Abstract
Background:
Results from neuroimaging studies suggest that disruptions in flexible decision-making functions in substance-dependent individuals are a consequence of drug-induced neural adaptations. In addicted populations, however, the causal relationship between biobehavioral phenotypes of susceptibility and addiction consequence are difficult to dissociate. Indeed, evidence from animals suggest that poor decision making due to pre-existing biological factors can independently enhance the risk for developing addiction-like behaviors. Neuroimaging studies in animals provide a unique translational approach for the identification of the neurobiological mechanisms that mediate susceptibility to addiction.
Methods:
Here, we used positron emission tomography in rats to quantify regional dopamine D2/3 receptors and metabotropic glutamate receptor 5 (mGluR5) and assessed decision making using a probabilistic reversal learning (PRL). Susceptibility to self-administer cocaine was then quantified for 21 days followed by tests of motivation and relapse-like behaviors.
Results:
We found that deficits specifically in reward-guided choice behavior on the PRL predicted greater escalation of cocaine self-administration behavior, greater motivation for cocaine, and, critically, was associated with higher midbrain D3 receptor availability. Additionally, individual differences in midbrain D3 receptor availability independently predicted the rate of escalation in cocaine-taking behaviors. No differences in mGlu5 receptor availability, responses during tests of extinction or cue-induced reinstatement were observed between the groups.
Conclusions:
These findings indicate that our identified D3-mediated decision-making phenotype can be used as a behavioral biomarker for the assessment of cocaine use susceptibility in human populations.
Keywords: dopamine D3 receptor, cocaine self-administration, decision making, reinforcement learning, metabotropic glutamate receptor 5, addiction susceptibility
Introduction
The ability to adapt choices in response to changes in the external or internal environment is disrupted in individuals dependent upon illicit substances (1–3). Studies in animals have found similar decision-making abnormalities in animals chronically exposed to drugs of abuse (4–7) suggesting that the aberrant decision-making processes observed in substance-dependent individuals are, in part, a consequence of drug exposure. However, decision-making problems that are present prior to any drug exposure are also associated with greater drug-taking behaviors (8, 9) suggesting that variability in decision making could be an informative phenotype for elucidating the neurobiology mediating addiction susceptibility.
Reinforcement learning is the process by which action-outcome associations are acquired, stored and updated to guide dynamic decision-making behavior. Theoretically, decisions are guided by action values generated in the brain through multiple computational steps based on previous actions and outcomes. For example, action values might be updated differently depending on whether that action was performed or not and whether the outcome of the chosen action was appetitive or aversive (10). Reinforcement-learning algorithms can quantify the degree to which these individual computational steps influence choice (11–13), and have been used to interrogate the decision-making processes that are affected in drug exposed humans and animals (7, 14, 15). The reinforcement-learning processes that underlie susceptibility to drug use, however, are not known.
We have reported that disruptions in reward-guided choice behavior during decision-making tasks are associated with greater methamphetamine self-administration (8) and that individual variation in reward-guided choice behavior is related to midbrain D3 receptor availability (16). Midbrain D3 receptor availability is higher in stimulant-dependent individuals compared to control subjects (17–19) and antagonism of D3 receptors reduces drug self-administration in rodents (20, 21). High midbrain D3 receptor availability prior to drug use may, therefore, enhance drug use susceptibility by disrupting reward-mediated decision-making processes.
To investigate this hypothesis and explore the role of metabotropic glutamate receptor 5 (mGluR5) in decision making and addiction susceptibility, we assessed decision making in adult, male rats using a probabilistic reversal learning (PRL) task and quantified in vivo dopamine D2/3 and mGlu5 receptor availability with positron emission tomography (PET). Rats were then trained to self-administer cocaine or saline in 6 h daily sessions for 21 days. We hypothesized that poor performance of rats in the PRL would be associated with reduced reward-guided choice behavior, higher [11C]-(+)-PHNO binding in the midbrain, where binding is exclusively due to D3 receptors (22, 23), and greater cocaine-taking behaviors. Together our study establishes putative links between decision-making performance, dual-tracer PET imaging of dopamine D3 and mGluR5 availability, and subsequent cocaine-taking behaviors.
Materials and methods
Animals
Adult, male Long Evans rats (N=50) were obtained from Charles River (Raleigh, NC) at approximately 6 weeks of age (Supplement).
Probabilistic reversal learning (PRL) training
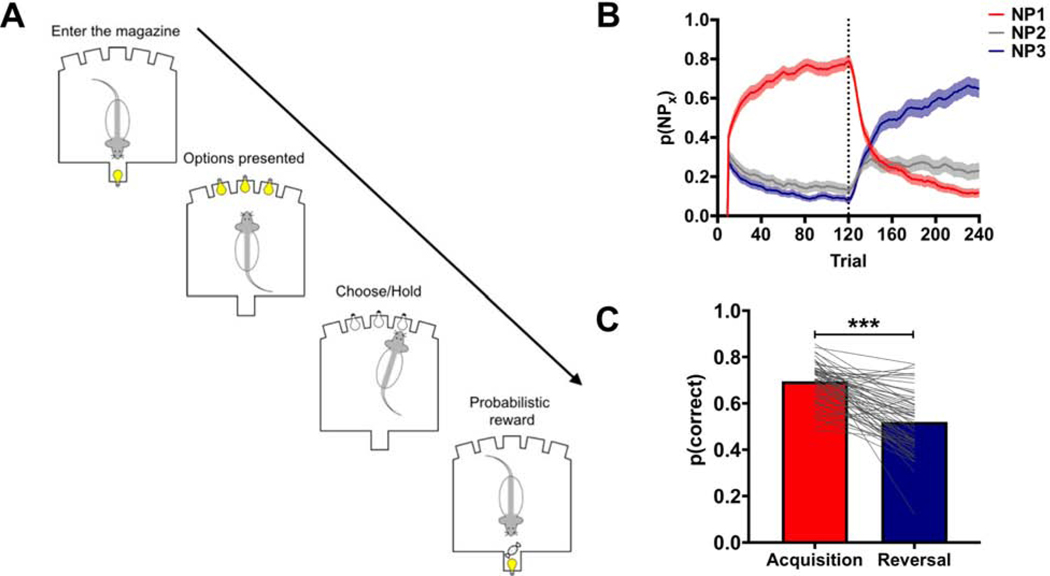
Decision making was assessed on a ‘three-armed bandit’ probabilistic reversal-learning (PRL) task using stochastic reward schedules (Figure 1A; Supplemental Figure 1). On each trial, three noseport apertures were illuminated with one of the apertures being associated with a higher probability of delivering reward than the other two apertures. Reinforcement probabilities assigned to each noseport were pseudo-randomly assigned at the start of each session for individual rats. Rats could make a single choice on each trial by making a noseport entry into the illuminated port. After completing 120 trials (referred to as the Acquisition phase), the probability of reward delivery changed and rats completed 120 additional trials under this new reward schedule (referred to as the Reversal phase).
