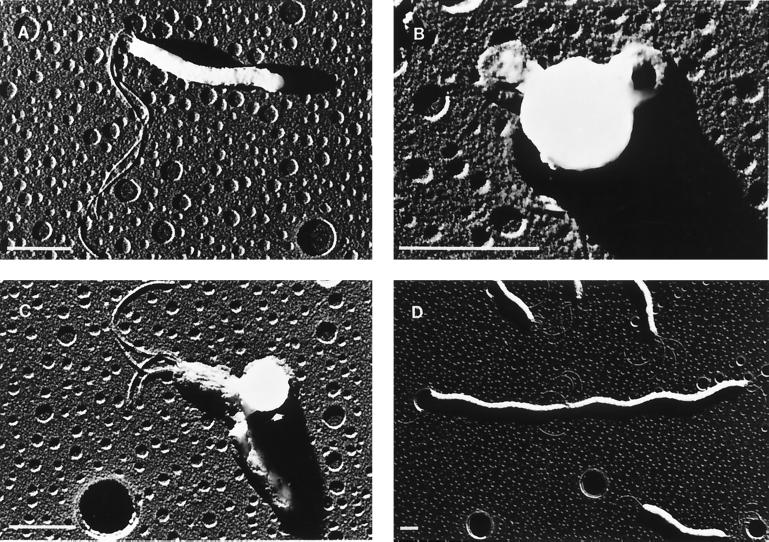
Having demonstrated that each of the eight β-lactam antibiotics was active against this H. pylori strain and bound, with differential preferences, to the major PBPs, we next examined the effect of each of these antibiotics on bacterial morphology. Table 1 summarizes the results of these experiments, and representative transmission electron micrographs are shown in Fig. 4. Each of these antibiotics, when used at one-half to one-fourth of the MIC, induced the formation of spherical cells (large cells with few cytoplasmic elements) and membrane blebbing. The addition of aztreonam induced similar morphologies but also led to pronounced filamentation, with many filaments 5 cells or greater in length and some spanning the length of the focal field (Fig. 4D). Interestingly, none of the penicillins or cephalosporins used in this study caused significant filamentation (>3 cells in length) at sub-MICs.
FIG. 4.
Transmission electron micrographs illustrating representative examples of log-phase H. pylori control bacteria (A), spherical cell formation typical of that seen after treatment with various β-lactam antibiotics (B), membrane blebbing (indicated by the arrowhead) seen after treatment with various β-lactam antibiotics (C), and significant filamentation, which was only observed in cultures exposed to sub-MICs of aztreonam (D). The bar in each graph represents 1 μm. Note that the spherical imperfections in the background are artifacts due to the formation of holes in the Formvar-coated support film.
Comparison of PBP profiles of helical and coccoid forms of H. pylori.
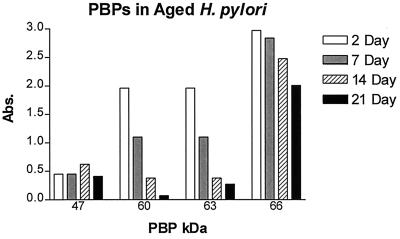
Continued incubation of H. pylori in liquid culture led to conversion of the helical form of H. pylori to the coccoid form within 3 to 5 days of incubation at 37°C, and after 7 to 14 days, >99% of the bacteria were observed to be coccoid, whereas by day 21, only coccoid forms were seen. Comparative binding studies using Bio-Amp and membranes obtained from bacteria grown for 2, 7, 14, and 21 days are shown in Fig. 5. The intensity of Bio-Amp labeling for both PBP60 and PBP63 was markedly reduced (by 44% on day 7 and 81% on day 14), and labeling all but disappeared by day 21 (PBP60 labeling was reduced by 96%, and PBP63 labeling was reduced by 86%). On the other hand, Bio-Amp labeling of PBP47 did not vary significantly from day 2 to day 21. PBP66, the PBP most abundantly labeled by Bio-Amp in helical H. pylori, was retained over the course of this 21-day study, with only modest reductions in intensity on days 7 and 14 and only a 32% decrease by day 21. Although the results are not shown here, control studies using β-lactamase pretreatment of Bio-Amp were performed to confirm that each of the proteins labeled with Bio-Amp in these aged cultures was a PBP.
FIG. 5.
Membrane fractions of H. pylori liquid cultures harvested after 2, 7, 14, or 21 days of incubation at 37°C were labeled with Bio-Amp, separated by SDS-PAGE, and visualized on Western blots. These blots were then quantitatively examined by laser densitometry, and the results of a representative experiment are shown. Abs. absorbance.
