Abstract
Neutrophils accumulate ciprofloxacin and other fluoroquinolones, a process that enhances the killing of intracellular pathogens and could facilitate the delivery of these agents to infection sites by migrating neutrophils. The mechanisms by which transport occurs have not been characterized. In the present study, quiescent neutrophils transported ciprofloxacin with an observed Km of 167 μg/ml (501 μM) and a maximum velocity of 25.2 ng/min/106 cells. When neutrophils were stimulated with phorbol myristate acetate (PMA), a second component of ciprofloxacin transport was induced. This pathway had an apparent Km of 9.76 μg/ml (29.3 μM) and a maximum velocity of 59.3 ng/min/106 cells. Transport by both pathways was Na+ independent. Ciprofloxacin transport by quiescent cells was relatively insensitive to pH and N-ethylmaleimide but was competitively inhibited by adenine (Ki = 1.55 mM). Papaverine, a benzylisoquinoline known to inhibit nucleobase transport, also inhibited ciprofloxacin transport by quiescent cells. In contrast, transport by PMA-stimulated cells was enhanced at pH 8.2, inhibited at pH 6.2, and blocked by N-ethylmaleimide. Cationic and neutral amino acids and cystine competitively inhibited ciprofloxacin transport by PMA-stimulated neutrophils (Ki = 158 μM for ornithine) but had little effect on quiescent cells. PMA-activated transport was not inhibited when the Na+ in the medium was replaced with K+ or Li+, and the pattern of inhibition by cationic and neutral amino acids was similar. In summary, neutrophils continuously transport ciprofloxacin via a transport pathway shared by adenine. Activation by PMA induces a separate, higher-affinity transport pathway shared by a broad scope of amino acids. Neutrophils utilize one or both of these mechanisms to transport other fluoroquinolones.
Fluoroquinolones are a class of antimicrobial agents that inhibit bacterial DNA topoisomerase II and produce bactericidal effects against a broad spectrum of bacteria. They are highly active against most aerobic and facultative gram-negative bacteria and exhibit good activity against gram-positive bacteria (18). In contrast to most widely used antimicrobial agents (e.g., cephalosporins and β-lactam antibiotics), ciprofloxacin and other fluoroquinolones can accumulate inside phagocytes and help eradicate bacteria that resist phagocytic killing. Neutrophils and macrophages take up ciprofloxacin so efficiently that steady-state intracellular levels of the agent can exceed plasma levels by severalfold (6, 7, 10, 17). When loaded with ciprofloxacin, neutrophils exhibit enhanced intracellular killing of bacteria relative to control cells that contain no antimicrobial agent (6, 20). If neutrophils could carry fluoroquinolones with them as they migrate to an infection site, they could potentially enhance the local concentration of these agents at these locations. Since neutrophils rapidly infiltrate these sites in large numbers, this could result in enhanced resolution of fluoroquinolone-susceptible infections.
Until recently, little was known about the mechanisms by which neutrophils take up fluoroquinolones. Previous studies had shown that uptake is dependent on cell viability (6), but there has been disagreement as to whether fluoroquinolone transport is an active process (16, 17). Our recent work demonstrated that ciprofloxacin uptake by human neutrophils is energy dependent, obeys Michaelis-Menten kinetics, and is strongly up-regulated when neutrophils are activated by phorbol esters (14). Similar to the purines, ciprofloxacin is a multiringed heterocyclic compound. Although ciprofloxacin’s structure is bulkier than that of common amino acids, it is an amphoteric compound with a pKa1 of 6.0 (carboxylic acid) and a pKa2 of 8.8 (amino). Since fluoroquinolones possess structural features that could potentially interact with systems that transport nucleobases or amino acids, we hypothesized that these systems play a role in fluoroquinolone uptake by neutrophils. In this report, we provide evidence to support this hypothesis.
MATERIALS AND METHODS
Neutrophil isolation.
Human neutrophils were isolated from citrated whole blood obtained from healthy volunteers, using Ficoll-Hypaque density gradient centrifugation and dextran sedimentation (2). Residual erythrocytes were eliminated by hypotonic lysis. The remaining neutrophils were washed three times with phosphate-buffered saline. For the assays described below, neutrophils (typically >98% pure and >98% viable) were resuspended in modified Hanks’ balanced salt solution (HBSS; 1.9 mM KH2PO4, 1.1 mM Na2HPO4, 5 mM KCl, 147 mM NaCl, 5.5 mM glucose, 1 mM MgCl2, 1 mM CaCl2 [pH 7.3]). The Bradford method (3) was used to assay cell protein.