Figure 1:
The probabilistic reversal learning (PRL) task. (A) A diagram outlining the events of a single trial in the PRL task. (B) The choice behavior of rats in the PRL. Rats were able to acquire (red line) and reverse (blue line) their choices in the PRL using a 10-trial moving average. The gray line is the probability of rats choosing the noseport associated with an intermediate probability of reinforcement. (C) The probability of choosing the highest reinforced option was significantly lower following the reversal. *** p<0.001. Related to Supplemental Figure 1.
Decision making in the PRL was assessed using two different schedules of reinforcement: the PRL high probability (referred to as the PRL-HP) schedule had a larger dynamic range than the PRL low probability (referred to as the PRL-LP) schedule (Supplemental Figure 1). Decision making was assessed in six sessions using either the PRL-HP schedule (session 1, 3, and 5) or the PRL-LP schedule (session 2, 4, and 6). Performance of rats in the PRL-HP was strongly correlated with that in the PRL-LP (Supplemental Figure 1), so dependent measures were collapsed across the two schedules of reinforcement.
Outcome-based measures
To characterize the decision-making processes responsible for differences in PRL performance, we examined how previous trial outcomes and choices influenced choice behavior on the current trial. First, we calculated the probability that rats would stay after a rewarded and correct response and the probability that rats would switch their choice after an unrewarded and incorrect response (24). To determine if the differences we observed using this single-trial back analysis extended to more distant trials in the past, choice behavior was then analyzed with a logistic regression that included choices and outcomes from trials in the recent history (t-1 through t-4). This model quantifies the degree to which choices and outcomes (rewarded or unrewarded) on the previous four trials influence current choice behavior (7, 24). Regression coefficients estimate the change in the likelihood of repeating the same choice relative to an arbitrary baseline. Positive regression coefficients indicate that the rats are more likely to persist with the same choice, whereas negative regression coefficients indicate that rats are more likely to shift their choice.
Reinforcement-learning model
The choice behavior of rats in the PRL also was analyzed with a differential forgetting reinforcement-learning model (10, 24, 25), which captures gradually decaying effects of previous choices and outcomes relationships more formally than those captured by the outcome-based measures described above. This model fits the choice behavior of rats significantly better than other reinforcement-learning models (see Supplemental methods and Supplemental Table 1). This reinforcement-learning model, described in detail in the Supplement, contains four free parameters: a decay rate for the action values of chosen options (γC), a decay rate for the action values of unchosen options (γU), a parameter for the appetitive strength of rewarded outcomes (Δ+), and a parameter for the aversive strength of unrewarded outcomes (Δ0).
PET Imaging and Processing
Once rats completed the PRL testing (~6–8 weeks), they underwent PET scans to quantify D2/3 receptor and mGluR5 availability using [11C]-(+)-PHNO and [18F]FPEB, respectively, during one serial PET scanning session which was collected ~1 week before starting the cocaine or saline self-administration procedure. The procedures for acquiring and processing the dynamic PET data are described in the Supplement. Activity concentration was extracted from six regions of interest (ROI): the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), ventral striatum (VS), dorsal striatum (DS), midbrain and cerebellum. These regions were selected based on previous work that has observed differences in [11C]-(+)-PHNO or [18F]FPEB binding in substance-dependent individuals (18, 19, 26, 27). Analyses of [11C]-(+)-PHNO binding were restricted to the VS, DS and midbrain given evidence of negligible specific binding in the prefrontal cortex (28). Time-activity curves from each ROI were fitted with the multilinear reference tissue model (MRTM) using activity from the cerebellum as the reference region (29, 30) to provide estimates of BPND, R1 and k2’. The primary outcome measure for both radiotracers was BPND, which is directly proportional to the number of receptor sites available for radioligand binding (31).
Self-administration procedures
Rats were implanted with intrajugular catheters as previously described (8) and were trained to self-administer cocaine (0.5 mg/kg/infusion) or saline in 6 h daily sessions for 21 days (Supplement). Rats were trained on a fixed ratio (FR) 1 schedule for three days to establish operant responding. The operant requirement was then changed to a FR3 schedule for the additional 18 self-administration days.
Characterizing latent drug-taking phenotypes
The number of drug infusions each rat earned across the 21 days of self-administration were fitted with a power function, as we have previously described (8):
where x is the session number during the self-administration procedure (1≤ × ≤ 21) and A and B are parameters estimating the scaling factor and rate of growth, respectively. The A parameter determines the initial strength of the drug-taking behavior and the B parameter determines the rate of growth of the function (Supplemental Figure 3).
Assessing addiction-relevant behaviors
Additional tests of drug-taking and -seeking behaviors were examined following the 21 d self-administration procedure using procedures previously described (32–34). Motivation to obtain an infusion of cocaine or saline was assessed under a progressive ratio schedule and drug-seeking behavior assessed in a cue-induced reinstatement test as described in the Supplement.
Statistical analyses
Statistical analyses are described in the Supplement.
Results
Poor reversal learning is associated with disruptions in reward-mediated updating
Decision making was assessed in adult, male rats (N=50) on a ‘three-armed bandit’ probabilistic reversal-learning (PRL) task (Figure 1A) using stochastic reward schedules (Supplemental Figure 1). Rats were able to track these dynamic reinforcement probabilities (Figure 1B) and, as expected, chose the most frequently reinforced option significant less following a change in reward probabilities compared to their performance in the acquisition phase (Figure 1C; χ2=62.05; p<0.001). Performance in the reversal phase, however, was still significantly above chance (t(49)=10.85; p<0.001) demonstrating that rats, on average, were able to adaptively adjust their choices following the change in reinforcement contingencies.