DISCUSSION
Previous studies by Dargis and Malouin (14) demonstrated that labeling of PBPs with Bio-Amp was comparable to that in studies using radiolabeled penicillin. Using this approach, we identified four major PBPs in helical H. pylori ATCC 43579—PBP47, PBP60, PBP63, and PBP66. We also noted the presence of an additional Bio-Amp-labeled protein at a molecular mass of 95 to 100 kDa which we consider to be a minor PBP. Some additional protein bands which were occasionally labeled with Bio-Amp proved to be due to nonspecific binding since these proteins were labeled even after the Bio-Amp had been treated with β-lactamase to destroy the normal PBP-binding site of ampicillin. Similar nonspecific labeling of proteins was also noted by Dargis and Malouin (14), who used several different bacterial species, as well as by Galleni et al. (19) who used Escherichia coli. To the best of our knowledge, Ikeda et al. (25) are the only other investigators who have examined PBPs in H. pylori; they used 14C-labeled penicillin G and identified three PBPs in H. pylori FP1532, although they did not report the molecular weights of these proteins.
Examination of the H. pylori 26695 genome sequence reported by Tomb et al. (37) revealed the presence of three genes with protein sequence homologies to E. coli PBPs: HP 597, which has 33.7% identity to PBP1A and a molecular mass of 74.23 kDa; HP 1556, which has 30.6% identity to FtsI (also known as PBP3) and a molecular mass of 69.07 kDa; and HP 1565, which has 35% identity to PBP2 and a molecular mass of 67.02 kDa. Based on this information, we predict that PBP66, PBP63, and PBP60 correspond to these three known gene sequences whereas the H. pylori gene corresponding to PBP47 remains to be identified. PBP47 may represent a “nontraditional” PBP with a sequence which is not closely homologous to the other three PBPs described above.
In order to further characterize the PBPs in H. pylori, we examined the relative binding affinities of each of the β-lactam antibiotics for the four major H. pylori PBPs and the subsequent effects of these antibiotics at sub-MICs on bacterial morphology. This approach mimics that of other investigators who characterized the various PBPs in E. coli and identified their roles in peptidoglycan formation. These investigators examined the varied effects of β-lactam antibiotics on cellular division, elongation, and shape in E. coli by comparing the binding affinities of different β-lactams for the known E. coli PBPs. These studies revealed that PBP1 of E. coli is involved in bacterial elongation, PBP2 is responsible for the rod shape, and PBP3 is involved in septum formation (12, 13, 36). These proteins have been called the “essential” PBPs of E. coli, while those with lower molecular weights appear to be dispensable (12, 13, 21, 36). The inhibition of these PBPs results in the various morphologies seen at the MICs of the respective β-lactams—e.g., inhibition of PBP2 results in round E. coli cells and inhibition of PBP3 results in filamentation (13, 36).
Kamimura et al. (27) noted that low concentrations of cefixime caused filamentation in E. coli by binding to PBP3 and bacteriolysis at high concentrations by binding to PBP1A and PBP1B. In contrast, Ikeda et al. (25) found that the same treatment of H. pylori caused rounded cells at low concentrations, with cefixime bound primarily to the protein they refer to as PBP B (molecular weight unreported). Armstrong et al. (2) treated Campylobacter pyloridis (since renamed H. pylori) with amoxicillin, benzylpenicillin, and cephalexin at concentrations above the determined MICs and found the normal bacilliform morphology to be replaced by bulging and dumbell-like profiles with cell wall blebbing and vesiculation and eventually by swollen forms with incomplete cell walls undergoing lysis. Berry et al. (4) noted that amoxicillin at 10 times the MIC was bactericidal for H. pylori but also induced the formation of coccoid forms.
In the present study, we compared relative binding intensities of various β-lactam antibiotics for the four major H. pylori PBPs with their effects on bacterial morphology. Each of the antibiotics was found to be bacteriocidal for H. pylori, although the concentrations required to achieve this effect differed significantly—these differences may be accounted for in part by the relative abilities of the antibiotics to diffuse across the outer membrane of H. pylori. This would explain the major difference in the MICs and MBCs of oxacillin and mezlocillin, while their relative abilities to bind each of the major PBPs were almost identical (note that the labeling studies were done with membrane fractions and not intact bacteria).