Fluoroquinolone transport.
Transport of ciprofloxacin and other fluoroquinolones was assayed by measuring cell-associated fluorescence as previously described (14). Neutrophils were resuspended in HBSS at 5 × 106 cells/ml and warmed to 37°C prior to incubation with a fluoroquinolone (5 to 200 μg/ml). In some experiments, cells were activated with 100 nM phorbol myristate acetate (PMA) immediately prior to exposure to fluoroquinolones. The assay was terminated before the end of the linear phase of transport (typically after 2 min for quiescent cells and after 5 min for PMA-activated cells). Aliquots of cell suspension were rapidly withdrawn, layered over 0.3 ml of canola oil-dibutyl phthalate (3:10), and centrifuged for 45 s at 15,000 × g in a microcentrifuge. The aqueous and oil layers were removed, and the cell pellet was recovered by cutting off the end of the microcentrifuge tube. The pellet was dispersed and lysed in 1.5 ml of 100 mM glycine-HCl (pH 3.0) by agitation at room temperature. The samples were centrifuged at 5,600 × g for 5 min, and the fluorescence of the supernatants was measured with a Perkin-Elmer LS-5B fluorescence spectrometer. For quantitation of ciproflixacin, excitation and emission wavelengths of 278 and 445 nm, respectively, were used. For norfloxacin, ofloxacin, and lomefloxacin, the excitation and emission wavelengths were 280 and 440 nm, 292 and 496 nm, and 330 and 452 nm, respectively. The sensitivity of detection of these fluoroquinolones was approximately 1 ng/ml, and their recovery from cell pellets was essentially quantitative.
Since the linear phase of initial uptake was relatively brief in quiescent cells, an inhibitor stop assay was used in kinetic analysis experiments (5). Quiescent cells were resuspended in HBSS at 2 × 107/ml and warmed to 37°C, and 0.125-ml aliquots of cell suspension were incubated with the appropriate fluoroquinolone. After 60 s, uptake was terminated by the rapid introduction of 0.875 ml of ice-cold 19 mM papavarine. This mixture was underlaid with 0.3 ml of canola oil-dibutyl phthalate (3:10) and centrifuged for 45 s at 15,000 × g in a microcentrifuge. The time between assay termination and centrifugation did not exceed 10 s. The cell pellet was recovered and processed as previously described. Lineweaver-Burk and Eadie-Hofstee analyses were used to determine the Km and Vmax of ciprofloxacin transport. Kinetic analysis of the inducible (PMA-activated) component of ciprofloxacin uptake was corrected by running parallel experiments with quiescent cells and subtracting quiescent ciprofloxacin uptake from the total uptake observed during the period of assay. Appropriate control experiments were performed to ensure that none of the inhibitors of fluoroquinolone transport interfered with the fluorescence measurements.
Ornithine and adenine transport.
Transport of ornithine and adenine was assayed my measuring cell-associated radioactivity. Experimental conditions were identical to those used for fluoroquinolone transport assays, except that l-[1-14C]ornithine (56 mCi/mmol) or [8-3H]adenine (24 Ci/mmol) (both from Amersham Pharmacia Biotech) was used as the substrate. Radioactivity was quantitated by liquid scintillation counting.
RESULTS
To begin the process of characterizing mechanisms of fluoroquinolone transport by quiescent and PMA-activated neutrophils, we compared the kinetics of ciprofloxacin transport with those of three other, structurally distinct fluoroquinolones. As shown in Table 1, quiescent neutrophils transported ciprofloxacin, norfloxacin, and ofloxacin with a relatively low affinity (apparent Km = 167 μg/ml, or 501 μM, for ciprofloxacin), and they transported lomefloxacin with an even lower affinity (Km = 988 μg/ml, or 2.47 mM). The maximum velocities of ciprofloxacin and norfloxacin transport were significantly lower than those observed for ofloxacin and lomefloxacin (P < 0.05, Tukey test). Neutrophil activation with PMA enhanced the uptake of ciprofloxacin, norfloxacin, and lomefloxacin but had no apparent effect on ofloxacin transport. When the PMA-induced component of the transport of ciprofloxacin, norfloxacin, and lomefloxacin was resolved from the activity of the low-affinity transport system, it was found to have a Km of approximately 9 to 15 μg/ml (28 to 40μM). This system transported ciprofloxacin at more than twice the maximum velocity observed in quiescent cells. However, the maximum velocity of norfloxacin transport by this mechanism was significantly lower than that for ciprofloxacin (P < 0.05, Tukey test), and the velocity of lomefloxacin transport was significantly lower than that for norfloxacin (P < 0.05, Tukey test).