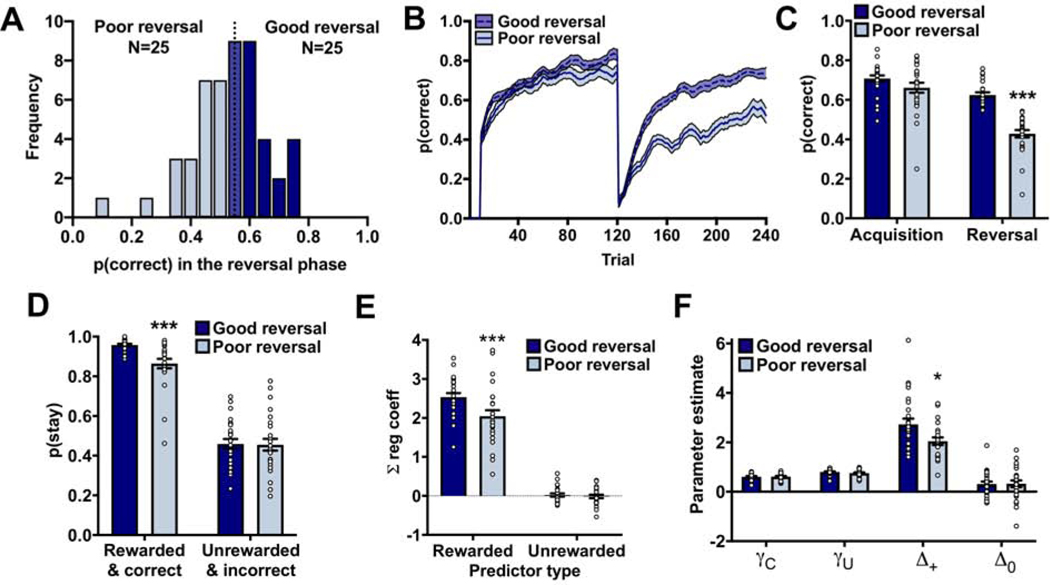
There was, however, a large amount of variation in the performance of rats during the reversal phase of the PRL (Figure 2A) that we hypothesized may be related to susceptibility to drug use. Rats were divided into two groups based on a median split of performance in the reversal phase (Figure 2A) into rats with good reversal performance (referred to as ‘good reversal’; N=25; mean: 0.63±0.01; dark blue) and rats with poor reversal performance (referred to as ‘poor reversal’; N=25; mean: 0.43±0.02; light blue). The performance of rats in the acquisition phase did not differ between these groups (χ2=2.70; p=0.10) indicating that rats in the good and poor reversal groups were able to acquire the discrimination to a similar degree. As expected when using a median split design, reversal performance was significantly worse in the poor reversal group compared to the good reversal group (group: χ2=65.68; p<0.001; Figure 2B-C).
Figure 2:
Differences in reversal performance are due to differences in action-value updating following positive feedback. (A) Frequency distribution of the performance of rats in the reversal phase. Rats were divided into two groups based on a mean-split: rats who chose the correct noseport with a probability greater than the group average (good reversal; dark bars; N=25) and rats who chose the correct noseport with a probability less than the group average (poor reversal; light bars; N=25). (B) The choice behavior of rats in the good and poor reversal group in the PRL using a 10-trial moving average. (C) Rats in the poor reversal group were able to acquire the initial discrimination, but had greater difficult following the reversal. (D) The poor reversal group were less likely to stay with a rewarded and correct response compared to the good reversal group. No group differences were observed in the probability that rats would stay with an unrewarded and incorrect response. (E) The logistic regression model containing a ‘Reward’ and ‘No reward’ predictor indicated that the poor reversal group were less likely to use positive feedback from recent trial history to guide their current choice compared to the good reversal group. No group differences were detected for the ‘No reward’ regression coefficients. (F) The reinforcement-learning algorithm indicated that the poor reversal group had a selective reduction in the Δ+, as no differences were detected in the other parameter estimates. * p<0.05; *** p<0.001. Related to Supplemental Figure 2.
This disparity in reversal performance observed between the good and poor reversal groups could be driven by impairments in select reinforcement-learning mechanisms. The probability that rats would persist with a rewarded and correct response and the probability that rats would switch after an unrewarded and incorrect response was compared between the groups (8). The poor reversal group was significantly less likely to persist with a rewarded and correct choice compared to the good reversal group (χ2=27.96; p<0.001; Figure 2D). No group differences were observed in the probability that rats would persist with an unrewarded and incorrect choice (χ2=0.01; p=0.92) indicating that poor reversal performance was specifically due to deficits in reward-based updating.
To determine whether this impairment extended into the recent history of choices and outcomes (e.g., t-1 to t-4) the regression coefficients for the ‘Reward’ and ‘No reward’ predictors in the logistic regression model were examined between the good and poor reversal groups. There was a significant group x outcome x trial interaction (χ2=10.69; p=0.01; Supplemental Figure 2) and post-hoc analyses indicated that the regression coefficients for the ‘Reward’ predictor were significantly lower in the poor reversal group compared the good reversal group (group: χ2=7.32; p=0.007; Figure 2E; Supplemental Figure 2). Consistent with the single-trial back analysis, the regression coefficients for the ‘No reward’ predictor did not differ between the groups (group × trial: χ2=0.37; p=0.55).
Reinforcement-learning models predict that choices based on action values are adjusted incrementally according to previous actions and outcomes over multiple trials. Such models might be more sensitive to subtle changes in the effect of previous outcomes on decision-making performance compared to the logistic regression analyses presented above. Choice data during the reversal phase of the PRL was fit with a differential forgetting reinforcement-learning model and parameter estimates compared between the groups. Post-hoc analyses of the group x parameter interaction (χ2=8.14; p=0.04) indicated that the Δ+ parameter was significantly lower in rats with poor reversal performance compared to those with good reversal performance (χ2=6.19; p=0.01; Figure 2F). No other significant group differences were detected (χ2<1.19; p>0.27). These results, collectively, indicate that the disparity in reversal performance observed between the good and poor reversal groups is due specifically to differences in updating of reward-guided choice behavior.