From these studies, we conclude that PBP66, PBP63, and PBP60 are the major PBPs bound by the β-lactams used in this study and that the interaction of these PBPs with sub-MICs of these antibiotics was responsible for producing the observed morphological changes in H. pylori. PBP63 was the only PBP to be significantly bound by each of the β-lactams studied (≥75% binding) and therefore may be an essential PBP for helical H. pylori. It is of note that aztreonam was the only β-lactam to preferentially bind to a single PBP, PBP63, and this antibiotic was the only one in the study which induced pronounced filamentation in H. pylori at sub-MICs. Satta et al. (32) found aztreonam to also saturate a single PBP, PBP3 (60 kDa) in E. coli, which has been determined to be involved in septum formation (12, 13, 36). Therefore, by analogy, it is possible that PBP63’s main function in H. pylori is in septum formation.
The four penicillins examined here showed rather similar binding patterns. The results obtained with mezlocillin are particularly impressive, with an IC50 of only 0.125 μg/ml for each PBP. The IC50s of amoxicillin and penicillin G were similar, except that amoxicillin bound more competitively to PBP66 than did penicillin G. With the exception of amoxicillin, the IC50s of all of the β-lactams were the lowest for PBP63, indicating that it is the preferred target of these antibiotics. As expected, unlabeled ampicillin competed very effectively with Bio-Amp for binding to each of the four major PBPs (data not shown).
With the exception of aztreonam, the other seven β-lactams bound significantly to more than one PBP and induced sphere formation and blebbing without significant filamentation at sub-MICs. While aztreonam also induced some sphere formation and blebbing at sub-MICs, filamentation was the dominant altered morphology observed. Thus, with most of the β-lactams, binding to one or more of the PBPs leads to inhibition of one or more of the enzymes involved in peptidoglycan biosynthesis, resulting in destabilization of cell structure and membrane disruption, as seen with membrane blebbing and sphere formation. In the case of aztreonam, the dominant enzymatic activity being inhibited is most likely that of PBP63, resulting in filamentation, while the other peptidoglycan-building enzymes are only somewhat inhibited, which resulted in some blebbing and sphere formation. PBP47 may not be as essential to the growth of H. pylori as PBP60, PBP63, and PBP66, since this protein was significantly bound by only two of the antibiotics studied, while the higher-molecular-weight PBPs were significantly bound by at least four of the β-lactam antibiotics. We also found an additional minor PBP of 95 to 100 kDa, which was somewhat affected by competitive binding with one or more of the β-lactam antibiotics (data not shown). The significance of this putative minor PBP for the growth and morphology of helical H. pylori is not known.
Aging of liquid cultures of H. pylori resulted in conversion of the helical form to the coccoid form. Examination of the Bio-Amp labeling of PBPs in these aged cultures demonstrated major reductions in PBP60 and PBP63, whereas PBP47 was only slightly affected. PBP66 seemed to be the only major PBP to be significantly retained, with only a 32% reduction in Bio-Amp staining intensity by day 21. A prominent band appeared in older cultures with a molecular mass of 28 kDa (data not shown); however, this band was not reduced in intensity with β-lactamase treatment of Bio-Amp and was therefore determined to be either the result of nonspecific binding or possibly a degradative product of one of the PBPs. We cannot distinguish between the possibilities that the decrease in the PBPs of H. pylori that accompanies aging is due to a decrease in the expression of these proteins or to degradation of the proteins. If the coccoid form is simply a degenerative form of the bacterium, then protein degradation would certainly be expected; however, the retention of PBP47 and PBP66 argues against this being the only explanation for these findings. Alternatively, since the coccoid form appears to be dormant, enzymes needed for septum formation and elongation are no longer needed. Conserving energy by no longer synthesizing unneeded enzymes would be a prudent strategy.
β-Lactam antibiotics, particularly amoxicillin, play a major role in the treatment of H. pylori infections. However, several investigators (23, 34) have found that continued exposure to amoxicillin can increase the MIC of amoxicillin, and Dore et al. recently (15) reported that one reason for the failure of amoxicillin-omeprazole treatment of H. pylori infection is the presence of amoxicillin-resistant strains. These studies point to the importance of continued surveillance for the presence and emergence of amoxicillin-resistant H. pylori strains. In addition, it is possible that relapses or recurrences of H. pylori infection arise because of the presence of dormant forms of the bacterium (coccoids) which are unlikely to be sensitive to β-lactam antibiotics because these coccoids are not actively dividing and have different PBP profiles than the helical, dividing forms. Additional studies focusing on the interaction of β-lactam antibiotics with the PBPs of H. pylori (both helical and coccoid forms) will provide more information important for guiding therapeutic interventions to prevent recurrent H. pylori infections.
ACKNOWLEDGMENTS
We thank Sacred Heart Medical Center of Spokane, Wash., for supplying the antibiotics at cost and F. Malouin of Microcide Pharmaceuticals for help with the biotinylation studies. We also greatly appreciate the assistance of J. Kitasako and P. Desjardins of the Plant Pathology Department at the University of California, Riverside, in preparing the transmission electron micrographs and the photographic assistance of R. Hatch.