TABLE 1.
Kinetics of fluoroquinolone transport by quiescent and PMA-activated neutrophilsa
| Fluoroquinolone | Pharmacokinetic parameters for:
|
|||
|---|---|---|---|---|
| Quiescent cells
|
Activated cells
|
|||
| Km (μg/ml) | Vmax (ng/min/106) | Km (μg/ml) | Vmax (ng/min/106) | |
| Ciprofloxacin | 167* (12.2) | 25.2+ (2.64) | 9.76*+ (0.82) | 59.3 (6.39) |
| Norfloxacin | 227* (18.3) | 35.2+ (2.82) | 8.96* (1.43) | 39.7 (4.05) |
| Ofloxacin | 340 (18.6) | 208* (27.1) | NAb | 0 |
| Lomefloxacin | 951 (22.0) | 257* (10.7) | 15.5+ (2.45) | 4.15 (1.03) |
Results are expressed as the means of five determinations, with standard errors of the means listed in parentheses. Within each column, the differences in the mean values are greater than would be expected by chance (P < 0.05, ANOVA). Pairs of identical superscripts within a column denote pairwise comparisons in which the means were not significantly different (P > 0.05, Tukey test). Kinetic analysis of the inducible (PMA-activated) component of ciprofloxacin transport was corrected by subtracting the ciprofloxacin uptake that occurred in quiescent neutrophils from the total uptake observed during the period of assay. The kinetics of ofloxacin transport were similar in quiescent and PMA-activated cells.
NA, not applicable.
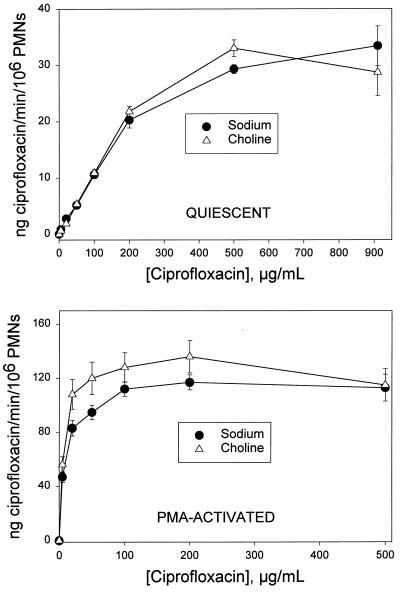
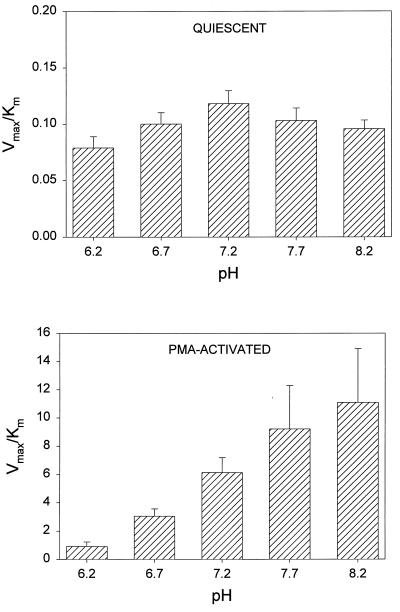
An Na+-free modification of HBSS, prepared by substituting K2HPO4 for Na2HPO4 and choline chloride for NaCl, was used to assess the Na+ dependence of ciprofloxacin transport. Transport velocity was measured in the presence and in the complete absence of Na+. Transport was saturable under both conditions (Fig. 1). Regardless of whether the cells were quiescent or activated by PMA, Na+ had no significant effect on the velocity of ciprofloxacin transport (P > 0.10, paired t test). To determine the effect of pH on ciprofloxacin transport, uptake kinetics were analyzed over the pH range of 6.2 to 8.2. In quiescent cells as well as PMA-activated cells, pH had a significant effect on the efficiency of transport (Vmax/Km ratio) (P ≤ 0.009, repeated-measures analysis of variance [ANOVA]). In quiescent cells, this influence was manifest as decreases in efficiency of 33% at pH 6.2 (P < 0.05, Tukey test) and 19% at pH 8.2 (Fig. 2, upper panel). There was no significant difference in the Vmax/Km ratios observed at pH 6.2 and 8.2 (P > 0.05, Tukey test). In PMA-activated neutrophils, the Vmax/Km ratio increased by more than 12-fold as the pH increased from 6.2 to 8.2 (Fig. 2, lower panel). The Vmax/Km ratios for ciprofloxacin transport at pH 7.7 and 8.2 were significantly higher than that at pH 6.2 (P = 0.042 and P = 0.012, respectively; Tukey test).