Poor reversal learning is associated with high midbrain D3 receptor availability
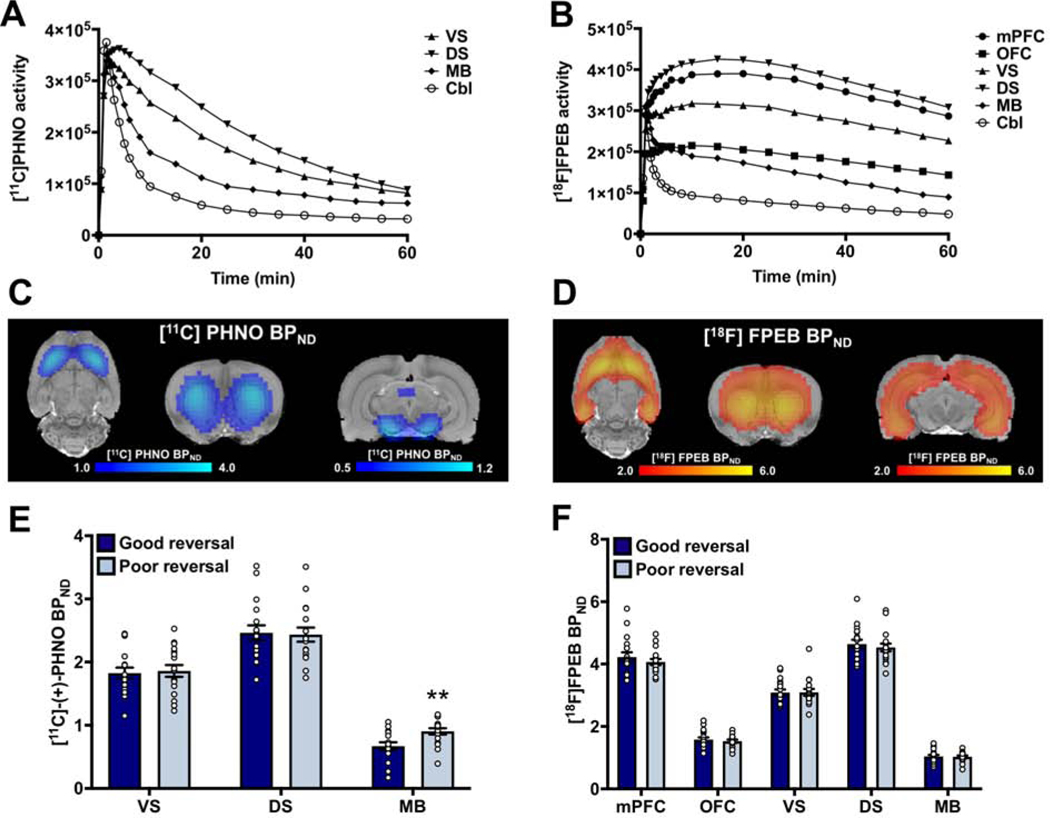
Reconstructed, three-dimensional [11C]-(+)-PHNO and [18F]FPEB PET images were co-registered to a rat MR template and activity extract from six regions of interest to generate time activity curves (Figure 3A,B). The distribution of [11C]-(+)-PHNO and [18F]FPEB observed in our rats was similar to that previously reported (16, 30) and binding potential maps revealed anatomical differences in non-displaceable binding potential (BPND) that are consistent with the known distribution of dopamine D3 receptors and mGluR5 (Figure 3C,D).
Figure 3:
Poor reversal performance is associated with higher midbrain D3 BPND. (A-B) Average time activity curves in the six regions of interest extracted from either (A) [11C]PHNO or (B) [18F]FPEB dynamic images. (C-D) Binding potential maps showing the distribution of (C) [11C]PHNO and (D) [18F]FPEB binding in the rat brain. (E) [11C]PHNO BPND in the midbrain was significantly greater in the poor reversal group compared to good reversal group. (F) No differences in [18F]FPEB BPND were observed between good and poor reversal groups. ** p<0.01; Abbreviations: mPFC – medial prefrontal cortex; OFC – orbitofrontal cortex; VS – ventral striatum; DS – dorsal striatum; MB – midbrain; Cbl – cerebellum.
We have previously reported that reversal performance is negatively correlated with midbrain D3 receptors (16). To determine if midbrain D3 BPND differed in this sample of rats, [11C]-(+)-PHNO BPND across the three regions of interest was compared between the good and poor reversal groups. Post-hoc analyses following a significant group x brain region interaction (χ2=8.75; p=0.01) indicated that midbrain [11C]-(+)-PHNO BPND was significantly higher in the poor reversal group compared to the good reversal group (group: χ2=9.45; p=0.002; Figure 3E), thus confirming our previous results. No significant differences were detected for the other brain regions (group: all χ2<0.07; p>0.79). We then examined whether mGluR5 BPND differed between the good and poor reversal groups. The group x brain region interaction and the effect of group, however, were not significant (group x brain region: χ2=3.56; p=0.47; group: χ2=0.20; p=0.66; Figure 3F).
Poor reversal learning is associated with greater cocaine self-administration
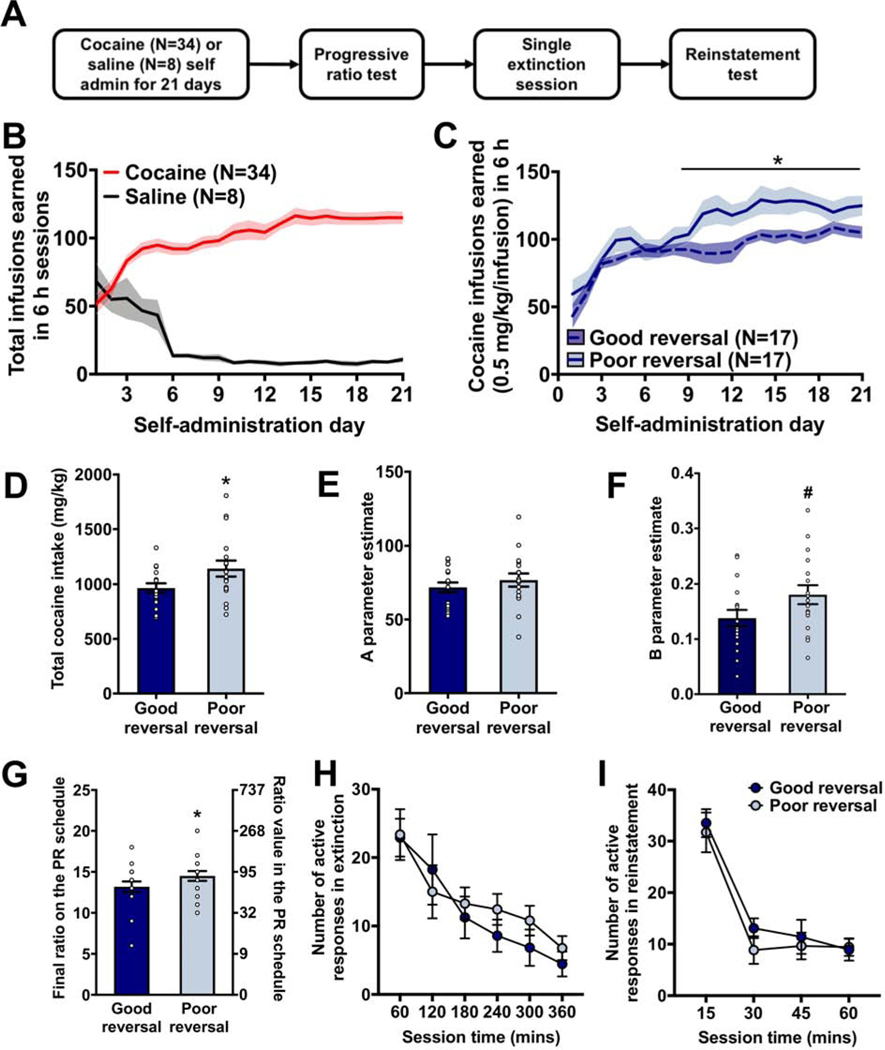
Following the dual-tracer PET imaging, rats were implanted with intrajugular catheters and trained to self-administer cocaine (N=34) or saline (N=8) in 6 h daily sessions for 21 days (Figure 4A). The number of infusions rats earned across the 21 days significantly increased in the cocaine group (χ2=180; p<0.001), whereas the number of infusions earned decreased in the saline group (χ2=971; p<0.001; Figure 4B).