ADDENDUM IN PROOF
After the manuscript was submitted for publication, Krishnamurthy et al. (J. Bacteriol. 181:5107–5110, 1999) reported the presence of four PBPs in H. pylori, including a novel PBP with significantly increased expression during mid- to late-log-phase growth, and Dore et al. (Helicobacter 4:154–161, 1999) reported the presence of four PBPs in amoxicillin-sensitive H. pylori, but only three of these PBPs were found in amoxicillin-resistant strains, suggesting a role for the small PBP in the amoxicillin-resistant phenotype of H. pylori.
REFERENCES
- 1.Andersen A P, Elliott D A, Lawson M, Barland P, Hatcher V B, Puszkin E G. Growth and morphological transformations of Helicobacter pylori in broth media. J Clin Microbiol. 1997;35:2918–2922. doi: 10.1128/jcm.35.11.2918-2922.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong J A, Wee S H, Goodwin C S, Wilson D H. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro—an ultrastructural study. J Med Microbiol. 1987;24:343–350. doi: 10.1099/00222615-24-4-343. [DOI] [PubMed] [Google Scholar]
- 3.Benaïssa M, Babin P, Quellard N, Pezennec L, Cenatiempo Y, Fauchère J L. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect Immun. 1996;64:2331–2335. doi: 10.1128/iai.64.6.2331-2335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:1859–1861. doi: 10.1128/aac.39.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg P M, Strominger J L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974;38:291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenciaglia M I, Fornara A M, Scaltrito M M, Braga P C, Dubini F. Activity of amoxicillin, metronidazole, bismuth salicylate and six aminoglycosides against Helicobacter pylori. J Chemother. 1996;8:52–54. doi: 10.1179/joc.1996.8.1.52. [DOI] [PubMed] [Google Scholar]
- 7.Cellini L, Allocati N, Angelucci D, Iezzi T, Di Campli E, Marzio L, Dainelli B. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38:843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 8.Cellini L, Allocati N, Di Campli E, Dainelli B. Helicobacter pylori: a fickle germ. Microbiol Immunol. 1994;38:25–30. doi: 10.1111/j.1348-0421.1994.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 9.Chan W-Y, Hui P-K, Leung K-M, Chow J, Kwok F, Ng C-S. Coccoid forms of Helicobacter pylori in the human stomach. Am J Clin Pathol. 1994;102:503–507. doi: 10.1093/ajcp/102.4.503. [DOI] [PubMed] [Google Scholar]
- 10.Cole S P, Cirillo D, Kagnoff M F, Guiney D G, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997;65:843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover T L, Blaser M J. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996;41:85–117. [PubMed] [Google Scholar]
- 12.Curtis N A C, Orr D, Ross G W, Boulton M G. Competition of β-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979;16:325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis N A C, Orr D, Ross G W, Boulton M G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979;16:533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dargis M, Malouin F. Use of biotinylated β-lactams and chemiluminescence for study and purification of penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1994;38:973–980. doi: 10.1128/aac.38.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dore M P, Piana A, Carta M, Atzei A, Are B M, Mura I, Massarelli G, Maida A, Sepulveda A R, Graham D Y, Realdi G. Amoxycillin resistance is one reason for failure of amoxycillin-omeprazole treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:635–639. doi: 10.1046/j.1365-2036.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty T J, Kennedy K, Kessler R E, Pucci M J. Direct quantitation of the number of individual penicillin-binding proteins per cell in Escherichia coli. J Bacteriol. 1996;178:6110–6115. doi: 10.1128/jb.178.21.6110-6115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton K A, Catrenich C E, Makin K M, Krakowa S. Virulence of coccoid and bacillary forms of Helicobacter pylori in gnotobiotic piglets. J Infect Dis. 1995;171:459–462. doi: 10.1093/infdis/171.2.459. [DOI] [PubMed] [Google Scholar]