FIG. 1.
The Na+ dependence of ciprofloxacin transport by quiescent and PMA-activated neutrophils. Neutrophils were suspended in HBSS containing NaCl or an Na+-free balanced salts solution containing choline chloride. (Upper panel) Ciprofloxacin was added to the indicated final concentrations, and uptake was assayed at 37°C. (Lower panel) Cells were activated with 100 nM PMA just prior to addition of ciprofloxacin. These data portray the total transport activity, consisting of PMA-induced activity superimposed on the activity that occurs in quiescent cells. In both panels, data are presented as the mean transport activity (± standard error of the mean) for three experiments. Under all of the indicated conditions, Na+ had no significant effect on transport (P > 0.10, paired t test). PMNs, polymorphonuclear leukocytes.
FIG. 2.
The pH dependence of ciprofloxacin transport by quiescent and PMA-activated neutrophils. Cells were suspended in HBSS adjusted to the appropriate pH. (Top panel) The kinetics of ciprofloxacin transport by quiescent cells were analyzed at 37°C. (Bottom panel) Cells were activated with 100 nM PMA just prior to assay. Data are presented as the means ± standard errors of the means for three experiments. Transport was significantly influenced by pH in both quiescent and activated cells (P ≤ 0.009, repeated-measures ANOVA). In the top panel, there was no difference in the Vmax/Km ratios observed at pH 6.2 and 8.2 (P = 0.312, Tukey test). In the bottom panel, however, the Vmax/Km ratios were significantly higher at pH 7.7 and 8.2 than at pH 6.2 (P = 0.042 and P = 0.012, respectively; Tukey test).
Cytochalasin B, which blocks the formation of actin microfilaments and inhibits pinocytosis, had no effect on ciprofloxacin transport by quiescent or PMA-activated neutrophils (P > 0.05, paired t test; n = 5). The thiol agent N-ethylmaleimide had little effect on ciprofloxacin transport by quiescent neutrophils (P = 0.59, paired t test; n = 5) but produced almost complete (>96%) inhibition of the PMA-induced component of transport (P = 0.01). N-Ethylmaleimide had similar effects on the transport of norfloxacin and lomefloxacin.
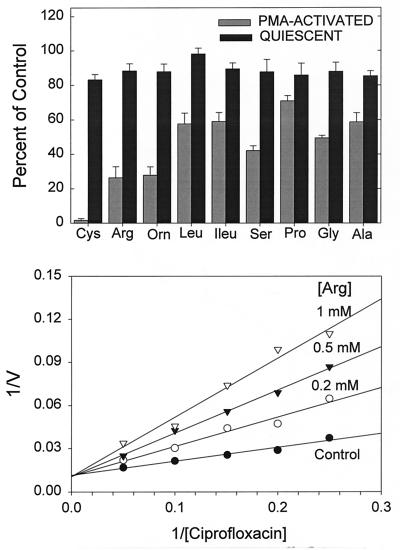
To further characterize the differences in ciprofloxacin transport in quiescent and activated neutrophils, we examined the pattern of inhibition by l-amino acids (Fig. 3, upper panel). Transport by quiescent cells was not significantly inhibited by 1 mM concentrations of several different neutral or cationic amino acids (P > 0.05, repeated-measures ANOVA). In PMA-activated cells, however, these same amino acids produced a significant effect on transport (P < 0.001, repeated-measures ANOVA). Each of the indicated amino acids produced significant inhibition compared to untreated controls (P < 0.05, Dunnett’s test). Cystine, arginine, and ornithine were among the most effective inhibitors, and serine was also relatively effective. Glycine, alanine, leucine, isoleucine, and proline, which possess nonpolar side chains, were less inhibitory. The mechanism of inhibition by arginine was competitive (Fig. 3, lower panel). Other amino acids utilized the same mechanism (data not shown). Arginine, lysine, and ornithine inhibited ciprofloxacin transport with apparent Ki values of 175, 164, and 158 μM, respectively, while serine and leucine inhibited uptake with Ki values of 247 and 460 μM, respectively. In parallel experiments, ciprofloxacin was found to competitively inhibit [14C]ornithine transport by quiescent neutrophils (Ki = 0.85 ± 0.14 mg/ml [mean ± standard deviation]; n = 4).