Figure 4:
Poor reversal performance is associated with a greater escalation in cocaine intake. (A) The behavioral experiments used for assessing addiction-relevant behaviors. (B) The number of infusions earned increased in the cocaine self-administration group and decreased in the saline self-administration group across the 21 6 h daily sessions. (C) The rate of increase in the number of cocaine infusions earned across the 21 self-administration sessions was significantly greater in the poor reversal group compared to the good reversal group. Group differences emerged on the 9th session and persisted until the 21st session. (D) The total number of infusions earned across the 21 sessions was significantly greater in the poor reversal group compared to the good reversal group. (E-F) The power function revealed that initial cocaine reinforcement was similar between the good and poor reversal groups but that the (E) rate of escalation in cocaine self-administration was higher in the poor reversal group. (G) The final schedule achieved in the progressive ratio (PR) test was higher in the poor reversal group. No group differences were observed in the number of responses rats made in the extinction test (H) or in the cue-induced reinstatement test (I). * p<0.05.
Cocaine self-administration was then compared between the good and poor reversal rats. The number of cocaine infusions animals earned in each daily session increased across the 21 days of self-administration but the rate of increase was significantly higher in the poor reversal group (group x day: χ2=60.63; p<0.001; Figure 4C). Rats in the poor reversal group took significantly more cocaine than rats in the good reversal group across the 21 d of self-administration (χ2=4.66; p=0.03; Figure 4D). Moreover, this effect was significant when reversal performance was entered as a covariate (day x reversal p(correct): χ2=37.35; p=0.01) rather than as a discrete factor.
The cocaine self-administration data for individual rats was fit with a power function to estimate the initial reinforcement of cocaine (e.g., A parameter) and the rate of escalation in drug use (e.g., B parameter) as we have previously described (8). The A parameter did not differ between the groups (χ2=0.84; p=0.36; Figure 4E), but a difference between the groups was observed for the B parameter (χ2=3.70; p=0.05; Figure 4F): rats in the poor reversal group escalated their cocaine intake at a rate greater than rats in the good reversal group.
We then examined whether there were differences in responding under a progressive ratio (PR) schedule between the good and poor reversal groups. Rats in the poor reversal group reached a higher final ratio on the PR schedule (χ2=3.37; p=0.06; Figure 4G) and made significantly more active lever responses compared to rats in the good reversal group (χ2=4.63; p=0.03). However, this heightened response rate was not specific to the cocaine-paired lever as we also observed more inactive lever responses in the poor reversal group compared to the good reversal group (χ2=5.15; p=0.02). These results indicate that rats in the poor reversal group had a greater level of motivation to obtain a cocaine infusion compared to rats in the good reversal group.
We then examined whether differences in reversal performance prior to drug use were associated with differences in responding under extinction and a reinstatement test. The number of active presses during the extinction test did not differ between the groups (χ2=0.06; p=0.81; Figure 4H). One rat in the good reversal group was excluded from the analysis of the reinstatement test because the number of active lever responses (e.g., 881) was more than 10 standard deviations from mean. Regardless, responding of rats in the cue-induced reinstatement test did not differ between the good and poor reversal groups (χ2=0.20; p=0.66; Figure 4I).
Midbrain D3 BPND is associated with reward-mediate updating of choices and rate of escalation in drug use
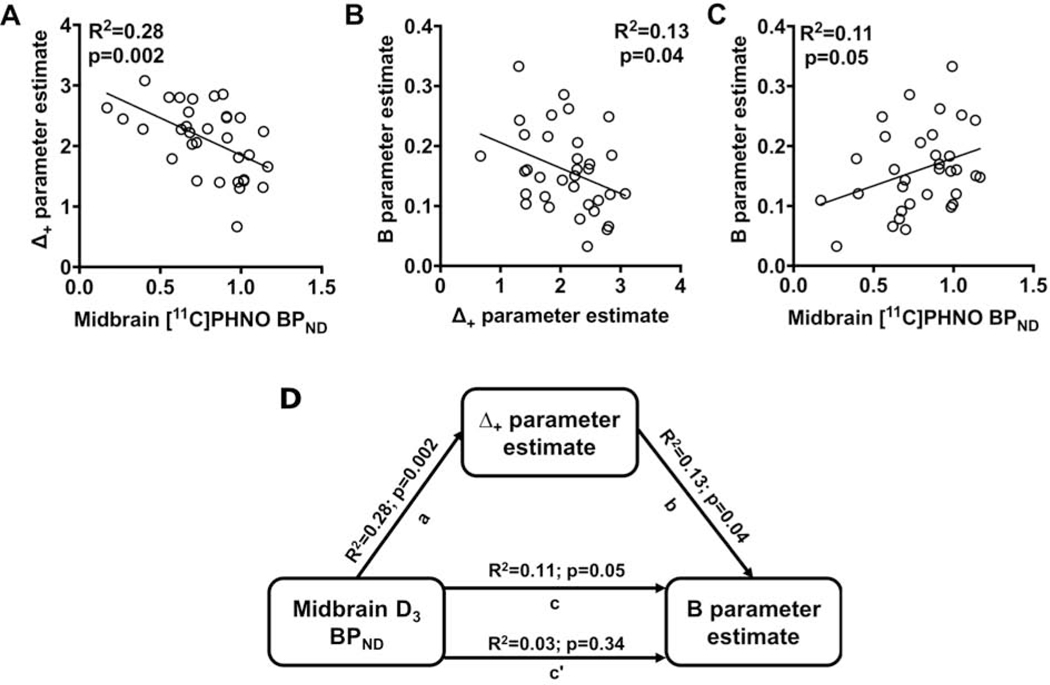
The results presented here indicate that poor reversal learning is associated with reduced reward-mediated updating of behavior, higher midbrain D3 BPND, and greater escalation in cocaine intake. These data suggested to us that midbrain D3 BPND may be a mechanistic point of convergence between reward-guided decision making and susceptibility to drug use. To investigate this possibility directly, we examined the relationships between midbrain [11C]-(+)-PHNO BPND, the Δ+ parameter and cocaine self-administration parameters. Consistent with our previous findings (16), midbrain [11C]-(+)-PHNO BPND was negatively related to the Δ+ parameter (R2=0.28; p=0.002): as midbrain [11C]-(+)-PHNO BPND increased across rats, the Δ+ parameter decreased (Figure 5A). We also observed a negative relationship between the Δ+ parameter and the B parameter estimate (R2=0.13; p=0.04; Figure 5B). As expected, midbrain D3 BPND was positively related to the B parameter estimate (R2=0.11; p=0.05; Figure 5C).