- 19.Galleni M, Lakaye B, Lepage S, Jamin M, Thamm L, Joris B, Frere J-M. A new, highly sensitive method for the detection and quantitation of penicillin binding proteins. Biochem J. 1993;291:19–21. doi: 10.1042/bj2910019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Rodríguez J A, García Sánchez J E, García García M I, García Sánchez E, Muñoz Bellido J L. In vitro activities of new oral β-lactams and macrolides against Campylobacter pylori. Antimicrob Agents Chemother. 1989;33:1650–1651. doi: 10.1128/aac.33.9.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgopapadakou N H. Penicillin-binding proteins and bacterial resistance to β-lactams. Antimicrob Agents Chemother. 1993;37:2045–2053. doi: 10.1128/aac.37.10.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibaldi M. Helicobacter pylori and gastrointestinal disease. J Clin Pharmacol. 1995;35:647–654. doi: 10.1002/j.1552-4604.1995.tb04103.x. [DOI] [PubMed] [Google Scholar]
- 23.Haas C E, Nix D E, Schentag J J. In vitro selection of resistant Helicobacter pylori. Antimicrob Agents Chemother. 1990;34:1637–1641. doi: 10.1128/aac.34.9.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua J, Ho B. Is the coccoid form of Helicobacter pylori viable? Microbios. 1996;87:103–112. [PubMed] [Google Scholar]
- 25.Ikeda F, Yokota Y, Mine Y, Tatsuta M. Activity of cefixime against Helicobacter pylori and affinities for the penicillin-binding proteins. Antimicrob Agents Chemother. 1990;34:2426–2428. doi: 10.1128/aac.34.12.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janas B, Czkwianianc E, Bak-Romaniszyn L, Bartel H, Tosik D, Planeta-Malecka I. Electron microscopic study of association of coccoid forms of Helicobacter pylori and gastric epithelial cells. Am J Gastroenterol. 1995;90:1829–1833. [PubMed] [Google Scholar]
- 27.Kamimura T, Kojo H, Matsumoto Y, Mine Y, Goto S, Kuwahara K. In vitro and in vivo antibacterial properties of FK027, a new orally active cephem antibiotic. Antimicrob Agents Chemother. 1984;25:98–104. doi: 10.1128/aac.25.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusters J G, Gerrits M M, Van Strijp J A G, Vandenbroucke-Grauls C M J E. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer J M, Ryu S, Pendland S L, Danziger L H. In vitro synergy testing of clarithromycin and 14-hydroxyclarithromycin with amoxicillin or bismuth subsalicylate against Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:1607–1608. doi: 10.1128/aac.41.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizoguchi H, Fujioka T, Kishi K, Nishizono A, Kodama R, Nasu M. Diversity in protein synthesis and viability of Helicobacter pylori coccoid forms in response to various stimuli. Infect Immun. 1998;66:5555–5560. doi: 10.1128/iai.66.11.5555-5560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilius M, Strohle A, Bode G, Malfertheiner P. Coccoid like forms (CLF) of Helicobacter pylori. Enzyme activity and antigenicity. Zentbl Bakteriol. 1993;280:259–272. doi: 10.1016/s0934-8840(11)80964-3. [DOI] [PubMed] [Google Scholar]
- 32.Satta G, Cornaglia G, Mazzariol A, Golini G, Valisena S, Fontana R. Target for bacteriostatic and bactericidal activities of β-lactam antibiotics against Escherichia coli resides in different penicillin-binding proteins. Antimicrob Agents Chemother. 1995;39:812–818. doi: 10.1128/aac.39.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahamat M, Mai U, Paszko-Kolva C, Kessel M, Colwell R R. Use of autoradiography to assess viability of Helicobacter pylori in water. Appl Environ Microbiol. 1993;59:1231–1235. doi: 10.1128/aem.59.4.1231-1235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorberg M, Hanberger H, Nilsson M, Bjorkman A, Nilsson L E. Risk of development of in vitro resistance to amoxicillin, clarithromycin, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:1222–1228. doi: 10.1128/aac.42.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorberg M, Nilsson M, Hanberger H, Nilsson L E. Morphologic conversion of Helicobacter pylori from bacillary to coccoid form. Eur J Clin Microbiol Infect Dis. 1996;15:216–219. doi: 10.1007/BF01591357. [DOI] [PubMed] [Google Scholar]
- 36.Spratt B G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 38.Van Zwet A A, Thijs J C, Schievink-DeVries W, Schiphuis J, Snijder J A. In vitro studies on stability and development of metronidazole resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1994;38:360–362. doi: 10.1128/aac.38.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijayakumari S, Khin M M, Jiang B, Ho B. The pathogenic role of the coccoid form of Helicobacter pylori. Cytobios. 1995;82:251–260. [PubMed] [Google Scholar]
- 40.Wang X, Sturegard E, Rupar R, Nilsson H-O, Aleljung P A, Carlen B, Willen R, Wadstrom T. Infection of Balb/c A mice by spiral and coccoid forms of Helicobacter pylori. J Med Microbiol. 1997;46:657–663. doi: 10.1099/00222615-46-8-657. [DOI] [PubMed] [Google Scholar]