FIG. 3.
Inhibition of ciprofloxacin transport by l-amino acids. (Upper panel) Quiescent and PMA-activated neutrophils were incubated with ciprofloxacin at 5 μg/ml and the indicated amino acids at 1 mM, and transport was monitored for 5 min. Results are expressed as the means ± standard errors of the means for three experiments. In PMA-activated cells, the amino acids produced a significant treatment effect (P < 0.001, repeated-measures ANOVA), and each amino acid produced significant inhibition relative to controls (P < 0.05, Dunnett’s test). In quiescent cells, the treatment effect was not statistically significant (P = 0.17, repeated-measures ANOVA). (Lower panel) Competitive inhibition of ciprofloxacin uptake by arginine in PMA-activated neutrophils. The figure was derived from one of four replicate experiments. The observed Ki for arginine was 175 ± 25.4 μM. V, velocity.
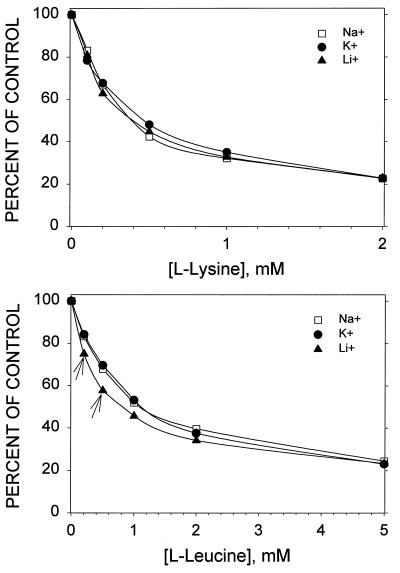
Some of the systems that transport cationic and neutral amino acids are modulated by inorganic monovalent cations in the extracellular medium (4). In our experiments, however, the efficiency (Vmax/Km) of ciprofloxacin transport by PMA-activated neutrophils was not altered significantly when the Na+ in the medium was replaced with K+ or Li+ (P > 0.05, Tukey test). Furthermore, there was little evidence that inorganic cations altered the competition between ciprofloxacin and amino acids (Fig. 4). Inhibition of ciprofloxacin transport by lysine was not significantly different in the presence of Na+, K+, or Li+ (P > 0.05, Tukey test). At concentrations below 1 mM, leucine produced a significantly greater degree of inhibition in medium containing LiCl (P < 0.05), but inorganic cations had no significant influence on inhibition by higher concentrations of leucine (P > 0.05). This suggests that PMA-activated neutrophils take up ciprofloxacin via a system possessing Na+-independent broad-scope amino acid transport activity.
FIG. 4.
The influence of inorganic monovalent cations on inhibition of ciprofloxacin transport by lysine and leucine. Neutrophils were resuspended in extracellular medium (37°C, pH 7.2) containing Na+ (154 mM NaCl, 5 mM sodium phosphate), K+ (154 mM KCl, 5 mM potassium phosphate), or Li+ (154 mM LiCl, 5 mM potassium phosphate). Cells were activated with 100 nM PMA just prior to exposure to ciprofloxacin (5 μg/ml) and the indicated concentrations of lysine or leucine. Transport of ciprofloxacin was monitored for 5 min. Results are expressed as the means of data from three experiments. The standard error of these measurements averaged 4.1% of the mean and did not exceed 9%. The arrows indicate points that were significantly different from the other two in pairwise multiple comparisons (P < 0.05, Tukey test). The efficiency of ciprofloxacin transport, as assessed by Vmax/Km determinations, was not altered significantly when the Na+ in the medium was replaced with K+ or Li+ (P > 0.05, Tukey test).