Figure 5:
Midbrain D3 receptor BPND is correlated with the Δ+ parameter and the rate of escalation in cocaine intake. (A) Individual differences midbrain [11C]-(+)-PHNO BPND are negatively correlated to variation in the Δ+ parameter. (B) Variation in the Δ+ parameter predicts the rate of escalation in cocaine intake (e.g., B parameter). (C) Midbrain [11C]-(+)-PHNO BPND predicts the rate of escalation in cocaine intake (e.g., B parameter). (D) A causal mediation analysis of midbrain [11C]-(+)-PHNO BPND, the Δ+ parameter, and the rate of escalation in cocaine intake (e.g., B parameter). When the Δ+ parameter is included as a mediator of the relationship between midbrain [11C]-(+)-PHNO BPND and the B parameter, the strength of this relationship was reduced (path c’). However, the indirect was not significant suggesting that the Δ+ parameter only partially mediated the relationship between [11C]-(+)-PHNO BPND and the B parameter.
To further explore the relationships between midbrain [11C]-(+)-PHNO BPND, reward-mediated updating and escalation in drug use (e.g., the B parameter), a mediation analysis was conducted using multiple regressions (35). The positive relationship between midbrain [11C]-(+)-PHNO BPND and the B parameter (R2=0.11; p=0.05) was attenuated when the Δ+ parameter was included in the model (R2=0.03; p=0.34). Although the total effect was significant (t=2.28; p=0.03), the indirect effect was not (95% CI: −0.025–0.3826) suggesting that the Δ+ parameter only partially mediated the relationship between midbrain [11C]-(+)-PHNO BPND and the B parameter.
Discussion
In the current study, we provide new evidence that heightened midbrain D3 BPND is associated with deficits in reward-guided decision making and predicts greater susceptibility to cocaine-taking behavior. Using a dynamic decision-making task and computational approaches, we demonstrate that poor reversal learning performance is associated with impairments in reward-mediated updating of choice behavior, higher midbrain D3 BPND and greater escalation in cocaine self-administration. Moreover, we provide direct evidence that relates midbrain D3 BPND with reward-mediated updating of choice and escalation in cocaine self-administration, which offers mechanistic insight into the biobehavioral mechanisms of addiction susceptibility. Together, these results indicate that the midbrain D3 receptor may be a unique biomarker of cocaine use susceptibility.
Disruptions in decision making predict greater susceptibility to drug use
Drug-induced neural adaptations are believed to be the mechanism by which disruptions in adaptive, flexible decision making emerge in substance-dependent individuals. Our data, however, indicate that poor decision-making prior to drug exposure is associated with an enhanced susceptibility to cocaine use. Specifically, we found that rats with poor reversal performance escalated their cocaine intake at a rate significantly greater than rats with good reversal performance. Higher rates of drug use escalation are associated with an increased risk for problematic drug use in humans (36–39) and, therefore, likely to be a meaningful predictor of addiction liability. These findings are consistent with previous work in mice (9) and suggest that the decision-making deficits observed in substance-dependent individuals may, in part, have been present prior to initiation of drug use.
The decision-making deficits observed in the poor reversal group was specific to reward-mediated updating: rats with poor reversal performance were less likely to persist with a rewarded, correct response and had a lower computationally-derived Δ+ parameter. Moreover, individual differences in the Δ+ parameter were negatively related to the rate of escalation in cocaine use, which was consistent with our previous findings (8). This work, collectively, suggests that individual differences in reward-guided choice behavior could serve a non-invasive behavioral phenotype for assessing psychostimulant susceptibility in humans. Indeed, a recent study in occasional recreational stimulant users reported that reward-mediated choice behavior in a Risky Gains Task predicted risk for future stimulant use disorder (40).
The precise decision-making factors that confer risk for cocaine use may differ from those decision-making processes that are disrupted by persistent drug use. Emerging evidence indicates that choice behavior following negative feedback is selectively disrupted in substance-dependent individuals (41–43), as well as in animals following psychostimulant self-administration (7, 8, 15). We propose, based on the current data, that individual differences in action-value updating that follows positive feedback mediates the initial stages of drug use by regulation of drug intake over time. In contrast, drug-induced changes in action-value updating that follows negative feedback may contribute to the development of compulsive drug-taking behaviors that emerge after extended drug use. We plan to test this hypothesis by examining the relationship between compulsive drug-taking behaviors and drug-induced changes in negative feedback updating measured by the PRL task. These select reinforcement-learning mechanisms are controlled by anatomically distinct orbitofrontal circuits (24) that may provide critical insights into the neural circuits underlying addiction.
Dopamine D3 receptors and susceptibility to drug use
We hypothesized that high D3 receptor density would increase drug use susceptibility by disrupting flexible, adaptive decision making (16). Here, we provide direct evidence supporting this hypothesis. Rats with poor reversal performance had greater midbrain D3 BPND and a higher rate of escalation in cocaine intake compared to those rats with good reversal performance. Our findings suggest that high midbrain D3 BPND that has been observed in stimulant-dependent populations (17–19) may have been present prior to drug use and contributed to the development of addiction. D3 selective antagonists reduce drug self-administration (21, 44, 45), conditioned place preference (20, 46) and relapse-like behaviors in rodent models (20, 47, 48) that also argues for a critical role of the D3 receptor in addiction-relevant behaviors. The mechanism by which midbrain D3 BPND impacts prefrontal-mediated, decision-making processes is unknown, but there is evidence that greater midbrain D3 receptor availability is associated with reduced functional connectivity between the orbitofrontal cortex and cognitive control networks (49). Midbrain D3 receptors act as autoreceptors and regulate the release of dopamine in striatal regions (50). Individuals with higher midbrain D3 receptor availability may have reduced dopamine tone in key dopaminergic circuits that regulate decision making (51). Blunted dopamine transmission has been hypothesized to mediate addiction liability (52), but there is evidence that amphetamine-induced dopamine release in the striatum is positively related to self-report measures of impulsivity (53), a known predictor of addiction. Studies that combine temporally discrete measures of dopamine dynamics with PET neuroimaging and drug self-administration in animals could provide critical insights into the role of midbrain D3 receptors and cocaine use susceptibility.