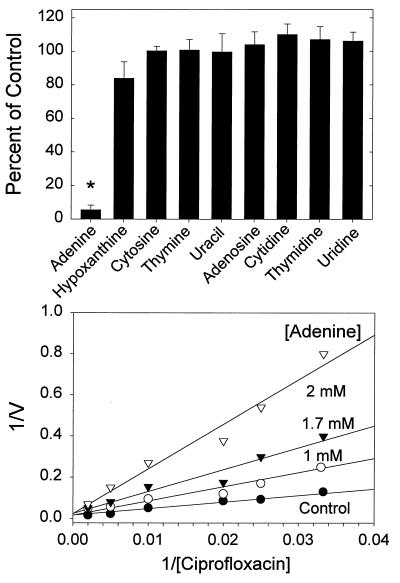
Since ciprofloxacin did not appear to share a major uptake pathway with amino acids in quiescent neutrophils, we examined the possibility that it is taken up by a nucleotide or nucleobase transport system (Fig. 5, upper panel). In quiescent cells, adenine significantly inhibited ciprofloxacin transport (P < 0.05, Tukey test). Hypoxanthine also inhibited ciprofloxacin transport, but its effects were not statistically significant (P > 0.05, Tukey test). Other nucleobases and nucleosides also failed to significantly inhibit ciprofloxacin transport (P > 0.05). Kinetic analysis demonstrated that the mechanism of inhibition by adenine was competitive (Ki = 1.55 ± 0.25 mM) (Fig. 5, lower panel). In parallel studies, ciprofloxacin acted as a competitive inhibitor of [3H]adenine transport by quiescent neutrophils (Ki = 2.47 ± 0.4 mg/ml; n = 4).
FIG. 5.
Inhibition of neutrophil ciprofloxacin transport by nucleobases and nucleosides. (Upper panel) Quiescent neutrophils were incubated with ciprofloxacin at 5 μg/ml and 5 mM concentrations of the indicated compounds, and transport was monitored over a 5-min period. Results are expressed as means ± standard errors of the means for five experiments. The asterisk denotes a condition under which ciprofloxacin transport was significantly different from that of untreated controls (P < 0.05, Dunnett’s test). (Lower panel) Competitive inhibition of ciprofloxacin transport in quiescent neutrophils by adenine. The figure was derived from one of five replicate experiments. The observed Ki for adenine was 1.55 ± 0.25 mM.
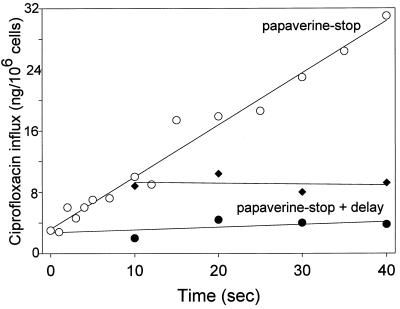
Cell pretreatment with papaverine (16.6 mM), a benzylisoquinoline known to inhibit nucleobase transport, completely blocked the transport of ciprofloxacin by neutrophils (Fig. 6). When papaverine was introduced immediately after the initiation of ciprofloxacin transport, it instantaneously blocked this process. Kinetic studies revealed that papaverine acted as a competitive inhibitor (Ki = 2.2 ± 0.17 mM; n = 4). Dipyridamole (1 μM) and [6-(4-nitrobenzyl)thio]-9-β-d-ribofuranosylpurine (1 μM), which both inhibit nucleoside transport, both produced minimal (10%) inhibition of ciprofloxacin transport (P = 0.191, repeated-measures ANOVA).
FIG. 6.
Inhibition of neutrophil ciprofloxacin transport by papaverine. Quiescent neutrophil suspensions were incubated with ciprofloxacin at 300 μg/ml for the indicated intervals (○). Transport was terminated by the addition of ice-cold papaverine to a final concentration of 16.6 mM, and the cells were pelleted through an oil cushion. In the papaverine plus delay experiments, cold papaverine was added just prior to addition of ciprofloxacin (●) or after 10 s of incubation of cells with ciprofloxacin (⧫). After the indicated delay periods, the cells were pelleted through oil. V, velocity.
DISCUSSION
Neutrophils possess at least two saturable transport systems that mediate the uptake of ciprofloxacin and other fluoroquinolones. Both systems are Na+ independent. One is a relatively low-affinity system that appears to operate continuously, while the other is a high-affinity system that is induced by the protein kinase C activator PMA. Previous work in our laboratory has shown that inhibitors of protein kinase C can block the activation of high-affinity ciprofloxacin accumulation (14). The neutrophil’s low-affinity system is less sensitive to extracellular pH, is not inhibited by N-ethylmaleimide, and is competitively inhibited by adenine. Neutrophils are known to possess a system for transporting adenine with micromolar affinity (12). These cells break down relatively large amounts of ATP in response to phagocytic stimuli (1). Circulating leukocytes have almost no capacity for de novo synthesis of adenine, the precursor of ATP (19, 21), so they must take it up from the extracellular environment. Similar to the purine nucleobase transport systems of human erythrocytes and HL-60 cells (5, 13), ciprofloxacin transport activity by quiescent neutrophils is profoundly inhibited by papaverine but is not significantly affected by pyrimidines, nucleosides, or agents that block nucleoside transport (e.g., dipyridamole). While some mammalian cells are capable of taking up nucleobases via their nucleoside transporters, this mechanism has an extremely low affinity for nucleobases and a low efficiency (11). Despite the relatively low affinity of this transporter for fluoroquinolones, it appears to play a major role in the intracellular accumulation of these compounds. When exposed to therapeutic levels (2 μg/ml) of ciprofloxacin, norfloxacin, lomefloxacin, or ofloxacin, quiescent neutrophils can attain intracellular fluoroquinolone levels that are five- to eightfold higher than the extracellular levels (6, 10, 17).
High-affinity fluoroquinolone transport by PMA-activated neutrophils is blocked by N-ethylmaleimide and is sensitive to pH. Fluoroquinolone transport by this pathway appears to be competitively inhibited by cationic amino acids, cystine, and neutral amino acids and is not inhibited when the Na+ in the medium is replaced with K+, Li+, or choline. These results suggest that ciprofloxacin uptake by PMA-activated neutrophils is mediated by a pH-sensitive, Na+-independent, broad-specificity amino acid transport system. The rBAT (related to b0,+ amino acid transport) family of transporters is one of the few that is capable of Na+-independent transport of cationic and neutral amino acids and cystine (4, 15). Broad-scope amino acid transport activity by this family is relatively insensitive to inorganic cations and is similar in isotonic NaCl, KCL, and LiCl (4, 15). Moreover, the observed Ki values for inhibition of ciprofloxacin uptake by amino acids reflect an affinity for amino acids that is similar to that reported for the rBAT family (4, 15).
The systems used by quiescent and activated neutrophils to transport fluoroquinolones differ in their preference for ciprofloxacin, norfloxacin, lomefloxacin, and ofloxacin. Ciprofloxacin and norfloxacin, which have slightly different N-1 substituents, are both transported with low affinity by quiescent neutrophils and with high affinity by PMA-activated cells. Lomefloxacin, which differs from norfloxacin by having a fluorine at position 8 and an N-substituted piperazine at position 7, is transported by quiescent neutrophils with a much lower affinity and a higher maximum velocity than norfloxacin. In PMA-activated cells, however, the maximum velocity of lomefloxacin transport is significantly lower than that for norfloxacin. Ofloxacin, which is similar to lomefloxacin but lacks a fluorine at position 8 and has a C3H6O group that is cyclic from N-1 and position 8, is transported with significantly higher affinity than lomefloxacin in quiescent neutrophils. However, ofloxacin doesn’t appear to interact with the high-affinity transporter in PMA-activated neutrophils. Thus, substituents at position 7 appear to influence the velocity of fluoroquinolone transport by quiescent cells, while fluorine or cyclic structures linked to position 8 appear to impair fluoroquinolone transport by PMA-activated cells.