The lack of group differences in striatal [11C]-(+)-PHNO binding was surprising based on our previous work (54, 55). We did, however, observe a non-significant negative relationship between [11C]-(+)-PHNO binding in the dorsal striatum – where binding is almost exclusively reflective of D2 receptors (22, 23) – and the rate of escalation in cocaine intake (R= −0.26; p=0.13; Supplemental Figure 4). Future [11C]-(+)-PHNO PET studies that use D2 selective antagonist to isolate the D3 receptor signaling could help determine the role that D3 receptors in other brain regions play in addiction-relevant behaviors.
We found that the decision-making processes that enhance susceptibility to cocaine use was specifically associated with greater midbrain D3 receptor availability, as mGluR5 BPND did not differ between the good and poor reversal groups. The lack of differences in mGluR5 BPND was somewhat surprising as pharmacological manipulations of mGluR5 signaling alter reversal learning (56) and spatial learning (57), as well as reducing drug self-administration (58, 59). Previous PET studies have reported that mGluR5 BPND is lower in cocaine-dependent individuals (26, 27) and decreases in rats following cocaine self-administration (60). Disruptions in mGluR5 signaling, therefore, could be the mechanism contributing to cocaine-induced decision-making deficits.
In summary, this study demonstrates that D3-mediated disruptions in reward-guided choice behavior predict susceptibility to cocaine use in rats. These results integrate human-based neuroimaging and computational approaches with measures of addiction-relevant behaviors in rats. This adds to a growing body of work indicating that D3 receptor dysregulation contributes to the behavioral mechanisms underlying addiction susceptibility and provides a rationale for translational investigations into vulnerable populations.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Chemical Compound or Drug | Cocaine hydrochloride | NIDA Drug Supply | ||
| Chemical Compound or Drug | [11C]-(+)-PHNO | Yale PET Center | Gallezot et al., 2012 | |
| Chemical Compound or Drug | [18F]FPEB | Yale PET Center | Hamill et al., 2015 | |
| Organism/Strain | Long Evans male rats | Charles River Laboratory | RRID:RGD_2308852 | |
| Software; Algorithm | Matlab (2019a) | Mathworks | RRID:SCR_001622 | |
| Software; Algorithm | Med PC | Med Associates | RRID:SCR_014721 |
Acknowledgements and Disclosures
We thank and acknowledge the exceptional technical assistance provided by Cynthia Santaniello, Courtney Chabina, Amanda Harsche, Jessica Pursi, and Dayshalis Ofray. We also thank the Yale PET Center and Dr. Irina Esterlis for radioligand support, as well as the Drug Supply Program at the National Institute on Drug Abuse for providing cocaine HCl. This research was supported by Public Health Service grants from the National Institute on Drug Abuse (Grant Numbers: DA041480 and DA043443 [to JRT]), the National Institute on Alcohol Abuse and Alcoholism (Grant Number: K01AA024788 [to ATH]), a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (to SMG) and funding provided by the State of Connecticut.
SMG and JRT were responsible for the conceptualization. SMG, KF, and DH were responsible for investigation. AH and HL were responsible for software. SMG was responsible for analysis and writing the original draft. SMG, AH, HL, KF, DH, EM, DL, and JRT were responsible for writing, reviewing and editing. All authors approved the final version of the manuscript.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ (2008): Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacol, 2008/01/25. 197: 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fillmore MT, Rush CR (2006): Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol, 2005/09/22. 20: 24–32. [DOI] [PubMed] [Google Scholar]
- 3.Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED (2011): Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology, 2011/02/04. 36: 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jentsch JD, Olausson P, De La Garza R 2nd, Taylor JR (2002): Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology, 2002/01/16. 26: 183–190. [DOI] [PubMed] [Google Scholar]
- 5.Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, et al. (2012): Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 32: 5843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B (2004): Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci, 2004/04/14. 19: 1997–2002. [DOI] [PubMed] [Google Scholar]
- 7.Groman SM, Rich KM, Smith NJ, Lee D, Taylor JR (2018): Chronic Exposure to Methamphetamine Disrupts Reinforcement-Based Decision Making in Rats. Neuropsychopharmacology. 43: 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groman SM, Massi B, Mathias SR, Lee D, Taylor JR (2019): Model-Free and Model-Based Influences in Addiction-Related Behaviors. Biol Psychiatry. 85: 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes MC, Laughlin RE, Jentsch JD (2013): Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology (Berl). 229: 515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barraclough DJ, Conroy ML, Lee D (2004): Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci, 2004/03/09. 7: 404–410. [DOI] [PubMed] [Google Scholar]
- 11.Rangel A, Camerer C, Montague PR (2008): A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 9: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dayan P, Daw ND (2008): Decision theory, reinforcement learning, and the brain. Cogn Affect Behav Neurosci. 8: 429–453. [DOI] [PubMed] [Google Scholar]
- 13.Lee D (2013): Decision making: from neuroscience to psychiatry. Neuron, 2013/04/30. 78: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlé KM, Zhang S, Schiff M, Mackey S, Paulus MP, Yu AJ (2015): Altered Statistical Learning and Decision-Making in Methamphetamine Dependence: Evidence from a Two-Armed Bandit Task. Front Psychol. 6: 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhukovsky P, Puaud M, Jupp B, Sala-Bayo J, Alsiö J, Xia J, et al. (2019): Withdrawal from escalated cocaine self-administration impairs reversal learning by disrupting the effects of negative feedback on reward exploitation: a behavioral and computational analysis. Neuropsychopharmacology. 44: 2163–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groman SM, Smith NJ, Petrullli JR, Massi B, Chen L, Ropchan J, et al. (2016): Dopamine D3 Receptor Availability Is Associated with Inflexible Decision Making. J Neurosci. 36: 6732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. (2012): Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 32: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, et al. (2014): Heightened D(3) dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [(11)C]-(+)-PHNO. Neuropsychopharmacology. 39: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matuskey D, Gallezot J-D, Pittman B, Williams W, Wanyiri J, Gaiser E, et al. (2014): Dopamine D₃ receptor alterations in cocaine-dependent humans imaged with [11C](+)PHNO. Drug Alcohol Depend. 139: 100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorel SR, Ashby CR, Paul M, Liu X, Hayes R, Hagan JJ, et al. (2002): Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 22: 9595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You Z-B, Gao J-T, Bi G-H, He Y, Boateng C, Cao J, et al. (2017): The novel dopamine D3 receptor antagonists/partial agonists CAB2–015 and BAK4–54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology. 126: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. (2010): Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 68: 392–9. [DOI] [PubMed] [Google Scholar]