Neutrophils are the first line of defense against bacterial infections. While they are capable of killing most of the bacteria they phagocytose, certain types of bacteria can evade their defensive measures (8). Intracellular accumulation of an appropriate microbicidal agent can help eradicate pathogens that resist phagocytic killing. Unfortunately, many otherwise effective antimicrobial agents (e.g., β-lactams) do not penetrate phagocytes as well as fluoroquinolones do. When loaded with ciprofloxacin, neutrophils exhibit enhanced intracellular killing of bacteria relative to control cells that contain no antimicrobial agent (6, 20). Neutrophils loaded with fluoroquinolones also have the potential to enhance the delivery of these agents to infection sites. Previous in vitro studies suggested that it is difficult for ciprofloxacin-loaded neutrophils to maintain elevated intracellular levels of the drug as they migrate through agar that does not contain ciprofloxacin (9). In humans taking systemic ciprofloxacin, however, neutrophils are constantly exposed to the agent during the process of chemotaxis. This could allow them to replenish ciprofloxacin lost by efflux. The results of the present study suggest that neutrophils could potentially accumulate fluoroquinolones more avidly as they approach an infection site, since they are known to undergo progressive activation by chemotactic agents and proinflammatory substances found at these locations. Because there is little information about the pathways by which cells take up antimicrobial agents, our findings provide a basis for a better understanding of the interaction between fluoroquinolones and neutrophils. If it were possible to up-regulate the accumulation of fluoroquinolones by neutrophils without adversely affecting functions that contribute to defense of the host, this could potentially enhance the effectiveness of antimicrobial chemotherapy. For this reason, further studies of the mechanisms that mediate fluoroquinolone flux across the neutrophil plasma membrane are warranted.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants DE00338, DE09851, and DE12601 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Investig. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyum A. Isolation of mononuclear cells and granulocytes from human peripheral blood. J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Deves R, Boyd C A R. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 5.Domin B A, Mahony W B, Zimmerman T P. Purine nucleobase transport in human erythrocytes. J Biol Chem. 1988;263:9276–9284. [PubMed] [Google Scholar]
- 6.Easmon C S F, Crane J P. Uptake of ciprofloxacin by human neutrophils. J Antimicrob Chemother. 1985;16:67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Easmon C S F, Crane J P. Uptake of ciprofloxacin by macrophages. J Clin Pathol. 1985;38:442–444. doi: 10.1136/jcp.38.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 9.Frank M O, Sullivan G W, Carper H T, Mandell G L. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1992;36:2584–2588. doi: 10.1128/aac.36.12.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garraffo R, Jambou D, Chichmanian R M, Ravoire S, Lapalus P. In vitro and in vivo ciprofloxacin pharmacokinetics in human neutrophils. Antimicrob Agents Chemother. 1991;35:2215–2218. doi: 10.1128/aac.35.11.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith D A, Jarvis S M. Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins R A, Berlin R D. Purine transport in polymorphonuclear leukocytes. Biochim Biophys Acta. 1969;173:324–337. doi: 10.1016/0005-2736(69)90115-1. [DOI] [PubMed] [Google Scholar]
- 13.Kraupp M, Paskutti B, Schon C, Marz R. Inhibition of purine nucleobase transport in human erythrocytes and cell lines by papaverine. Biochem Pharmacol. 1994;48:41–47. doi: 10.1016/0006-2952(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 14.Loo K C, Cario A C, Zhang F, Walters J D. Regulation of ciprofloxacin uptake in human promyelocytic leukemia cells and polymorphonuclear leukocytes. J Leukoc Biol. 1997;61:619–623. doi: 10.1002/jlb.61.5.619. [DOI] [PubMed] [Google Scholar]
- 15.Malandro M S, Kilberg M S. Molecular biology of mammalian amino acid transporters. Annu Rev Biochem. 1996;65:305–336. doi: 10.1146/annurev.bi.65.070196.001513. [DOI] [PubMed] [Google Scholar]
- 16.Pascual A, Garcia I, Perea E J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1989;33:653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perea E J, Garcia I, Pascual A. Comparative penetration of lomefloxacin and other quinolones into human phagocytes. Am J Med. 1992;92(Suppl. 4A):48–51. doi: 10.1016/0002-9343(92)90309-y. [DOI] [PubMed] [Google Scholar]
- 18.Sanders C C. Microbiology of fluoroquinolones. In: Sanders W E, Sanders C C, editors. Fluoroquinolones in the treatment of infectious diseases. Glenview, Ill: Physicians and Scientists Publishing; 1990. pp. 1–27. [Google Scholar]
- 19.Scott J L. Human leukocyte metabolism in vitro. 1. Incorporation of adenine-8-C14 and formate-C14 into the nucleic acids of leukemic leukocytes. J Clin Investig. 1962;41:67–79. doi: 10.1172/JCI104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rensburg C E J, Joone G, Anderson R. Interactions of the oxygen-dependent antimicrobial system of the human neutrophil with difloxacin, ciprofloxacin, perfloxacin, and fleroxacin in the intraphagocytic eradication of Staphylococcus aureus. J Med Microbiol. 1990;32:15–17. doi: 10.1099/00222615-32-1-15. [DOI] [PubMed] [Google Scholar]
- 21.Williams A M. Nucleic acid metabolism in leukemic human leukocytes. 1. In vitro incorporation by leukocytes from chronic granulocytic leukemia. Cancer Res. 1962;22:314–321. [PubMed] [Google Scholar]