- 23.Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. (2009): In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 63: 782–93. [DOI] [PubMed] [Google Scholar]
- 24.Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, et al. (2019): Orbitofrontal Circuits Control Multiple Reinforcement-Learning Processes. Neuron. 103: 734–746.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M, Doya K (2009): Validation of decision-making models and analysis of decision variables in the rat basal ganglia. J Neurosci, 2009/08/07. 29: 9861–9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NBL, Perez A, et al. (2014): Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry. 75: 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milella MS, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, et al. (2014): Limbic system mGluR5 availability in cocaine dependent subjects: A high-resolution PET [11C]ABP688 study. Neuroimage. 98: 195–202. [DOI] [PubMed] [Google Scholar]
- 28.Egerton A, Hirani E, Ahmad R, Turton DR, Brickute D, Rosso L, et al. (2010): Further evaluation of the carbon11-labeled D(2/3) agonist PET radiotracer PHNO: reproducibility in tracer characteristics and characterization of extrastriatal binding. Synapse. 64: 301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groman SM, Smith NJ, Petrullli JR, Massi B, Chen L, Ropchan J, et al. (2016): Dopamine D 3 Receptor Availability Is Associated with Inflexible Decision Making. J Neurosci. 36: 6732–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Laat B, Leurquin-Sterk G, Celen S, Bormans G, Koole M, Van Laere K, Casteels C (2015): Preclinical Evaluation and Quantification of 18F-FPEB as a Radioligand for PET Imaging of the Metabotropic Glutamate Receptor 5. J Nucl Med. 56: 1954–9. [DOI] [PubMed] [Google Scholar]
- 31.Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. (2003): Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab, 2003/09/16. 23: 1096–1112. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009): Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology, 2008/08/19. 56 Suppl 1: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR (2010): Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 30: 4401–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torregrossa MM, Sanchez H, Taylor JR (2010): D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 30: 10526–10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron RM, Kenny DA (1986): The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 36.Worhunsky PD, Dager AD, Meda SA, Khadka S, Stevens MC, Austad CS, et al. (2016): A Preliminary Prospective Study of an Escalation in ‘Maximum Daily Drinks’, Fronto-Parietal Circuitry and Impulsivity-Related Domains in Young Adult Drinkers. Neuropsychopharmacology. 41: 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Vergara HI, Spillane NS, Merrill JE, Jackson KM (2016): Developmental trends in alcohol use initiation and escalation from early to middle adolescence: Prediction by urgency and trait affect. Psychol Addict Behav. 30: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucker JS, Ellickson PL, Orlando M, Martino SC, Klein DJ (2005): Substance use Trajectories from Early Adolescence to Emerging Adulthood: A Comparison of Smoking, Binge Drinking, and Marijuana use. J Drug Issues. 35: 307–332. [Google Scholar]
- 39.Derefinko KJ, Charnigo RJ, Peters JR, Adams ZW, Milich R, Lynam DR (2016): Substance Use Trajectories From Early Adolescence Through the Transition to College. J Stud Alcohol Drugs. 77: 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair MA, Stewart JL, May AC, Reske M, Tapert SF, Paulus MP (2018): Blunted Frontostriatal Blood Oxygen Level–Dependent Signals Predict Stimulant and Marijuana Use. Biol Psychiatry Cogn Neurosci Neuroimaging. 3: 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LHE, Luijten M, et al. (2016): Carrots and sticks fail to change behavior in cocaine addiction. Science. 352: 1468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parvaz MA, Konova AB, Proudfit GH, Dunning JP, Malaker P, Moeller SJ, et al. (2015): Impaired Neural Response to Negative Prediction Errors in Cocaine Addiction. J Neurosci. 35: 1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA (2003): Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 53: 65–74. [DOI] [PubMed] [Google Scholar]
- 44.Xi Z-X, Gilbert JG, Pak AC, Ashby CR, Heidbreder CA, Gardner EL (2005): Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 21: 3427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thanos PK, Katana JM, Ashby CR, Michaelides M, Gardner EL, Heidbreder CA, Volkow ND (2005): The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav. 81: 190–7. [DOI] [PubMed] [Google Scholar]
- 46.Ashby CR, Paul M, Gardner EL, Heidbreder CA, Hagan JJ (2003): Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 48: 154–6. [DOI] [PubMed] [Google Scholar]
- 47.Song R, Bi G-H, Zhang H-Y, Yang R-F, Gardner EL, Li J, Xi Z-X (2014): Blockade of D3 receptors by YQA14 inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology. 77: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Hagan JJ, et al. (2004): Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl). 176: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, et al. (2012): Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 22: 2784–93. [DOI] [PubMed] [Google Scholar]
- 50.Koeltzow TE, Xu M, Cooper DC, Hu X-T, Tonegawa S, Wolf ME, White FJ (1998): Alterations in Dopamine Release But Not Dopamine Autoreceptor Function in Dopamine D3 Receptor Mutant Mice. J Neurosci. 18: 2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gobert A, Lejeune F, Rivet J-M, Cistarelli L, Millan MJ (2002): Dopamine D3 (Auto)receptors Inhibit Dopamine Release in the Frontal Cortex of Freely Moving Rats In Vivo. J Neurochem. 66: 2209–2212. [DOI] [PubMed] [Google Scholar]
- 52.Trifilieff P, Ducrocq F, van der Veldt S, Martinez D (2017): Blunted Dopamine Transmission in Addiction: Potential Mechanisms and Implications for Behavior. Semin Nucl Med. 47: 64–74. [DOI] [PubMed] [Google Scholar]
- 53.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. (2010): Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci, 2010/03/17. 13: 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, et al. (2011): Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 31. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groman SM, James AS, Seu E, Tran S, Clark TA, Harpster SN, et al. (2014): In the Blink of an Eye: Relating Positive-Feedback Sensitivity to Striatal Dopamine D2-Like Receptors through Blink Rate. J Neurosci. 34: 14443–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Zhu Y, Kraniotis S, He Q, Marshall JJ, Nomura T, et al. (2013): Potentiating mGluR5 function with a positive allosteric modulator enhances adaptive learning. Learn Mem. 20: 438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fowler SW, Walker JM, Klakotskaia D, Will MJ, Serfozo P, Simonyi A, Schachtman TR (2013): Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents. Neurobiol Learn Mem. 99: 25–31. [DOI] [PubMed] [Google Scholar]
- 58.Paterson NE, Semenova S, Gasparini F, Markou A (2003): The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl). 167: 257–64. [DOI] [PubMed] [Google Scholar]
- 59.Platt DM, Rowlett JK, Spealman RD (2008): Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl). 200: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Laat B, Weerasekera A, Leurquin-Sterk G, Bormans G, Himmelreich U, Casteels C, Van Laere K (2018): Glutamatergic Biomarkers for Cocaine Addiction: A Longitudinal Study Using MR Spectroscopy and mGluR5 PET in Self-Administering Rats. J Nucl Med. 59: 952–959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.